Abstract
Objective
Diet-induced obesity (DIO) causes several pathophysiological changes in adipose tissue. Increased inflammation reduces white adipose tissue (WAT) insulin sensitivity and contributes to the development of diabetes. However, little is known about how DIO alters the function of brown adipose tissue (BAT), an organ that consumes calories by β3-adrenergic receptor (AR)-mediated thermogenesis and helps regulate energy balance.
Methods
To test the effects of DIO on BAT, we fed 6 week-old C57BL/6 mice either a normal chow diet (NCD) or a high-fat diet (HFD). After 16 additional weeks, we measured body fat, WAT and BAT mRNA expression, glucose tolerance, and rates of glucose uptake in response to insulin and the β3-AR agonist mirabegron.
Results
Compared with NCD, HFD increased body fat and impaired glucose tolerance. Both WAT and BAT had higher mRNA levels of markers of inflammation, including TNFα and F4/80. Insulin signaling in BAT and WAT was reduced, with decreased Akt phosphorylation. Diet-normalized BAT glucose uptake rates were lower in response to mirabegron.
Conclusions
These results support a model in which DIO leads to BAT inflammation and insulin resistance, leading to a broader impairment of BAT function.
Keywords: Brown Adipose Tissue, Insulin resistance, Obesity, β3-Adrenergic Receptor Agonist
Introduction
There are two distinct functions for adipose tissue. White adipose tissue (WAT) stores energy, while brown adipose tissue (BAT) and the related beige adipocytes contribute to energy expenditure through activation of the thermogenic tissue-specific uncoupling protein 1 (UCP1) (1). When energy intake exceeds energy expenditure, WAT accumulates and can eventually lead to obesity. Diet-induced obesity (DIO) has been consistently shown to cause resistance to insulin-mediated glucose uptake in liver, muscle, and WAT (2–5). Preliminary evidence suggests that this process may also occur in BAT (6), but this process has not been demonstrated at the molecular level. Recently it was discovered that a β3-adrenergic receptor (AR) agonist can stimulate human BAT (7), making it a potential therapeutic treatment for obesity. It is therefore critical to determine the physiological parameters affecting signal transduction underlying BAT thermogenesis. In this study, we used a mouse model to determine if DIO causes BAT insulin resistance at the molecular level. We then assessed whether DIO affects the ability of insulin or a β3-AR agonist to stimulate BAT glucose uptake.
Methods
Mice and diets
Animal care and study protocols were approved by the Animal Care Committee of the Joslin Diabetes Center (JDC). Beginning at 6 weeks of age, C57BL/6J male mice from Jackson Labs were fed either chow diet (Research Diet Inc, 10% kcal fat, 70% carbohydrate) or high-fat diet (HFD) (Research Diets Inc., 60% kcal fat, 20% carbohydrate). At 16 weeks, mice were transferred to JDC and maintained on either normal chow diet (NCD), with 21% kcal from fat, or HFD, with 60% kcal from fat. All mice had free access to food and water and were kept in temperature-controlled facilities at 23°C on a 12-hour light/dark cycle for 8 weeks. Body composition was measured at 22 weeks of age by DXA (GE Lunar PIXImus 2 Series Densitometer).
Immunoblotting and Histology
Immunoblotting was carried out as described (8) with antibodies listed in Table S1. Densitometry of the bands was quantified using ImageJ software. Interscapular BAT (iBAT) and perigonadal WAT (pgWAT) were fixed in Bouin’s fixative, embedded in paraffin, and stained with hematoxylin and eosin at the Harvard Medical School Histology Core (http://www.dfhcc.harvard.edu/core-facilities/rodent-histopathology-pathology/).
