Abstract
Objective
Caffeine and ephedrine was an effective combination therapy for weight loss until ephedrine was removed from the market due to safety concerns. We investigated the combination of caffeine and albuterol as a possibly safer alternative to ephedrine.
Design and Methods
In a series of experiments using cultured adipocytes, rat models, and humans, we evaluated the effects of caffeine and albuterol on lipolysis, metabolic rate, food intake, and body composition.
Results
Both caffeine and albuterol enhanced lipolysis in cultured adipocytes. Acute treatment of humans with caffeine and/or albuterol increased resting metabolic rate. Longer-term studies of rats revealed a trend for increased metabolic rate with albuterol treatment. There was increased lean mass gain concurrent with decreased fat mass gain with caffeine/albuterol treatment that was greater than albuterol treatment alone.
Conclusions
In rats, albuterol with caffeine produced significantly greater increases in lean body mass and reductions in fat mass without changes in food intake after 4-8 weeks of treatment. Since caffeine and albuterol are approved for the treatment of asthma in children and adolescents at the doses tested and change body composition without changing food intake, this combination may deserve further exploration for use in treating pediatric obesity.
Keywords: obesity, lipolysis, energy expenditure
Introduction
Chronic diseases such as hypertension and diabetes are commonly treated with combination drug therapies, but the development of such treatments for obesity is still in its infancy (1). Recent obesity research has focused on finding drugs that will increase lipolysis and metabolic rate. One such combination, caffeine and ephedrine, initially showed much promise. Studies showed that subjects treated with a caffeine/ephedrine combination exhibited greater loss of body fat compared to those treated with placebo (1, 2). This combination was subsequently sold as an unregulated dietary supplement using ephedra, a naturally occurring food consisting of 4 isomers, the most potent being ephedrine. However, in 2004, the FDA banned the sale of ephedra-containing supplements due to concerns over the cardiovascular risks associated with its use (3, 4)[FDA]. In order to find an optimal replacement, the mechanism of action for both caffeine and ephedra/ephedrine needs to be considered.
Caffeine is a well-known stimulator of lipolysis and metabolic rate. It increases intracellular cyclic adenosine monophosphate (cAMP) levels, which are key to regulating lipolysis in white adipose tissue (WAT). Elevated cAMP results in increased triacylglyceride breakdown and increased circulating free fatty acids (FFA) (5). Caffeine acts as a competitive inhibitor of phosphodiesterase, the enzyme that breaks down cAMP within the adipocyte (6). Caffeine also stimulates the sympathetic nervous system (5) and activates the β2 adrenergic receptors on WAT through the neural release of norepinephrine. The activation of the β2 adrenergic receptors results in activation of adenylate cyclase, promotion of cAMP production, and lipolysis. While caffeine has proven to raise resting energy expenditure and increase fatty acid turnover, most of the mobilized FFAs are re-esterified and significant weight loss cannot be achieved (5). To solve this problem, researchers focused their attention on finding a drug that could increase metabolic rate to complement the effects of caffeine.
Ephedrine, an alpha and beta-adrenergic receptor agonist, was originally marketed as a bronchodilator. It was later found to increase thermogenesis and metabolism in asthmatic patients (7), and researchers began investigating its potential as a weight loss treatment. Ephedrine stimulates the release of norepinephrine from nerve terminals, which then binds to β-adrenergic receptors on adipocytes, ultimately resulting in lipolysis.
A possible substitute for ephedrine is albuterol, a common and accessible bronchodilator used to treat asthma and a selective β2 adrenergic agonist. It was previously shown to increase metabolic rate (oxygen consumption) and lipolysis when given by inhalation at four times the therapeutic dose of 200 μg (8, 9). Unfortunately, albuterol given at this dose in the aerosolized form invariably causes tachycardia. However, the swallowed fraction does not cause this side effect, and thus an orally given pill form of albuterol seems to be a safer form of administration (10). A comprehensive investigation into the metabolic effects of a combination therapy of caffeine and albuterol has not been completed. Therefore, the aim of these studies was to determine whether the combination treatment of albuterol and caffeine would act synergistically to effectively stimulate lipolysis and increase resting metabolic rate.
