Abstract
Objective
Environmental endocrine disrupting chemicals (EDCs) are increasingly implicated in the pathogenesis of obesity. Evidence implicates various EDCs as being pro-adipogenic, including tributyltin (TBT), which activates the peroxisome proliferator activated receptor-γ (PPARγ). However, the conditions required for TBT-induced adipogenesis and its functional consequences are incompletely known.
Methods
The co-stimulatory conditions necessary for preadipocyte-to-adipocyte differentiation were compared between TBT and the pharmacological PPARγ agonist troglitazone (Trog) in the 3T3-L1 cell line; basal and insulin-stimulated glucose uptake were assessed using radiolabeled 2-deoxyglucose.
Results
TBT enhanced expression of the adipocyte marker C/EBPα with co-exposure to either isobutylmethylxanthine or insulin in the absence of other adipogenic stimuli. Examination of several adipocyte-specific proteins revealed that TBT and Trog differentially affected protein expression despite comparable PPARγ stimulation. In particular, TBT reduced adiponectin expression upon maximal adipogenic stimulation. Under submaximal stimulation, TBT and Trog differentially promoted adipocyte-specific gene expression despite similar lipid accumulation. Moreover, TBT attenuated Trog-induced adipocyte gene expression under conditions of co-treatment. Finally, TBT-induced adipocytes exhibited altered glucose metabolism, with increased basal glucose uptake.
Conclusions
TBT-induced adipocytes are functionally distinct from those generated by a pharmacological PPARγ agonist, suggesting that obesogen-induced adipogenesis may generate dysfunctional adipocytes with the capacity to deleteriously affect global energy homeostasis.
Keywords: adipocyte, adipogenesis, adiponectin, endocrine disrupting chemicals, PPARγ, tributyltin, troglitazone
Introduction
Global metabolic health has deteriorated dramatically over the last several decades with the emergence of the dual obesity and diabetes epidemics. While increased caloric consumption and physical inactivity are central drivers in the pathogenesis of metabolic diseases, these factors alone fail to fully account for the rapid rise in obesity and diabetes rates. The Environmental Obesogen Hypothesis posits that the burgeoning obesity epidemic is partially a consequence of the modulation of adipose development and function by synthetic chemicals (1). Significant attention has focused on the capacity of environmental endocrine disrupting chemicals (EDCs) to promote adipogenesis (2), particularly through stimulation of the peroxisome proliferator activated receptor-γ (PPARγ). Because PPARγ is a principle regulator of adipocyte differentiation and function (3), compounds with the capacity to activate PPARγ signaling are of great interest for understanding how synthetic chemicals might promote adipose accumulation. Indeed, several EDCs have been shown to act as PPARγ agonists, including alkylated tin compounds, phthalates, flame retardants, and fungicides (reviewed in ref. (2)), suggesting that diverse exposures may alter adipose development and function.
The best studied of these environmental obesogens is tributyltin (TBT), which functions as a nanomolar agonist of both PPARγ and retinoid X receptor-α (RXRα) (4). A number of studies have shown that TBT augments adipocyte differentiation in cell lines (1,5,6), while also promoting fat deposition (7) and increased body weight (8) following in vivo exposure. Furthermore, in utero exposure to TBT increases adiposity postnatally (1,9). Based on this strong data, studies of TBT form the foundation of the environmental obesogen hypothesis. Because of the metabolic benefits of smaller, more metabolically active adipocytes and the salutary metabolic effects of pharmacological PPARγ agonists, e.g. thiazolidinediones (TZDs) (10), the pro-adipogenic effects of TBT would be predicted to improve energy homeostasis. However, in some experimental animal models, acute and chronic exposure to TBT resulted in metabolically deranged phenotypes (11,12). This apparent paradox raises questions about the precise effects of TBT on adipose tissue development; therefore, studies were undertaken to delineate the contextual effects of TBT on adipocyte differentiation and to characterize the metabolic consequences of TBT-induced differentiation on mature adipocyte function.
Methods
3T3-L1 Culture and Differentiation
3T3-L1 preadipocytes were cultured in 10% calf serum as previously described (13). After reaching confluence, cells were refed for an additional two days at which point differentiation was initiated by the addition of Dulbecco’s modified Eagle’s medium (DMEM; Mediatech, Manassas, VA) containing 10% fetal bovine serum (FBS; Aleken Biologicals, Nash, TX) and components of the differentiation cocktail: 167 nM insulin, a glucocorticoid receptor agonist (100 nM corticosterone (Cort) or 250 nM dexamethasone (Dex)), and/or 0.5 mM isobutylmethylxanthine (MIX) (all from Sigma, St. Louis, MO). After three days, cells were cultured for two additional days in DMEM plus 10% FBS and 167 nM insulin, after which assays were performed. The effects of TBT (5 or 50 nM) or the TZD troglitazone (Trog; 2.5 μM) on 3T3-L1 differentiation were determined by incorporating TBT and/or Trog into the first 3 days of the differentiation protocol. All compounds were dissolved in 100% ethanol as vehicle (Sigma), with cells exposed to a final ethanol concentration of ≤0.1%. All co-exposure studies utilized TBT 50 nM and Trog 2.5 μM.
Luciferase Assays
PPARγ activity in undifferentiated 3T3-L1 preadipocytes was determined by luciferase assay as previously described (13). Briefly, subconfluent 3T3-L1 preadipocytes were transiently transfected with 2 μg of luciferase construct containing two copies of the phosphoenolpyruvate carboxykinase PPARγ response element into the pGL2-Promoter vector (Promega, Madison, WI) and 2 μg of PPARγ using Lipofectamine Plus (Invitrogen, Carlsbad, CA) over 16– 18 h. Cells were then washed with PBS prior to 24 hour treatment with vehicle, TBT, or Trog in DMEM plus 10% calf serum. Cells were harvested and lysed, and luciferase activity determined as previously described (14).
