Abstract
Aims
To find specific magnetic resonance imaging (MRI) features to differentiate metastatic ovarian tumors from primary epithelial ovarian cancers.
Methods
Eleven cases with metastatic ovarian tumors and 26 cases with primary malignant epithelial ovarian cancers were retrospectively studied. All features such as patient characteristics, MRI findings and biomarkers were evaluated. The differences including laterality, configuration, uniformity of locules, diffusion weighted imaging (DWI) signal of solid components and enhancement of solid portions between metastatic ovarian tumors and primary epithelial ovarian cancers were compared by Fisher’s exact test. Median age of patients, the maximum diameter of lesions and biomarkers were compared by the Mann-Whitney test.
Results
Patients with metastatic ovarian tumors were younger than patients with primary epithelial ovarian cancers in the median age (P = 0.015). Patients with bilateral tumors in metastatic ovarian tumors were more than those of primary epithelial ovarian cancers (P = 0.032). The maximum diameter of lesions in metastatic ovarian tumors was smaller than that of primary epithelial ovarian cancers (P = 0.005). The locules in metastatic ovarian tumors were more uniform than those of primary epithelial ovarian cancers (P = 0.024). The enhancement of solid portions in metastatic ovarian tumors showed more moderate than that of primary epithelial ovarian cancers (P = 0.037). There was no statistically significant difference between the two groups in configuration, DWI signal of solid components and ascites. Biomarkers such as CA125 and human epididymis protein 4 (HE4) in metastatic ovarian tumors showed less elevated than that of primary epithelial ovarian cancers.
Conclusions
Significant differences between metastatic ovarian tumors and primary epithelial ovarian cancers were found in the median age of patients, laterality, the maximum diameter of lesions, uniformity of locules, enhancement patterns of solid portions and biomarkers. Metastatic ovarian tumors usually presented in the younger patients, smaller-sized, more bilateral lesions, more uniform of locules, more moderate enhancement of solid portions, and less elevated levels of CA125 and HE4 than those of primary epithelial ovarian cancers.
Keywords: Ovary, Primary epithelial ovarian cancer, Metastatic, Magnetic resonance imaging
Background
The optimal management and prognosis of metastatic ovarian tumors depend on the origin of the primary tumor [1, 2]. For primary ovarian cancers, management will be based on cytoreductive surgery and systemic therapy (depending on stage). Therefore, preoperative discrimination is very critical. However, by now, it is difficult to discriminate these tumors by imaging, or even by histopathology in some cases, as macroscopic and microscopic features of metastatic ovarian tumors and primary epithelial ovarian cancers are often similar. Therefore, they cannot be definitively classified without further clinical evaluations [3, 4]. Histological preoperative diagnosis is now and then impossible because there is a risk of dissemination of primary ovarian cancer on an early stage otherwise. In general, the therapy methods and prognoses of metastatic ovarian tumors are different from those of primary epithelial ovarian cancers [5]. Therefore, discriminations between them are very critical. However, there is a lack of comprehensive imaging studies concerning the distinctions. Magnetic resonance imaging (MRI) is a useful tool for investigation and description of characteristic signs for the preoperative diagnosis of an ovarian lesion [1]. The object of this study is to detect specific MRI features of metastatic ovarian tumors that can be discriminated from primary epithelial ovarian cancers.
