Abstract
Background
Blocking CD40-CD40L costimulatory signals induces transplantation tolerance. While B cell depletion prevents alloantibody formation, non-humoral functions of B cells in tolerance have not been well characterized. We investigated whether specific subsets of B cell or B cell derived IL-10 contribute to tolerance.
Methods
Wild type C57BL/6, or B cell specific IL-10−/− (CD19-Cre+/−::IL-10fl/fl) mice, received vascularized BALB/c cardiac allografts. BALB/c donor-specific splenocyte transfusion (DST) and anti-CD40L mAb were used as tolerogen. B cells were depleted with anti-mouse CD20 mAb. Various B cell subsets were purified and characterized by flow cytometry, RT-PCR, and adoptive transfer.
Results
B cell depletion prevented co-stimulatory blockade induced allogeneic tolerance. Costimulatory blockade increased IL-10 in marginal zone precursor (MZP) B cells, but not other subsets. In particular, costimulatory blockade did not change other previously defined regulatory B cell subsets (Breg), including CD5+CD1dhi Breg or expression of TIM1 or TIM4 on these Breg or other Breg cell subsets. Costimulatory blockade also induced IL-21R expression in MZP B cells, and IL-21R+ MZP B cells expressed even more IL-10. B cell depletion or IL-10 deficiency in B cells prevented tolerance in a cardiac allograft model, resulting in rapid acute cardiac allograft rejection. Adoptive transfer of wild type MZP B cells but not other subsets to B cell specific IL-10 deficient mice prevented graft rejection.
Conclusion
CD40 costimulatory blockade induces MZP B cell IL-10 which is necessary for tolerance. These observations have implications for understanding tolerance induction and how B cell depletion may prevent tolerance.
Introduction
Many T cell costimulatory receptor-ligand interactions have been identified (CD28-CD80, CD28-CD86, CTLA-4-ICOS, CD27-CD70, CD134-OX40L and CD40L-CD40), and costimulatory blockade has been used to induce tolerance in murine as well as in non-human primate models (1). In particular, blockade of CD40-CD40L suppresses alloimmunity and induces long-term tolerance to skin, islet, bone marrow, heart, kidney, myoblast and limb allografts (1). CD40 is expressed on B cells, DC, macrophages, epithelial cells, hematopoietic progenitors and activated T cells; whereas CD40L (CD154) is expressed on activated T cells, activated B cells and activated platelets (2). During inflammation, peripheral blood monocytes, human vascular endothelial cells, smooth muscle cells and mononuclear phagocytes may also express CD40L (2). Costimulatory blockade induced tolerance can be potentiated through administration of alloantigen, such as DST, to induce peripheral tolerance to alloantigen (3). It has been proposed that CD40-CD40L blockade induces peripheral tolerance by inhibiting APC maturation, T cell activation, and allo- and auto-antibody production while promoting the generation of regulatory T cells (1). Based on these observations, some investigators have shown that B cell depletion also partially inhibits alloantigen presentation and alloantibody production, thereby promoting graft survival (4, 5). In contrast, others have found evidence that B cells may promote graft survival or tolerance (6–8). The role of B cells in co-stimulatory blockade induced transplantation tolerance is not fully understood.
B cell functions include antibody production, antigen presentation to T cells, secretion of pro- and anti-inflammatory cytokines, help for T cell repertoire development and maintenance, and lymphoid organogenesis. Alloantibodies produced by B cells are clearly involved in the pathogenesis of graft rejection, and depletion of B cells has been suggested as a therapeutic approach to prevent or treat rejection (9). However, there are additional ways B cells may influence tolerance. i) B cells can tolerize antigen specific CD8+ T cells directly via CD95-mediated activation induced deletion (10). ii) Activated B cells presenting antigen via MHC class I can induce anergy in CD8+ T cells (11). iii) B cells help in the induction of Foxp3+Treg (12). iv) Activated B cells with increased surface expression of B7-2 inhibit proliferation of self-reactive CD4+ T cells in a CD40-CD40L dependent manner (13). v) B cells control the antigen presenting function of DCs in a cytokine dependent manner, increasing tolerogenic responses (14). vi) B cell secretion of IgG linked with latent TGFβ (IgG-TGFβ) inhibits CTL function in an antigen nonspecific manner (15). vii) A subset of IL-10 producing CD1dhiCD5+ B cells in mice (16, 17) and CD19+CD24+CD38+ B cells in humans (18) has protective function in autoimmune diseases (19). However, how the non-humoral functions of B cells contribute to the generation of costimulatory blockade induced alloantigen specific tolerance is not known.
In the present study we showed that depletion of B cells inhibited the development of costimulatory blockade induced transplantation tolerance, leading to acute cellular rejection of allogeneic cardiac allografts. Costimulatory blockade specifically induced greater numbers of IL-10+ MZP cells, but not other putatively tolerogenic Breg subsets, and IL-21R+ MZP B cells produced even more IL-10. MZP B cells were required for tolerance, and transfer of this subset specifically restored graft survival.
Materials and Methods
Mice
BALB/c (H-2d), C57BL/6 (H-2b), CD19-cre (H-2b) and CCR6−/− (H-2b) mice 8–10 weeks old were purchased from The Jackson Laboratory. Human CD20 transgenic (hCD20Tg; H-2d) mice were from Dr. Mark I Shlomchik (Yale University, New Haven, CT). IL-10fl/fl (H-2b) mice were from Dr. Christopher Karp (Cincinnati Children’s Hospital Research Foundation, Cincinnati, OH). CD19Cre+/−:IL-10fl/fl (B-IL-10−/−) mice were generated by breeding CD19-Cre+/+ with IL-10fl/fl mice. All mice were housed in a specific pathogen-free facility in microisolator cages. All experiments used age- and sex-matched mice in accordance with protocols approved by the Institutional Animal Care and Utilization Committee.
Antibodies and reagents
Anti-mouse CD4 (GK1.5), anti-mouse CD8 (53.67), anti-mouse B220 (RA3-6B2), PE-anti-mouse CD19 (MB19-1), APC anti-mouse B220 (RA3-6B2), APC-Cy7 anti-mouse CD11c (N418), APC-Cy7 anti-mouse CD1d (1B1), PE-Cy7 anti-mouse CD5 (53-7.3), PE anti-mouse GR-1 (RB6-8C5), Pacific Blue anti-mouse CD45 (30F-11), PE-anti-mouse IgM (II/41), PE anti-mouse CD11b (M1/70), PE anti-mouse CD8 (53-6.7), APC anti-mouse CD4 (GK1.5), FITC anti-mouse CD25 (PC61.5), biotin anti-mouse TIM-1 (RMT1-4), PE-anti-mouse TIM-4 (RMT4-54), PE anti-mouse/rat Foxp3 (FKJ-16s) antibodies and isotype control antibodies were purchased from eBioscience (San Diego, CA). FITC anti-mouse CD93 (AA4.1), PE/Cy7 anti-mouse CD21/CD35 (7E9), PE-anti-mouse CD23 (B3-B4), Pacific Blue anti-mouse IgD (11-26c 2A), BV421 anti-mouse IL-10 (JES5-16E3), and biotin anti-mouse IgM (MRM-47) were purchased from Biolegend, (San Diego, CA). Rabbit anti-mouse Foxp3 polyclonal antibody and MOMA-1 were purchased from Abcam (San Francisco, CA). Anti-mouse CD20 mAb (clone 5D2, mouse IgG2a) was received from Genentech, Inc. (San Francisco, CA). Cy3-donkey anti-rat, FITC-donkey anti-rat, Cy5-donkey anti-rat, Cy3-goat anti-hamster, Cy5-anti-hamster, Cy5-goat anti-rabbit antibodies and fluorochrome conjugated streptavidin were purchased from Jackson Immunoresearch Laboratory, Inc. (West Grove, PA). Anti-mouse CD40L (MR-1) and control isotype antibodies were purchased from BioXcell (West Lebanon, NH). 4′,6-diamidino-2-phenylindole (DAPI) were purchased from Invitrogen (Carlsbad, CA). Anti-mouse CD19 (clone 1D3; American Type Culture Collection, Manassas, VA) and mouse anti-human CD20 mAb (clone 2H7; from Dr. Mark I Schlomchik, Yale University) were purified in our laboratory.
