Abstract
Amplitude-integrated EEG (aEEG) is a commonly-used predictor of outcome after hypoxic ischemic encephalopathy (HIE). Cerebral and systemic near-infrared spectroscopy (NIRS) and acute kidney injury (AKI) might also have prognostic value. We monitored neonates with aEEG, cerebral and systemic NIRS during therapeutic hypothermia, assigned an AKI stage, and measured neurodevelopmental outcome. For 18 infants, cerebral NIRS variables did not differentiate between those with favorable (N=13) versus adverse (death or moderate-severe disability; N=5) 18-month outcomes. However, systemic rSO2 variability was higher during hours 48–72 of cooling among those with favorable outcomes (0.02<p<0.03). Mean aEEG amplitude during hours 24–48 of cooling was higher among those with good outcomes (0.027<p<0.032). The aEEG lower margin was also higher during hours 12–48 for those with good outcomes (0.014<p<0.035). AKI did not predict outcome (p>0.05). aEEG is a useful prognostic tool for outcomes after neonatal HIE, but the role of NIRS in the hypothermia-treated population remains uncertain.
Keywords: hypoxic-ischemic encephalopathy, amplitude-integrated EEG, near-infrared spectroscopy, acute kidney injury, developmental outcome
Introduction
Therapeutic hypothermia is now the standard of care for term or near-term neonates with hypoxic ischemic encephalopathy (HIE). Despite the most advanced neonatal intensive care, including hypothermia, up to 40% of neonates with HIE have adverse 18-month neurodevelopmental outcomes1–3, and their deficits persist into school age.4–7 There remains a pressing need for optimal risk stratification of these infants in order to effectively test novel therapeutic strategies.
The prognostic utility of amplitude-integrated EEG (aEEG) in the first few hours of life for neonates with HIE is well established8–10, but is altered by therapeutic hypothermia.11–14 Interrater reliability for subjective aEEG background interpretation is not perfect15, and few have evaluated quantitative measures of the aEEG signal for neonates with HIE.
Changes in cerebral oxygen metabolism have been proposed as potential biomarkers for brain injury in neonatal HIE. The rSO2 measured by cerebral near-infrared spectroscopy (NIRS) reflects cerebral blood flow and brain oxygen metabolism and might provide prognostic data for 18-month outcomes after HIE.16,17 NIRS recordings can also be employed to measure renal18, splanchnic19, and peripheral tissue20 perfusion in critically-ill infants.
Our previous work suggested that systemic NIRS provided better short-term prognostic value than cerebral NIRS data in neonates with HIE.21 Additionally, emerging evidence suggests that markers of systemic illness are closely associated with short-term neurological outcomes after therapeutic hypothermia for HIE.22,23 For example, acute kidney injury (AKI) is a major risk factor for brain MRI abnormalities after neonatal HIE.22
We hypothesized that markers of systemic illness, such as abnormal systemic perfusion, recorded by NIRS, and the presence of renal or hepatic dysfunction may augment the prognostic value of measures of cerebral dysfunction (e.g. aEEG and cerebral NIRS) in first days of life for neonates with HIE. Our aim, therefore, was to identify systemic and cerebral risk factors for adverse (or favorable) long-term neurodevelopmental outcomes after neonatal HIE.
Methods
All term neonates admitted to the University of Michigan neonatal intensive care unit whose parents consented to whole-body hypothermia for HIE treatment between January 2009 and February 2011 were enrolled in this study, after written informed consent was provided by the parent. This study was approved by our Institutional Review Board. Details of this cohort’s short-term outcomes were analyzed and published previously.21
Clinical Care
All infants were cooled according to a published protocol, using a servo-controlled cooling blanket, to 33.5°C ± 0.5°C for 72 hours, and were rewarmed using the blanket at 0.5°C per hour until they reached normal body temperature.2 Demographic information and clinically-indicated laboratory testing results (e.g. renal and hepatic function tests) were recorded. Pulse oximetry was monitored for each infant, and values were recorded every 5 seconds in a research computer. Blood pressure was measured as clinically required, and values were recorded in a research computer (frequency ranged from every 5 seconds for those who required arterial lines to every 4 hours for those who were hemodynamically stable at the end of the therapeutic hypothermia protocol).