Methods
Experiment 1: Lipolysis assay
Primary human adipose tissue was obtained from patients undergoing liposuction, paniculectomy, or bariatric surgery. Tissue was processed by LaCell LLC (New Orleans, LA) to isolate and cryopreserve preadipocytes. At the time of assay, preadipocytes were differentiated into adipocytes. Differentiated human adipocytes adherent to the bottom of a 96-well plate were washed and treated with media containing 1) albuterol 7 ng/mL, 2) albuterol 17 ng/mL, 3) caffeine 3 μg/mL 4) caffeine 10 μg/mL 5) albuterol 7 ng/mL and caffeine 3 μg/mL 6) albuterol 17 ng/mL and caffeine 3 μg/mL 7) albuterol 7 ng/mL and caffeine 10 μg/mL 8) albuterol 17 ng/mL and caffeine 10 μg/mL. Cells were incubated with their respective treatments at 37°C, 5% CO2 for 3 hours. Isoproterenol 1 μM was used as a positive control. After incubation, a 50 μL aliquot was removed and combined with 50 μL glycerol reagent to create a colorimetric assay dependent on the amount of glycerol released during incubation with test compounds. Samples were read at 540 nm and results were calculated using a standard curve. Eight replicates were conducted for each condition.
Experiment 2: Metabolic rate in humans
The study was approved by the Pennington Biomedical Research Center Institutional Review Board. Eight healthy males and females between the ages of 18 and 50 years with a body mass index between 19 and 40 kg/m2 were recruited from the greater Baton Rouge, LA area. Subjects who were pregnant, breast feeding, smoking, using nicotine products, taking regular medication or taking any medication known to alter metabolic rate like asthma medications or beta-adrenergic blocking drugs were specifically excluded. Women of child-bearing potential who did not agree to use an effective method of contraception (abstinence, barrier methods, intrauterine devices, or hormonal methods of contraception) were also excluded. All subjects signed a written informed consent prior to initiation of study procedures.
The study was a randomized, double-blind, crossover study comparing the effects of: 1) albuterol 2 mg-placebo, 2) albuterol 4 mg-placebo, 3) placebo-caffeine 100 mg, 4) placebo- caffeine 200 mg, 5) albuterol 2 mg-caffeine 100 mg, 6) albuterol 2 mg-caffeine 200 mg, 7) albuterol 4 mg-caffeine 100 mg, 8) albuterol 4 mg-caffeine 200 mg. Subjects enrolled in the trial completed eight test days, each separated by one week. Before each visit the subjects fasted from 9 pm the prior night, refrained from strenuous physical activity for 24 hours, ate their normal diet, and refrained from alcohol or caffeine-containing beverages for 48 hours. On each visit, the subject had blood pressure, pulse and temperature recorded and rested for 30 minutes prior to measurement of the resting metabolic rate (RMR) and respiratory quotient (RQ) at 0, 60, 120 and 180 minutes. Resting metabolic rate and respiratory quotient were measured by indirect calorimetry using a ventilated hood system (DeltaTrac II metabolic monitor, Datex Inc. Helsinki, Finland). During this procedure a transparent hood was placed over the subject’s head and the amount of oxygen consumed and carbon dioxide exhaled was analyzed by the system. After the baseline measurements were taken, subjects were given their treatment consisting of 2 pills to swallow, and the baseline measurements were repeated the last 30 minutes out of each hour for 3 hours.
Experiments 3 and 4: Weight gain and body composition in rats
The Pennington Biomedical Research Center Animal Care and Use Committee approved the animal protocols. Rats were individually housed in shoe-box cages under controlled conditions (12 h light-dark cycle, 22°C, 55% humidity). Male Sprague-Dawley (Harlan, Inc., Indianapolis, IN) rats (8-9 weeks old) were fed high fat (60% kcal from fat, D12492, Research Diets, New Brunswick, NJ) diet for 4 weeks. For experiment 3, forty rats were randomized to 4 treatment groups and continued on high-fat diet for 4 weeks: 1) saline/saline, 2) saline/albuterol, 3) saline/caffeine, and 4) caffeine/albuterol. For experiment 4, sixty rats were randomized to 2 treatment groups and continued on high-fat diet for 8 weeks: 1) saline/albuterol and 2) caffeine/albuterol. All treatments were administered by intraperitoneal injection twice a day. Albuterol was administered at 0.125 mg/kg and caffeine was administered at 3.12 mg/kg. The dose of albuterol and caffeine was the rodent equivalent of the human dose of albuterol 4 mg and caffeine 100 mg three times a day based on the metabolic mass equation (3). Body composition was measured by Nuclear Magnetic Resonance (Minispec LF90 NMR analyzer, Bruker Optics, Billerica, MA). Weight and food intake were recorded every 2 days. In experiment 3, the animals were placed in a metabolic chamber (Phenomaster, TSE Systems, Chesterfield, MO) at the end of the 4 week treatment period for 3 days to measure oxygen consumption, respiratory exchange ratio, and activity levels while the treatment to which they were assigned was continued. Total activity was measured using the Phenomaster system (TSE Systems, Chesterfield MO). Chambers had evenly spaced infrared beam grids emitted along the X, Y, and Z axis, and the system sensed and quantified total beam breaks caused by movements of the animal.