Cardiac transplantation and treatment protocols
Transplantation tolerance was induced by administering 1 X 107 BALB/c DST on day −7 prior to transplant and anti-CD40L mAb (days −7, −4, 0 and +4; 250 μg/injection i.v.) to C57BL/6 recipients of BALB/c donor vascularized cardiac allografts (3). B cells were depleted by one i.v. injection (d +1; 100μg/mouse) of anti-mouse CD20 mAb (5D2), anti-mouse CD19 mAb (1D3) or isotype control mAb in C57BL/6 mice or anti-human CD20 mAb (2H7) in hCD20Tg mice. Graft function was monitored every other day by abdominal palpation.
Isolation of cells from LN, spleen and grafts
Cardiac grafts were perfused with PBS, minced and digested with 1 mg/ml collagenase D (Roche, Indianapolis, IN) in RPMI medium for 45 minutes at 37°C. Single cell suspensions from spleen, lymph nodes (LN) and graft were made, and RBC lysed using ACK lysis buffer (Lonza, Walkerville, MD).
Cell staining and flow cytometric analysis
Staining of cells was performed with the indicated antibodies with 1μg/106 cells at 4°C for 30 minutes. We purified MZP B cells using a 100 μm nozzle at an event rate of 10,000–12,000 cells/second using a FACS ARIA II sorter (BD Bioscience, Mountainview, CA). Trypan Blue staining of cells showed more than 98% viable cells, and purity was found to be more than 98% for each subset. Data were acquired using the FACSCantoII, LSR Fortessa, or LSRII flow cytometer (BD Biosciences), and analyzed using FlowJo software (Tree Star Inc., Ashland, OR).
Quantitative real-time RT PCR (qRT-PCR)
Cells were lysed in TRIzol reagent (Invitrogen), total RNA was purified, and cDNA was made using oligo d(T)12-14 primer and Omniscript RT kit (Invitrogen) as per manufacture’s guidelines. mRNA expression was quantified by CFX96 thermal cycler (Bio-Rad, Hercules, CA) or 7900 fast real-time PCR (Applied Biosystem, Carlsbad, CA) using SYBR Green PCR kit (Qiagen, Valencia, CA). PCR consisted of a 15 minutes at 95°C denaturation step, followed by 40 cycles of 15sec at 94°C, 20sec at 56°C, 20sec at 72°C. Relative mRNA expression of specific gene was calculated as: 2(Ct of cyclophilin-A - Ct of IL-10). The primers used for real-time PCR: mouse IL-10, forward 5′-GGGTTGCCAAGCCTTATCGGAAAT-3′ and reverse 5′-CCTTGATTTCTGGGCCATGCTTCT-3′; mouse cyclophilin-A, forward 5′-AGGGTGGTGACTTTACACGC-3′ and reverse 5′-ATCCAGCCATTCAGTCTTGG-3′.
Histopathological analysis
Cardiac grafts, spleens and LN were harvested, frozen directly in Optimal Cutting Temperature (OCT) compound (Sacura Finetek, Torrance, CA), and stored at −80°C. 8 μm sections were cut with a Leica 1900CM cryomicrotome, fixed with chilled acetone, blocked with 2.5% normal horse serum (Vector Laboratories, Burlingame, CA), stained with the indicated primary antibodies for 30 minutes, stained with conjugated secondary reagents, and mounted with VectaShield mounting solution (Vector Laboratories) with or without DAPI. Images were acquired with a Leica fluorescence microscope (Leica Mikrosysteme, Vertrieb, Germany) and a digital Hamamatsu CCD camera (Hamamatsu Corporation, Bridgewater, NJ). Separate images were collected on the CY3, GFP, and DAPI channels, overlaid, and analyzed with Openlab software (Improvision, Lexington, MA) and Leica MMF software. Quantification of graft infiltrating cells was performed by counting 4–5 fields per tissue section and 3–4 sections per graft.
For hematoxylin and eosin (H&E) staining, PBS buffered formalin fixed tissues were embedded in paraffin, sectioned at 5 μm and H&E staining performed. Parenchymal rejection score of graft was performed as described earlier (20).
Based on modified protocol published from International Society for Heart and Lung Transplantation, severity of histopathology of allograft can be accessed with hematoxylin and eosin (20–22). The Parenchymal rejection (PR) Scoring was performed as: 0= No rejection; 1= Focal mononuclear cell infiltration without necrosis; 2= Focal mononuclear cell infiltrates with necrosis; 3= Multi-focal infiltrates with necrosis; 4= Wide spread infiltrates with hemorrhage and/or vasculitis.
Luminex ELISA
Sera were collected and stored at −80 °C. Cytokines were quantified using the Bio-Plex Pro Mouse Cytokine Th1/Th2 assay kit (Bio-Rad). Assays were performed according to the manufacturer’s instructions and analyzed on a Bio-Plex 200 System (Bio-Rad).
Adoptive transfer of MZP B cells
Different subsets of B cells were purified from wild-type C57BL/6 or B-IL-10−/− spleens using flow cytometry and gating on specific populations as shown in Figure S1A. Purity of B cell subset was typically 90–99%. Sorted cells were used either for adoptive transfer or analysis of mRNA expression.
Intracellular cytokine staining
Single cell suspension from spleen was prepared, RBCs removed by ACK lysis buffer treatment. Splenocytes (2 X 106 cells/well) were stimulated with PMA (50 ng/ml), ionomycin (500 ng/ml) and monensin (2 μM) for 5 hours in 24 well plates in complete RPMI medium containing 10% FBS at 37°C in 5% CO2 incubator. Cells were stained for surface markers, fixed with fixation buffer (Biolegend) and permeabilized with 1X permeabilization buffer (Biolegend). Intracellular IL-10 staining was performed, washed with PBS, and cells acquired by flow cytometry (FACS Canto, BD Bioscience). Data were analyzed using FlowJo software.
Statistics
Graft survivals were graphically expressed using the Kaplan-Meier method and statistical differences were assessed by Log-rank (Mantel-Cox) test using Prism 6 software (GraphPad software, La Jolla, CA). Unpaired, two tailed Student t test was performed and p < 0.05 was considered statistically significant.
