Abstract
Background
Culicoides biting midges are biological vectors of internationally important arboviruses of livestock and equines. Insecticides are often employed against Culicoides as a part of vector control measures, but systematic assessments of their efficacy have rarely been attempted. The objective of the present study is to determine baseline susceptibility of multiple Culicoides vector species and populations in Europe and Africa to the most commonly used insecticide active ingredients. Six active ingredients are tested: three that are based on synthetic pyrethroids (alpha-cypermethrin, deltamethrin and permethrin) and three on organophosphates (phoxim, diazinon and chlorpyrifos-methyl).
Methods
Susceptibility tests were conducted on 29,064 field-collected individuals of Culicoides obsoletus Meigen, Culicoides imicola Kieffer and a laboratory-reared Culicoides nubeculosus Meigen strain using a modified World Health Organization assay. Populations of Culicoides were tested from seven locations in four different countries (France, Spain, Senegal and South Africa) and at least four concentrations of laboratory grade active ingredients were assessed for each population.
Results
The study revealed that insecticide susceptibility varied at both a species and population level, but that broad conclusions could be drawn regarding the efficacy of active ingredients. Synthetic pyrethroid insecticides were found to inflict greater mortality than organophosphate active ingredients and the colony strain of C. nubeculosus was significantly more susceptible than field populations. Among the synthetic pyrethroids, deltamethrin was found to be the most toxic active ingredient for all species and populations.
Conclusions
The data presented represent the first parallel and systematic assessment of Culicoides insecticide susceptibility across several countries. As such, they are an important baseline reference to monitor the susceptibility status of Culicoides to current insecticides and also to assess the toxicity of new active ingredients with practical implications for vector control strategies.
Electronic supplementary material
The online version of this article (doi:10.1186/s13071-015-1042-8) contains supplementary material, which is available to authorized users.
Keywords: Culicoides imicola, Culicoides obsoletus, Culicoides nubeculosus, Insecticide susceptibility, Pyrethroids, Organophosphates, Vector control
Background
Culicoides Latreille (Diptera: Ceratopogonidae) are small haematophagous insects implicated worldwide as primary biological vectors of arboviruses causing important diseases of livestock [1]. These arboviruses include bluetongue (BTV), African horse sickness (AHSV), epizootic haemorrhagic disease (EHDV) and Schmallenberg (SBV) viruses [2]. Culicoides-borne arboviruses have a severe economic impact through direct loses due to the morbidity and mortality that occurs in susceptible animals. Additional losses also occur, however, due to the imposition of animal movement restrictions to limit BTV spread that inhibit animal trade [3, 4] and the indirect costs of monitoring and surveillance measures during outbreaks. Culicoides are also notorious as a biting nuisance in some regions, causing discomfort in humans and livestock and seasonal recurrent allergic dermatitis in horses [5–7].
In attempts to control Culicoides-borne arboviruses such as BTV and AHSV outside of their endemic range, compulsory vaccination campaigns and livestock movement restrictions are usually employed as the most effective way of controlling outbreaks [8]. Where safe and effective vaccines to Culicoides-borne viruses are either not initially available or economically unviable, control measures against Culicoides have been recommended by veterinary authorities to reduce host-vector contact and thus mitigate against arbovirus transmission. The use of insecticide residual spraying within stables and during transport when livestock is moved outside a restricted movement zone has been recommended in protecting animals with high economic value (e.g. prize rams and racehorses). Additional physical measures have also been suggested to reduce Culicoides populations such as the mechanical removal and/or reduction of larval breeding sites on farms and housing livestock during periods of high Culicoides activity [9].
To date, no insecticidal products have been authorized specifically against Culicoides in the European Union (EU), although a wide range of products are available, licensed and in use against other arthropods of veterinary importance [9]. Worldwide, the most commonly used method to protect livestock from Culicoides is the application of insecticides to livestock at risk of infection. Synthetic pyrethroid (SP) active ingredients are most often used in this role, but certain organophosphate (OP) products are also still available and licensed for use in Europe [7]. Insecticidal pour-on products exert their effect in two ways: primarily they are highly toxic to insects landing on the treated animal, often killing them within minutes of their landing on the host; secondarily they exert a contact irritation that may reduce the probability of the insect successfully initiating or completing a blood meal from the host. While some effort has been made to assess the efficiency of pour-on products against Culicoides, results vary greatly between studies according to different experimental designs [7]. Methodologies used include the exposure of Culicoides to hair clippings from treated animals [10–12] or direct exposure to a treated animal [13]. The variability of results across studies highlights the importance of using a standardized method to obtain comparable and reliable data.
Following the European Food Safety Authority’s recommendation to assess susceptibility of Culicoides to insecticides using standardized procedures [9], a World Health Organisation (WHO) standardized technique in adult mosquitoes has been adapted for use with Culicoides [13, 14]. This baseline information is essential for recommending the most effective insecticides and in detecting and monitoring the development of resistance. Insecticide resistance to earlier classes of insecticides including organochlorine-based larval treatments such as dieldrin and lindane was documented in Culicoides in the late 1950’s [15], but has not been examined for either OP or SP use. This risk exists considering that products based on single classes of insecticide have been used on a wide scale on livestock to control other arthropods including ticks, horn flies and stable flies in addition to often being used on crops.
Standardised information concerning the susceptibility of Culicoides to insecticides is at present limited to small scale studies [13, 14]. This study aims to assess the susceptibility of multiple populations of Culicoides species in different countries to the most frequently used insecticide active ingredients in Europe (SP: alpha-cypermethrin, deltamethrin and permethrin; OP: diazinon/dimpylate and phoxim) and Africa/Latin America (OP: chlorpyrifos-methyl). The main objective of the study is to generate reference baseline data regarding the efficiency of insecticidal products in killing Culicoides under laboratory conditions. Implementation of such insecticidal treatments into control programmes against Culicoides-borne diseases is then discussed.
