Abstract
Coumarin derivatives are an important class of C6–C3 plant metabolites that show a variety of bioactivities. Currently, most clinical anticoagulant agents are coumarins, such as warfarin, dicoumarol and acenocoumarol, and patients taking these drugs must be monitored for adverse reactions. In a search for safe and effective anticoagulant compounds from Chinese herbal medicine, a screening procedure on the whole plant of Ainsliaea fragrans was performed. The phytochemical investigation of this plant afforded five new coumarin derivatives, including a pair of natural 4-hydroxycoumarin enantiomers (1), a pair of coumarin enantiomers with a rare polycyclic pyrano[3-2c] carbon skeleton (2) and a 7-hydroxycoumarin derivative (3), together with 5 known biogenetically related compounds (4–8). Enantioseparation of 1 and 2 produced optically pure compounds 1a, 1b, 2a and 2b. The absolute configurations of the new compounds were confirmed by single-crystal X-ray diffraction analysis. In addition, we evaluated the anticoagulant activity of all isolates via activated partial thromboplastin time (APTT), thrombin time (TT) and prothrombin time (PT) assays in vitro and in vivo. Of note, compound 3 displayed potent anticoagulant activity and no significant hepatic or renal toxicity, which could make it a promising agent for further preclinical evaluation for preventing abnormal blood clotting.
Coumarins are a well-known class of secondary metabolites in plants1,2,3 and fungi4,5,6 Owing to their structural features, coumarins are an important type of substrate in the areas of natural product modification and synthetic chemistry7,8,9. Among the various coumarin derivatives, 4-hydroxycoumarins, which have a special enol moiety, have shown particularly high activity in chemical synthesis and can act as potent metal ligands and starting material10,11. The C3-substituted 4-hydroxycoumarins in particular have attractive biological activities12, especially anticoagulant activity.
Cardio-cerebrovascular disease caused by thromboembolism poses a serious threat to human health. Coumarins are widely used in the clinic for antithrombotic therapy; for example, warfarin, which used to be a rodenticide, is now used as an anticoagulant13,14. However, the therapeutic use of coumarin agents is severely limited by their associated adverse reactions, such as platelet disease and haemorrhages.
In order to search for novel, highly efficient anticoagulant compounds with low toxicity from Chinese herbal medicine, a study was conducted on extracts of Ainsliaea fragrans Champ (Compositae).
Ainsliaea fragrans, also known as “xing-xiang-tu-er-feng”, is mainly distributed in the south of China15 and is still applied as a prescription medicine for treating chronic cervicitis. A few compounds have been reported to be found in this plant, including seven sesquiterpenoids16,17,18, six sesquiterpene glycosides19 and two phenolic compounds20,21. Herein, we report five new coumarin derivatives and five other known, biogenetically related coumarin derivatives isolated from this plant. Two pairs of new natural C3-substituted 4-hydroxycoumarin enantiomers 1 and 2 were enantioseparated successfully. A preliminary assay was carried out to evaluate the anticoagulant activity of all of the isolates.
Results and Discussion
This study is focused on identifying the structure of coumarin derivatives from Ainsliaea fragrans and investigating the anticoagulant activity of these isolates in vitro and in vivo.
Structural elucidation
Compound 1 was obtained as colourless needles. Its molecular formula, C20H26O4, was determined by HRESIMS at m/z 331.1894 [M + H+] [calcd. for C20H27O4+ m/z 331.1904], indicating eight degrees of unsaturation. The IR spectrum of 1 showed absorption bands assignable to a benzene group and a conjugated ester group (1601 and 1645 cm−1). The 1H NMR spectrum showed three aromatic olefinic protons at δH 7.01 (br d, J = 7.4 Hz, H-6), 7.34 (dd, J = 7.4, 8.0 Hz, H-7) and 7.24 (br d, J = 8.0 Hz, H-8); five methyl signals at δH 1.58 (s, H3-17), 1.67 (s, H3-18), 2.78(s, H3-21), 1.54 (d, J = 7.3 Hz, H3-20) and 1.50 (s, H3-19); and two methylene signals at δH 1.98 (m, H-13) and 2.26 (m, H-14). (Table 1) The 13C NMR and DEPT spectra revealed the presence of 20 carbon resonances, including one conjugated ester, ten olefinic carbons, five methyls, and two methylenes. The presence of a 1, 2, 3-trisubstituted phenyl moiety was supported by the NMR information as follows: δH 7.01 (H-6) to δC 128.8 (C-6), δH 7.34 (H-7) to δC 132.2 (C-7), and δH 7.24 (H-8) to δC 116.1 (C-8). A characteristic single peak (δH 2.78, δC 24.9) suggested an aromatic methyl. The 1D NMR data of 1 were similar to those of cyclobrachycoumarin22,23,24, which indicated that 1 was a 5-methylcoumarin derivative. Of the 20 carbon resonances, ten were typical of a 5-methylcoumarin moiety, whereas the additional ten carbons consisted of two isoprene moieties, as shown by HMBC correlations from H-11 to C-12/C-20/C-19, from H3-20 to C-11/C-3/C-12, from H3-19 to C-11/C-12/C-13, and from H3-18/H3-17 to C-16/C-15. The two isoprene moieties were connected by C-13 and C-14, as shown by the 1H-1H COSY spectrum vicinal couplings between H-14 (δ 2.26) and H-13 (δ 1.98). Furthermore, the 5-methylcoumarin moiety and the isoprene side-chain were connected at C-3, as shown by the HMBC correlations from H-11 to C-3/C-4 and from H3-20 to C-3/C-11. All of these signals suggested that 1 was a C-3 substituted 5-methylcoumarin. (Fig. 1)
Table 1. 1H and 13C NMR Data of Compounds 1, 2 and 3 a.