Measures of Systemic Illness
A neonatal modification of the Kidney Disease: Improving Global Outcomes guidelines was used to classify AKI in the first week of life based on an absolute rise in serum creatinine (SCr) from a previous trough: Stage 1 = rise of SCr 0.3 mg/dL or SCr 150–<200%, Stage 2= rise of SCr 200–<300%, and Stage 3 = rise of SCr ≥300%, absolute SCr ≥ 2.5 mg/dL or dialysis.24 This classification has been used previously for neonates with HIE treated with therapeutic hypothermia.22,23
Liver function was classified as definitely abnormal in infants with liver enzyme levels ≥300% of normal during each 24 hour interval for the 3 days of therapeutic hypothermia. Normal values were defined as 7–35 IU/L for alanine aminotransferase (ALT) and 2–70 IU/L for aspartate aminotransferase (AST).
Amplitude-integrated EEG monitoring
Bilateral aEEG recording commenced as soon as possible after consent was obtained and continued throughout the cooling and rewarming protocol (BRM-2, BraiNZ Instruments, Auckland, New Zealand). The infants’ clinicians reviewed, and acted upon, the aEEG traces during bedside rounds and as needed per the NICU clinical treatment protocol. If seizures were suspected, conventional video-EEG monitoring was initiated. Off-line aEEG analyses were performed using proprietary software (Analyze Research version 1.4, BraiNZ Instruments, Auckland, New Zealand) to facilitate objective and reproducible analyses. The mean and standard deviations for the minimum, maximum, and mean amplitudes, for the left and right aEEG traces were calculated, as well as the validated spectral edge frequency, for each 12-hour interval during the 72 hour cooling period. These variables, rather than classification of aEEG background though pattern recognition, were selected in order to allow quantitative, objective, and reproducible statistical analyses of continuous variables, instead of ordinal or dichotomous regression models, thereby improving overall statistical power.
Near-infrared Spectroscopy Monitoring
Neonatal NIRS sensors (Invos 5100c, Somanetics, Troy, MI) were placed over bilateral parietal regions, with one additional sensor over the quadriceps for systemic measurements, and recording started as soon as possible after obtaining consent. NIRS-measured rSO2 was collected every five seconds and data were extracted with proprietary software (Somanetics Corp, Troy, Michigan). The averages and standard deviations for the left and right cerebral and the systemic measurements rSO2 were calculated for each 12-hour interval during the 72 hour cooling period. Since the clinical implications of NIRS data are uncertain in this patient population, the results were analyzed off-line and were not available to the treating clinicians.
Developmental Follow-Up Assessments
Bayley Scales of Infant Development (BSID) version 3 were recorded at 18-months. Adverse outcomes were defined as death or BSID cognitive and language score <85.25 Gross motor function classification system (GMFCS)26 scores were also recorded at 18-months.
Statistical Analyses
T-tests, Chi squared, and Fischer’s exact tests were employed to assess for differences in AKI and liver function among infants with favorable vs. adverse outcomes. The relationships between NIRS and aEEG data at each 12-hour interval and 18-month outcomes were assessed with independent sample t-tests for the dichotomous (favorable vs. adverse) outcome. For this pilot study, we did not adjust for multiple comparisons; p<0.05 was taken to be significant. STATA was used for all analyses (College Station, TX).
Results
Twenty-one infants were enrolled and outcomes are available for 18 (3 were lost to follow-up). Details of this cohort’s acute NICU course were previously reported.21 Demographic, clinical, and follow-up data are presented in Table 1. Five of the patients had adverse outcomes (3 deaths). Four patients had neonatal seizures, of whom 2 had adverse outcome, and 2 had good outcome. Two patients had abnormal GMFCS scores (N=1 Level II and N=1 Level III); both met additional criteria for adverse outcomes. None of the patients had epilepsy, blindness, or severe hearing impairment at the time of follow-up.