Results
B cell depletion prevents tolerance
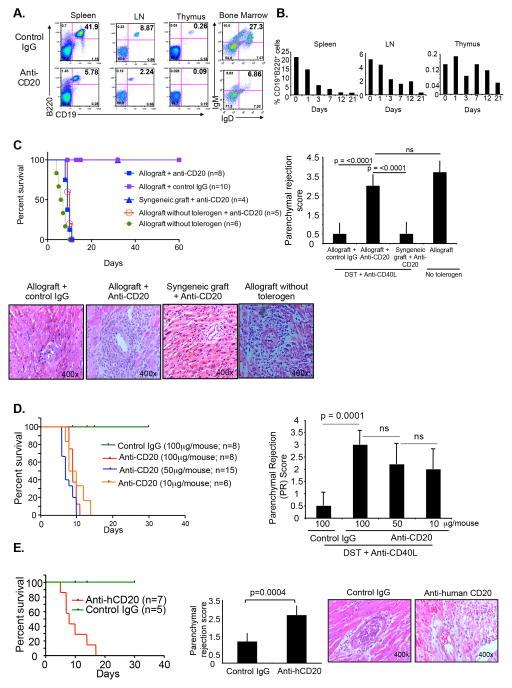
To understand the function of the B cells in costimulatory blockade induced tolerance, we investigated different B cell depleting strategies. A single i.v. injection of 100 μg of rat anti-mouse CD20 mAb (5D2) into C57BL/6 mice depleted ~75% of total CD19+ B cells in the spleen and LN and CD19+IgM+IgD+ B cells in bone marrow within 3 days (Figure 1A). This depletion persisted for at least 3 weeks (Figure 1B). Compared to isotype control mAb, even as little as 10 μg anti-CD20 depleted CD19+ B cells among total lymphocytes in spleen within 48 hours (Figure S1B). Depletion with 10μg anti-CD20 mAb was distributed across all subsets of B cells in the spleen and LN (Figure S1B). Injection of 25 μg or 100 μg anti-CD20 mAb also uniformly depleted all B cell subsets in the spleen and LN (Figure S1B, S1C, and data not shown). Depletion of B cells in presence of the allograft and tolerogen also gave a similar B cell depletion profile (not shown). A single injection of anti-human CD20 mAb (2H7) in hCD20Tg mice also depleted B cells in spleen and LN (Figure S2A). B cell depletion did not induce significant changes in the numbers of CD11c+ DCs, plasmacytoid DCs, CD4+ T cells or Foxp3+ regulatory CD4 T cells in the spleen or LN (Figure S2B).
Figure 1. B cell depletion prevents tolerance.
(A) C57BL/6 mice i.v. injected with anti-mCD20 mAb (100μg/mouse) or isotype control IgG. After 3 days, CD19+B220+ cells in the spleen, LN and thymus analyzed by flow cytometry. In bone marrow, IgM+IgD+ cells analyzed by gating on CD19+ cells. Based on FSC-A vs. SSC-A profile, all the cells gated on the lymphocytic population. (B) C57BL/6 mice i.v. injected with anti-mCD20 mAb (100μg/mouse). CD19+B220+ cells as a percentage of total lymphocytes in spleen, LN and thymus analyzed by flow cytometry at different time points after gating on lymphocytic population. (C) C57BL/6 recipient mice given tolerogen (DST, 1 X 107 cells/mouse d−7 relative to transplantation; and anti-CD40L mAb 250 μg/mouse d−7, −4, 0 and +4) and received BALB/c cardiac allografts or C57BL/6 syngeneic grafts on d0. Recipients given anti-mCD20 mAb (100μg/mouse) d+1. Graft survival monitored (left). After 5 days, grafts harvested, sections made and stained with hematoxylin and eosin (H & E), and parenchymal rejection scores calculated (right). H & E staining of graft (bottom). (D) C57BL/6 recipients given tolerogen and BALB/c allografts as described above, and various doses of anti-mCD20 mAb on d +1. Graft survival plotted (left). After 5 days, grafts harvested, sections stained with H & E, and parenchymal rejection scores calculated (right). (E) Human CD20 transgenic (H-2d) mice given tolerogen and C57BL/6 (H-2b) cardiac allografts. Anti-human CD20 mAb (100μg/mouse) given i.v. on d+1. Graft survival (left), Masson’s trichrome staining and parenchymal rejection score calculated (middle), and H & E staining on d+5 (right).
To investigate the role of B cells in tolerance, C57BL/6 mice received DST day −7; anti-CD40L mAb days −7, −4, 0, +4; and BALB/c vascularized cardiac allografts on day 0. Depletion of B cells with anti-mouse CD20 mAb 1 day after transplantation prevented tolerance and lead to acute rejection with increased parenchymal rejection (PR) scores in the allograft, while B cell depletion did not cause rejection of syngeneic grafts (Figure 1C). Dose-response analysis showed that as little as 10 μg of anti-mouse CD20 mAb induced rejection and increased PR scores (Figure 1D). Similarly, hCD20Tg (H-2d, BALB/c background) mice received the tolerogenic regimen (C57BL/6 DST and anti-CD40L mAb) and C57BL/6 vascularized grafts. Depletion of B cells with anti-human CD20 mAb in hCD20Tg mice also resulted in acute rejection (mean survival time (MST) = 8 days, n=7 mice) (Figure 1E). Injection of anti-mouse CD19 mAb (1D3), which caused partial B cell depletion, also prevented tolerization of C57BL/6 recipients (MST=12.5 days, n=8 mice) (data not shown). Together, these results demonstrated that B cell depletion with different antibodies and in different strains prevented costimulatory blockade induced tolerance and led to acute rejection.
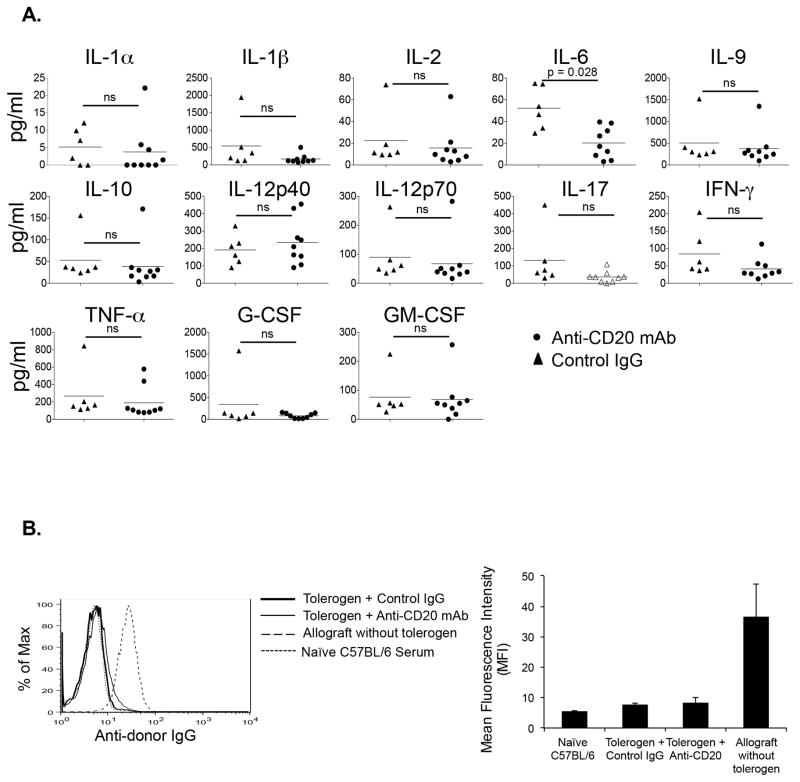
B cell depletion induced graft rejection is not due to serum cytokine storm or alloantibodies
To test if depletion of B cell lead to strong inflammatory cytokine production, we administered tolerogen, transplanted allografts, and depleted B cells as above. Five days after transplantation, various cytokines were analyzed in the serum. The results showed no significant change in almost all inflammatory or anti-inflammatory cytokines tested (Figure 2A). There was reduction in serum IL-6 after B cell depletion compared to control IgG treated animals, which would not be expected to impair graft survival since IL-6 promotes Th17 and interferes with Treg induction (23).
Figure 2. B cell depletion does not cause serum cytokine storm or alloantibody responses.
(A) C57BL/6 recipients given tolerogen and BALB/c allografts or C57BL/6 syngeneic grafts on d0, and anti-mCD20 mAb (100μg/mouse) d+1. 5 days after transplantation, sera collected and cytokine levels quantitated by Luminex bead assay. (B) Sera from animals in Figure 1C collected 5 days after transplantation and alloantibody responses measured by flow cytometry. Representative histogram (left) and mean fluorescence intensity (MFI) (right). n= 3–5 mice/group.
We also measured the presence of alloantibody in B cell depleted mice. The results showed that, as expected, B cell depletion did not result in altered alloantibody levels compared to nondepleted mice (Figure 2B). Together, these results showed that B cell depletion in presence of costimulatory blockade did not induce an inflammatory cytokine storm or alloantibody in the serum.