Quantification of Adipocyte Lipid Accumulation
Lipid accumulation in differentiated 3T3-L1 adipocytes was determined by quantitative ORO staining. ORO (Sigma) was dissolved in isopropanol overnight at a concentration of 0.35% followed by 0.2 μm filtration, dilution in water to a final concentration of 0.2%, and refiltration. Adipocytes were washed with PBS followed by 10% formalin fixation for 60 min. Cells were then washed with 60% isopropanol, allowed to dry, and stained with ORO for 10 min. Following multiple water washes, plates were dried at room temperature, ORO was eluted in 100% isopropanol, and 500 nm absorbance of the isopropanol solution measured using a Synergy H1 Hybrid Reader (BioTek, Winooski, VT).
Protein and Gene Expression Analyses
Preparation of whole-cell lysates, SDS-PAGE, and immunoblotting were performed as previously described (15). Western blots were probed with anti-adiponectin (Millipore, Billerica, MA), anti-β-actin, anti-CCAAT/enhancer binding protein-α (C/EBP-α), anti-perilipin, and anti-PPARγ (Cell Signaling Technology, Beverly, MA) antibodies. Next, blots were probed with horseradish peroxidase-conjugated goat anti-mouse (adiponectin) or anti-rabbit (all other proteins) secondary antibodies (Bio-Rad, Hercules, CA). Relative protein expression was evaluated by densitometry using ImageJ version 1.47 (National Institutes of Health), with β-actin used to control for total protein recovery. RNA isolation, cDNA synthesis, and quantitative real time-PCR (qRT-PCR) were performed as previously described (16). Relative gene expression was evaluated by the ΔCt method (17), with β-actin used to control for total mRNA recovery. Primers were designed using Primer-BLAST (National Center for Biotechnology Information) and obtained from Integrated DNA Technologies (Supplemental Table 1).
Glucose Uptake Assay
Following differentiation, glucose transport was assessed by the uptake of 2-deoxy-D-[3H]-glucose (3H-2-DG). Cells were incubated in assay media consisting of DMEM, 25 mM Hepes (pH 7.4), 0.5% FBS, and 5 mM glucose for 2.5 hours. Next, cells were washed with assay media lacking glucose, and incubated in this media in the presence or absence of 100 nM insulin for 30 min. The media were then spiked with 0.4 μCi 3H-2-DG in 200 μM unlabeled 2-DG and incubated for 5 minutes. Probe uptake was terminated by placing the cells on ice and adding 200 mM unlabeled 2-DG, followed by washing with cold PBS. Cells were then scraped into ddH20, and cellular 3H-2-DG content was quantified by liquid scintillation counting.
Statistical Analyses
Between group differences were identified by repeated measures one-way analysis of variance (ANOVA) followed by Fisher’s least significant difference post hoc test using GraphPad Prism version 6.0e (La Jolla, CA). All results represent N ≥3 independent experiments, each performed in triplicate. P<0.05 was considered significant for all analyses.
Results
TBT and Trog Stimulate PPARγ Activity
To determine appropriate TBT and Trog concentrations for comparison, PPARγ luciferase assays were performed. Relative to a vehicle-induced activity of 1.0, Trog 2.5 μM increased luciferase expression to 1.8 ± 0.09 (P<0.001); TBT 5 nM increased expression to 1.2 ± 0.12 (P=0.29); TBT 50 nM increased expression to 1.6 ± 0.08 (P<0.01); and TBT 100 nM increased expression to 1.6 ± 0.04 (P<0.01). There was no statistically significant difference in luciferase activity between Trog 2.5 μM and TBT 50 nM (P=0.20); thus, these two treatments were considered comparable with regard to PPARγ activation for the described studies, while also utilizing the lowest TBT concentration to achieve equivalent activation of PPARγ signaling.
TBT and Trog Augment Lipid Accumulation
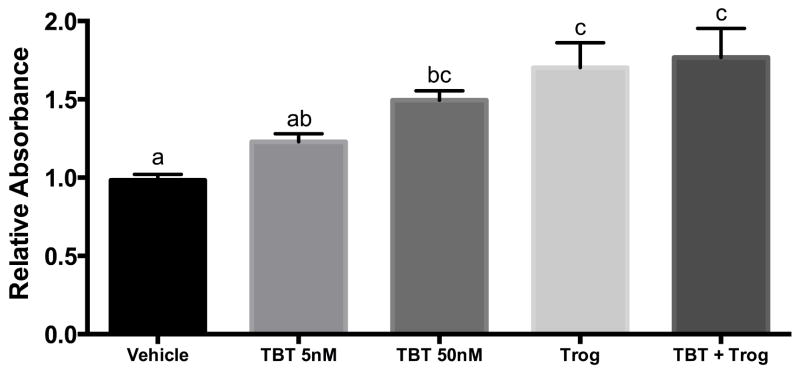
The well-characterized 3T3-L1 preadipocyte cell line is a model of adipogenesis with adipocyte differentiation induced using a cocktail that includes MIX to raise intracellular cAMP levels, the pharmacologic glucocorticoid Dex, and insulin (MDI). Adipocyte differentiation results in cellular lipid accumulation and expression of adipocyte-specific proteins. PPARγ agonists induce or augment 3T3-L1 differentiation (18). To assess the relative capacity of TBT and Trog to promote adipocyte differentiation, these compounds were included in a submaximal differentiation cocktail, substituting the active, but less potent, murine glucocorticoid Cort for Dex (MCI). PPARγ agonists were present only during the first 3 days of differentiation. Although TBT 5 nM did not increase lipid accumulation relative to the control (P=0.24), TBT 50 nM and Trog comparably increased lipid accumulation (Figure 1). Interestingly, the combination of Trog and TBT 50 nM did not promote further lipid accumulation relative to TBT or Trog alone.