Statistical Analyses
All data were analyzed using the PROC MIXED procedure of the statistical software package SAS® version 9.3 (SAS Institute, Inc., Cary, NC). Data from experiment 1 were analyzed by analysis of variance. Experiment 2 investigated 8 treatments (dose combinations of albuterol and caffeine) in an 8×8 Latin square study design with 8 subjects observed across 8 weeks in a balanced arrangement of the 8 treatment-dose combinations whereby each subject received each of the treatment-dose combinations exactly once and each treatment-dose combination was given exactly once in each week. Since this study was a pilot trial, no calculations were done to determine the number of participants required to provide nominal 80% power for detecting specific treatment differences. Changes from assessment time 0 were viewed as repeated measurements across assessment times (60, 120, 180 minutes) and modeled as effects of albuterol doses, caffeine doses, weeks and assessment times. Demographics, adverse events and any other non-normally distributed data were analyzed by chi squared test. Data from experiments 3 and 4 were analyzed similarly using analogous mixed effects statistical models. Energy expenditure data were analyzed using analysis of covariance with lean body mass as a covariate. Statistical significance was defined as p ≤ 0.05.
Results
Experiment 1: Lipolysis assay
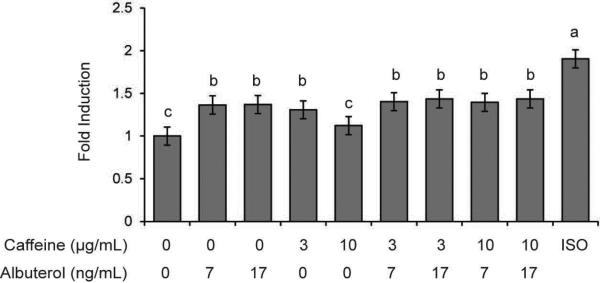
Treatment of adipocytes with any dose of caffeine, albuterol, or their combination resulted in a 30-40% increase in lipolysis over buffer-treated cells (p<0.01) with the curious exception of the 10 μg/mL of caffeine treatment (p=0.83) as shown in Figure 1. These increases were smaller than 90% increase in lipolysis seen with isoproteronol treatment p<0.001).
Figure 1.
Caffeine and albuterol increase lipolysis in vitro. Values are means ± 95% confidence interval, n=8. Bars that are statistically different (p<0.05) are denoted by a different letter (a, b or c). Bars with the same letter are not significantly different.
Experiment 2: Metabolic rate in humans
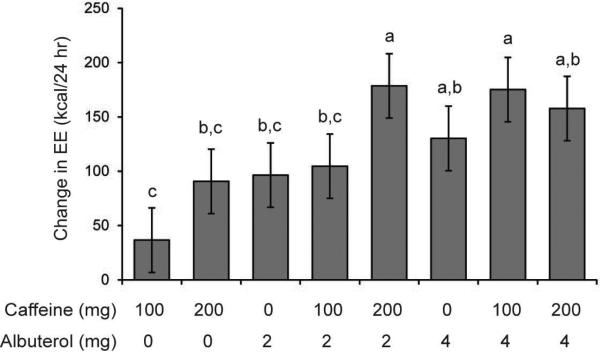
Eight subjects completed this eight-condition cross-over study, and their characteristics are shown in Table 1. Treatment with caffeine and/or albuterol, irrespective of treatment-dose combination, resulted in significant increase in energy expenditure over the baseline metabolic rate of 1368 ± 153 kcal/d (p<0.02 to p<0.001 for monotherapy, see Figure 2).
