Abstract
We investigated the role of Drosophila larva olfactory system in identification of congeners and aliens. We discuss the importance of these activities in larva navigation across substrates, and the implications for allocation of space and food among species of similar ecologies. Wild type larvae of cosmopolitan D. melanogaster and endemic D. pavani, which cohabit the same breeding sites, used species-specific volatiles to identify conspecifics and aliens moving toward larvae of their species. D. gaucha larvae, a sibling species of D. pavani that is ecologically isolated from D. melanogaster, did not respond to melanogaster odor cues. Similar to D. pavani larvae, the navigation of pavani female x gaucha male hybrids was influenced by conspecific and alien odors, whereas gaucha female x pavani male hybrid larvae exhibited behavior similar to the D. gaucha parent. The two sibling species exhibited substantial evolutionary divergence in processing the odor inputs necessary to identify conspecifics. Orco (Or83b) mutant larvae of D. melanogaster, which exhibit a loss of sense of smell, did not distinguish conspecific from alien larvae, instead moving across the substrate. Syn 97CS and rut larvae of D. melanogaster, which are unable to learn but can smell, moved across the substrate as well. The Orco (Or83b), Syn 97CS and rut loci are necessary to orient navigation by D. melanogaster larvae. Individuals of the Trana strain of D. melanogaster did not respond to conspecific and alien larval volatiles and therefore navigated randomly across the substrate. By contrast, larvae of the Til-Til strain used larval volatiles to orient their movement. Natural populations of D. melanogaster may exhibit differences in identification of conspecific and alien larvae. Larval locomotion was not affected by the volatiles.
Introduction
The identification of congeners and aliens is an essential capability enabling animals to efficiently use ecological resources [1]. In species as diverse as insects, mice and humans, behaviors linked with recognition of individuals have evolved toward the formation of societies to improve access to ecological resources and increase individual fitness [2, 3]. In Drosophila, most studies on identification of individuals have focused on adults due to the importance of recognition for mating and reproduction [4]. How Drosophila larvae distinguish and navigate toward congeners merits particular attention because of the significance of these behaviors in the allocation of space and food among species with similar ecologies and their consequences for the expansion and evolution of populations [5–9]. Therefore, studies of species-specific cues that guide and orient the movement of Drosophila larvae in the wild are of great importance.
Drosophila larvae develop in dynamic and variable environments. Moreover, the gradual desiccation of Drosophila breeding sites is a selective pressure that acts on larval behavior [7, 10, 11]. The process of decay in fruit where a number of Drosophila species live produces volatiles as alcohols, esters and fatty acids that surround the larvae [12]. The fruit itself, the microorganisms responsible for fermentation and conspecific and alien larvae also emit odors [13, 14]. The proportions of these compounds change over time [15]. To live in these changing environments larvae are equipped with highly sophisticated olfactory and gustatory receptors and brain structures that respond to a variety of stimuli [16, 17], indicating their ecological importance for the larvae. Detailed information is available on the cellular biology, genetics and development of the structure and functioning of the olfactory and gustatory systems of Drosophila larva [18]. However, the role of those structurally complex sensorial systems in the ecology of Drosophila at breeding sites is unclear because very little is known regarding Drosophila larva routines in the wild [19]. Here we examine the role of Drosophila larva olfactory system in the identification of congeners and aliens and in navigation across substrates. Our study may contribute to an understanding of neurobiology, genetics, ecology and evolution of Drosophil+a larval behavior.
We hypothesized that identification at distance of conspecifics and aliens by Drosophila larvae might be based on species-specific volatiles [6]. As these olfactory cues are processed by the nervous system, the larvae would navigate toward congeners, avoiding larvae of other species and grouping in proximity to the conspecific source of volatile emissions. This function might be detected under laboratory conditions in which third-instar larvae searching for pupation sites are incited to distribute themselves in space according larval odors of the same and other species. We conducted this essay under illuminated conditions. Thus, we presumed that larvae used primarily chemosensory cues to explore the environment. To verify this assumption, we tested Orco (Or83b) mutant larvae of D. melanogaster, which are unable to smell but can taste and see [17].
We also reasoned that the relatively short duration of the breeding sites caused principally by loss of water [20], could make it essential for the larvae to be able to rapidly scrutinize the fruit. The larvae could save time associating conspecific larval odors with appropriate places to feed and pupate. This behavior could be amalgamated with the detection of alien larval odors. Such odors might indicate locations colonized by other species. We tested these hypotheses by using mutant larvae of D. melanogaster that are unable to learn but can smell and see (Syn 97CS and rut larvae) [17, 21–26].
We also addressed the larval olfactory responses of the endemic sibling species Drosophila pavani and Drosophila gaucha. Although larvae of D. melanogaster and D. pavani cohabit on the same fruits, the larvae of D. gaucha are ecologically isolated from these two species [6]. We tested the response of larvae of the two sibling species to D. melanogaster larval odors. We also investigated the olfactory responses of D. pavani x D. gaucha reciprocal hybrids to conspecific and D. melanogaster larval odors. These studies can provide valuable insight into the olfactory world of Drosophila.
Materials and Methods
Collection of decaying fruits and larval behavior in the wild
We observed the activities of Drosophila larvae on a variety of decaying substrates (grape, apple, pear, peach, prickly pear and cactus cladode tissue (Opuntia ficus-indica) in Til-Til, 33°05’00”S, and Trana, 35° 52’ 00” S, Central Valley of Chile. To substantiate that larvae of several species cohabit in the same fruits, we randomly collected decaying fruits (N = 502 fruits), and taxonomically identifying the adults as they emerged from each of the fruits.