B cell depletion leads to acute cellular rejection of allograft
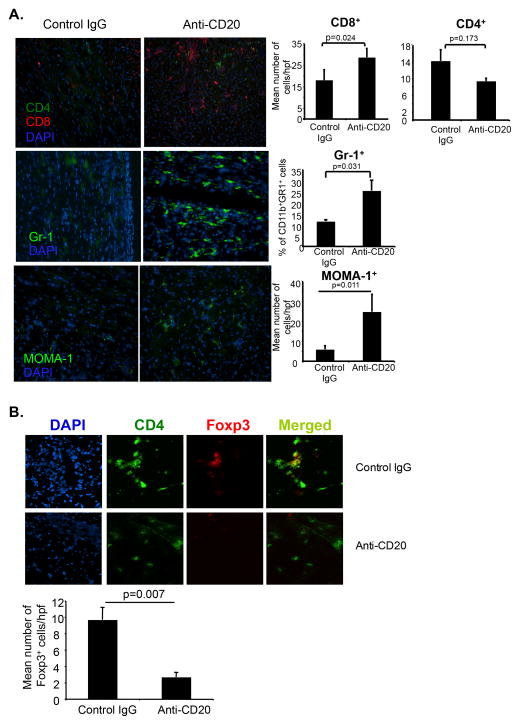
Since histopathological analysis of rejecting donor allografts showed mononuclear cell infiltration and increased PR scores in the allografts (Figures 1C and 1D), we further characterized the allograft infiltrating cells. Flow cytometric analysis revealed a significantly increased leukocytic infiltration (193.8±32.9 X 104 vs. 73.7±18.7 X 104 total leukocytes/heart, p=0.019). Immunohistological analysis showed increased infiltration of GR1+ monocytes, MOMA-1+ cells and CD8+ T cells (Figure 3A). There were no significant changes in the number of CD4+ T cells, but rejecting grafts had decreased numbers of Foxp3+Treg compared to tolerated grafts (Figure 3B). No infiltration of B cells, as expected, or CD11c+ DCs was observed in the allograft (not shown). Flow cytometric analysis of MOMA-1+ cells showed a phenotype of FSChiSSChiB220−CD11c−CD11b+Gr1int/lo (Figure S1C) suggesting a monocytic lineage. Microscopic analysis of isolated cells showed increased cytoplasmic content indicative of activated macrophages (Figure S1D). It has been reported that under inflammatory conditions CD11b+Gr1+ myeloid cells promote Th17 cell differentiation (24); and MOMA-1+ (CD169) macrophages cross-presents antigen from dead cells to CD8+ T cells (25), suggesting that this population might contribute to alloantigen presentation and graft rejection. Together these results showed that B cell depletion induced increased infiltration of CD8+ T cells and MOMA-1+ macrophages in the rejecting allografts.
Figure 3. B cell depletion causes acute cellular rejection with increased infiltration in the allograft.
(A) C57BL/6 recipients given tolerogen, BALB/c allografts and anti-mCD20 mAb as in Figure 1C. Grafts harvested 5 days after transplantation. Immunohistochemistry for graft infiltrating cells. Magnification 200× (left). Quantitative data (right). (B) Immunohistochemistry and quantitation of graft infiltrating CD4+ and Foxp3+ cells. Magnification 400×. 3 grafts/group, 3 sections/graft, 3–4 fields/section.
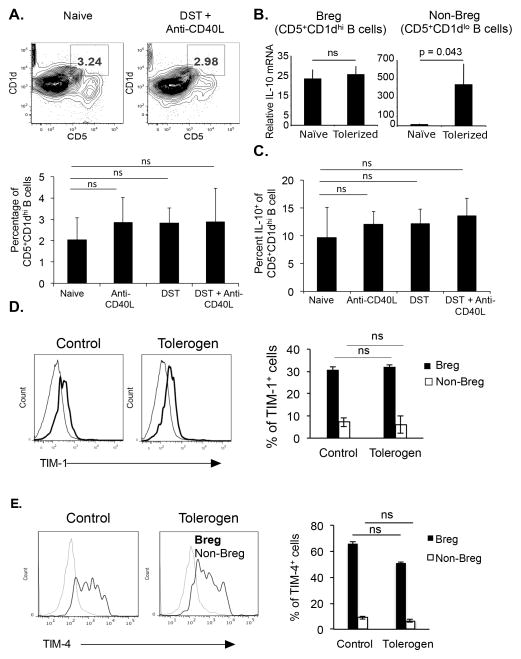
Costimulatory blockade does not change CD19+CD1dhiCD5+ Breg
It has been shown that IL-10 producing CD1dhiCD5+ B cells function as suppressor Breg to prevent the development of autoimmune diseases (16). Tolerogen treatment did not induce significant changes in the percentage of CD1dhiCD5+ Breg cells in spleen (Figure 4A), LN, or bone marrow (data not shown). The expression of IL-10 in these cells did not change with tolerogen treatment (Figures 4B and 4C). In contrast, tolerogen increased IL-10 mRNA expression in non-Breg cells (B220+CD19+CD5−CD1dlo B cells) (Figure 4B). It has been shown that TIM-1+ B cells produce IL-10 and help in the maintenance of tolerance (26). TIM-1 and TIM-4 expression on CD19+CD5+CD1dhi B cells was detected, however their expression did not change after tolerogenic treatment in Breg or non-Breg cells (Figures 4D and 4E). Thus, the tolerogenic regimen did not change the percentage of CD5+CD1dhi Breg nor their expression of molecules known for their suppressive function.
Figure 4. Costimulatory blockade does not increase the CD19+CD5+CD1dhi Breg.
(A) C57BL/6 given no treatment (naïve), DST, anti-CD40L mAb, or DST + anti-CD40L mAb; and after 5 days frequency of CD5+CD1dhi B cells among all lymphocytes in the spleen analyzed after gating on B220+CD19+ cells (upper). Mean percentage of CD5+CD1dhi cells among all CD19+ cells (lower). 5 mice/group. (B) C57BL/6 mice given tolerogen, and after 5 days, B220+CD19+CD5+CD1d+ Breg and B220+CD19+CD5+CD1d− non-Breg cells purified from spleen, and IL-10 mRNA expression assessed by qRT-PCR. (C) Splenocytes were stimulated with PMA and ionomycin, intracellular IL-10 stained, and analyzed by gating on CD19+CD5+CD1dhi cells. 5 mice/group. (D and E) C57BL/6 mice given tolerogen, and after 5 days TIM-1 and TIM-4 expression analyzed on CD19+CD5+CD1d+ Breg or CD19+CD5+CD1d− non-Breg in spleen. 4 mice/group.
