Abstract
Objective
This study examined the prognostic significance of depressive symptoms in bariatric surgery patients over 24 months of follow-ups.
Method
Three hundred fifty-seven patients completed a battery of assessments before and at 6, 12, and 24 months following gastric bypass surgery. In addition to weight loss and depressive symptoms, the assessments targeted eating disorder psychopathology and quality of life.
Results
Clinically significant depressive symptoms, defined as a score of 15 or greater on the Beck Depression Inventory, characterized 45% of patients prior to surgery, and 12% at 6-month follow-up, 13% at 12-month follow-up, and 18% at 24-month follow-up. Preoperative depressive symptoms did not predict postoperative weight outcomes. In contrast, post-surgery depressive symptoms were predictive of weight loss outcomes. Higher post-surgery depressive symptoms at each time point predicted a greater degree of concurrent and subsequent eating disorder psychopathology and lower quality of life.
Conclusions
The frequency of elevated depressive symptoms decreases substantially following gastric bypass surgery but increases gradually over 24-months. Postoperative depressive symptoms are significantly associated with poorer weight outcomes at 6-months and 12-months following surgery but does not predict longer-term weight outcomes at 24-months. Post-operative depressive symptoms prospectively predict greater eating disorder psychopathology and poorer quality of life through 24-months. Elevated depressive symptoms, readily assessed by self-report, may signal a need for clinical attention after surgery.
Bariatric surgery is the most effective treatment for severe obesity, resulting in significant weight loss for people who have been unsuccessful in multiple nonsurgical interventions 1,2. Research has also found that, in addition to weight loss, various medical and psychosocial outcomes improve following surgery 3,4. Mental health professionals frequently conduct psychosocial evaluations prior to bariatric surgery to evaluate whether patients are candidates for the surgery. The American Society for Metabolic & Bariatric Surgery 5 and best practice recommendations 6 suggest that pre-surgical evaluations include screening for cognitive and emotional functioning, and evaluation for current and lifetime psychopathology, to determine whether problems in such areas might predict a worsened postsurgical outcome.
Research with bariatric surgery candidates has found that mood disorders are frequently co-morbid, although the reported rates of lifetime and current mood diagnoses are somewhat variable across studies. Among bariatric surgery candidates evaluated prior to surgery, rates of lifetime mood disorder range from 22.4% 7 to 45% 8, and rates of current mood disorder range from 11% 7 to 33% 9. Similarly, depressive symptoms among bariatric patients are frequently high and appear elevated relative to population data and to persons with severe obesity electing non-surgical interventions 4,10. Converging lines of research suggest that bariatric surgery is associated with improvements in depression 11–14.
The clinical significance of mood disorders and depressive symptoms on bariatric surgeries outcomes, however, is not well understood and the few available studies have reported mixed findings. Research has reported that a diagnosis of pre-surgical depression was not associated with post-surgery depression at 6–12 months following surgery, but did predict depression 24–36 months after surgery 9, while other analyses suggested that patients with pre-surgical depression features had elevated risk for post-surgery depression features relative to those with low depression levels before surgery 14. Some research has found that lifetime mood disorder comorbidity is associated with poorer weight loss outcomes at post-surgery. Kalarchian et al. 15 reported 25.1% weight loss at 6 months post-surgery among patients with lifetime mood disorders, which was significantly less than the 29.5% weight loss achieved among patients without histories of psychiatric disorders. Mitchell and colleagues reported that patients with elevated pre-operative levels of depression (based on scores of 10 or greater on the Beck Depression Inventory) had higher rates of post-operative adverse events14. Other research, however, found that post-surgery depression, but not pre-surgical depression, predicted weight outcomes at 12 and 24 months 16 and 24–36 months 9 following surgery.
It could be that the influence of depressive features on weight and psychosocial outcomes is obscured by the potent influence of the surgery itself. It is possible that the influence of pre-surgery depressive symptoms will emerge for long-term postoperative outcomes, when the influence of surgery has diminished. The current study was designed to examine prospectively the prognostic significance of depressive symptoms on weight loss and psychosocial outcomes of gastric bypass surgery. Depressive symptoms (assessed both pre- and post-surgery) were examined in relation to 6- 12- and 24-month post-surgical outcomes.
Method
Participants
Participants were 357 (50 male and 307 female) extremely obese patients who underwent gastric bypass surgery at 2 academic medical centers. In terms of surgical site, 53.2% (n=190) were assessed at the Yale University School of Medicine, and 46.8% (n=167) were assessed at the Western Psychiatric Institute and Clinic. Mean age was 43.7 years (SD=10.0) and mean body mass index (BMI) was 51.2 (SD=8.3). Of the participants, 81.8% (n=292) were white, 9.0% (n=32) were African-American, 7.0% (n=25) were Hispanic-American, 0.3% (n=1) was Asian, and 2.0% (n=7) were of another ethnicity or unknown. Educationally, 67.8% (n=242) attended at least some college, and an additional 26.1% (n=93) completed high school. The 2 sites did not differ in BMI, distribution of gender, or educational attainment.