| 1b |
2c |
3c |
||||||
|---|---|---|---|---|---|---|---|---|
| NO | δH | δC | NO | δH | δC | NO | δH | δC |
| 1 | 1 | 1 | ||||||
| 2 | 166.6 | 2 | 163.2 | 2 | 162.9 | |||
| 3 | 108.8 | 3 | 103.5 | 3 | 6.23 (d, 9.5) | 113.0 | ||
| 4 | 165.5 | 4 | 161.9 | 4 | 7.85 (d, 9.5) | 146.4 | ||
| 5 | 139.1 | 5 | 137.2 | 5 | 7.29 (s) | 119.9 | ||
| 6 | 7.01 (d, 7.4) | 128.8 | 6 | 7.02 (d, 7.5) | 127.9 | 6 | 130.7 | |
| 7 | 7.34 (dd, 7.4, 8.0) | 132.2 | 7 | 7.34 (dd, 7.5, 8.0) | 131.7 | 7 | 155.1 | |
| 8 | 7.24 (d, 8.0) | 116.1 | 8 | 7.15 (d, 8.0) | 115.2 | 8 | 132.6 | |
| 9 | 155.6 | 9 | 154.1 | 9 | 149.2 | |||
| 10 | 117.9 | 10 | 114.5 | 10 | 115.6 | |||
| 11 | 3.99 (q, 7.3) | 41.2 | 11 | 2.69 (s) | 23.6 | 11 | 4.06 (s) | 61.3 |
| 12 | 77.3 | 12 | 123.3 | 1′ | ||||
| 13 | 1.97 (dd, 1.8, 6.8) 1.99 (dd, 1.8, 6.6) | 42.2 | 13 | 4.16 (d, 3.8) | 70.7 | 2′ | 4.41 (d, 6.0) | 93.3 |
| 14 | 2.26 (m, overlap) 2.26 (m, overlap) | 25.2 | 14 | 70.9 | 3′ | 5.37 (d, 6.0) | 72.5 | |
| 15 | 5.20 (ddd, 1.3, 7.0, 8.3) | 125.8 | 15 | 1.72 (ddd, 1.0, 2.9, 6.6, 13.5) 1.94 (ddd, 6.2, 9.8, 13.5) | 29.6 | 4′ | 73.1 | |
| 16 | 133.0 | 16 | 2.07 (ddd, 2.9, 6.2, 18.0) 2.33 (ddd, 6.6, 9.8, 18.0) | 22.3 | 5′ | 1.48 (s) | 26.5 | |
| 17 | 1.58 (s) | 18.8 | 17 | 132.0 | 6′ | 1.51 (s) | 27.1 | |
| 18 | 1.67 (s) | 27.0 | 18 | 82.1 | ||||
| 19 | 1.50 (s) | 26.7 | 19 | 1.59 (s) | 25.5 | |||
| 20 | 1.54 (d, 7.3) | 14.0 | 20 | 1.42 (s) | 24.8 | |||
| 21 | 2.78 (s) | 24.9 | 21 | 2.69 (s) | 23.6 | |||
aRecorded at 400 and 100 MHz for 1H and13C. J values (Hz) are shown in parentheses.
bSpectra obtained in Pyridine-d5.
cSpectra obtained in CD3OD.
Figure 1. Isolated compounds from extracts of Ainsliaea fragrans.
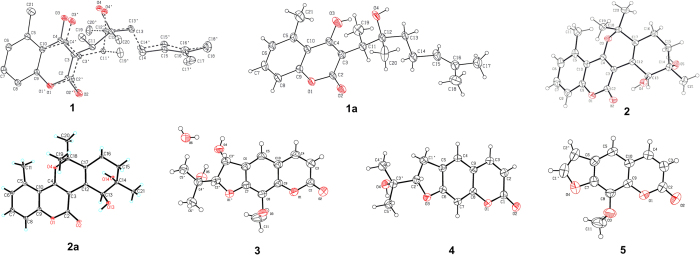
The relative configuration of the two ortho-position chiral carbon atoms (C-11 and C-12) could not be unambiguously determined by NOE correlations. After many attempts, a suitable crystal of 1 was obtained from a solvent system of CH2Cl2/MeOH/H2O (Fig. 2). However, the single-crystal X-ray diffraction experiment showed that compound 1 was a mixture of two enantiomers in a ratio of 62.7%:37.3%. Therefore, the planar structure of 1 was constructed as a natural C3-substituted 4-hydroxy coumarin.
Figure 2. ORTEP drawings of compounds 1, 1a, 2, 2a, 3, 4, and 5.
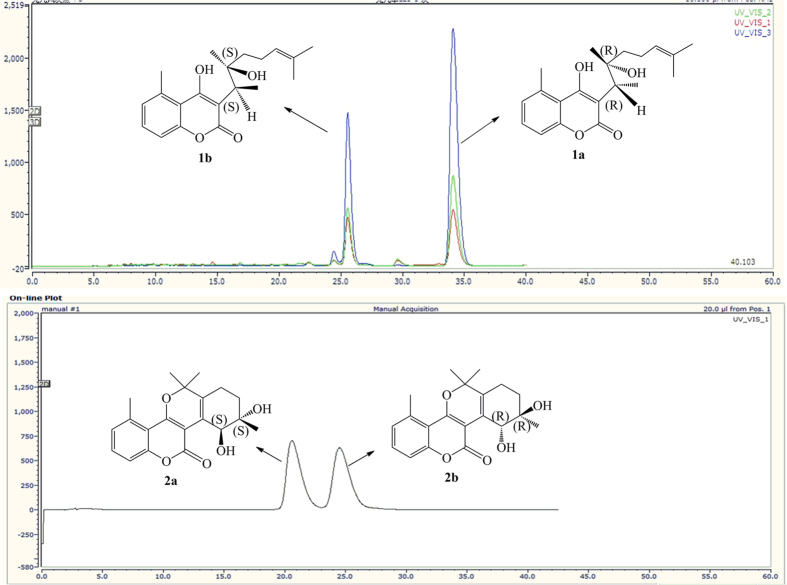
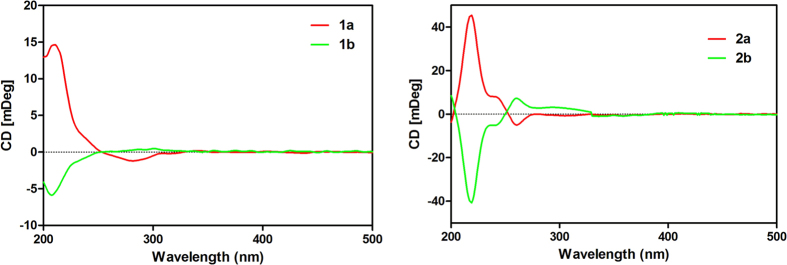
Chiral analysis and optical resolution of 1 were achieved by HPLC with a CHIRALPAK AS-H chiral column (n-hexane/i-PrOH, 95:5) at 0.8 ml/min, which afforded compounds 1a and 1b. The relative peak area ratio of 1a to 1b was approximately 2:1, consistent with the X-ray result (62.7%:37.3%). (Fig. 3) Similarly, a suitable crystal of 1a for the single-crystal X-ray diffraction experiment was obtained. Remarkably, the melting point of 1 was at 184 °C, but the melting points of 1a and 1b were increased to 191 °C and 190 °C, respectively. Finally, the absolute configuration of 1a was confirmed to be 11R, 12R, and the absolute configuration of 1b was confirmed as 11S, 12S, by combining the X-ray result of 1 and the CD spectrum of 1a (Fig. 4). Thus, the structures of 1a and 1b were assigned and named ainsliaeasin A1 and ainsliaeasin A2.