Subjects
We tested wild-type larvae of natural Chilean populations (Til-Til and Trana strains) and laboratory stocks (Oregon R-c and Canton–Special strains) of D. melanogaster. We also examined larvae of the vestigial (vg) strain. The vg strain and the Oregon R-c strain differ in certain larval behaviors. For example, Oregon R-c larvae dig deeper into the substratum than vg larvae [27]. We also tested three neurological mutants derived from the Canton-Special (CS) strain of D. melanogaster. We reasoned that the olfactory-mediated behaviors require normal olfactory receptor functioning. The larval perception of odorants in the Orco (Or83b) mutant strain is blocked because the dendritic localization of the receptors is lost [17, 21]. Thus, the Orco mutation disrupts behavioral and electrophysiological responses to many odorants [17]. To obtain clues regarding social larval odor-based learning, we tested the Syn 97CS and rut learning mutant larvae. The Syn 97CS mutation affects presynaptic vesicle release in the entire larval brain, and olfactory associative learning is reduced in approximately 50% of these larvae compared with the CS larvae; however, the responsiveness to stimuli and motor performance in untrained animals are normal [22–24]. The rut locus participates in olfactory conditioning learning in D. melanogaster, and it is expressed in the neurons located in the larval and adult mushroom bodies; rut does not affect larval locomotion or responsiveness to stimuli [25, 26].
The wild type Trana strain of D. melanogaster was established with adults that emerged from grape (Vitis vinifera, País Variety); no other Drosophila species emerged from the decaying fruit. The wild-type Til-Til strain of D. melanogaster was formed with adults that emerged from decaying prickly pear fruits collected. The Til–Til and Trana larvae used in the laboratory experiments were fourth-generation.
We are indebted Don Rodrigo Pica owner of the Fundo Trana in Cauquenes, VIII Region of Chile, who kindly issued the permission for our field studies in that land. We are also grateful to Don Juan Ignacio Herrera and Don Gonzalo Herrera owners of the Fundo La Capilla in Til-Til who issued the permission to collect flies and fruits, and for their tolerance while we invaded their land. We thank Dr Bertram Gerber, University of Würzburg in Germany. He sent us the Canton-Special (CS), Orco (Or83b), Syn 97CS and rut strains to our laboratory.
We also investigated the effect of larval olfactory cues on navigation of D. pavani (La Florida strain, 33° 33’ 00”S), its sibling D. gaucha (Buenos Aires strain, 34°20’00”S) and the F1 reciprocal hybrid larvae. The emergency of adults of D. melanogaster and D. pavani from the same decaying fruit unit in the wild, suggested that D. pavani larval odors play a role in the orientation of the movement of D. melanogaster larvae. These two endemic South American sibling species belong to Subgenus Drosophila, mesophragmatica group [28–30]. D. pavani is predominantly Andean in distribution, whereas D. gaucha is distributed in Argentina, Uruguay and southern Brazil [29]. Under laboratory conditions, the two species can produce viable but sterile hybrids [31]. D. pavani and D. gaucha have similar development durations of molting, wandering and pupating [32].
The strains were maintained by mass culture at 24 ± 1°C 70% (D. melanogaster) and 18 ± 1°C, 80% humidity (D. pavani and D. gaucha). D. pavani and D. gaucha grow better at this temperature and humidity than at 24°C. All stocks were maintained under constant light because the laboratory was not equipped to change the light/dark period.
Crosses
Fifteen-day-old D. pavani (La Florida strain) and D. gaucha (Buenos Aires strain) males and females were reciprocally crossed. At this post-emergence age, individuals are sexually competent [32]. Homogametic mating within strains served as controls for the interspecific crosses. Crosses between the La Florida (D. pavani) and Buenos Aires (D. gaucha) strains provided abundant hybrid larvae of both sexes [31, 32].
Larva collection
Groups of 40–50 inseminated females of D. melanogaster, D. pavani, D. gaucha, and D. pavani females and D. gaucha females crossed with males of the other species were allowed to oviposit for 2–3 h on plastic spoons containing the culture medium [31]. Thirty eggs of the species, strains and hybrids were randomly collected with a dissecting needle. Each batch of eggs was incubated on fresh spoons for 96–100 h at 24°C (D. melanogaster strains) and for 168–172 h at 18°C (D. pavani, D. gaucha and the hybrids).
One hour before an experiment, third-instar larvae were collected from the glass wall of rearing bottles, washed twice with distilled water, and identified by the presence of protruded anterior spiracles [33]. All larvae were raised in half-pint bottles at 24°C (D. melanogaster) and 18°C (D. pavani and D. gaucha) on Burdick’s medium [34].
Treatments
We used two 2 x 2 cm pieces of Whatman cellulose filter papers. In the first treatment, one of the papers was moistened in sterile Burdick’s medium, whereas the other was moistened in Burdick’s medium used for 4–5 days by Oregon R-c larvae of D. melanogaster (or D. pavani) larvae. Before transfer to Petri dishes, the two filter paper types were carefully examined under stereomicroscope to verify that no food had adhered to the surface. In the second treatment, one of the papers was moistened in food used by larvae of the strain (Canton-Special, Til-Til, Trana, vestigial, Orco (Or83b), Syn 97CS and rut strains of D. melanogaster) and the other filter paper was moistened in food processed by the Oregon R-c larvae. The test for Oregon R-c larvae was an Oregon R-c filter paper, and a Canton-Special filter paper. In the third treatment, we used one filter paper moistened in food occupied by larvae of D. melanogaster strains, another moistened in food used by D. pavani larvae. These treatments were also applied to D. pavani, D. gaucha and the interspecific reciprocal hybrid larvae.
For each treatment, 10-cm Petri dishes were filled with 10 ml of 3% agar gel. The two different filter papers were deposited on opposite sides (6 cm of separation) of the agar in Petri dish. Batches of 20 third-instar larvae of each species and strain were introduced into the Petri dishes and gently deposited onto the middle of the agar, 3 cm from each piece of paper. Once the larvae actively moving, we recorded the observed number of larvae on each type of filter paper every 2 min for 20 min. Some larvae only approached the papers. Larvae detected on agar within 1 cm of the border of each paper were counted as belonging to that paper. Replicate measurements (10 replicates, N = 200 larvae) were performed for each strain. To decrease possibility that differences in illumination conditions would interfere with orientation of the larvae in each treatment, we recorded every 5 min the amount of light in four opposite locations near the edge of each of the Petri dishes. The possibility that the substances present in the papers could diffuse through the agar was addressed by testing Orco mutant larvae. This mutation does not affect gustatory neurons [17].
