Abstract
Objective
To examine whether maternal reports of infant eating behaviors are stable over time and whether eating behaviors are prospectively associated with weight gain.
Methods
In an ongoing study of infant growth, weight and length were measured at 2-weeks, 3-months, and 5-months of age. Food responsiveness (FR), satiety responsiveness (SR), enjoyment of feeding (EF), and slow eating (SE) were assessed with the Baby Eating Behavior Questionnaire. Repeated measures ANOVA were used to examine changes in eating behaviors from 2-weeks to 5-months. Simple Pearson correlations examined associations among eating behaviors across time, and associations of eating behaviors with subsequent change in weight-for-length z-scores.
Results
Among 31 infants studied from 2-weeks to 3-months, FR and SR remained consistent (P<0.05), and among 21 infants studied from 3- to 5-months, FR, EF, and SE were consistent (P<0.01). Infants ate more quickly (P<0.01), and tended to have greater SR with age (P=0.09). Only SE at 3-months was associated with subsequent gain in weight-for-length (P<0.05).
Conclusions
Consistent with previous research, SE was predictive of weight gain during infancy. Given that eating behaviors were largely consistent after 3-months of age, it may be important to encourage the development of healthy eating behaviors during early infancy.
Keywords: eating behaviors, weight gain, childhood obesity
Introduction
Almost 17% of children in the United States have a body mass index (BMI) greater than the 95th percentile, characterizing them as obese (1). Minority children are particularly burdened by obesity, with 20% prevalence in African American children and 22% prevalence in Hispanic children, compared to 14% among Caucasians (1). Childhood obesity is associated with serious metabolic health conditions such as high blood pressure, type 2 diabetes, and non-alcoholic fatty liver disease (2). Furthermore, deleterious psychological consequences, such as low self-esteem, depression, anxiety, and behavior problems, are also associated with obesity (3). Children who are obese have a much greater risk of becoming obese as adults, as well as having more severe obesity than those who were not obese during childhood (4, 5). It is important, therefore, to identify potential contributors to obesity early in life.
One of the most consistent predictors of childhood obesity is rapid infant weight gain. Studies have consistently shown that rapid weight gain in the first six months is associated with overweight and obesity during childhood (6-9), adolescence (10, 11), and adulthood (12, 13) One study found that children who had rapid weight gain in infancy were nearly 10 times more likely to be obese at 4 years old than children who had not experienced rapid infant weight gain, irrespective of children's ethnicity or history of breast- versus formula-feeding (14). Furthermore, excess weight gain during the first, but not the second, six months of life, has been associated with relatively greater trunk fat among 1-year-olds (15), and among 4 – 20 year old individuals (16). This is particularly important given the association between abdominal obesity and adverse metabolic health (for review see (17, 18)). Less is known about the more immediate consequences of rapid weight gain during infancy, but at least one study has shown an association between activity level and fat mass during infancy (19), which may suggest that infants who have experienced a rapid rate of weight gain may be less active, which could potentially contribute to more weight gain thereafter. Additionally, ethnic minority infants are at an increased risk for rapid infant weight gain, and this may be at least partially attributable to a shorter duration of exclusive breastfeeding and the earlier introduction of solid foods (20).
Eating behavior may play a role in rapid infant weight gain and subsequent obesity. In studies of older children, those who are obese show less responsiveness to internal satiety signals (21, 22) eat more quickly during meals (23, 24) and show greater sensitivity to food cues than healthy weight children (25). There are few studies among infants, but in a longitudinal study, parental report of their infants' response to food cues and enjoyment of food were associated with subsequent weight gain (26, 27). However, one limitation of this study was that parents were asked when their children were 8 months of age to report the feeding behavior of their infants for the first 3 months of life, and so it is possible that the parents' recall was influenced by the child's current weight status. In an observational study of infants, Stunkard and colleagues (28) found that infants with vigorous sucking subsequently gained more weight up to 3 years of age. Together these findings suggest that infant eating behaviors may be an important predictor of subsequent weight gain.
