Abstract
Objectives
Focal dystonia of the head, neck are associated with a loss of kinaesthetic acuity at muscles distant from the dystonic sites. That is, while the motor deficits in focal dystonia are confined, the associated somatosensory deficits are generalized. This is the first systematic study to examine, if patients diagnosed with spasmodic dystonia (SD) show somatosensory impairments similar in scope to other forms of focal dystonia.
Methods
Proprioceptive acuity (ability to discriminate between two stimuli) for forearm position and motion sense was assessed in 14 spasmodic dystonia subjects and 28 age-matched controls using a passive motion apparatus. Psychophysical thresholds, uncertainty area and a proprioceptive acuity index were computed based on the subjects’ verbal responses.
Results
The main findings are: First, the SD group showed significantly elevated thresholds and uncertainty areas for forearm position sense when compared to the control group. Second, 9 out of 14 dystonia subjects (64%) exhibited an acuity index for position sense above the control group maximum. Three SD subjects had a motion sense acuity index above the control group maximum.
Conclusion
The results indicate that impaired limb proprioception is a common feature of SD. Like other forms of focal dystonia, spasmodic dystonia does affect the somatosensation of non-dystonic muscle systems. That is, SD is associated with a generalized somatosensory deficit.
Keywords: Basal ganglia, Focal dystonia, Human, Kinaesthesia, Somatosensation
INTRODUCTION
Spasmodic dysphonia (SD) is a chronic voice disorder characterized by involuntary, random movement of laryngeal muscles causing disruption of fluent speech with strained-strangled voice quality. Vocal interruptions or spasms, periods of no sound, and periods of near normal voice all co-occur in SD. SD signs and symptoms are task specific, occurring during speech but not during other phonatory (e.g., prolonging vowels) or non-phonatory tasks (e.g., breathing). Current therapeutic options are limited. SD does not respond to current forms of behavioral speech therapy and is treated primarily with Botulinum toxin injections (Botox) to provide temporary symptom relief. There is no cure and misdiagnosis with other idiopathic voice disorders collectively termed muscle-tension dysphonia is not uncommon and can lead to inappropriate treatment.
Although its exact aetiology is unknown, SD has been considered a form of focal dystonia. SD shares several abnormal neurologic signs with focal dystonia of the head, neck and hand. For example, abnormal blink reflexes were observed in SD, torticollis, and blepharospasm 1–4 and abnormal long-latency responses to peripheral nerve stimulation have been observed in SD 5, blepharospasm and oromandibular dystonia 6.
Substantial evidence indicates that basal ganglia-related diseases such as Parkinson’s disease and certain forms of dystonia are associated with somatosensory and specifically proprioceptive abnormalities which are closely linked to the observed motor deficits 7–17 (for reviews see: 18–20). With respect to focal dystonia, the kinaesthetic impairments are generalized and not restricted to the dystonic musculature 17. Given that muscle spindles in dystonic muscles are intact 21 and focal dystonia is not associated with known proprioceptive receptor or peripheral nervous system damage, the most plausible explanation is that the observed kinaesthetic deficits in focal dystonia are of central origin and not solely caused by the abnormal tone in the affected musculature or abnormal reflex circuitry in the brainstem. This assessment is corroborated by recordings of somatosensory evoked potentials (SEPs) and transcranial magnetic stimulation documenting that abnormal processing of somatosensory information in focal dystonia is associated with abnormally enhanced cortical excitability and decreased intracortical inhibition 22, 23, which recently has also been confirmed for SD 24.
The purpose of this study is to determine whether the proprioceptive acuity of non-speech motor systems is altered in patients with the voice disorder SD. Acuity refers to one’s ability to perceive the smallest, just noticeable difference between two detectable stimuli 25. We measured proprioceptive acuity by determining a) the psychophysical threshold and b) the uncertainty around the threshold. Specifically, we examined the acuity of the position and passive motion sense of the forearm in SD patients and compared the results to the perceptual performance of a healthy control group. Controlled passive motion of the forearm, not requiring any active muscular contractions, was induced by a custom-built apparatus described in detail previously 10. The systematic testing of two proprioceptive senses important for motor control allowed us to obtain a proprioceptive profile of each participant. Showing that SD patients have reduced proprioceptive acuity similar to patients with other forms of focal dystonia would provide additional evidence for classifying SD as a form of focal dystonia. More importantly, it could provide an justification for creating behavioral treatments that aim to alter proprioceptive inputs to the laryngeal muscles in order to overcome the SD symptoms. This would be especially relevant for patients who do not respond well to botox injections and who currently have no viable treatment option.
METHOD
Participants
The study was approved by the institutional review boards of the University of Minnesota and Chang Gung Memorial Hospital, Taiwan. A total of 43 subjects participated, including 15 SD patients (mean age 55.4±10.6 yrs.; 6 male, 9 female), and 28 healthy controls (mean age 61.1±11.6 yrs.; 13 male, 15 female). All SD patients and 13 control subjects were recruited in Minnesota. Fifteen additional controls were recruited in Taiwan. All patients were seen at the end of their Botox cycle when voice symptoms were most pronounced and the effect of medication was minimized. Prior to testing, clinical status was assessed through a questionnaire and clinical speech evaluation.
