Abstract
Background
We evaluated the clinicopathologic features and outcomes of Taiwanese patients with right-sided versus left-sided colon cancer according to various cancer stages.
Methods
A total of 1095 patients with primary colorectal cancer (CRC) undergoing surgery at a single-institution were enrolled. We analyzed patient differences in terms of clinicopathologic features, overall survival (OS) and cancer-specific survival (CSS) of right- versus left-sided colon cancer.
Results
Right-sided colon cancers were noted in 249 (22.7 %) patients, and left-sided colon cancers were noted in 846 (77.3 %) patients. Right-sided colon cancers were found to be significantly larger (P = 0.003) and poorly differentiated (P < 0.001), while also exhibiting advanced depth of tumor invasion (P = 0.002) and advanced UICC/AJCC stage (P = 0.016). Patients with right-sided colon cancers had both poorer OS and CSS than those with left-sided colon cancers (P = 0.021 and 0.023, respectively). However, analysis by various stages revealed significant OS and CSS differences (P = 0.002 and 0.002, respectively) between right-sided and left-sided colon cancers only in stage III patients.
Conclusions
This study demonstrated poorer OS and CSS in patients with right-sided versus those with left-sided colon cancers, but significant differences were noted only in stage III patients.
Keywords: Right-sided colon, Left-sided colon, Stage III, Overall survival, Cancer-specific survival
Background
Colorectal cancer (CRC) is the third most common cancer and the third leading cause of cancer death in the United States, where an estimated 142,820 newly diagnosed cases of CRC and an estimated 50,830 cancer deaths from CRC were reported in 2013 [1]. In Taiwan, CRC is the most common cancer type, having increased rapidly in prevalence in recent years and was third leading cause of cancer-related death as of 2012. The incidence of CRC was 32.38 per 100,000 (7,213 new diagnoses of CRC) in 2000 and 60.72 per 100,000 (14,040 new diagnoses of CRC) in 2010, and in 2012, 5131 people died from CRC, resulting in a death rate of 22.0 per 100,000 [2].
There were various embryological and biological differences between the proximal and distal colon. The proximal segment of the large intestine, including the cecum, ascending colon, and proximal two-thirds of the transverse colon, arises from embryonic midgut and receives blood perfusion from the superior mesentery artery. The distal segment of the large intestine, from the splenic flexure to the upper anal canal, arises from embryonic hindgut and receives blood perfusion from the inferior mesentery artery. Bufill [3] reported that there were differences in the epidemiologic, pathologic, cytogenetic, and molecular features of proximal and distal CRC, as well as indications of differing carcinogenetic mechanisms. Right-sided colon cancers tend to be bulky, exophytic, polypoid lesions growing into the colon lumen and causing anemia. However, left-sided colon cancers tend to be infiltrating, constricting lesions encircling the colorectal lumen and causing obstruction. Sex and age disparities have also been reported [4, 5], and different clinicopathologic features of the right colon and left colon have likewise been noted. Meguid et al. [6] reported a higher proportion of poorly differentiated tumors, larger tumors, and stage III disease in right-sided colon cancers than in left-sided colon cancers. Similar results have also been presented in other studies [7–10].
In addition to different clinicopathologic features, there are genetic differences between the right colon and left colon. Microsatellite instability (MSI) and chromosomal instability (CIN) are the two most frequently mentioned of these genetic differences. MSI-high tumors have been reported to be more frequent in the right colon [11–13]. Nevertheless, CIN has been reported to be more frequent in the left colon [11–13]. MSI-high tumors have also been shown to present aggressive histological types, such as poor differentiation.
In spite of the aforementioned clinicopathologic and genetic differences, there are still controversies regarding differences in the clinical outcomes of patients with right colon and left colon [6–10, 14–19]. In order to help resolve these controversies, we conducted a retrospective study to evaluate the clinicopathologic features and clinical outcomes of Taiwanese colorectal cancer (CRC) patients with right colon cancer versus those with left colon cancer according to the various cancer stages.
Methods
Patients
There were 1198 consecutive patients with histologically proven CRC (adenocarcinoma) who received surgical treatment with curative intent from a single-institution- Kaohsiung Medical University Hospital from January 2002 to December 2008. Twenty-two patients without complete follow-up data were excluded. Moreover, eighty-one patients with second cancers were also excluded. In total, then, this retrospective study included 1095 patients with CRC. The present study was approved by the Institutional Review Board of the Kaohsiung Medical University Hospital. Patients’ clinical outcomes and survival statuses were regularly followed up. Available variables included the following: age at diagnosis, sex, tumor size, histological type, TNM classification, vascular invasion, perineural invasion, preoperative serum level of albumin, preoperative and postoperative serum level of CEA (Carcinoembryonic antigen), comorbidity of diabetes mellitus (DM), comorbidity of cardiac disease, comorbidity of renal disease, whether or not the patient received chemotherapy, and body mass index (BMI). Right-sided colon cancers were defined as those located in the cecum, ascending colon, hepatic flexure, and transverse colon, while left-sided cancers were defined as those located in the splenic flexure, descending colon, sigmoid, and rectum. This definition was similar to previous studies [4, 5, 13, 20–22]. Diagnoses of DM were made according to chart records indicating a history of DM or the use of medicines for DM. Preoperative serum levels of albumin and CEA were checked within 1 week before the operation, and postoperative serum levels of CEA were checked at least 4 weeks after. The cut-off values of serum albumin and CEA were set at 3.5 gm/dl and 5 ng/ml, respectively. The existence of comorbidity was determined by chart record according to the International Classification of Diseases (ICD, 9th version), that is 390 ~ 398, 410 ~ 414, and 420 ~ 429 for cardiac disease, and 584 ~ 588 for renal disease. The TNM classification was defined according to the criteria of the American Joint Commission on Cancer/International Union Against Cancer (AJCC/UICC) [23]. All patients were followed up until their deaths, their last follow-up, or December 31, 2010. The median follow-up time was 37 months (range: 4–97 months). Overall survival (OS) was defined as the time from the date of primary treatment to the date of death from any cause or until the date of the last follow-up. Cancer-specific survival was defined as the time from primary surgery and death from CRC or until the date of the last follow-up.
Statistical analysis
All data were statistically analyzed using the Statistical Package for the Social Sciences, version 19.0 (SPSS Inc., Chicago, IL, USA). The correlation between clinicopathological features and tumor location (right-sided colon vs left-sided colon) was calculated using a Chi-square test (for categorical variables) and Student t-test (for continuous variables). The Cox proportional-hazards model was used for univariate and multivariate analyses to identify the independent prognostic factors for OS and CSS. OS and CSS were calculated by the Kaplan-Meier method, and the differences in survival rates were analyzed by the log-rank test. A P value of less than 0.05 was considered to be statistically significant.