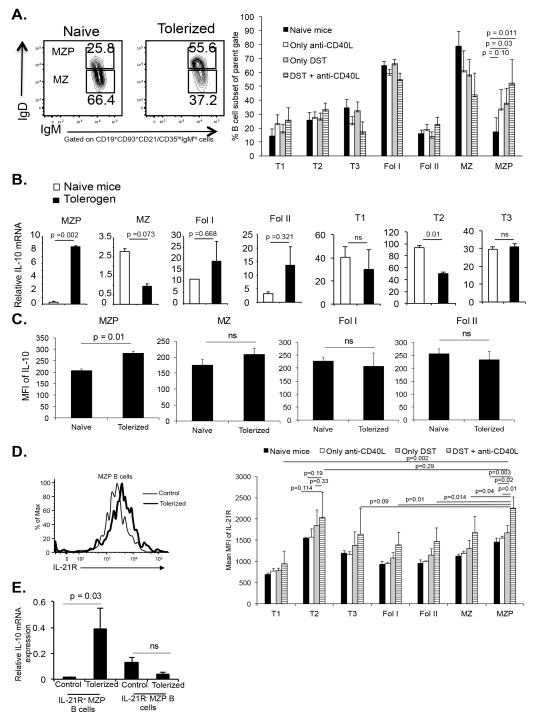
Co-stimulatory blockade increases the percentage of MZP B cells and their IL-10 expression
Since there was no change in CD5+CD1dhi Breg after toleorgen administration, and since there was increased IL-10 mRNA expression in non-Breg cells, we next enumerated multiple other B cell subsets in naïve and tolerized mice. The various B cell subsets were gated and defined as shown in Figure S1A. The results showed that there were significant increases in the percentage of splenic marginal zone precursor (MZP) B cell subsets comparing naïve controls and nontolerized mice (Figure 5A), while other subsets were unchanged and there was a nonsignificant decrease in marginal zone (MZ) B cells. Further analysis of these non-Breg cells showed an increase in IL-10 in the MZP B cell subset (CD19+CD23+sIgMhisIgDhiCD21/CD35hi) at both the transcriptional and translational levels, but no increases in IL-10 in other subsets (Figures 5B and 5C). Anti-CD20 caused depletion of this subset and all other B cell subsets without preferential subset effects (Figure S1B). These results revealed that costimulatory blockade increased both the percentage of the MZP B cell subset and its IL-10 expression.
Figure 5. Costimulatory blockade increases MZP B cells and their IL-10 expression.
C57BL/6 mice given no treatment (naïve), DST, anti-CD40L or DST + anti-CD40L (tolerogen), and after 5 days different subsets of B cells from spleen were analyzed. (A) Contour plots show the changes in MZP and MZ cells (left). B cell subset percentages (right) calculated based on gating scheme in Supplementary Figure S1A. 4 mice/group. (B) IL-10 mRNA expression assessed by qRT-PCR. Error bar is standard deviation. (C) Splenocytes (2 X 106 cells/well) stimulated with PMA and ionomycin, intracellular IL-10 stained, and flow gated on various B cell subsets. 5 mice/group. (D) IL-21R expression analyzed on MZP and other B cell subsets. Representative histogram of IL-21R expression on MZP B cells (left). MFI of IL-21R expression on various subsets (right). 4 mice/group. (E) IL-21R+ and IL-21R− B cells purified from control or tolerized mice and IL-10 mRNA analyzed by qRT-PCR. Error bar is standard deviation.
It has been reported that IL-21 together with CD40 regulate B cell expression of IL-10 (27, 28). MZP B cells from tolerogen treated mice had significantly increased IL-21R expression compared to other B cell subsets and compared to nontolerogenic treatment conditions (Figure 5D). The IL-21R+ MZP B cells expressed enhanced IL-10 mRNA compared to IL-21R− MZP B cells (Figure 5E), suggesting that IL-21 signaling in the MZP B cells was involved in IL-10 expression.
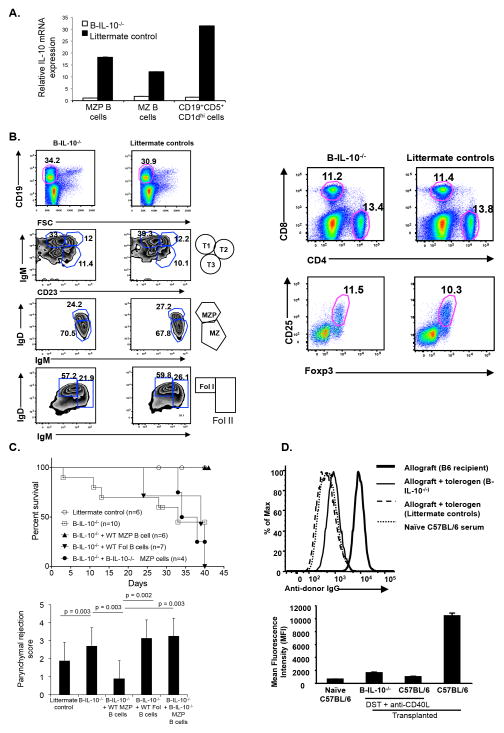
Deficiency of IL-10 in B cells prevents costimulatory blockade induced tolerance
To test if IL-10 producing B cells were involved in DST plus anti-CD40L mAb induced tolerance, we generated CD19Cre+/−::IL-10fl+/+ (B-IL-10−/−) mice which specifically lacked IL-10 expression in B cells (Figure 6A). These mice had normal B cell development (Figure 6B) and normal CD4+, CD8+, and Foxp3+ CD4 Treg compared to littermate controls (Figure 6B). B-IL-10−/− mice were transplanted and received tolerogen, yet deficiency of IL-10 in B cells prevented tolerance in B-IL-10−/− mice, but not in littermate controls (Figure 6C). Rejecting B-IL-10−/− mice had increased parenchymal rejection scores suggestive of T cell mediated rejection, but did not have enhanced alloantibody responses to account for the increased incidence of rejection (Figure 6D). Importantly, adoptive transfer of MZP B cells at the time of transplantation only from IL-10 sufficient littermate mice, but not IL-10 deficient MZP B cells or IL-10 sufficient follicular B cells into B-IL-10−/−, rescued graft survival (Figure 6C). Parenchymal rejection scores of the allograft were also significantly reduced with the adoptive transfer of wild-type MZP B cells compared to either IL-10−/− MZP or wild-type follicular B cells (Figure 6C). Transfer of follicular B cells from tolerized wild type mice also did not prevent rejection in B-IL-10−/− recipients (data not shown). Together, these data demonstrated that IL-10 producing MZP B cells were necessary for induction of costimulatory blockade transplantation tolerance.
Figure 6. B cell specific IL-10 is required for tolerance.
(A) MZP, MZ and CD19+CD5+CD1dhi+ B cells characterized from B-IL-10−/− or littermates by flow cytometry. IL-10 mRNA expression monitored by qRT-PCR. (B) Splenic B cell subsets (left), CD4+ and CD8+ cells subsets (upper right), and CD25 and Foxp3 subsets gated on CD4+ cells (lower right) analyzed. (C) B-IL-10−/− and littermate control recipients given tolerogen and BALB/c grafts. MZP B cells or follicular (Fol) B cells (4 X 105 cells/mouse) from wild-type or B-IL-10−/− naïve mice adoptively transferred on the day of transplant. Graft survival (upper) and parenchymal rejection score (lower). Littermate control or B-IL-10−/− + WT MZP B cells vs B-IL-10−/− mice (p<0.05); B-IL-10−/− vs B-IL-10−/− + WT Fol B cells or B-IL-10−/− + B-IL-10−/− MZP B cells (p ns). (D) Alloantibody responses of allograft recipients with the indicated genotypes and treatments measured 14 days after transplantation.
Discussion
The results here demonstrated that costimulation blockade induced a subset of IL-10+IL-21R+ MZP B cells in the secondary lymphoid organs. Tolerogen did not induce similar changes in other previously characterized suppressive or regulatory B cell subsets. B cells and B cell IL-10 in the MZP subset were required for costimulatory blockade induced, alloantigen specific tolerance, while other B cell subsets were not. We conclude that B cell produced IL-10 specifically in the MZP subset determines the outcome of costimulatory blockade induced tolerance.
The role of B cells in immunity and tolerance is varied and diverse. B cells play an important role in the induction of peripheral tolerance (29). Conversely, as revealed by B cell deficient μMT mice, B cells are required for acute rejection (30). However, μMT animals have several immune abnormalities including decreased size of secondary lymphoid organs, decreased diversity in the T cell repertoire and number, imbalance of Th1/Th2 cytokines (14), and lack of macrophages and follicular dendritic cell subsets. It has been shown that tolerated grafts have increased infiltration of B cells compared to rejecting grafts (31, 32). Depletion of B cells with anti-CD19 mAb slightly increases graft survival, whereas anti-CD20 mAb leads to acute rejection of minor histocompatibility-mismatched skin, cardiac and renal allografts (5). In a kidney transplant clinical trial, depletion of B cells induced acute cellular rejection (8), yet others have shown that B cell depletion increases graft survival (4). Thus, the depleting strategy affects different subsets of B cells which have diverse immunomodulatory functions. Together, these observations show that no general consensus about the role of B cells in tolerance exists, and suggest that simple depletion of B cells may be of limited therapeutic value or even detrimental.