Informed Consent and Study Procedures
Institutional review board approval was granted at each site, and written informed consent was obtained from all participants. Patients were informed that they were participating in research studies to learn about the effects of bariatric surgery on weight, eating behaviors, psychological functioning, and quality of life. Patients were informed that their participation would not influence the type of care provided by the surgical team. Patients were told there would be no direct medical benefit to them, although it was hoped that the knowledge gained might ultimately benefit other bariatric patients in the future. Patients were also informed that the findings would only be shared with the treatment team if they so desired and provided consent. No compensation was provided.
Patients completed a battery of assessments prior to surgery and at 6-month, 12-month, and 24-month follow-up points. Of the patients who completed the baseline (preoperative) assessment, 85% (n=303) completed the 6-month follow-up, 80% (n=285) completed the 12-month follow-up, and 47% (n=167) completed the 24-month follow-up. To be included in the current study, participants had to have completed at least 1 follow-up assessment. Participants who completed the follow-up assessments did not differ from those who did not in terms of preoperative BMI or psychological functioning (Beck Depression Inventory, Short-Form 36, or Eating Disorder Examination Questionnaire scores). The group that participated in the 24-month assessment did not differ from the group that did not on weight loss, BDI, or on any of the other clinical outcome measures (described below) at 12 months.
Measures
Weight and Height
Percent weight loss from baseline was the primary outcome variable. BMI (weight in kilograms/height in meters2) was calculated from self-reported weight and height as part of a larger questionnaire battery and completed at the same time as the psychosocial measures. Concurrently measured (i.e., by clinic staff) weight data were available for a subsample (n=183). In this subsample, the measured (mean=52.0, SD=7.9) and self-reported (mean=51.8, SD=8.3) BMIs did not differ (t=0.67, p=.50). Further, the degree of misreport was unrelated to increasing BMI (r=0.12, p=.10).
Assessment of Depressive Features
The Beck Depression Inventory (BDI; Revised edition) 17 21-item version assesses the cognitive, affective, and somatic symptoms of depression. The BDI is a widely used and well-established measure of depressive symptoms with demonstrated reliability and validity 18. Higher scores reflect higher symptom levels of depression and, more broadly, negative affect 19,20. The BDI is used as a measure for evaluating bariatric surgery patients 21,22 and in research studies of depression before and after obesity treatment 23 and bariatric surgery 14,24,25. Evidence generally supports the use of the BDI in this patient group, but studies have reported variable findings regarding the utility and optimal cut-point scores25,26. A recent study investigating the clinical utility of the BDI in relation to the gold standard clinical interview (i.e., the Structured Clinical Interview for DSM-IV: SCID-I/P 27) for determining a diagnosis of mood disorder reported ROC curve analysis indicating the BDI had moderate discriminating accuracy (AUC= 0.788, 95% Confidence Interval= 0.681 to 0.896) and that a BDI score of 15 optimized sensitivity and specificity in a group of bariatric surgery candidates.28. Thus in the current study, the presence of clinically significant depressive features was determined by a score of 15 or greater on the BDI 29.
Eating Disorder Psychopathology
The Eating Disorder Examination – Questionnaire (EDE-Q) 30, the self-report version of the Eating Disorder Examination Interview 31, was used to assesses eating disorder psychopathology. The EDE-Q yields a global score (mean of four subscales) that reflects overall severity. The EDE-Q has been found to perform adequately in bariatric surgery candidates 32; studies with obese patient groups have found that global scores generated by the EDEQ and EDE are significantly and highly correlated 33,34.
The Medical Outcomes Study Short Form-36 Health Survey (SF-36) 35 is a 36-item, widely used, self-report instrument to assess health-related quality of life (HRQL). The SF-36 has well-established reliability 36 and validity 37. The SF-36 generates 2 summary scores (summarizing 8 subscales): the Physical Component Summary (PCS) and the Mental Component Summary (MCS) 38. Scores reflect frequency (i.e., 6-point scale from 1 [all of the time] to 6 [none of the time]), severity (i.e., 3-point scale from 1 [yes, limited a lot] to 3 [no, not limited at all]), or forced choice (i.e., dichotomous scale for yes or no) items. The SF-36 raw scores are transformed to scale scores ranging from 0 (lowest level of HRQL) to 100 (highest level of HRQL) with a standard deviation of 15, and the PCS and MCS scores are such that the means are 50 and standard deviations are 10 for the general US population.
Statistical Analyses
Data were analyzed using SAS v9.1 and SPSS v19. A series of binary logistic regression analyses was used to test the hypothesis that timepoint-specific post-surgery depressive symptoms would be predicted by clinically significant depressive symptoms at baseline. One-way analyses of variance were used to test the hypothesis that pre-surgery depression groups would differ on BMI and psychosocial outcomes at baseline. Regression analyses tested whether depressive symptoms (BDI scores) were associated concurrently and prospectively (i.e., with subsequent timepoints) with weight loss, eating disorder psychopathology (EDEQ global scores), and quality of life (SF-36 composite scores).