Table 1.
Demographic and clinical profile of the 18 subjects with 18-month neurodevelopmental outcome data available for analysis
| Gestational age | 39.9 ± 1.6 wk |
| Sex | 12 M, 6 F |
| Birth weight | 3,501 ± 445 g |
| Head circumference | 35.6 ± 2.0 cm |
| Median 5-min Apgar score (range) | 3 (0–7) |
| Initial pH | 6.94 ± 0.18 |
| Initial base deficit | 19.2 ± 6.0 |
| Outcome | 5 adverse 13 favorable |
| Acute Kidney Injury | Present in 6 Absent in 11 |
| Bayley Cognitive Score (range), n=15 | 94 ± 19.3 (60–125) |
| Bayley Language Score (range), n=15 | 94.2 ± 20.6 (59–138) |
| Bayley Motor Score (range), n=14 | 96.5 ± 16.8 (46–112) |
Systemic variables
AKI was documented in 6 subjects, while 11 had no AKI (insufficient data were available for one infant). The presence of AKI did not distinguish those who went on to have favorable versus adverse outcomes (3/12 with good outcome had AKI vs. 3/5 with adverse outcome; p=0.28). We lacked power to evaluate associations according to the grade of AKI. There was no association between significantly elevated liver function tests (>300% of normal AST or ALT) on day of life 1, 2, or 3 and developmental outcome (p>0.9 for all comparisons).
Amplitude-integrated EEG variables
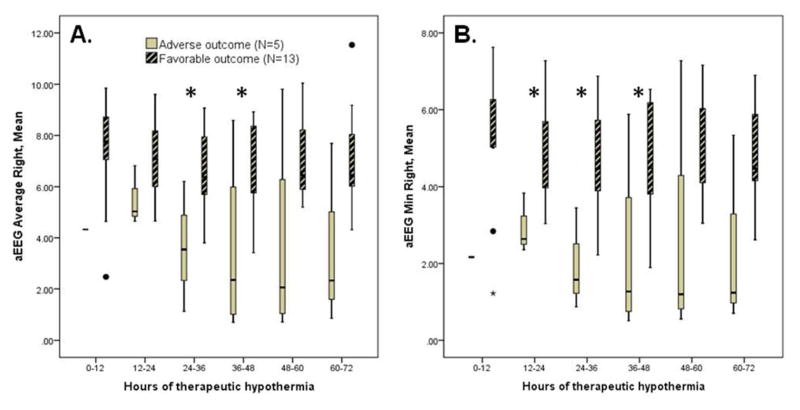
Several aEEG variables were predictive of outcomes. There were insufficient data to analyze aEEG from the first 12 hours of hypothermia, but aEEG data from hours 12 through 48 of cooling were associated with outcomes. Mean aEEG amplitude during hours 24–48 of cooling was higher among those with good outcomes (p=0.027 to 0.032; figure 1a). The aEEG lower margin was also higher during hours 12–48 for those with good outcomes (p=0.014 to 0.035; figure 1b). The spectral edge frequency data showed no significant relationship with outcome (p>0.05 for all time points).
Figure 1.
The average aEEG amplitude was significantly higher during hours 24–48 (Panel A; *p=0.027 to 0.032), as was the average lower margin of the aEEG during hours 12–48 of cooling (Panel B; *p=0.014 to 0.035), among those with favorable vs. adverse 18-month outcomes. There were insufficient aEEG data for the first 12 hours for infants with adverse outcomes. The boxplots present median, 25th, and 75th percentiles, while the whiskers extend to 1.5 x interquartile range. Open circles represent outliers.
Near-infrared spectroscopy variables
Both cerebral and systemic rSO2 remained within the expected normal range (>70) at all times for all infants. There was no relationship between cerebral rSO2 or and outcome (p>0.05 at all time-points; figure 2a). Absolute values of systemic rSO2 did not distinguish between those with favorable versus adverse outcomes. However systemic rSO2 variability was highest during hours 48–72 of cooling among those with favorable outcomes (p=0.02 to 0.03; Figure 2b).