Clinical evaluation was based on audio recordings of vowel prolongation and connected speech with the latter using the ten ‘adductor’ sensitive sentences 26. Sentence audio recordings were used for rating dysphonia severity and for identifying voice breaks. Two judges, blinded to the diagnosis, were given access to the secured audio files and listened to them online. Both were certified speech-language pathologists who work exclusively with voice-disordered patients. Judges used a visual-analog scale (VAS) to rate dysphonia severity. The scale was a line of 100 mm in length, where the 0 mm mark represented no voice impairment and the most severely impaired status was by the 100 mm mark. Based on the same recordings, acoustic analysis was performed to determine the presence of voice breaks using PRAATsoftware 27. Voice (phonation) breaks were identified for a voiced component (usually a vowel) within a word and only when the break was greater than 50 msec following established guidelines 28. A voice break was identified as an absence of voicing occurring during a voiced segment, and lasting 50 ms or more. Nasoendoscopic exams were available for 10 patients to confirm the diagnosis of SD. The remaining 5 patients were referrals and the original diagnosis had been made elsewhere. Clinically, they were treated as SD and did respond to Botox. Table 1 lists the patient characteristics in detail. Data sets of one SD patient and five control subjects were incomplete (subjects did not perform both tests). For the analysis of the position sense acuity the data of 14 SD and 25 controls were considered. For the analysis of motion sense acuity the data of all 15 SD patients and 26 controls were included.
Table 1.
Clinical characteristics and basic demographics of spasmodic dysphonia patients. Note: For SD06 no audio/video data could be obtained and thus no data on voice quality could be derived. Phonation (voice) breaks were identified for a voiced component (usually a vowel) within a word and only when the break was greater than 50 ms.
| ID | Age (years) |
Gender | Reported symptom duration (years) |
Duration since official diagnosis (years) |
Voice symptom severity (0–100) |
Number of voice breaks |
|---|---|---|---|---|---|---|
| SD01 | 40 | F | 15 | 8 | 40 | 0 |
| SD06 | 68 | M | 47 | 27 | ||
| SD10 | 50 | F | 1.9 | 0.2 | 35 | 0 |
| SD09 | 67 | F | 37 | 22 | 34 | 1 |
| SD16 | 48 | M | 3.5 | 2 | 55 | 2 |
| SD21 | 47 | F | 2.5 | 0.75 | 61.0 | 5 |
| SD27 | 67 | F | 1.5 | 1.2 | 47.7 | 0 |
| SD25 | 60 | M | 15 | 8 | 61.3 | 5 |
| SD28 | 57 | M | 9 | 1.5 | 72.3 | 10 |
| SD29 | 49 | M | 22 | 2 | 21.3 | 2 |
| SD34 | 46 | F | 3.5 | 3 | 62.7 | 0 |
| SD36 | 45 | M | 3 | 2.5 | 92.7 | 0 |
| SD37 | 70 | F | 1.5 | 1 | 61.7 | 12 |
| SD43 | 70 | F | 11 | 9 | 38.0 | 0 |
| SD44 | 47 | F | 1.4 | 0.4 | 64.0 | 0 |
Instrumentation
A passive motion apparatus was used to test motion and position sense acuity (see Figure 1A). Subjects placed their forearm on a rotatable aluminium splint that was moved passively by a DC five phase stepping motor (precision: 5466 steps per 1° = 0.00018°/step; Nyden Inc., San Jose, USA). The splint was padded with 16 cm thick foam to attenuate possible vibration effects of the DC motor. Control of the apparatus was realized through customized software routines coded in Matlab Technical Programming Language.
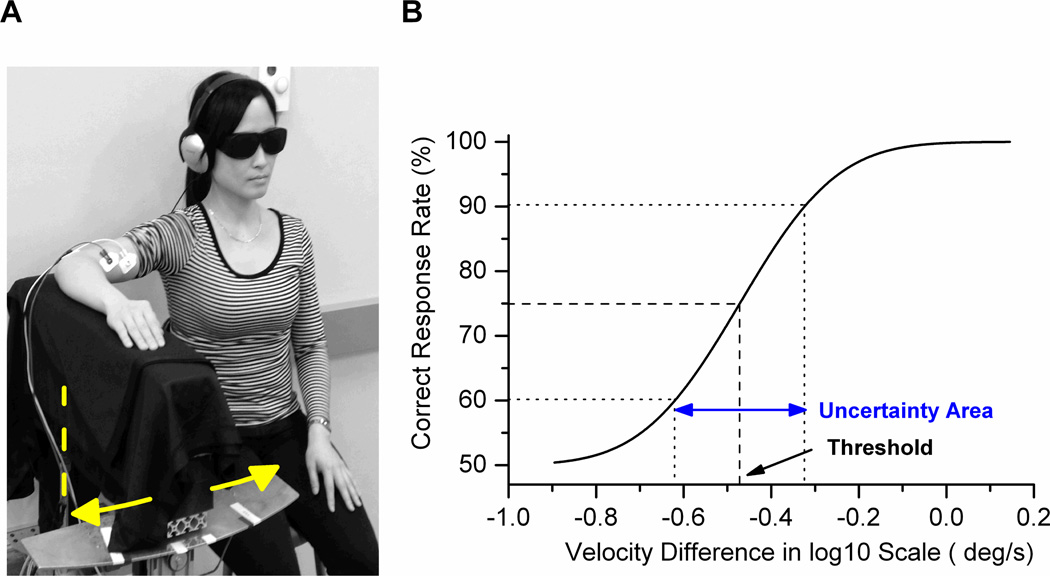
Fig. 1.
A. Experimental setup with passive motion apparatus. The arm was rotated horizontally towards flexion or extension (axis of rotation = dashed line). EMG of the biceps and triceps brachii was recorded to monitor muscle activation. B. Exemplar psychometric function obtained during motion sense testing. The JND corresponds to the stimulus size at 75% correct response level. The uncertainty area is the standard deviation of the Gaussian function fit.