Results
Prior to surgery, 45% (N=160) of patients reported clinically significant depressive symptoms (BDI score ≥ 15). At follow up points, this frequency was substantially lower, with 11.5% (N=35) at 6-months, 13.3% (N=38) at 12-months, and 17.5% (N=29) at 24-months reporting symptoms of depression in the clinically significant range.
The hypothesis that post-surgery depressive symptoms would be predicted by pre-surgery depressive symptoms was tested using simple binary logistic regression analyses for each follow-up time point. At each follow up point, the presence of clinically significant depressive symptoms was predicted by the presence of clinically significant depressive symptoms at baseline. However, the proportion of patients reporting elevated depressive symptoms was significantly lower at all follow up points: among those who reported depression at baseline, 22.1%, 23.4%, and 30.3% reported clinically significant depressive symptoms at 6-, 12-, and 24-months respectively. Despite the proportionately lower number of participants reporting significant depressive features at 6-months as compared to baseline, the presence of baseline depressive symptoms was highly associated with clinically significant depressive symptoms at 6 months, OR=9.2, p<.001, 95% CI: 3.4–24.5. A similar pattern was observed for the 12-months (OR=5.7, p<.001, 95% CI: 2.5–13.0) and 24-months evaluations (OR=6.1, p<.001, 95% CI: 2.3–15.9). The figure demonstrates the percentage of patients reporting post-surgery depressive symptoms (i.e., BDI≥15) as a function of pre-surgery depressive symptoms.
Although the proportion of the sample reporting clinically significant depressive symptoms decreased immediately following surgery (χ2 (N=304, df=1)=135.4, p<.001), the prevalence of clinically significant depressive symptoms remained stable from 6 to 12 months following surgery. A chi-square goodness-of-fit test found that compared to the 6-month point, the prevalence of depressive symptomatology did not differ at the 12-month follow up (χ2 (N=285, df=1)=0.94, p=.33). Compared to the 12 month assessment, the prevalence of clinically significant depressive symptoms remained stable at the 24-month assessment (χ2 (N=166, df=1)=2.5, p=.11). When comparing the 24-month outcomes to the 6 month outcomes, the prevalence of clinically significant symptoms increased significantly during that time frame (χ2 (N=166, df=1)=5.8, p=.02).
Baseline values for BMI and psychosocial outcomes appear in Table 1; patients with and without clinically significant depressive symptoms at baseline were compared on BMI and psychosocial measures with one-way ANOVAs. The groups did not differ in terms of pre-surgical BMI. Significant differences were observed such that the group experiencing clinically significant depressive symptoms reported higher scores on the EDE-Q global score and diminished quality of life as measured by the SF-36. Table 2 presents mean values on outcome variables based on the presence or absence of pre-surgical depressive symptoms. Clinically significant depressive symptoms at baseline were not related to weight outcomes at any follow up point but were associated with higher EDE-Q scores and lower SF-36 Mental Health component scores at each follow up point.
Table 1.
Baseline BMI and psychosocial measures as a function of pre-surgery depression
| No Depression (BDI<15) (n=197) | Clinically Significant Depression (BDI≥15) (n=160) | F | p | η2 | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| M | sd | M | sd | ||||
| BMI – baseline | 50.7 | (7.9) | 51.7 | (8.8) | 1.35 | .25 | .004 |
| Total EDE-Q (avg) | 2.8 | (0.9) | 3.5 | (0.8) | 68.50 | .00 | .163 |
| SF-36 Physical Component | 33.0 | (10.3) | 30.8 | (9.5) | 4.27 | .04 | .012 |
| SF-36 Mental Component | 52.7 | (7.8) | 38.6 | (10.0) | 221.86 | .00 | .387 |
Table 2.
Post-surgery outcomes as a function of pre-surgery depression
| No Depression (BDI<15) (n=197) | Clinically Significant depression (BDI≥15) (n=160) | F | p | η2 | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| M | (sd) | M | (sd) | ||||
| Percent BMI loss at 6 months | 27.80 | (5.9) | 27.80 | (6.0) | 0.01 | .95 | .000 |
| Total EDE-Q (avg) at 6 months | 1.60 | (0.9) | 2.11 | (1.11) | 20.13 | .00 | .062 |
| Phys Component 6 months | 48.70 | (9.2) | 46.80 | (10.0) | 2.67 | .10 | .009 |
| Mental Component 6 months | 56.40 | (6.0) | 49.20 | (11.4) | 49.25 | .00 | .141 |
| Percent BMI loss at 12 months | 36.50 | (7.3) | 35.80 | (7.6) | 0.56 | .45 | .002 |
| Total EDE-Q (avg) at 12 months | 1.49 | (0.9) | 2.03 | (1.1) | 21.88 | .00 | .071 |
| Phys Component 12 months | 49.90 | (9.3) | 48.90 | (10.5) | 0.80 | .37 | .003 |
| Mental Component 12 months | 55.40 | (8.1) | 46.50 | (13.2) | 48.77 | .00 | .147 |
| Percent BMI loss at 24 months | 37.40 | (9.7) | 38.30 | (8.7) | 0.42 | .52 | .003 |
| Total EDE-Q (avg) at 24 months | 1.53 | (1.0) | 2.15 | (1.3) | 12.42 | .00 | .070 |
| Phys Component 24 months | 49.30 | (10.9) | 46.70 | (11.4) | 2.13 | .15 | .013 |
| Mental Component 24 months | 54.00 | (10.4) | 45.20 | (12.9) | 23.36 | .00 | .125 |
The low rates of clinically significant depressive symptoms at follow up points resulted in very imbalanced cell sizes. To examine the association of post-surgery depressive symptoms on postsurgical outcomes, BDI scores were tested using linear regression. We chose to use BDI scores, rather than depression categories, for the following reasons. First, as shown in Figure 1, we observed relatively low rates of clinically significant depressive symptoms at the early follow-ups following surgery. Second, in addition to the greater statistical power afforded by continuous data, we emphasize that research has demonstrated the superiority of BDI levels over categorical mood diagnoses for identifying severity and impairment 20. Third, this approach has greater ecological validity because while pre-surgical evaluations might involve the use of structured diagnostic interviews (as suggested by Rosenberger et al., 7), bariatric surgical practices are more likely to use the BDI than interviews following surgery.