The overall objectives of this study were first, to examine whether maternal reports of infant eating behaviors are consistent across infancy, and second, to examine whether maternal reports of infant eating behaviors are predictive of subsequent weight gain. We hypothesized that mothers would report their infants eating faster and becoming more responsive to food cues as they got older. Furthermore, we hypothesized that maternal reports of low satiety responsiveness, rapid eating, and greater food responsiveness and enjoyment at 2-weeks and 3-months of age would be associated with greater weight gain thereafter. These hypotheses were tested in data collected from a cohort of primarily African American bottle-feeding mother-infant dyads enrolled in an ongoing growth study.
Methods and Procedures
Participants
Mothers were recruited in the third trimester of pregnancy as part of a study to investigate metabolic health during pregnancy and infant growth to 3-months of age. Those who completed the 3-month visit were invited to attend another follow-up visit at 5-months of age. Women were excluded from participation in the parent study if they had pre-existing diabetes, had previously delivered a preterm (i.e. <37.0 weeks) or growth restricted (i.e. <2500 gram) infant, or had any medical condition during pregnancy that is believed to interfere with normal fetal growth. Infants were excluded if they were born prior to 37.0 weeks' gestation or were exclusively fed directly from the breast for the first 3-months of life. Only data from infants who attended at least two follow-up visits were included.
Protocol
Mothers and infants attended three study related visits at 2 weeks (17 ± 6 days), 3 months (93 ± 4 days) and 5 months (153 ± 3 days) of age. At each visit, infant length (cm) and weight (g) was obtained using standard clinical procedure, and the Baby Eating Behavior Questionnaire (BEBQ) (29) was administered to examine infant eating behaviors. Additional data retrieved from prenatal records included: maternal date of birth, race, BMI in early pregnancy, gestational weight gain, infant sex, gestational age at delivery, birth weight, and birth length.
Baby Eating Behavior Questionnaire (BEBQ)
The BEBQ questionnaire was adapted from the Child Eating Behavior Questionnaire (CEBQ) used for older children, which was originally validated against objective behavioral measures (29, 30). The BEBQ asks parents to rate 18 statements on a 5-point scale of never, rarely, sometimes, often or always. Items load onto four subscales: Enjoyment of Eating (EF; e.g., My baby enjoys feeding time); Food Responsiveness (FR; e.g., My baby frequently wants more milk than I have given him/her); Slowness in Eating (SE; e.g., My baby finishes feeding quickly); Satiety Responsiveness (SR; e.g., My baby sucks/drinks more and more slowly during a feed). There is also a fifth subscale, General Appetite, which consists of one item (GA; e.g., My baby has a big appetite). The BEBQ was verbally administered to mothers during the study visits.
Statistical analysis
Infant weight-for-length was derived using the World Health Organization (WHO) Child Growth Standards (31), which are based on international growth charts of healthy infants growing under optimal conditions. Change in weight-for-length z-scores was calculated by subtracting the earlier z-score from the later (i.e. 3-month – 2-week and 5-month – 3-month). Repeated measures analysis of variance (ANOVA) was used to examine changes in BEBQ scores from 2-weeks to 3-months and from 3-months to 5-months. Simple Pearson correlations were used to assess associations among maternal reports of infant eating behaviors at 2-weeks and 3-months, and at 3-months and 5-months. Simple Pearson correlations were also used to examine whether BEBQ scores at one time point were associated with subsequent change in weight-for-age and weight-for-length z-scores.