Procedure
Participants completed two separate tasks. One task consisted of subjects providing verbal judgments regarding the angular velocity about the elbow joint (motion sense) and the other regarding angular displacement about the elbow joint (position sense). For both tasks, participants sat on a height adjustable stool and comfortably rested their forearm on the foam padded splint (see Fig. 1A). The elbow joint axis was aligned with the rotational axis of the apparatus. The arm was abducted so that passive motion of the apparatus induced primarily motion of the forearm segment by rotating around elbow movement (flexion/extension) and to a minimal extent shoulder movement (medial/lateral rotation). During passive arm motion subjects maintained a “loose fist” throughout testing to exclude the possibility of obtaining haptic information from the sensitive palmar surface of the hand. They wore goggles to exclude vision and headphones (AKG K-66 series, AKG AG, Vienna, Austria) emanating low volume pink noise to mask possible auditory cues. Only the dominant arm was tested in each subject. Subjects were randomized as to whether they first completed the motion sense or the position sense task. Within each task, subjects completed a minimum of 62 trials. Electromyography (EMG) of arm flexor and extensors was monitored online to detect muscle activation. Surface EMG silver chloride electrodes (Biopac, EL 507) were placed on m. biceps brachii and triceps brachii and activity was sampled at 250Hz. Signals were amplified using a Biopac MP36 system (BIOPAC Systems, Inc., Goleta, USA). Trials in which muscle activation was detected by the experimenter during a trial were removed and immediately repeated to assure the same number of trials across subjects.
Motion Sense Task
Starting position was an elbow joint angle of 90°. During each trial subjects were randomly presented with a sequence of two velocity stimuli separated by a 500 ms interstimulus interval. Employing a 2-interval, forced-choice procedure 29, 30, subjects were instructed to discriminate between the presentation of one fixed (standard = 1.5°/s) and one variable comparison stimulus (1.5°/s < comparison < 2.9°/s). Within a single trial, the two stimuli to be discriminated moved in the same direction (flexion or extension), with stimuli of a subsequent trial moving in the opposite direction (e.g. flexion-flexion; extension-extension; flexion-flexion; etc.). After each trial the subject indicated verbally which of the two movements was faster. Based on this judgment, the comparison velocity in the subsequent trial was adjusted using an adaptive QUEST algorithm procedure 31. This adaptive procedure assured that the sequence of velocity values converged to the threshold almost monotonically. Each trial was initiated by the experimenter by pressing a button and accompanied by an acoustic signal transmitted to the subject over the headphones. Time between trials was kept variable, so that subjects could not predict the onset of the subsequent trial.
Position Sense Task
Testing followed the same format as described above with the exception that two positions, not motions had to be discriminated. Starting position was an elbow joint angle of approximately 97°. During each trial subjects were randomly presented with a sequence of two joint position stimuli separated by a 500 ms interstimulus interval. Employing the same forced-choice procedure as above, subjects were instructed to discriminate between a fixed standard (= 10° - relative to starting elbow joint angle) and a variable comparison stimulus (2° < comparison < 10°). In each trial the subject’s arm was passively displaced toward the subject’s body midline (elbow flexion) at a fixed velocity (2°/s) and then returned to the initial starting location. After 500 ms, the subject’s arm moved to a second angular position before returning to the starting position. Subsequently, the subject indicated verbally which of the two positions was “farther away” from the initial starting location. Based on this judgment, the comparison displacement in the subsequent trial was adjusted using the same adaptive procedure as described above 31. Each trial was initiated by the experimenter by pressing a button and accompanied by an acoustic signal transmitted to the subject over the headphones.
For both tasks the size of standard and comparison stimuli was chosen to be above the known forearm detection thresholds for motion and position for basal-ganglia related disease such as focal dystonia or Parkinson’s disease 10, 13.
Measurements and Analysis
The percentage of trials, where the comparison was judged as faster (motion sense) or “farther away” (position sense) than the standard, was computed for each task and participant. Subsequently, a cumulative Gaussian function was fitted (see Fig.1B). Based on this psychophysical function, the just-noticeable-difference threshold (JND) was determined as the stimulus intensity at the 75% correct response level. The corresponding uncertainty area (UA) was defined as the range between the +1 and −1 standard deviation of the JND (see Fig. 1B). While the JND reflects the participant’s discrimination threshold, the UA provides a measure of how certain a subject was around this threshold. To obtain a single acuity measure for a given proprioceptive sense that reflects both threshold and uncertainty, we determined a proprioceptive acuity index (AI) for each sense as follows:
, where i = position or motion sense, JND and UA representing the respective thresholds and uncertainty areas. The PAI expresses JND and UA against the standard stimulus (i.e. 10° or 1.5°/s), thus, providing a relative measure of overall acuity.