Larva locomotion
We tested four treatments to measure locomotion of the larvae on agar: (i) no odor, except the aroma of agar, (controls); (ii) the aroma of Burdick’s medium; (iii) conspecific larval odors; and (iv) D. pavani (or D. gaucha and hybrid) larval cues. For the treatments (ii), (iii) and (iv), a 2 x 2 cm piece of paper impregnated in Burdick’s medium (or the corresponding strains of D. melanogaster, D. pavani, D. gaucha or hybrids) was deposited onto the center of a Petri dish filled with 3% agar gel for 1 hour. The paper was subsequently removed, and a third-instar larva of the strains and species indicated above was gently deposited on the Petri dish agar (N = 50 per strains and species and hybrids). Once the larva started to actively move, locomotion was recorded for 2 min. Locomotion was measured as the number of waves of segmental contraction per minute passing in series along the body [31]. A new Petri dish was used for each larva tested.
Statistical analysis
Larval orientation
We applied a generalized linear mixed model (GLZM) to analyze number of larvae on the filter papers. The explicatory variables (strains, treatments, observation times and their interactions) were treated as categorical variables and fixed factors. For this purpose we used the Poisson distribution. The link function was a log-linear model [35]:
In the model, treatments correspond to the two tests conducted with each strain. For example, we compared number of larvae on the papers in the sterile medium/conspecific medium treatment versus the sterile medium/heterospecific medium treatment (for an example see Fig 1A–1H ). We nested replicates within each strain because the number of larvae on the papers could vary randomly across strains of D. melanogaster and across genotypes within a strain. We also conducted a similar analysis of D. pavani, D. gaucha and the hybrid larvae. The Deviance, Pearson χ 2 and Akaike Information Criterion (AIC) values were also determined to assess how closely the model-based fitted values approximated the observed values [35]. We examined the statistically significant interactions to analyze the effect of each treatment on larval behavior of each strain and species. We applied Wald χ 2 to assess statistical significance of the explanatory variables [35]; the G-test for heterogeneity of the replicates (D. gaucha and the gaucha female x pavani male hybrid larvae) was also applied.
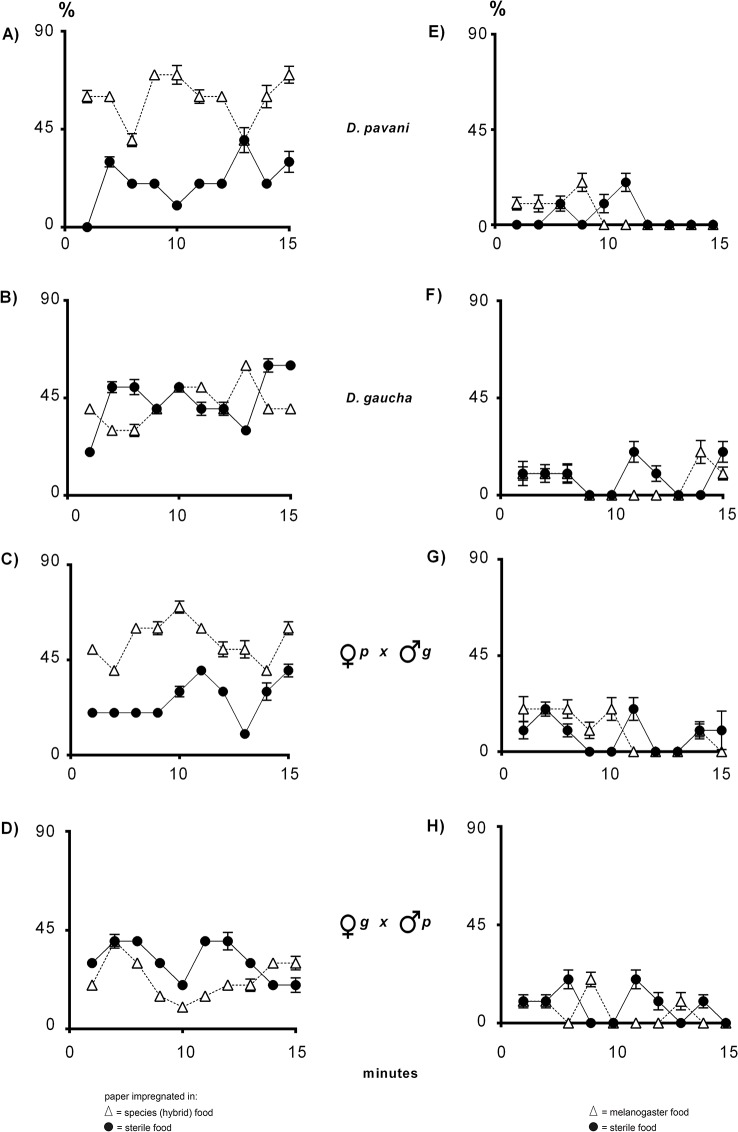
Fig 1. Navigation of third-instar larvae of D. melanogaster stimulated by conspecific odors and D. pavani odors.
(A–H), response to sterile food aroma and food processed by Oregon R-c larvae (D. melanogaster). (I–P), response to sterile food aroma and food worked by La Florida larvae (D. pavani ). Navigation toward sterile food, congeners and alien odors is shown as percentage of larvae ± SE arriving at the papers. Black circle (A–P), filter paper moistened in sterile food. White triangle (A–H), filter paper moistened in food used by Oregon R-c larvae (D. melanogaster) or La Florida larvae (D. pavani) (I–P). When standard errors are not shown is because they are too small.
Larva locomotion analysis
We applied the t-test to larval locomotion data from the strains and species examined [35] (N = 50 larvae per strain (species) and treatment).