There are several B cell surface receptors such as CD19, CD20, CD22 and CD79 that have been targeted to deplete B cells in autoimmune disease models. Functional deficiency of B cells or depletion of B cells can exacerbate inducible autoimmune disease due to increased inflammatory cytokine production (33) or infiltration of mononuclear cells in autoimmune prone organs (34, 35), although B cell derived IL-10 was not protective in at least one model (36). Recently, it was shown that IL-10 producing cells that belong to the MZP B cell population play a protective role in experimental arthritis (37), and mice lacking these populations have increased inflammatory Th1/Th17 and decreased Treg differentiation leading to exacerbation of disease (37). B cell costimulatory and regulatory function is required for anti-CD45RB induced cardiac transplantation tolerance (38, 39). However, absence of IL-10 in B cells increased anti-CD45RB mAb mediated graft survival and tolerance induction (40). In the CD45RB model there are increased regulatory CD4+ T cells in the secondary lymphoid tissues as well as reduced alloantibody production. The cellular and molecular mechanisms of how IL-10 deficiency in B cells prolonged anti-CD45RB mAb mediated graft survival requires further exploration. Our data showed that depletion of B cells under tolerogenic conditions lead to acute rejection with migration of inflammatory effector cells into the graft. In models in which B cell depletion is protective, combination immunosuppressive regimens employing tacrolimus or rapamycin that control the differentiation of T cells are used (5–8). Thus, the timing of B cell depletion, the subset depleted, and the precise combination of other immunosuppressive variables in the model may result in either pathogenic or protective B cell function.
Blocking of co-stimulatory CD40-CD40L signals provides a robust approach to induce tolerance, and several mechanisms have been proposed to explain CD40-CD40L blockade induced transplantation tolerance. The mechanisms depend on the type of transplant, subsequent therapy, and the relative importance of CD4+ and CD8+ T cell responses (41–43). However, the cellular and molecular mechanistic contributions of B cells in costimulatory blockade induced tolerance have not been well studied. We found here that costimulatory blockade induced an IL-10+IL-21R+ MZP B cell subset, while others have shown that IL-21R deficient mice do not expand the IL-10 producing B cells following antigenic stimulation in an experimental autoimmune encephalomyelitis mouse model (44). We did not find increased CD19+CD5+CD1d+TIM-1+ B cells or IL-10 production in these cells during tolerization as reported in a different model of allograft tolerance (26). We also did not find the well-described IL-10 producing (B10) Breg subset (45). However, it is noteworthy that B10 Breg rely on CD40 for their induction or expansion (44), so that the tolerogen used in our model may have prevented their induction. Our results and others suggest there are multiple discrete regulatory B cells subsets that rely on IL-10 or other immunosuppressive mechanisms, and that the immunosuppressive regimen may dictate which one(s) are functional in a particular setting.
A theme is emerging in studies of lymphoid organ structure that there are distinct microdomains which dictate the regulation of immune responses, so that Th1, Th2, Th17, Tfh, Treg, Tfr, Tc1, and Tc2 responses take place in different places and times (46). Interventions or stimuli that alter lymphoid organ structure or lymphocyte migration may have profound effects on immune interactions and the net result of the immune response (46). The roles of regulatory B cells and how they shape these microdomains in diverse immune responses, including transplantation tolerance, are not well defined. Our work showed that IL-10 sufficient MZP B cells help in the establishment of tolerance Since MZ and MZP B cells are found in proximity to the follicles and germinal centers (47, 48), our findings suggest that the B cell subsets may contribute to tolerance through effects on T follicular helper and regulatory subsets (49, 50). Indeed our preliminary data point to such an effect (not shown). Therapeutic regimens that disrupt the regulatory network in the germinal center, including lymphoid organ domain structure, inhibit tolerance induction leading to acute inflammation and allograft rejection (51). The fine balance among cytokine and chemokine expression and precise localization in the germinal center dictates the subsequent fate of T cells and the inhibition or generation of tolerance.
Supplementary Material
(A) Gating strategies for analysis of different B cell subsets. (B) C57BL/6 mice given DST (day −7) and anti-CD40L mAb (days −7, −4 and 0; 250 μg/injection, i. v.). Mice given anti-CD20 mAb (day +1, 10 or 25 μg/mouse; i.v.) or control IgG (10 or 25μg/mouse). 48 hours after anti-CD20 mAb administration, depletion of B cell subsets analyzed in spleen and LN. B cell subset percentages calculated based on gating scheme in Supplementary Figure S1A. 5 mice/group. (C) Absolute number of B cell subsets in spleen. 5 mice/group.
(A) hCD20Tg mice were given tolerogen, C57BL/6 cardiac allografts and anti-human CD20 mAb (100 μg/mouse i.v., d +1). Five days after transplantation, spleens and LN were harvested, stained for indicated markers, and analyzed by flow cytometry. (n= 6–8 mice/group). (B) C57BL/6 mice were given tolerogen, BALB/c cardiac allografts and anti-mouse CD20 mAb (100 μg/mouse i.v., d +1). Five days after transplantation, spleens were harvested, stained for indicated markers, and analyzed by flow cytometry. Absolute number of cells were calculated and average number per spleen plotted. (n= 3–5 mice/group). (C) C57BL/6 recipients given tolerogen, BALB/c allografts and anti-mCD20 mAb as in Figure 1C. After 10 days, mononuclear cells purified from allografts. Surface staining for GR1, B220, CD11b and CD11c, and intracellular staining for MOMA-1. Cells analyzed gating on MOMA-1+ cells. (D) Wright’s stain of purified CD45+CD11c−B220−CD11b+GR1+MOMA-1+ and CD45+CD11c−B220−CD11b+GR1+MOMA-1− graft infiltrating cells.
Acknowledgments
We thank Drs. Christopher Karp, Axel Roers and Mark Shlomchik for reagents. This work was supported by NIH grants AI41428, AI62765 and AI72039 (all to JSB); Department of Biotechnology, Government of India grants, Ramalingaswami Fellowship, BT/03/IYBA/2010 and BT/PR4610/MED/30/720/2012 to GL. NK received junior research fellowship from Council of Scientific and Industrial Research, Government of India. We acknowledge the help from University of Maryland Marlene and Stewart Greenebaum Cancer Center Flow Cytometry Shared Service, and National Centre for Cell Science FACS core facility for help.
Abbreviations
- APC
antigen presenting cells
- Breg
regulatory B cell
- DC
dendritic cell
- DST
donor-specific splenocyte transfusion
- H&E
hematoxylin and eosin staining
- i.v
intravenous
- LN
lymph node
- mAb
monoclonal antibody
- MOMA-1
metallophilic macrophages
- MST
median survival time
- MZ
marginal zone B cell
- MZP
marginal zone precursor B cell
- PR
parenchymal rejection
- Tfh
T follicular helper cell
- Tfr
follicular regulatory CD4+ T cell
Footnotes
Disclosure: The authors of this manuscript have no conflicts of interest to disclose.
GL, YN, AS, AKS, BEB, NK and CCB performed the experiments, analyzing data; DI and TZ performed the cardiac transplantation; GL and JSB design the experiments, interpreting data, and wrote the manuscript.
