Abstract
Objective
Sjögren’s syndrome (SS) is an autoimmune disease targeting salivary and lacrimal glands. While all patients demonstrate inflammatory infiltration and abnormal secretory function in target tissues, disease features, pathology and clinical course can vary. Activation of distinct inflammatory pathways may drive disease heterogeneity. We investigated whether interferon (IFN) pathway activation correlates with key phenotypic features.
Methods
Clinical data and one frozen labial salivary gland were obtained from each of 82 participants (53 primary SS, 29 controls) in the Sjögren’s International Collaborative Clinical Alliance registry. Salivary gland lysates were immunoblotted with markers of type I or II IFN and patterns of IFN activity were determined by hierarchical clustering. Correlations were defined between SS phenotypic features and IFN activity in the salivary gland.
Results
58% of SS participants had high IFN activity and differed significantly from those with low activity (higher prevalence of abnormal sialometry, leukopenia, hyperglobulinemia, high titer ANA, anti-SSA, and high focus score). Furthermore, distinct patterns of IFN were evident: type I-predominant; type II-predominant; and type I/II IFN. These groups were clinically indistinguishable except for focus score which was highest in type II-predominant participants.
Conclusion
The SS phenotype includes distinct molecular subtypes, segregated by the magnitude and pattern of IFN responses. Associations between IFN pathways and disease activity suggest that IFNs are relevant therapeutic targets in SS. Patients with distinct patterns of high IFN activity are clinically similar, demonstrating that IFN-targeting therapies must be selected based on prior analyses of which specific pathway(s) are active in vivo in individual patients.
Keywords: Sjogren’s syndrome, interferon, molecular diagnostics
Primary Sjögren’s Syndrome (SS) is a chronic, autoimmune inflammatory disease which is characterized by lymphocytic infiltration of the salivary and lacrimal glands, resulting in abnormal tear and saliva secretion (1–3). Although all SS patients have abnormal secretory function and inflammatory infiltration of their salivary glands, there is significant heterogeneity in disease features, pathology and clinical course (4, 5). This heterogeneity is a feature of all rheumatic autoimmune diseases and likely reflects distinct patient subsets within a primary disease phenotype, driven by unique pathophysiologic mechanisms.
While substantial evidence indicates that interferons (IFNs) play significant roles in the pathogenesis of rheumatic diseases including SS (6–13), there is striking heterogeneity in IFN activity amongst different individuals and diseases. Indeed, it still remains to be determined whether type I or type II IFNs are the primary drivers of the IFN signature seen in patients with SS and other rheumatic diseases (14) and whether IFN expression in target tissue is associated with disease activity. In recent studies (12), we defined and validated specific markers of type I and II IFN activity, and used these probes in a small study to investigate the distinct IFN pathways active in patient tissues. We examined relevant target tissues in patients with SS and dermatomyositis and determined that different patterns of IFN activity were apparent between rheumatic diseases and the magnitude of the IFN effects varied significantly amongst patients.
While heterogeneity in the IFN signatures exists in SS, the frequency and clinical associations of the different patterns are unclear. To better understand this, we investigated the IFN expression patterns in labial salivary glands (LSG) from a large cohort of well-characterized SS participants and controls. All subjects were enrolled in the Sjögren’s International Collaborative Clinical Alliance (SICCA) registry, which systematically collected extensive phenotypic data and biospecimens across 9 sites internationally between 2003 and 2013 (15). Based on our recent findings (12), we selected to use interferon-induced protein with tetratricopeptide repeats (IFIT3) to readout type I IFN, and interferon inducible guanylate binding proteins 1 and 2 (GBP1 and GBP2), as markers of type II IFN activity (for immunoblotting and immunohistochemistry, respectively) in the current study. We show that high levels of IFN activity are associated with a more severe disease phenotype, and that distinct IFN patterns are apparent in the group with high IFN activity. Although SS participants in this group are clinically indistinguishable, those with type II IFN activity have higher LSG focus scores, and the presence of inflammatory infiltrates correlates well with type II IFN activity, but not with type I IFN.
As therapies targeting immune effector pathways become increasingly available, it will be helpful to develop approaches which quantitatively define inflammatory pathway activity in patient tissues to assess their activity prior to initiating treatment. These studies demonstrate that analysis of patient-derived target tissues can identify distinct molecular subgroups. These analyses provide opportunities to identify optimal candidates for participation in clinical trials, monitor therapeutic responses, and to determine the efficacy of novel agents in SS and possibly other autoimmune rheumatic diseases.
Materials and Methods
Study Participants
A single frozen LSG and corresponding clinical data were obtained from each of 82 participants in the SICCA registry (16). Salivary gland paraffin sections were obtained from a subset of 6 of these SICCA participants. SICCA participants each underwent a LSG gland biopsy that was independently examined by two histopathologists (17) and had the following phenotypic characteristics (Supplementary Table 1): (i) 53 had SS, as defined by American College of Rheumatology (ACR) criteria (18). We selected SS participants who all had a diagnosis of focal lymphocytic sialadenitis (FLS) in a LSG biopsy with ≥1 focus/4 mm2 (i.e. focus score≥1). Furthermore, we selected those with a broad range of salivary gland lymphocytic infiltration as defined arbitrarily by a focus score ranging from 1 to <2 = pSS1, mild; 2 to <3 = pSS2, moderate and ≥3 = pSS3 severe); (ii) 14 individuals had dry eye disease (ocular surface staining (OSS) ≥3 for either eye) occurring in the absence of serologic (SSA/SSB or [ANA≥1:320 and rheumatoid factor]) or histopathologic evidence of SS (no FLS). We refer to this control group in the results as “non-SS dry-eye disease”; (iii) 15 individuals had symptoms suggestive of SS, but had no objective evidence of dry eyes (OSS<3 for both eyes), absence of FLS, and negative serologies (SSA/SSB or [ANA≥1:320 and rheumatoid factor]). We refer to this other control group in the results as “non-SS, no dry-eye disease”. We excluded participants with confirmed diagnoses of RA, SLE, and other autoimmune connective tissue diseases. No participant had evidence of lymphoma or hepatitis C. Informed consent was obtained from all participants in compliance with the Helsinki Declaration. The SICCA registry was approved by the institutional review boards of the study center (University of California, San Francisco) and of each of the participating research sites.