Figure 1. Exposure to the environmental and pharmacological PPARγ agonists TBT and Trog during adipocyte differentiation promotes adipocyte lipid accumulation.
Two days after reaching confluency, 3T3-L1 preadipocytes treated with a submaximal MCI differentiation cocktail: 10% FBS in DMEM containing 167 nM insulin, 0.5 mM isobutylmethylxanthine, and 100 nM corticosterone in the presence of either vehicle, TBT (5 or 50 nM), 2.5 μM Trog, or 50 nM TBT plus 2.5 μM Trog. Following three days in differentiation media, media was replenished with 10% FBS in DMEM containing 167 nM insulin. Following two additional days in insulin-containing media, cells were assessed for lipid accumulation by ORO staining, with absorbance measured at 500 nM and increased absorbance indicative of increased lipid accumulation. Three independent experiments were performed in triplicate and normalized to a null condition. Differences were determined by ANOVA, and treatments not sharing a letter were significantly different with P<0.05. Data are presented as means ± S.E.M. TBT, tributyltin; Trog, troglitazone.
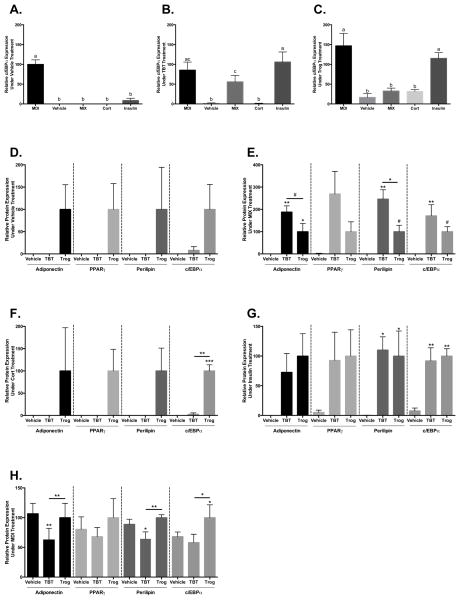
TBT and Trog Have Distinct Hormonal Requirements for Inducing Adipocyte-Specific Protein Expression
To characterize the conditions under which Trog and TBT promote adipocyte-specific protein expression, adipocyte development was assessed in the presence of each individual component of the MCI cocktail in isolation. Adipocyte differentiation was determined 7 days post-induction by the expression of adipocyte-specific proteins, including perilipin (lipid droplet-associated protein), C/EBPα, PPARγ (transcription factors), and adiponectin (adipokine). Expression of C/EBPα, an important early promoter of adipocyte differentiation (19), provides a measure of relative adipogenesis within and across conditions at the time point investigated (Figures 2A (Vehicle), 2B (TBT), and 2C (Trog)). In the presence of vehicle alone, neither Trog nor TBT significantly increased expression of adipocyte-specific proteins, although there were trends toward increased expression with Trog (Figure 2D). In the presence of MIX, however, TBT and Trog both increased adipocyte-specific protein expression, although the effect of TBT was more pronounced; TBT increased expression of adiponectin, perilipin, and C/EBPα while Trog only increased adiponectin expression (Figure 2E). In contrast, in the presence of Cort, Trog upregulated expression of C/EBPα while TBT did not (Figure 2F). In the presence of insulin, both Trog and TBT had similar effects on protein expression, increasing levels of perilipin and C/EBPα (Figure 2G). In addition, protein expression was interrogated following inclusion of Trog or TBT into the full MDI cocktail to assess effects under maximally stimulatory conditions (Figure 2H). Trog increased expression of PPARγ, C/EBPα, and adiponectin relative to TBT. Additionally, while Trog increased C/EBPα expression relative to vehicle, TBT co-exposure actually reduced adiponectin and perilipin expression relative to controls (Figure 2H).
Figure 2. TBT and Trog promote adipocyte differentiation in a context-dependent manner.
Two days after reaching confluency, 3T3-L1 preadipocytes were treated with differentiation media: 10% FBS in DMEM containing either the full MDI differentiation cocktail (0.5 mM MIX, 250 nM dexamethasone, and 167 nM insulin) or individual components of the submaximal MCI differentiation cocktail (0.5 mM MIX, 100 nM Cort, or 167 nM insulin) in the presence of absence of 50 nM TBT or 2.5 μM Trog as indicated. Following three days in differentiation media, media was replenished with 10% FBS in DMEM containing 167 nM insulin. Following two additional days in insulin-containing media, whole cell lysates were collected, processed, and resolved by immunoblotting. Relative protein expression of C/EBPα across conditions provides a measure of adipogenesis within vehicle (Panel A), TBT (Panel B), and Trog (Panel C) treatment groups. Expression of adiponectin, PPARγ, perilipin, and C/EBPα were assessed in vehicle (D), MIX (E), Cort (F), insulin (G), and MDI conditions (H). Relative protein expression was determined by densitometry and normalized to β-actin to control for total protein recovery, with three independent experiments performed in triplicate. Differences in protein expression were determined by ANOVA; data are presented as means ± S.E.M. Panels A–C were normalized to the mean of the MDI-vehicle treatment condition, and conditions not sharing a letter were significantly different at P<0.05. Panels D–H were normalized to the mean of Trog treatment for each protein investigated. # P<0.10; * P<0.05; ** P<0.01; *** P<0.001. C/EBPα, CCAAT/enhancer binding protein-α; Cort, corticosterone; MIX, isobutylmethylxanthine; PPARγ, peroxisome proliferator activated receptor-γ; TBT, tributyltin; Trog, troglitazone.