Figure 2.
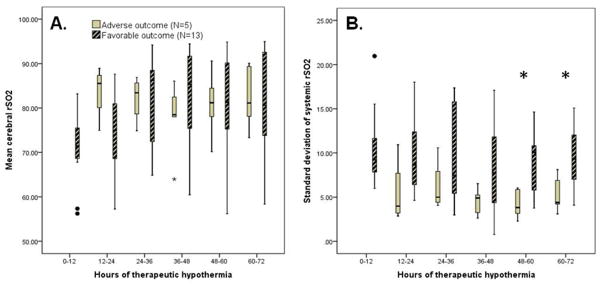
Mean cerebral rSO2 was not different between those with favorable versus adverse 18-month outcomes (Panel A), but those with favorable outcomes had higher systemic rSO2 variability during hours 48–72 of cooling (Panel B; *p=0.02–0.03).
Discussion
Continuous monitoring with aEEG and NIRS is feasible during therapeutic hypothermia for neonatal HIE. We demonstrate here that objective, quantitative aEEG data recorded during the first 48 hours of hypothermia have prognostic value – higher amplitudes (less discontinuity) are associated with favorable 18-month neurodevelopmental outcomes. We echo our previous report that cerebral NIRS data do not distinguish between neonates who go on to have good versus poor outcomes. We also add that decreased systemic rSO2 variability during the third day of hypothermia may be a marker of illness and is associated with adverse 18-month outcomes. However, in this pilot study, neither liver nor kidney dysfunction was associated with outcomes.
Others have reported that normalizing aEEG patterns within the first 48 hours of life are associated with favorable outcomes among infants with who receive therapeutic hypothermia.12 Our quantitative aEEG results confirm this observation and validate aEEG as a useful prognostic monitoring device for this patient population. Of note, our previous work suggested that aEEG recorded during and after rewarming did not distinguish those with adverse short-term neurologic outcomes21, and the present work confirms that aEEG data from the third day of cooling do not add prognostic value.
The presence of acute kidney injury (AKI) was reported to be a predictor of abnormal brain MRI at 7–10 days of life in asphyxiated newborns (OR 3.2, 95% confidence interval 1.3–8.2)22, however, there was no correlation between the stage of AKI and the degree of brain MRI abnormalities. Short-term markers of abnormal neurologic outcome can be imprecise predictors of long-term neurodevelopment. We did not uncover the same relationship between AKI and 18-month outcomes, possibly because of our small sample size (in our study, 25% of those with favorable outcome had AKI, versus 60% with adverse outcome, but p=0.28). Hepatic dysfunction was not associated with outcomes in our cohort, either. However, we speculate that a lack of systemic rSO2 variability is marker of systemic illness severity and demonstrate that this variable – measured on the third day of therapeutic hypothermia – is predictive of both short-term21 and 18-month neurologic abnormalities. Neonates with birth asphyxia commonly have substantial multiorgan dysfunction. Hepatic and renal dysfunction could simply serve as markers of severe HIE rather than specifically contributing to adverse outcomes. Conversely, severe systemic illness may predispose to complications, such as fluid and electrolyte abnormalities or coagulopathy, that compound risk for adverse outcome.