Laboratory testing
Complete blood counts were performed at the local site and all other laboratory testing of SICCA registrants was performed centrally by Quest Diagnostics (Madison, NJ). This included testing for ANA, anti-SSA and anti-SSB antibodies, rheumatoid factor, quantitative immunoglobulins, and C4 levels.
Immunoblotting
Frozen labial salivary glands were thawed on ice and homogenized in buffer containing Nonidet P-40, 20 mM Tris pH 7.4, 150 mM NaCl, 1 mM EDTA and a protease inhibitor cocktail, as described previously (19). Equivalent amounts of protein (4 micrograms) were electrophoresed on SDS-polyacrylamide gels, transferred onto nitrocellulose membranes and immunoblotted with antibodies against GBP1 (Santa Cruz Biotechnology) and IFIT3 (Sigma Aldrich), which we have previously validated as precise probes of distinct IFN pathways (12). CD45 (BD Transduction Laboratories) was used as a pan-leukocyte marker and vinculin (Sigma Aldrich) and β-actin (Sigma Aldrich) were used as loading controls. Visualization was performed using horseradish peroxidase-conjugated secondary antibodies (Jackson Immunoresearch) and developed using an enhanced chemiluminescence detection system (Pierce). A calibrator sample (lysates made from IFN-treated cultured human salivary gland cells for IFIT3 and GBP1, or PBMCs isolated from a healthy donor for CD45) was included on each gel for normalizing exposure times to ensure accurate quantitation across gels. For densitometry, X-ray films were scanned using an AGFA Arcus II scanner, and densities were quantified using Bio-Rad Quantity One software. To define patterns of IFN-induced protein expression in an unbiased fashion in individual patients, loading control-normalized expression values were median-centered, subjected to unsupervised hierarchical clustering in GenePattern (Broad Institute) using the Hierarchical Clustering algorithm (20), and visualized using JavaTreeView (21).
Immunohistochemistry
LSG paraffin sections from SS patients were processed for immunohistochemistry as described (12). Briefly, after rehydration, antigen retrieval and blocking, sections were incubated overnight at 4°C with antibodies against IFIT3 (12.5 µg/ml, Novus Biologicals) or GBP2 (30 µg/ml; Novus Biologicals). HRP-conjugated secondary antibody incubations were performed for 1 hour at RT, and staining was visualized with diaminobenzidine (Dako) per the manufacturer’s directions. Nuclei were counterstained with Mayer’s hematoxylin. All images were captured using a Zeiss Axioskop 50 with a Zeiss AxioCam HRc camera and AxioVision 4 software.
Statistical analysis
Descriptive statistics were used to describe the demographic features of the cohort of participants. We utilized a cross-sectional study design to investigate the correlation of IFN protein expression with SS clinical phenotype. Differences in phenotypic characteristics were compared between (i) patients with SS and controls, (ii) the two control groups (non-SS dry eye disease and non-SS, no dry eye disease), (iii) SS patients with high versus low IFN activity in salivary gland lysates, and (iv) SS patients with predominantly type I, type II or a mixed type I/II IFN signature. Differences in categorical variables were assessed using a Fisher’s exact test, and in continuous variables by a Wilcoxon rank sum or Kruskal Wallis test as appropriate. Because of the exploratory nature of these analyses, no formal adjustment was made for multiple hypothesis testing.
We hypothesized that IFN activity was primarily related to the degree of salivary gland lymphocytic infiltration. To test this, we performed simple and multivariable logistic regression analyses to explore the explanatory role of key phenotypic features of SS in relation to the outcome of high versus low IFN activity in the salivary gland. Covariates examined included the following histologic, serologic and clinical measures of disease activity based on existing literature: focus score, hyperglobulinemia, positive serology defined by ACR classification criteria [(anti-SSA or anti-SSB antibodies) or (ANA≥1:320 and rheumatoid factor)], ocular surface staining score, and salivary flow rate (15, 17).
All statistical analyses were performed using JMP (Cary, NC) and STATA version 13 (College Station, TX) software.
Results
Characteristics of SS participants and controls
We obtained a single frozen LSG and corresponding clinical data from each of 82 participants in the SICCA registry (16). The participants included 53 with SS and 29 controls with either non-SS dry eye disease or non-SS, no dry eye disease. The demographic and key phenotypic features of the 82 subjects are shown in Supplementary Table 1 and those of the control populations, listed as two separate groups, are shown in Supplementary Table 2. The SS and control subject groups did not differ significantly in terms of age, gender, or ethnicity. Of the 29 controls, 14 individuals had idiopathic dry eye disease (see Methods). Since the OSS score is the single feature that distinguishes the two control groups, for all subsequent studies we have combined these 29 individuals into a single group. The majority of the SS participants were women, with a median age of 56 years. The ethnic distribution of the cohort reflected the global nature of the SICCA registry. As expected, the majority of SS participants had symptoms of dry eyes and dry mouth, OSS ≥3, unstimulated whole saliva flow (UWSF) rates of <0.5 ml/5 min, hyperglobulinemia, as well as high titer ANA, rheumatoid factor, and SSA and/or SSB antibodies.