Results
Characteristics of patients at all stages
The clinical and pathologic data regarding all 1095 CRC patients are summarized in Table 1. Of the 1095 patients, 249 (22.7 %) had right-sided colon cancers, including cecal cancers (1.5 %), ascending colon cancers (13.9 %), and transverse colon cancers (7.4 %), and 846 (77.3 %) had left-sided colon cancers, including descending colon cancers (9.2 %), sigmoid colon cancers (34.9 %), and rectal cancers (33.2 %). The proportion of tumors with sizes ≥ 5 cm was significant higher and poorly differentiated cancers were more common in patients with right-sided colon cancers than in those with left-sided colon cancers. Meanwhile, locally advanced cancers were also more common in patients with right-sided colon cancers than in those with left-sided colon cancers (T3 + T4: 85.5 % vs 76.5 %, P = 0.017). Patients with right-sided colon cancers had less stage I CRC and more stage II and III CRC (P = 0.016) than those with left-sided colon cancers. Higher postoperative serum CEA levels were more frequently observed in patients with right-sided colon cancers than in those with left-sided colon cancers. However, there were no significant differences found in terms of age at diagnosis, gender, lymph node metastasis, vascular invasion, perineural invasion, pre-operative serum CEA level, pre-operative serum albumin level, the percentages of patients with diabetes mellitus, the percentages of patients receiving chemotherapy, the frequencies of cardiac disease comorbidity, the frequencies of renal disease comorbidity, or BMI. There is no difference in the quality of the operations for the left-sided colon and right-sided colon in this study (data not shown).
Table 1.
Baseline characteristics of 1095 colorectal cancer patients by tumor location
| Characteristic | Right colon (%) | Left colon (%) | P value |
|---|---|---|---|
| N = 249 (22.7 %) | N = 846 (77.3 %) | ||
| Age (years, mean ± SD) | 64.94 ± 13.49 | 63.82 ± 12.85 | 0.233 |
| Gender | 0.118 | ||
| Female | 119 (47.8) | 357 (42.2) | |
| Male | 130 (52.2) | 489 (57.8) | |
| Tumor size | 0.003* | ||
| < 5 cm | 121 (48.8) | 495 (59.4) | |
| ≥ 5 cm | 127 (51.2) | 339 (40.6) | |
| Histology | <0.001* | ||
| Well | 20 (8.2) | 51 (6.2) | |
| Moderately | 181 (74.5) | 704 (85.7) | |
| Poorly | 42 (17.3) | 66 (8.0) | |
| AJCC Stagea (Initial diagnosis) | 0.016* | ||
| I | 28 (11.2) | 164 (19.4) | |
| II | 101 (40.6) | 286 (33.8) | |
| III | 80 (32.1) | 251 (29.7) | |
| IV | 40 (16.1) | 145 (17.1) | |
| Tumor depth | 0.017* | ||
| T1 | 12 (4.8) | 59 (7.0) | |
| T2 | 24 (9.7) | 139 (16.5) | |
| T3 | 199 (80.3) | 591 (70.4) | |
| T4 | 13 (5.2) | 51 (6.1) | |
| Lymph Node metastasis | 0.677 | ||
| N0 | 142 (57.3) | 491 (58.7) | |
| N1 | 62 (25.0) | 217 (25.9) | |
| N2 | 44 (17.7) | 129 (15.4) | |
| Lymph Node metastasis | 0.694 | ||
| No | 142 (57.3) | 491 (58.7) | |
| Yes | 106 (42.7) | 346 (41.3) | |
| Vascular invasion | 0.529 | ||
| No | 163 (66.5) | 561 (68.7) | |
| Yes | 82 (33.5) | 256 (31.3) | |
| Perineural invasion | 0.833 | ||
| No | 157 (63.8) | 516 (63.1) | |
| Yes | 89 (36.2) | 302 (36.9) | |
| Pre-op serum CEAb level | 0.496 | ||
| < 5 ng/ml | 121 (51.5) | 424 (54.0) | |
| ≥ 5 ng/ml | 114 (48.5) | 361 (46.0) | |
| Post-op serum CEAb level | 0.023* | ||
| < 5 ng/ml | 158 (68.1) | 594 (75.6) | |
| ≥ 5 ng/ml | 74 (31.9) | 192 (24.4) | |
| Pre-op serum Albumin level (g/dl) | 3.56 ± 0.44 | 3.63 ± 0.45 | 0.062 |
| Diabetes mellitus | 0.731 | ||
| Yes | 60 (24.1) | 195 (23.0) | |
| No | 189 (75.9) | 651 (77.0) | |
| Chemotherapy | 0.427 | ||
| Yes | 161 (64.9) | 570 (67.6) | |
| No | 87 (35.1) | 273 (32.4) | |
| Cardiac disease | 0.764 | ||
| Yes | 103 (41.4) | 359 (42.4) | |
| No | 146 (58.6) | 487 (57.6) | |
| Renal disease | 0.690 | ||
| Yes | 13 (5.2) | 39 (4.6) | |
| No | 236 (94.8) | 807 (95.4) | |
| BMIc | 23.53 ± 4.25 | 23.63 ± 3.62 | 0.758 |
aAJCC American Joint Commission on Cancer
bCEA Carcinoembryonic antigen
cBMI Body mass index
*Indicated P < 0.05
Univariate and multivariable analyses of Overall Survival (OS) and Cancer-specific Curvival (CSS) for patients at all stages
Univariate and multivariate analyses were performed to investigate independent prognostic factors for OS and CSS using the Cox proportional-hazards model (Table 2). Old age, poor differentiation, vascular invasion, perineural invasion, post-operative serum CEA level, and comorbidity of renal disease were demonstrated to be independent negative prognostic factors for OS and CSS.
Table 2.