Quantitative RT-PCR
RNA was extracted from iBAT and pgWAT tissue using Qiazol and RNeasy mini-kit following manufacturer’s protocols (Qiagen) and reverse transcribed using High Capacity cDNA kit (Applied Biosystems). 2.5 μL of 4 ng/μL cDNA was used in a 10 μL reaction with intercalating SYBR Green dye (Bio-Rad) and quantified using the ABI Prism 7900 sequence detection system. Values were normalized to TATA-binding protein (TBP). Primers (IDT) are listed in Table S2.
Insulin Tolerance and Glucose Uptake Rate Assays
A separate group of 36 mice was raised as above. At age 26 weeks, mice were fasted for four hours and then anesthetized with 0.3 μg/μL Avertin (2,2,2-tribromoethanol)(Sigma-Aldrich) dissolved in PBS, at 10 μL/g. Mice were given a retro-orbital injection of 2-deoxy-D-[1,2-C14(N)]glucose (10 μCi/30g of body weight) as previously described (8) with of one of three treatments: 1μL/g body weight of 10% DMSO/saline (vehicle), 0.75 mU/g body weight of insulin diluted in vehicle, or 0.1μg/g body weight mirabegron (Santa Cruz) dissolved in vehicle. Mice were sacrificed by cervical dislocation, and iBAT, pgWAT, inguinal WAT (ingWAT), and skeletal muscle (gastrocnemius) were resected. Tissues were snap-frozen in liquid nitrogen and stored at −80 °C. The amount of glucose radiotracer was determined as described (9).
Statistical Analyses
Data are expressed as the mean ± SEM and were compared using independent t-tests or two-way ANOVA, with P values <0.05 considered statistically significant.
Results
DIO changed iBAT lipid content and mRNA expression
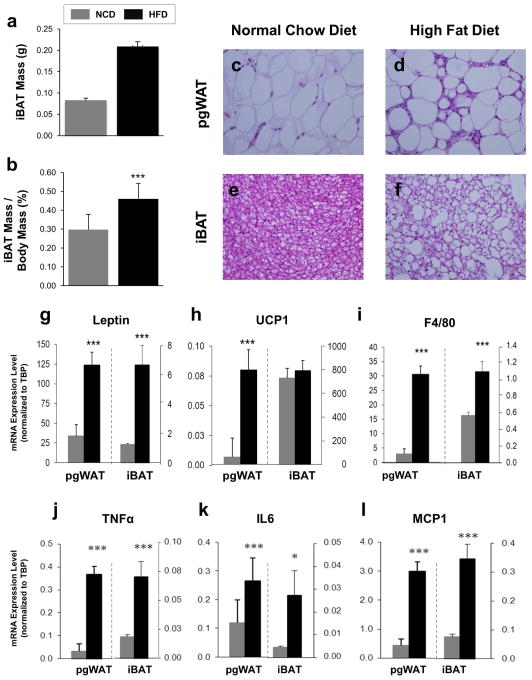
Compared to the NCD, mice fed 16 weeks of a HFD had higher body weight, fat percentage, and impaired fasting glucose and glucose tolerance (Figure S1a–d). In particular, the iBAT from HFD mice was larger, both in absolute terms, and when normalized to body weight (Figure 1a–b). At the cellular level, pgWAT from DIO mice showed adipocytes that were larger and had an abundance of crown-like structures consistent with macrophage infiltration, and the brown adipocytes had larger lipid droplets (Figure 1c–f).
Figure 1. Effects of DIO on pgWAT and iBAT Anatomy and mRNA Expression.
(a) iBAT mass was recorded after sacrifice. (b) iBAT mass was normalized by dividing by whole body mass. Hematoxylin and eosin stained sections of perigonadal WAT from mice who ate (c) NCD or (d) HFD and iBAT from mice who ate (e) NCD or (f) HFD; shown at 400X magnification. mRNA expression analysis was performed on pgWAT and iBAT taken from C57BL/6J male mice fed NCD or HFD for 18 weeks. Expression levels are shown as averages normalized to TBP for the following genes (g) leptin; (h) UCP1; (i) F4/80; (j) TNFa; (k) IL6; (l) MCP1. n=12 per group. *P < 0.05, ***P < 0.001.