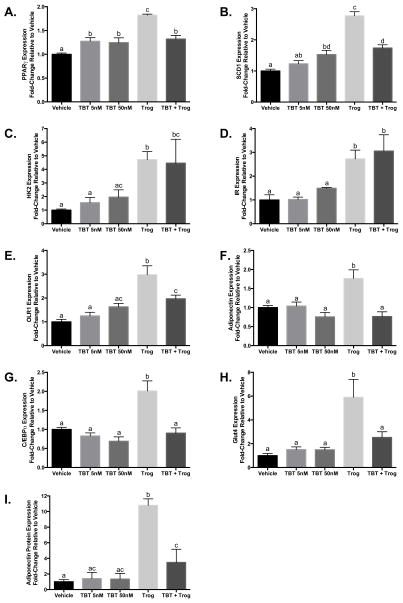
TBT and Trog Differentially Induce Adipocytic Gene and Protein Expression
Structurally distinct pharmacological PPARγ agonists differentially regulate transcription of PPARγ-responsive genes (20,21). Because TBT lacks structural similarity to pharmacological PPARγ agonists and because proper adipocyte function requires the expression of a multitude of PPARγ-responsive genes, studies were undertaken to determine whether TBT and Trog differentially altered expression of several PPARγ-regulated adipocytic genes in the presence of a submaximal MCI differentiation cocktail. While both TBT and Trog stimulated expression of some genes (e.g. PPARγ, Figure 3A; stearoyl-CoA desaturase-1 (SCD1), Figure 3B), the majority of genes investigated were responsive to Trog but not TBT (e.g. hexokinase 2 (HK2), insulin receptor (IR), oxidized low-density lipoprotein receptor-1 (OLR1), adiponectin, C/EBPα, and glucose transporter-4 (Glut4), Figures 3C, 3D, 3E, 3F, 3G, and 3H, respectively). Importantly, while TBT co-treatment did not affect Trog-stimulated gene expression for some genes (e.g. HK2, Figure 3C; IR, Figure 3D), TBT co-treatment attenuated or completely abolished Trog-stimulated expression of several genes (e.g. PPARγ, SCD1, adiponectin, OLR1, C/EBPα, and Glut4; Figures 3A, 3B, 3E, 3F, 3G, and 3H, respectively). Protein expression of the beneficial adipokine adiponectin was also assessed under MCI stimulation. Unlike Trog, TBT did not increase expression relative to vehicle; furthermore, co-treatment with TBT markedly reduced Trog-induced adiponectin expression (Figure 3I). These results suggest that although TBT promotes adipocyte differentiation and lipid accumulation, the resultant gene and protein expression profile of adipocytes is distinct from the pharmacological PPARγ agonist Trog; moreover, TBT alters Trog-induced expression in a gene-dependent manner.
Figure 3. TBT and Trog exposure during differentiation promote distinct gene and protein expression profiles in mature adipocytes.
Two days after reaching confluency, 3T3-L1 preadipocytes were provided submaximal MCI differentiation media: 10% FBS in DMEM containing 167 nM insulin, 0.5 mM MIX, and 100 nM corticosterone in the presence or absence of TBT (5 or 50 nM), 2.5 μM Trog, or 50 nM TBT plus 2.5 μM Trog. Following three days in differentiation media, media was replenished with 10% FBS in DMEM containing 167 nM insulin. Following two additional days in insulin-containing media, either RNA or whole cell lysates were collected. Gene expression of PPARγ (Panel A), SCD1 (Panel B), HK2 (Panel C), IR (Panel D), OLR1 (Panel E), adiponectin (Panel F), C/EBPα (Panel G), and Glut4 (Panel H), were assessed by qRT-PCR, and normalized to β-actin to control for RNA recovery. Whole cell lysates were processed and resolved by immunoblotting, with relative protein expression of adiponectin assessed by densitometry and normalized to β-actin to control for total protein recovery (I). N=3–10 performed in triplicate and normalized to a vehicle average of 1. Differences in gene and protein expression were determined by ANOVA; treatments not sharing a letter were significantly different at P<0.05. Data are presented as means ± S.E.M. C/EBPα, CCAAT/enhancer binding protein-α; Glut1, glucose transporter 1; Glut4, glucose transporter 4; HK2, hexokinase 2; IR, insulin receptor; OLR1, oxidized low-density lipoprotein receptor 1; PPARγ, peroxisome proliferator activated receptor-γ; SCD1, stearoyl-CoA desaturase-1; TBT, tributyltin; Trog, troglitazone.
