Abstract
The authors report two cases of women with Preiser disease treated with dorsal distal radius vascularized grafts. In the first case, after minor trauma, the patient had pain in the left wrist of insidious onset and evolution with significant worsening. The radiographic examination showed increased density of the proximal pole of the scaphoid, and magnetic resonance imaging (MRI) showed partial necrosis. Intraoperatively, as the integrity of the cartilage of the proximal pole of the scaphoid was observed, dorsal vascularized distal radius graft was performed using the 1,2 intercompartmental supraretinacular artery. In 4 months postoperatively, MRI showed almost total integration of the graft, and 1 year after surgery, the patient was asymptomatic, with normal mobility of the operated wrist and imaging showing a normal scaphoid. The second case had similar history and clinical picture, but the radiographs showed narrowing and diffuse sclerosis and also osteolytic areas in the proximal pole of the scaphoid; MRI showed diffuse necrosis. The same graft technique was used, considering that there was a good cartilaginous coverage of the scaphoid. After 9 years of follow-up, the patients remain free of pain or functional limitations. In such cases, the vascularized graft technique was effective and, therefore, a good therapeutic option, provided that there is no degenerative changes in the carpus and, especially, the cartilage of the proximal pole is viable.
Introduction
Idiopathic avascular necrosis of the scaphoid was initially described by Preiser [16] in 1910, and its pathogenesis, progression, and treatment are still under much discussion. The reduction of dorsal blood supply seems to jeopardize the vascularity of the proximal two thirds of the scaphoid, with consequent osteonecrosis [7, 15]. Prolonged use of corticosteroids, chemotherapy, trauma, collagen diseases, and alcoholism are associated with osteonecrosis [2]. Wrist position can also influence the vasculature, as suggested by Buttermann et al. [3], who noted that the blood supply to the scaphoid is interrupted at the position of 60° of palmar flexion and 15° of ulnar deviation after compression of the extensor carpi radialis brevis muscle.
The condition occurs more frequently in dominant hands, of women aged between 20 and 70 years [12]. There is only one case described below the age of 10 years [10]. The clinical picture is characterized by localized pain on the lateral aspect of the wrist (anatomical snuff box). On palpation, there is a hypersensitive region of the scaphoid and, sometimes, slight swelling and loss of strength. The decrease in the amplitude of motion occurs only in advanced disease.
Early diagnosis is needed, before the scaphoid suffers fragmentation and collapse and degenerative disease installs in the radiocarpal and mediocarpal joint surfaces. X-rays and computed tomography (CT) scans may show sclerosis and fragmentation in the scaphoid, especially in the proximal region [9, 11].
There is no consensus regarding the best treatment of the disease, as there are no relevant prospective studies. In this report, with the aim of presenting another therapeutic option, we describe two cases treated with vascularized grafts of the dorsal distal radius bone.
Case 1
The patient, a 47-year-old real estate broker, complained of pain and limitation of flexion in the left wrist (nondominant) for 3 years, due to minor trauma events, and had no improvement with the use of anti-inflammatory drugs and physiotherapy. Radiographic examination showed narrowing, diffuse sclerosis, and osteolytic areas in the proximal pole of the scaphoid. Radiographic images (Fig. 1) and MRI allowed diagnosis and the classification, respectively, as in stage III according to Herbert and Lanzetta [9] and type 2 Kalainov et al. [11].