Methods
Culicoides collection and identification
Susceptibility tests were performed on three Culicoides species. Laboratory-reared Culicoides nubeculosus Meigen were provided from a colony maintained in an insectary (temperature: 24 °C ±1 °C; relative humidity: 70 ± 10 %; light:dark: 12:12) at Cirad (Montpellier, France). This colony was established in Cirad during 2012 from eggs and larvae provided by The Pirbright Institute (UK). Field populations of Culicoides obsoletus Meigen and Culicoides imicola Kieffer were collected from multiple locations in two European countries (France, Spain) and two African countries (Senegal, South Africa) (Additional file 1: Table S1). Collection sites were privately owned farms characterized by abundant populations of Culicoides target species and reduced use of insecticides on the animals or pesticides on crops.
Culicoides were collected using a modified suction UV-light trap (OVI model, South Africa) [16] with the collection beaker replaced by a fine mesh netted cage to enable live collections. To prevent desiccation of Culicoides during the collection period, wet paper was placed on aluminium foil and rolled around the mesh cages. Traps were set before sunset and retrieved at dawn. Culicoides collection cages were stored in an isothermal container with an ice pack during transport to the insecticide trials room. Following completion of insecticide trials, field-collected individuals were morphologically identified to species level for C. imicola or to Obsoletus complex [17] using a binocular microscope and Obsoletus complex individuals were further identified to species level using a diagnostic multiplex polymerase chain reaction (PCR) assay [18].
Selection of insecticides and production of impregnated papers
Insecticide active ingredients were selected from those used most frequently in pour-on formulations within Europe. All active ingredients were used at > 98 % purity (Pestanal®, a registered trademark Sigma-Aldrich Laborchemikalien Gmbh, London, UK). Test papers (Whatman n°1 filter paper, 90 g/m2, 12 × 15 cm) were impregnated following training provided by a WHO collaborative centre (LIN-IRD, France). Insecticide active ingredients were applied at different concentrations (Additional file 2: Table S2) to papers in a silicone oil as the carrier agent (2 ml per paper, 67 % acetone, and 33 % silicone oil). Control papers were impregnated with 2 ml of acetone-silicone oil mix only. Impregnations were conducted by the same person (RV) to ensure consistency. Papers were impregnated a few days before the testing period, wrapped in aluminium foil and then stored at 4 °C. Impregnated papers were sent to each country in a polystyrene cooler box with ice cooler packs for maintaining the temperature at 4 °C during transport. Each paper was used three times in assays and stored at 4 °C between trials.
Insecticide susceptibility tests
Insecticide susceptibility tests were performed following the standardized WHO protocol for adult mosquito bioassay using test tubes (WHO/VBC/81.806) [19]) adapted for Culicoides [13]. Because insecticide susceptibility could be age specific [20], and physiological status and sex dependant [21, 22], bioassays were performed with 2–3 day old laboratory-reared C. nubeculosus unfed females. Due to the difficulties of colonizing C. obsoletus and C. imicola, adults were collected from the field and used one day after collection in bioassays. For field-collected Culicoides, only unpigmented females that were believed to have not previously taken a blood meal were used in data analysis, as determined through observation of abdominal pigmentation [23].
Culicoides were exposed for 1 h to either insecticide-impregnated papers or a control paper with the carrier compound only. For each replicate carried out on field-collected Culicoides, approximately 100 unsorted individuals were placed in each tube to obtain at least 25 unfed females of the target population. Following this exposure period, all Culicoides (including incapacitated individuals) were transferred using a motorised aspirator from exposure to observation tubes. Observation tubes were then stored vertically for 24 h and Culicoides within tubes were given access to a 10 % sugar solution provided on cotton wool pads through the top of the tube. Following the observation period, live and dead individuals were recorded and placed in 96 % ethanol. A replicate within the trials consisted of one complete set of serial dilutions and one negative control (untreated paper). Four replicates were performed for each active ingredient and target population. All susceptibility tests were performed in each country in a dedicated laboratory by the same trained person following standard protocols and at a temperature of 21 ± 3 °C and relative humidity of 70 ± 10 %.
Statistical analysis
Dose–response analysis for Culicoides mortality followed WHO recommendations [22]. Mortality rates were calculated by pooling the total number of dead Culicoides by active ingredient concentration across all replicates and expressed as a percentage of the total number of exposed individuals. When control mortality exceeded 20 % of the Culicoides exposed the replicate was discarded, while at rates of 5 to 20 % control mortality, rates were corrected using Abbott’s method (corrected mortality = 100 x (% observed mortality - % control mortality)/(100 - % control mortality) [24]). Abbott’s method reduces the estimated mortality effect of the treatment by the non-treatment mortality, as measured by the control. Data were analysed by a probit regression analysis [25] using PriProbit ver. 1.63 to obtain susceptibility values (LC50 and LC90) and sigmoidal curves of dose–response estimations of each insecticide active ingredient for each target population.
A second insecticide susceptibility analysis was performed to determine the effect of species origin (country and population), active ingredient concentration and their interactions. The two families of insecticide active ingredients were analysed separately as the concentrations used in testing differed. Initially, a generalised linear model with a binomial distribution was fitted, leading to an over dispersion of data (goodness of fit, p < 0.05). To assess the fixed effects (species and origin), the differences in deviation between the complete model including fixed effects (species, origin and doses without interaction) and without the fixed effect were calculated, taking into account the dispersion factor. R freeware (R Development Core Team 2012) and additional packages (aods3, lattice) were used for data analysis and graphics [26].