Results
Behavior of Drosophila larvae in the breeding sites
Drosophila larvae explore the entire breeding site in the wild, suggesting that they invest a substantial amount of time and energy searching for microorganisms to consume and sites to pupate. To better understand the significance of this behavior, we carefully examined the fruits and tissues. The rearing materials consisted of several connected microhabitats. Some sections of a fruit were in advanced states of decay and dryness, whereas desiccation and fermentation were only beginning in other parts of the same fruit. Other locations in the rearing sites were not fermented (S1 and S2 Figs and Fig 2). These different stages of decay represent changes in ecological conditions that are rapid and difficult to predict. The microhabitats differed also in acidity/alkalinity; acetic acid tended to accumulate in the fruit zones in which decay was advanced. We found D. melanogaster larvae feeding at pH 3.0; larvae of other species were not observed. Thus, larvae of different Drosophila species seem to detect and select chemically different microhabitats within a fruit unit. The desiccation was also heterogeneous. Some parts of a fruit unit were drier than other. At drier sites, we did not find Drosophila larvae but instead identified pupae, corroborating the observations of Brncic [19] and Beltramí et al. [14].
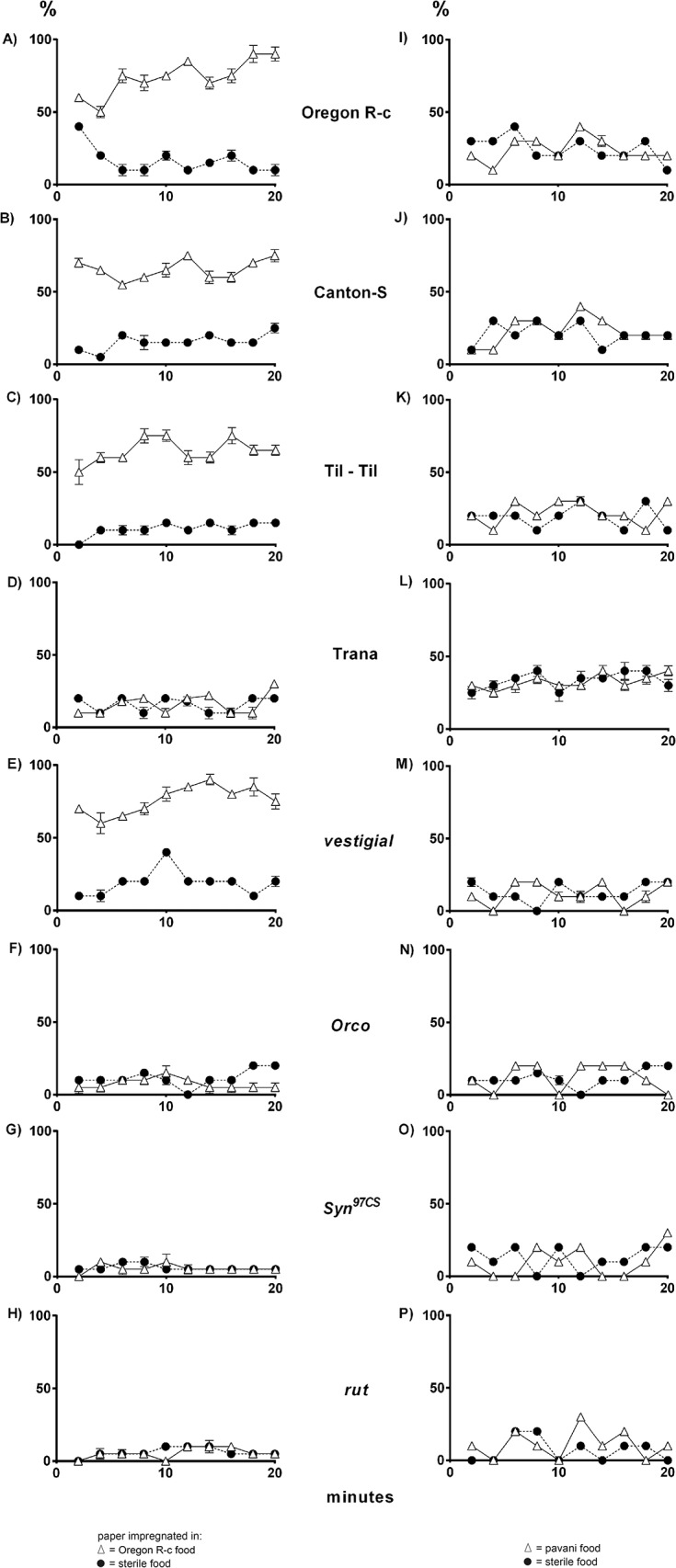
Fig 2. Navigation of D. melanogaster larvae stimulated by conspecific larval odors of different strains and conspecific and D. pavani odors.
Percentage of larvae on papers impregnated, respectively, in (i) food used by larvae of the strain, white triangles, and (ii) food worked by Oregon R-c larvae, black circles (A–H). For Oregon R-c larvae the papers were moistened, respectively, in Oregon R-c food and Canton-Special food. (I-P), white triangles, filter paper moistened in food of the strain; black circles, filter paper moistened in D. pavani food. For further details see Fig 1.
Behavior toward other larvae in the wild
Two or more Drosophila species emerged from 79.83% of the collected fruits (N = 502). We identified the sibling D. melanogaster and Drosophila simulans, Drosophila buzzatii, Drosophila hydei, Drosophila busckii, Drosophila immingrans, D. pavani, and Drosophila nigricruria. We observed the behavior of larvae toward other individuals. In some instances, navigation was toward other larvae; in other cases, they moved away from other individuals, avoiding contact with them. We conjectured that the larvae were able to identify congeners and aliens at distance, as suggested by Medina-Muñoz and Godoy-Herrera [36]. Some Drosophila larvae occasionally touched the spiracles and/or cephalic region of other larvae. Other larvae also scrutinized other individuals by touching them from the cephalic region to the spiracles and/or vice versa. Thus, tactile signals might also be necessary to confirm the identity of congeners and aliens, as in the case of larvae of sibling species.