Figure 3. Enantioseparation of compounds 1 and 2 by chiral columns.
Figure 4. CD spectra of compounds 1a, 1b, 2a and 2b.
Compound 2 was obtained as colourless needles. Its formula C20H22O5 was determined by HRESIMS at m/z 343.1531 [M + H+] [calcd. for C20H23O5+ m/z 331.1540]. The IR bands at 1671.2 and 1605.9 suggested a benzene group and a conjugated ester group. Its 1H NMR spectrum (Table 1) showed three phenyl protons at δH 7.02 (br d, J = 7.5 Hz, H-6), 7.34 (dd, J = 7.5, 8.0 Hz, H-7), and 7.15 (br d, J = 8.0 Hz, H-8), which indicated a 1, 2, 3-substituted phenyl moiety. A characteristic methyl singlet (δH 2.69, δC 23.1) implied that 2 was also a 5-methylcoumarin. The 13C NMR of 2 showed 20 carbon resonances comprising a conjugated ester carbonyl, ten sp2 carbons, two quaternary sp3, two sp3 methine, one sp3 methylene, and four methyls. The 1H and 13C NMR spectra of 2 were extremely similar to those of gerberlin B25, as further supported by the 2D NMR spectroscopic spectra, including 1H-1H COSY, HSQC, and HMBC spectra. However, the specific rotatory and CD spectra of 2 could not be detected. To confirm the planar structure of 2, a single-crystal X-ray diffraction experiment was performed, which indicated that the space group of 2 was a mixture of two enantiomers.
Like 1, 2 was resolved to 2a and 2b using a chiral column (CHIRALPAK AD) under reverse phase conditions (Acetonitrile/H2O = 40/60) at 0.5 ml/min. The relative retention times for 2a and 2b were 20.8 min and 24.9 min, respectively. The relative peak areas of 2a and 2b were approximately 1:1, which was highly consistent with the X-ray result (ee 50%). In addition, the melting point of 2 was 208 °C, but the melting points of 2a and 2b increased to 214 °C and 213 °C, respectively. The successful X-ray diffraction with Cu-Kα, which resulted in a Flack parameter of 0.11(15), allowed an unambiguous assignment of the absolute configuration of 2a as 12S, 13S. Based on the X-ray of 2 and CD spectrum of 2a, the absolute configuration of compound 2b was confirmed as 12R, 13R. Finally, the structures of 2a and 2b were assigned and named ainsliaeasin B1 and ainsliaeasin B2.
Compound 3 was obtained as colourless needles. Its molecular formula C15H16O6 was determined by HRESIMS at m/z 293.1013 [M + H+] [calcd. for C15H17O6+ m/z 293.1020]. The UV spectrum exhibited λmax at 323 nm and 211 nm, suggesting the presence of a benzene conjugated system and an unsaturated ester moiety, as shown by IR bands at 1695.3, 1609.3, and 1578.1. The NMR spectrum of 3 was similar to that of 4 nodakenetin26, except for the presence of one more methoxy group and one more hydroxy group. This observation was supported by the HMBC data, which showed correlations of H3-11 (δH 4.06, s) to C-8 (δC 132.6) and H-2′ (δH 4.41, d) to C-3′ (δC 72.5). The hydroxy group was also observed in the 1H-1H COSY spectrum from H-2′ to H-3′ (δH 5.37, d). The absolute configuration of 3 was determined as 2′S and 3′R by X-ray diffraction with a Flack parameter of 0.09(12). As a result, the structure of 3 was assigned and named ainsliaeasin C.
To date, only two polycyclic pyrano [3-2c] coumarins (gerberlin A and gerberlin B) have been isolated from Gerbera saxatilis25, so 2 is the third example of this carbon skeleton. In nature, chiral natural products are usually produced in optically pure forms; however, occasionally, both enantiomers are formed27. In this study, the enantiomers of 1 and 2 were isolated then enantioseparated to obtain compounds 1a, 1b, 2a, and 2b.
Other compounds found included xanthotoxin (5)28, bothrioclinin (6)29, nodakenin (7)30, and gerberinside (8)31.
In vitro coagulation studies
All of the compounds isolated from this plant were analysed for their anticoagulant activities by monitoring the activated partial thromboplastin time (APTT), thrombin time (TT) and prothrombin time (PT). Coumarins usually interfere with the intrinsic coagulation process by inhibiting the vitamin K conversion cycle, but not with the extrinsic process32. Consistent with this finding, compound 4, without 3′-hydroxyl, and compound 5, without 2′-isopropyl, presented no anticoagulation effects. However, compound 3, with a hydroxyl group at the 3′-position, showed moderate anticoagulant activity in vitro by significantly increasing the PT, exceeding the full scale of the instrument. The other compounds were inactive according to these three parameters. (Table 2)
Table 2. PT, APTT, and TT of normal human platelet-poor plasma.
| Reference/Fraction/Compounds | PT | APTT | TT |
|---|---|---|---|
| Heparin (3U)a | _ _ _._**c | _ _ _._** | _ _ _._** |
| Normal salineb | 10.7 ± 0.02 | 25.8 ± 0.03 | 16.3 ± 0.04 |
| 1 (1 mg/ml) | 10.6 ± 0.02 | 26.1 ± 0.04 | 18.1 ± 0.03 |
| 2 (1 mg/ml) | 10.6 ± 0.00 | 25.7 ± 0.02 | 17.6 ± 0.04 |
| 3 (1 mg/ml) | _ _ _._** | 25.8 ± 0.05 | 17.3 ± 0.03 |
| 4 (1 mg/ml) | 10.8 ± 0.03 | 28.0 ± 0.06 | 17.8 ± 0.03 |
| 5 (1 mg/ml) | 10.7 ± 0.00 | 26.1 ± 0.04 | 17.3 ± 0.05 |
| 6 (1 mg/ml) | 10.6 ± 0.03 | 25.4 ± 0.02 | 17.1 ± 0.00 |
| 7 (1 mg/ml) | 10.7 ± 0.03 | 26.3 ± 0.04 | 17.9 ± 0.01 |
| 8 (1 mg/ml) | 11.0 ± 0.06 | 27.2 ± 0.07 | 17.7 ± 0.03 |
| 3 (2 mg/ml) | _ _ _._** | 26.9 ± 0.07 | 18.4 ± 0.03 |
| 3 (1 mg/ml) | _ _ _._** | 26.5 ± 0.05 | 18.4 ± 0.04 |
| 3 (0.5 mg/ml) | 17.4 ± 0.04* | 26.3 ± 0.03 | 17.7 ± 0.06 |
| 3 (0.25 mg/ml) | 11.3 ± 0.01 | 25.8 ± 0.08 | 17.9 ± 0.01 |
| 3 (0.125 mg/ml) | 11.2 ± 0.06 | 25.2 ± 0.05 | 17.3 ± 0.02 |
aPositive control
bnegative control (1% DMSO)
c_ _ _._ exceeded the full scale of the instrument.