Results
A total of 29,064 unpigmented females were used in bioassays (11,761 C. nubeculosus, 11,975 C. imicola and 5,328 C. obsoletus). Among the 5,516 individuals collected belonging to the Obsoletus group: 5,328 (96.6 %) were molecularly identified as C. obsoletus, 152 (2.8 %) as C. scoticus and 36 specimens (0.6 %) were not identified and were excluded from the analysis. Amongst the 4,973 individuals from Obsoletus group collected in Corrèze, France; 4,815 (96.8 %) were identified as C. obsoletus, 149 (3.0 %) as C. scoticus and 9 (0.2 %) were unidentified; from the 543 individuals collected in Mallorca Island, Spain, 513 (94.5 %) were identified as C. obsoletus, 3 (0.5 %) as C. scoticus and 27 (5.0 %) were not identified. Data analysis was only performed with C. obsoletus as numbers of C. scoticus were not sufficient for examination.
Mortality recorded 24 h after 1 h exposure to insecticide active ingredients indicated that all Culicoides populations were susceptible to the active ingredients tested. The lethal concentrations (LC50 and LC90) calculated by probit analysis are presented in percentage of active ingredient (Tables 1, 2, 3, 4) and in its equivalent in mg/m2 (Additional file 3: Tables S3, Additional file 4: Table S4, Additional file 5: Table S5a, S5b). Within SPs, permethrin elicited the highest LC50 and LC90 values indicating less sensitivity, whereas deltamethrin gave the lowest values for all the populations studied. Among the OPs, chlorpyrifos-methyl produced the lowest LC50 and LC90 values, whereas diazinon gave the highest values.
Table 1.
Susceptibility values (LC50 and LC90 expressed in % of active ingredient) of Culicoides nubeculosus from French colony to different active ingredients. Mortality was recorded 24 h after 1 h exposure to different concentrations
| Active ingredient | No. test | LC50 % | LC90 % |
|---|---|---|---|
| (n) | (95 % CI) | (95 % CI) | |
| Deltamethrin | 4 | 0.0003 | 0.0019 |
| (2,528) | (0.0001–0.0004) | (0.0012–0.0043) | |
| Alpha-cypermethrin | 3 | 0.0016 | 0.0199 |
| (1,883) | NA | NA | |
| Permethrin | 3 | 0.0102 | 0.1045 |
| (2,055) | (0.0080–0.0117) | (0.0849–0.1337) | |
| Chlorpyrifos-methyl | 4 | 0.0725 | 0.1661 |
| (1,803) | NA | NA | |
| Phoxim | 3 | 0.1532 | 0.2759 |
| (1,965) | (0.1143–0.2091) | (0.2036–0.5761) | |
| Diazinon | 4 | 0.1839 | 0.3317 |
| (1,527) | (0.0800–0.2747) | (0.2347–0.4333) |
No test number of tests performed, n number of individuals tested, CI confidence interval, NA confidence interval not computed due to a large variability in the dose/response effect
Table 2.
Susceptibility values (LC50 and LC90 expressed in % of active ingredient) of different populations of Culicoides obsoletus to different active ingredients. Mortality was recorded 24 h after 1 h exposure to different concentrations
| Active ingredient | C. obsoletus (Corrèze, France) | C. obsoletus (Mallorca, Spain) | ||||
|---|---|---|---|---|---|---|
| No. test | LC50 % | LC90 % | No. test | LC50 % | LC90 % | |
| (n) | (95 % CI) | (95 % CI) | (n) | (95 % CI) | (95 % CI) | |
| Deltamethrin | 3 | 0.0001 | 0.0008 | 4 | 0.0005 | 0.0032 |
| (1,491) | (0.0000–0.0002) | (0.0005–0.0018) | (382) | (0.0002–0.0011) | (0.0013–0.1246) | |
| Alpha-cypermethrin | 3 | 0.0012 | 0.0102 | |||
| (502) | NA | NA | ||||
| Permethrin | 2 | 0.0207 | 0.0668 | 4 | 0.0147 | 0.0840 |
| (527) | (0.0188–0.0229) | (0.0565–0.0822) | (131) | (0.0101–0.0203) | (0.0502–0.2445) | |
| Chlorpyrifos-methyl | 5 | 0.0182 | 0.0769 | |||
| (615) | (0.0142–0.0222) | (0.0632–0.0980) | ||||
| Phoxim | 6 | 0.0273 | 0.1177 | |||
| (763) | (0.0238–0.0312) | (0.0944–0.1564) | ||||
| Diazinon | 2 | 0.0848 | 0.2944 | |||
| (917) | NA | NA | ||||
No test number of tests performed, n number of individuals tested, CI confidence interval, NA confidence interval not computed due to a large variability in the dose/response effect
Table 3.
Susceptibility values (LC50 and LC90 expressed in % of active ingredient) of different European populations of Culicoides imicola to different active ingredients. Mortality was recorded 24 h after 1 h exposure to different concentrations
| Active ingredient | Corsica, France | Catalonia, Spain | ||||
|---|---|---|---|---|---|---|
| No. test | LC50 % | LC90 % | No. test | LC50 % | LC90 % | |
| (n) | (95 % CI) | (95 % CI) | (n) | (95 % CI) | (95 % CI) | |
| Deltamethrin | 3 | 0.0002 | 0.0008 | 3 | 0.0003 | 0.0023 |
| (2,525) | (0.0001–0.0002) | (0.0005–0.0014) | (378) | (0.0002–0.0004) | (0.0016–0.0037) | |
| Alpha-cypermethrin | 4 | 0.0008 | 0.0034 | |||
| (2.084) | NA | NA | ||||
| Permethrin | 4 | 0.0194 | 0.0812 | 2 | 0.0175 | 0.1071 |
| (1.562) | (0.0171–0.0219) | (0.0695–0.0975) | (123) | NA | NA | |
| Chlorpyrifos-methyl | 1 | 0.0274 | 0.1273 | |||
| (122) | (0.0176–0.0388) | (0.0784–0.3535) | ||||
| Phoxim | 2 | 0.1053 | 0.2441 | |||
| (2,207) | NA | NA | ||||
| Diazinon | 2 | 0.1031 | 0.3424 | |||
| (1,704) | NA | NA | ||||
No test number of tests performed, n number of individuals tested, CI confidence interval, NA confidence interval not computed; due to a large variability in the dose/response effect
Table 4.