The histological changes were corroborated by mRNA expression profiling. Leptin, a measure of white adipocyte triglyceride stores, was expressed ten-fold higher in the pgWAT compared to iBAT, and DIO led to higher levels in both tissues, reflecting a shift toward a white adipocyte-like phenotype in iBAT (Figure 1g). Expression of UCP1, the marker of brown adipocytes, was several orders of magnitude higher in iBAT compared to pgWAT, and DIO led to higher expression in pgWAT but not iBAT (Figure 1h). In both pgWAT and iBAT, DIO produced higher expression levels of the macrophage markers F4/80 and CD68 (Figure 1i and S2) as well as the inflammatory cytokines genes TNFα, IL6, and MCP1 (Figure 1j–l). For all five markers of inflammation, the relative expression levels were higher in pgWAT compared with iBAT by 10- to 100-fold.
HFD impaired iBAT insulin signaling
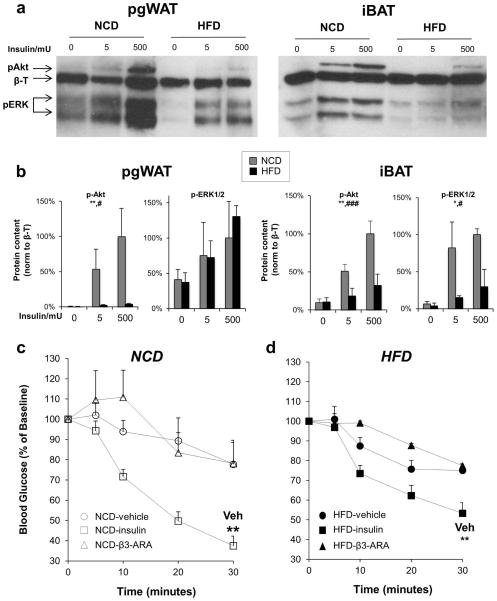
In mice fed a NCD, both the pgWAT and iBAT demonstrated insulin dose-dependent increases in phosphorylation of Akt and ERK, two kinases central to insulin receptor signal transduction, with Akt responsible for glucose uptake (10). In response to HFD, insulin signaling through both Akt and ERK was significantly blunted in iBAT (Figure 2a–b).
Figure 2. High fat diet reduces insulin signaling in pgWAT and iBAT.
(a) Representative immunoblots of phospho-Akt (S473) and phospho-ERK in pgWAT and iBAT from mice on NCD or HFD for 18 weeks treated via IVC injection with vehicle, 5 mU insulin, or 500 mU insulin for 5 minutes before tissue harvest. β-Tubulin (β-T) was used as a loading control. Data shown are representative of three independent experiments. (b) Densitometry of p-Akt (n=4) and p-ERK (n=2). Two-way ANOVA *P < 0.05, **P < 0.01 for comparing NCD and HFD, and #P < 0.05, ###P < 0.001, for comparing insulin doses. Blood glucose levels were determined after injection of vehicle (circles), 0.75 mU/kg insulin (squares), 0.1 μg/g body weight of the β3-adrinergic agonist mirabegron (triangles) in mice fed (c) NCD (white symbols) or (d) HFD (black symbols). n=5–6 per treatment. **P < 0.01, compared with vehicle.
A different set of mice fed NCD or HFD for 16 weeks were fasted for 4 hours and then injected with 14C-deoxy-D-glucose mixed with vehicle, insulin, or mirabegron, a β3-AR agonist. Thirty minutes after injection with either vehicle or mirabegron, there was no change in blood glucose levels, but they were significantly lower with administration of insulin (Figure 2c). DIO did not affect the change in blood glucose after injection with vehicle or mirabegron, but insulin was not as effective at lowering blood glucose in HFD-treated mice (Figure 2d).