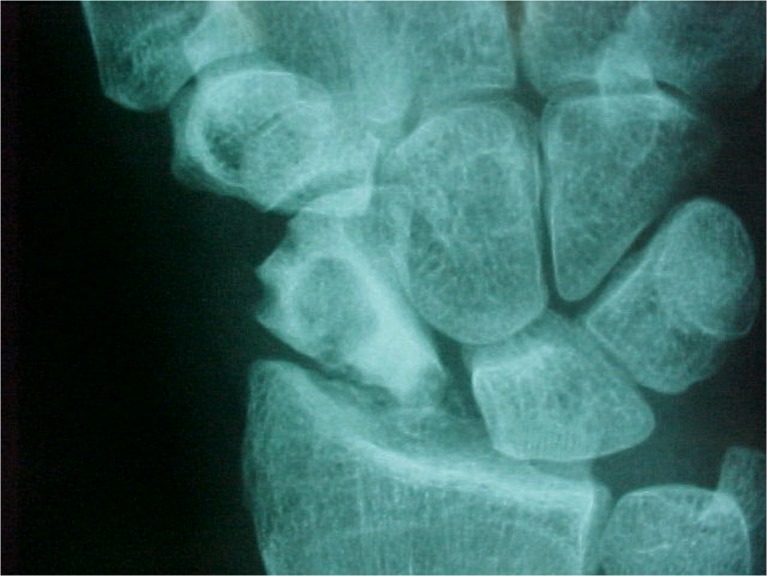
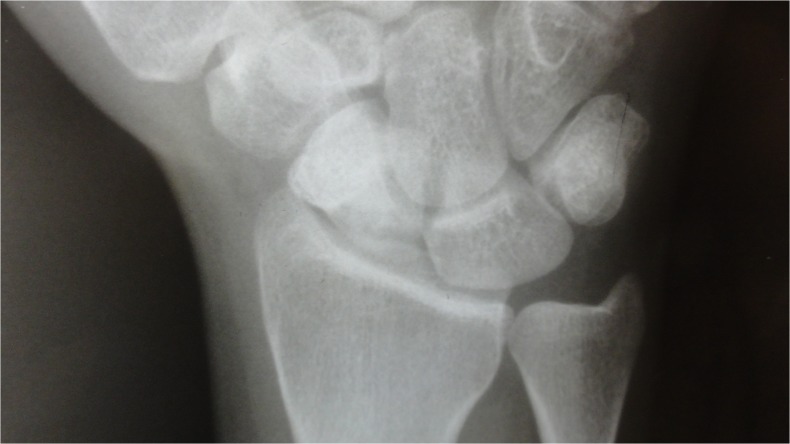
Fig. 1.
Preoperative radiograph in case 1: sclerosis and irregularity in the scaphoid, stage III of Herbert and Lanzetta [8]
The patient was operated on in February 2003. During surgery (Fig. 2), we observed that the proximal pole had a good cartilaginous cap. Therefore, we decided to use the dorsal vascularized graft of the distal radius using the 1,2 intercompartmental supraretinacular artery. Figure 3 shows the 1-month postoperative radiograph, with signs of graft integration. The patient was immobilized for 6 weeks, when she started occupational physiotherapy. After 6 months, the MRI findings showed bone graft integration. Radiographs 2 years after surgery showed a preserved scaphoid, without pieces of evidence of fragmentation (Fig. 4).
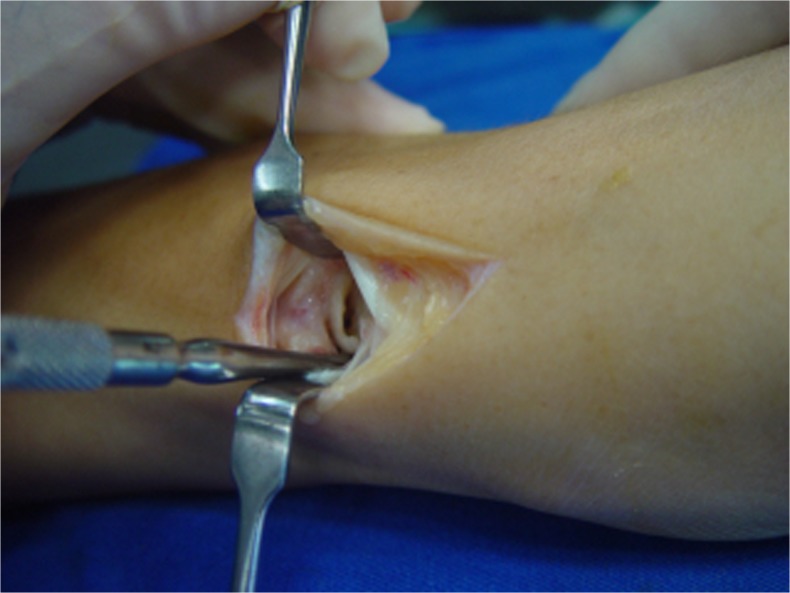
Fig. 2.