The prognostic utility of NIRS data has been evaluated previously for neonates with HIE. Prior to the era of therapeutic hypothermia, cerebral rSO2 was reported to distinguish ten infants with adverse outcome (all but one of whom died after redirection of care) from eight with favorable 18-month neurodevelopment.16 When the same research team repeated a similar study with 39 infants treated with therapeutic hypothermia, they reported similar results. rSO2 values were significantly higher among the 13 infants with adverse outcome (12 of whom died in the neonatal period) than the 26 with good outcomes from hour 24 of life onward. The authors also commented that aEEG appeared more robust than NIRS as a predictive tool.17 Another small study reported similar findings (N=12).27
The prior studies’ methods were slightly different from ours, which might explain the divergent conclusions. First, the previously published studies utilized pediatric NIRS sensors which were placed over the frontal region, while we employed the Food and Drug Administration-approved neonatal sensors and applied them over the parietal regions (overlying the cerebrovascular watershed zones). The different sensors are reported to provide somewhat divergent data.28 Second, we speculate that the culture surrounding withdrawal of intensive care for critically ill neonates may be differ and note that in the Dutch studies, the majority of those with adverse outcomes died after redirection of the goals of care during the neonatal period.16,17 Third, the other studies utilized Griffiths’ Mental Development Scale while our long-term outcomes were defined by BSID-III results.
Strengths of our study include the use of NIRS data recorded with neonatal sensors placed over the cerebrovascular watershed zone. This type of sensor is most likely to be employed both in American clinical practice and in future clinical trials. Unique in the extant literature is our assessment of systemic NIRS data for this patient population. Additionally, we employed objective aEEG data analysis. Objective analysis of aEEG should be more reproducible between institutions than subjective analyses of background patterns and ought, therefore, to be of value for future multicenter studies.
The primary limitation of this work is the small sample size, a problem which has affected much of the comparable literature.17,27 Because of the limited sample size, we are unable to assess for effects of medication (e.g. phenobarbital) or other factors that could, in theory, affect aEEG and/or NIRS data. Additionally, there were limited aEEG and NIRS data for hours 0–12 of hypothermia, due to delays in obtaining consent and beginning neuromonitoring, so we are unable to comment on the utility of the aEEG and NIRS data during the first hours of therapeutic hypothermia. However, others have shown that aEEG during these first hours of life lack predictive value for infants treated with therapeutic hypothermia.11–13 All of our data were analyzed off-line; additional evidence is required before such data can be applied in real-time in clinical practice.
Conclusion
Improving methods for early identification of hypothermia-treated infants with HIE who remain at elevated risk for adverse outcomes is a priority. Such methods might be suitable for selection of subjects for future trials which evaluate adjuvant interventions. Although NIRS is increasingly used to monitor cerebral perfusion and oxygen metabolism in the NICU, its role for neonates with HIE remains uncertain. However, we confirm that some objective aEEG variables during the first two days of cooling distinguish those who go on to experience good versus adverse outcomes after HIE. Additional study of systemic variables, such as markers of renal or hepatic dysfunction is warranted. Use of NIRS data as a biomarker of systemic illness may be of interest for future studies.
Acknowledgments
The authors are grateful to the patients and families who participated in this research, as well as to the NICU nurses who facilitated the monitoring protocols.
Footnotes
Author Contributions:
R. Shellhaas designed the study, interpreted the data, and drafted the manuscript.
J. Kushwaha extracted data, interpreted the data, and assisted with drafting and revising the manuscript.
M. Plegue analyzed and interpreted the data and revised the manuscript.
D. Selewski interpreted the data and revised the manuscript.
J. Barks assisted with study design, interpreted the data, and revised the manuscript.
Ethical Approval: The University of Michigan Institutional Review Board approved this study. The parents of each enrolled infant provided written informed consent.
Declaration of Conflicts of Interest:
Dr. Shellhaas receives research funding from NIH, the Child Neurology Foundation, and intramural grants from the University of Michigan’s Department of Pediatrics and Communicable Diseases. She serves on the editorial boards of Pediatric Neurology and Journal of Child Neurology.
J. Kushwaha and M. Plegue have nothing to disclose.
D. Selewski receives research funding from the Renal Research Institute and intramural grants from the University of Michigan’s Department of Pediatrics and Communicable Diseases.
J. Barks receives research funding from NIH.
Financial Disclosure: This research was supported by the Child Neurology Foundation’s Shields Fellowship Award (to RAS) and Swaiman Medical Student Scholarship (to JSK), the University of Michigan’s Charles Woodson Pediatric Biostatistics Fund, and the Michigan Institute for Clinical & Health Research (CTSA: UL1RR024986).