All of the data are expressed as mean ± SD (n = 3). *P < 0.05,**P < 0.01, compared with the negative control group.
By comparing the structures of compounds 3, 4 and 5, it was concluded that the anticoagulant activity is closely related to the 3′-hydroxy and 2′-isopropyl moieties. Subsequently, compound 3 was found to exert anticoagulant activity at a minimum concentration of 1 mg/ml. (Table 2)
In vivo coagulation studies
To estimate the putative in vivo efficacy, we performed studies in Wistar rats to measure anticoagulant activity. Initially, the dose and sampling time of warfarin were tested on rats at 1 mg/kg, 0.5 mg/kg, and 0.2 mg/kg after 1 day, 2 days, and 3 days.
To evaluate the in vivo anticoagulant activity of compounds 1–8, the APTT, PT, and TT were determined on the third and fifth days after administration. After treating for 3 days, compound 7 markedly prolonged the TT (P < 0.01), whereas compound 8 extended it to an insignificant degree (P > 0.05). However, compounds 1, 2, 3, 4, 5, and 6 did not change the APTT, PT or TT on the third day compared with the negative control group. (Table 3)
Table 3. Effects of compounds 1–8 on the APTT, PT, and TT clotting assays.
| Groups | Dose (mg/kg) | 3 days later after the last administration |
5 days later after the last administration |
||||||
|---|---|---|---|---|---|---|---|---|---|
| n | APTT(S) | PT(S) | TT(S) | n | APTT(S) | PT(S) | TT(S) | ||
| CMC-Na | – | 0 | 18.8 ± 0.7 | 29.1 ± 5.1 | 77.4 ± 10.6 | 0 | 19.1 ± 0.5 | 30.1 ± 4.1 | 78.4 ± 9.7 |
| Warfarin | 0.2 | 1 | 28.7 ± 2.3*** | 53.7 ± 7.3*** | 79.6 ± 15.8 | 1 | 29.5 ± 2.2*** | 55.7 ± 6.6*** | 80.6 ± 14.7 |
| 1 | 1.0 | 1 | 19.9 ± 1.1 | 28.7 ± 3.5 | 87.1 ± 25.8 | 1 | 19.6 ± 2.4 | 26.5 ± 5.1 | 63.8 ± 6.3 |
| 2 | 1.0 | 0 | 18.4 ± 1.1 | 28.8 ± 6.9 | 80.4 ± 21.3 | 0 | 18.7 ± 1.0 | 30.0 ± 4.2 | 73.1 ± 10.1 |
| 3 | 1.0 | 0 | 17.5 ± 1.3 | 26.7 ± 4.2 | 75.5 ± 18.4 | 0 | 19.4 ± 1.6 | 41.2 ± 4.7*** | 128.5 ± 30.6** |
| 4 | 1.0 | 0 | 18.7 ± 1.2 | 30.1 ± 3.9 | 82.5 ± 12.7 | 1 | 18.9 ± 1.2 | 40.1 ± 8.0* | 210.2 ± 67.2* |
| 5 | 1.0 | 0 | 17.8 ± 0.7 | 29.3 ± 4.8 | 75.7 ± 11.6 | 0 | 18.4 ± 1.3 | 31.0 ± 6.1 | 87.0 ± 34.8 |
| 6 | 1.0 | 0 | 18.6 ± 1.1 | 28.0 ± 6.6 | 74.8 ± 25.0 | 0 | 18.3 ± 0.9 | 31.1 ± 3.8 | 143.5 ± 106.4 |
| 7 | 1.0 | 1 | 19.3 ± 0.6 | 31.8 ± 3.8 | 101.1 ± 12.9** | 0 | 17.9 ± 0.3 | 27.9 ± 2.4 | 72.9 ± 3.6 |
| 8 | 1.0 | 0 | 18.8 ± 1.3 | 29.1 ± 4.5 | 108.8 ± 54.7 | 1 | 18.2 ± 0.7 | 28.8 ± 4.5 | 75.9 ± 9.7 |
n = the number of death of rats caused by haemorrhage.
All of the data are expressed as mean ± SD (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001; compared with the negative control group.
To better characterize the anticoagulant profile, changes in the APTT, PT, and TT values were also determined on the fifth day. The PT and TT were prolonged in rats treated with compounds 3 and 4, whereas compounds 5 and 6 increased the TT value but not to a significant degree (P > 0.05). The TT values of group 7 and group 8 reached normal levels on the fifth day. (Table 3) The result showed that compound 3 exhibited anticoagulant activity at 1 mg/kg in Wistar rats.
Moreover, no death was found in rats treated with compound 3. However, one death caused by haemorrhage was observed in each of the groups treated with warfarin, compound 1 and compound 7 on the third day, and another death was observed in each of the groups treated with warfarin, compound 1, compound 4 and compound 8 on the fifth day. As a result, compound 3 was shown to be less toxic than the other compounds.
After the dissection of the rats on the seventh day, no significant liver or renal toxicity was observed in any of the groups of rats.
Conclusion
In this study, five new coumarins were isolated from Ainsliaea fragrans Champ. The chiral resolution of enantiomers (1 and 2) led to optically pure compounds 1a, 1b, 2a and 2b. Their planar structures and absolute configurations were determined by NMR, X-ray and CD analysis. Biologically, the anticoagulant activity of all of the isolates was evaluated. Compound 3 had anticoagulant activity both in vitro and in vivo. Additionally, compound 3 proved to be less toxic than warfarin and showed no significant liver or kidney toxicity. However, it is important to note that the results of the current study are preliminary, pending confirmation of the anticoagulant activity in vitro and in vivo. Further research is necessary to evaluate the action and mechanism of action of compound 3.