IFN activity is heterogeneous in LSG biopsies amongst SS participants
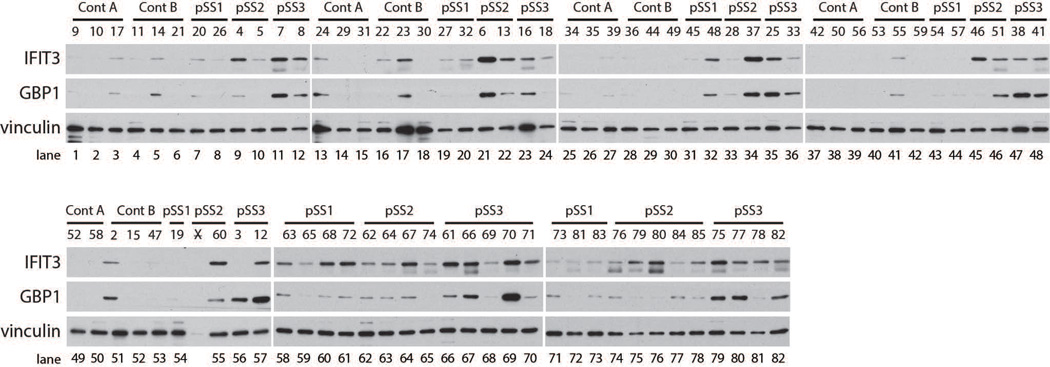
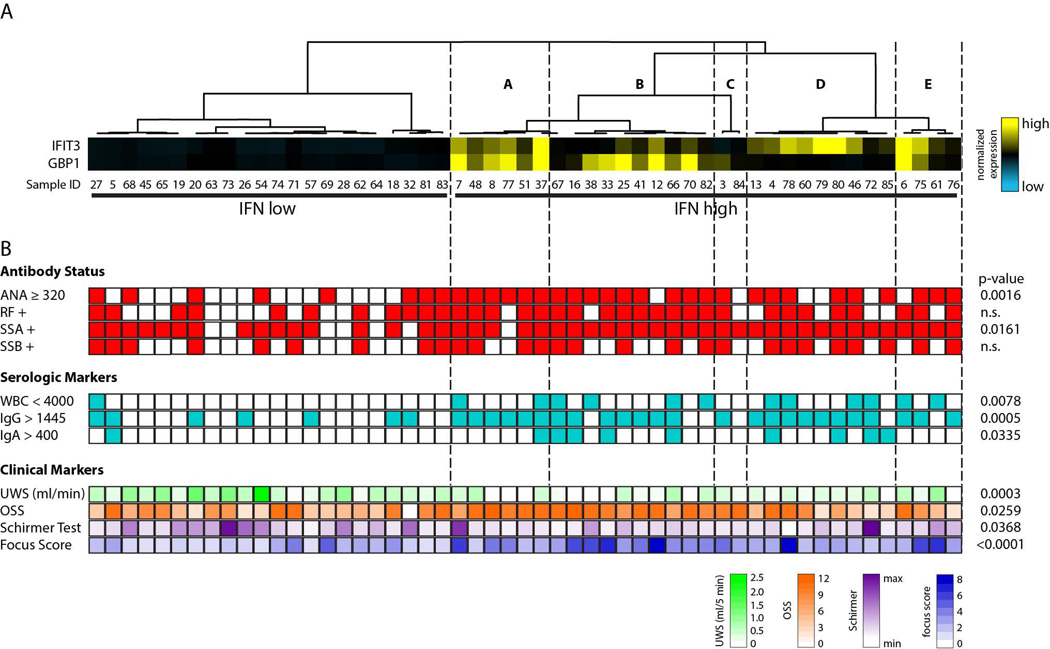
In a recent study, we extensively validated probes specific for type I or type II IFN pathways, and addressed the complexity of the system in great detail (12). We now use these well-defined probes as tools to quantitatively analyze salivary gland biopsy lysates obtained from 53 SS patients and 29 controls (note that a single frozen gland was used to generate each lysate). Equivalent amounts of SS and control salivary gland protein lysates were separated by SDS-PAGE and immunoblotted with antibodies against IFIT3 or GBP1, probes which read out type I and type II IFN activity, respectively. Data from all participants and controls are presented in Figure 1. To address the relationship between glandular lymphocytic infiltration and IFN activity, SS participants were selected for analysis based on focus score (see Methods). Interestingly, higher levels of IFN-induced protein expression were evident in participants with greater salivary gland focus scores (pSS2 and pSS3 groups). While most exhibited evidence of type I and type II IFN activity concomitantly, patterns consistent with type I IFN-predominant (Figure 1; lanes 45, 55, 75 and 76) and type II IFN-predominant (Figure 1; lanes 36 and 56) responses were also apparent. Compared to SS patient tissues, the levels of IFN-inducible protein expression were low or absent in all control salivary gland lysates. In order to objectively classify IFN expression in SS biopsies, the data were quantified by densitometry and the expression of IFIT3 and GBP1 were normalized to the level of a loading control, vinculin, in the same sample. The data were median centered and subjected to unsupervised hierarchical clustering. Two major subgroups were identified based on IFN pathway activity: (i) IFN-low and (ii) IFN-high (Figure 2A). This unbiased approach showed robust IFN pathway activity in 31 of 53 (58%) pSS participants. Evidence of IFN activity was robust in most samples, while others (e.g. 76 and 84) clustered with the IFN high group but had levels which were only modestly above the IFN low group (see Fig 1). Amongst the IFN-high patients, different patterns were evident, with 9/31 (29%) demonstrating a type I IFN-predominant pattern (Fig 2A – branch D), 11/31 (35.5%) having a type II IFN-predominant pattern (Fig 2A – branches B and C), and 11/31 (35.5%) having evidence of type I and type II IFN activity (Fig 2A – branches A and E).
Figure 1. Distinct patterns of IFN activity are evident in lysates made from LSG biopsies from SS participants.
Protein lysates made from control (n=29) or SS (n=53) LSG biopsies were probed for IFN activity by Western blotting. A marker of type I IFN (IFIT3) and type II IFN (GBP1) is included. Vinculin was analyzed as a loading control.
Figure 2. Correlation of clinical features with IFN activity in LSG biopsies from SS participants.