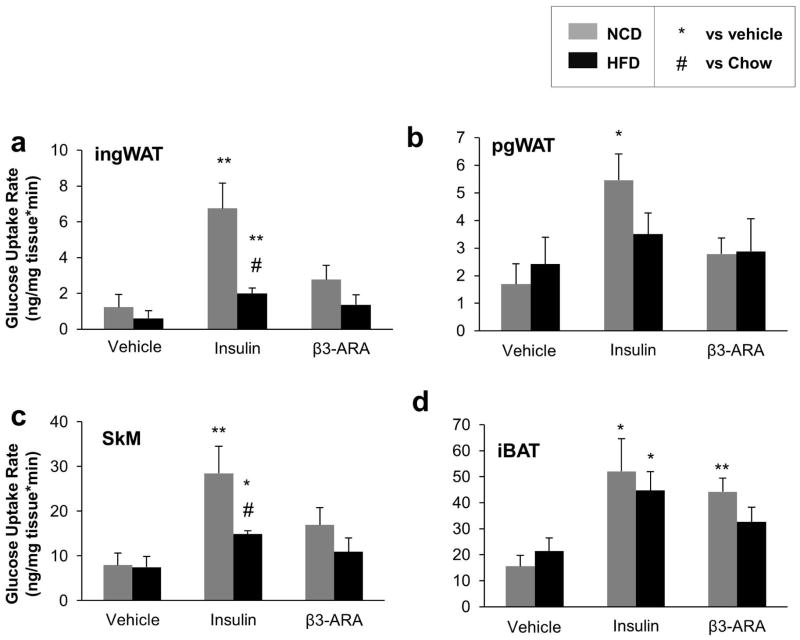
To quantify the effect of DIO on the specific insulin- and β3-AR agonist-responsive tissues, we also measured the rates of glucose uptake in iBAT, pgWAT, ingWAT, and skeletal muscle after treatment with vehicle, insulin, and mirabegron. Rates were higher in response to insulin in all four tissues, but the β3-AR agonist mirabegron significantly increased glucose uptake only in iBAT (Figure 3a–d). To compare the effects of diet on the fold change of insulin- and mirabegron-mediated glucose uptake, we normalized the rates in each diet to what was seen in the mice treated with vehicle. In all four tissues, DIO reduced the rates of glucose uptake, but they were significantly lower only in the pgWAT (P=0.02) and skeletal muscle (P=0.05). In contrast, mirabegron-induced glucose uptake was impaired only in iBAT (P=0.01)(Figure S3a–d). Thus, iBAT was comparatively resistant to DIO-associated decreases in insulin-mediated glucose uptake.
Figure 3. Tissue Glucose Uptake Rates in Mice Fed NCD or HFD.
Rates of 14C-2-deoxy glucose uptake were determined in (a) ingWAT, (b) pgWAT, (c) skeletal muscle (SkM), and (d) iBAT after a retro-orbital injection of tracer with one of three treatments: vehicle, 0.75 mU/kg insulin, or 0.1 μg/g body weight of the β3-adrinergic agonist mirabegron (β3-ARA). n=5–6 per group. *P < 0.05, **P < 0.01 vs. vehicle.
Discussion
Despite the extensive body of work showing how DIO produces insulin resistance in liver, muscle, and WAT, very little is known about its effects on BAT. Given the multiple roles that human BAT may have in regulating energy balance and metabolism (11, 12), it has become essential that we better understand its unique molecular physiology. In this study, we found that DIO causes resistance to insulin signaling in both of the principal PI3K and ERK signaling pathways, and our findings suggest the process was mediated by inflammation within the BAT itself.