Methods
General
The melting point (uncorrected) was determined on an apparatus made by Beijing TECH INSTRUMENT CO. LTD. Optical rotations were measured with a Perkin Elmer spectropolarimeter. The UV spectra were measured on a VARIAN SARY 50 spectrophotometer. The IR spectra were recorded using a BRUKER VERTEX 70 spectrometer. The NMR experiments were run on a Bruker AM-400 spectrometer. The HRFABMS data were obtained on a VG 7070-HF spectrometer. Column chromatographic separations were carried out using silica gel H60 and ODS as packing materials. HSGF254 silica gel TLC plates were used for analytical TLC. The HPLC columns consisted of a Welch Material column (XB-C18, 10 μm, 10 × 250 mm), a normal phase chiral column (CHIRALPAK AS-H, 10 μm, 4.6 mm × 250 mm, part no. 20325), and a reversed phase chiral column (CHIRALPAK AD-RH, 10 μm, 4.6 mm × 150 mm, part no. 19724). The automatic coagulative instrument (Sysmex CA-7000), activated partial Thromborel S, Thrombin and Dade Actin Activated Cephaloplastin Reagent were commercial reagents from Siemens Healthcare Diagnostics Products GmbH. The semi-automatic biochemical analyser (MC-4000, Germany).
Plant material
The Ainsliaea fragrans Champ whole plants were collected from Shiyan City, Hubei Province, P. R. C., in 2013 and identified by Dr. Jian-Ping Wang, Tongji Medical Collage, Huazhong University of Science and Technology. A voucher specimen (No. 20130701) was deposited at Hubei Key Laboratory of Natural Medicinal Chemistry and Resource Evaluation, School of Pharmacy, Tongji Medical College, Huazhong University of Science and Technology.
Extraction and isolation
The whole plant of Ainsliaea fragrans (20 kg) was percolated with 95% industrial ethanol at room temperature. The filtrate was concentrated in vacuo. The residue was partitioned with petroleum ether (200 g), EtOAc (120 g), and n-BuOH (150 g), successively.
The petroleum ether portion was subjected to column chromatography over silica gel eluted with a petroleum-acetone gradient to afford five fractions (A–E). Fraction B was separated into three subfractions, B1, B2, and B3. Compound 1 (15.0 mg, tR 46.2 min) was separated from B2 by semipreparative HPLC (80:20 MeOH–H2O, 230 nm, 2.0 ml/min), while 1a and 1b were obtained from a chiral column (CHIRALPAK AS-H) under normal phase conditions (n-hexane : isopropanol = 9:1) at 0.5 ml/min using a UV detector at 230 nm, 254 nm and 210 nm; the relative retention times of 1a and 1b were 34.3 min and 25.5 min, respectively. Compound 5 (50 g) was recrystallized from fraction C, while compound 6 (15 g) was recrystallized from fraction D.
The EtOAc portion was subjected to column chromatography over silica gel eluted with a gradient system of CH3Cl–MeOH (100:1–1:100) to give five fractions F–J. Fraction F was separated into three subfractions, F1A, F1B and F1C, by Sephadex LH-20 using MeOH. Fraction F1B was also separated into two subfractions, F1BA and F1BB, by silica gel using a system of MeOH–CHCl3 (40:1–30:1). Compound 7 (4.0 mg) was separated from fraction F1BA on silica gel using a system of MeOH–CHCl3 (30:1–20:1). Similarly, compounds 3 (11.3 mg) and 4 (25.6 mg) were separated from fraction G. Compound 2 (19 mg) was separated from fraction H on silica gel using a system of MeOH–CHCl3 (20:1–10:1), and compounds 2a (1.2 mg) and 2b (1.1 mg) were separated on a chiral column (CHIRALPAK AD) under reverse phase conditions (acetonitrile : H2O = 40 : 60) at 0.5 ml/min using a UV detector at 230 nm; the relative retention times were 20.8 min and 24.9 min, respectively.
The n-BuOH portion was separated using a silica gel column eluted with a CH3Cl-MeOH gradient to afford four fractions to obtain 7 (22 mg) and 8 (180 mg).
Spectroscopic data of the isolated compounds
Compound 1: colourless needles; m.p. 184.0–185.0 °C; 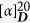 + 1.0 (c, 0.6, MeOH); UV (MeOH) νmax (log ε) 208 (3.52) nm, 296 (4.52) nm; IR(KBr) νmax 3222.1, 2923, 1644.8, 1600.9, 1563.7, 1332.2, 784.5, 744.8; 1H (Py-d5, 400 MHz) and 13C(Py-d5, 100 MHz) NMR data, see Table 1; HRESIMS m/z 331.1849 [M + H+] [calcd. for C20H27O4+ m/z 331.1904].
+ 1.0 (c, 0.6, MeOH); UV (MeOH) νmax (log ε) 208 (3.52) nm, 296 (4.52) nm; IR(KBr) νmax 3222.1, 2923, 1644.8, 1600.9, 1563.7, 1332.2, 784.5, 744.8; 1H (Py-d5, 400 MHz) and 13C(Py-d5, 100 MHz) NMR data, see Table 1; HRESIMS m/z 331.1849 [M + H+] [calcd. for C20H27O4+ m/z 331.1904].
Compound 1a: colourless needles; m.p. 191.0–192.0 °C; 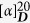 + 18.0 (c, 0. 3, MeOH); CD (CH3OH, 3.0 mM) λextr. (θ [mdeg cm2 dmol−1]) 282 (−1.2), 210 (+14.6).
+ 18.0 (c, 0. 3, MeOH); CD (CH3OH, 3.0 mM) λextr. (θ [mdeg cm2 dmol−1]) 282 (−1.2), 210 (+14.6).
Compound 1b: colourless tiny needles; m.p. 190.0–191.0 °C; 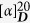 − 12.0 (c, 0.3, MeOH); CD (CH3OH, 1.0 mM) λextr. (θ [mdeg cm2 dmol−1]) 301 (+0.5), 208 (−5.9).
− 12.0 (c, 0.3, MeOH); CD (CH3OH, 1.0 mM) λextr. (θ [mdeg cm2 dmol−1]) 301 (+0.5), 208 (−5.9).
Compound 2: colourless needles; m.p. 208.0 °C; 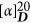 0 (c, 0.8, MeOH); UV (MeOH) νmax (log ε) 214 (3.46) nm, 265 (3.44) nm, 342 (4.40) nm; IR(KBr) νmax 3425.6, 2921.1, 1671.1, 1605.8, 1584.9, 1368.7, 1009.0, 786.7, 740.3; 1H (CDCl3, 400 MHz) and 13C(CDCl3, 100 MHz) NMR data, see Table 1; HRESIMS m/z 343.1531 [M + H+] [calcd. for C20H23O5+ m/z 331.1540].