Intraoperative aspect of case 1: preserved scaphoid proximal pole cartilage
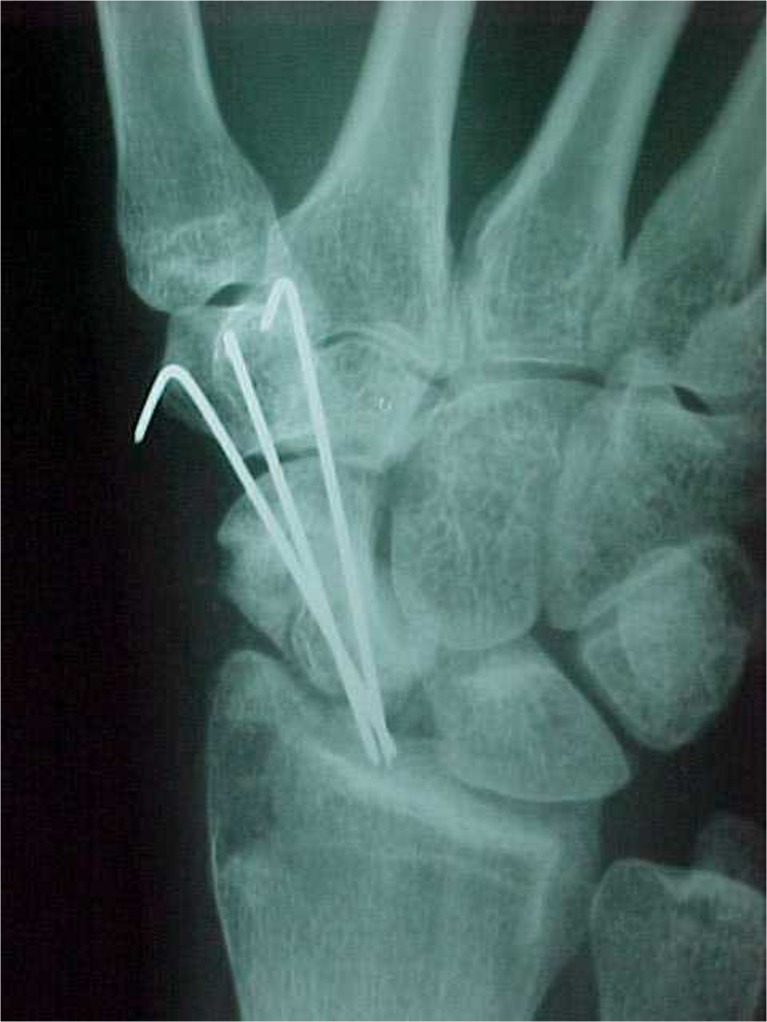
Fig. 3.
Radiograph at 30 days postoperatively in case 1: signs of graft integration with fixation
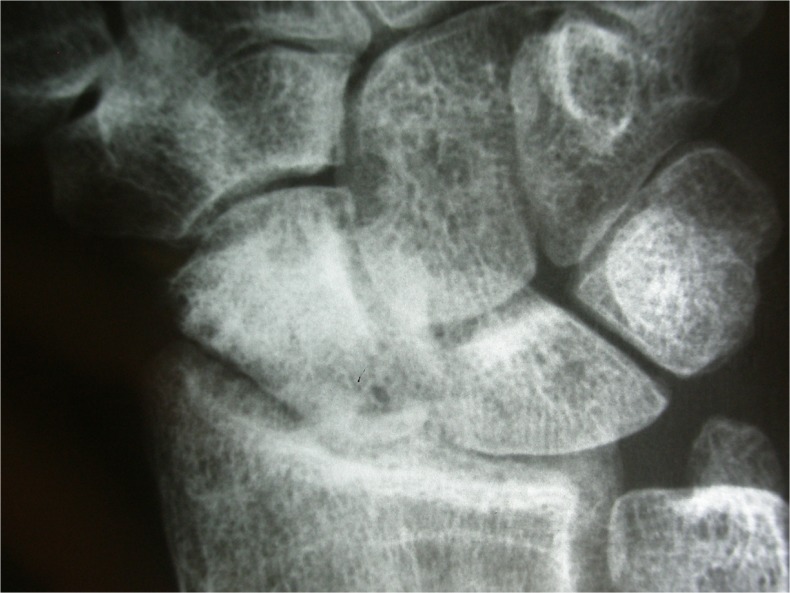
Fig. 4.
Radiograph at 2 years postoperatively in case 1: preserved scaphoid shape, without fragmentation
Seven years after surgery, the graft was fully integrated (Fig. 5). Currently, in the 9-year follow-up, the patient presents for clinical consult without pain or functional limitation and she has resumed her sports activities with little differences in range of motion and grip strength compared to the contralateral side—dorsal flexion of 60°, palmar flexion of 60°, ulnar deviation of 32°, radial deviation of 28°, and a grip strength of 92.8 % of the contralateral side (Figs. 6, 7, and 8 and Table 1).