RESULTS
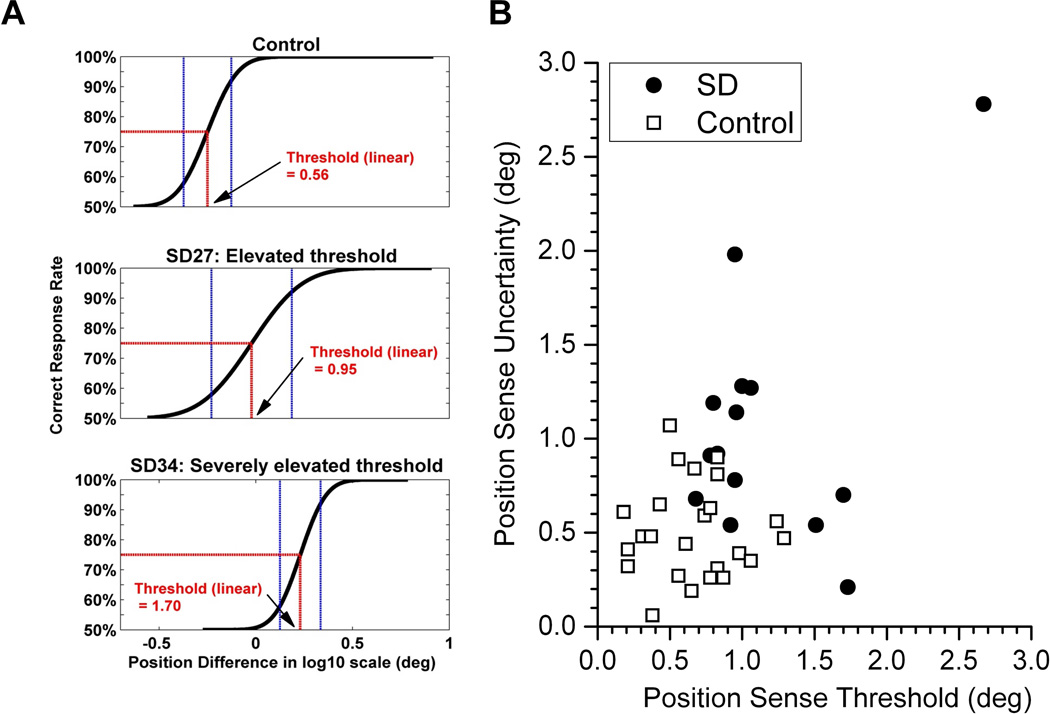
The analysis of the psychophysical data revealed that SD is associated with a loss of limb proprioception as evidenced by enhanced thresholds and greater uncertainty, especially for position sense. Exemplar psychometric data of a control subject and two patients shown in Figure 2A serve to illustrate the range in position sense acuity seen in the SD patient sample. While some patients had measures of proprioceptive acuity within the range of the control group, other patients revealed proprioceptive impairments that became manifest in terms of a higher threshold and/or an enlarged uncertainty area. To illustrate the degree of the perceptual impairment of each patient, Fig. 2B expresses individual thresholds as a function of the corresponding uncertainty. These data indicate that 64% of the patients exhibited abnormalities in arm position sense, i.e. these patients exhibited thresholds and/or uncertainty areas outside the range of the control group.
Fig. 2.
A. Exemplar acuity functions of one control and two SD subjects. Note that with respect to the control, the functions of the SD subjects are shifted to the right giving rise to higher thresholds. Also note that next to an elevated threshold, subjects may also exhibited less certainty in discriminating stimuli around the threshold resulting in a larger uncertainty area (e.g. SD 27). B. JND as a function of UA for position sense. Each data point reflects a single subject. Note that the SD sample revealed a left and upward shift with respect to the controls. Eight SD subjects showed UA and/or JND values above the maximum of the control group.
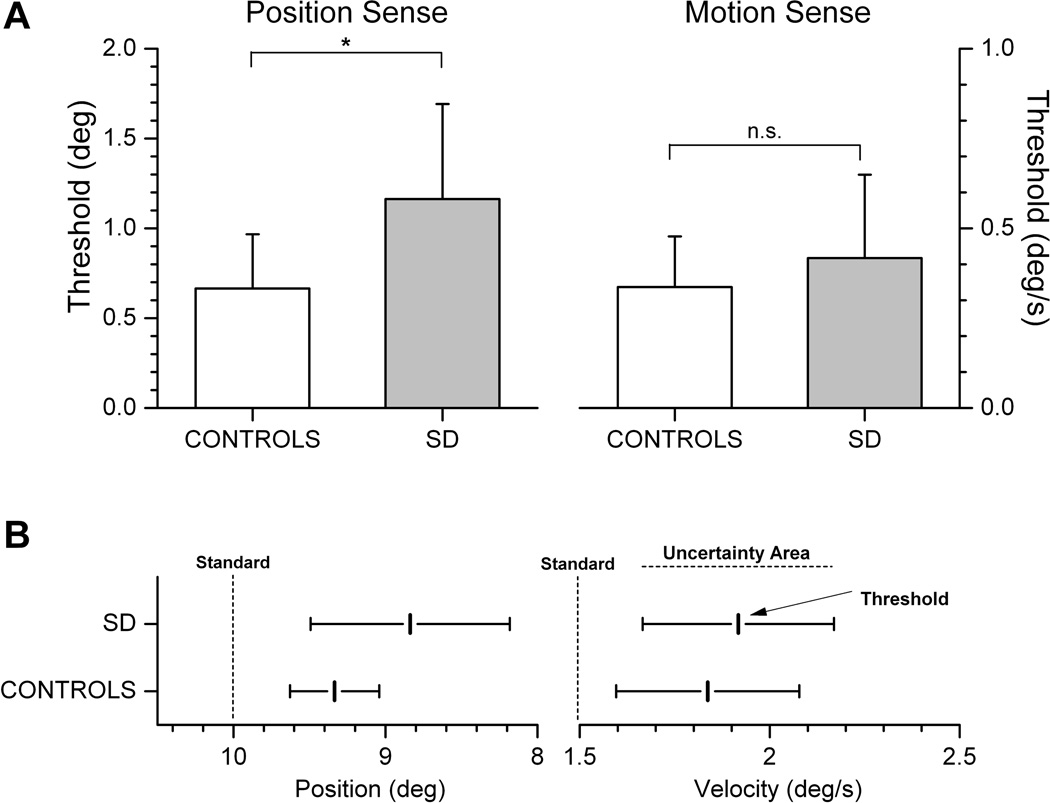
One-way Analysis of Variance procedures performed on the complete sample revealed that mean thresholds and uncertainty areas for position sense were significantly higher for the SD when compared to the control group (JNDpos of SD group: 1.16° ± Std 0.53° vs. Controls: 0.66 ° ± Std 0.31°; p = 0.00076; and UApos of SD group: 1.01° ± Std 0.66° vs. Controls: 0.51 ° ± Std 0.25°; p = 0.0025). In contrast, the respective values for motion sense failed to reach significance (p values > 0.05; see Fig. 3A). Only two patients showed a motion sense threshold or uncertainty area (SD09, SD27) that fell outside the range of the control group.