Results
Forty-seven mothers enrolled in the parent study. Two infants were excluded from follow-up because they were born preterm, and data from one infant who was exclusively fed from the breast was removed. Three infants did not return for any post-partum follow-up visits, and six attended only one visit. Final analyses for the 2-week to 3-month assessments were conducted on data from 31 infants who attended both of these study visits. This number reflects 70% retention of eligible infants from the parent study. There was no difference in birth weight-for-length z-score or gestational age among infants retained for the 2-week to 3-month analyses versus those who were lost to follow-up. Further loss to follow-up was experienced after 3-months which may be partly due to the fact that this extra follow-up was not part of the original study. Data from 21 infants were included in the 3-5 month assessments, which represents 64% of the sample who were informed of this follow-up study. There were no differences in weight-for-length z-scores at birth, 2-weeks, or 3-months among those who were included in the 3-5-month analyses as compared to those lost to follow-up. These groups also did not differ in terms of gestational age at delivery and BEBQ scores at 2-weeks and 3-months, or feeding mode at 2-weeks. Characteristics of the study participants are shown in Table 1. All except one infant was African American and 61% of the sample was male. The majority of the infants received formula only across the duration of the study, but at 2-weeks of age, 12 were receiving some breastmilk and 1 received rice cereal. By 3-months of age, 1 infant was still receiving some breastmilk and 12 infants were receiving complementary foods (e.g., cereal, baby food, or other table foods). The type of formula used at 2-weeks was reported for all but six of the mothers, with the majority using Similac Advance (N=12) or Similac Sensitive (N=6). The remainder used another type of Similac formula or did not specify which type it was. Two mothers reported that they had changed formula by the 3-month visit, and were now using Similac Advance instead of Similac SpitUp or Similac Sensitive. The majority of the infants were healthy, with only 5 (16%) mothers reporting use of medication for common gastrointestinal disturbances, such as reflux or gas, at some point in the study.
Table 1.
Characteristics of the study sample (N=31 unless noted).
| Variable | Mean ± SD |
|---|---|
| Maternal BMI in early pregnancy (kg/m2) | 29.57 ± 9.11 |
| Gestational age at delivery (weeks) | 39.46 ± 1.20 |
| Birth weight (kg) | 3.22 ± 0.36 |
| Birth length (cm) | 50.19 ± 2.05 |
| Birth weight-for-length z-score | -0.63 ± 1.17 |
| 2 weeks | |
| Weight (kg) | 3.57 ± 0.37 |
| Length (cm) | 51.44 ± 1.94 |
| Weight-for-length z-score | -0.39 ± 1.28 |
| 3 months | |
| Weight (kg) | 6.08 ± 0.69 |
| Length (cm) | 60.49 ± 1.90 |
| Weight-for-length z-score | -0.03 ± 0.94 |
| 5 months1 | |
| Weight (kg) | 7.14 ± 1.85 |
| Length (cm) | 64.27 ± 5.36 |
| Weight-for-length z-score | 0.36 ± 1.15 |
N=21
Table 2 shows associations among the infant eating behaviors from 2-weeks to 3-months, and from 3-5-months of age. From 2-weeks to 3-months, maternal reports of FR and SR, but not EF and SE, remained consistent (P<0.05), and there was a trend for general appetite to also be consistent (P=0.06). Partial correlations were also run to examine whether these associations were independent of the exclusivity of formula feeding at 2 weeks and results were similar (not shown). From 3-months to 5-months, maternal reports of FR, FE, SE, and general appetite, but not SR, remained consistent (P<0.01). Similar to the 2-week to 3-month associations, the results did not change when the associations were adjusted for the presence or absence of complementary foods in the diet (not shown). Given that the analyses from 3-5-months differed both in terms of the age of the infants and the sample size, the correlations between scores at 2-weeks and 3-months were re-ran with only the those infants who also were included in the 3-5-month comparison (not shown). Results were similar to those described above, with FR being the only feeding behavior that remained consistent from 2-weeks to 3-months (r = 0.62, P<0.05).For the 31 infants who attended the first two visits, there was no significant change reported for FR, EF, SR, or general appetite. However, mothers reported a decrease in infant SE over time (F (30, 1) = 10.01, P ≤ 0.01), indicating that the infants ate more quickly as they got older (Figure 1A). Among the 21 infants who attended the 3- and 5-month visits, mothers reported that SR increased as the infants got older (F (20, 1) = 5.18, P < 0.05; Figure 1B), but the other variables did not change significantly.
Table 2. Simple Pearson correlations of the associations among eating behaviors across time.
| Eating Behavior | 2 weeks × 3 months1 | 3 months × 5 months2 |
|---|---|---|
| Enjoyment of Food | 0.16 | 0.72*** |
| Food Responsiveness | 0.63** | 0.83*** |
| Slowness in Eating | 0.21 | 0.64** |
| Satiety Responsiveness | 0.38* | 0.30 |
| General Appetite | 0.34+ | 0.66** |
N=31;
N=21;
0.05<P<0.10;
P<0.05;
P<0.01;
P≤0.001
Figure 1.