Recently it was shown that DIO increased WAT inflammation, but BAT was resistant to macrophage infiltration (13), suggesting that BAT may also be comparatively resistant to DIO-mediated insulin resistance. Our results are in fact consistent with these findings; specifically, expression of inflammatory markers was 10–100 fold lower in iBAT compared to pgWAT, regardless of diet. In this study, the mice consumed a higher percentage of dietary fat for a longer duration, which led to higher fasting glucose levels and a greater degree of glucose intolerance. Others have reported similar findings to ours in the setting of HFD with the same percentage of dietary fat (14). Thus, our results demonstrate that iBAT is indeed more resistant to DIO-induced inflammation than WAT depots, but inflammatory changes eventually are evident in iBAT when subjected to a higher burden of fat calories.
A limitation of this study is that while DIO led to insulin resistance in iBAT, we do not yet know the entire signaling pathway by which it occurred. Potential sources include both direct and indirect effects of inflammatory mediators on the insulin-signaling pathway (15, 16), and future studies should be directed toward defining the underlying processes and the potential effects on energy homeostasis. In addition, our data suggest that DIO may also impair β3-AR signaling (10, 17). If indeed β3-AR signaling is impaired in iBAT in response to DIO, this could reduce thermogenesis as has been implicated in human models of BAT function (18, 19). A more pronounced effect may be seen with lower doses of mirabegron, and a dose-response assessment will better-characterize the β3-AR effect.
In summary, we show that DIO causes BAT insulin resistance and reduced glucose uptake in a mouse model, which is associated with increased tissue inflammation. DIO-induced changes in BAT may also reduce adrenergic signaling, which could impair its ability to help prevent obesity and metabolic disease.
Supplementary Material
Study Importance Questions.
What is already known about this subject?
Diet induced obesity (DIO) causes inflammation and insulin resistance in muscle, liver, and white adipose tissue, but little is known about its effect on brown adipose tissue (BAT).
Mirabegron is the first β3-adrenergic receptor (AR) agonist found to activate human BAT.
What does your study add?
DIO causes insulin resistance in BAT.
The insulin resistance is likely from increased BAT inflammation.
Insulin resistance in BAT may also be accompanied by reduced β3-AR-mediated glucose uptake, which suggests that DIO-induced insulin resistance in BAT leads to a broader impairment of BAT function.
Acknowledgments
Funding: This work was supported by National Institutes of Health (NIH) grants K23 DK081604 (A.M.C.), K08 DK100543 (B.T.O.), Joslin DRC, the Clinical Translational Science Award UL1RR025758 to Harvard University and BIDMC from the National Center for Research Resources (NCRR), and the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK).
We thank Murray Mittleman for biostatistical advice; C. Ronald Kahn, Marc L. Reitman, and Raymond H. Cypess for their input in preparing this manuscript; Maura Mulvey and Allen Clermont of the Joslin Diabetes Center Animal Core; Tanya Genest from the JDC histology core; and Christie Sass and Lindsay McDougall for their laboratory support.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIDDK, NCRR, or the NIH.
Disclosure Statement: Dr. Cypess was a recipient of sponsored research grants from Chugai Pharmaceutical Co., Ltd, and Molecular Metabolism, LLC, both through Joslin Diabetes Center; he has received payment for lecturing about clinical diabetes on behalf of Joslin Diabetes Center to employees of Sanofi and Genentech; and was paid an honorarium by Pfizer, Inc. to give a lecture to its research unit.
Author Contributions: All authors contributed to the experimental design; manuscript writing; data analysis; and editing of the manuscript. Aaron M. Cypess is the guarantor who takes full responsibility for the work as a whole, including study design, access to data, and the decision to submit and publish the manuscript.