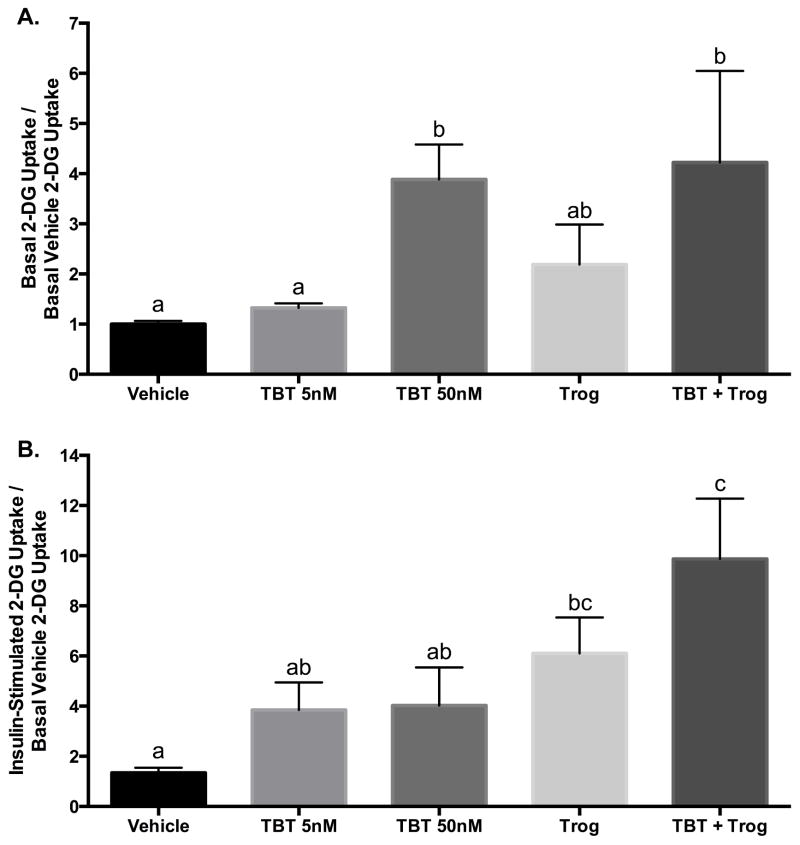
Exposure to TBT during Differentiation Augments Basal Glucose Uptake
Adipose tissue is essential for metabolic homeostasis, storing energy in times of caloric surfeit and mobilizing energy in times of caloric scarcity. To assess how exposure to TBT and Trog during differentiation affect adipocyte metabolism, glucose uptake was interrogated in mature adipocytes under basal and insulin-stimulated conditions after exposure during the first three days of differentiation. Despite similar PPARγ luciferase activity and lipid accumulation (Figure 1), TBT 50 nM increased basal glucose uptake, whereas Trog exerted no effect; co-exposure maintained the TBT effect (Figure 4A). Under insulin-stimulated conditions, exposure to Trog, but not TBT, augmented insulin-stimulated glucose uptake; the effect of Trog was maintained with co-exposure (Figure 4B). At the concentrations tested, these findings suggest that TBT and Trog differentially alter adipocyte glucose handling despite similar activation of PPARγ signaling and lipid accumulation.
Figure 4. TBT exposure during differentiation augments basal glucose uptake in mature adipocytes.
Two days after reaching confluency, 3T3-L1 preadipocytes were provided submaximal MCI differentiation media: 10% FBS in DMEM containing 167 nM insulin, 0.5 mM MIX, and 100 nM corticosterone in the presence or absence of TBT (5 or 50 nM), 2.5 μM Trog, or 50 nM TBT plus 2.5 μM Trog. Following three days in differentiation media, media was replenished with 10% FBS in DMEM containing 167 nM insulin. Following two additional days in insulin media, glucose uptake was assessed under basal (Panel A) or insulin-stimulated (100 nM) conditions (Panel B) by supplementing media with 3H-2-DG for 5 minutes and glucose uptake determined by liquid scintillation counting, with four independent experiments performed in triplicate and normalized to basal uptake under vehicle treatment. Differences in glucose uptake were determined by ANOVA; treatments not sharing a letter were significantly different at P<0.05. Data are presented as means ± S.E.M. 2-DG, 2-deoxy-D-glucose; TBT, tributyltin; Trog, troglitazone.
Discussion
TBT is an organotin that has historically been used as a booster biocide in marine paints, a fungicide on fruit crops and in wood preservatives, a disinfectant in textiles, and a polyvinylchloride (PVC) resin stabilizer (22). Human exposure principally occurs through contaminated water and seafood (23) as well as PVC-containing devices (24). While its use as a booster biocide has been curtailed by international treaties (25), its environmental persistence and continued use in developing countries render it an ongoing human health threat. A variety of studies have shown that TBT has the capacity to induce adipocyte differentiation (1,5,6); however, the effects of this chemical on adipocyte development and physiology are more complex and nuanced than initially appreciated and do no simply recapitulate the action of other PPARγ agonists.
In the present study, both TBT and Trog increased adipocyte differentiation (Figures 1 and 2); however, TBT exposure generated a unique gene expression profile relative to Trog (Figures 3A–H). Moreover, these studies show for the first time that the co-stimulatory conditions under which TBT promotes adipogenesis differ from those under which a TZD increases adipocyte development (Figure 2). Most notably, TBT increased expression of three adipocyte-specific proteins in the presence of elevated levels of cAMP brought about by the phosphodiesterase inhibitor MIX (Figure 2E). Increases in intracellular cAMP stimulate adipogenic differentiation by activating cAMP-responsive element-binding protein (CREB) (26), which induces a temporally regulated transcriptional cascade, increasing levels of multiple transcription factors, including C/EBPβ, C/EBPα, and PPARγ (19). Additionally, intracellular increases in cAMP may increase production of endogenous PPARγ ligands (27). Importantly, unlike Trog, TBT exhibits dual agonism on both RXRα and PPARγ (4). Because RXRα is a permissive partner of PPARγ, activation of RXRα independently increases PPARγ transcriptional activity (28). Indeed, in a murine bone marrow stem cell line, the RXRα agonist bexarotene promoted adipocyte differentiation (29). In models of acute myeloid leukemia, RXR agonist-induced cellular differentiation was contingent upon coordinate stimulation of cAMP signaling (30,31). Similar crosstalk between cAMP signaling and RXRα may explain the potentiation of TBT-induced adipogenesis with co-treatment with MIX. Importantly, increased sympathetic tone increases cAMP production through adrenergic signaling (32), suggesting that pathophysiologic states characterized by increased adrenergic tone, e.g. obstructive sleep apnea (32,33), may augment TBT-induced adipocyte development. Finally, because RXR signaling appears to be more important for human adipogenesis compared to mice (6), TBT agonism of RXRα in promoting human adipogenesis may be understated in murine studies.