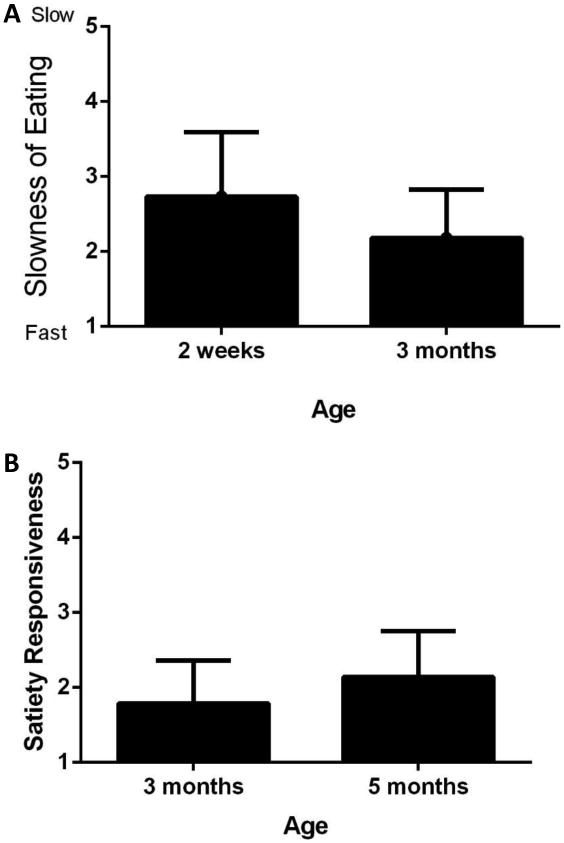
From 2-weeks to 3-months, speed of eating increased among infants (A: P≤0.01), and from 3-months to 5-months, satiety responsiveness also increased (B: P<0.05).
Eating behaviors at 2-weeks of age were not found to be associated with change in weight-for-length z-score from 2-weeks to 3-months. However, SE at 3-months was inversely associated with change in weight-for-length from 3-months to 5-months (r = -0.51, P < 0.05; Figure 2A) and there was a trend for FR at 3-months to be positively associated with change in weight-for-length z-score from 3-months to 5-months (r = 0.39, P = 0.08; Figure 2B). Results were similar when the analyses were repeated without data from the single Caucasian child (i.e. SE × change in weight-for-length: r = -0.56, P < 0.05; FR × change in weight-for-length: r = 0.42, P = 0.07), and when adjusted for the exclusivity of formula feeding at 2-weeks or the addition of complementary foods at 3-months (not shown).
Figure 2.
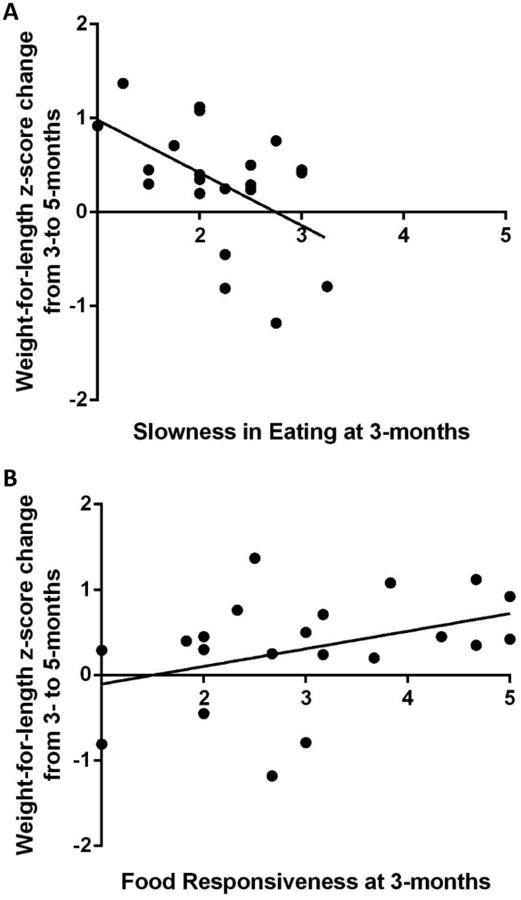
Weight-for-length z-score gain from 3- to 5-months was greater for infants who were reported to be quicker eaters at 3-months of age (A: r = -0.51, P<0.05), and also tended to be greater among infants who were reported to be relatively more responsive to food cues at 3-months of age (B: r = 0.39, P=0.08).