References
- 1.Pilat N, Sayegh MH, Wekerle T. Costimulatory pathways in transplantation. Seminars in immunology. 2011;23 doi: 10.1016/j.smim.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quezada SA, Jarvinen LZ, Lind EF, Noelle RJ. CD40/CD154 interactions at the interface of tolerance and immunity. Annual review of immunology. 2004;22 doi: 10.1146/annurev.immunol.22.012703.104533. [DOI] [PubMed] [Google Scholar]
- 3.Ochando JC, Homma C, Yang Y, Hidalgo A, Garin A, Tacke F, et al. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat Immunol. 2006;7 doi: 10.1038/ni1333. [DOI] [PubMed] [Google Scholar]
- 4.Kelishadi SS, Azimzadeh AM, Zhang T, Stoddard T, Welty E, Avon C, et al. Preemptive CD20+ B cell depletion attenuates cardiac allograft vasculopathy in cyclosporine-treated monkeys. J Clin Invest. 2010;120 doi: 10.1172/JCI41861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DiLillo DJ, Griffiths R, Seshan SV, Magro CM, Ruiz P, Coffman TM, et al. B Lymphocytes Differentially Influence Acute and Chronic Allograft Rejection in Mice. The Journal of Immunology. 2011;186 doi: 10.4049/jimmunol.1002983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sagoo P, Perucha E, Sawitzki B, Tomiuk S, Stephens DA, Miqueu P, et al. Development of a cross-platform biomarker signature to detect renal transplant tolerance in humans. J Clin Invest. 2010;120 doi: 10.1172/JCI39922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Newell KA, Asare A, Kirk AD, Gisler TD, Bourcier K, Suthanthiran M, et al. Identification of a B cell signature associated with renal transplant tolerance in humans. J Clin Invest. 2010;120 doi: 10.1172/JCI39933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clatworthy MR, Watson CJ, Plotnek G, Bardsley V, Chaudhry AN, Bradley JA, et al. B-cell-depleting induction therapy and acute cellular rejection. The New England journal of medicine. 2009;360 doi: 10.1056/NEJMc0808481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu C, Noorchashm H, Sutter JA, Naji M, Prak EL, Boyer J, et al. B lymphocyte-directed immunotherapy promotes long-term islet allograft survival in nonhuman primates. Nat Med. 2007;13 doi: 10.1038/nm1673. [DOI] [PubMed] [Google Scholar]
- 10.Bennett SR, Carbone FR, Toy T, Miller JF, Heath WR. B cells directly tolerize CD8(+) T cells. J Exp Med. 1998;188 doi: 10.1084/jem.188.11.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hollsberg P, Batra V, Dressel A, Hafler DA. Induction of anergy in CD8 T cells by B cell presentation of antigen. J Immunol. 1996;157 [PubMed] [Google Scholar]
- 12.Reichardt P, Dornbach B, Rong S, Beissert S, Gueler F, Loser K, et al. Naive B cells generate regulatory T cells in the presence of a mature immunologic synapse. Blood. 2007;110 doi: 10.1182/blood-2006-10-053793. [DOI] [PubMed] [Google Scholar]
- 13.Mizoguchi E, Mizoguchi A, Preffer FI, Bhan AK. Regulatory role of mature B cells in a murine model of inflammatory bowel disease. Int Immunol. 2000;12 doi: 10.1093/intimm/12.5.597. [DOI] [PubMed] [Google Scholar]
- 14.Moulin V, Andris F, Thielemans K, Maliszewski C, Urbain J, Moser M. B lymphocytes regulate dendritic cell (DC) function in vivo: increased interleukin 12 production by DCs from B cell-deficient mice results in T helper cell type 1 deviation. J Exp Med. 2000;192 doi: 10.1084/jem.192.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rowley DA, Stach RM. B lymphocytes secreting IgG linked to latent transforming growth factor-beta prevent primary cytolytic T lymphocyte responses. Int Immunol. 1998;10 doi: 10.1093/intimm/10.3.355. [DOI] [PubMed] [Google Scholar]
- 16.Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28 doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 17.Matsushita T, Horikawa M, Iwata Y, Tedder TF. Regulatory B cells (B10 cells) and regulatory T cells have independent roles in controlling experimental autoimmune encephalomyelitis initiation and late-phase immunopathogenesis. J Immunol. 2010;185 doi: 10.4049/jimmunol.1001307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blair PA, Norena LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR, et al. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity. 2010;32 doi: 10.1016/j.immuni.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 19.Candando KM, Lykken JM, Tedder TF. B10 cell regulation of health and disease. Immunol Rev. 2014;259 doi: 10.1111/imr.12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagano H, Mitchell RN, Taylor MK, Hasegawa S, Tilney NL, Libby P. Interferon-gamma deficiency prevents coronary arteriosclerosis but not myocardial rejection in transplanted mouse hearts. J Clin Invest. 1997;100 doi: 10.1172/JCI119564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cooper JD, Billingham M, Egan T, Hertz MI, Higenbottam T, Lynch J, et al. A working formulation for the standardization of nomenclature and for clinical staging of chronic dysfunction in lung allografts. International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 1993;12 [PubMed] [Google Scholar]
- 22.Shimizu K, Schonbeck U, Mach F, Libby P, Mitchell RN. Host CD40 ligand deficiency induces long-term allograft survival and donor-specific tolerance in mouse cardiac transplantation but does not prevent graft arteriosclerosis. J Immunol. 2000;165 doi: 10.4049/jimmunol.165.6.3506. [DOI] [PubMed] [Google Scholar]
- 23.Korn T, Mitsdoerffer M, Croxford AL, Awasthi A, Dardalhon VA, Galileos G, et al. IL-6 controls Th17 immunity in vivo by inhibiting the conversion of conventional T cells into Foxp3+ regulatory T cells. Proc Natl Acad Sci U S A. 2008;105 doi: 10.1073/pnas.0809850105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yi H, Guo C, Yu X, Zuo D, Wang XY. Mouse CD11b+Gr-1+ myeloid cells can promote Th17 cell differentiation and experimental autoimmune encephalomyelitis. J Immunol. 2012;189 doi: 10.4049/jimmunol.1200086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Asano K, Nabeyama A, Miyake Y, Qiu CH, Kurita A, Tomura M, et al. CD169-Positive Macrophages Dominate Antitumor Immunity by Crosspresenting Dead Cell-Associated Antigens. Immunity. 2011;34 doi: 10.1016/j.immuni.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 26.Ding Q, Yeung M, Camirand G, Zeng Q, Akiba H, Yagita H, et al. Regulatory B cells are identified by expression of TIM-1 and can be induced through TIM-1 ligation to promote tolerance in mice. J Clin Invest. 2011;121 doi: 10.1172/JCI46274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Good KL, Bryant VL, Tangye SG. Kinetics of human B cell behavior and amplification of proliferative responses following stimulation with IL-21. J Immunol. 2006;177 doi: 10.4049/jimmunol.177.8.5236. [DOI] [PubMed] [Google Scholar]
- 28.Spolski R, Kim HP, Zhu W, Levy DE, Leonard WJ. IL-21 mediates suppressive effects via its induction of IL-10. J Immunol. 2009;182 doi: 10.4049/jimmunol.0802978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kirk AD, Turgeon NA, Iwakoshi NN. B cells and transplantation tolerance. Nat Rev Nephrol. 6 doi: 10.1038/nrneph.2010.111. [DOI] [PubMed] [Google Scholar]
- 30.Gareau A, Hirsch GM, Lee TD, Nashan B. Contribution of B cells and antibody to cardiac allograft vasculopathy. Transplantation. 2009;88 doi: 10.1097/TP.0b013e3181b076cc. [DOI] [PubMed] [Google Scholar]