Methods
Study subjects
This retrospective study was approved by the institutional review boards of Shanghai First People’s Hospital, Shanghai, China. The informed consent requirement was waived. We searched for data of patients with ovarian tumors from January 2012 to December 2014 on a hospital information system—a picture archiving and communication system (PACS). We encountered 11 consecutive cases (median age, 42 years; range, 21–58 years) of metastatic ovarian tumors confirmed by pathology. Six gastric cancers, two colon cancers, one cervical cancer, one breast cancer and one thyroid cancer were found among the patients. It is obvious that the most common site of primary origin of metastatic ovarian cancers was stomach. Meanwhile, we detected 26 consecutive cases (median age, 56 years; range, 17–82 years) with pathologically confirmed primary epithelial ovarian cancers. None of these cases witnessed the history of malignant tumors except the present cancers. All the primary epithelial ovarian cancers had undergone surgery. Then the cancers were confirmed by histopathological pathologists in Shanghai First People’s Hospital. Consequently, ten serous cystadenocarcinoma, six clear cell cancers, five borderline malignancy, three mucinouscystadenocarcinoma and two endometrioid adenocarcinomas were found (Table 1).
Table 1.
Summary of the cases
| Tumor type | Total cases | Histopathologically confirmed cases | |
|---|---|---|---|
| Metastatic ovarian tumors | Gastric cancer | 6 | 6 |
| Colon cancer | 2 | 2 | |
| Thyroid cancer | 1 | 1 | |
| Uterine cervical cancer | 1 | 1 | |
| Breast cancer | 1 | 1 | |
| Subtotal | 11 | 11 | |
| Primary ovarian tumors | Serous cystadenocarcinoma | 10 | 10 |
| Clear cell carcinoma | 6 | 6 | |
| Borderline malignancy | 5 | 5 | |
| Mucinous cystadenocarcinoma | 3 | 3 | |
| Endometrioid adenocarcinoma | 2 | 2 | |
| Subtotal | 26 | 26 | |
| Total | 37 | 37 | |
MRI scanning
MRI examinations were performed on a 3T system (Signa HD, General Electric Healthcare, Milwaukee, WI, USA). The scan scope was from the umbilicus to the pubic symphysis in the caudocranial direction. For too large lesions to be totally scanned on axial imaging, a sagittal scanning sequence was adopted to contain as much of the total lesion as possible. First, routine MRI protocols were performed for the detection of the lesions, which contained fast spin-echo (FSE) T1-weighted images (T1WI), sagittal FSE T2-weighted images (T2WI) and fat-suppressed T2WI (FS T2WI) on the axial imaging. A diffusion weighted imaging (DWI)-MRI sequence contained an echo-planar imaging sequence with an array spatial-sensitivity-encoding technique (ASSET). The settings of the T1WI MRI protocol were: repetition time (TR) = 540 ms; echo time (TE) = 7 ms; number of excitations (NEX) = 2 and thickness = 7 mm. The parameters of the T2WI MRI protocol were: TR = 2400 ms; TE = 85 ms; NEX = 1 and thickness = 7 mm. The settings of the FS T2WI MRI protocol were: TR = 3800 ms; TE = 90 ms; NEX = 2 and thickness = 7 mm. The settings of the DWI MRI protocol were: TR = 4300 ms; TE = 63 ms; NEX = 6; thickness = 7 mm and the b value = 0 or 800 s/mm2. Second,a liver acquisition with a volume acceleration (LAVA) sequence was adopted for contrast-enhanced pelvic imaging, and a power injector (Missouri Ulrich, Ulm, Germany) was used to inject the contrast medium (Magnevist, Bayer Schering Pharma AG, Germany). The parameters of the LAVA MRI protocol were: TR = 3.5 ms; TE = 1.6 ms; NEX = 1; flip angle = 15°; band width =125 kHz and thickness = 2 mm. Subsequently, the images were obtained in multiple phases of contrast agent enhancement among the sagittal and axial planes—precontrast sagittal and axial oblique, postcontrast at 20 s, 40 s, 60 s, 80 s in the axial plane, and 120 s in the sagittal plane. The details of the scanning parameters of imaging are presented in Table 2.
Table 2.