Figure 1.
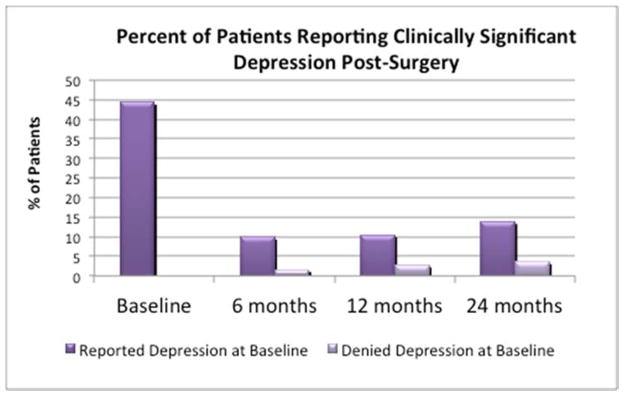
Table 3 presents the results for regression analyses separately by time point. Depression score at 6 months was significantly associated with BMI loss at 6 months and at 12 months, although the magnitude of the associations was somewhat modest (accounting for 4.2% of the variance at 6 months and 2.2% of the variance at 12 months). Depression scores at 6 months did not predict weight outcomes at 24 months. Depression scores at 6 months were highly associated with concurrent and subsequent eating disorder psychopathology, although the magnitude of the associations decreased with time. Depression scores at 6 months were highly predictive of both physical and mental component scores at 6, 12, and 24 months. The pattern of influence was such that depression was associated with mental component scores to a greater degree than physical component scores, and the magnitude of associations diminished over time while still remaining highly significant.
Table 3.
Regressions testing the prediction of depression (BDI scores) on concurrent and prospective weight loss, quality of life, and eating pathology
| Beta | R2 | F | p | |
|---|---|---|---|---|
| BDI score at 6 months | ||||
| Percent BMI loss 6 months | -.205 | .042 | 12.84 | <.001 |
| Total EDE-Q (avg) at 6 months | .486 | .237 | 93.90 | <.001 |
| Physical Component 6 months | .417 | .174 | 62.66 | <.001 |
| Mental Component 6 months | .696 | .485 | 279.76 | <.001 |
| Percent BMI loss 12 months | -.149 | .022 | 5.69 | .018 |
| Total EDE-Q (avg) at 12 months | .340 | .116 | 32.62 | <.001 |
| Physical Component 12 months | .385 | .145 | 42.77 | <.001 |
| Mental Component 12 months | .608 | .369 | 144.16 | <.001 |
| Percent BMI loss 24 months | -.110 | .012 | 1.56 | .214 |
| Total EDE-Q (avg) at 24 months | .329 | .108 | 16.99 | <.001 |
| Physical Component 24 months | .317 | .101 | 15.57 | <.001 |
| Mental Component 24 months | .546 | .298 | 59.08 | <.001 |
| BDI score at 12 months | ||||
| Percent BMI loss 12 months | .136 | .019 | 5.15 | .024 |
| Total EDE-Q (avg) at 12 months | .350 | .122 | 39.61 | <.001 |
| Physical Component 12 months | .368 | .135 | 44.01 | <.001 |
| Mental Component 12 months | .688 | .473 | 252.64 | <.001 |
| Percent BMI loss 24 months | .105 | .011 | 1.42 | .235 |
| Total EDE-Q (avg) at 24 months | .252 | .063 | 9.41 | .003 |
| Physical Component 24 months | .262 | .068 | 10.07 | .002 |
| Mental Component 24 months | .528 | .278 | 52.88 | <.001 |
| BDI score at 24 months | ||||
| Percent BMI loss 24 months | .107 | .011 | 1.77 | .186 |
| Total EDE-Q (avg) at 24 months | .472 | .223 | 47.68 | <.001 |
| Physical Component 24 months | .410 | .168 | 33.35 | <.001 |
| Mental Component 24 months | .771 | .594 | 241.14 | <.001 |
Depression scores at 12 months were significantly but modestly associated with concurrent (i.e., 12 month) weight loss and were not predictive of weight loss at 24 months. Depression scores at 12 months were associated concurrently and prospectively with eating disorder psychopathology and with physical and mental component scores.