Navigation in D. melanogaster larvae
In the treatment in which one paper was impregnated in sterile food and the other in food processed by Oregon R-c larvae, more than 65% of wild type (the Oregon R-c, Canton-Special and Til–Til strains) and vg larvae of D. melanogaster were detected on the conspecific paper, indicating that when stimulated by conspecific odor cues, the larvae navigated toward them (Fig 1A–1C and 1E ). The response was relatively rapid. At 2–4 min of observation time, approximately 65% of the larva were on or near the conspecific paper; this percentage tended to remain constant over time until the end of the 20 min observation time (Fig 1A–1C and 1E ), suggesting a robust response to conspecific larval odor. In this same assay, approximately 20% of the wild-type Trana larvae were on the sterile food paper and on the paper moistened in food previously exposed to larvae of the same strain; these percentages tended to maintain throughout the entire observation time. The results suggest that Trana larvae did not respond to conspecific odors to navigate across the substrate (Fig 1D ).
Stimulated by odors emanating from the sterile food paper and Oregon R-c food-impregnated paper, approximately 18% of Orco larvae were detected on the papers and 70% on Petri dish agar (Fig 1F ), suggesting that these larvae did not respond to larval volatiles. In this same essay, approximately 15% of Syn 97CS and rut larvae were detected on each of the papers, and more than 65% moved across agar during the observation time of 20 min (Fig 1G and 1H ). This behavior contrast with that of wild type Canton-Special larvae (Fig 1B ). We conclude that in D. melanogaster, Orco, Syn 97CS and rut loci are necessary to orient the movement of larvae toward congeners.
Stimulated by the aroma of sterile food and D. pavani larval odors more than 60% of wild-type (the Oregon R-c, Canton-Special, Til–Til and Trana strains) and vg larvae of D. melanogaster moved on agar; fewer than 15% of the larvae were on each of the papers (Fig 1I–1M ). The percentage of wild type and vg larvae on agar tended to remain constant between 2 and 20 min of observation time, suggesting that the larvae identified the odors early (Fig 1I–1M ). Approximately 17% of the Orco, Syn 97CS and rut mutant larvae were detected on each of the papers throughout the entire observation time (20 min; Fig 1N–1P ). These findings suggest that volatiles emitted by pavani larvae influence navigation of wild-type D. melanogaster larvae and, again, indicate that the Orco, Syn 97CS and rut loci are necessary for identification of congeners and strangers.
In the presence of conspecific odors only, most of the wild type and vg larvae of D. melanogaster moved across the agar, and approximately 15% were recorded on each of the papers (Fig 2A–2E ). Notably, this pattern changed greatly in the presence of congener and D. pavani odors. The Oregon R-c, Canton-Special, Til-Til and vg larvae oriented their movement toward the conspecific paper and approximately 10% of the larvae were on D. pavani paper (Fig 2I–2K and 2M ). Remarkably, Trana larvae again were principally observed on agar in Petri dishes (Figs 1L, 1D , 2L and 2D ), indicating that these larvae did not respond to congener or pavani odors. The Orco, Syn 97CS and rut mutant larvae were also principally detected on Petri dish agar (Fig 2F, 2G, 2H, 2N, 2O and 2P ).
Statistical analysis for D. melanogaster larval navigation
The ratio of deviance and generalized Pearson χ2 calculated values to the degrees of freedom were 1.11 and 1.09, respectively, close to the expected value/df 1.00. We concluded that the model effectively describes the response of larvae of D. melanogaster to congener and alien larval odors. AIC was 12.35, a relatively small value, suggesting that over-dispersion was absent from data [35].
The calculated Wald χ2 values for those explanatory variables were (i) strains, 49.62, df = 7, P < 0.0001; (ii) treatments, 58.81, df = 2, P < 0.0001; (iii) strains x treatments, 34.09, df = 14, P = 0.035. These results show that differences between strains, treatments and interactions between these two variables contribute to variance between and within the strains used. The Wald χ2 values for observation times, 7.89, df = 9, P = 0.996, NS; strains x observation times, 10.32, df = 63, P = 1.00, NS); treatments x observation times, 17.36, df = 18, P = 0.922, NS; and strains x treatments x observation times, 21.54, df = 126, P = 1.0, NS, are not statistically important. Based on the significance of the strain x treatment interaction, we also infer strong genotype-by-environment interaction at the level of individual genotypes in the strains of D. melanogaster tested.
Navigation of D. pavani, D.gaucha and D. pavani x D. gaucha reciprocal hybrid larvae
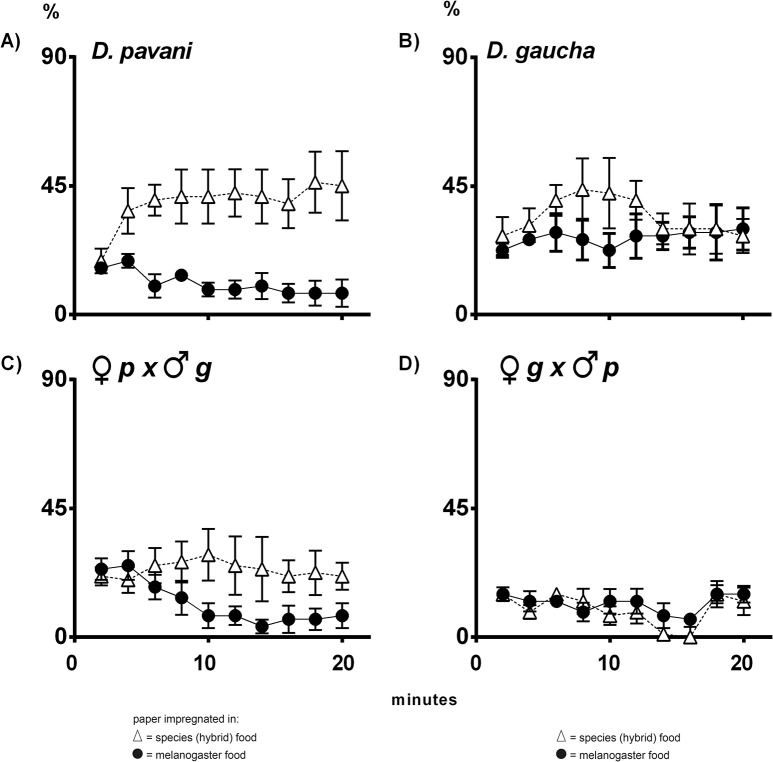
In the sterile food/conspecific food treatment, more than 65% of the D. pavani and the pavani female x gaucha male hybrid larvae oriented their movements toward the conspecific paper (Fig 3A and 3C ). Under the same condition, D. gaucha and the gaucha female x pavani male hybrid larvae were principally on agar and about a 12% of the larvae were detected on the two papers in the Petri dishes, suggesting that the larvae did not respond to the signals (Fig 3B and 3D ). We assessed these responses by using one paper impregnated in sterile food and the other in D. melanogaster food. D. pavani larvae and the pavani female x gaucha male hybrid larvae moved across the agar in the Petri dish (Fig 3E and 3G ). These results confirm that in D. pavani, olfaction is required to orient the movement of the larvae. D. gaucha and the gaucha female x pavani male hybrid larvae exhibited a similar response to the behavior displayed in the sterile food/conspecific food treatment, suggesting again that they did not use larval volatiles to direct their movements (Fig 3F and 3H ).