Fig. 3.
A. Mean thresholds for position and motion sense for both groups. Mean JND was significantly larger for position, but not for motion sense. Error bars represent 1 standard deviation (* = p < 0.01; n.s. = not significant). B. Mean JND and mean UA for position and motion sense in relation to the tested standards (10° and 1.5°/s). The comparison stimuli for position sense testing were always smaller than 10°, for motion sense testing stimuli were always larger than 1.5°/s.
In order to appreciate the group differences in position sense, it is important to consider the size of the standard stimuli used in this experiment. When group thresholds are expressed against the standard amplitude of 10°, the above result implies that SD patients, on average, needed a forearm position difference of 11.6% to correctly differentiate between the comparison and the standard, while controls only required a 6.6% stimulus difference (see Fig. 3B). In absolute terms, the 0.5° difference between the mean position sense thresholds of both groups corresponds to a 76% increase in threshold for the SD group with respect to control group.
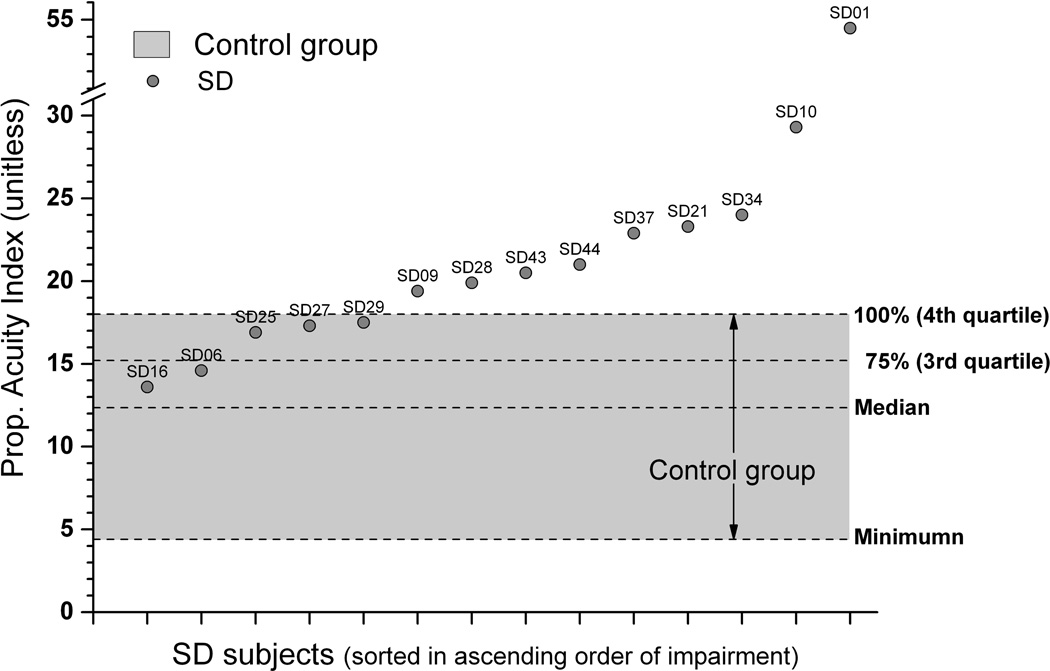
To obtain an overall measure of proprioceptive acuity the respective PAI values were computed for each participant. For position sense, the respective data in Figure 4 illustrate that PAI values of 9 out of 14 SD patients (64%) were above the observed maximum of the control participants, and another three SD subjects had values in the upper half of the 4th quartile. That is, 12/14 SD patients (86%) revealed PAI values that were above the 75% range of the control group sample. For motion sense, 6 patients showed PAI values above the 75% range with three revealing PAIs above the observed maximum of the control group. SD voice symptom severity did not correlate significantly with either threshold measure (rJNDposition = −0.31; rJNDmotion = −0.39, nor with either uncertainty area measure (rUA-position = −0.05; rUA-motion = −0.34).
Fig. 4.
Proprioceptive acuity index for position sense. Data points represent PAI values of each SD subject (for better readability sorted in ascending order). The shaded area represents range of observed PAIs for the control group. Note that 9 SD subjects had PAIs above the maximum of the control group. No SD subject had PAI values below the control group median, indicating that the SD sample revealed an overall elevated shift in acuity.
DISCUSSION
The aim of this study was to determine, if patients with spasmodic dysphonia, a voice disorder affecting the laryngeal muscles, exhibit deficits in forearm proprioception. While, on a first glance, it seems counterintuitive that a voice disorder is associated with deficient limb proprioception, increasing scientific evidence exists indicating that basal ganglia related diseases such as Parkinson’s disease and focal dystonia show a range of generalized somatosensory deficits 18, 32. The main finding of this study is that impaired limb proprioception is a common feature in spasmodic dysphonia. That is, proprioceptive deficits were present in non-dystonic, non-symptomatic limb motor systems. When quantified in terms of position sense thresholds, the proprioceptive loss in SD corresponded to a 43% decrease in acuity with respect to control group.
Is spasmodic dysphonia a disorder of somatosensory processing?