Discussion
This study examined the prognostic significance of depressive symptoms on postoperative outcomes in gastric bypass surgery patients followed for 24 months after surgery. Clinically significant depressive symptoms, defined as a BDI score greater than or equal to 15, were reported by nearly half of the participants prior to surgery. Substantial improvements in depressive symptoms were observed following surgery, with 12%, 13%, and 18% of the participants meeting the threshold for clinically significant depression at 6, 12, and 24 months respectively. Depressive symptoms following surgery were significantly predicted by depressive symptoms at baseline. Consistent with previous research with bariatric surgery patients 9,14, the dramatic improvements in depressive symptoms began to diminish as the time since surgery increased, such that by 24 months the frequency of clinically significant depressive symptoms had increased significantly although it was still lower than the frequency observed before surgery.
Depressive symptoms prior to surgery were unrelated to BMI prior to surgery and did not predict percent BMI loss at any post-surgical follow up time point. Post-surgery depressive symptoms were generally associated with percent BMI loss at the concurrent time point as well as prospectively. Percent BMI loss at 24 months, however, was unrelated to depressive symptoms at any follow up point. Depressive symptoms were associated with psychosocial outcomes (eating disorder psychopathology and quality of life) at baseline and at all follow up points. Post-surgery depressive symptoms were associated with these poorer psychosocial outcomes concurrently and prospectively (i.e., at subsequent time points) throughout each wave of follow up.
Taken together, the current findings suggest that gastric bypass surgery, by way of the considerable weight losses achieved, leads to subsequent improvements in psychosocial functioning. Following gastric bypass surgery, participants reported substantial and clinically significant improvements in depression symptoms. Although the percentage of participants reporting clinically significant depressive symptoms began to increase as the time since surgery increased, the frequency through 24-months remained lower than that at baseline. To date, research investigating psychosocial functioning prior to surgery has generally reported little prognostic impact of these variables on postsurgical outcomes. The current findings support and extend previous research showing a limited prognostic effect of pre-surgery depression on weight outcomes 9,16 but suggest that depressive symptoms following surgery have significant prognostic impact on weight outcomes, at least until 24 months. Alternatively, the current results could be interpreted to indicate that depressive features may be a consequence – rather than a cause – of poorer weight outcomes. Furthermore, it is possible that dysregulated biological pathways, such as immune inflammation39, oxidative stress 40,41, or neuroprogression, could be implicated in both the suppressed weight loss and depressive features.42 Collectively, these findings might suggest that pre-surgery depression, although common, may not require specific additional clinical intervention prior to gastric bypass unless it is severe enough to interfere with a patient’s ability to adhere to self-care 43. The current findings, however, suggest that elevated depressive symptoms following surgery may have negative prognostic influence on weight loss and psychosocial functioning outcomes and therefore may signal the need for additional clinical support or intervention. Additional research employing longitudinal modeling may be indicated to determine whether depressive features following surgery are a cause versus a consequence of poorer weight outcomes.
This study has some potential limitations that should be considered when interpreting the findings. Participants were extremely obese gastric bypass surgery patients; the findings may not generalize to patients seeking other types of bariatric surgery, which appear to vary in outcomes 2. That is, the results may not extend to participants undergoing gastric banding, which is associated with lower rates of post-surgery complications but yields lower weight losses than gastric bypass and the sleeve gastrectomy.44 The findings are further limited by the amount of missing data, particularly for the 24-month follow-up point, although our analyses indicated no differences at baseline between patients who participated in all assessments as compared to those who did not. Unfortunately it remains possible that attrition may signal worsened psychosocial functioning at follow up among those participants. The study design precluded clinical follow up with these patients; as such it remains unknown whether these patients sought and/or received psychological and/or psychiatric treatments during the follow-up period.12,45 We note, however, that Hayden and colleagues11 found that antidepressant therapy had little to no impact on either weight loss outcomes or depressive symptomatology in an outcome study of bariatric surgery patients. It is also possible that follow-up care, or the lack thereof, could have confounded the results. Additional longitudinal studies should closely monitor the nature and duration of follow up care to understand the complexities of the emergence of mood symptoms following surgery, and their interactions with weight outcomes. Another potential limitation is the possibility that extremely obese patients seeking bariatric surgery may minimize the existence of certain problems (e.g., depression) in order to appear psychologically healthy (e.g., appropriate to meet eligibility for surgery). Indeed, research has shown that patients undergoing psychological evaluation prior to surgery have elevated scores on social desirability and commonly deny active problems. Although the possibility of impression management must be considered, the research study procedures and informed consent methods should have served to minimize this likelihood.46 Specifically, participants completed the assessments as part of a research study and were informed that the results would not be shared with the clinical treatment team unless the patients specifically requested it. It was stressed that the assessments would have no medical benefit to patients and were intended solely to advance knowledge regarding psychosocial needs and outcomes of bariatric surgery patients. Since the study sample included only those patients who received surgery, it is possible that surgery candidates suffering from severe depression at baseline would have been excluded from the study sample, resulting in a truncated distribution of depression scores. However, the range of depression scores in this sample included individuals scoring in the moderate and severe ranges of depressive symptomatology, thus reducing this concern to some degree. In addition, we note the possibility that the measure of eating-related psychopathology, the EDE-Q, may not be the optimal measure for this patient group.