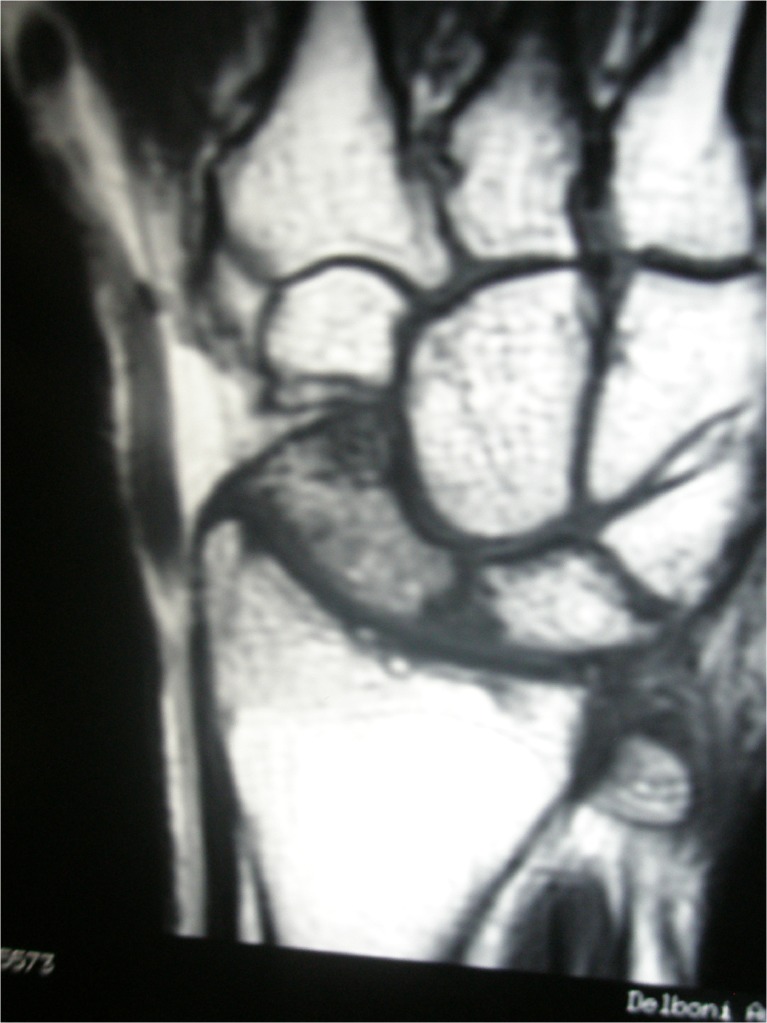
Fig. 5.
Magnetic resonance imaging 7 years after surgery in case 1 showing integration of the vascularized bone graft
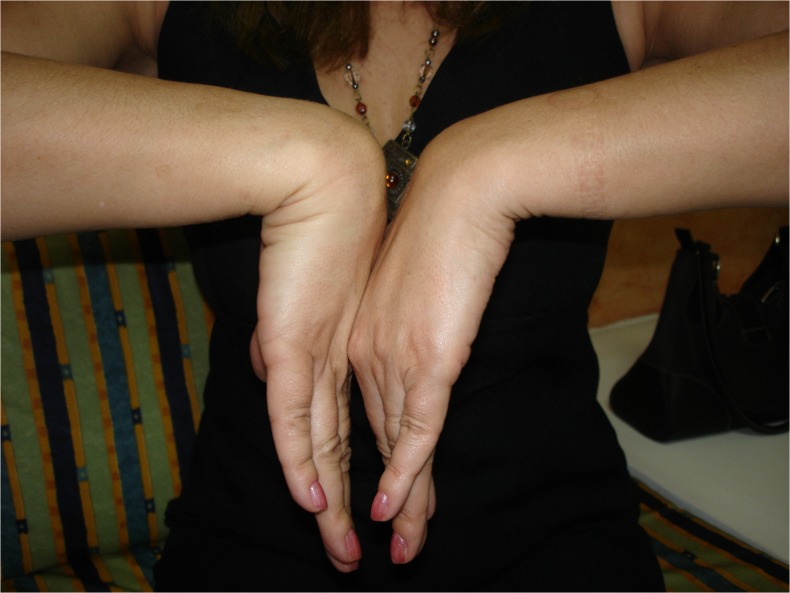
Fig. 6.
Postoperative dorsal flexion in case 1
Fig. 7.
Postoperative volar flexion in case 1
Fig. 8.
Scar aspect in the radial side in case 1
Table 1.