Univariate and multivariable analysis of prognostic indicators on overall survival and cancer-specific survival for 1095 colorectal cancer patients
| Parameters | Overall survival | Cancer-specific survival | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariable analysis | Univariate analysis | Multivariable analysis | |||||
| HRd (95 % CIe) | P value | HRd (95 % CIe) | P value | HRd (95 % CIe) | P value | HRd (95 % CIe) | P value | |
| Age (years) | 1.352 (1.099 – 1.664) | 0.002* | 1.347 (1.008 – 1.801) | 0.044* | 1.349 (1.096 – 1.601) | 0.005* | 1.362 (1.018 – 1.822) | 0.037* |
| ≥65 vs <65 (576/519) | ||||||||
| Gender | 1.098 (0.893 – 1.349) | 0.377 | 1.036 (0.795 – 1.506) | 0.792 | 1.096 (0.891 – 1.347) | 0.386 | 1.032 (0.792 – 1.345) | 0.817 |
| Male vs Female (619/476) | ||||||||
| Location | 1.309 (1.041 – 1.647) | 0.021* | 1.172 (0.870 – 1.580) | 0.296 | 1.303 (1.036 – 1.639) | 0.024* | 1.168 (0.867 – 1.572) | 0.308 |
| Right vs Left (249/846) | ||||||||
| Tumor size | 1.477 (1.200 – 1.817) | <0.001* | 0.981 (0.745 – 1.292) | 0.893 | 1.480 (1.203 – 1.821) | <0.001* | 0.979 (0.744 – 1.289) | 0.880 |
| ≥5 cm vs <5 cm (466/612) | ||||||||
| Tumor depth | 2.959 (2.088 – 4.193) | <0.001* | 1.385 (0.596 – 3.220) | 0.449 | 2.867 (2.094 – 4.205) | <0.001* | 1.379 (0.593 – 3.205) | 0.455 |
| T3 + T4 vs T1 + T2 (853/235) | ||||||||
| Lymph Node metastasis | 2.464 (1.996 – 3.042) | <0.001* | 1.055 (0.644 – 1.730) | 0.831 | 2.460 (1.992 – 3.037) | <0.001* | 1.060 (0.647 – 1.738) | 0.816 |
| Yes vs No (452/633) | ||||||||
| AJCCa stage | 3.441 (2.753 – 4.302) | <0.001* | 1.375 (1.032 – 1.760) | 0.015* | 3.436 (2.748 – 4.295) | <0.001* | 2.022 (1.065-2.099) | <0.001* |
| III + IV vs I + II (516/579) | ||||||||
| Histology | 1.997 (1.510 – 2.642) | <0.001* | 1.655 (1.153 – 2.275) | 0.006* | 2.012 (1.521 – 2.662) | <0.001* | 1.687 (1.177 – 2.419) | 0.004* |
| PD vs MD + WDb (108/952) | ||||||||
| Vascular invasion | 2.796 (2.261 – 3.457) | <0.001* | 1.642 (1.228 – 2.149) | 0.001* | 2.783 (2.251 – 3.441) | <0.001* | 1.623 (1.226 – 2.418) | 0.001* |
| Yes vs No (338/724) | ||||||||
| Perineurial invasion | 2.437 (1.971 – 3.013) | <0.001* | 1.787 (1.358 – 2.351) | <0.001* | 2.423 (1.960 – 2.996) | <0.001* | 1.765 (1.341 – 2.322) | <0.001* |
| Yes vs No (391/673) | ||||||||
| Pre-op CEAc (ng/ml) | 3.047 (2.423 – 3.832) | <0.001* | 1.249 (0.902 – 1.729) | 0.180 | 3.053 (2.428 -–3.840) | <0.001* | 1.245 (0.899 – 1.723) | 0.186 |
| ≥5/ vs <5 (475/545) | ||||||||
| Post-op CEA c (ng/ml) | 5.567 (4.456 – 6.956) | <0.001* | 2.492 (1.817 – 3.418) | <0.001* | 5.591 (4.474 – 6.986) | <0.001* | 2.514 (1.832 – 3.449) | <0.001* |
| ≥5 vs <5 (266/752) | ||||||||
| Pre-op albumin (g/dl) | 1.847 (1.474 – 2.314) | <0.001* | 1.245 (0.933 – 1.662) | 0.137 | 1.828 (1.460 – 2.290) | <0.001* | 1.221 (0.915 – 1.630) | 0.175 |
| <3.5 vs ≥3.5 (324/572) | ||||||||
| Diabetes mellitus | 1.012 (0.795 – 1.288) | 0.923 | 0.962 (0.710 – 1.305) | 0.804 | 1.012 (0.795 – 1.288) | 0.924 | 0.949 (0.700 – 1.288) | 0.738 |
| Yes vs No (255/840) | ||||||||
| Cardiac disease | 1.162 (0.947 – 1.427) | 0.150 | 1.016 (0.772 – 1.337) | 0.909 | 1.162 (0.947 – 1.426) | 0.151 | 1.019 (0.774 – 1.342) | 0.895 |
| Yes vs No (462/633) | ||||||||
| Renal disease | 2.583 (1.777 – 3.754) | <0.001* | 3.139 (1.854 – 5.317) | <0.001* | 2.588 (1.780 – 3.761) | <0.001* | 3.101 (1.830 – 5.254) | <0.001* |
| Yes vs No (52/1043) | ||||||||
| Chemotherapy | 1.509 (1.188 – 1.916) | <0.001* | 1.233 (0.831 – 1.828) | 0.298 | 1.580 (1.188 – 1.915) | 0.001* | 1.230 (0.830 – 1.824) | 0.302 |
| Yes vs No (731/360) | ||||||||
aAJCC American Joint Commission on Cancer, bPD Poorly differentiated, MD Moderately differentiated, WD Well differentiated
cCEA Carcinoembryonic antigen, dHR Hazard ratio, eConfidence interval
*Indicated P < 0.05
The Kaplan-Meier survival analysis demonstrated that patients with right-sided colon cancer had poorer OS (P = 0.021) and CSS (P = 0.023) than patients with left-sided colon cancer (Fig. 1a and b). The median OS times of patients with right-sided colon cancer and those with left-sided colon cancer were 60.33 and 67.37 months (P = 0.021; 95 % CI, 54.982-65.679 and 64.528-70.204), respectively. The 5-year OS rates of patients with right-sided colon cancer and those with left-sided colon cancer were 47 % and 59 %, respectively. The median CSS times of patients with right-sided colon cancer and those with left-sided colon cancer were 42.94 and 45.88 months (P = 0.023; 95 % CI, 40.114-45.765 and 44.412-47.346), respectively. The 5-year CSS rates of patients with right-sided colon cancer and those with left-sided colon cancer were 42 % and 55 %, respectively.
Fig. 1.
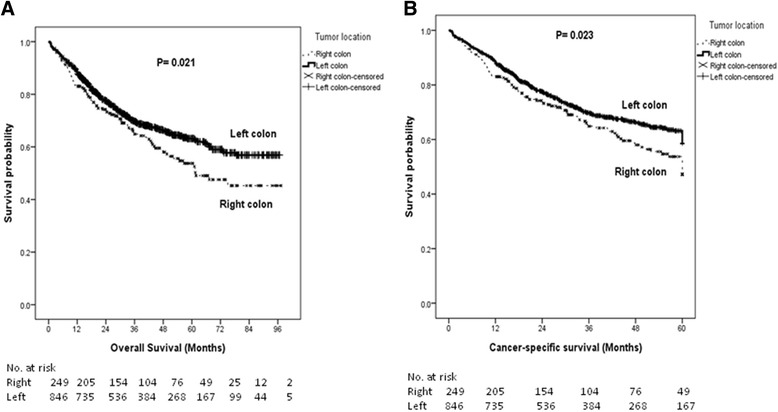
The Kaplan-Meier survival curves for patients with right- and left-sided colon cancers (all stages combined). a Overall survival; (b) Cancer-specific survival
Characteristics of 331 patients with stage III disease
Subgroup analyses by stage revealed significant differences in terms of OS and CSS only in patients with stage III CRC, but not in patients with stages I, II, or IV CRC. The clinical and pathological data were analyzed by stage and are summarized in Table 3. Of the 331 patients with stage III CRC, eighty patients (24.2 %) were right-sided colon cancers and 251 patients (75.8 %) were left-sided colon cancers. Poorly differentiated cancers were more common and the proportion of tumors with sizes ≥ 5 cm was higher in patients with right-sided colon cancers than in those with left-sided colon cancers. Higher pre-operative and postoperative serum CEA levels were more frequently observed in patients with right-sided colon cancers than in those with left-sided colon cancers. However, there were no significant differences found in terms of age at diagnosis, gender, tumor depth, lymph node metastasis, vascular invasion, perineural invasion, pre-operative serum albumin level, the percentages of patients with diabetes mellitus, the percentages of patients receiving chemotherapy, the frequencies of cardiac disease comorbidity, the frequencies of renal disease comorbidity, or BMI.