Susceptibility values (LC50 and LC90 expressed in % of active ingredient) of different African populations of Culicoides imicola to different active ingredients. Mortality was recorded 24 h after 1 h exposure to different concentrations
| Rufisque, Senegal | Pretoria, South Africa | |||||
|---|---|---|---|---|---|---|
| Active ingredient | No. test | LC50 % | LC90 % | No. test | LC50 % | LC90 % |
| (n) | (95 % CI) | (95 % CI) | (n) | (95 % CI) | (95 % CI) | |
| Deltamethrin | 4 | 0.0005 | 0.0015 | 3 | 0.0003 | 0.0020 |
| (458) | (0.0004–0.0005) | (0.0012–0.0018) | (291) | NA | NA | |
| Permethrin | 3 | 0.0031 | 0.0168 | |||
| (521) | (0.0018–0.0045) | (0.0105–0.0431) | ||||
No test number of tests performed, n number of individuals tested, CI confidence interval, NA confidence interval not computed; due to a large variability in the dose/response effect
Sigmoidal curves of dose–response obtained after data analysis indicated that SPs were 1–3 log-fold more effective/unit weight than OPs (Fig. 1). Within SPs, there was no significant impact according to the species tested for deltamethrin (p = 0.14), alpha-cypermethrin (p = 0.26) or permethrin (p = 0.65). In contrast, within OPs tested significant species effects were recorded in all three cases (p < 0.001). Chlorpyriphos-methyl and diazinon elicited LC50 values in C. nubeculosus which were 61.5–73.7 and 44.5–54.6 % higher than the other two species tested, whereas the phoxim LC50 of C. obsoletus was 74.4–82.1 % lower than the other two species (Fig. 1). Diagnostic concentrations (defined as twice the value of LC99) for the insecticides tested are presented in Table 5 and Additional file 6: S6. In addition, the statistical analysis showed that there was no difference between species in the diagnostic concentrations for deltamethrin (p = 0.98), alpha-cypermethrin (p = 0.26) or permethrin (p = 0.16). In contrast, OP active ingredients were significantly different in species effects (p < 0.001) between chlorpyriphos-methyl, phoxim and diazinon.
Fig. 1.
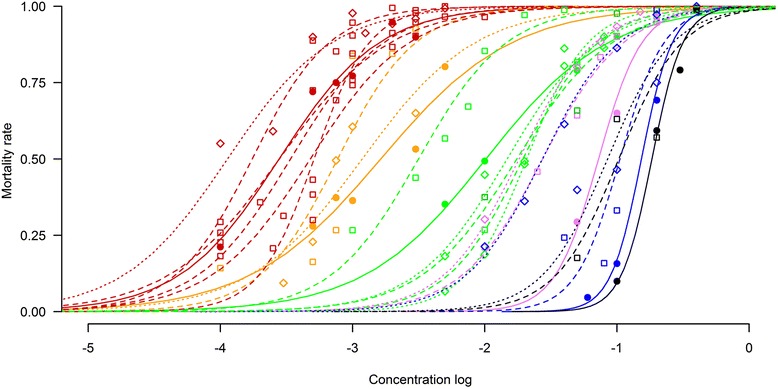
Sigmoidal curves of concentration-response estimations of different Culicoides populations exposed to different insecticides active ingredients. Dots represent pooled data obtained for each tested concentration (filled circle = C. nubeculosus; square = C. obsoletus; diamond = C. imicola) and lines represent the logistic regression of each population (straight: C. nubeculosus, dotted: C. obsoletus; dashed: C. imicola) for each active ingredient (red = deltamethrin; orange = alpha-cypermethrin; green = permethrin; purple = chlorpyriphos-methyl; blue = phoxim; black = diazinon). Data was analysed with PriProbit ver. 1.63, based on the mortality recorded at 24 h after 1 h exposure to different concentrations of active ingredients
Table 5.
Insecticide diagnostic concentrations expressed in % of active ingredient of different populations of Culicoides to different active ingredients
| Active ingredient | Population (origin) | ||||||
|---|---|---|---|---|---|---|---|
| C. nubeculosus | C. obsoletus | C. imicola | |||||
| (Cirad, FR) | (Corrèze, FR) | (Mallorca, ES) | (Corsica, FR) | (Catalonia, ES) | (Rufisque, SEN) | (Pretoria,SA) | |
| Pyrethroids | |||||||
| Deltamethrin | 0.03 | 0.01 | 0.04 | 0.01 | 0.04 | 0.01 | 0.03 |
| Alpha-cypermethrin | 0.61 | 0.21 | 0.03 | ||||
| Permethrin | 2.65 | 0.48 | 1.12 | 0.77 | 1.55 | 0.20 | |
| Organophosphates | |||||||
| Chlorpyrifos-methyl | 0.82 | 0.74 | 1.36 | ||||
| Phoxim | 1.05 | 1.16 | 1.22 | ||||
| Diazinon | 1.26 | 2.29 | 2.54 | ||||
Most of diagnostic concentrations likely overestimated due to classical dose/response analysis (see discussion)
FR France, ES Spain, SEN Senegal, SA South Africa
When the effect of the variable origin (country) was tested, a significant effect was found for deltamethrin (p <0.001) and permethrin (p <0.001). LC50 and LC90 values of field-collected Culicoides populations from France to deltamethrin were significantly lower than those from other countries (Tables 1, 2, 3, 4). Similarly, when the effect of origin population was tested, a significant effect was found for deltamethrin (p < 0.001) and permethrin (p < 0.001) responses with the French population of C. obsoletus and the Senegal population of C. imicola eliciting lower LC50 and LC90 values for deltamethrin and permethrin, respectively (Figs. 1 and 2).