0 (c, 0.8, MeOH); UV (MeOH) νmax (log ε) 214 (3.46) nm, 265 (3.44) nm, 342 (4.40) nm; IR(KBr) νmax 3425.6, 2921.1, 1671.1, 1605.8, 1584.9, 1368.7, 1009.0, 786.7, 740.3; 1H (CDCl3, 400 MHz) and 13C(CDCl3, 100 MHz) NMR data, see Table 1; HRESIMS m/z 343.1531 [M + H+] [calcd. for C20H23O5+ m/z 331.1540].
Compound 2a: colourless needles; m.p. 214.0 °C; 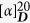 + 22 (c, 0.5, MeOH); CD (CH3OH, 3.0 mM) λextr. (θ [mdeg cm2 dmol−1]) 261 (−4.8), 240 (+8.5), 218 (+45.1).
+ 22 (c, 0.5, MeOH); CD (CH3OH, 3.0 mM) λextr. (θ [mdeg cm2 dmol−1]) 261 (−4.8), 240 (+8.5), 218 (+45.1).
Compound 2b: colourless needles; m.p. 213.0 °C; 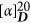 − 20 (c, 0.5, MeOH); CD (CH3OH, 3.0 mM) λextr. (θ [mdeg cm2 dmol−1]) 256 (+7.8), 240 (−4.5), 218 (−39.5).
− 20 (c, 0.5, MeOH); CD (CH3OH, 3.0 mM) λextr. (θ [mdeg cm2 dmol−1]) 256 (+7.8), 240 (−4.5), 218 (−39.5).
Compound 3: colourless needles; m.p. 106.0–107.0 °C; 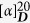 − 4.0 (c, 0.3, MeOH); UV (MeOH) νmax (log ε) 211 (3.77) nm, 323 (4.70) nm; CD (CH3OH, 3.0 mM) λextr. (θ [mdeg cm2 dmol−1]) 301 (+7.5), 256 (−3.8), 228 (−19.5), 207 (+32.8); IR(KBr) νmax 3425.6, 2921.9, 1695.3, 1609.3, 1576.1, 1407.9, 1151.1, 775.5; 1H(CD3OD, 400 MHz) and 13C(CD3OD, 100 MHz) NMR data, see Table 1. HRESIMS m/z 293.1013 [M + H+] [calcd. for C20H23O5+ m/z 293.1020].
− 4.0 (c, 0.3, MeOH); UV (MeOH) νmax (log ε) 211 (3.77) nm, 323 (4.70) nm; CD (CH3OH, 3.0 mM) λextr. (θ [mdeg cm2 dmol−1]) 301 (+7.5), 256 (−3.8), 228 (−19.5), 207 (+32.8); IR(KBr) νmax 3425.6, 2921.9, 1695.3, 1609.3, 1576.1, 1407.9, 1151.1, 775.5; 1H(CD3OD, 400 MHz) and 13C(CD3OD, 100 MHz) NMR data, see Table 1. HRESIMS m/z 293.1013 [M + H+] [calcd. for C20H23O5+ m/z 293.1020].
Single-Crystal X-ray Diffraction Analysis and Crystallographic Data of Compounds 1, 1a, 2, 2a, 3, 4, and 5.
Diffraction intensity data for compounds 1, 1a, 2, 2a, 3, 4, and 5 were acquired on a Bruker APEX-II diffractometer employing graphite-monochromatized Cu Kα radiation (λ = 1.54178 Å) at 298(2) K or Mo Kα radiation (λ = 0.71073 Å) at 298(2) K. The data were collected by Bruker APEX2 software and reduced with Bruker SAINT. Structure solution and refinement were performed with the SHELXTL program package. All of the non-hydrogen atoms were refined anisotropically. The hydrogen atom positions were geometrically idealized and allowed to ride on their parent atoms. The crystal structures of compounds 1, 1a, 2, 2a, 3, 4, and 5 were drawn by ORTEP 3 for Windows (version 2.02). All of the data can be obtained free of charge from the CCDC via http://www.ccdc.cam.ac.uk/Community/Requestastructure/Pages/DataRequest.aspx.
Crystal data for 1: colourless needles, C20H26O4; MW = 330.41; Cu Kα (λ = 1.54178 Å); temperature = 296 (2) K; triclinic; space group P-1; a = 7.2247(2) Å, b = 7.6916(2) Å, c = 16.5807 (3) Å, α = 79.1020(10)°, β = 82.8350(10)°, γ = 89.8210 (10)°; V = 897.50(4) Å3, Z = 2, Dcalcd = 1.223 mg/m3, crystal size 0.20 × 0.10 × 0.10 mm3, Final R indices: R1 = 0.0482, wR2 = 0.1293; reflections collected: 13646. (CCDC No. 1028802).
Crystal data for 1a: colourless needles, C20H26O4; MW = 330.41; Cu Kα (λ = 1.54178 Å); Temperature = 296 (2) K; triclinic; space group P1; a = 7.2264(2) Å, b = 7.6852(2) Å, c = 16.5860(3) Å, α = 100.8970(10)°, β = 96.9830(10)°, γ = 90.1240(10)°; V = 897.51(4) Å3, Z = 2, Dcalcd = 1.223 mg/m3, crystal size 0.20 × 0.10 × 0.10 mm3, Final R indices: R1 = 0.0681, wR2 = 0.1914; reflections collected: 19991; Flack parameter = 0.3 (3). (CCDC No. 981339).
Crystal data for 2: colourless needles, C20H22O5; MW = 342.38; Cu Kα (λ = 1.54178 Å); temperature = 298 (2) K; monoclinic; space group P2(1)/c; a = 5.65620(10) Å, b = 13.7286 (2) Å, c = 21.7344(4) Å, α = 90°, β = 91.2250(10)°, γ = 90°; V = 1687.33 (5) Å3, Z = 4, Dcalcd = 1.348 mg/m3, crystal size 0.30 × 0.30 × 0.20 mm3, Final R indices: R1 = 0.0352, wR2 = 0.0949; reflections collected: 36236. (CCDC No. 1028800).