(A) IFN-induced protein expression in SS participants from Fig. 1 was quantified by densitometry and normalized to the level of vinculin expression in the same sample. The vinculin-normalized expression values were subjected to unsupervised hierarchical clustering to define patterns of IFN activity in each patient. (B) The clinical features for each individual patient are presented in line with the respective clustering data. White represents a negative value and colored blocks represent a positive value. Serologic marker values and units are as follows: WBC < 4000/µL, IgG > 1445 mg/dL and IgA > 400 mg/dL. P-values for clinical features which satisfy statistical significance between IFN low and IFN high groups are given.
Immunohistochemical evidence of IFN pathway heterogeneity in patient tissues
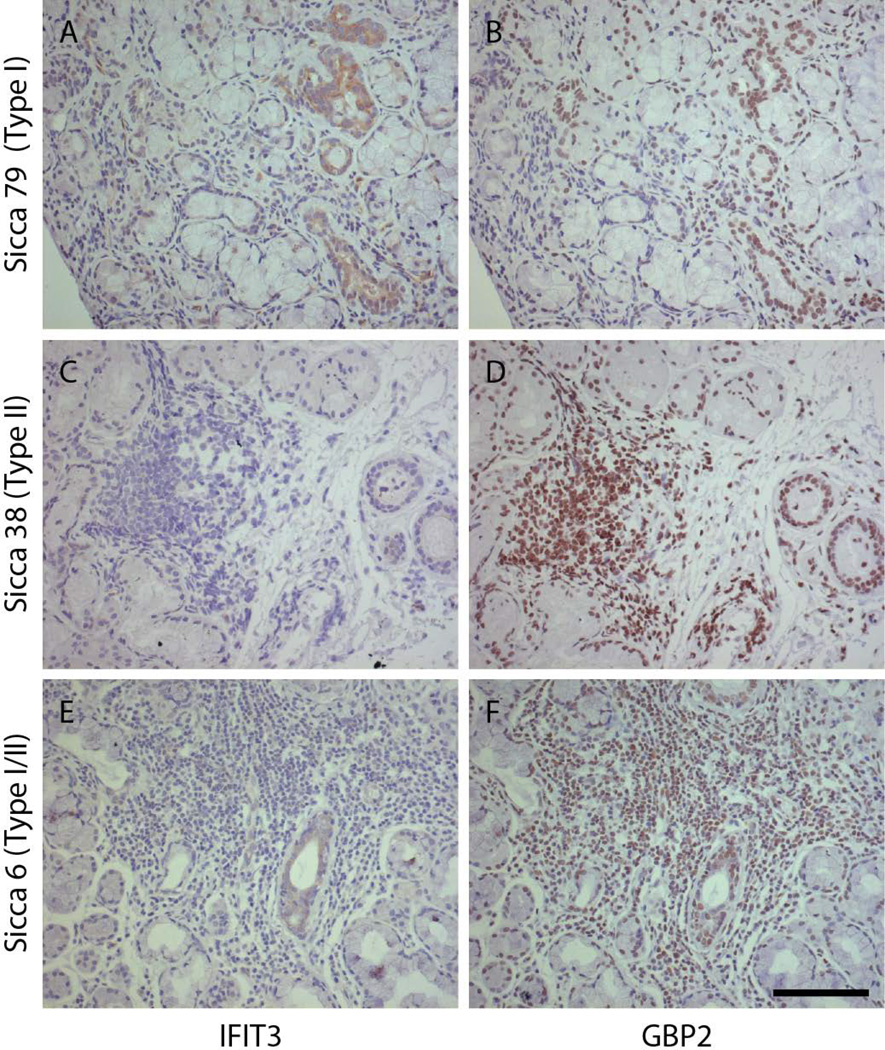
We next determined whether the 3 patterns defined by immunoblotting (type I IFN-predominant, type II IFN-predominant and type I and type II IFN) were evident by immunohistochemistry in tissue biopsies. For this, we selected representative patients from each of the 3 distinct biochemically defined IFN patterns. Of note, since we showed previously that markers of these IFN-induced proteins did not stain LSG from control individuals, we purposefully focused our immunohistochemical comparison on the SS patient spectrum described above. Serial paraffin sections of LSG biopsies were stained with antibodies against IFIT3 and GBP2 (both previously validated and used as markers of types 1 and II IFN activity, respectively, on LSG paraffin sections (12)). The immunohistochemical staining correlated with our biochemical findings, and was consistent with our previously reported observations (12). Representative data from patients with type I IFN-predominant (Sicca 79 – Fig. 1, lane 75), type II IFN-predominant (Sicca 38 – Fig. 1, lane 47 ) and type I and II IFN activity (Sicca 6 – Fig. 1, lane 21) are shown in Figure 3. IFIT3 staining (Fig 3 A, C & E) was seen in salivary duct epithelial cells, while GBP2 staining (Fig 3 B, D & F) was prominent within the nuclei of lymphoid cells and duct epithelia which were surrounded by inflammatory infiltrates. The most pronounced IFIT3 staining was seen in a patient identified as type I IFN-predominant in regions without significant infiltrates (Figure 3A), suggestive of a plasmacytoid DC driven IFN process. Consistent with the biochemical findings, IFIT3 staining was not detected in salivary gland biopsies from patients with a type II IFN pattern (Figure 3C). In contrast, GBP2 was robust in type II and type I/II IFN tissues, where it was prominent in the nuclei of infiltrating inflammatory cells and duct epithelium, but was only seen at low levels in type I IFN tissue.
Figure 3. Distinct patterns of IFN activity are evident in LSG paraffin sections from SS patients.
LSG biopsies from 6 patients, each with distinct biochemically defined patterns of IFN activity (type I-predominant, type II-predominant and type I and type II equal), were stained with antibodies against IFIT3 (A, C and E) and GBP2 (B, D and F). Representative images from one patient with each pattern (A&B, type I- predominant; C&D, type II-predominant and E&F, type I/II equal) are shown. Scale bar = 100 µm.