Adipose tissue plays a central role in metabolic homeostasis through the regulated storage and mobilization of energy stores and the secretion of metabolic hormones (i.e. adipokines). Endogenous hormones modulate shifts in metabolic states, with insulin primarily stimulating the transition to nutrient uptake and storage (34). In the present study, TBT increased adipogenesis in the presence of insulin alone (Figure 2G), suggesting that TBT may be an especially potent adipogenic signal under hyperinsulinemic conditions, such as the postprandial state or in type 2 diabetes. Additionally, exposure to TBT during differentiation generated mature adipocytes with increased capacity for basal glucose uptake, an effect not seen with Trog (Figure 4A). If recapitulated in vivo, this increase in non-insulin-mediated glucose uptake could promote continuous expansion of adipose fat stores even under fasting conditions.
Adipose also modulates global energy homeostasis through the secretion of adipokines such as adiponectin. Among its many physiological effects, adiponectin increases global insulin sensitivity (35) and promotes β-cell survival (36). The present studies demonstrate that TBT, in contrast to Trog, decreases adiponectin protein expression when differentiation is maximally stimulated (Figure 2H), suggesting that TBT has the capacity to modulate adiponectin production in a manner expected to promote metabolic dysfunction. This suggests that the insulin resistance (12) and β-cell apoptosis (37) observed following in vivo TBT exposure could arise, at least partially, as a consequence of direct TBT disruption of adipose adiponectin production. Additionally, co-treatment with TBT completely abolished Trog-induced adiponectin gene expression (Figure 3F) and drastically blunted Trog-induced adiponectin protein expression (Figure 3I). In human studies, Trog increased circulating adiponectin levels, and concentrations of this beneficial adipokine both before and after treatment correlated with glucose disposal rates, suggesting that adiponectin may be an important mediator of the salutary effects of Trog on systemic glucose metabolism (38). Thus, it is possible that exposure to TBT may antagonize the anti-diabetic efficacy of Trog, and perhaps other TZDs. Finally, several EDCs have been shown to reduce adiponectin expression, including polychlorinated biphenyl-77 (39), bisphenol A (40), and tolylfluanid (16), suggesting that adiponectin may be a common target of metabolically disruptive EDCs.
Although the present studies have identified several novel aspects of TBT-induced adipogenesis, there are several limitations. First, these studies were conducted in the 3T3-L1 cell line, which models preadipocyte-to-adipocyte differentiation; potential effects on earlier stages of differentiation were not explored, e.g. mesenchymal stem cell commitment to the adipocyte lineage. Second, TBT and Trog exposure were restricted to the first three days of the differentiation protocol during which the cells undergo tremendous changes in gene expression. Whether the effects of TBT would be modulated by continued exposure throughout differentiation requires further investigation. Third, this investigation focused on a single time point for assessing markers of adipocyte differentiation and effects on glucose uptake. As such, it is possible that alternative exposure paradigms, modeling other concentrations and durations of treatment, may yield further insights into the differential effects of TBT and Trog in modulating adipocyte development and physiology. Fourth, the present studies employ an in vitro model to replicate an in vivo phenomenon. In vivo exposure is complicated by the interplay of multiple metabolic tissues in the regulation of energy homeostasis; however, specific interrogation of the functional state of adipose tissue after in vivo TBT exposure is warranted given the present results, as is more comprehensive metabolic profiling of TBT-exposed mice. Finally, Trog was utilized as the model pharmacological PPARγ agonist; however, given the ligand-specificity of PPARγ activity (20,21), interrogation of other pharmacological TZDs and EDC-agonists of PPARγ may further illuminate the unique adipocytic disruptions resulting from TBT exposure.
These novel findings suggest that all adipocytes generated by activation of PPARγ signaling are not created equal. Furthermore, these studies suggest that differences in the resulting adipocyte may have profound effects on the function of EDC-induced adipose tissue, and consequentially, global energy homeostasis. This adipocyte heterogeneity supports a more expansive approach to characterizing environmental obesogens, with directed interrogation of the myriad pathways by which adipocytes regulate metabolism. Such analyses will greatly expand our understanding of the marked adipose heterogeneity that results from exposure to environmental toxicants.
Supplementary Material
Study Importance.
Prior studies have shown that tributyltin (TBT) is a dual agonist of the peroxisome proliferator activated receptor-γ (PPARγ) and the retinoid X receptor-α, with the capacity to augment adipocyte differentiation ex vivo and adipose accumulation in vivo.
The present study delineates the adipogenic conditions under which TBT promotes preadipocyte-to-adipocyte differentiation and demonstrates novel differences between adipocytes exposed during differentiation to TBT or the pharmacological PPARγ agonist troglitazone, including differential gene and protein expression as well as alterations in glucose uptake.
Tributyltin exposure during differentiation, in contrast to troglitazone, produces an adipocyte with metabolically deleterious characteristics, including reduced expression of the insulin-sensitizing adipokine adiponectin.
Acknowledgments
Funding: This work was supported by a Junior Investigator Award from the Brinson Foundation, an Early Career Development Award from the Central Society for Clinical and Translational Research, and the National Institutes of Health (K08-ES019176 and R21-ES021354 to RMS and T32-HD007009 supporting SMR).
Footnotes
Disclosures: The authors declare they have no conflicts of interest related to this work.