Details of parameters for MRI scanning protocols
| Parameters | FSE-T1WI | FSE-T2WI | FS T2WI | EPI-DWI | LAVA |
|---|---|---|---|---|---|
| Repetition/echo time (ms) | 540/7 | 2400/85 | 3800/90 | 4300/63 | 3.5/1.6 |
| NEX | 2 | 1 | 2 | 6 | 1 |
| Thickness(mm) | 7 | 7 | 7 | 7 | 2 |
| Field of view (mm) | 36 | 36 | 36 | 40 | 38 |
| Matrix | 320 × 224 | 256 × 224 | 256 × 224 | 96 × 130 | 320 × 224 |
| Flip angle (degrees) | 15 |
MRI image analysis
The following lesion parameters were evaluated: 1) the maximum diameter of lesions; 2) laterality; 3) uniformity of locules (uniform or not uniform); 4) configuration: cystic-solid (less than half of solid component), solid (more than half of solid component); 5) DWI signal of solid components (moderate or high); 6) enhancement of solid portions (moderate or prominent enhancement, referring to the enhancement of myometrium); 7) ascites. The preoperative MRI diagnoses were correlated with histopathological results.
Statistical analyses
Statistical analyses were performed with SPSS 19.0 for Windows (SPSS, Chicago, IL). The differences between metastatic ovarian tumors and primary epithelial ovarian cancers in laterality, configuration, uniformity of locules, DWI signal of solid components, and enhancement of solid portions were compared by Fisher’s exact test, Median age of patients, the maximum diameter of lesions and biomarkers (CA125, human epididymis protein 4 (HE4)) were compared by the Mann-Whitney test. The sensitivity, specificity, accuracy, positive predictive value (PPV), and negative predictive value (NPV) of the significant MRI features of metastatic ovarian tumors were calculated. P < 0.05 was considered statistically significant.
Results
Metastatic ovarian tumors and primary epithelial ovarian cancers were found in the median patients who were 42 and 56 years old respectively (P = 0.015). Totally, seventeen tumors were found in 11 patients with metastatic ovarian tumors—bilateral tumors in six patients and unilateral tumors in five patients; thirty-one tumors were found in 26 patients with primary epithelial ovarian cancers—bilateral tumors in five patients and unilateral tumors in 21 patients (P = 0.032). The maximum tumor diameter was generated from 25 to 200 mm (median, 67 mm) in metastatic ovarian tumors versus 16 to 285 mm (median, 122 mm) in primary epithelial ovarian cancers (P = 0.005). The locules were uniform in 59 % of metastatic ovarian tumors versus 26 % of primary epithelial ovarian cancers (P = 0.024). The enhancement of solid portions was moderate in 76 % of metastatic ovarian tumors versus 45 % in primary epithelial ovarian cancers (P = 0.037). Figure 1 showed that the locules of a serous cystadenocarcinoma on the right ovary were not uniform on FS T2WI and the solid portion was prominent enhancement on LAVA dynamic contrast-enhanced MRI (DCE-MRI) (A and C). In contrast, the locules of a metastatic ovarian tumors on the right ovary were uniform on FS T2WI and the solid portion was moderate enhancement on LAVA DCE-MRI (B and D). CA125 and HE4 of metastatic ovarian tumors and primary epithelial ovarian cancers were significantly different (P = 0.033, 0.006, respectively). In all, there was a statistically significant difference in the median age of patients, the maximum diameter of lesions, laterality, uniformity of locules, enhancement of solid components, CA125 and HE4 between metastatic ovarian tumors and primary epithelial ovarian cancers. There was no statistically significant difference between the two groups in terms of configuration, DWI signal of solid components and ascites (P = 0.272, 0.428 and 0.108, respectively). The MRI features of metastatic ovarian tumors compared with primary epithelial ovarian cancers are shown in Table 3.
Fig. 1.