Table 3.
Baseline characteristics of 1095 colorectal cancer patients stratified by stage and tumor location
| Characteristic | Stage I | Stage II | Stage III | Stage IV | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Right-sided colon (%) | Left-sided colon (%) | P value | Right-sided colon (%) | Left-sided colon (%) | P value | Right-sided colon (%) | Left-sided colon (%) | P value | Right-sided colon (%) | Left-sided colon (%) | P value | |
| N = 28 (14.6 %) | N = 164 (85.3 %) | N = 101 (26.1 %) | N = 286 (73.9 %) | N = 80 (24.2 %) | N = 251 (75.8 %) | N = 40 (21.4 %) | N = 145 (78.4 %) | |||||
| Age (years, mean ± SD) | 67.36 ± 10.05 | 65.04 ± 13.20 | 0.289 | 65.90 ± 13.67 | 65.30 ± 12.15 | 0.678 | 61.99 ± 14.86 | 61.90 ± 13.21 | 0.959 | 66.70 ± 11.57 | 62.85 ± 12.77 | 0.087 |
| Gender | 0.603 | 0.244 | 0.751 | 0.011* | ||||||||
| Female | 12 (42.9) | 79 (48.2) | 52 (47.3) | 128 (40.9) | 37 (46.3) | 111 (44.2) | 23 (57.5) | 51 (35.2) | ||||
| Male | 16 (57.1) | 85 (51.8) | 58 (52.7) | 185 (59.1) | 43 (53.8) | 140 (55.8) | 17 (42.5) | 94 (64.8) | ||||
| Tumor size | 1.000 | 0.060 | 0.070 | 0.921 | ||||||||
| < 5 cm | 24 (85.7) | 139 (85.8) | 42 (41.6) | 150 (52.4) | 37 (46.3) | 144 (57.8) | 18 (46.2) | 62 (45.3) | ||||
| ≥ 5 cm | 4 (14.3) | 23 (14.2) | 59 (58.4) | 136 (47.6) | 43 (53.8) | 105 (42.2) | 21 (53.8) | 75 (54.7) | ||||
| Histology | 0.195 | 0.002* | 0.036* | 0.517 | ||||||||
| Well | 10 (35.7) | 32 (20.3) | 6 (6.1) | 11 (3.9) | 3 (3.9) | 5 (2.0) | 1 (2.6) | 3 (2.2) | ||||
| Moderately | 17 (60.7) | 120 (75.9) | 76 (76.8) | 255 (90.1) | 57 (74.0) | 212 (86.5) | 31 (79.5) | 117 (86.7) | ||||
| Poorly | 1 (3.6) | 6 (3.8) | 17 (17.2) | 17 (6.0) | 17 (22.1) | 28 (11.4) | 7 (17.9) | 15 (11.1) | ||||
| Tumor depth | 0.872 | 0.197 | 0.457 | 0.858 | ||||||||
| T1 | 10 (35.7) | 56 (34.1) | 0 (0.0) | 0 (0.0) | 2 (2.5) | 2 (0.8) | 0 (0.0) | 1 (0.7) | ||||
| T2 | 18 (64.3) | 108 (65.9) | 0 (0.0) | 0 (0.0) | 5 (6.3) | 25 (10.0) | 1 (2.6) | 6 (4.3) | ||||
| T3 | 0 (0.0) | 0 (0.0) | 101 (100.0) | 279 (97.6) | 67 (83.8) | 209 (83.3) | 31 (79.5) | 103 (74.1) | ||||
| T4 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 7 (2.4) | 6 (7.5) | 15 (6.0) | 7 (17.9) | 29 (20.9) | ||||
| Lymph Node metastasis | 0.158 | 0.813 | ||||||||||
| N0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 13 (33.3) | 41 (30.1) | ||||
| N1 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 49 (61.3) | 175 (69.7) | 13 (33.3) | 42 (30.9) | ||||
| N2 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 31 (38.8) | 76 (30.3) | 13 (33.3) | 53 (39.0) | ||||
| Vascular invasion | 0.695 | 0.133 | 0.110 | 0.510 | ||||||||
| No | 27 (96.4) | 146 (92.4) | 83 (82.2) | 211 (74.8) | 39 (48.8) | 145 (58.9) | 14 (38.9) | 59 (45.0) | ||||
| Yes | 1 (3.6) | 12 (7.6) | 18 (17.8) | 71 (25.2) | 41 (51.2) | 101 (41.1) | 22 (61.1) | 72 (55.0) | ||||
| Perineural invasion | 0.473 | 0.861 | 0.512 | 0.790 | ||||||||
| No | 27 (96.4) | 143 (90.5) | 67 (66.3) | 185 (65.4) | 47 (59.5) | 136 (55.3) | 16 (42.1) | 52 (39.7) | ||||
| Yes | 1 (3.6) | 15 (9.5) | 34 (33.7) | 98 (34.6) | 32 (40.5) | 110 (44.7) | 22 (57.9) | 79 (60.3) | ||||
| Pre-op serum CEAa level | 0.560 | 0.237 | 0.043* | 0.410 | ||||||||
| < 5 ng/ml | 22 (81.5) | 127 (85.8) | 62 (62.6) | 150 (55.8) | 28 (38.4) | 122 (51.9) | 9 (25.0) | 25 (18.8) | ||||
| ≥ 5 ng/ml | 5 (18.5) | 21 (14.2) | 37 (37.4) | 119 (44.2) | 45 (61.6) | 113 (48.1) | 27 (75.0) | 108 (81.2) | ||||
| Post-op serum CEAa level | 0.697 | 0.158 | 0.025* | 0.764 | ||||||||
| < 5 ng/ml | 24 (96.0) | 140 (92.1) | 75 (79.8) | 232 (85.9) | 49 (64.5) | 185 (77.4) | 10 (27.0) | 37 (29.6) | ||||
| ≥ 5 ng/ml | 1 (4.0) | 12 (7.9) | 19 (20.2) | 38 (14.1) | 27 (35.5) | 54 (22.6) | 27 (73.0) | 88 (70.4) | ||||
| Pre-op serum Albumin level (g/dl) | 3.72 ± 0.37 | 3.76 ± 0.39 | 0.664 | 3.46 ± 0.47 | 3.58 ± 0.46 | 0.030 | 3.68 ± 0.37 | 3.69 ± 0.40 | 0.887 | 3.48 ± 0.45 | 3.46 ± 0.50 | 0.885 |
| Diabetes mellitus | 0.307 | 0.841 | 0.920 | 0.844 | ||||||||
| Yes | 9 (32.1) | 38 (23.2) | 24 (23.8) | 69 (24.1) | 19 (23.8) | 61 (24.3) | 8 (20.0) | 27 (18.6) | ||||
| No | 19 (67.9) | 126 (76.8) | 77 (76.2) | 217 (75.9) | 62 (76.2) | 190 (75.7) | 32 (80.0) | 118 (81.4) | ||||
| Chemotherapy | 0.030* | 0.852 | 0.817 | |||||||||
| Yes | 53 (52.5) | 185 (64.7) | 72 (91.1) | 227 (90.4) | 33(82.6) | 121 (84.0) | ||||||
| No | 48 (47.5) | 101 (35.3) | 7 (8.9) | 24 (9.6) | 7 (17.5) | 23 (16.0) | ||||||
| Cardiac disease | 0.257 | 0.365 | 0.772 | 0.976 | ||||||||
| Yes | 15 (53.6) | 69 (42.1) | 41 (40.6) | 131 (45.8) | 32 (40.0) | 105 (41.8) | 15 (37.5) | 54 (37.2) | ||||
| No | 13 (46.4) | 95 (57.9) | 60 (59.4) | 156 (54.2) | 48 (60.0) | 146 (58.2) | 25 (62.5) | 91 (62.8) | ||||
| Renal disease | 0.127* | 0.693 | 0.450 | 1.000 | ||||||||
| Yes | 3 (10.7) | 6 (3.7) | 6 (5.9) | 14 (4.9) | 2 (2.5) | 11 (4.4) | 2 (5.0) | 8 (5.5) | ||||
| No | 25 (89.3) | 158 (96.3) | 95 (94.1) | 272 (95.1) | 78 (97.5) | 240 (95.6) | 38 (95.0) | 137 (94.5) | ||||