Fig 3. Navigation of D. pavani, D. gaucha and hybrid larvae stimulated by conspecific or D. melanogaster odors.
(A—D), percentage of larvae on the paper impregnated in sterile food (black circles) and on the paper moistened in food used by larvae of the species or the respective hybrid (white triangles). (E–H), percentage of larvae on papers impregnated in sterile food and Oregon R-c (D. melanogaster) food. See also Fig 1 for further details.
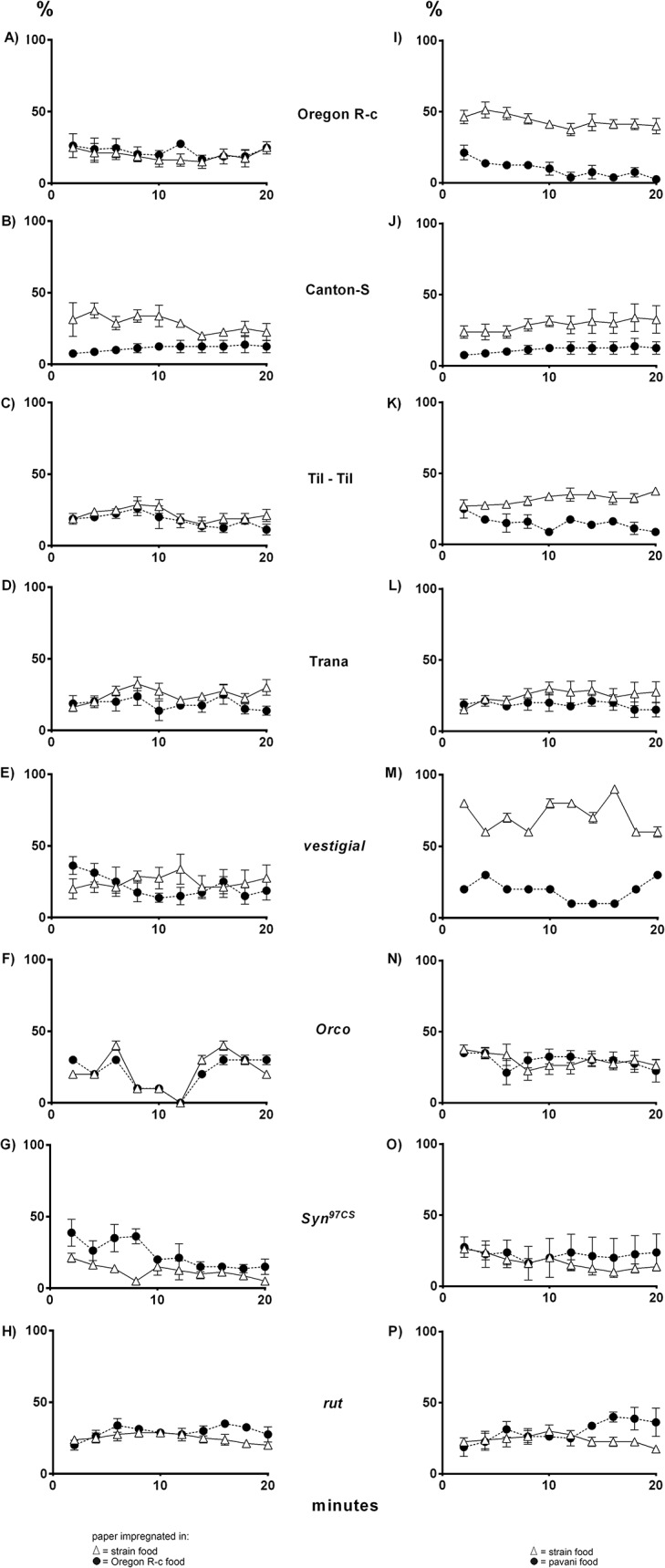
Stimulated at the same time by food of the species (hybrid) and Oregon R-c food, larvae of D. pavani and the pavani female x gaucha male hybrids navigated toward the conspecific paper (Fig 4A and 4C ). By contrast, D. gaucha and the gaucha female x pavani male hybrid larvae were on the agar of the Petri dishes (Fig 4B and 4D ). We conclude that in the two sibling species, divergent evolutionary changes associated with the processing of odor volatiles necessary to identify conspecifics and aliens have occurred in the organization and function of the nervous system.
Fig 4. Navigation of D. pavani, D. gaucha and the hybrid larvae stimulated at the same time by conspecific and D. melanogaster odors.
White triangles, percentage of larvae on the paper moistened in food used by the species or the respective hybrid. Black circles, percentage of larvae on the paper impregnated in food used by Oregon R-c larvae (D. melanogaster). (A) D. pavani larvae; (B) D. gaucha larvae; (C) pavani female x gaucha male hybrid larvae; (D) gaucha female x pavani male hybrid larvae.