Fig. 2.
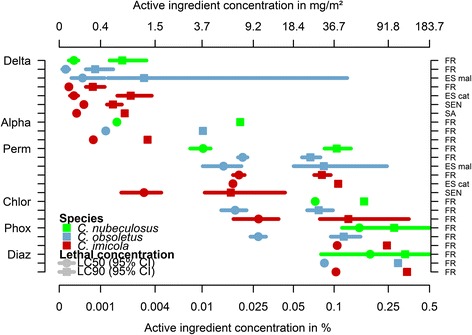
Lethal concentrations LC50, LC90 and 95 % confidence intervals for different populations exposed to six active ingredients: deltamethrin (Delta); alpha-cypermethrin (Alpha), permethrin (Perm), Chlorpyriphos-methyl (Chlor); phoxim (Phox) and diazinon (Diaz). Populations origin FR: France; ES mal: Mallorca Island, Spain; ES cat: Catalonia, Spain; SA: South Africa and SEN: Senegal. Lethal concentrations were calculated with PriProbit ver. 1.63, based on the mortality recorded at 24 h after 1 h exposure to different concentrations
A steeper slope of sigmoidal curves (Fig. 1), and the small gap between LC50 and LC90 for deltamethrin and permethrin (Fig. 2), demonstrated the efficacy of pyrethroids to induce higher mortality in Culicoides by only a slight increase in dose. Despite the general robustness and low variability of the response within each population against each active ingredient, which can be inferred from the small size of gaps between lower and upper 95 % confidence intervals (95 % CI) (length of LC lines in Fig. 2), the results from the C. obsoletus population in Mallorca (Spain) were highly variable.
Discussion
This study presents the first systematic survey of insecticide susceptibility of Culicoides species of veterinary interest on a wide geographic scale in Europe and Africa. More than 25,000 Culicoides were tested to obtain a robust and reliable dataset demonstrating that species within the genus varied significantly in their susceptibility to SP and OP active ingredients according to their origin. The use of the standardized method enabled the assessment of the Culicoides susceptibility to current insecticides, providing important baseline information, including reference values for the laboratory reared-species, C. nubeculosus. SPs were more toxic to the three Culicoides species tested than OPs, confirming previous studies conducted in wind tunnel trials carried out in USA with other Culicoides vector species [27–29]. This differential toxicity between insecticide families has also previously been highlighted in other insect species such as mosquitoes [30–32] and termites [33].
The most toxic insecticides tested against Culicoides in the study were deltamethrin and alpha-cypermethrin, both of which are synthetic 2nd generation type II α-cyano SPs. During our trials, no excito-repellency effect was observed, even when Culicoides were exposed to the highest concentration of permethrin, which has a documented repellence effect in mosquitoes [31]. Further work is required, however, to define methods of testing the impact of repellency versus toxicity in Culicoides as this factor might influence their efficacy in the field.
The WHO recommendations for adult mosquito susceptibility tests advises the use of insects of standardized ages for analysis [19, 22]. This restricts material in use to either adult females derived from larval collections (the preferred option) or, if larval collections are not possible, the F1 progeny of field collected females. These cohorts were not used in the present study as colonization of C. imicola or C. obsoletus has not been achieved and larval rearing does not provide sufficient numbers of adults for use [34]. As a more logistically feasible alternative to circumvent this issue, field-collected unpigmented females were assumed to be of a similar age [23]. While this raises the issues of repeatability of the study when a population with a different age structure is assessed, the restriction to unpigmented individuals at least reduces this source of error to a likely variation of days rather than weeks, which could occur if both unpigmented and pigmented individuals were used. These issues highlight the requirement for further development of accurate age grading methods for Culicoides, which, at present, are lacking.
The classical approach (probit regression analysis) for assessment of dose–response data as LC50, LC90 and their 95 % confidence intervals has been used for decades [19]. However, the probit regression method used in the current study for data analysis of mortality data has limitations. Mainly, this method doesn’t take into account over-dispersion of the data and when this occurs it cannot calculate associated confidence intervals. The probit method also predicts beyond data observed limits and outputs reference values as LC90 or LC99 using this prediction, which may underestimate the effect of insecticides. Two examples illustrate this point: i) using 0.005 % of deltamethrin, 99 % of exposed C. obsoletus died, but the prediction for the LC99 was 0.007 % (1.4-fold greater than the observed data); ii) a 99 % mortality was observed with 0.4 % of permethrin, but the prediction was LC99 = 1.32 % (3.3-fold greater than the observed data). Thirdly, results obtained after analyses are aggregated and comparison between individual trials is difficult. This is in part due to the fact that the probit regression method was designed to demonstrate if a field population of a given species is less sensitive than a susceptible reference population of the same species when both populations are exposed to the same range of concentrations of an active ingredient.
The advantage of the second method of analysis used was that over-dispersion in the datasets is taken into account in the calculation method. In addition, the predictions remain inside the data limits, no pre-determined shape was imposed on the regressions and results are more detailed. This approach highlighted no species-specific differences in toxicity of the three SP active ingredients tested. Results with OPs highlighted species effects, however, suggesting natural species-specific susceptibility as previously reported for mosquitoes [35, 36]. This could also represent specific resistance mechanisms, although diagnostic concentrations are similar to those of susceptible mosquito strains of Anopheles gambiae [22].