References
- 1.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84(1):277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 2.Rossmeisl M, Rim JS, Koza RA, Kozak LP. Variation in type 2 diabetes--related traits in mouse strains susceptible to diet-induced obesity. Diabetes. 2003;52(8):1958–66. doi: 10.2337/diabetes.52.8.1958. [DOI] [PubMed] [Google Scholar]
- 3.Yamauchi T, Waki H, Kamon J, Murakami K, Motojima K, Komeda K, et al. Inhibition of RXR and PPARgamma ameliorates diet-induced obesity and type 2 diabetes. J Clin Invest. 2001;108(7):1001–13. doi: 10.1172/JCI12864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Viljanen AP, Iozzo P, Borra R, Kankaanpaa M, Karmi A, Lautamaki R, et al. Effect of weight loss on liver free fatty acid uptake and hepatic insulin resistance. J Clin Endocrinol Metab. 2009;94(1):50. doi: 10.1210/jc.2008-1689. [DOI] [PubMed] [Google Scholar]
- 5.Petersen KF, Dufour S, Befroy D, Lehrke M, Hendler RE, Shulman GI. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes. 2005;54(3):603–8. doi: 10.2337/diabetes.54.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kraegen EW, James DE, Storlien LH, Burleigh KM, Chisholm DJ. In vivo insulin resistance in individual peripheral tissues of the high fat fed rat: assessment by euglycaemic clamp plus deoxyglucose administration. Diabetologia. 1986;29(3):192–8. doi: 10.1007/BF02427092. [DOI] [PubMed] [Google Scholar]
- 7.Cypess AM, Weiner LS, Roberts-Toler C, Elia EF, Kessler SH, Kahn PA, et al. Activation of Human Brown Adipose Tissue by a beta3-Adrenergic Receptor Agonist. Cell Metab. 2015;21(1):33–8. doi: 10.1016/j.cmet.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Emanuelli B, Vienberg SG, Smyth G, Cheng C, Stanford KI, Arumugam M, et al. Interplay between FGF21 and insulin action in the liver regulates metabolism. J Clin Invest. 2014;124(2):515–27. doi: 10.1172/JCI67353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferre P, Burnol AF, Leturque A, Terretaz J, Penicaud L, Jeanrenaud B, et al. Glucose utilization in vivo and insulin-sensitivity of rat brown adipose tissue in various physiological and pathological conditions. Biochem J. 1986;233(1):249–52. doi: 10.1042/bj2330249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 2006;7(2):85–96. doi: 10.1038/nrm1837. [DOI] [PubMed] [Google Scholar]
- 11.Chechi K, Nedergaard J, Richard D. Brown adipose tissue as an anti-obesity tissue in humans. Obes Rev. 2014;15(2):92–106. doi: 10.1111/obr.12116. [DOI] [PubMed] [Google Scholar]
- 12.Bartelt A, Heeren J. Adipose tissue browning and metabolic health. Nat Rev Endocrinol. 2014;10(1):24–36. doi: 10.1038/nrendo.2013.204. [DOI] [PubMed] [Google Scholar]
- 13.Fitzgibbons TP, Kogan S, Aouadi M, Hendricks GM, Straubhaar J, Czech MP. Similarity of mouse perivascular and brown adipose tissues and their resistance to diet-induced inflammation. Am J Physiol Heart Circ Physiol. 2011;301(4):H1425–37. doi: 10.1152/ajpheart.00376.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112(12):1821–30. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwon H, Pessin JE. Adipokines mediate inflammation and insulin resistance. Front Endocrinol (Lausanne) 2013;4:71. doi: 10.3389/fendo.2013.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chakrabarti SK, Cole BK, Wen Y, Keller SR, Nadler JL. 12/15-lipoxygenase products induce inflammation and impair insulin signaling in 3T3-L1 adipocytes. Obesity (Silver Spring) 2009;17(9):1657–63. doi: 10.1038/oby.2009.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chernogubova E, Cannon B, Bengtsson T. Norepinephrine increases glucose transport in brown adipocytes via beta3-adrenoceptors through a cAMP, PKA, and PI3-kinase-dependent pathway stimulating conventional and novel PKCs. Endocrinology. 2004;145(1):269–80. doi: 10.1210/en.2003-0857. [DOI] [PubMed] [Google Scholar]
- 18.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360(15):1509–17. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360(15):1500–8. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.