Discussion
The overall goals of this study were to explore whether maternal reports of infant eating behaviors were consistent over time and whether infant eating behaviors, as reported by the mothers, were predictive of subsequent weight gain. With the exception of SR, maternal reports of their infants' eating behaviors were more consistent between 3- and 5- months than they were from 2-weeks to 3-months. However, mothers reported that their infants ate more quickly by 3-months of age, as compared to 2-weeks, and they became more responsive to satiety from 3-5 months. Although reported eating behaviors at 2-weeks were not predictive of subsequent gain in weight-for-length z-score to 3-months of age, SE at 3-months was inversely associated with weight-for-length z-score gain to 5-months of age.
In this cohort, mothers reported that their infants' eating behaviors become relatively stable after the neonatal phase, although exactly when and how this occurs is not clear. To our knowledge, there are no other studies that have examined the stability of maternal reports of infant eating behaviors over time, but a study of older children showed that eating behaviors were established by the time children were four years of age, and remained relatively consistent through 10 years of age, albeit with modest associations (r = 0.29 – 0.55) (32). In the current study, the correlation coefficients among most of the eating behaviors were relatively robust (i.e., 0.66 – 0.83) between 3- and 5- months of age, but it is not known whether this pattern will persist over a longer duration of time, particularly in light of the food transitions that occur during the first few years of life. It is possible that greater consistency is seen among infants in comparison to older children because of the more limited diet of infants. However, given that this association was independent of whether the children were receiving complementary foods at 3-months of age, results from this cohort suggest that, at least during infancy, the mode of feeding may not influence how consistently mothers perceive their children's feeding behavior to be. It would be of interest in the future to examine whether eating behaviors remain stable into early childhood despite transitioning to table food.
Maternal report of infant response to satiety was the only eating behavior that was not consistent from 3- to 5-months of age, with mothers reporting increased satiety responsiveness with age. Although little is known about satiety responsiveness among very young infants, there is evidence from previous research that satiety regulation is established after the first month or two of life. In survey data from a nationally representative cohort, 4-5 month-old infants consumed smaller meals when they had a greater frequency of meals (33). Another study found that by 7- to 14-weeks of age, infants are able to regulate the amount of milk they drink in response to the amount of time that has passed since the previous meal (34). To our knowledge, however, no previous studies have examined whether younger infants respond to internal satiety cues from the first days of life.
Mothers in this cohort also reported that infants ate more quickly as they got older. It is perhaps not surprising that infants would become more efficient at eating as they mature. It is interesting to note, however, that despite the overall change in speed of eating, infants who were reported to be relatively quick at eating at a young age remained so as they aged. Speed of eating among adults has been associated with obesity (35, 36), and this is consistent with the Stunkard et al. finding that infants with more vigorous sucking had a greater body weight at three years of age (28). We found a similar trend in the current study for greater weight gain from 3-5 months of age among those who were reported to be more rapid eaters at 3-months of age. If these findings are confirmed in a larger prospective cohort, it may be interesting in the future to examine whether an intervention to slow infant intake could reduce the risk for rapid weight gain.
In the current study, there was also a trend for responsiveness to food cues at 3-months to be prospectively associated with greater gain in weight-for-length. Although our sample is small, this finding is consistent with previous research among infants (26, 27), and with studies among adults and older children showing that response to food cues is associated with obesity (21, 25, 37-40). In addition, given that response to food cues was reported to be relatively consistent across the ages examined, these data suggest that food responsiveness may be established during the early neonatal phase. It will be of interest in the future to examine reasons for individual differences in infant response to food cues and mechanisms that underlie the generalization of this behavior from breast to bottle and to cues associated with table food.