Comparison of range of motion and grip strength between cases 1 and 2
| Case | Range of motiona | Grip strengthb |
|---|---|---|
| 1 | 60/60/32/28 | 26/28 |
| 2 | 62/55/22/23 | 27/32 |
aRange of motion in the following order: volar flexion, dorsal flexion, ulnar deviation, and radial deviation
bGrip strength in kilograms in the following order: diseased wrist and contralateral wrist
Case 2
The second patient, an 18-year-old nonsmoker lady, a college student, presented with pain in the left wrist (dominant side), of insidious onset 4 months before. Since then, she had worsening of pain and significant disability. She reported mild trauma 2 years ago in the affected wrist. After evaluation of imaging studies (Fig. 9), she was classified as stage II of Herbert and Lanzetta [9] and type 1 according to the classification of Kalainov et al. [11]. The MRI showed low signal on T1 and T2, with impregnation of the bone marrow after injection of contrast (gadolinium) and also a chondropathy in the volar region of the proximal scaphoid (Fig. 10). CT showed a fracture in the subchondral joint surface of the proximal pole of the scaphoid, with fragmentation and irregularity of the bony edges, associated with a focus of adjacent medullary sclerosis.
Fig. 9.
Preoperative radiograph in case 2: sclerosis and irregularity in the scaphoid, without fragmentation, stage II of Herbert and Lanzetta [8]
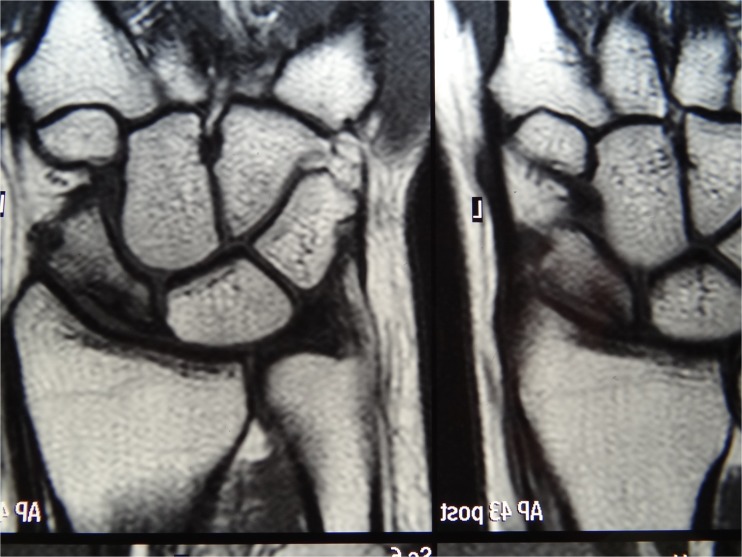
Fig. 10.
Coronal cut in the preoperative magnetic resonance imaging in case 2: stage II of Kalainov [9], showing partial necrosis
The patient was operated on August 2, 2010, and during surgery (Fig. 11), it was possible to observe the cartilage integrity of the proximal pole of the scaphoid, which allowed us to conduct dorsal vascularized grafting of the distal radius using the 1,2 intercompartmental supraretinacular artery. Grafting was performed under pressure, without bone fixation.
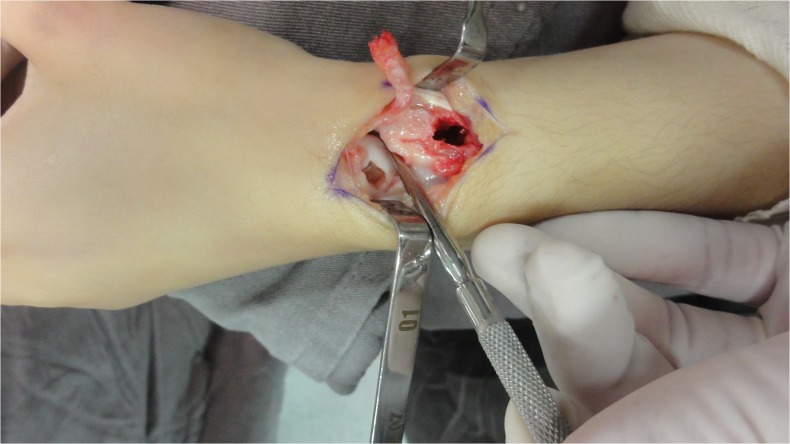
Fig. 11.