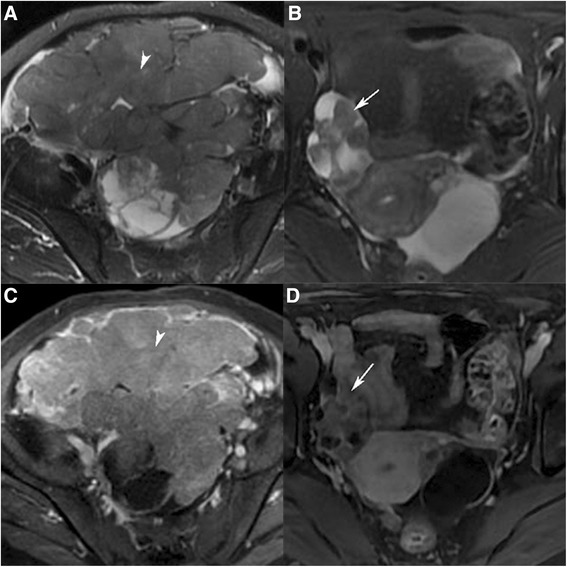
a and c A 58-year-old woman with serous cystadenocarcinoma on the right ovary. a Axial T2WI with fat saturation image showed the locules were not uniform. c Axial LAVA dynamic contrast-enhanced MRI image showed the solid portion was prominent enhancement (arrowhead). b and d: A 49-year-old woman with metastatic ovarian tumors on the right ovary. b Axial T2WI with fat saturation image showed the locules were uniform. d Axial LAVA dynamic contrast-enhanced MRI image showed the solid portion was moderate enhancement (arrow)
Table 3.
Comparison of each parameter between metastatic and primary ovarian tumors
| Metastatic | Primary | P value | ||
|---|---|---|---|---|
| Patients’ age (years) | Median | 42 | 56 | 0.015 |
| Laterality | Unilateral cases | 5 | 21 | 0.032 |
| Bilateral cases | 6 | 5 | ||
| Maximum diameter of lesions (mm) | Median | 67 | 122 | 0.005 |
| Uniformity of locules | Uniform | 10 | 8 | 0.024 |
| Not uniform | 7 | 23 | ||
| Configuration | Cystic-solid | 10 | 23 | 0.272 |
| Solid | 7 | 8 | ||
| DWI signal of solid components | Intermediate | 5 | 6 | 0.428 |
| High | 12 | 25 | ||
| Enhancement of solid portions | Moderate | 13 | 14 | 0.037 |
| Prominent | 4 | 17 | ||
| CA125 | Elevated cases | 3 | 17 | 0.033 |
| HE4 | Elevated cases | 1 | 15 | 0.006 |
| Ascites | 5 | 19 | 0.108 |
Diagnostic parameters for the characterization of the metastatic ovarian tumors are listed in Table 4. The combination of ovarian lesions with any one of the following three features, patients’ age, small size, and bilaterality, yielded sensitivity, specificity, accuracy, PPV, and NPV for identifying metastatic ovarian tumors of 100, 87, 92, 89, and 100 %, respectively.
Table 4.
Accuracy of MRI in characterizing ovarian lesions as metastatic ovarian tumors
| MRI features | Sensitivity (%) | Specificity (%) | Accuracy (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|
| Patients’ age(<50 years) | 65 (11/17) | 81 (25/31) | 75 (36/48) | 65 (11/17) | 81 (25/31) |
| Small size (<100 mm) | 82 (14/17) | 61 (19/31) | 69 (33/48) | 54 (14/26) | 86 (19/22) |
| Bilaterality | 71 (12/17) | 68 (21/31) | 69 (33/48) | 55 (12/22) | 81 (21/26) |
| Uniformity of locules | 59 (10/17) | 74 (23/31) | 69 (33/48) | 50 (10/20) | 77 (23/30) |
| Moderate enhancement | 76 (13/17) | 55 (17/31) | 63(30/48) | 48 (13/27) | 81 (17/21) |
PPV positive predictive value, NPV negative predictive value
Discussion
It is important to discriminate between metastatic ovarian tumors and primary ovarian cancers to select the most appropriate management which influences the prognosis. However, it is difficult to distinguish those two groups of tumors because they both show the imaging features of malignant tumors. There are some studies concerning the imaging features of metastatic ovarian tumors. Metastatic ovarian tumors and primary epithelial ovarian cancers are difficult to distinguish by CT as they both display mixed cystic and solid lesions [6]. Bilaterality has been reported as a specific feature of metastatic ovarian tumors [7]. However, La Fianza et al concluded that bilaterality was not significantly different between secondary and primary ovarian cancers after reviewing more than eighty cases of ovarian tumors [8]. In our study, 54.5 % of metastatic tumors showed bilaterality in contrast to 23.8 % of primary epithelial ovarian cancers (P = 0.032), roughly in agreement with Kim et al. [7]. Therefore, bilaterality still seems to be an important factor in diagnosing metastatic ovarian tumors by imaging.