Somanetics, Inc (Troy, MI) donated the NIRS equipment for research conducted in our neonatal intensive care unit, but had no input into this study’s conception, design, data analysis, writing of the manuscript, or the decision to submit the manuscript.
References
- 1.Azzopardi DV, Strohm B, Edwards AD, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. New England Journal of Medicine. 2009;361:1349–58. doi: 10.1056/NEJMoa0900854. [DOI] [PubMed] [Google Scholar]
- 2.Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole body hypothermia for neonates with hypoxic-ischemic encephalopathy. New England Journal of Medicine. 2005;353:1574–84. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 3.Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365:663–70. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- 4.Guillet R, Edwards AD, Thoresen M, et al. Seven- to eight-year follow-up of the CoolCap trial of head cooling for neonatal encephalopathy. Pediatric Research. 2012;71:205–9. doi: 10.1038/pr.2011.30. [DOI] [PubMed] [Google Scholar]
- 5.Azzopardi D, Strohm B, Marlow N, et al. Effects of hypothermia for perinatal asphyxia on childhood outcomes. New England Journal of Medicine. 2014;371:140–9. doi: 10.1056/NEJMoa1315788. [DOI] [PubMed] [Google Scholar]
- 6.Natarajan G, Shankaran S, Pappas A, et al. Functional status at 18 months of age as a predictor of childhood disability after neonatal hypoxic-ischemic encephalopathy. Dev Med Child Neurol. 2014;56:1052–8. doi: 10.1111/dmcn.12512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shankaran S, Pappas A, McDonald SA, et al. Childhood outcomes after hypothermia for neonatal encephalopathy. New England Journal of Medicine. 2012;366:2085–92. doi: 10.1056/NEJMoa1112066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.al Naqeeb N, Edwards AD, Cowan FM, Azzopardi D. Assessment of neonatal encephalopathy by amplitude-integrated electroencephalography. Pediatrics. 1999;103:1263–71. doi: 10.1542/peds.103.6.1263. [DOI] [PubMed] [Google Scholar]
- 9.Hellstrom-Westas L, Rosen I, Svenningsen NW. Predictive value of early continuous amplitude integrated EEG recordings on outcome after severe birth asphyxia in full term infants. Arch Dis Child. 1995;72:F34–F8. doi: 10.1136/fn.72.1.f34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toet MC, Hellstrom-Westas L, Groenendaal F, Eken P, de Vries LS. Amplitude-integrated EEG 3 and 6 hours after birth in full term neonates with hypoxic-ischaemic encephalopathy. Arch Dis Child Fetal Neonatal Ed. 1999;81:19–23. doi: 10.1136/fn.81.1.f19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hallberg B, Grossmann K, Bartocci M, Blennow M. The prognostic value of early aEEG in asphyxiated infants undergoing systemic hypothermia treatment. Acta Paediatr. 2010;99:531–6. doi: 10.1111/j.1651-2227.2009.01653.x. [DOI] [PubMed] [Google Scholar]
- 12.Thoresen M, Hellstrom-Westas L, Liu X, de Vries LS. Effect of hypothermia on amplitude-integrated electroencephalogram in infants with asphyxia. Pediatrics. 2010;126:e131–e9. doi: 10.1542/peds.2009-2938. [DOI] [PubMed] [Google Scholar]