Clinical characterization of SS participants with activated IFN pathways
To determine whether specific phenotypic characteristics of SS were associated with IFN activity, we compared clinical measures of disease activity between SS patients with high versus low IFN activity (Table 1). The high IFN group had lower UWSF rates (median 0.164 ml/5 min vs 0.549 ml/5 min, p=0.0003), higher maximum OSS score (median 10 vs 6, p=0.0259), and a lower mean Schirmer test value (median for both eyes 4 mm/5 min vs 6.5 mm/5 min, p=0.0368), indicating an overall greater disruption of secretory function in IFN-positive participants. In addition, laboratory and serologic markers which were more prevalent among the high IFN group included high titer ANA (≥ 1:320; 81% vs 36%, p=0.0016), and anti-SSA antibodies (97% vs 73%, p=0.0161), hyperglobulinemia (IgG >1445 mg/dL; 81% vs 32%, p=0.0005 and IgA >400 mg/dL; 29% vs 5%, p=0.0335), and leukopenia (WBC <4000/µL; 39% vs 5%, p=0.0078). The focus score was also significantly higher in the high IFN group (median 3.1 vs 1.45, p<0.0001). Significant findings are presented at the level of the individual patient (Figure 2B).
Table 1.
Comparison of phenotypic characteristics in SS participants with high and low IFN activity
| SS phenotypic features | IFN High (n=31) | IFN Low (n=22) |
P value |
|---|---|---|---|
| Categorical variables1 | |||
| Female | 28 (90) | 19 (86) | 0.683 |
| Caucasian | 17 (55) | 14 (64) | 0.581 |
| Asian/Pacific Islander | 9 (29) | 5 (23) | 0.755 |
| WBC < 4000/µL | 12 (39) | 1 (5) | 0.008 |
| IgG > 1445 mg/dL | 25 (81) | 7 (32) | 0.0005 |
| IgA > 400 mg/dL | 9 (29) | 1 (5) | 0.034 |
| ANA ≥ 1:320 | 25 (81) | 8 (36) | 0.002 |
| Rheumatoid Factor | 23 (74) | 11 (50) | 0.088 |
| Anti-SSA | 30 (97) | 16 (73) | 0.016 |
| Anti-SSB | 18 (58) | 9 (41) | 0.271 |
| C4 < 16 mg/dL | 7 (23) | 7 (32) | 0.534 |
| Dry eye symptoms | 30 (97) | 17 (77) | 0.071 |
| Dry mouth symptoms | 30 (97) | 20 (91) | 0.563 |
| Continuous variables2 | |||
| Age | 56 (45, 64) | 53.5 (43.5, 62.25) | 0.56 |
| Focus score | 3.1 (2.4, 5.7) | 1.45 (1.08, 2.55) | <0.0001 |
| Unstimulated whole saliva flow rate (ml/5 min) | 0.164 (0, 0.415) | 0.549 (0.256, 0.978) | 0.0003 |
| Ocular surface staining score (maximum of both eyes) | 10 (7,11) | 6 (4, 9.25) | 0.026 |
| Schirmer test (mean of both eyes) | 4 (3, 6.5) | 6.5 (3.5, 16.6) | 0.037 |
Values for categorical variables are numbers (percentage). Analyses performed with Fisher’s exact test
Values for continuous variables are listed as median (Q1, Q3) unless otherwise noted. Analyses performed with Wilcoxon rank sum test
We postulated that high IFN activity was determined primarily by glandular lymphocytic infiltration, as measured semi-quantitatively by the focus score. To test this hypothesis and to examine whether other factors remained predictive of high versus low IFN activity after adjusting for focus score, we fitted a logistic regression model using variables that we considered most relevant as markers of disease activity, based on the literature. These included focus score, hyperglobulinemia, positive serology defined by ACR classification criteria [(anti-SSA or anti-SSB antibodies) or (ANA ≥1:320 and rheumatoid factor)], OSS, and UWSF. In the adjusted model, both focus score (OR=3.3, 95%CI 1.3–8.4) and IgG hyperglobulinemia (OR=12.1, 95%CI 1.7–85.5) were statistically significant predictors of high glandular IFN activity (Supplementary Table 3).
To determine whether patterns of IFN activity correlate with clinical features of the disease, we segregated IFN-positive SS participants into the three groups based on predominant IFN pathway [type I IFN-predominant (n=9), type II IFN-predominant (n=11), and type I and type II IFN-mixed (n=11)] and compared the expression of key phenotypic features between the groups (Table 2). Although the numbers in the groups are small, it is noteworthy that the differences in focus score reached statistical significance, with focus scores lowest in the type I-predominant group and highest in the type II predominant group (p=0.024, Kruskal Wallis rank sum test). Two additional measures of disease activity were worse in the type II IFN-predominant group: the frequency of C4 hypocomplementemia (p=0.0488) and OSS (p=0.0416), albeit with marginal levels of statistical significance.
Table 2.