Khunamornpong et al reported that the maximum diameter of unilateral ovarian carcinomas less than 100 mm were considered as metastases, and more than 100 mm as primary ovarian cancers [4]. Jung et al advocated that a cutoff of 150 mm for classifying unilateral tumors resulted in a higher diagnostic accuracy [5]. In our study, the median maximum tumor diameter is 101 mm in unilateral metastatic ovarian tumors versus 154 mm in primary epithelial ovarian cancers. Perhaps, the maximum diameter of tumors ranged from100 to 150 mm may be an overlap area between unilateral metastatic ovarian tumors and primary ovarian cancers.
Tanaka et al. reported that metastatic ovarian tumors tended to be composed of uniform cysts compared with primary mucinous tumors [2]. Our results suggested that metastatic ovarian tumors tend to be composed of uniform locules in contrast to primary epithelial ovarian cancers, which is similar to Tanaka et al. [2]. Histopathological findings show that metastatic tumors proliferate in a more uniform manner than primary ovarian cancers [2]. We think that features such as mucus productivity of tumor cells may be more uniform in metastatic tumors. Consequently, this could explain that the size of locules with metastatic ovarian tumors would be more uniform than those of primary epithelial ovarian cancers.
Primary ovarian neoplasms often appeared as a prominent enhancement [9–12]. Our study was also in accordance with previously reported studies. Our study advocated that metastatic ovarian tumors often appeared as a moderate enhancement versus primary epithelial ovarian cancers with a prominent enhancement.
DWI-MRI evaluation of ovarian tumors has yet been reported as a useful tool for differentiating malignant tumors from benign ovarian lesions [13–18]. However, there is no helpful role for distinguishing two types of malignant tumors by DWI-MRI. Moreover, there was no statistically significant difference between the two groups in terms of DWI signal of solid components. Besides, there was also no statistically significant difference between the two groups in terms of presence of ascites.
For other features, the patients of metastatic ovarian tumors were younger than those of primary epithelial ovarian cancers. This is possibly due to the younger population of some kinds of cancers recently. In one series of gastric cancers in young women (age < 36 years), 55 % had ovarian involvement [19]. Thus, in a woman with a known gastric carcinoma, the development of bilateral ovarian masses on imaging will be considered as highly likely to be secondary metastases rather than primary ovarian cancers. CA125 and HE4 are significantly higher in primary ovarian cancers than metastatic ovarian tumors. But, the level of CA125 and HE4 are not correlated to the size of the lesions, which is corresponded by Liao et al. [20].
There were some limitations in our study. First, a limited number of patients were studied. Therefore, more cases are needed to further investigate the value of these imaging features for diagnosing metastatic ovarian tumors. Second, the interreader variability was not evaluated. Third, a selection bias was inevitably appeared as the retrospective inherent nature of the study.