Crystal data for 2a: colourless needles, C20H22O5; MW = 342.38; Cu Kα (λ = 1.54178 Å); temperature = 100 (2) K; orthorhombic; space group P21212; a = 13.7242(3) Å, b = 22.2290(5) Å, c = 5.5733(10) Å, α = 90°, β = 90°, γ = 90°; V = 1700.28 (6) Å3, Z = 4, Dcalcd = 1.337 mg/m3, crystal size 1.60 × 0.18 × 0.11 mm3, Final R indices: R1 = 0.0376, wR2 = 0.1143; reflections collected: 12603; Flack parameter = 0.11(15). (CCDC No. 1028801).
Crystal data for 3: colourless needles, C15H18O7; MW = 310.29; Cu Kα (λ = 1.54178 Å); temperature = 196 (2) K; monoclinic; space group P2(1); a = 5.5649(10) Å, b = 12.7775(3) Å, c = 10.2937(2) Å, α = 90°, β = 93.9340(10)°, γ = 90°; V = 730.21 (3) Å3, Z = 2, Dcalcd = 1.411 mg/m3, crystal size 0.12 × 0.12 × 0.10 mm3, Final R indices: R1 = 0.0265, wR2 = 0.0734; reflections collected: 22485; flack parameter = 0.09(12). (CCDC No. 981338).
Crystal data for 4 (CCDC No. 981340) and 5 (CCDC No. 981341), see supporting information.
Anticoagulant activity assay in vitro 33
Assays were performed for each sample using an automatic coagulative instrument (Sysmex CA-7000 System) according to the instructions provided by the biological reagent provider (Siemens Healthcare Diagnostics Products Gmbh).
Fresh whole blood (50 ml), collected in sodium citrate coagulation test tubes, was donated by the first author of this paper, which was approved by the ethics committee of Puai Hospital. After centrifugation (3000 rpm, 8 min), the supernatants (270 μL) were divided into containers. Compounds 1–8 were diluted for use with normal saline (with 1% DMSO) at 1 mg/ml. Then, all of the isolates (30 μL) were added into the plasma sequentially. After incubation in a 37 °C thermostatic water bath for 10 minutes, all of the samples were analysed with the automatic coagulative instrument, which had been supplied with activated partial Thromborel S, Throbin, Dade Actin Activated Cephaloplastin Reagent (Siemens Healthcare Diagnostics Products Gmbh) and calcium-chloride solutions. All of the tests were performed in an automated environment.
Anticoagulant activity assay in vivo 34
The methods were carried out in Wistar rats accordance the European Community guidelines for the use of experimental animals and all experimental protocols were approved by the ethics committee of Puai Hospital. Rats were kept in polyethylene cages with wood shavings as bedding and maintained in a temperature controlled room at 20 ± 1 °C with a 12/12 h lighting schedule (lights on at 08:00 h, off at 20:00 h) and a relative humidity of 50% for at least 2 weeks prior to use.
All of the experiments were performed using adult male Wistar rats (250–300 g, body wt, Institute of Laboratory Animals of Sichuan Academy of Medical Sciences, SCXK 2013-24.) The animals were grouped and housed with seven per cage/group. The warfarin (0.2 mg/kg) (WUHAN XIANGHESHUNDA FINE CHEMICAL CO. LTD, XH20150206) and the tested compounds (1 mg/kg) were dissolved in sodium carboxymethyl cellulose and administered to animals by gavage for three days. Citrated blood was collected from the eye socket on the third day and the fifth day.
Platelet-poor plasma was prepared by centrifugation for measuring the APTT, PT, and TT on a semi-automatic biochemical analyser (MC-4000, Germany). All of the data are expressed in relative fold values, compared with the values obtained with the control group. The data were tested for statistical significance by nonparametric two-tailed Mann-Whitney test using the SPSS 17.0 software. A value of P < 0.05 was considered significant.
Additional Information
How to cite this article: Lei, L. et al. Coumarin derivatives from Ainsliaea fragrans and their anticoagulant activity. Sci. Rep. 5, 13544; doi: 10.1038/srep13544 (2015).
Supplementary Material
Acknowledgments
The authors thank the Clinical Laboratory of Puai Hospital for providing the detecting instruments for the anticoagulant activity assay. We also thank the Analysis and Measurement Centre at HUST for the IR, MS and ECD analyses. This work was financially supported by the National Science and Technology Project of China (Nos. 2011ZX09102-004 and 2013ZX09103001-020) and Health and Family Planning Commission of Wuhan Municiplity (WZ15B06).
Footnotes
Author Contributions Y.H.Z. and H.P.S. designed the experimental scheme; L.L., S.S.P. and Y.B.X. carried out the phytochemical study and identification of compounds; J.W.Z., G.Z. and J.P.W. collected and identified the plant; Z.L. and Y.Z. carried out the anticoagulant activity assay in vitro; R.F. and Y.H. carried out the anticoagulant activity assay in vivo; G.M.Y. and W.L.Z. revised the manuscript.