Statistical analysis for navigation by D. pavani, D. gaucha and reciprocal hybrid larvae
The deviance and generalized Pearson χ2 values were 108.45, df = 27, and 184.08, df = 27, respectively, yielding ratio of deviance and Pearson χ2 values to degrees of freedom greater than the expected value 1.00. AIC was also high, 228.06. Thus, the binomial GLMM model applied did not fit to the collected data. We examined the four groups of genotypes to identify heterogeneity of the replicates [35]. The D. gaucha and D. gaucha female x D. pavani male hybrid data showed replicate heterogeneity (Fmax(3,9)—test = 7.56, P = 0.002), suggesting over-dispersion. Thus, we analyzed D. pavani and D. pavani female x D. gaucha male hybrid data separately from D. gaucha and D. gaucha female x D. pavani male hybrid data.
The GLMM applied to D. pavani and D. pavani female x D.gaucha male data yielded ratios of the deviance and Pearson χ2 values to degrees of freedom near the expected value 1.0, suggesting good agreement between the binary model applied to the data and the number of larvae on the conspecific paper. The AIC value was now 21.02, also suggesting a satisfactory goodness of fit between the model and the data [35]. The explanatory variables treatments and species/hybrid x treatments interaction were statistically significant. The calculated Wald χ2 values were12.08, df = 2, P = 0.0070, and 7.93, df = 2, P = 0.0475, respectively. We did not find statistical significant differences between the behavior of D. pavani larvae and D. pavani female x D. gaucha male hybrid larvae (Wald χ2 = 2.35, df = 1, P = 0.1253, NS).
Locomotion
Locomotion is essential to move across substrates. We did not observe differences in locomotion between larvae of the corresponding strains and species in the four treatments (Table 1). For statistical significance of those differences see S1 Table (t-test). We conclude that larval volatiles do not modify the function of the neurological circuits that control locomotion at central level.
Table 1. Larval locomotion of D. melanogaster (the Oregon R-c, Canton-Special, Til-Til, Trana, vestigial, Orco, Syn 97CS and rut strains), D.pavani (La Florida strain), D.gaucha (the Buenos Aires strain), and the pavani female x gaucha male and gaucha female x pavani male hybrids.
Locomotion was measured in four conditions: (i) on agar (agar aroma, controls), (ii) agar plus synthetic food aroma, (iii) on agar plus conspecific odors, and (iv) on agar plus alien food aroma. For D. melanogaster larvae, D. pavani food was used as alien food. To D. pavani, D. gaucha and the hybrid larvae, alien food means D. melanogaster (Oregon R-c) food. Locomotion of N = 50 third instar larvae of each strain and species was measured by 2 min (see Materials and Methods).
| Species and strains | larval locomotion on agar (controls) (cpm/min) | larval locomotion on agar plus | ||
|---|---|---|---|---|
| steril food (cpm/min) | conspecific food (cpm/min) | alien food (cpm/min) | ||
| D. melanogaster | ||||
| Oregon R-c | 108.93 ± 3.27 | 99.72 ± 8.07 | 102.92 ± 4.71 | 103.21 ± 5.03 |
| Canton-Special | 96.59 ± 3.86 | 97.14 ± 4.73 | 99.62 ± 5.15 | 97.64 ± 3.92 |
| Til–Til | 96.09 ± 7.21 | 97.69 ± 3.48 | 96.84 ± 3.82 | 99.74 ± 3.92 |
| Trana | 66.72 ± 6.48 | 64.35 ± 5.71 | 70.13 ± 6.19 | 68.07 ± 5.74 |
| vestigial | 112.33 ± 5.49 | 110.85 ± 6.29 | 109.22 ± 4.73 | 110.41 ± 3.67 |
| Orco | 94.61 ± 5.43 | 96.71 ± 5.03 | 97.19 + 4.37 | 95.45 ± 4.76 |
| Syn 97CS | 107.96 ± 4.22 | 99.53 ± 6.46 | 101.05 ± 2.18 | 107.48 ± 5.72 |
| rut | 98.86 ± 5.41 | 99.06 + 5.79 | 97.42 ± 6.73 | 99.76 ± 3.25 |
| D. pavani | 60.31 ± 4.79 | 61.75 ± 3.25 | 64.32 ± 4.71 | 63.93 ± 4.21 |
| D. gaucha | 57.48 ± 5.81 | 58.12 ± 8.19 | 56.76 ± 3.15 | 58.35 ±6.05 |
| The F 1 hybrids | ||||
| pavani x gaucha | 59.58 ± 9.61 | 55.63 ± 7.46 | 60.21 ± 5.34 | 56.31 ± 4.76 |
| gaucha x pavani | 62.79 ± 8.72 | 65.28 ± 6.39 | 64.07 ± 7.15 | 60.32 ± 5.39 |
Discussion
In this study we asked where do Drosophila larvae go? The absence of information on the behavior of larvae in the wild and on techniques for studying their behavior in the field are partially responsible for the general ignorance of distribution of larvae at the breeding sites, although Godoy-Herrera previously highlighted the potential importance of larval digging behavior in the use of space [27]. We hypothesized that larvae identify other larvae at distance approaching congeners and avoiding aliens. To facilitate this behavior, Drosophila larvae would emit species-specific volatiles.
Our study confirms that D. melanogaster and D. pavani larvae have the ability to identify other larvae at a distance, orienting their movements toward congeners while avoiding and moving away from aliens. The mechanism of this recognition is the emission of species-specific volatiles (Figs 1 and 2). Del Pino et al. [6] reported that the selection of pupation sites in D. melanogaster is also significantly affected by Orco, suggesting the importance of this locus in the allocation of space and habitat selection. Other olfactory mutations such as poxn ΔΧBs6 [37], may also participate in recognition.
The identification of and orientation toward congeners is flexible and variable. Plasticity of identification and navigation toward congeners while avoiding aliens may be a means by which larvae cope with and adapt to changing and essentially unpredictable environments. The presence of larvae of two or more species within a decaying fruit unit and, consequently, of alien larval odors, is difficult to predict. We conjecture that the presence/absence of alien larval volatiles in a fruit unit is a circumstance that promotes flexibility and plasticity in the navigation of Drosophila larvae. More specifically, the environmental uncertainty created by female egg-laying site selection could act as an evolutionary pressure on the organization and function of the nervous system of Drosophila larva.