Notable strengths include the use of the BDI, a widely-used, valid, and efficient measure of depressive levels and features. Clinicians working with post-surgery patients can quickly score the BDI to identify patients in need of intervention. Moreover, the BDI provides a scaled score (as opposed to a single threshold), and severity of depressive symptoms is indicated by a higher score. Therefore although clinicians can use a score of 15 or greater to identify clinical significance, higher scores on the BDI after surgery are associated with worse outcomes and would therefore indicate greater need for intervention. Additional strengths include repeated measures over a multi-wave follow up and the use of data from two independent research groups.
In summary, this study found that pre-surgery depressive symptoms do not predict weight loss through 24-months of follow up after gastric bypass surgery. Post-surgery depressive symptoms are associated with poorer weight outcomes and with poorer psychosocial outcomes. Future research should include longer-term follow-ups to examine the durability of these findings, as well as the potential for worsening or reemergence of depressive symptoms over time.
Acknowledgments
This research was supported in part by grants K23 DK062291, K23 DK071646, R01 DK098492, and K24 DK070052 from the NIH/NIDDK.
Footnotes
Conflict of Interest Statement
Dr. Kalarchian reports a grant from the National Institute of Health, during the conduct of the study and grants from TOS/Nutrisystem, grants from NIH/NIDDK, and grants from ASMBS Foundation, outside the submitted work.
Dr. Grilo reports grants from National Institutes of Health, during the conduct of the study; grants from National Institutes of Health and Medical research Foundations, personal fees from Shire, honoraria from American Psychological Association, various scientific conferences and universities for delivering research lectures and grand rounds, and various CME-related educational talks, book royalties from Guilford Press; Taylor Francis Publishers, outside the submitted work.
Statement of Informed Consent
Informed consent was obtained from all individual participants included in the study.
Statement of Human and Animal Rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Contributor Information
Marney A. White, Email: Marney.white@yale.edu.
Melissa A. Kalarchian, Email: kalarchianm@duq.edu.
Michele D. Levine, Email: levinem@upmc.edu.
Robin M. Masheb, Email: robin.masheb@yale.edu.
Marsha D. Marcus, Email: marcusmd@upmc.edu.
Carlos M. Grilo, Email: carlos.grilo@yale.edu.
References
- 1.Adams TD, Davidson LE, Litwin SE, et al. Health benefits of gastric bypass surgery after 6 years. JAMA : the journal of the American Medical Association. 2012 Sep 19;308(11):1122–1131. doi: 10.1001/2012.jama.11164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang SH, Stoll CR, Song J, Varela JE, Eagon CJ, Colditz GA. The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003–2012. JAMA surgery. 2014 Mar;149(3):275–287. doi: 10.1001/jamasurg.2013.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Courcoulas AP, Christian NJ, Belle SH, et al. Weight change and health outcomes at 3 years after bariatric surgery among individuals with severe obesity. JAMA : the journal of the American Medical Association. 2013 Dec 11;310(22):2416–2425. doi: 10.1001/jama.2013.280928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ryden A, Torgerson JS. The Swedish Obese Subjects Study--what has been accomplished to date? Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery. 2006 Sep-Oct;2(5):549–560. doi: 10.1016/j.soard.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 5.Mechanick JI, Youdim A, Jones DB, et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient--2013 update: cosponsored by American Association of Clinical Endocrinologists, the Obesity Society, and American Society for Metabolic & Bariatric Surgery. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery. 2013 Mar-Apr;9(2):159–191. doi: 10.1016/j.soard.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 6.Greenberg I, Sogg S, FMP Behavioral and psychological care in weight loss surgery: best practice update. Obesity (Silver Spring) 2009 May;17(5):880–884. doi: 10.1038/oby.2008.571. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberger PH, Henderson KE, Grilo CM. Psychiatric disorder comorbidity and association with eating disorders in bariatric surgery patients: a cross-sectional study using structured interview-based diagnosis. The Journal of clinical psychiatry. 2006;67:1080–1085. doi: 10.4088/jcp.v67n0710. [DOI] [PubMed] [Google Scholar]