Table 1.
Characteristics of study subjects (mean ± SD)
| Gender | 7 female: 1 male |
| Ethnicity | 5 Caucasian: 3 Black |
| Age (yrs) | 32 ± 7 |
| Weight (kg) | 71.8 ± 11.8 |
| Body Mass Index (kg/m2) | 25.0 ± 4.9 |
| Systolic blood pressure (mm Hg) | 110 ± 7 |
| Diastolic blood pressure (mm Hg) | 74 ± 8 |
| Pulse (beats per minute) | 67 ± 12 |
Figure 2.
Effect of caffeine and albuterol on human metabolic rate. Values are means ± 95% confidence interval, n=8. Bars that are statistically different (p<0.05) are denoted by a different letter (a, b or c). Bars with the same letter are not significantly different.
Two doses of caffeine and albuterol increased energy expenditure more than two times the respective caffeine monotherapy. Increased energy expenditure was 2.87 fold higher with combination therapy of 2 mg albuterol and 100 mg caffeine compared to 100 mg caffeine alone (104.7 kcal versus 36.5 kcal). Increased energy expenditure was 4.80 fold higher with combination therapy of 4 mg albuterol and 100 mg caffeine compared to 100 mg caffeine alone (175.2 kcal versus 36.5, p<0.0001). Although there was evidence that combination therapy was significantly better than monotherapy, there was no clear evidence of a dose-response of the combinations or evidence of an albuterol/caffeine synergy on metabolic rate (see Figure 2).
No statistically significant changes in systolic blood pressure, diastolic blood pressure, heart rate, or temperature were observed (data not shown).
Experiment 3: Weight gain and body composition in rats
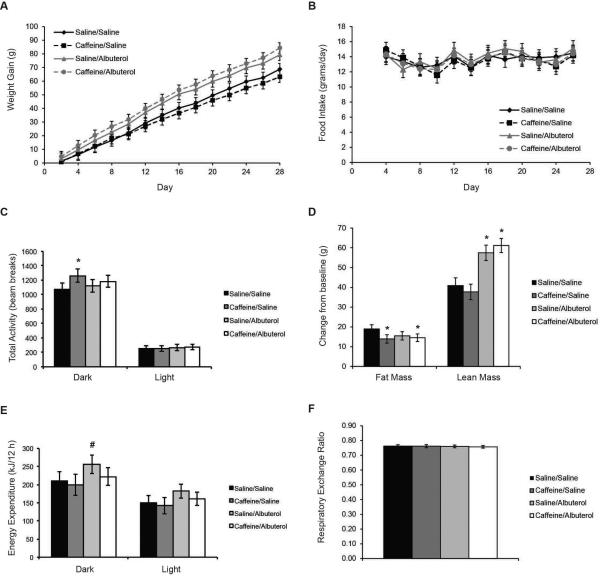
Rats treated with saline/albuterol or caffeine/albuterol gained significantly more weight compared to the saline control group (p=0.04, Figure 3A). This was not explained by changes in food intake or activity as only the caffeine/saline group experienced a significant increase in activity (p=0.02, Figure 3B and 3C). The differences in weight were largely explained by changes in body composition. Rats treated with saline/albuterol or caffeine/albuterol gained an additional 16.6 and 20.2 g of lean mass, respectively, compared to the saline control group (p<0.001, Figure 3D). Treatment with caffeine or caffeine/albuterol also reduced fat accretion (p=.006, Figure 3D). Rats treated with albuterol alone exhibited a trend for increased energy expenditure during the dark phase (p=0.07, Figure 3E). Respiratory exchange ratio was similar among all groups (Figure 3F).
Figure 3.
Impact of caffeine and albuterol on body composition and metabolic rate in rats. (A) Weight gain, (B) Food intake, (C) Total activity, (D) Body composition, (E) Energy expenditure, and (F) Respiratory exchange ratio. Values are means ± 95% confidence interval, n=8-10. *p<0.05. #p=0.07.