Comparison of demographic and phenotypic features of participants with SS and predominant type I, type II, or type I/II mixed IFN activity
| Demographic and phenotypic features |
Type I IFN (n=9) |
Type II IFN (n=11) |
Type I and type II IFN-mix (n=11) |
P value |
|---|---|---|---|---|
| Categorical variables1 | ||||
| Female | 9 (100) | 10 (91) | 9 (82) | 0.758 |
| Caucasian | 6 (67) | 4 (36) | 7 (64) | 0.401 |
| Asian/PI | 3 (33) | 4 (36) | 2 (18) | 0.692 |
| WBC < 4000/µL | 4 (44) | 3 (27) | 5 (45) | 0.721 |
| IgG >1445 mg/dL | 8 (89) | 7 (64) | 10 (91) | 0.315 |
| IgA >400 mg/dL | 4 (44) | 3 (27) | 2 (18) | 0.541 |
| ANA ≥1:320 | 6 (67) | 9 (82) | 10 (91) | 0.453 |
| Rheumatoid Factor | 5 (56) | 9 (82) | 9 (82) | 0.393 |
| Anti-SSA | 9 (100) | 11 (100) | 10 (91) | 1.0 |
| Anti-SSB | 6 (67) | 5 (45) | 7 (64) | 0.660 |
| C4 <16 mg/dL | 0 | 5 (45) | 2 (18) | 0.067 |
| Dry eye symptoms | 9 (100) | 10 (91) | 11 (100) | 1.0 |
| Dry mouth symptoms | 9 (100) | 10 (91) | 11 (100) | 1.0 |
| Continuous variables2 | ||||
| Age | 52 (45–69.5) | 58 (52–64) | 56 (39–63) | 0.587 |
| Focus score | 2.6 (2.1, 2.85) | 4.3 (3.5, 4.7) | 2.9 (2.3, 5.8) | 0.024 |
| UWSF (ml/5 min) | 0.229 (0.0975, 0.297) | 0.114 (0, 0.491) | 0.164 (0, 0.552) | 0.863 |
| OSS (max of both eyes) | 5 (2.5, 10) | 11 (9,11) | 7 (2.6, 11) | 0.042 |
| Schirmer test (mean of both eyes) | 3.75 (2.625, 6.25) | 4 (3, 6.5) | 5 (3,9) | 0.716 |
Values are numbers (percentage). Analyses performed with Fisher Exact test
Values for continuous variables are listed as median (Q1, Q3) unless otherwise noted. Analyses performed with Kruskal Wallis rank sums test
Type II IFN activity is associated with the presence of CD45+ infiltrates
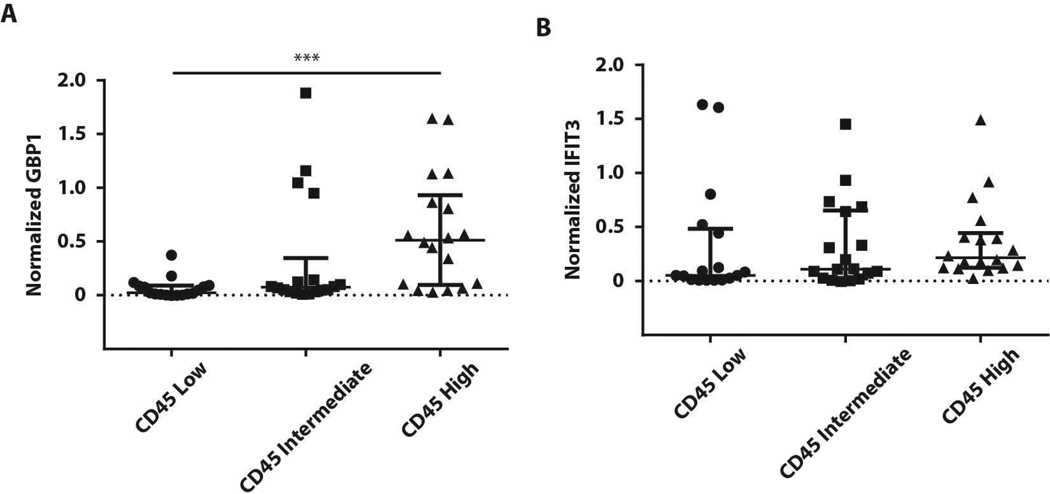
To directly analyze associations between inflammation and IFN activity, we interrogated the aggregate level of inflammation in each tissue. Since the components of the inflammatory infiltrate in SS tissues are heterogeneous (22), we analyzed the expression of a pan-leukocyte marker, CD45, in each sample by immunoblotting (Supplementary Figure 1). There was striking heterogeneity in CD45 protein expression amongst SS participants. CD45 expression was normalized to the level of β-actin (included as a loading control) in the same sample, and the population was divided into tertiles of CD45 expression for analysis. We compared normalized CD45 expression with normalized IFIT3 or GBP1 in each patient. GBP1 levels were highest in participants with the greatest CD45 expression (Figure 4A); however, IFIT3 expression could be found at high levels in the absence or presence of CD45 expression (Figure 4B).
Figure 4. CD45+ infiltrates in tissues are associated with high type II IFN activity.
CD45 expression from Supplemental Figure 1 was quantified by densitometry and normalized to the level of β-actin in the same sample. Participants were divided roughly into tertiles based on normalized CD45 expression (CD45 low (n=17), CD45 intermediate (n=18) and CD45 high (n=18)). Normalized-GBP1 (A), and normalized-IFIT3 (B) expression levels were compared for each CD45 group. Error bars represent median, IQR. *** p=0.0004; Kruskal-Wallis test.
Discussion
The significant heterogeneity amongst SS patients complicates disease classification, assignment of mechanism and selection of therapy. These challenges underscore the need to discover novel approaches to classify disease pathophysiology. As treatment for SS and other rheumatic diseases moves towards a personalized approach, developing tools which can reliably define inflammatory pathway activity is a major priority. In this study, we quantified the patterns of IFN activity in LSGs from a large cohort of well-characterized SS participants and controls without SS to determine whether IFN activity was associated with clinical phenotypes. We were able to achieve this using a single frozen LSG from each participant. We demonstrated that IFN activity was high in 31 of 53 SS patients and was associated with a more severe disease phenotype (characterized by more prevalent salivary hypofunction and ocular dryness, higher focus score, leukopenia, SSA antibodies, high titer ANA and hyperglobulinemia). Both focus score and hyperglobulinemia were the most significant predictors of high IFN activity in a multivariate model adjusted for the effects of focus score, positive SS serology, OSS score, UWSF, and hyperglobulinemia.
The patterns of IFN activity were heterogeneous and SS patients in the high IFN group could be further stratified by the IFN pathways which were most active in their salivary gland tissue, including type I-predominant, type II-predominant and mixed type I/II IFN activity. Interestingly these participants were indistinguishable in their key SS phenotypic features except for focus score, which was highest in type II-predominant participants. The lack of a difference in clinical phenotype between these different IFN patterns could be due to our relatively small sample size and the imprecision of certain clinical measures; however, the lack of differentiating clinical features between different IFN patterns suggests the need to interrogate inflammatory pathways directly in target tissues to determine which pathways are active. Our data show that subsets based on molecular signatures enriched in LSG can be defined, thus providing a quantitative, standardizable approach to classify inflammatory pathway activity in patient tissues. The definition of patient subsets, within a group of patients with a similar clinical profile, may potentially be useful in the setting of disease treatment.