There are several limitations of this study that should be addressed. First, this study was conducted in a small, homogenous sample, and as such, it is not known whether the results will generalize to breastfed infants and to those of other racial or ethnic groups. Further, significant loss to follow-up was experienced across the duration of this study which, although not uncommon in studies of low income families, raises questions about whether data from the later visits was biased. In exploratory analyses, however, those who remained versus those who dropped out of this study did not differ in terms of their feeding behavior scores or weight-for-length prior to drop-out. Another limitation of this study is the use of a parent-report measure rather than objective behavioral measurements, which raises the possibility that parental perception of infant feeding behavior became more consistent with time, rather than there being an actual change in infant feeding behavior. However, the child version of the questionnaire that was used to develop the BEBQ has shown good validity when compared with behavioral measures (30), suggesting that parental report does reflect actual feeding behavior. This study was strengthened by the use of a prospective, longitudinal design, which to our knowledge, has not previously been undertaken.
Together with previous research, the results of this exploratory study suggest that potentially obesogenic feeding behaviors, such as rapid eating, may be established at a very young age and may play an important role in the development of obesity. These implications warrant further study in a larger, less homogenous sample. It will also be of interest in the future to examine whether interventions can change these feeding behaviors during infancy and possibly reduce the risk for overeating and subsequent obesity.
What is already known about this subject
Rapid weight gain during infancy is a consistent predictor of subsequent obesity.
Infants of ethnic minority are at an increased risk for rapid infant weight gain.
Parental recall of infants' response to food cues and enjoyment of eating has been associated with infant weight gain.
What this study adds
In this cohort, maternal reports of eating behaviors were relatively stable from 3 to 5 months of age.
Maternal report of infants' slowness in eating at 3-months of age was prospectively associated with subsequent weight gain.
Acknowledgments
Both authors were involved in writing the paper and had final approval of the submitted and published versions. The authors thank Britney Blackstock for data collection. This project was supported by Award Numbers P30DK079626, P30DK056336, and K01DK090126, from the National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Conflicts of Interest Statement: The authors declare no conflict of interest related to this study.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311(8):806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daniels SR. The consequences of childhood overweight and obesity. The Future of Children. 2006;16(1):47–67. doi: 10.1353/foc.2006.0004. [DOI] [PubMed] [Google Scholar]
- 3.Pulgarón ER. Childhood Obesity: A Review of Increased Risk for Physical and Psychological Comorbidities. Clin Ther. 2013;35(1):A18–A32. doi: 10.1016/j.clinthera.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biro FM, Wien M. Childhood obesity and adult morbidities. Am J Clin Nutr. 2010;91(5):1499S–1505S. doi: 10.3945/ajcn.2010.28701B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH. Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med. 1997;337(13):869–873. doi: 10.1056/NEJM199709253371301. [DOI] [PubMed] [Google Scholar]
- 6.Dubois L, Girard M. Early determinants of overweight at 4.5 years in a population-based longitudinal study. Int J Obes (Lond) 2006;30(4):610–617. doi: 10.1038/sj.ijo.0803141. [DOI] [PubMed] [Google Scholar]