This is the first report showing that SD affects the somatosensation of non-voice related sensorimotor systems. Extrapolating from our limited sample of SD patients, the prevalence of a reduced or abnormally low proprioceptive limb acuity in SD is high with 64% of patients exhibiting PAI values outside the range of the control group. This finding of impaired limb proprioception is in line with research documenting somatosensory deficits in other task-specific focal dystonia (for review see 32). For example, proprioceptive-based finger position sense thresholds and the perception of arm motion are abnormal in patients with cervical dystonia or blepharospasm 17, 33. Related abnormalities in tactile and proprioceptive processing have been reported for dystonic and non-symptomatic body regions in focal 34, 35 but not in generalized dystonia 36. All these findings indicate that a generalized somatosensory deficit is common in focal dystonia, but is not prevalent in generalized dystonia.
Evidence for a neural correlate of the observed decrement in somatosensory function in focal dystonia comes from recordings of somatosensory evoked potentials and transcranial magnetic stimulation documenting that abnormal processing of somatosensory information in focal dystonia is associated with abnormally enhanced cortical excitability and decreased intracortical inhibition 22, 23. This was also recently confirmed for SD 24. With respect to processing of efferent motor commands, SD subjects were found to show increased activation of the primary sensorimotor cortices comprising both somatosensory (areas 3a, 3b, 1, and 2) and motor cortical regions (areas 4a, 4p, and 6) during symptomatic vocalization tasks 37.
While these studies clearly delineate abnormal processing of somatosensory processing at the cortical level, we still have an incomplete understanding how such abnormal sensory processing is linked to abnormal motor patterns in focal dystonia. Our group 20 recently proposed that musician’s dystonia, where non-volitional muscle contractions interfere with the motor control of playing a musical instrument, may be understood as a process where a primary somatosensory deficit interacts with intact mechanisms of sensorimotor integration (i.e. those processes where sensory information is used to plan and execute volitional movement). That is, at least in the initial stages of the disease, motor control mechanisms are intact, but become faulty, because the motor system receives noisy or degraded afferent proprioceptive signals that ultimately lead to “smeared” motor cortical representations 38, 39. Given the fact that both focal dystonia and SD exhibit a generalized somatosensory deficit, it is plausible that SD is associated with overlapping or “smeared” task-specific motor cortical representations of speech while non-speech related activation of laryngeal muscles remain spared.
Is position sense more affected than motion sense?
Our results indicate that limb position sense is seemingly more affected than motion sense in SD. There is no apparent neurophysiological reason why this should be the case, unless one assumes that input from dynamic muscle spindle afferents and its central processing is somehow spared by the disease. A more plausible and parsimonious answer is that the chosen motion stimulus standard (1.5 °/s) was close to the detection threshold for some participants, making it difficult for them to reliably discriminate between the two stimuli. Unfortunately, we did not examine detection thresholds (i.e. the minimum velocity that can reliably be detected) and there is no research available that has documented any limb position thresholds in SD. From our own work we know that forearm motion stimuli as small as 0.9 °/s are reliably detected by healthy young adults 40 – a value well below the 1.5°/s standard here. However, given our older SD and the control sample populations, we cannot exclude the possibility that the standard was too low to allow for a consistent comparison of two similar stimuli and thus gave rise to more variable data as reported here. Another possible reason for the differences between motion sense and position is that passive motion detection requires integration of proprioceptive information over time, while position sense testing does not.
Somatosensory stimulation as a potential treatment option for SD
The finding of a proprioceptive deficit in SD may provide a justification for creating behavioral treatments that aim to alter proprioceptive inputs to the laryngeal muscles in order to overcome its clinical signs. This would be especially relevant for patients who do not respond well to botox injections and who currently have no viable treatment option. The susceptibility of focal dystonia to somatosensory stimulation has long been known, because patients with task-specific dystonia may use sensory tricks (geste antagoniste) to temporarily alleviate dystonic symptoms by touching or pressing areas of or near the dystonic musculature 41, 42. Research on cervical dystonia documented that effective sensory tricks are associated with pallidal and motor cortical desynchronization at low neuronal firing frequencies (6–8Hz) 43. It has further been shown that focal dystonia is sensible to vibro-tactile muscle stimulation. For example, vibrating dystonic neck muscles of patients with torticollis, who exhibit abnormally tilted head postures, induced head righting and nearly restored normal head posture 44. At present, no studies exist that investigated the effects of vibro-tactile vibration in SD. However, the physiological similarities between the speech and limb motor systems make the use of vibro-tactile stimulation for treating SD plausible. In addition, recent advances in light-weight wearable transducers and sensors make this approach technologically feasible. Finally, there has been a recent report on modulating laryngeal muscle spindle responses in SD patients through implanted electrical stimulators 45, which resulted in measurable symptom improvements. This finding, again, underlines the notion that modulating proprioceptive afferents of laryngeal muscles may become a feasible treatment option for SD.
Summary and conclusion
Our findings establish that reduced limb proprioceptive acuity is a prevalent sign of the voice disorder spasmodic dysphonia. That is, spasmodic dysphonia does affect the somatosensation of non-dystonic muscle systems. SD shares this clinical feature of a generalized somatosensory deficit with other forms of task-specific dystonia affecting muscles around the eyes, the neck, and hand.