Resistance to insecticides has been reported in the New World for Haematobia irritans irritans Linnaeus (Diptera: Muscidae), a large biting fly which has been specifically targeted by insecticide treatment of ruminants using pour-on applications. This resistance has been demonstrated to occur through several complex resistance mechanisms, including target site insensitivity [37, 38] and metabolic detoxification [39]. In order to detect the development of resistance mechanisms in Culicoides, as in mosquitoes, it is recommended to test the vector susceptibility by bioassay over time throughout the year to assess temporal trends in resistance, and also to compare multiple sites in order to assess geographical distribution of resistance. Similar surveys of broadly distributed species such as C. imicola and C. obsoletus could provide helpful information about resistance according to the different insecticide pressure and environmental context across countries. While Culicoides populations investigated in the current study present no evidence of resistance, low variability between results was observed except for the Mallorca (Spain) population of C. obsoletus. A key consideration is that multicentric insecticide trials are subject to extrinsic factors that can influence results, misleading the real effect of an insecticide to a given population [22, 40]. In the current study significant efforts were made to control conditions during testing including ambient temperature and humidity, the origin of impregnated papers and the bioassay procedure. However, one could not exclude the possibility that some differences in the procedures persist between countries.
Conclusions
In conclusion, this study has defined the baseline susceptibility status of different Culicoides vector populations against six SP and OP insecticide active ingredients. The information regarding lethal concentrations will be used in future studies with the aim of testing the efficiency of insecticidal products applied directly on animals (e.g. pour-on and baths/dips), or insecticide impregnated materials (e.g. nets and paints) and also to monitor the potential emergence of resistance in field populations. This will improve our understanding of the efficacy of control measures against Culicoides in the field and enable better policy recommendations for their use in Europe and Africa.
Acknowledgments
This study was funded by the “Direction générale de l’alimentation du Ministère francais en charge de l’Agriculture” and the EU grant FP7-261504. This manuscript is catalogued by the EDENext steering committee as EDENext404 (http:www.edenext.eu).
The authors are grateful to farmers: Jacques Virolles and family (Corrèze, France), Albert Jungen and family (Corsica, France), Joan Capó and family (Catalonia, Spain), Andreu Cosme Oliver Ramón and family (Mallorca Island, Spain), Samba Coura Diop and family (Rufisque, Senegal) and the ARC-OVI and OBP (Onderstepoort Biological Products) (Pretoria, South Africa), who permitted us to enter into their farms and made their stables available for Culicoides collection. Authors are also grateful to Marie Laure Setier-Rio from EID-Med (France) and Lassana Konaté from LEVP/UCAD (Senegal) for technical help.
Additional files
Culicoides collections sites and date of collection during trials. (DOCX 16 kb)
Serial dilutions of insecticides used in trials to assess susceptibility of different populations of Culicoides. (DOCX 16 kb)
Susceptibility values (LC50 and LC90 expressed in mg/m² of active ingredient) of Culicoides nubeculosus from French colony to different active ingredients. Mortality recorded 24 h after 1 h exposure to different concentrations. (DOCX 15 kb)
Susceptibility values (LC50 and LC90 expressed in mg of active ingredient/m²) of different populations of Culicoides obsoletus to different active ingredients. Mortality was recorded 24 h after a 1 h exposure to insecticides. (DOCX 16 kb)
Susceptibility values (LC50 and LC90 expressed in mg of active ingredient/m²) of different European populations of Culicoides imicola to different active ingredients. Mortality was recorded 24 h after 1 h exposure to different concentrations. Table S5b. Susceptibility values (LC50 and LC90 expressed in mg of active ingredient/m²) of different African populations of Culicoides imicola to different active ingredients. Mortality was recorded 24 h after 1 h exposure to different concentrations. (DOC 54 kb)
Insecticide diagnostic concentrations expressed in mg of active ingredient/m² of different populations of Culicoides to different active ingredients. (DOCX 16 kb)
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
RV, ThiB, ThoB, SC designed the study. RV, JL, MF, RdR, ST, KL, MM, NP, GV, IG, XA, BS, LG, GG, MM carried out the bioassays. RV, ThiB, GG, ThoB, RL, CG, CC-S analysed the data. RV, ThiB, GG ThoB, SC wrote the manuscript, which was revised by all the authors. All authors read and approved the final manuscript.
Contributor Information
Roger Venail, Email: rvenail@yahoo.com.
Jonathan Lhoir, Email: jonathanlhoir@hotmail.com.
Moussa Fall, Email: moussafall08@yahoo.fr.
Ricardo del Río, Email: ricardo.delrio@uib.es.
Sandra Talavera, Email: sandra.talavera@cresa.uab.cat.
Karien Labuschagne, Email: labuschagnek@arc.agric.za.
Miguel Miranda, Email: ma.miranda@uib.es.
Nonito Pagès, Email: nitu.pages@cresa.uab.cat.
Gert Venter, Email: VenterG@arc.agric.za.
Ignace Rakotoarivony, Email: ignace.rakotoarivony@cirad.fr.
Xavier Allène, Email: xavier.allene@cirad.fr.
Bethsabée Scheid, Email: bethsabee.scheid@gmail.com.
Laëtitia Gardès, Email: laetitia.gardes@cirad.fr.
Geoffrey Gimonneau, Email: geoffrey.gimonneau@cirad.fr.
Renaud Lancelot, Email: renaud.lancelot@cirad.fr.
Claire Garros, Email: claire.garros@cirad.fr.