- 7.Stettler N, Zemel BS, Kumanyika S, Stallings VA. Infant weight gain and childhood overweight status in a multicenter, cohort study. Pediatrics. 2002;109(2):194–199. doi: 10.1542/peds.109.2.194. [DOI] [PubMed] [Google Scholar]
- 8.Taveras EM, Rifas-Shiman SL, Sherry B, Oken E, Haines J, Kleinman K, et al. Crossing growth percentiles in infancy and risk of obesity in childhood. Arch Pediatr Adolesc Med. 2011;165(11):993–998. doi: 10.1001/archpediatrics.2011.167. [DOI] [PubMed] [Google Scholar]
- 9.Lamb M, Dabelea D, Yin X, Ogden L, Klingensmith G, Rewers M, et al. Early-life predictors of higher body mass index in healthy children. Ann Nutr Metab. 2010;56(1):16–22. doi: 10.1159/000261899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ekelund U, Ong K, Linné Y, Neovius M, Brage S, Dunger DB, et al. Upward weight percentile crossing in infancy and early childhood independently predicts fat mass in young adults: the Stockholm Weight Development Study (SWEDES) Am J Clin Nutr. 2006;83(2):324–330. doi: 10.1093/ajcn/83.2.324. [DOI] [PubMed] [Google Scholar]
- 11.Eriksson M, Tynelius P, Rasmussen F. Associations of birthweight and infant growth with body composition at age 15--the COMPASS study. Paediatr Perinat Epidemiol. 2008;22(4):379–388. doi: 10.1111/j.1365-3016.2008.00944.x. [DOI] [PubMed] [Google Scholar]
- 12.Ekelund U, Ong K, Linné Y, Neovius M, Brage S, Dunger DB, et al. Upward weight percentile crossing in infancy and early childhood independently predicts fat mass in young adults: the Stockholm Weight Development Study (SWEDES) Am J Clin Nutr. 2006;83:324–330. doi: 10.1093/ajcn/83.2.324. [DOI] [PubMed] [Google Scholar]
- 13.Oyama M, Saito T, Nakamura K. Rapid weight gain in early infancy is associated with adult body fat percentage in young women. Environ Health Prev Med. 2010;15(6):381–385. doi: 10.1007/s12199-010-0152-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dennison BA, Edmunds LS, Stratton HH, Pruzek RM. Rapid infant weight gain predicts childhood overweight. Obes. 2006;14(3):491–499. doi: 10.1038/oby.2006.64. [DOI] [PubMed] [Google Scholar]
- 15.Chandler-Laney PC, Gower BA, Fields DA. Gestational and early life influences on infant body composition at 1 year. Obesity (Silver Spring) 2013;21(1):144–148. doi: 10.1002/oby.20236. [DOI] [PubMed] [Google Scholar]
- 16.Chomtho S, Wells JC, Williams JE, Davies PS, Lucas A, Fewtrell MS. Infant growth and later body composition: evidence from the 4-component model. Am J Clin Nutr. 2008;87(6):1776–1784. doi: 10.1093/ajcn/87.6.1776. [DOI] [PubMed] [Google Scholar]
- 17.Vega GL, Adams-Huet B, Peshock R, Willett D, Shah B, Grundy SM. Influence of body fat content and distribution on variation in metabolic risk. J Clin Endocrinol Metab. 2006;91(11):4459–4466. doi: 10.1210/jc.2006-0814. [DOI] [PubMed] [Google Scholar]
- 18.Goran MI, Gower BA. Relation between visceral fat and disease risk in children and adolescents. Am J Clin Nutr. 1999;70(1):149S–156S. doi: 10.1093/ajcn/70.1.149s. [DOI] [PubMed] [Google Scholar]
- 19.Li R, O'Connor L, Buckley D, Specker B. Relation of activity levels to body fat in infants 6 to 12 months of age. J of Ped. 1995;126(3):353–357. doi: 10.1016/s0022-3476(95)70447-7. [DOI] [PubMed] [Google Scholar]
- 20.Taveras EM, Gillman MW, Kleinman K, Rich-Edwards JW, Rifas-Shiman SL. Racial/ethnic differences in early-life risk factors for childhood obesity. Pediatrics. 2015;125(4):686–695. doi: 10.1542/peds.2009-2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fisher JO, Cai GW, Jaramillo SJ, Cole SA, Comuzzie AG, Butte NF. Heritability of hyperphagic eating behavior and appetite related hormones among Hispanic children. Obes. 2007;15:1484–1495. doi: 10.1038/oby.2007.177. [DOI] [PubMed] [Google Scholar]
- 22.Moens E, Braet C. Predictors of disinhibited eating in children with and without overweight. Behav Res Ther. 2007;45:1357–1638. doi: 10.1016/j.brat.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Barkeling B, Ekman S, Rossner S. Eating behavior in obese and normal weight 11-year-old children. Int J Obes. 1992;16:355–360. [PubMed] [Google Scholar]