ACKNOWLEDGMENT
The authors sincerely thank all subjects for their participation in this study. We would like to extend our gratitude to the professionals at the University of Minnesota Lion’s Voice Clinic for identifying interested patients and to the research staff in the Human Sensorimotor Control Laboratory for their help the data collection. This research was funded by grant 1R21DC011841-01 of U.S. National Institutes of Health to J.K. and P.J.W.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Cohen LG, Ludlow C, Warden M, et al. Blink reflex excitability recovery curves in patients with spasmodic dysphonia. Neurology. 1989;39:572. doi: 10.1212/wnl.39.4.572. [DOI] [PubMed] [Google Scholar]
- 2.Tolosa E, Montserrat L. Depressed blink reflex habituation in dystonic blepharospasam. Neurology. 1985;35:251–260. [Google Scholar]
- 3.Tolosa E, Montserrat L, Bayes A. Blink reflex studies in focal dystonias: enhanced excitability of brainstem interneurons in cranial dystonia and spasmodic torticollis. Mov. Disord. 1988;3:61–69. doi: 10.1002/mds.870030108. [DOI] [PubMed] [Google Scholar]
- 4.Topka H, Hallett M. Perioral reflexes in orofacial dyskinesia and spasmodic dysphonia. Muscle & Nerve. 1992;15:1016–1022. doi: 10.1002/mus.880150906. [DOI] [PubMed] [Google Scholar]
- 5.Ludlow CL, Schulz GM, Yamashita T, Deleyiannis FW. Abnormalities in long latency responses to superior laryngeal nerve stimulation in adductor spasmodic dysphonia. Ann. Otol. Rhinol. Laryngol. 1995;104:928–935. doi: 10.1177/000348949510401203. [DOI] [PubMed] [Google Scholar]
- 6.Berardelli A, Rothwell JC, Day BL, Marsden CD. Pathophysiology of blepharospasm and oromandibular dystonia. Brain. 1985;108(Pt 3):593–608. doi: 10.1093/brain/108.3.593. [DOI] [PubMed] [Google Scholar]
- 7.Adamovich SV, Berkinblit MB, Hening W, Sage J, Poizner H. The interaction of visual and proprioceptive inputs in pointing to actual and remembered targets in Parkinson's disease. Neuroscience. 2001;104:1027–1041. doi: 10.1016/s0306-4522(01)00099-9. [DOI] [PubMed] [Google Scholar]
- 8.Contreras-Vidal JL, Gold DR. Dynamic estimation of hand position is abnormal in Parkinson's disease. Parkinsonism Relat. Disord. 2004;10:501–506. doi: 10.1016/j.parkreldis.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Demirci M, Grill S, McShane L, Hallett M. A mismatch between kinesthetic and visual perception in Parkinson's disease. Ann. Neurol. 1997;41:781–788. doi: 10.1002/ana.410410614. [DOI] [PubMed] [Google Scholar]
- 10.Konczak J, Krawczewski K, Tuite PJ, Maschke M. The perception of passive motion in Parkinson's disease. J. Neurol. 2007;254:655–663. doi: 10.1007/s00415-006-0426-2. [DOI] [PubMed] [Google Scholar]
- 11.Konczak J, Li K, Tuite PJ, Poizner H. Haptic perception of object curvature in Parkinson's Disease. PLoS ONE. 2008;3:e2625. doi: 10.1371/journal.pone.0002625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Konczak J, Sciutti A, Avanzino L, et al. Parkinson's disease accelerates age-related decline in haptic perception by altering somatosensory integration. Brain. 2012;135:3371–3379. doi: 10.1093/brain/aws265. [DOI] [PubMed] [Google Scholar]
- 13.Maschke M, Gomez CM, Tuite PJ, Konczak J. Dysfunction of the basal ganglia, but not the cerebellum, impairs kinaesthesia. Brain. 2003;126:2312–2322. doi: 10.1093/brain/awg230. [DOI] [PubMed] [Google Scholar]
- 14.Maschke M, Tuite PJ, Krawczewski K, Pickett K, Konczak J. Perception of heaviness in Parkinson's disease. Mov. Disord. 2006;21:1013–1018. doi: 10.1002/mds.20876. [DOI] [PubMed] [Google Scholar]
- 15.Maschke M, Tuite PJ, Pickett K, Wächter T, Konczak J. The effect of subthalamic nucleus stimulation on kinaesthesia in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry. 2005;76:569–571. doi: 10.1136/jnnp.2004.047324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Suilleabhain P, Bullard J, Dewey RB. Proprioception in Parkinson's disease is acutely depressed by dopaminergic medications. J. Neurol. Neurosurg. Psychiatry. 2001;71:607–610. doi: 10.1136/jnnp.71.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Putzki N, Stude P, Konczak J, Graf K, Diener H-C, Maschke M. Kinesthesia is impaired in focal dystonia. Mov. Disord. 2006;21:754–760. doi: 10.1002/mds.20799. [DOI] [PubMed] [Google Scholar]
- 18.Patel N, Jankovic J, Hallett M. Sensory aspects of movement disorders. Lancet Neurol. 2014;13:100–112. doi: 10.1016/S1474-4422(13)70213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Konczak J, Corcos DM, Horak F, et al. Proprioception and motor control in Parkinson's disease. J. Mot. Behav. 2009;41:543–552. doi: 10.3200/35-09-002. [DOI] [PubMed] [Google Scholar]
- 20.Konczak J, Abbruzzese G. Focal dystonia in musicians: linking motor symptoms to somatosensory dysfunction. Front. Hum. Neurosci. 2013;7:297. doi: 10.3389/fnhum.2013.00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swash M, Fox KP. Normal muscle spindles in idiopathic torsion dystonia. J. Neurol. Sci. 1976;27:525–527. doi: 10.1016/0022-510x(76)90218-5. [DOI] [PubMed] [Google Scholar]