Experiment 4: Weight gain and body composition in rats
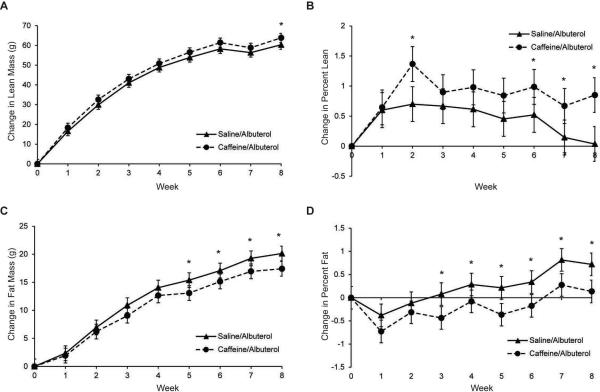
Because rats treated with caffeine/albuterol appeared to gain more lean mass than rats treated with albuterol alone in Experiment 3, an eight week study was undertaken to assess whether these effects would be more pronounced with longer-term treatment. Rats treated with caffeine albuterol gained more lean mass by week 8 compared to albuterol alone (63.9g vs. 60.3g, p=0.03, Figure 4A). When analyzed by the change in percent lean mass, significant differences were detected at weeks 2, 6, 7, and 8 (Figure 4B). Rats treated with caffeine albuterol also gained less fat mass compared to albuterol alone (Figure 4C). This difference was statistically significant by week 5 and persisted through week 8 (17.4 g vs 20.1g, p=.004). When analyzed by change in percent fat mass, significant differences could be detected beginning at week 3 (Figure 4D).
Figure 4.
The combination of caffeine and albuterol reduces fat mass gain compared to albuterol alone. (A) Fat mass gain, (B), Change in percent fat mass, (C) Lean mass gain, (D) Change in percent lean mass. Values are means ± 95% confidence interval, n=30. *p<0.05 to p<0.004
Discussion
Previous studies on adipocytes have shown that the combined treatment of catecholamines and caffeine display synergy and yield substantial increases in cAMP production (6). Furthermore, it has been established that α2-adrenergic receptors inhibit WAT lipolysis, which would give a selective β-agonist an advantage in stimulating lipolysis (11). Thus, we wanted to determine if the combination of a more selective β-agonist, albuterol, and caffeine would display synergy through a larger increase in cAMP resulting in a greater increase in glycerol production, an end product of lipolysis, than albuterol alone. Our first experiment measured glycerol production of in vitro adipocytes treated with different doses of caffeine, albuterol, and a caffeine/albuterol combination (Figure 1). Other than the 10 μg/ml dose of caffeine, all of the treatments yielded an increase in glycerol of ~30%. Surprisingly, treatment with caffeine/albuterol combinations did not result in any additive or synergistic effects on glycerol production compared to caffeine alone. This result is inconsistent with an experiment conducted by Butcher et al. (6), which found that cultured adipocytes treated with a combination of caffeine and the α and β-agonist epinephrine yielded a synergistic increase in cAMP. Because the mechanism of lipolysis relies on cAMP, we expected to see greater results in glycerol production with the use of a more selective β-agonist combined with caffeine than with the β-agonist alone. It is important to note that this in vitro model does not account for caffeine’s potential effects on metabolic rate in a whole organism. Thus, conclusions on the potential of albuterol/caffeine as a weight loss therapy cannot be drawn from this experiment alone.
It was previously reported that the combination of 20 mg ephedrine and 200 mg caffeine yielded synergy during measurement of energy expenditure in six healthy and lean human subjects (2). For this reason, it was expected that the combination of albuterol and caffeine would also have synergistic properties in increasing resting metabolic rate in humans. According to our data, the resting metabolic rate for all treatments increased from baseline. However, our results did not indicate synergy between albuterol and caffeine in increasing metabolic rate. Response trends were inconsistent across combined doses of albuterol and caffeine. Our study was limited in that it only examined a small number of subjects due to the exploratory nature of the study. Differences in the mechanism of action between ephedrine and albuterol may have also contributed to the lack of synergy. Ephedrine and albuterol differ in that ephedrine stimulates both α- and β-adrenergic receptors, while albuterol is a selective β2 adrenergic receptor agonist. Since α-adrenergic stimulators cause vasoconstriction inducing an acute increase in pulse rate and blood pressure, we anticipated and saw an absence of this effect with albuterol. Due to this difference in receptor stimulation, it is possible that synergy between ephedrine and caffeine on energy expenditure may be due to additional stimulation of α1 or β1 adrenergic receptors.