Intraoperative aspect of the vascularized graft and pedicle, the distal radius, and the scaphoid prepared to receive the graft, in case 2
The patient was immobilized after surgery for 6 weeks and with bracing for another 4 weeks. In the sixth week, she was referred for physiotherapy, with improvements in pain and joint range of motion. MRI after 6 weeks showed areas of contrast enhancement in both the graft and in the original bone marrow of the scaphoid, indicating the start of graft integration.
In 4 months postoperatively, new MRI exam showed the almost total integration of the graft. One year after surgery (Fig. 12), the patient was asymptomatic, with normal mobility of the operated wrist and imaging showing a normal scaphoid. Currently, at 2 years of follow-up (Fig. 13), she is asymptomatic and without restrictions in work and leisure. The range of motion of the wrist and the grip strength showed little difference compared to the contralateral side—dorsal flexion of 55°, palmar flexion of 62°, ulnar deviation of 22°, radial deviation of 23°, and a grip strength of 87.5 % of the contralateral side (Figs. 14, 15, and 16 and Table 1).
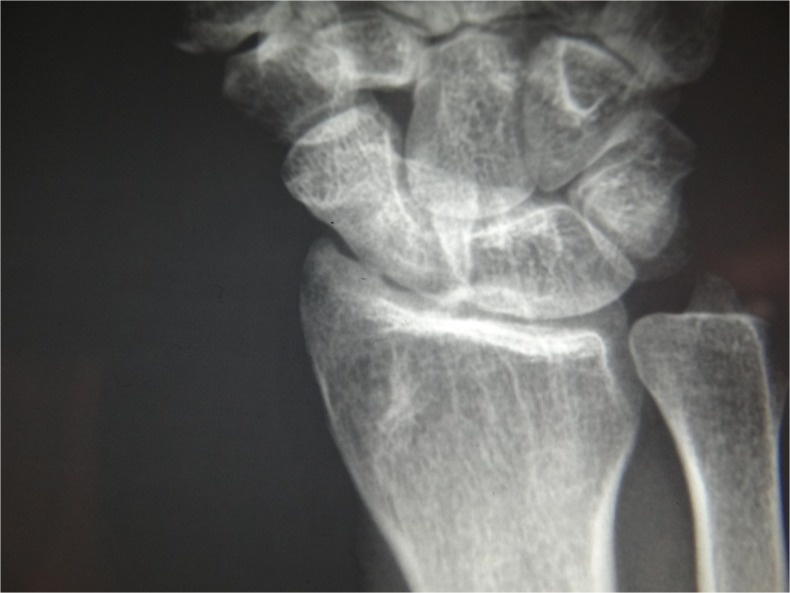
Fig. 12.
Radiograph of case 2 2 years postoperatively
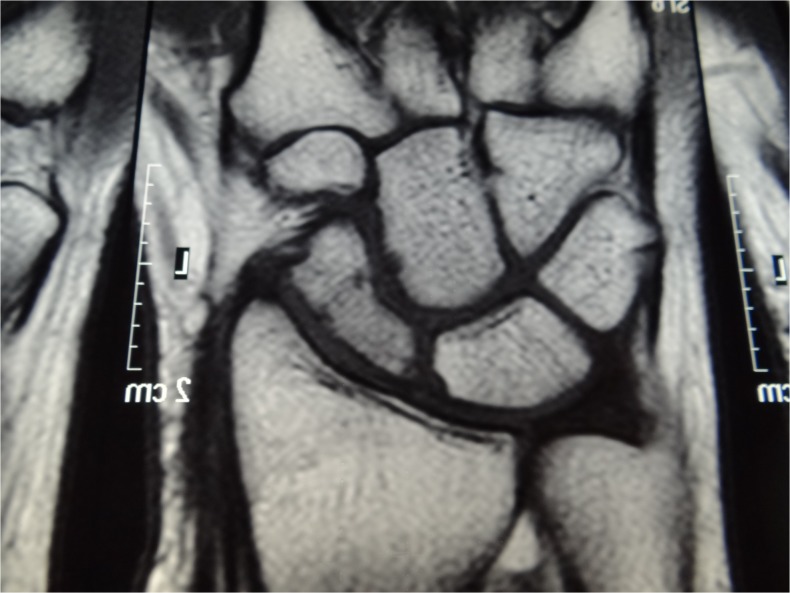
Fig. 13.
Magnetic resonance imaging 1 year postoperatively in case 2, with normalization of the signal evidencing revascularization
Fig. 14.
Postoperative dorsal flexion in case 2
Fig. 15.
Postoperative volar flexion in case 2
Fig. 16.