The focus score is a count of discrete lymphocytic infiltrates normalized to 4 mm2 of gland tissue. It does not measure the percentage of gland tissue infiltrated by lymphocytes, and is thus not an accurate measure of total glandular inflammation. In contrast, analysis of whole glands/tissues (rather than tissue sections, as is the case with immunohistochemical scoring of disease in tissue) enables in vivo events to be viewed in aggregate. Whole gland biochemistry also provides a more integrated analysis of gland tissue, with areas of the gland not examined histologically included in the biochemical analysis. Expression of proteins will also be influenced by salivary gland tissue heterogeneity amongst SS patients, including extent and nature of infiltrate, the amount of epithelial structure destruction, healing and replacement of glandular tissue by fat or fibrosis. Biochemical analysis of whole glands integrates these additional sources of variation, and future studies using specific markers will also allow these additional processes to be analyzed.
To date, most clinical trial selection criteria in rheumatic diseases have been based on broad phenotypic features, and the results of these trials have not been striking in terms of clinical response {targeting B-cells and BAFF in SS and SLE, and IFN in SLE} (23–25). Defining the activity of inflammatory pathways in disease-relevant target tissues prior to initiating a controlled clinical trial and examining response of such pathways to therapy may provide important stratification tools and pharmacodynamic markers. Similar approaches have provided important tools for the study of novel cancer therapies, where inclusion in a clinical trial requires the presence of a genetically defined marker (e.g. ALK gene rearrangements, BRAF V600E mutation, HER2/neu) which identifies the active pathway (26–28).
Our data demonstrate that significant heterogeneity occurs in IFN pathway activation in SS patients. The approach defined here to quantify inflammatory pathways in tissues uses tiny amounts of patient material (4 micrograms of protein lysate was sufficient to assay the relevant proteins). Using such tools to stratify patients and select therapies could provide a novel method for selecting patients for clinical trials, and improve the chances of identifying disease subgroups in which specific IFN inhibition might be beneficial. Of note, this approach is readily applicable to other inflammatory pathways and autoimmune diseases, especially those with well-defined, accessible target tissues.
Supplementary Material
Protein lysates made from control (n=29) or SS (n=53) LSG biopsies were analyzed for the presence of inflammatory infiltrates by measuring CD45 expression by Western blotting. β-actin is included as a loading control.
Acknowledgements
Mi Lam provided phenotypic data for each participant in this study from the SICCA registry database. The authors thank the investigators and study participants from the SICCA registry.
None of the authors has received any financial support or other benefits from commercial sources for the work reported in this manuscript. JCH, LC-R and AR are authors on a pending patent application entitled “Specific markers of type II interferon activity help to define the origin of interferon signatures in human rheumatic diseases” (P11883-03 (US) and P1183-04 (Europe) that was filed with the United States Patent and Trademark Office.
Source of financial support: These studies were supported by NIH grants R01 DE12354-15A1 (AR), and R01-AR-44684 and R56 AR062615-01A1 (LCR). The Johns Hopkins authors and their research are supported by the Jerome L. Greene Foundation. LAC is affiliated with the Rosalind Russell / Ephraim P. Engleman Rheumatology Research Center. The Johns Hopkins Rheumatic Disease Research Core Center, where the immunohistochemistical staining was performed, is supported by NIH P30-AR-053503. The Sjogren’s International Collaborative Clinical Alliance (SICCA) Biorepository is funded under contract #HHSN26S201300057C by the National Institute of Dental and Craniofacial Research, and was supported (2003–2013) by National Institute for Dental and Craniofacial Research, National Eye Institute, and Office of Research on Women’s Health contract N01 DE32636.
Footnotes
Conflicts of interest: The other authors declare no competing interests.
References
- 1.Ramos-Casals M, Tzioufas AG, Font J. Primary Sjogren's syndrome: new clinical and therapeutic concepts. Ann Rheum Dis. 2005 Mar;64(3):347–354. doi: 10.1136/ard.2004.025676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fox PC. Autoimmune diseases and Sjogren's syndrome: an autoimmune exocrinopathy. Ann N Y Acad Sci. 2007 Mar;1098:15–21. doi: 10.1196/annals.1384.003. [DOI] [PubMed] [Google Scholar]
- 3.Jonsson R, Bolstad AI, Brokstad KA, Brun JG. Sjogren's syndrome--a plethora of clinical and immunological phenotypes with a complex genetic background. Ann N Y Acad Sci. 2007 Jun;1108:433–447. doi: 10.1196/annals.1422.046. [DOI] [PubMed] [Google Scholar]
- 4.Mariette X, Gottenberg JE. Pathogenesis of Sjogren's syndrome and therapeutic consequences. Curr Opin Rheumatol. 2010 Sep;22(5):471–477. doi: 10.1097/BOR.0b013e32833c36c5. [DOI] [PubMed] [Google Scholar]
- 5.Daniels TE, Whitcher JP. Association of patterns of labial salivary gland inflammation with keratoconjunctivitis sicca Analysis of 618 patients with suspected Sjogren's syndrome. Arthritis Rheum. 1994 Jun;37(6):869–877. doi: 10.1002/art.1780370615. [DOI] [PubMed] [Google Scholar]
- 6.Bave U, Nordmark G, Lovgren T, Ronnelid J, Cajander S, Eloranta ML, et al. Activation of the type I interferon system in primary Sjogren's syndrome: a possible etiopathogenic mechanism. Arthritis Rheum. 2005 Apr;52(4):1185–1195. doi: 10.1002/art.20998. [DOI] [PubMed] [Google Scholar]