- 31.Le Texier L, Thebault P, Lavault A, Usal C, Merieau E, Quillard T, et al. Long-term allograft tolerance is characterized by the accumulation of B cells exhibiting an inhibited profile. Am J Transplant. 2011;11 doi: 10.1111/j.1600-6143.2010.03336.x. [DOI] [PubMed] [Google Scholar]
- 32.Chesneau M, Pallier A, Braza F, Lacombe G, Le Gallou S, Baron D, et al. Unique B cell differentiation profile in tolerant kidney transplant patients. Am J Transplant. 2014;14 doi: 10.1111/ajt.12508. [DOI] [PubMed] [Google Scholar]
- 33.Moritoki Y, Lian ZX, Lindor K, Tuscano J, Tsuneyama K, Zhang W, et al. B-cell depletion with anti-CD20 ameliorates autoimmune cholangitis but exacerbates colitis in transforming growth factor-beta receptor II dominant negative mice. Hepatology. 2009;50 doi: 10.1002/hep.23238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hjelmstrom P, Juedes AE, Fjell J, Ruddle NH. B-cell-deficient mice develop experimental allergic encephalomyelitis with demyelination after myelin oligodendrocyte glycoprotein sensitization. J Immunol. 1998;161 [PubMed] [Google Scholar]
- 35.Dhirapong A, Lleo A, Yang GX, Tsuneyama K, Dunn R, Kehry M, et al. B cell depletion therapy exacerbates murine primary biliary cirrhosis. Hepatology. 2011;53 doi: 10.1002/hep.24044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Teichmann LL, Kashgarian M, Weaver CT, Roers A, Muller W, Shlomchik MJ. B cell-derived IL-10 does not regulate spontaneous systemic autoimmunity in MRL.Fas(lpr) mice. J Immunol. 2012;188 doi: 10.4049/jimmunol.1102456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carter NA, Vasconcellos R, Rosser EC, Tulone C, Muñoz-Suano A, Kamanaka M, et al. Mice Lacking Endogenous IL-10-Producing Regulatory B Cells Develop Exacerbated Disease and Present with an Increased Frequency of Th1/Th17 but a Decrease in Regulatory T Cells. The Journal of Immunology. 2011;186 doi: 10.4049/jimmunol.1100284. [DOI] [PubMed] [Google Scholar]
- 38.Lee KM, Kim JI, Stott R, Soohoo J, O’Connor MR, Yeh H, et al. Anti-CD45RB/anti-TIM-1-induced tolerance requires regulatory B cells. Am J Transplant. 2012;12 doi: 10.1111/j.1600-6143.2012.04055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deng S, Moore DJ, Huang X, Lian MM, Mohiuddin M, Velededeoglu E, et al. Cutting edge: transplant tolerance induced by anti-CD45RB requires B lymphocytes. J Immunol. 2007;178 doi: 10.4049/jimmunol.178.10.6028. [DOI] [PubMed] [Google Scholar]
- 40.Zhao G, Moore DJ, Lee KM, Kim JI, Duff PE, O’Connor MR, et al. An unexpected counter-regulatory role of IL-10 in B-lymphocyte-mediated transplantation tolerance. Am J Transplant. 2010;10 doi: 10.1111/j.1600-6143.2010.03027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Y, Li XC, Zheng XX, Wells AD, Turka LA, Strom TB. Blocking both signal 1 and signal 2 of T-cell activation prevents apoptosis of alloreactive T cells and induction of peripheral allograft tolerance. Nat Med. 1999;5 doi: 10.1038/15256. [DOI] [PubMed] [Google Scholar]
- 42.Monk NJ, Hargreaves RE, Marsh JE, Farrar CA, Sacks SH, Millrain M, et al. Fc-dependent depletion of activated T cells occurs through CD40L-specific antibody rather than costimulation blockade. Nat Med. 2003;9 doi: 10.1038/nm931. [DOI] [PubMed] [Google Scholar]
- 43.Nanji SA, Hancock WW, Luo B, Schur CD, Pawlick RL, Zhu LF, et al. Costimulation blockade of both inducible costimulator and CD40 ligand induces dominant tolerance to islet allografts and prevents spontaneous autoimmune diabetes in the NOD mouse. Diabetes. 2006;55 [PubMed] [Google Scholar]
- 44.Yoshizaki A, Miyagaki T, DiLillo DJ, Matsushita T, Horikawa M, Kountikov EI, et al. Regulatory B cells control T-cell autoimmunity through IL-21-dependent cognate interactions. Nature. 2012;491 doi: 10.1038/nature11501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matsushita T, Horikawa M, Iwata Y, Tedder TF. Regulatory B Cells (B10 Cells) and Regulatory T Cells Have Independent Roles in Controlling Experimental Autoimmune Encephalomyelitis Initiation and Late-Phase Immunopathogenesis. The Journal of Immunology. 2010;185 doi: 10.4049/jimmunol.1001307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burrell BE, Ding Y, Nakayama Y, Park KS, Xu J, Yin N, et al. Tolerance and lymphoid organ structure and function. Frontiers in immunology. 2011;2 doi: 10.3389/fimmu.2011.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cerutti A, Cols M, Puga I. Marginal zone B cells: virtues of innate-like antibody-producing lymphocytes. Nat Rev Immunol. 2013;13 doi: 10.1038/nri3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kanayama N, Cascalho M, Ohmori H. Analysis of marginal zone B cell development in the mouse with limited B cell diversity: role of the antigen receptor signals in the recruitment of B cells to the marginal zone. J Immunol. 2005;174 doi: 10.4049/jimmunol.174.3.1438. [DOI] [PubMed] [Google Scholar]
- 49.Baumjohann D, Preite S, Reboldi A, Ronchi F, Ansel KM, Lanzavecchia A, et al. Persistent antigen and germinal center B cells sustain T follicular helper cell responses and phenotype. Immunity. 2013;38 doi: 10.1016/j.immuni.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 50.Linterman MA, Pierson W, Lee SK, Kallies A, Kawamoto S, Rayner TF, et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med. 2011;17 doi: 10.1038/nm.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakayama Y, Bromberg JS. Lymphotoxin-beta receptor blockade induces inflammation and fibrosis in tolerized cardiac allografts. Am J Transplant. 2012;12 doi: 10.1111/j.1600-6143.2012.04090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Gating strategies for analysis of different B cell subsets. (B) C57BL/6 mice given DST (day −7) and anti-CD40L mAb (days −7, −4 and 0; 250 μg/injection, i. v.). Mice given anti-CD20 mAb (day +1, 10 or 25 μg/mouse; i.v.) or control IgG (10 or 25μg/mouse). 48 hours after anti-CD20 mAb administration, depletion of B cell subsets analyzed in spleen and LN. B cell subset percentages calculated based on gating scheme in Supplementary Figure S1A. 5 mice/group. (C) Absolute number of B cell subsets in spleen. 5 mice/group.
(A) hCD20Tg mice were given tolerogen, C57BL/6 cardiac allografts and anti-human CD20 mAb (100 μg/mouse i.v., d +1). Five days after transplantation, spleens and LN were harvested, stained for indicated markers, and analyzed by flow cytometry. (n= 6–8 mice/group). (B) C57BL/6 mice were given tolerogen, BALB/c cardiac allografts and anti-mouse CD20 mAb (100 μg/mouse i.v., d +1). Five days after transplantation, spleens were harvested, stained for indicated markers, and analyzed by flow cytometry. Absolute number of cells were calculated and average number per spleen plotted. (n= 3–5 mice/group). (C) C57BL/6 recipients given tolerogen, BALB/c allografts and anti-mCD20 mAb as in Figure 1C. After 10 days, mononuclear cells purified from allografts. Surface staining for GR1, B220, CD11b and CD11c, and intracellular staining for MOMA-1. Cells analyzed gating on MOMA-1+ cells. (D) Wright’s stain of purified CD45+CD11c−B220−CD11b+GR1+MOMA-1+ and CD45+CD11c−B220−CD11b+GR1+MOMA-1− graft infiltrating cells.