Experiment 3 yielded some interesting and unexpected changes in body composition. The saline/albuterol and caffeine/albuterol treatment groups actually gained more weight than the saline control group. The caffeine/saline and caffeine/albuterol groups had reduced body fat gain. These data indicate that the differences in body weight were largely due to an increase in lean body tissue with albuterol treatment. The combination of caffeine and albuterol did not increase the lean body tissue significantly more than the albuterol treatment individually in experiment 3, but did in experiment 4 where group size was larger and treatment duration was longer (8 weeks vs. 4 weeks). These data provide further support for the muscle building effect of albuterol that has been found in previous studies (13, 14). The increase in skeletal muscle mass is believed to be a result of both an increase in muscle protein synthesis and a decrease in protein degradation (15, 16). A previous investigation of clenbuterol, a similar β2 agonist, proposed that the increase in muscle protein synthesis is mediated by an increase in muscle sensitivity to insulin which has a primary role in protein synthesis (16). Further study is still needed to better evaluate the potential of albuterol as a treatment to change body composition in humans.
The combination of caffeine and albuterol might be most relevant to treatment of pediatric obesity. Currently, orlistat is the only medication approved for obesity treatment in adolescents, and it has embarrassing side effects such as fecal urgency and oily stools. Albuterol is an inexpensive generic medication approved for the treatment of asthma in children age ≥6 years and caffeine is not only a food, but is an inexpensive approved non-prescription medication for the treatment of drowsiness in children ≥12 years. Our data indicate that the combination of caffeine and albuterol can increase lean mass and decrease fat mass during growth and weight gain. Thus, a medication combination that increases lean mass, decreases fat mass, and does so without changing food intake might be a welcome option for physicians confronted with the challenge of treating childhood and adolescent obesity. Parents may be reluctant to act as food police and many pediatricians are concerned about restricting food intake during growth. Further research would be needed to determine safety and efficacy of caffeine and albuterol.
In summary, caffeine and albuterol increased lipolysis in vitro and metabolic rate in humans, but not synergistically. Albuterol treatment resulted in a trend for increased metabolic rate in rats, and caffeine did not increase the effect of albuterol alone. However, the addition of caffeine to albuterol enhanced the treatment’s ability to increase lean body mass while decreasing fat mass without changing food intake in rats, and deserves further exploration as a potential treatment for pediatric obesity.
What is already known about this subject:
Caffeine and ephedrine was an effective combination therapy for weight loss until ephedrine was removed from the market due to safety concerns.
Albuterol, a common bronchodilator used to treat asthma and a selective β2 adrenergic agonist, may serve as a possible substitute for ephedrine.
Albuterol has previously been shown in increase metabolic rate and lipolysis.
What this study adds:
Caffeine and albuterol increased lipolysis in vitro and metabolic rate in humans, but not synergistically.
Caffeine/albuterol treatment increased lean body mass while decreasing fat mass more than albuterol alone without changing food intake in rats.
Acknowledgements
This work was funded by Pennington Biomedical Research Center of the Louisiana State University, and in part by a Nutrition Obesity Research Center grant NIH 2P30DK072476 from NIDDK, Botanical Research Center grant P50AT002776 from NCCAM and ODS, and 1 U54 GM104940 from NIGMS which funds the Louisiana Clinical and Translational Science Center. FLG, WDJ, and SDP conceived and carried out experiments, contributed to data analysis and interpretation, and were involved in writing the manuscript. KPA, ALS, and YY carried out experiments. JTC, GJ, and RCT reviewed the literature and were involved in writing the manuscript. AGL contributed to data analysis and interpretation, generated figures, and was involved in writing the manuscript. All authors reviewed and approved the final manuscript.
Footnotes
CLINICAL TRIAL REGISTRY: This trial is registered at ClinicalTrials.gov #NCT02135965
Conflicts of Interest: The authors declare no conflicts of interest.