- 7.Hjelmervik TO, Petersen K, Jonassen I, Jonsson R, Bolstad AI. Gene expression profiling of minor salivary glands clearly distinguishes primary Sjogren's syndrome patients from healthy control subjects. Arthritis Rheum. 2005 May;52(5):1534–1544. doi: 10.1002/art.21006. [DOI] [PubMed] [Google Scholar]
- 8.Baechler EC, Batliwalla FM, Karypis G, Gaffney PM, Ortmann WA, Espe KJ, et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci U S A. 2003 Mar 4;100(5):2610–2615. doi: 10.1073/pnas.0337679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bennett L, Palucka AK, Arce E, Cantrell V, Borvak J, Banchereau J, et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med. 2003 Mar 17;197(6):711–723. doi: 10.1084/jem.20021553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walsh RJ, Kong SW, Yao Y, Jallal B, Kiener PA, Pinkus JL, et al. Type I interferon-inducible gene expression in blood is present and reflects disease activity in dermatomyositis and polymyositis. Arthritis Rheum. 2007 Nov;56(11):3784–3792. doi: 10.1002/art.22928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gottenberg JE, Cagnard N, Lucchesi C, Letourneur F, Mistou S, Lazure T, et al. Activation of IFN pathways and plasmacytoid dendritic cell recruitment in target organs of primary Sjogren's syndrome. Proc Natl Acad Sci U S A. 2006 Feb 21;103(8):2770–2775. doi: 10.1073/pnas.0510837103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall JC, Casciola-Rosen L, Berger AE, Kapsogeorgou EK, Cheadle C, Tzioufas AG, et al. Precise probes of type II interferon activity define the origin of interferon signatures in target tissues in rheumatic diseases. Proc Natl Acad Sci U S A. 2012 Oct 23;109(43):17609–17614. doi: 10.1073/pnas.1209724109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mavragani CP, Crow MK. Activation of the type I interferon pathway in primary Sjogren's syndrome. J Autoimmun. 2010 Nov;35(3):225–231. doi: 10.1016/j.jaut.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 14.Chiche L, Jourde-Chiche N, Whalen E, Presnell S, Gersuk V, Dang K, et al. Modular transcriptional repertoire analyses of adults with systemic lupus erythematosus reveal distinct type I and type II interferon signatures. Arthritis Rheumatol. 2014 Jun;66(6):1583–1595. doi: 10.1002/art.38628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malladi AS, Sack KE, Shiboski SC, Shiboski CH, Baer AN, Banushree R, et al. Primary Sjogren's syndrome as a systemic disease: a study of participants enrolled in an international Sjogren's syndrome registry. Arthritis Care Res (Hoboken) 2012 Jun;64(6):911–918. doi: 10.1002/acr.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daniels TE, Criswell LA, Shiboski C, Shiboski S, Lanfranchi H, Dong Y, et al. An early view of the international Sjogren's syndrome registry. Arthritis Rheum. 2009 May 15;61(5):711–714. doi: 10.1002/art.24397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daniels TE, Cox D, Shiboski CH, Schiodt M, Wu A, Lanfranchi H, et al. Associations between salivary gland histopathologic diagnoses and phenotypic features of Sjogren's syndrome among 1,726 registry participants. Arthritis Rheum. 2011 Jul;63(7):2021–2030. doi: 10.1002/art.30381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shiboski SC, Shiboski CH, Criswell L, Baer A, Challacombe S, Lanfranchi H, et al. American College of Rheumatology classification criteria for Sjogren's syndrome: a data-driven, expert consensus approach in the Sjogren's International Collaborative Clinical Alliance cohort. Arthritis Care Res (Hoboken) 2012 Apr;64(4):475–487. doi: 10.1002/acr.21591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Casciola-Rosen L, Nagaraju K, Plotz P, Wang K, Levine S, Gabrielson E, et al. Enhanced autoantigen expression in regenerating muscle cells in idiopathic inflammatory myopathy. J Exp Med. 2005 Feb 21;201(4):591–601. doi: 10.1084/jem.20041367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Hoon MJ, Imoto S, Nolan J, Miyano S. Open source clustering software. Bioinformatics. 2004 Jun 12;20(9):1453–1454. doi: 10.1093/bioinformatics/bth078. [DOI] [PubMed] [Google Scholar]
- 21.Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci U S A. 1998 Dec 8;95(25):14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Christodoulou MI, Kapsogeorgou EK, Moutsopoulos HM. Characteristics of the minor salivary gland infiltrates in Sjogren's syndrome. J Autoimmun. 2010 Jun;34(4):400–407. doi: 10.1016/j.jaut.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 23.Petri M, Wallace DJ, Spindler A, Chindalore V, Kalunian K, Mysler E, et al. Sifalimumab, a human anti-interferon-alpha monoclonal antibody, in systemic lupus erythematosus: a phase I randomized, controlled, dose-escalation study. Arthritis Rheum. 2013 Apr;65(4):1011–1021. doi: 10.1002/art.37824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Furie R, Petri M, Zamani O, Cervera R, Wallace DJ, Tegzova D, et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum. 2011 Dec;63(12):3918–3930. doi: 10.1002/art.30613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Merrill JT, Neuwelt CM, Wallace DJ, Shanahan JC, Latinis KM, Oates JC, et al. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum. 2010 Jan;62(1):222–233. doi: 10.1002/art.27233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gianni L, Pienkowski T, Im YH, Roman L, Tseng LM, Liu MC, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012 Jan;13(1):25–32. doi: 10.1016/S1470-2045(11)70336-9. [DOI] [PubMed] [Google Scholar]
- 27.Solomon B, Varella-Garcia M, Camidge DR. ALK gene rearrangements: a new therapeutic target in a molecularly defined subset of non-small cell lung cancer. J Thorac Oncol. 2009 Dec;4(12):1450–1454. doi: 10.1097/JTO.0b013e3181c4dedb. [DOI] [PubMed] [Google Scholar]
- 28.Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011 Jun 30;364(26):2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Protein lysates made from control (n=29) or SS (n=53) LSG biopsies were analyzed for the presence of inflammatory infiltrates by measuring CD45 expression by Western blotting. β-actin is included as a loading control.