Conclusions
In conclusion, metastatic ovarian tumors seem to be smaller in size, more bilateral, more uniform in locules and more moderate enhancement in solid portions than those of primary ovarian cancers. Metastatic ovarian tumors were with less elevated levels of CA125 and HE4 in contrast with primary ovarian cancers. Although the sensitivity, specificity, and accuracy of any features are not sufficient for diagnoses, the combination of three key features (patients’ age, small size, and bilaterality) tends to have a high sensitivity, specificity, and accuracy for identifying metastatic ovarian tumors. The radiologist must depend on a combination of the imaging features as well as the clinical examinations in order to diagnose metastatic ovarian tumors.
Acknowledgments
This research is financially supported by Technology Commission of Shanghai Municipality (11JC1410500).
Published disclosure
The authors report no disclosure of interest in this work.
Footnotes
Yanhong Xu and Jia Yang contributed equally to this work.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
Guarantor of integrity of entire study, GFZ; study design or data acquisition or data interpretation, all authors; manuscript drafting or manuscript revision for important intellectual content, GFZ, YHX; approval of final version of submitted manuscript, all authors; literature research, YHX; clinical studies, GFZ, YHX; statistical analysis, YHX; and manuscript editing, YHX, JY. All authors read and approved the final manuscript.
References
- 1.Willmott F, Allouni KA, Rockall A. Radiological manifestations of metastasis to the ovary. J Clin Pathol. 2012;65:585–90. doi: 10.1136/jclinpath-2011-200390. [DOI] [PubMed] [Google Scholar]
- 2.Tanaka YO, Okada S, Satoh T, Matsumoto K, Oki A, Saida T, Yoshikawa H, Minami M. Diversity in size and signal intensity in multilocular cystic ovarian masses: new parameters for distinguishing metastatic from primary mucinous ovarian neoplasms. J Magn Reson Imaging. 2013;38:794–801. doi: 10.1002/jmri.24058. [DOI] [PubMed] [Google Scholar]
- 3.Pinto PB, Derchain SF, Andrade LA. Metastatic mucinous carcinomas in the ovary: a practical approach to diagnosis related to gross aspects and to immunohistochemical evaluation. Int J Gynecol Pathol. 2012;31:313–8. doi: 10.1097/PGP.0b013e31823f844d. [DOI] [PubMed] [Google Scholar]
- 4.Khunamornpong S, Suprasert P, Pojchamarnwiputh S, Na Chiangmai W, Settakorn J, Siriaunkgul S. Primary and metastatic mucinous adenocarcinomas of the ovary: Evaluation of the diagnostic approach using tumor size and laterality. Gynecol Oncol. 2006;101:152–7. doi: 10.1016/j.ygyno.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 5.Jung ES, Bae JH, Lee A, Choi YJ, Park JS, Lee KY. Mucinous adenocarcinoma involving the ovary: comparative evaluation of the classification algorithms using tumor size and laterality. J Korean Med Sci. 2010;25:220–5. doi: 10.3346/jkms.2010.25.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi HJ, Lee JH, Seo SS, Lee S, Kim SK, Kim JY, Lee JS, Park SY, Kim YH. Computed tomography findings of ovarian metastases from colon cancer: comparison with primary malignant ovarian tumors. J Comput Assist Tomogr. 2005;29:69–73. doi: 10.1097/01.rct.0000149958.86165.ca. [DOI] [PubMed] [Google Scholar]