Scar aspect in radial side in case 2
Discussion
Herbert and Lanzetta [9] proposed a staging system of scaphoid avascular necrosis, based on the progression of the disease:
Type I—normal radiographs and CT scans showing abnormal findings
Type II—increased density of the proximal pole with generalized osteopenia
Type III—fragmentation with or without pathologic fracture
Type IV—fragmentation and collapse with osteoarthritis
Magnetic resonance imaging (MRI) helps in the disease staging. Kalainov et al. [11] identified two types of avascular necrosis of the scaphoid as seen in MRI:
Type I—necrosis or diffuse ischemia
Type II—partial necrosis
Preiser disease treatment is still much discussed and not well established. The literature offers only retrospective case series with low numbers of patients. A recent literature review [12] cites 126 cases in 30 articles published from 1980 to 2012.
In accordance with the literature, showing a higher frequency of Preiser disease in females, our two cases were women aged 18 and 47 years (the case subjects in the published reports are aged between 20 and 70 years). Although the case series report mainly on Preiser disease on dominant hands, we had a case in a nondominant hand. Both had a history of minor traumas, probably involved in the pathogenesis of the disease.
Conservative treatment has been attempted by several authors. Immobilization, infiltration with corticosteroids or hyaluronic acid, and electrical stimulation, among others, did not show satisfactory results, not prevented disease progression, or improved symptoms [6, 9, 12, 19].
Several surgical techniques have been described: replacement by silicone prosthesis [9], proximal carpectomy [4], partial or total arthrodesis of the carpal [1, 6, 11], arthroscopic drilling [13], osteotomy of the radius [8], conventional graft [11], and vascularized bone graft [5, 12, 14, 17] among others, none of which showed good results.
The graft for revascularization of the scaphoid was first described by de Smet [5], in 2000. Moran et al. [14] reviewed eight cases of Preiser disease without fragmentation or degenerative changes of the scaphoid, treated with a distal radius vascularized graft of dorsal retrograde flow (six cases based on the 1,2 supraretinacular artery and two cases based on the 2,3 supraretinacular artery). All patients had improvement in pain and range of motion. MRI postoperative findings showed signs of revascularization of the proximal pole in all cases. These authors, as well as Lenoir et al. [12], indicate revascularization in early stages (I and II Herbert and Lanzetta [9]) and in patients without radiocarpal arthrosis.
Although Sokolow et al. [18] always recommend the use of vascularized graft to preserve the morphology of the carpus, the indication of this technique is not clear at stage III, when the architecture of the scaphoid is already compromised. In the two cases reported here, the use of distal radius vascularized graft showed satisfactory results, even in Herbert stage III, as with our case 2. After 9 years of follow-up, the patient in case 2 performs her professional and leisure activities without symptoms. In case 1, the patient also performs their daily activities without restrictions. In both, the MRI findings showed complete revascularization of the scaphoid.
The use of a vascularized bone graft seems to be intuitive and based on the potential of revascularization. In our two cases, the technique was effective and, therefore, a good therapeutic option, provided that there is no degenerative changes in the carpus and, especially, the cartilage of the proximal pole is viable.
Acknowledgments
Conflicts of Interest
Sérgio Augusto Machado da Gama declares that he has no conflict of interest.
Marcelo Rosa de Rezende declares that he has no conflict of interest.
Samuel Ribak declares that he has no conflict of interest.
Statement of Human and Animal Rights
As a case report, this is a retrospective description of the cases of two patients treated in our institution. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008. This article does not contain any studies with human or animal subjects. Therefore, their privacy has been protected and all ethic principles have been followed.
Statement of Informed Consent
The patients have signed informed consent forms at the time of treatment, and they are not identified by any means in this paper.