References
- 1.Grün F, Watanabe H, Zamanian Z, Maeda L, Arima K, Cubacha R, et al. Endocrine-disrupting organotin compounds are potent inducers of adipogenesis in vertebrates. Mol Endocrinol. 2006 Sep;20(9):2141–55. doi: 10.1210/me.2005-0367. [DOI] [PubMed] [Google Scholar]
- 2.Regnier SM, Sargis RM. Adipocytes under assault: environmental disruption of adipose physiology. Biochim Biophys Acta. 2014 Mar;1842(3):520–33. doi: 10.1016/j.bbadis.2013.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spiegelman BM. PPAR-gamma: adipogenic regulator and thiazolidinedione receptor. Diabetes. 1998 Apr;47(4):507–14. doi: 10.2337/diabetes.47.4.507. [DOI] [PubMed] [Google Scholar]
- 4.Le Maire A, Grimaldi M, Roecklin D, Dagnino S, Vivat-Hannah V, Balaguer P, et al. Activation of RXR–PPAR heterodimers by organotin environmental endocrine disruptors. EMBO Rep. 2009 Apr;10(4):367–73. doi: 10.1038/embor.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li X, Ycaza J, Blumberg B. The environmental obesogen tributyltin chloride acts via peroxisome proliferator activated receptor gamma to induce adipogenesis in murine 3T3-L1 preadipocytes. J Steroid Biochem Mol Biol. 2011 Oct;127(1–2):9–15. doi: 10.1016/j.jsbmb.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirchner S, Kieu T, Chow C, Casey S, Blumberg B. Prenatal exposure to the environmental obesogen tributyltin predisposes multipotent stem cells to become adipocytes. Mol Endocrinol. 2010 Mar;24(3):526–39. doi: 10.1210/me.2009-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meador JP, Sommers FC, Cooper KA, Yanagida G. Tributyltin and the obesogen metabolic syndrome in a salmonid. Environ Res. 2011 Jan;111(1):50–6. doi: 10.1016/j.envres.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 8.Si J, Wu X, Wan C, Zeng T, Zhang M, Xie K, et al. Peripubertal exposure to low doses of tributyltin chloride affects the homeostasis of serum T, E2, LH, and body weight of male mice. Environ Toxicol. 2011 Jun;26(3):307–14. doi: 10.1002/tox.20560. [DOI] [PubMed] [Google Scholar]
- 9.Chamorro-García R, Sahu M, Abbey RJ, Laude J, Pham N, Blumberg B. Transgenerational inheritance of increased fat depot size, stem cell reprogramming, and hepatic steatosis elicited by prenatal exposure to the obesogen tributyltin in mice. Environ Health Perspect. 2013 Mar;121(3):359–66. doi: 10.1289/ehp.1205701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lafontan M. Adipose tissue and adipocyte dysregulation. Diabetes Metab. 2014 Feb;40(1):16–28. doi: 10.1016/j.diabet.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Matsui H, Wada O, Manabe S, Ushijima Y, Fujikura T. Species difference in sensitivity to the diabetogenic action of triphenyltin hydroxide. Experientia. 1984 Apr 15;40(4):377–8. doi: 10.1007/BF01952561. [DOI] [PubMed] [Google Scholar]
- 12.Zuo Z, Chen S, Wu T, Zhang J, Su Y, Chen Y, et al. Tributyltin causes obesity and hepatic steatosis in male mice. Environ Toxicol. 2011 Feb;26(1):79–85. doi: 10.1002/tox.20531. [DOI] [PubMed] [Google Scholar]
- 13.Sargis RM, Johnson DN, Choudhury RA, Brady MJ. Environmental endocrine disruptors promote adipogenesis in the 3T3-L1 cell line through glucocorticoid receptor activation. Obesity (Silver Spring) 2010 Jul;18(7):1283–8. doi: 10.1038/oby.2009.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Temple KA, Cohen RN, Wondisford SR, Yu C, Deplewski D, Wondisford FE. An intact DNA-binding domain is not required for peroxisome proliferator-activated receptor gamma (PPARgamma) binding and activation on some PPAR response elements. J Biol Chem. 2005 Feb 4;280(5):3529–40. doi: 10.1074/jbc.M411422200. [DOI] [PubMed] [Google Scholar]
- 15.Sargis RM, Neel BA, Brock CO, Lin Y, Hickey AT, Carlton DA, et al. The novel endocrine disruptor tolylfluanid impairs insulin signaling in primary rodent and human adipocytes through a reduction in insulin receptor substrate-1 levels. Biochim Biophys Acta. 2012 Jun;1822(6):952–60. doi: 10.1016/j.bbadis.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Regnier SM, Kirkley AG, Ye H, El-Hashani E, Zhang X, Neel BA, et al. Dietary exposure to the endocrine disruptor tolylfluanid promotes global metabolic dysfunction in male mice. Endocrinology. 2015 Mar;156(3):896–910. doi: 10.1210/en.2014-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–8. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 18.Grün F, Blumberg B. Endocrine disrupters as obesogens. Mol Cell Endocrinol. 2009 May 25;304(1–2):19–29. doi: 10.1016/j.mce.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang QQ, Zhang JW, Lane MD. Sequential gene promoter interactions by C/EBPbeta, C/EBPalpha, and PPARgamma during adipogenesis. Biochem Biophys Res Commun. 2004 May 21;318(1):213–8. doi: 10.1016/j.bbrc.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 20.Camp HS, Li O, Wise SC, Hong YH, Frankowski CL, Shen X, et al. Differential activation of peroxisome proliferator-activated receptor-gamma by troglitazone and rosiglitazone. Diabetes. 2000 Apr;49(4):539–47. doi: 10.2337/diabetes.49.4.539. [DOI] [PubMed] [Google Scholar]