| BMIb | 23.94 ± 3.88 | 23.70 ± 3.73 | 0.748 | 23.80 ± 4.39 | 23.73 ± 3.73 | 0.092 | 23.71 ± 4.13 | 23.95 ± 3.44 | 0.616 | 22.23 ± 4.31 | 22.77 ± 3.50 | 0.418 |
aCEA Carcinoembryonic antigen, bBMI Body mass index, *Indicated P < 0.05
Univariate and multivariable analyses of Overall Survival (OS) and Cancer-specific Survival (CSS) for patients with stage III CRC
Univariate and multivariate analyses were performed to investigate independent prognostic factors for OS and CSS using the Cox proportional-hazards model (Table 4). Vascular invasion, perineural invasion, post-operative serum CEA level, and comorbidity of renal disease were demonstrated to be independent negative prognostic factors for OS and CSS.
Table 4.
Univariate and multivariable analysis of prognostic indicators on overall survival and cancer-specific survival for 331 stage III colorectal cancer patients
| Parameters | Overall survival | Cancer-specific survival | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariable analysis | Univariate analysis | Multivariable analysis | |||||
| HRc (95 % CId) | P value | HRc (95 % CId) | P value | HRc (95 % CId) | P value | HRc (95 % CId) | P value | |
| Age (years) | 1.157 (0.801 – 1.671) | 0.437 | 1.032 (0.607 – 1.754) | 0.909 | 1.157(0.801 – 1.670) | 0.438 | 1.032 (0.607 – 1.757) | 0.906 |
| ≥65 vs <65 (151/180) | ||||||||
| Gender | 1.347 (0.926 – 1.960) | 0.119 | 1.348 (0.795 – 1.356) | 0.227 | 1.336 (0.918 – 1.942) | 0.130 | 1.341 (0.827 – 2.176) | 0.235 |
| Male vs Female (183/148) | ||||||||
| Location | 1.828 (1.242 – 2.681) | 0.002* | 1.536 (0.939 – 2.513) | 0.087 | 1.842 (1.253 – 2.703) | 0.002* | 1.548 (0.946 – 2.532) | 0.082 |
| Right vs Left (80/251) | ||||||||
| Tumor size | 1.625 (1.120 – 2.359) | 0.011 | 1.557 (0.951 – 2.548) | 0.078 | 1.617 (1.114 – 2.345) | 0.011* | 1.544 (0.944 – 2.527) | 0.084 |
| ≥5 cm vs <5 cm (148/181) | ||||||||
| Tumor depth | 1.639 (0.799 – 3.363) | 0.178 | 0.935 (0.347 – 2.515) | 0.894 | 1.651 (0.805 – 3.388) | 0.171 | 0.937 (0.348 – 2.520) | 0.897 |
| T3 + T4 vs T1 + T2 (297/34) | ||||||||
| Histology | 2.171 (1.378 – 3.419) | 0.001* | 1.761 (0.969 – 3.201) | 0.063 | 2.185 (1.387 – 3.442) | 0.001* | 1.767 (0.972 – 3.210) | 0.062 |
| PD vs MD + WDa (45/277) | ||||||||
| Vascular invasion | 2.307 (1.565 – 3.399) | <0.001* | 1.784 (1.091 – 2.915) | 0.021* | 2.291 (1.555 – 3.376) | <0.001* | 1.777 (1.087 – 2.904) | 0.022* |
| Yes vs No (142/184) | ||||||||
| Perineural invasion | 1.866 (1.286 – 2.765) | 0.001* | 1.822 (1.112 – 2.986) | 0.017* | 1.880 (1.282 – 2.757) | 0.001* | 1.820 (1.111 – 2.983) | 0.017* |
| Yes vs No (142/183) | ||||||||
| Pre-op CEAb (ng/ml) | 1.925 (1.288 – 2.875) | 0.001* | 1.237 (0.697 – 2.195) | 0.468 | 1.945 (1.301 – 2.908) | 0.001* | 1.253 (0.706 – 2.224) | 0.441 |
| ≥5/ vs <5 (158/150) | ||||||||
| Post-op CEAb (ng/ml) | 3.253 (2.198 – 4.816) | <0.001* | 2.053 (1.187 – 3.550) | 0.010* | 3.285 (2.218 – 4.864) | <0.001* | 2.064 (1.193 – 3.571) | 0.010* |
| ≥5 vs <5 (81/234) | ||||||||
| Pre-op albumin (g/dl) | 1.480 (0.965 – 2.269) | 0.072 | 1.237 (0.740 – 2.067) | 0.417 | 1.474 (0.961 – 2.259) | 0.075 | 1.226 (0.734 – 2.046) | 0.437 |
| <3.5 vs ≥3.5 (85/185) | ||||||||
| Diabetes mellitus | 1.331 (0.888 – 1.996) | 0.166 | 1.103 (0.633 – 1.924) | 0.729 | 1.325 (0.884 –1.987) | 0.173 | 1.091 (0.626 – 1.903) | 0.758 |
| Yes vs No (80/251) | ||||||||
| Cardiac disease | 1.337 (0.926 – 1.931) | 0.121 | 0.796 (0.464 – 1.366) | 0.407 | 1.342 (0.929 – 1.938) | 0.117 | 0.799 (0.465 – 1.372) | 0.415 |
| Yes vs No (137/194) | ||||||||
| Renal disease | 4.151 (2.164 – 7.963) | <0.001* | 2.981 (1.089 – 8.157) | 0.033* | 4.165 (2.171 – 7.999) | <0.001* | 2.979 (1.088 – 8.159) | 0.034* |
| Yes vs No (13/318) | ||||||||
| Chemotherapy | 0.437 (0.257 – 0.742) | 0.002* | 1.061 (0.434 – 2.594) | 0.897 | 0.435 (0.256 – 0.738) | 0.002* | 1.078 (0.440 – 2.639) | 0.869 |
| Yes vs No (31/299) | ||||||||
aPD Poorly differentiated, MD Moderately differentiated, WD Well differentiated, b CEA Carcinoembryonic antigen, cHR Hazard ratio,
dConfidence interval
*Indicated P < 0.05
The Kaplan-Meier survival analysis demonstrated that patients with right-sided colon cancer had poorer OS (P = 0.002) (Fig. 2c) and CSS (P = 0.002) (Fig. 3c). The median OS times of patients with right-sided colon cancer and those with left-sided colon cancer were 54.06 and 69.57 months (P = 0.002; 95 % CI, 45.207-62.903 and 64.505-74.633), respectively. The 5-year OS rates of patients with right-sided colon cancer and those with left-sided colon cancer were 39 % and 60 %, respectively. The median CSS times of patients with right-sided colon cancer and those with left-sided colon cancer were 40.20 and 47.53 months (P = 0.002; 95 % CI, 35.328-45.069 and 44.985-50.068), respectively. The 5-year CSS rates of patients with right-sided colon cancer and those with left-sided colon cancer were 32 % and 57 %, respectively.