Catherine Cêtre-Sossah, Email: catherine.cetre-sossah@cirad.fr.
Thomas Balenghien, Email: thomas.balenghien@cirad.fr.
Simon Carpenter, Email: simon.carpenter@pirbright.ac.uk.
Thierry Baldet, Email: thierry.baldet@cirad.fr.
References
- 1.Purse BV, Carpenter S, Venter GJ, Bellis G, Mullens BA. Bionomics of temperate and tropical culicoides midges: knowledge gaps and consequences for transmission of culicoides-borne viruses. Annu Rev Entomol. 2015;60:373–392. doi: 10.1146/annurev-ento-010814-020614. [DOI] [PubMed] [Google Scholar]
- 2.Carpenter S, Groschup MH, Garros C, Felippe-Bauer ML, Purse BV. Culicoides biting midges, arboviruses and public health in Europe. Antiviral Res. 2013;100:102–113. doi: 10.1016/j.antiviral.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 3.Sinclair M, Bührmann G, Gummow B. An epidemiological investigation of the African horsesickness outbreak in the Western Cape Province of South Africa in 2004 and its relevance to the current equine export protocol. J S Afr Vet Assoc. 2006;77:191–196. doi: 10.4102/jsava.v77i4.376. [DOI] [PubMed] [Google Scholar]
- 4.Hoogendam K. International study on the economic consequences of outbreaks of bluetongue serotype 8 in north-western Europe. University of Van Hall-Larenstein. Leewarden, Netherlands; 2007.
- 5.Riek RF. Studies on allergic dermatitis (“Queensland itch”) of the horse. Aust Vet J. 1953;29:177–184. doi: 10.1111/j.1751-0813.1953.tb13937.x. [DOI] [Google Scholar]
- 6.Anderson GS, Belton P, Kleider N. The hypersensitivity of horses to Culicoides bites in British Columbia. Can Vet J. 1988;29:718–723. [PMC free article] [PubMed] [Google Scholar]
- 7.Carpenter S, Mellor PS, Torr SJ. Control techniques for Culicoides biting midges and their application in the U.K. and northwestern Palaearctic. Med Vet Entomol. 2008;22:175–187. doi: 10.1111/j.1365-2915.2008.00743.x. [DOI] [PubMed] [Google Scholar]
- 8.Savini G, al e. Vaccines against bluetongue in Europe. 2007. [DOI] [PubMed]
- 9.EFSA Scientific opinion of the panel on animal health and welfare on a request from the European commission (DG SANCO) on bluetongue. The EFSA Journal. 2008;735:1–69. doi: 10.2903/j.efsa.2008.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmahl G, Walldorf V, Klimpel S, Al-Quraishy S, Mehlhorn H. Efficiency of Oxyfly on Culicoides species -the vectors of Bluetongue virus- and other insects. Parasitol Res. 2008;103:1101–1103. doi: 10.1007/s00436-008-1098-x. [DOI] [PubMed] [Google Scholar]
- 11.Mehlhorn H, Schmahl G, D’Haese J, Schumacher B. Butox ® 7.5 pour on: a deltamethrin treatment of sheep and cattle: pilot study of killing effects on Culicoides species (Ceratopogonidae) Parasitol Res. 2008;102:515–518. doi: 10.1007/s00436-007-0841-z. [DOI] [PubMed] [Google Scholar]
- 12.Papadopoulos E, Bartram D, Carpenter S, Mellor PS, Wall R. Efficacy of alphacypermethrin applied to cattle and sheep against the biting midge Culicoides nubeculosus. Vet Parasitol. 2009;163:110–114. doi: 10.1016/j.vetpar.2009.03.041. [DOI] [PubMed] [Google Scholar]
- 13.Venail R, Mathieu B, Setier-Rio ML, Borba C, Alexandre M, Viudes G, et al. Laboratory and field-based tests of deltamethrin insecticides against adult Culicoides biting midges. J Med Entomol. 2011;48:351–357. doi: 10.1603/ME10178. [DOI] [PubMed] [Google Scholar]
- 14.del Rio R, Venail R, Calvete C, Barceló C, Baldet T, Lucientes J, et al. Sensitivity of Culicoides obsoletus (Meigen) (Diptera: Ceratopogonidae) to deltamethrin determined by an adapted WHO standard susceptibility test. Parasitology. 2014;141:542–546. doi: 10.1017/S0031182013001935. [DOI] [PubMed] [Google Scholar]
- 15.Smith CN, Davis AN, Weidhaas DE, Seabrook EL. Insecticide resistance in the salt-marsh sand Fly culicoides furens. J Econ Entomol. 1959;52:352–353. doi: 10.1093/jee/52.2.352. [DOI] [Google Scholar]
- 16.Venter GJ, Labuschagne K, Hermanides KG, Boikanyo SNB, Majatladi DM, Morey L. Comparison of the efficiency of five suction light traps under field conditions in South Africa for the collection of Culicoides species. Vet Parasitol. 2009;166:299–307. doi: 10.1016/j.vetpar.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 17.Delécolle JC, De La Rocque S. Contribution à l’étude des Culicoides de Corse. Liste des espèces recensées en, 2000/2001 et redescription du principal vecteur de la fièvre catarrhale ovine : C. imicola Kieffer, 1913 (Diptera, Ceratopogonidae) Bull Soc Entomol Fr. 2002;107:371–379. [Google Scholar]
- 18.Nolan V, Carpenter S, Barber J, Mellor PS, Dallas J, Mordue JA, et al. Rapid diagnostic PCR assays for members of the Culicoides obsoletus and Culicoides pulicaris species complexes, implicated vectors of bluetongue virus in Europe. Vet Microbiol. 2007;124:82–94. doi: 10.1016/j.vetmic.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 19.WHO . World Health Organization. Instructions for determining the susceptibility or resistance of adult mosquitoes to organochlorine, organophosphate and carbamate insecticides. Diagnostic test. Geneva: WHO/VBC; 1981. p. 81.806. [Google Scholar]
- 20.Rajatileka S, Burhani J, Ranson H. Mosquito age and susceptibility to insecticides. Trans R Soc Trop Med Hyg. 2011;105:247–253. doi: 10.1016/j.trstmh.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 21.Chareonviriyaphap T, Kongmee M, Bangs MJ, Sathantriphop S, Meunworn V, Parbaripai A, et al. Influence of nutritional and physiological status on behavioral responses of Aedes aegypti (Diptera: Culicidae) to deltamethrin and cypermethrin. J Vector Ecol. 2006;31:89–101. doi: 10.3376/1081-1710(2006)31[89:IONAPS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 22.WHO. Test procedures for insecticide resistance monitoring in malaria vector mosquitoes. 2013.