- 7.Kim SHKW, Park KH, Lee JK, Kim JS. CT and MR findings of Krukenberg tumors: comparison with primary ovarian tumors. J Comput Assist Tomogr. 1996;20:393–8. doi: 10.1097/00004728-199605000-00013. [DOI] [PubMed] [Google Scholar]
- 8.La Fianza AAE, Pistorio A, Generoso P. Differential diagnosis of Krukenberg tumors using multivariate analysis. Tumori. 2002;88:284–7. doi: 10.1177/030089160208800408. [DOI] [PubMed] [Google Scholar]
- 9.Bazot M, Darai E, Nassar-Slaba J, Lafont C, Thomassin-Naggara I. Value of magnetic resonance imaging for the diagnosis of ovarian tumors: a review. J Comput Assist Tomogr. 2008;32:712–23. doi: 10.1097/RCT.0b013e31815881ef. [DOI] [PubMed] [Google Scholar]
- 10.Thomassin-Naggara I, Balvay D, Aubert E, Darai E, Rouzier R, Cuenod CA, Bazot M. Quantitative dynamic contrast-enhanced MR imaging analysis of complex adnexal masses: a preliminary study. Eur Radiol. 2012;22:738–45. doi: 10.1007/s00330-011-2329-6. [DOI] [PubMed] [Google Scholar]
- 11.Ma FH, Cai SQ, Qiang JW, Zhao SH, Zhang GF, Rao YM. MRI for differentiating primary fallopian tube carcinoma from epithelial ovarian cancer. J Magn Reson Imaging. 2014 doi: 10.1002/jmri.24740. [DOI] [PubMed] [Google Scholar]
- 12.Cai SQ, Ma FH, Qiang JW, Zhao SH, Zhang GF, Rao YM. Primary Fallopian Tube Carcinoma: Correlation Between Magnetic Resonance and Diffuse Weighted Imaging Characteristics and Histopathologic Findings. J Comput Assist Tomogr 2014, Published Online First. [DOI] [PubMed]
- 13.Zhang H, Zhang GF, Wang TP. Value of 3.0 T diffusion-weighted imaging in discriminating thecoma and fibrothecoma from other adnexal solid masses. J Ovarian Res. 2013;6:58. doi: 10.1186/1757-2215-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang H, Zhang GF, He ZY, Li ZY, Zhu M, Zhang GX. Evaluation of primary adnexal masses by 3T MRI: categorization with conventional MR imaging and diffusion-weighted imaging. J Ovarian Res. 2012;5:33. doi: 10.1186/1757-2215-5-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang H, Zhang GF, He ZY, Li ZY, Zhang GX. Prospective evaluation of 3T MRI findings for primary adnexal lesions and comparison with the final histological diagnosis. Arch Gynecol Obstet. 2014;289:357–64. doi: 10.1007/s00404-013-2990-x. [DOI] [PubMed] [Google Scholar]
- 16.Zhao SH, Qiang JW, Zhang GF, Ma FH, Cai SQ, Li HM, Wang L. Diffusion-weighted MR imaging for differentiating borderline from malignant epithelial tumours of the ovary: pathological correlation. Eur Radiol. 2014;24:2292–9. doi: 10.1007/s00330-014-3236-4. [DOI] [PubMed] [Google Scholar]
- 17.Zhao SH, Qiang JW, Zhang GF, Boyko OB, Wang SJ, Cai SQ, Wang L. MRI appearances of ovarian serous borderline tumor: pathological correlation. J Magn Reson Imaging. 2014;40:151–6. doi: 10.1002/jmri.24339. [DOI] [PubMed] [Google Scholar]
- 18.Ma FH, Zhao SH, Qiang JW, Zhang GF, Wang XZ, Wang L. MRI appearances of mucinous borderline ovarian tumors: pathological correlation. J Magn Reson Imaging. 2014;40:745–51. doi: 10.1002/jmri.24408. [DOI] [PubMed] [Google Scholar]
- 19.Sohaib SA, Sahdev A, Van Trappen P, Jacobs IJ, Reznek RH. Characterization of adnexal mass lesions on MR imaging. AJR Am J Roentgenol. 2003;180:1297–304. doi: 10.2214/ajr.180.5.1801297. [DOI] [PubMed] [Google Scholar]
- 20.Liao XY, Huang GJ, Gao C, Wang GH. A meta-analysis of serum cancer antigen 125 array for diagnosis of ovarian cancer in Chinese. J Cancer Res Ther. 2014;10(Suppl):C222–4. doi: 10.4103/0973-1482.145884. [DOI] [PubMed] [Google Scholar]