Fig. 2.
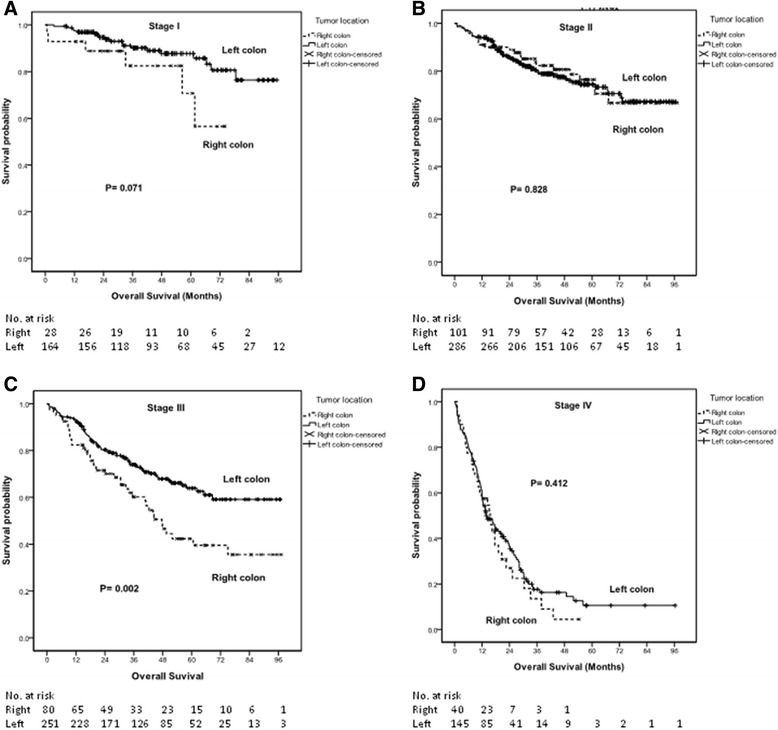
The Kaplan-Meier survival curves for overall survival of patients with right- and left-sided colon cancers (according to stage). a Stage I disease; (b) Stage II disease; (c) Stage III disease; and (d) Stage IV disease
Fig. 3.
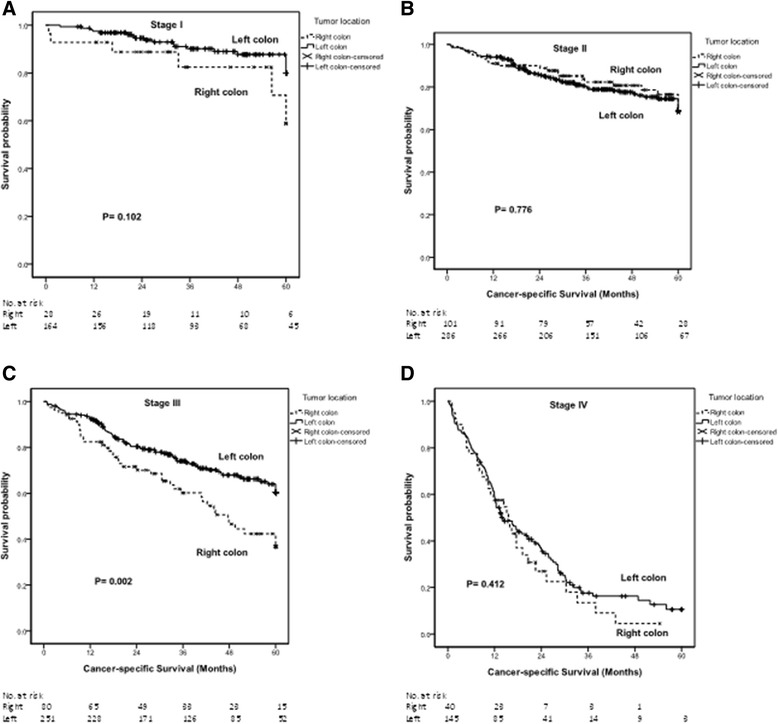
The Kaplan-Meier survival curves for cancer-specific survival of patients with right- and left-sided colon cancers (according to stage). a Stage I disease; (b) Stage II disease; (c) Stage III disease; and (d) Stage IV disease
Discussion
In the present study, 22.7 % (249) patients had right-sided colon cancers and 77.3 % (846) patients had left-sided colon cancers. We showed that patients with right-sided colon cancer had significantly worse OS and CSS than patients with left-sided colon cancer. We also demonstrated that the differences in OS and CSS were still significant only in patients with stage III CRC.
The large intestine can be differentiated to proximal and distal part in relation to the splenic flexure because the two parts have two different embryologic origins, with differing physiological and biological characteristics [13]. On the basis of underlying genetic mutational pathways, Gervaz P et al. [13] reported that CRC could be differentiated into two phenotypes. They also emphasized the new paradigm of two colons-two cancers, which declared the connection between embryology, physiology, and molecular biology. They also suggested that location of the neoplasm in relation to the splenic flexure should be considered before group stratification in future trials of adjuvant chemotherapy. In the present study, therefore, we divided the large intestine into two segments in relation to the splenic flexure. The right-sided colon includes the cecum, ascending colon, hepatic flexure, and transverse colon, and the left-sided colon includes the splenic flexure, descending colon, sigmoid and rectum.