- 24.Drabman RS, Cordua GD, Hammer D, Jarvie GJ, Horton W. Developmental-trends in eating rates of normal and overweight preschool-children. Child Dev. 1979;50:211–216. [PubMed] [Google Scholar]
- 25.Ludwig DS, Peterson KE, Gortmaker SL. Relation between consumption of sugar-sweetened drinks and childhood obesity: a prospective, observational analysis. Lancet. 2001;357:505–508. doi: 10.1016/S0140-6736(00)04041-1. [DOI] [PubMed] [Google Scholar]
- 26.van Jaarsveld C, Llewellyn C, Johnson L, Wardle J. Prospective associations between appetitive traits and weight gain in infancy. Amer J Clin Nutr. 2011;94(6):1562–1567. doi: 10.3945/ajcn.111.015818. [DOI] [PubMed] [Google Scholar]
- 27.van Jaarsveld C, Boniface D, Llewellyn C, Wardle J. Appetite and Growth: A longitudinal sibling analysis. JAMA Pediatrics. 2014;168(4):345–350. doi: 10.1001/jamapediatrics.2013.4951. [DOI] [PubMed] [Google Scholar]
- 28.Stunkard AJ, Berkowitz RI, Stallings VA, Schoeller DA. Energy intake, not energy output, is a determinant of body size in infants. Amer J Clin Nutr. 1999;69:524–530. doi: 10.1093/ajcn/69.3.524. [DOI] [PubMed] [Google Scholar]
- 29.Llewellyn CH, van Jaarsveld CHM, Johnson L, Carnell S, Wardle J. Development and facture structure of the Baby Eating Behaviour Questionnaire in the Gemini birth cohort. Appetite. 2011;57:388–396. doi: 10.1016/j.appet.2011.05.324. [DOI] [PubMed] [Google Scholar]
- 30.Carnell S, Wardle J. Measuring behavioural susceptibility to obesity: validation of the child eating behaviour questionnaire. Appetite. 2007;48(1):104–113. doi: 10.1016/j.appet.2006.07.075. [DOI] [PubMed] [Google Scholar]
- 31.WHO Multicenter Growth Reference Study Group. Methods and development. World Health Organization; Geneva: 2006. WHO Child Growth Standards: Length/height-for age, weight-for-age, weight-for-length, weight-for-height, and body mass index-for-age; p. 312. [Google Scholar]
- 32.Ashcroft J, Semmler C, Carnell S, van Jaarsveld CH, Wardle J. Continuity and stability of eating behaviour traits in children. Eur J Clin Nutr. 2008;62(8):985–990. doi: 10.1038/sj.ejcn.1602855. [DOI] [PubMed] [Google Scholar]
- 33.Fox MK, Reidy K, Novak T, Ziegler P. Sources of energy and nutrients in the diets of infants and toddlers. J Am Diet Assoc. 2006;106(1 Suppl):S28–S42. doi: 10.1016/j.jada.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 34.Lumeng JC, Patil N, Blass EM. Social influences on formula intake via suckling in 7 to 14-week old-infants. Dev Psychobiol. 2007;49:351–361. doi: 10.1002/dev.20221. [DOI] [PubMed] [Google Scholar]
- 35.Laessle RG, Lehrke S, Duckers S. Laboratory eating behavior in obesity. Appetite. 2007;49:399–404. doi: 10.1016/j.appet.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 36.Westerterp-Plantenga MS, Wouters L, Ten Hoor F. Restrained eating, obesity, and cumulative food intake curves during four course meals. Appetite. 1991;16:149–158. doi: 10.1016/0195-6663(91)90040-y. [DOI] [PubMed] [Google Scholar]
- 37.Birch LL, Fisher JO, Davison KK. Learning to overeat: maternal use of restrictive feeding practices promotes girls' eating in the absence of hunger. Amer J Clin Nutr. 2003;78:215–220. doi: 10.1093/ajcn/78.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DelParigi A, Pa DN, Tataranni PA. In pursuit of neural risk factors for weight gain in humans. Neurobiol Aging. 2005;Suppl 1:50–55. doi: 10.1016/j.neurobiolaging.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 39.Tataranni PA, DelParigi A. Functional neuroimaging: a new generation of human brain studies in obesity research. Obes Rev. 2003;4(4):229–238. doi: 10.1046/j.1467-789x.2003.00111.x. [DOI] [PubMed] [Google Scholar]
- 40.Schachter S. Obesity and Eating. Science. 1968;161(3843):751–756. doi: 10.1126/science.161.3843.751. [DOI] [PubMed] [Google Scholar]