- 8.Kalarchian MA, Marcus MD, Levine MD, et al. Psychiatric disorders among bariatric surgery candidates: relationship to obesity and functional health status. The American journal of psychiatry. 2007 Feb;164(2):328–334. doi: 10.1176/ajp.2007.164.2.328. quiz 374. [DOI] [PubMed] [Google Scholar]
- 9.de Zwaan M, Enderle J, Wagner S, et al. Anxiety and depression in bariatric surgery patients: A prospective, follow-up study using structured clinical interviews. Journal of affective disorders. 2011 Apr 17; doi: 10.1016/j.jad.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 10.Karlsson J, Sjostrom L, Sullivan M. Swedish obese subjects (SOS)--an intervention study of obesity. Two-year follow-up of health-related quality of life (HRQL) and eating behavior after gastric surgery for severe obesity. Int J Obes Relat Metab Disord. 1998 Feb;22(2):113–126. doi: 10.1038/sj.ijo.0800553. [DOI] [PubMed] [Google Scholar]
- 11.Hayden MJ, Dixon JB, Dixon ME, Shea TL, O’Brien PE. Characterization of the improvement in depressive symptoms following bariatric surgery. Obesity surgery. 2011 Mar;21(3):328–335. doi: 10.1007/s11695-010-0215-y. [DOI] [PubMed] [Google Scholar]
- 12.Rutledge T, Braden AL, Woods G, Herbst KL, Groesz LM, Savu M. Five-year changes in psychiatric treatment status and weight-related comorbidities following bariatric surgery in a veteran population. Obesity surgery. 2012 Nov;22(11):1734–1741. doi: 10.1007/s11695-012-0722-0. [DOI] [PubMed] [Google Scholar]
- 13.Lier HO, Biringer E, Hove O, Stubhaug B, Tangen T. Quality of life among patients undergoing bariatric surgery: associations with mental health-A 1 year follow-up study of bariatric surgery patients. Health and quality of life outcomes. 2011;9:79. doi: 10.1186/1477-7525-9-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitchell JE, King WC, Chen JY, et al. Course of depressive symptoms and treatment in the longitudinal assessment of bariatric surgery (LABS-2) study. Obesity. 2014 doi: 10.1002/oby.20738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalarchian MA, Marcus MD, Levine MD, Soulakova JN, Courcoulas AP, Wisinski MS. Relationship of psychiatric disorders to 6-month outcomes after gastric bypass. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery. 2008 Jul-Aug;4(4):544–549. doi: 10.1016/j.soard.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dixon JB, Dixon ME, O’Brien PE. Depression in association with severe obesity: changes with weight loss. Arch Intern Med. 2003 Sep 22;163(17):2058–2065. doi: 10.1001/archinte.163.17.2058. [DOI] [PubMed] [Google Scholar]
- 17.Beck AT, Steer R. Manual for Revised Beck Depression Inventory. New York: Psychological Corporation; 1987. [Google Scholar]
- 18.Beck AT, Steer R, Garbin MG. Psychometric Properties of the Beck Depression Inventory - 25 Years of Evaluation. Clinical Psychology Review. 1988;8(1):77–100. [Google Scholar]
- 19.Watson D, Clark LA. Negative affectivity: the disposition to experience aversive emotional states. Psychol Bull. 1984 Nov;96(3):465–490. [PubMed] [Google Scholar]
- 20.Grilo CM, Masheb RM, Wilson GT. Subtyping binge eating disorder. Journal of consulting and clinical psychology. 2001 Dec;69(6):1066–1072. doi: 10.1037//0022-006x.69.6.1066. [DOI] [PubMed] [Google Scholar]
- 21.Bauchowitz AU, Gonder-Frederick LA, Olbrisch ME, et al. Psychosocial evaluation of bariatric surgery candidates: a survey of present practices. Psychosomatic medicine. 2005 Sep-Oct;67(5):825–832. doi: 10.1097/01.psy.0000174173.32271.01. [DOI] [PubMed] [Google Scholar]
- 22.Walfish S, Vance D, Fabricatore AN. Psychological evaluation of bariatric surgery applicants: procedures and reasons for delay or denial of surgery. Obesity surgery. 2007 Dec;17(12):1578–1583. doi: 10.1007/s11695-007-9274-0. [DOI] [PubMed] [Google Scholar]
- 23.Rubin RR, Wadden TA, Bahnson JL, et al. Impact of intensive lifestyle intervention on depression and health-related quality of life in type 2 diabetes: the Look AHEAD Trial. Diabetes care. 2014 Jun;37(6):1544–1553. doi: 10.2337/dc13-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayden MJ, Dixon JB, Dixon ME, O’Brien PE. Confirmatory factor analysis of the Beck Depression Inventory in obese individuals seeking surgery. Obesity surgery. 2010 Apr;20(4):432–439. doi: 10.1007/s11695-009-9977-5. [DOI] [PubMed] [Google Scholar]
- 25.Krukowski RA, Friedman KE, Applegate KL. The utility of the Beck Depression Inventory in a bariatric surgery population. Obesity surgery. 2010 Apr;20(4):426–431. doi: 10.1007/s11695-008-9717-2. [DOI] [PubMed] [Google Scholar]
- 26.Hayden MJ, Brown WA, Brennan L, O’Brien PE. Validity of the Beck Depression Inventory as a screening tool for a clinical mood disorder in bariatric surgery candidates. Obesity surgery. 2012 Nov;22(11):1666–1675. doi: 10.1007/s11695-012-0682-4. [DOI] [PubMed] [Google Scholar]