Recent reports have indicated an increasing percentage of right-sided colon cancers, with the percentage rising from 33 % to 67 % [5–8, 10, 13, 17–19]. However, only 22.7 % of the patients in the present study had right-sided colon cancers. Stratified by stage, 14.6 %, 26.1 %, 24.2 %, and 21.4 % of those patients had right-sided colon cancers in stages I, II, III, and IV, respectively.
Right-sided colon cancers significantly tended to be larger in the present study, consistent with previous reports [6, 9]. Higher proportions of poorly differentiated tumors were noted in right-sided colon cancers than in left-sided colon cancers in all stages of the disease, in accordance with previous studies [6–10, 17, 20]. We also found similar results in stage II disease and stage III disease. Meanwhile, local advanced tumors were more common in right-sided colon cancers than left-sided colon cancers, consistent with previous studies [7, 8, 10, 14, 19, 24]. In addition, we also demonstrated higher proportion of stage II and III diseases and a lower proportion of stage I diseases in patients with right-sided colon cancers, consistent with the result reported by Meguid et al., Benedix et al. and Weiss et al. [6–8].
The 5-year OS rate of patients with right-sided colon cancer was 12 % lower than the rate of those with left-sided colon cancer. In addition, the 5-year CSS rate of patients with right-sided colon cancer was 13 % lower than the rate of those with left-sided colon cancer. Meguid et al. [6] reported a 3.4 % lower 5-year OS rate, 4.1 % lower 10-year OS rate, and 4 % lower 15-year OS rate in patients with right-sided colon cancer compared with patients with left-sided colon cancer. Benedix et al. [7] reported a 4 % lower 5-year OS rate in patients with right-sided colon cancer and the difference in the 5-year disease-free survival rates was only marginal. Suttie et al. [14] presented a worse prognosis for patients with right-sided colorectal cancers than those with left-sided and rectal cancers. Wong et al. [15] reported that patients diagnosed with proximal cancers were 13 % less likely than those with distal cancers to survive in 5 years. Derwinger et al. [17] reported that right colon cancer resulted in a worse OS rate compared to left colon cancer, but not a worse CSS rate or disease-free survival rate. In contrast, Weiss et al. [8] reported no difference in mortality between right- and left-sided cancers for all stages combined. Relatedly, Powell et al. [10] showed no significant differences between tumor locations with regard to cancer-specific survival.
We found significant differences of OS and CSS only in patients with stage III CRC, but not for patients in stages I, II, and IV CRC. The 5-year OS rate of patients with stage III right-sided colon cancer was 21 % lower compared with the rate for those with stage III left-sided colon cancer. In addition, the 5-year CSS rate of patients with stage III right-sided colon cancer was 25 % lower than the rate for those with stage III left-sided colon cancer. The two differences were more prominent in patients with stage III disease than they were for all of the stages combined. Meguid et al. [6] showed a 4.2 % increased mortality risk in patients with right-sided colon cancer compared with those with left-sided colon cancer. They also reported that patients with stage III and IV disease right-sided colon cancer had a significantly increased mortality risk. However, patients with stage II disease right-sided colon cancer had a significanlyt decreased mortality risk. Benedix et al. [7] reported a 6 % lower 5-year OS rate in stage I disease and a 5 % lower OS rate in stage III disease in patients with right-sided colon cancer. However, they failed to show a significant difference in 5-year disease-free survival rates between patients with right- and left-sided colon cancer by sub-analysis with respect to various stages. Weiss et al. [8] reported no difference in mortality between right- and left-sided cancers for CRCs of all stages. However, they did report a lower mortality for stage II right-sided cancers specifically, in contrast with a higher mortality for stage III right-sided cancers.
In the present study, old age, poor differentiation, vascular invasion, perineural invasion, post-operative serum CEA level, and comorbidity of renal disease were demonstrated to be independent negative prognostic factors for OS and CSS in patients at all stages. Although tumor location was not found to be an independent prognostic factor, patients with right-sided cancers had a higher proportion of poorly differentiated tumors, a higher percentage of postoperative serum CEA levels ≧ 5 ng/ml, more locally advanced tumors and more stage II and III diseases. The similar results were also noted for patients with stage III CRC. Conversely, we also found no significant survival differences in stages I, II, and IV. The above results suggest that the factors that influence the prognosis of patients with CRC might be clinicopathologic characteristics other than tumor location. In addition, variations in tumor biology might be another potential factor related to the prognosis of patients with CRC [8]. For example, patients who are MSI-positive have a better OS and MSI status has been shown to be an independent favorable predictor of survival [8].
There are several limitations to the present study. First, the present study was a retrospective study of patients at only a single-institution. Second, for a retrospective study, the sample size was relatively small. Third, we did not examine the MSI statuses of the CRC patients studied; hence, any relevant information regarding the correlation between MSI status and the prognoses of patients with CRC is lacking.
Conclusions
We have demonstrated that patients with right-sided colon cancer have poorer OS and CSS compared with those with left-sided colon cancer for all stages combined, with these survival differences being especially prominent in stage III disease. These survival differences may be the result of clinicopathologic characteristics, while aspects of tumor biology, such as MSI status, may be another crucial factor. Additional studies are needed to verify the relationship between tumor biology and prognosis for patients with CRC.
Acknowledgements
The authors acknowledge the contribution to data collection made by the Colorectal Cancer Group of the Cancer Center of Kaohsiung Medical University Hospital. This study was supported by grants from the Excellence for Cancer Research Center Grant through funding from the Ministry of Health and Welfare, Executive Yuan, Taiwan, ROC (MOHW104-TDU-B-212-124-003), the Kaohsiung Medical University Hospital (KMUH98-8G08), and the Grant of Biosignature in Colorectal Cancers, Academia Sinica, Taiwan, ROC. The authors also thank the assistence from the Statistical Analysis Laboratory, Department of Medical Research, Kaohsiung Medical University Hospital, Kaohsiung Medical University. The authors thank the help from the Statistical Analysis Laboratory, Department of Medical Research, Kaohsiung Medical University Hospital, Kaohsiung Medical University
Abbreviations
- BMI
body mass index
- CSS
cancer-specific survival
- CRC
colorectal cancer
- OS
overall survival
- PFS
progression-free survival
- CEA
Carcinoembryonic antigen
- AJCC
American Joint Commission on Cancer
- PD
Poorly differentiated
- MD
Moderately differentiated
- WD
Well differentiated
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
CWH analyzed the data and wrote the manuscript. HLT, MYH, CMH, YSY and CJM made substantial contributions in data acquisition, statistical analyses, and data interpretation, and helped in manuscript preparation. JYW participated in study design and coordination. All authors have read and approved the final manuscript.