References
- 1.Allen PR. Idiopathic avascular necrosis of the scaphoid. A report of two cases. J Bone Joint Surg (Br) 1983;65(3):333–5. doi: 10.1302/0301-620X.65B3.6841406. [DOI] [PubMed] [Google Scholar]
- 2.Amillo-Garayoa S, Romero-Muñoz LM, Pons-DeVillanueva J. Bilateral Preiser’s disease: a case report and review of the literature. Musculoskelet Surg. 2011;95(2):131–3. doi: 10.1007/s12306-011-0095-x. [DOI] [PubMed] [Google Scholar]
- 3.Buttermann GR, Putnam MD, Shine JD. Wrist position affects loading of the dorsal scaphoid: possible effect on extrinsic scaphoid blood flow. J Hand Surg (Br) 2001;26(1):34–40. doi: 10.1054/jhsb.2000.0475. [DOI] [PubMed] [Google Scholar]
- 4.De Smet L, Aerts P, Fabry G. Avascular necrosis of the scaphoid: report of three cases treated with a proximal row carpectomy. J Hand Surg [Am] 1992;17(5):907–9. doi: 10.1016/0363-5023(92)90466-3. [DOI] [PubMed] [Google Scholar]
- 5.de Smet L. Avascular nontraumatic necrosis of the scaphoid. Preiser’s disease? Chir Main. 2000;19(2):82–5. doi: 10.1016/S1297-3203(00)73464-0. [DOI] [PubMed] [Google Scholar]
- 6.Ferlic DC, Morin P. Idiopathic avascular necrosis of the scaphoid: Preiser’s disease? J Hand Surg [Am] 1989;14(1):13–6. doi: 10.1016/0363-5023(89)90053-1. [DOI] [PubMed] [Google Scholar]
- 7.Gelberman RH, Menon J. The vascularity of the scaphoid bone. J Hand Surg [Am] 1980;5(5):508–13. doi: 10.1016/S0363-5023(80)80087-6. [DOI] [PubMed] [Google Scholar]
- 8.Hayashi O, Sawaizumi T, Ito H. Closed radial wedge osteotomy for Preiser’s disease: a report of four cases. Hand Surg. 2011;16(3):347–52. doi: 10.1142/S0218810411005679. [DOI] [PubMed] [Google Scholar]
- 9.Herbert TJ, Lanzetta M. Idiopathic avascular necrosis of the scaphoid. J Hand Surg (Br) 1994;19(2):174–82. doi: 10.1016/0266-7681(94)90159-7. [DOI] [PubMed] [Google Scholar]
- 10.Jensen CH, Leicht P. Idiopathic avascular necrosis of the scaphoid in a child. Scand J Plast Reconstr Hand Surg. 1995;29(4):359–60. doi: 10.3109/02844319509008973. [DOI] [PubMed] [Google Scholar]
- 11.Kalainov DM, Cohen MS, Hendrix RW, Sweet S, Culp RW, Osterman AL. Preiser’s disease: identification of two patterns. J Hand Surg [Am] 2003;28(5):767–78. doi: 10.1016/S0363-5023(03)00260-0. [DOI] [PubMed] [Google Scholar]
- 12.Lenoir H, Coulet B, Lazerges C, Mares O, Croutzet P, Chammas M. Idiopathic avascular necrosis of the scaphoid: 10 new cases and a review of the literature. Indications for Preiser’s disease. Orthop Traumatol Surg Res. 2012;98(4):390–7. doi: 10.1016/j.otsr.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 13.Menth-Chiari WA, Poehling GG. Preiser’s disease: arthroscopic treatment of avascular necrosis of the scaphoid. Arthroscopy. 2000;16(2):208–13. doi: 10.1016/S0749-8063(00)90038-0. [DOI] [PubMed] [Google Scholar]
- 14.Moran SL, Cooney WP, Shin AY. The use of vascularize grafts from the distal radius for the treatment of Preiser’s disease. J Hand Surg [Am] 2006;31(5):705–10. doi: 10.1016/j.jhsa.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Panagis JS, Gelberman RH, Taleisnik J, Baumgaertner M. The arterial anatomy of the human carpus. Part II: the intraosseous vascularity. J Hand Surg Am. 1983;8(4):375–82. doi: 10.1016/S0363-5023(83)80195-6. [DOI] [PubMed] [Google Scholar]
- 16.Preiser G. Eine typische posttraumatische und zur spontanfraktur führende ostitis des naviculare carpi. Fortschr Geb Roentgenstr. 1910;15:189–97. [Google Scholar]
- 17.Sawaizumi T, Nanno M, Ito H. Vascularized second metacarpal-base bone graft in scaphoid non-union by the palmar approach. J Reconstr Microsurg. 2003;19(2):99–106. doi: 10.1055/s-2003-37814. [DOI] [PubMed] [Google Scholar]
- 18.Sokolow C, Theron P, Saffar P. Preiser’s disease. J Am Soc Surg Hand. 2004; 4(2):103-8. Available from: http://www.sciencedirect.com/science/article/pii/S1531091404000464. Accessed in 2013 (Jun 5).
- 19.Vidal MA, Linscheid RL, Amadio PC, Dobyns JH. Preiser's disease. Ann Chir Main Memb Super. 1991;10(3):227–35. doi: 10.1016/S0753-9053(05)80287-X. [DOI] [PubMed] [Google Scholar]