References
- Murray R. D. H. Coumarins. Nat. Prod. Rep. 6, 591–624 (1989). [DOI] [PubMed] [Google Scholar]
- Murray R. D. H. Coumarins. Nat. Prod. Rep. 6, 477–505 (1995). [DOI] [PubMed] [Google Scholar]
- Estévez-Braun A. & González G. A. Coumarins. Nat. Prod. Rep. 5, 465–475 (1997). [DOI] [PubMed] [Google Scholar]
- Yang X. L., Awakawa T., Wakimoto T. & Abe I. Induced production of novel prenyldepside and coumarins in endophytic fungi Pestalotiopsis acacia. Tetrahedron Lett. 54, 5814–5817 (2013). [Google Scholar]
- Xu J. et al. Cytosporones, coumarins, and an alkaloid from the endophytic fungus Pestalotiopsis sp. isolated from the Chinese mangrove plant Rhizophora mucronata. Bioorg. Med. Chem. 17, 7362–7367 (2009). [DOI] [PubMed] [Google Scholar]
- Sandjo L. P. et al. Coumarin derivatives from Pedilanthus tithymaloides as inhibitors of conidial germination in Magnaporthe oryzae. Tetrahedron Lett. 53, 2153–2156 (2012). [Google Scholar]
- Khan A. T., Das D. K., Islam K. & Das P. A simple and expedient synthesis of functionalized pyrido[2, 3-c] coumarin derivatives using molecular iodine catalyzed three-component reaction. Tetrahedron Lett. 53, 6418–6422 (2012). [Google Scholar]
- Moshkin V. S., Sosnovskikh V. Y. & Röschenthaler G. V. Synthesis of benzopyranopyrrolidines via 1, 3-dipolar cycloaddition of nonstabilized azomethine ylides with 3-substituted coumarins. Tetrahedron 69, 5884–5892 (2013). [Google Scholar]
- Biswas B., Sen P. K. & Venkateswaran R. V. Bargellini condensation of coumarins. Expeditious route to o-carboxyvinylphenoxyisobutyric acids and application to the synthesis of sesquiterpenes helianane, heliannuol A and heliannuol C. Tetrahedron 63, 12026–12036 (2007). [Google Scholar]
- Rodríguez S. A., Nazareno M. A. & Baumgartner M. T. Effect of different C3-aryl substituents on the antioxidant activity of 4-hydroxycoumarin derivatives. Bioorg. Med. Chem. 19, 6233–6238 (2011). [DOI] [PubMed] [Google Scholar]
- Rajale T. et al. An efficient synthesis of 4-substituted coumarin derivatives via a palladium-catalyzed Suzuki cross-coupling reaction. Tetrahedron Lett. 55, 6627–6630 (2014). [Google Scholar]
- Yakushiji F. et al. Palladium-catalyzed C3-selective mono-arylation of 4-hydroxycoumarin. Tetrahedron Lett. 55, 3316–3318 (2014). [Google Scholar]
- Hayes W. J. & Gaines T. B. Control of Norway Rats With Residual Rodenticide Warfarin. Public Health Reports 65, 1537–1555 (1950). [PMC free article] [PubMed] [Google Scholar]
- Wadelius M. & Pirmohamed M. Pharmacogenetics of warfarin: current status and future challenges J. Pharmacogenomics 7, 99–111 (2007). [DOI] [PubMed] [Google Scholar]
- State Administration of Traditional Chinese Medicine, Chinese Materia Medica, Shanghai, Shanghai Scientific and Technical Publishers 6682 (1999).
- Bohlmann F. & Chen Z. L. Naturally occurring terpene derivatives. Part 426. Guaianolides from Ainsliaea fragrans. Phytochemistry 21, 2120–2122 (1982). [Google Scholar]
- Li X. S., Liu J. N. & Cai P. L. Complete 1H and 13C data assignments of two new guaianolides isolated from Ainsliaea fragrans. Magn. Reson. Chem. 46, 1070–1073 (2008). [DOI] [PubMed] [Google Scholar]
- Feng F., Chen M. H., Xing C. X., Liu W. Y. & Xie N. Two novel sesquiterpenoids from Ainsliaea fragrans Champ. J. Asian Nat. Prod. Res. 11, 856–860 (2009). [DOI] [PubMed] [Google Scholar]
- Wang H. et al. Sesquiterpenes from Ainsliaea fragrans and their inhibitory activities against cyclooxygenases-1 and 2. Chem. Pharm. Bull. 57, 597–599 (2009). [DOI] [PubMed] [Google Scholar]
- Wang Y. F. & Liu B. Preparative Isolation and Purification of Dicaffeoylquinic Acids from the Ainsliaea fragrans Champ by High-speed Counter-current Chromatography. Phytochem. Anal. 18, 436–440 (2007). [DOI] [PubMed] [Google Scholar]
- Su D., Huang J., Song Y. G. & Feng Y. L. Comparative pharmacokinetics and tissue distribution study of mono- and di- caffeoylquinic acids isomers of Ainsliaea fragrans Champ by a fast UHPLC–MS/MS method. Fitoterapia 99, 139–152 (2014). [DOI] [PubMed] [Google Scholar]
- Zdero C., Bohlmann F., King R. M. & Robinson H. Further 5-Methyl coumarins and other constituents from the subtribe mutisiinae. Phytochemistry 25, 509–516 (1986). [Google Scholar]
- Bohlmann F. & Steinmeyer A. Synthesis of brachycoumarin and cyclobrachycoumarin. Tetrahedron Lett. 27, 5359–5362 (1986). [Google Scholar]
- Lee Y. R., Kim B. S. & Wang H. C. Silver(I)/celite promoted oxidative cycloaddition of 4-hydroxycoumarin to olefins. A facile synthesis of dihydrofurocoumarins and furocoumarins. Tetrahedron 54, 12215–12222 (1998). [Google Scholar]
- Qiang Y., Chen Y. J., Li Y. & Gao K. Coumarin Derivatives from Gerbera saxatilis. Planta Med. 77, 175–178 (2011). [DOI] [PubMed] [Google Scholar]
- Ishii H., Sekiguchi F. & Ishikawa T. Studies on the chemical constituents of Rutaceous plants. XLI. Absolute configuration of rutaretin methyl ether. Tetrahedron 37, 285–290 (1981). [Google Scholar]
- Finefield J. M., Sherman D. H., Kreitman M. & Williams R. M. Enantiomeric Natural Products: Occurrence and Biogenesis. Angew. Chem. Int. Ed. 51, 4802–4836 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlmann F. & Zdero C. Neue 5-alkylcumarine und chromone aus Bothriocline laxa. Phytochemistry 16, 1261–1263 (1977). [Google Scholar]
- Razdan T. K., Qadri B., Harkar S. & Waight E. S. Chromones and coumarins from Skimmia laureola. Phytochemistry 26, 2063–2069 (1987). [Google Scholar]
- Gu Z. M., Zhang D. X., Yang X. W., Hattori M. & Namba T. Isolation of Two New coumarin Glycosides from Notopterygium froibesii and Evaluation of a Chinese Crude Drug, Qiang-Huo, the Underground Parts of N. incisum and N. Forbesii, by High-Performance Liquid Chromatography. Chem. Pharm. Bull. 38, 2498–2502 (1990). [DOI] [PubMed] [Google Scholar]
- Cesari I. et al. Extensive phytochemical investigation of the polar constituents of Diospyros bipindensis Gürke traditionally used by Baka pygmies. Phytochemistry 96, 279–287 (2013). [DOI] [PubMed] [Google Scholar]
- Hirsh J. et al. Oral Anticoagulants: Mechanism of Action, Clinical Effectiveness, and Optimal Therapeutic Range. Chest. 119, 8S–21S (2001). [DOI] [PubMed] [Google Scholar]
- Dietrich K., Stang L., Ryn van J. & Mitchell L. G. Assessing the anticoagulant effect of dabigatran in children: An in vitro study. Thromb. Res. 135, 630–635 (2015). [DOI] [PubMed] [Google Scholar]
- Correia-da-Silva M. et al. Flavonoids with an Oligopolysulfated Moiety: A New Class of Anticoagulant Agents. J. Med. Chem. 54, 95–106 (2011). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.