Contributor Information
Ching-Wen Huang, Email: baseball5824@yahoo.com.tw.
Hsiang-Lin Tsai, Email: chunpin870132@yahoo.com.tw.
Ming-Yii Huang, Email: miyihu@gmail.com.
Chun-Ming Huang, Email: 930321@mail.kmuh.org.tw.
Yung-Sung Yeh, Email: 920055@gmail.com.
Cheng-Jen Ma, Email: aisa.hsieh@msa.hinet.net.
Jaw-Yuan Wang, Phone: +886-7-3122805, Email: cy614112@ms14.hinet.net.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Ministry of Health and Welfare, the Executive Yuan, Republic of China. Health and Vital Statistics. [http://www.mohw.gov.tw/CHT/Ministry/]
- 3.Bufill JA. Colorectal cancer: evidence for distinct genetic categories based on proximal or distal tumor location. Ann Intern Med. 1990;113:779–88. doi: 10.7326/0003-4819-113-10-779. [DOI] [PubMed] [Google Scholar]
- 4.Yamauchi M, Lochhead P, Morikawa T, Huttenhower C, Chan AT, Giovannucci E, Fuchs C, Ogino S. Colorectal cancer: a tale of two sides or a continuum? Gut. 2012;61:794–7. doi: 10.1136/gutjnl-2012-302014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iacopetta B. Are there two sides to colorectal cancer? Int J Cancer. 2002;101:403–8. doi: 10.1002/ijc.10635. [DOI] [PubMed] [Google Scholar]
- 6.Meguid RA, Slidell MB, Wolfgang CL, Chang DC, Ahuja N. Is there a difference in survival between right- versus left-sided colon cancers? Ann Surg Oncol. 2008;15:2388–94. doi: 10.1245/s10434-008-0015-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benedix F, Kube R, Meyer F, Schmidt U, Gastinger I, Colon/Rectum Carcinomas (Primary Tumor) Study Group. Lippert H. Comparison of 17,641 patients with right-and left-sided colon cancer: differences in epidemiology, perioperative course, histology, and survival. Dis Colon Rectum. 2010;53:57–64. doi: 10.1007/DCR.0b013e3181c703a4. [DOI] [PubMed] [Google Scholar]
- 8.Weiss JM, Pfau PR, O'Connor ES, King J, LoConte N, Kennedy G, Smith MA. Mortality by stage for right- versus left-sided colon cancer: analysis of surveillance, epidemiology, and end results--Medicare data. J Clin Oncol. 2011;29:4401–9. doi: 10.1200/JCO.2011.36.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishihara S, Watanabe T, Akahane T, Shimada R, Horiuchi A, Shibuya H, Hayama T, Yamada H, Nozawa K, Matsuda K, Maeda K, Sugihara K. Tumor location is a prognostic factor in poorly differentiated adenocarcinoma, mucinous adenocarcinoma, and signet-ring cell carcinoma of the colon. Int J Colorectal Dis. 2012;27:371–9. doi: 10.1007/s00384-011-1343-0. [DOI] [PubMed] [Google Scholar]
- 10.Powell AG, Wallace R, McKee RF, Anderson JH, Going JJ, Edwards J, Horgan PG. The relationship between tumour site, clinicopathological characteristics and cancer-specific survival in patients undergoing surgery for colorectal cancer. Colorectal Dis. 2012;14:1493–9. doi: 10.1111/j.1463-1318.2012.03048.x. [DOI] [PubMed] [Google Scholar]
- 11.Markowitz SD, Bertagnolli MM. Molecular origins of cancer: Molecular basis of colorectal cancer. N Engl J Med. 2009;361:2449–60. doi: 10.1056/NEJMra0804588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pritchard CC, Grady WM. Colorectal cancer molecular biology moves into clinical practice. Gut. 2011;60:116–29. doi: 10.1136/gut.2009.206250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gervaz P, Bucher P, Morel P. Two colons-two cancers: paradigm shift and clinical implications. J Surg Oncol. 2004;88:261–6. doi: 10.1002/jso.20156. [DOI] [PubMed] [Google Scholar]
- 14.Suttie SA, Shaikh I, Mullen R, Amin AI, Daniel T, Yalamarthi S. Outcome of right- and left-sided colonic and rectal cancer following surgical resection. Colorectal Dis. 2011;13:884–9. doi: 10.1111/j.1463-1318.2010.02356.x. [DOI] [PubMed] [Google Scholar]
- 15.Wong R. Proximal tumors are associated with greater mortality in colon cancer. J Gen Intern Med. 2010;25:1157–63. doi: 10.1007/s11606-010-1460-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cucino C, Buchner AM, Sonnenberg A. Continued rightward shift of colorectal cancer. Dis Colon Rectum. 2002;45:1035–40. doi: 10.1007/s10350-004-6356-0. [DOI] [PubMed] [Google Scholar]
- 17.Derwinger K, Gustavsson B. Variations in demography and prognosis by colon cancer location. Anticancer Res. 2011;31:2347–50. [PubMed] [Google Scholar]
- 18.Sjo OH, Lunde OC, Nygaard K, Sandvik L, Nesbakken A. Tumour location is a prognostic factor for survival in colonic cancer patients. Colorectal Dis. 2008;10:33–40. doi: 10.1111/j.1463-1318.2007.01302.x. [DOI] [PubMed] [Google Scholar]
- 19.Hemminki K, Santi I, Weires M, Thomsen H, Sundquist J, Bermejo JL. Tumor location and patient characteristics of colon and rectal adenocarcinomas in relation to survival and TNM classes. BMC Cancer. 2010;10:688. doi: 10.1186/1471-2407-10-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Distler P, Holt PR. Are right- and left-sided colon neoplasms distinct tumors? Dig Dis. 1997;15:302–11. doi: 10.1159/000171605. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez EC, Roetzheim RG, Ferrante JM, Campbell R. Predictors of proximal vs. distal colorectal cancers. Dis Colon Rectum. 2001;44:251–8. doi: 10.1007/BF02234301. [DOI] [PubMed] [Google Scholar]
- 22.McCashland TM, Brand R, Lyden E, de Garmo P, CORI Research Project Gender differences in colorectal polyps and tumors. Am J Gastroenterol. 2001;96:882–6. doi: 10.1111/j.1572-0241.2001.03638.x. [DOI] [PubMed] [Google Scholar]
- 23.Edge SB, Byrd DR, Compton CC, Fritz AG. AJCC Cancer Staging Manual. 7. New York: Springer-Verlag; 2010. [Google Scholar]
- 24.Nawa T, Kato J, Kawamoto H, Okada H, Yamamoto H, Kohno H, Endo H, Shiratori Y. Differences between right- and left-sided colon cancer in patient characteristics, cancer morphology and histology. J Gastroenterol Hepatol. 2008;23:418–23. doi: 10.1111/j.1440-1746.2007.04923.x. [DOI] [PubMed] [Google Scholar]