- 21.Zhang F, Lavan BE, Gregoire FM. Selective modulators of PPAR-gamma activity: molecular aspects related to obesity and side-effects. PPAR Res. 2007;2007:32696. doi: 10.1155/2007/32696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grün F. The obesogen tributyltin. Vitam Horm. 2014;94:277–325. doi: 10.1016/B978-0-12-800095-3.00011-0. [DOI] [PubMed] [Google Scholar]
- 23.Antizar-Ladislao B. Environmental levels, toxicity and human exposure to tributyltin (TBT)-contaminated marine environment. A review. Environment International. 2008 Feb;34(2):292–308. doi: 10.1016/j.envint.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 24.Muncke J. Endocrine disrupting chemicals and other substances of concern in food contact materials: an updated review of exposure, effect and risk assessment. J Steroid Biochem Mol Biol. 2011 Oct;127(1–2):118–27. doi: 10.1016/j.jsbmb.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 25.Champ MA. Economic and environmental impacts on ports and harbors from the convention to ban harmful marine anti-fouling systems. Mar Pollut Bull. 2003 Aug;46(8):935–40. doi: 10.1016/S0025-326X(03)00106-1. [DOI] [PubMed] [Google Scholar]
- 26.Zhang JW, Klemm DJ, Vinson C, Lane MD. Role of CREB in transcriptional regulation of CCAAT/enhancer-binding protein beta gene during adipogenesis. J Biol Chem. 2004 Feb 6;279(6):4471–8. doi: 10.1074/jbc.M311327200. [DOI] [PubMed] [Google Scholar]
- 27.Tzameli I, Fang H, Ollero M, Shi H, Hamm JK, Kievit P, et al. Regulated production of a peroxisome proliferator-activated receptor-gamma ligand during an early phase of adipocyte differentiation in 3T3-L1 adipocytes. J Biol Chem. 2004 Aug 20;279(34):36093–102. doi: 10.1074/jbc.M405346200. [DOI] [PubMed] [Google Scholar]
- 28.Mangelsdorf DJ, Evans RM. The RXR heterodimers and orphan receptors. Cell. 1995 Dec 15;83(6):841–50. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- 29.Yanik SC, Baker AH, Mann KK, Schlezinger JJ. Organotins are potent activators of PPARγ and adipocyte differentiation in bone marrow multipotent mesenchymal stromal cells. Toxicol Sci. 2011 Aug;122(2):476–88. doi: 10.1093/toxsci/kfr140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Altucci L, Rossin A, Hirsch O, Nebbioso A, Vitoux D, Wilhelm E, et al. Rexinoid-triggered differentiation and tumor-selective apoptosis of acute myeloid leukemia by protein kinase A-mediated desubordination of retinoid X receptor. Cancer Res. 2005 Oct 1;65(19):8754–65. doi: 10.1158/0008-5472.CAN-04-3569. [DOI] [PubMed] [Google Scholar]
- 31.Benoit G, Altucci L, Flexor M, Ruchaud S, Lillehaug J, Raffelsberger W, et al. RAR-independent RXR signaling induces t(15;17) leukemia cell maturation. EMBO J. 1999 Dec 15;18(24):7011–8. doi: 10.1093/emboj/18.24.7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silveira WA, Gonçalves DA, Graça FA, Andrade-Lopes AL, Bergantin LB, Zanon NM, et al. Activating cAMP/PKA signaling in skeletal muscle suppresses the ubiquitin-proteasome-dependent proteolysis: implications for sympathetic regulation. Journal of Applied Physiology. 2014 Jul 1;117(1):11–9. doi: 10.1152/japplphysiol.01055.2013. [DOI] [PubMed] [Google Scholar]
- 33.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995 Oct;96(4):1897–904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arner P. The adipocyte in insulin resistance: key molecules and the impact of the thiazolidinediones. Trends Endocrinol Metab. 2003 Apr;14(3):137–45. doi: 10.1016/s1043-2760(03)00024-9. [DOI] [PubMed] [Google Scholar]
- 35.Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001 Aug;7(8):947–53. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- 36.Brown JEP, Conner AC, Digby JE, Ward KL, Ramanjaneya M, Randeva HS, et al. Regulation of beta-cell viability and gene expression by distinct agonist fragments of adiponectin. Peptides. 2010 May;31(5):944–9. doi: 10.1016/j.peptides.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 37.Zuo Z, Wu T, Lin M, Zhang S, Yan F, Yang Z, et al. Chronic exposure to tributyltin chloride induces pancreatic islet cell apoptosis and disrupts glucose homeostasis in male mice. Environ Sci Technol. 2014 May 6;48(9):5179–86. doi: 10.1021/es404729p. [DOI] [PubMed] [Google Scholar]
- 38.Yu JG, Javorschi S, Hevener AL, Kruszynska YT, Norman RA, Sinha M, et al. The Effect of Thiazolidinediones on Plasma Adiponectin Levels in Normal, Obese, and Type 2 Diabetic Subjects. Diabetes. 2002 Oct 1;51(10):2968–74. doi: 10.2337/diabetes.51.10.2968. [DOI] [PubMed] [Google Scholar]
- 39.Arsenescu V, Arsenescu RI, King V, Swanson H, Cassis LA. Polychlorinated biphenyl-77 induces adipocyte differentiation and proinflammatory adipokines and promotes obesity and atherosclerosis. Environ Health Perspect. 2008 Jun;116(6):761–8. doi: 10.1289/ehp.10554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hugo ER, Brandebourg TD, Woo JG, Loftus J, Alexander JW, Ben-Jonathan N. Bisphenol A at environmentally relevant doses inhibits adiponectin release from human adipose tissue explants and adipocytes. Environ Health Perspect. 2008 Dec;116(12):1642–7. doi: 10.1289/ehp.11537. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.