- 23.Dyce A. The recognition of nulliparous and parous Culicoides (Diptera: Ceratopogonidae) without dissection. Aust J Entomol. 1969;8:11–15. doi: 10.1111/j.1440-6055.1969.tb00727.x. [DOI] [Google Scholar]
- 24.Abbott WS. A method of computing the effectiveness of an insecticide. J Econ Entomol. 1925;18:265–267. doi: 10.1093/jee/18.2.265a. [DOI] [Google Scholar]
- 25.Finney D. Probit analysis: a statistical treatment of the sigmoid response curve. Oxford, England: Macmillan; 1947. [Google Scholar]
- 26.R Development Core Team (2013). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org
- 27.Kline D, Haile D, Baldwin K. Wind tunnel tests with seven insecticides against adult Culicoides mississippiensis Hoffman. Mosq News. 1981;41:745–747. [Google Scholar]
- 28.Floore T. Laboratory wind tunnel tests of nine insecticides against adult Culicoides species. Fla Entomol. 1985;68:678–682. doi: 10.2307/3494873. [DOI] [Google Scholar]
- 29.Holbrook FR. Wind tunnel evaluations of insecticides applied to colonized Culicoides variipennis (Diptera: Ceratopogonidae) J Fla Anti-Mosq Assoc. 1986;57:1–3. [Google Scholar]
- 30.Bansal SK, Singh KV. Efficacy of different organophosphate and synthetic pyrethroid insecticides to the larvae of malaria vector Anopheles stephensi, Liston. J Environ Biol. 2004;25:485–488. [PubMed] [Google Scholar]
- 31.Mosha F, Lyimo I, Oxborough R, Matowo J, Malima R, Feston E, et al. Comparative efficacies of permethrin-, deltamethrin- and alpha-cypermethrin-treated nets, against Anopheles arabiensis and Culex quinquefasciatus in northern Tanzania. Ann Trop Med Parasitol. 2008;102:367–376. doi: 10.1179/136485908X278829. [DOI] [PubMed] [Google Scholar]
- 32.Juntarajumnong W, Pimnon S, Bangs MJ, Thanispong K, Chareonviriyaphap T. Discriminating lethal concentrations and efficacy of six pyrethroids for control of Aedes aegypti in Thailand. J Am Mosq Control Assoc. 2012;28:30–37. doi: 10.2987/11-6203.1. [DOI] [PubMed] [Google Scholar]
- 33.Su N-Y, Scheffrahn RH, Ban PM. Barrier efficacy of pyrethroid and organophosphate formulations against subterranean termites (Isoptera : Rhinotermitidae) J Econ Entomol. 1993;86:772–776. doi: 10.1093/jee/86.3.772. [DOI] [Google Scholar]
- 34.Nayduch D, Cohnstaedt LW, Saski C, Lawson D, Kersey P, Fife M, et al. Studying Culicoides vectors of BTV in the post-genomic era: Resources, bottlenecks to progress and future directions. Virus Res. 2014;182:43–49. doi: 10.1016/j.virusres.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Somboon P, Prapanthadara LA, Suwonkerd W. Insecticide susceptibility tests of Anopheles minimus s.l., Aedes aegypti, Aedes albopictus, and Culex quinquefasciatus in northern Thailand. Southeast Asian J Trop Med Public Health. 2003;34:87–93. [PubMed] [Google Scholar]
- 36.Pridgeon JW, Pereira RM, Becnel JJ, Allan SA, Clark GG, Linthicum KJ. Susceptibility of Aedes aegypti, Culex quinquefasciatus Say, and Anopheles quadrimaculatus Say to 19 pesticides with different modes of action. J Med Entomol. 2008;45:82–87. doi: 10.1603/0022-2585(2008)45[82:soaacq]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 37.Foil LD, Guerrero F, Alison MW, Kimball MD. Association of the kdr and superkdr sodium channel mutations with resistance to pyrethroids in Louisiana populations of the horn fly, Haematobia irritans irritans (L.) Vet Parasitol. 2005;129:149–158. doi: 10.1016/j.vetpar.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 38.Oyarzún MP, Li AY, Figueroa CC. High levels of insecticide resistance in introduced horn fly (Diptera: Muscidae) populations and implications for management. J Econ Entomol. 2011;104:258–265. doi: 10.1603/EC10188. [DOI] [PubMed] [Google Scholar]
- 39.Szalanski AL, Black WC, Broce AB. Esterase staining activity in pyrethroid-resistant horn flies (Diptera: Muscidae). J Kans Entomol Soc 1991;68:303–312.
- 40.WHO. World Health Organization. Test procedures for insecticide resistance monitoring in malaria vectors, bio-efficacy and persistence of insecticides on treated surfaces. 1998.