- 27.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, research version, patient edition. New York: Biometrics Research, New York State Psychiatric Institute; 1996. [Google Scholar]
- 28.Ivezaj V, Barnes RD, Grilo CM. The utility and validity of the Beck Depression Inventory in weight loss surgery patients. Paper presented at: The Obesity Society; 2014; Boston, MA. [Google Scholar]
- 29.Seggar LB, Lambert MJ, Hansen NB. Assessing clinical significance: Application to the Beck Depression Inventory. Behavior Therapy. 2002;33(2):253–269. [Google Scholar]
- 30.Fairburn CG, Beglin SJ. Assessment of eating disorders: interview or self-report questionnaire? The International journal of eating disorders. 1994 Dec;16(4):363–370. [PubMed] [Google Scholar]
- 31.Fairburn CG, Cooper Z. The Eating Disorder Examination. In: Fairburn CG, Wilson GT, editors. Binge eating: nature, assessment, and treatment. 12. New York: Guilford Press; 1993. pp. 317–360. [Google Scholar]
- 32.Kalarchian MA, Wilson GT, Brolin RE, Bradley L. Assessment of eating disorders in bariatric surgery candidates: self-report questionnaire versus interview. The International journal of eating disorders. 2000 Dec;28(4):465–469. doi: 10.1002/1098-108x(200012)28:4<465::aid-eat17>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 33.Grilo CM, Masheb RM, Wilson GT. A comparison of different methods for assessing the features of eating disorders in patients with binge eating disorder. Journal of consulting and clinical psychology. 2001 Apr;69(2):317–322. doi: 10.1037//0022-006x.69.2.317. [DOI] [PubMed] [Google Scholar]
- 34.Grilo CM, Masheb RM, Wilson GT. Different methods for assessing the features of eating disorders in patients with binge eating disorder: a replication. Obesity research. 2001 Jul;9(7):418–422. doi: 10.1038/oby.2001.55. [DOI] [PubMed] [Google Scholar]
- 35.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Med Care 30. 1992;6:473–483. [PubMed] [Google Scholar]
- 36.McHorney CA, Ware JE, Jr, Lu JF, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Medical care. 1994 Jan;32(1):40–66. doi: 10.1097/00005650-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 37.McHorney CA, Ware JE, Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Medical care. 1993 Mar;31(3):247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 38.Ware JE, Jr, Kosinski M, Keller SD. SF-36 Physical and Mental Health Summary Scales -- A User’s Manual. Boston: New England Medical Center, The Health Institute; 1994. [Google Scholar]
- 39.Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends in immunology. 2004;25(1):4–7. doi: 10.1016/j.it.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 40.Nunes SOV, Vargas HO, Prado E, et al. The shared role of oxidative stress and inflammation in major depressive disorder and nicotine dependence. Neuroscience & Biobehavioral Reviews. 2013;37(8):1336–1345. doi: 10.1016/j.neubiorev.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 41.Maes M, Leonard B, Fernandez A, et al. (Neuro) inflammation and neuroprogression as new pathways and drug targets in depression: From antioxidants to kinase inhibitors. Progress in neuro-psychopharmacology and biological psychiatry. 2011;35(3):659–663. doi: 10.1016/j.pnpbp.2011.02.019. [DOI] [PubMed] [Google Scholar]
- 42.Lopresti AL, Drummond PD. Obesity and psychiatric disorders: commonalities in dysregulated biological pathways and their implications for treatment. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2013;45:92–99. doi: 10.1016/j.pnpbp.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 43.LeMont D, Moorehead MK, Parish MS, Reto CS, Ritz SJ. Suggestions for the pre-surgical psychological assessment of bariatric surgery candidates. American Society for Bariatric Surgery; Oct, 2004. [Google Scholar]
- 44.Hutter MM, Schirmer BD, Jones DB, et al. First report from the American College of Surgeons Bariatric Surgery Center Network: laparoscopic sleeve gastrectomy has morbidity and effectiveness positioned between the band and the bypass. Annals of surgery. 2011 Sep;254(3):410–420. doi: 10.1097/SLA.0b013e31822c9dac. discussion 420–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cunningham JL, Merrell CC, Sarr M, et al. Investigation of antidepressant medication usage after bariatric surgery. Obesity surgery. 2012 Apr;22(4):530–535. doi: 10.1007/s11695-011-0517-8. [DOI] [PubMed] [Google Scholar]
- 46.Mitchell JE, Selzer F, Kalarchian MA, et al. Psychopathology before surgery in the longitudinal assessment of bariatric surgery-3 (LABS-3) psychosocial study. Surgery for Obesity and Related Diseases. 2012;8(5):533–541. doi: 10.1016/j.soard.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]