References
- 1.Greenway FL. The safety and efficacy of pharmaceutical and herbal caffeine and ephedrine use as a weight loss agent. Obes Rev. 2001;2:199–211. doi: 10.1046/j.1467-789x.2001.00038.x. [DOI] [PubMed] [Google Scholar]
- 2.Astrup A, Toubro S, Cannon S, Hein P, Madsen J. Thermogenic synergism between ephedrine and caffeine in healthy volunteers: a double-blind, placebo-controlled study. Metabolism. 1991;40:323–329. doi: 10.1016/0026-0495(91)90117-f. [DOI] [PubMed] [Google Scholar]
- 3.Bray GA, Greenway FL. Pharmacological treatment of the overweight patient. Pharmacol Rev. 2007;59:151–184. doi: 10.1124/pr.59.2.2. [DOI] [PubMed] [Google Scholar]
- 4.Haller CA, Benowitz NL. Adverse cardiovascular and central nervous system events associated with dietary supplements containing ephedra alkaloids. N Engl J Med. 2000;343:1833–1838. doi: 10.1056/NEJM200012213432502. [DOI] [PubMed] [Google Scholar]
- 5.Acheson KJ, Gremaud G, Meirim I, Montigon F, Krebs Y, Fay LB, et al. Metabolic effects of caffeine in humans: lipid oxidation or futile cycling? Am J Clin Nutr. 2004;79:40–46. doi: 10.1093/ajcn/79.1.40. [DOI] [PubMed] [Google Scholar]
- 6.Butcher RW, Baird CE, Sutherland EW. Effects of lipolytic and antilipolytic substances on adenosine 3′,5′-monophosphate levels in isolated fat cells. J Biol Chem. 1968;243:1705–1712. [PubMed] [Google Scholar]
- 7.Diepvens K, Westerterp KR, Westerterp-Plantenga MS. Obesity and thermogenesis related to the consumption of caffeine, ephedrine, capsaicin, and green tea. Am J Physiol Regul Integr Comp Physiol. 2007;292:R77–85. doi: 10.1152/ajpregu.00832.2005. [DOI] [PubMed] [Google Scholar]
- 8.Amoroso P, Wilson SR, Moxham J, Ponte J. Acute effects of inhaled salbutamol on the metabolic rate of normal subjects. Thorax. 1993;48:882–885. doi: 10.1136/thx.48.9.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldberg R, van As M, Joffe BI, Krut L, Bersohn I, Seftel HC. Metabolic responses to selective beta-adrenergic stimulation in man. Postgrad Med J. 1975;51:53–58. doi: 10.1136/pgmj.51.592.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collier JG, Dobbs RJ, Williams I. Salbutamol aerosol causes a tachycardia due to the inhaled rather than the swallowed fraction. Br J Clin Pharmacol. 1980;9:273–274. doi: 10.1111/j.1365-2125.1980.tb04837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wenkeova J, Kuhn E, Wenke M. Adrenergic lipolysis in human adipose tissue in vitro. Eur J Pharmacol. 1975;30:49–55. doi: 10.1016/0014-2999(75)90201-0. [DOI] [PubMed] [Google Scholar]
- 12.Dulloo AG, Geissler CA, Horton T, Collins A, Miller DS. Normal caffeine consumption: influence on thermogenesis and daily energy expenditure in lean and postobese human volunteers. Am J Clin Nutr. 1989;49:44–50. doi: 10.1093/ajcn/49.1.44. [DOI] [PubMed] [Google Scholar]
- 13.Carter WJ, Lynch ME. Comparison of the effects of salbutamol and clenbuterol on skeletal muscle mass and carcass composition in senescent rats. Metabolism. 1994;43:1119–1125. doi: 10.1016/0026-0495(94)90054-x. [DOI] [PubMed] [Google Scholar]
- 14.Skura CL, Fowler EG, Wetzel GT, Graves M, Spencer MJ. Albuterol increases lean body mass in ambulatory boys with Duchenne or Becker muscular dystrophy. Neurology. 2008;70:137–143. doi: 10.1212/01.WNL.0000287070.00149.a9. [DOI] [PubMed] [Google Scholar]
- 15.Rogers KL, Fagan JM. Effect of beta agonists on protein turnover in isolated chick skeletal and atrial muscle. Proc Soc Exp Biol Med. 1991;197:482–485. doi: 10.3181/00379727-197-4-rc1. [DOI] [PubMed] [Google Scholar]
- 16.Bates PC, Pell JM. Action and interaction of growth hormone and the beta-agonist, clenbuterol, on growth, body composition and protein turnover in dwarf mice. Br J Nutr. 1991;65:115–129. doi: 10.1079/bjn19910074. [DOI] [PubMed] [Google Scholar]