- 13.Shankaran S, Pappas A, McDonald SA, et al. Predictive value of an early amplitude integrated electroencephalogram and neurologic examination. Pediatrics. 2011;128:e112–e20. doi: 10.1542/peds.2010-2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Azzopardi D Group ObotTS. Predictive value of the amplitude integrated EEG in infants with hypoxic ischaemic encephalopathy: data from a randomised trial of therapeutic hypothermia. Arch Dis Child Fetal Neonatal Ed. 2014;99:F80–F2. doi: 10.1136/archdischild-2013-303710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shellhaas RA, Gallagher PR, Clancy RR. Assessment of neonatal electroencephalography (EEG) background by conventional and two amplitude-integrated EEG classification systems. Journal of Pediatrics. 2008;153:369–74. doi: 10.1016/j.jpeds.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Toet MC, Lemmers PMA, van Schelven LJ, van Bel F. Cerebral oxygenation and electrical activity after birth asphyxia: their relation to outcome. Pediatrics. 2006;117:333–9. doi: 10.1542/peds.2005-0987. [DOI] [PubMed] [Google Scholar]
- 17.Lemmers PMA, Zwanenburg RJ, Benders MJ, et al. Cerebral oxygenation and brain activity after perinatal asphyxia: does hypothermia change their prognostic value? Pediatric Research. 2013;74:180–5. doi: 10.1038/pr.2013.84. [DOI] [PubMed] [Google Scholar]
- 18.Owens GE, King K, Gurney JG, Charpie JR. Low renal oximetry correlates with acute kidney injury after infant cardiac surgery. Pediatric Cardiology. 2011;32:183–8. doi: 10.1007/s00246-010-9839-x. [DOI] [PubMed] [Google Scholar]
- 19.Cortez J, Gupta M, Amaram A, Pizzino J, Sawhney M, Sood BG. Noninvasive evaluation of splanchnic tissue oxygenation using near-infrared spectroscopy in preterm neonates. The Journal of Maternal-Fetal and Neonatal Medicine. 2011;24:574–82. doi: 10.3109/14767058.2010.511335. [DOI] [PubMed] [Google Scholar]
- 20.Urlesberger B, Grossauer K, Pocivalnik M, Avian A, Muller A, Pichler G. Regional oxygen saturation of the brain and peripheral tissue during birth transition of term infants. Journal of Pediatrics. 2010;157:740–7. doi: 10.1016/j.jpeds.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 21.Shellhaas RA, Thelen BJ, Bapuraj JR, et al. Limited short-term prognostic utility of cerebral NIRS during neonatal therapeutic hypothermia. Neurology. 2013;81:249–55. doi: 10.1212/WNL.0b013e31829bfe41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarkar S, Askenazi DJ, Jordan BK, et al. Relationship between acute kidney injury and brain MRI findings in asphyxiated newborns after therapeutic hypothermia. Pediatric Research. 2014;75:431–5. doi: 10.1038/pr.2013.230. [DOI] [PubMed] [Google Scholar]
- 23.Selewski DT, Jordan BK, Askenazi DJ, Dechert RE, Sarkar S. Acute kidney injury in asphyxiated newborns treated with therapeutic hypothermia. Journal of Pediatrics. 2013;162:725–9. doi: 10.1016/j.jpeds.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Jetton JG, Askenazi DJ. Update on acute kidney injury in the neonate. Current Opinion in Pediatrics. 2012;24:191–6. doi: 10.1097/MOP.0b013e32834f62d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson S, Moore T, Marlow N. Using the Bayley-III to assess neurodevelopmental delay: which cut-off should be used? Pediatric Research. 2014;75:670–4. doi: 10.1038/pr.2014.10. [DOI] [PubMed] [Google Scholar]
- 26.Palisano R, Rosenbaum P, Walter S, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Developmental medicine and child neurology. 1997;39:214–23. doi: 10.1111/j.1469-8749.1997.tb07414.x. [DOI] [PubMed] [Google Scholar]
- 27.Ancora G, Maranella E, Grandi S, et al. Early predictors of short term neurodevelopmental outcome in asphyxiated cooled infants. A combined brain amplitude integrated electroencephalography and near infrared spectroscopy study. Brain & Development. 2013;35:26–31. doi: 10.1016/j.braindev.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 28.Dix LM, van Bel F, Baerts W, Lemmers PMA. Comparing near-infrared spectroscopy devices and their sensors for monitoring cerebral oxygen saturation in the neonate. Pediatric Research. 2013;74:557–63. doi: 10.1038/pr.2013.133. [DOI] [PubMed] [Google Scholar]