- 22.Kanovsky P, Bares M, Streitova H, Klajblova H, Daniel P, Rektor I. Abnormalities of cortical excitability and cortical inhibition in cervical dystonia Evidence from somatosensory evoked potentials and paired transcranial magnetic stimulation recordings. J. Neurol. 2003;250:42–50. doi: 10.1007/s00415-003-0942-2. [DOI] [PubMed] [Google Scholar]
- 23.Zeuner KE, Molloy FM. Abnormal reorganization in focal hand dystonia--sensory and motor training programs to retrain cortical function. NeuroRehab. 2008;23:43–53. [PubMed] [Google Scholar]
- 24.Samargia S, Schmidt R, Kimberley TJ. Shortened cortical silent period in adductor spasmodic dysphonia: Evidence for widespread cortical excitability. Neurosci. Lett. 2014;560:12–15. doi: 10.1016/j.neulet.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gescheider GA. Psychophysics: method, theory, and application. Second ed. Lawrence Erlbaum Associates, Inc. Publishers; 1985. [Google Scholar]
- 26.Ludlow CL, Adler CH, Berke GS, et al. Research priorities in spasmodic dysphonia. Otolaryngol. Head Neck Surg. 2008;139:495–505. doi: 10.1016/j.otohns.2008.05.624. e491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boersma P, Weenik W. Version 4.4.30. Amsterdam: Institute of Phonetic Sciences; 2005. Praat: A system for doing phonetics by computer. [Google Scholar]
- 28.Sapienza CM, Cannito MP, Murry T, Branski R, Woodson G. Acoustic variations in reading produced by speakers with spasmodic dysphonia pre-botox injection and within early stages of post-botox injection. J. Speech. Lang. Hear. Res. 2002;45:830–843. doi: 10.1044/1092-4388(2002/067). [DOI] [PubMed] [Google Scholar]
- 29.Ehrenstein WH, Ehrenstein A. Psychophysical methods. In: Windhorst U, Johansson H, editors. Modern Techniques in Neuroscience Research. Heidelberg: Springer Verlag; 1999. pp. 1211–1241. [Google Scholar]
- 30.Fechner GT. Elemente der Psychophysik. Leipzig: Breitkopf & Härtel; 1889. [Google Scholar]
- 31.Watson AB, Pelli DG. QUEST: a Bayesian adaptive psychometric method. Percept. Psychophys. 1983;33:113–120. doi: 10.3758/bf03202828. [DOI] [PubMed] [Google Scholar]
- 32.Stamelou M, Edwards MJ, Hallett M, Bhatia KP. The non-motor syndrome of primary dystonia: clinical and pathophysiological implications. Brain. 2012;135:1668–1681. doi: 10.1093/brain/awr224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grünewald RA, Yoneda Y, Shipman JM, Sagar HJ. Idiopathic focal dystonia: a disorder of muscle spindle afferent processing? Brain. 1997;120(Pt 12):2179–2185. doi: 10.1093/brain/120.12.2179. [DOI] [PubMed] [Google Scholar]
- 34.Fiorio M, Tinazzi M, Scontrini A, et al. Tactile temporal discrimination in patients with blepharospasm. J Neurol Neurosurg Psychiatry. 2008;79:796–798. doi: 10.1136/jnnp.2007.131524. [DOI] [PubMed] [Google Scholar]
- 35.Fiorio M, Weise D, Onal-Hartmann C, Zeller D, Tinazzi M, Classen J. Impairment of the rubber hand illusion in focal hand dystonia. Brain. 2011;134:1428–1437. doi: 10.1093/brain/awr026. [DOI] [PubMed] [Google Scholar]
- 36.Molloy FM, Carr TD, Zeuner KE, Dambrosia JM, Hallett M. Abnormalities of spatial discrimination in focal and generalized dystonia. Brain. 2003;126:2175–2182. doi: 10.1093/brain/awg219. [DOI] [PubMed] [Google Scholar]
- 37.Simonyan K, Ludlow CL. Abnormal activation of the primary somatosensory cortex in spasmodic dysphonia: an fMRI study. Cereb. Cortex. 2010;20:2749–2759. doi: 10.1093/cercor/bhq023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bara-Jimenez W, Catalan MJ, Hallett M, Gerloff C. Abnormal somatosensory homunculus in dystonia of the hand. Annals of neurology. 1998;44:828–831. doi: 10.1002/ana.410440520. [DOI] [PubMed] [Google Scholar]
- 39.Meunier S, Garnero L, Ducorps A, et al. Human brain mapping in dystonia reveals both endophenotypic traits and adaptive reorganization. Annals of neurology. 2001;50:521–527. doi: 10.1002/ana.1234. [DOI] [PubMed] [Google Scholar]
- 40.Pickett K, Konczak J. Measuring kinaesthetic sensitivity in typically developing children. Dev. Med. Child Neurol. 2009;51:711–716. doi: 10.1111/j.1469-8749.2008.03229.x. [DOI] [PubMed] [Google Scholar]
- 41.Kägi G, Katschnig P, Fiorio M, et al. Sensory tricks in primary cervical dystonia depend on visuotactile temporal discrimination. Mov. Disord. 2013;28:356–361. doi: 10.1002/mds.25305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poisson A, Krack P, Thobois S, et al. History of the 'geste antagoniste' sign in cervical dystonia. J. Neurol. 2012;259:1580–1584. doi: 10.1007/s00415-011-6380-7. [DOI] [PubMed] [Google Scholar]
- 43.Tang JK, Mahant N, Cunic D, et al. Changes in cortical and pallidal oscillatory activity during the execution of a sensory trick in patients with cervical dystonia. Exp. Neurol. 2007;204:845–848. doi: 10.1016/j.expneurol.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 44.Karnath H, Konczak J, Dichgans J. Effect of prolonged neck muscle vibration on lateral head tilt in severe spasmodic torticollis. J. Neurol. Neurosurg. Psychiatry. 2000;69:658–660. doi: 10.1136/jnnp.69.5.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pitman MJ. Treatment of spasmodic dysphonia with a neuromodulating electrical implant. Laryngoscope. 2014 doi: 10.1002/lary.24749. [DOI] [PubMed] [Google Scholar]