Olfaction is not required for navigation in larvae of a natural population of D. melanogaster
A key finding in this study is the apparent absence of olfactory perception for navigation in larvae of the Trana isolate (Figs 1 D, 1L , 2D and 2L ). These findings contrast with the behavior of the Til-Til isolate larvae of the same species (Figs 1C, 1K , 2C and 2K ). The Trana strain was obtained from adults that emerged from grapes of the País variety. Only D. melanogaster adult flies emerged from these grapes. The decay process in grapes of the País variety produces relatively high ethanol, acetaldehyde and acetic acid concentrations, and acidity may reach pH 3.0 (unpublished data). D. melanogaster larvae are exceptional for their ability to utilize and tolerate high concentrations of these substances, whereas larvae of other species exhibit less or no tolerance [38]. By contrast, from the decaying semitropical prickly pear fruits, at pH 6.31, the ancestors of the Til-Til strain of D. melanogaster emerged together with D. simulans, D. buzzatii and a few D. pavani. Thus, Til-Til larvae developed with larvae of other species of Drosophila. The deprivation of odor experience may lead to a loss of sensitivity and acuity for distinguishing odorants [39]. However, we cannot exclude the possibility that the Til-Til and Trana isolates also differ in the frequency of certain genes involved in the processing of olfactory cues [40, 41].
The above conjectures cannot be completely correct. In the presence of conspecific and D. pavani larval odors, pupae of the Trana strain were preferentially observed near the conspecific paper [6]. Taken together, these contrasting findings suggest that D. melanogaster larvae emit a variety of chemically different volatiles. Each odor could have a different function. Some of these aromas might be necessary to identify conspecifics and aliens at a distance. Other chemically different odor cues may be required to select pupation sites. Independent neurological circuits may be involved in processing these different larva odor inputs at a central level. Larvae of different natural populations of D. melanogaster may also exhibit quantitative differences in emission rates of these scents.
Navigation of learning mutant larvae of D. melanogaster
Our data show that the Syn 97CS and rut learning mutant larvae are unable to identify congeners and aliens (Figs 1F–1H, 1N–1P , 2F–2H and 2N–2P ). These findings suggest social larval odor-based learning in identification and orientation of the larvae toward congeners. Additionally, the results for the rut mutation indicate that the neuronal circuits that control the identification of congeners and aliens are located at the larva brain. Wild type larvae of D. simulans and D. buzzatii reared in isolation away from their congeners pupated randomly across the substrate. These larvae form pupa aggregations when reared with congeners [14]. Larvae of Drosophila hydei, subgenus Drosophila, repleta group, hydei subgroup, and Drosophila busckii, subgenus Dorsilopha, also form pupa aggregations away from D. melanogaster and D. pavani pupae [14, 36]. These findings suggest that social larval odor-based learning seem to be widely distributed in Drosophila genus having an essential role in the identification of congeners and aliens. Future experiments should be focused on the navigation of wild type D. melanogaster larvae reared in isolation from congeners.
The sibling species D. pavani and D. gaucha
The sibling species D. pavani and D. gaucha exhibited notable differences in olfactory sensorial system function in larvae (Figs 3 and 4), confirming and extending the results reported by Del Pino et al. [6]. In nature, Chilean populations of D. pavani and D. melanogaster coexist in the same orchards, whereas the Buenos Aires strain of D. gaucha is allopatric with respect to D. pavani and its larvae are ecologically isolated from those of D. melanogaster (unpublished data). This isolation from other species produces delays in processing and transmitting information to central structures [39]. We have not examined whether D. gaucha larvae respond to odor cues emitted by D. pavani larvae. In addition, adults of D. simulans and D. buzzatii may emerge from the same decaying cladodes of prickly pear used by D. gaucha as breeding sites. For definitive conclusions, D. gaucha larvae must be tested against larval volatiles of each of those three species.
D. pavani x D. gaucha hybrid larvae
The divergent results for the pavani x gaucha F1 reciprocal hybrids confirm that the two sibling parental species differ remarkably in olfactory sensitivity for the identification of larvae of the same and other Drosophila species (Figs 3 and 4). The results suggest that the X chromosome of each of the two parental species include loci for the identification of congener and alien larvae, which also affect the orientation and navigation of the larvae. These loci may be linked with those involved in the selection of pupation sites [6]. The genes seem to exhibit behavioral pleiotropy for navigation across the substrates and selection of pupation sites.
Alternative alleles appear to be linked on the X chromosome of each species. However, these reciprocal differences in behavior in crosses between species might also reflex cytoplasmic heredity. Relatively little attention has been paid to olfactory responses implicated in identification and spatial orientation in inter- and intraspecific hybrid larvae of Drosophila. Studies of this type may provide valuable information about neurogenetics and the evolution of behavior. Definitive proof will require a comparative cellular and molecular analysis of chemosensory receptors and brain circuits in larvae of D. pavani and D. gaucha and the reciprocal hybrids.
Locomotion
Locomotion reflects the functional state of the nervous system [42]. Thoracic and abdominal neuronal circuits control freely moving larvae [43]. Our data revealed no differences in the locomotion rate of larvae that freely move on agar (controls) and those moving in the presence of synthetic food aroma and of conspecific and alien larval odors (Table 1 and S1 Table). We conclude that the conspecific and alien larval odors studied principally affect larval brain circuit performance.
Concluding Remarks
We have not chemically identified the nature of the molecules emitted by Drosophila larvae and recognized by their olfactory receptors. However, it is clear that these emissions perform an essential ecological function related to the partition and allocation of space and habitat selection and, presumably, food preferences.
The mechanism by which the absence of larvae of other species blocks the identification of congeners and aliens remains unclear. Combinations of behavior, electrophysiology, and genetic approaches should make it feasible to better decipher the role of the olfactory cues emitted by Drosophila larvae in those behaviors.
We propose that Drosophila larvae emit a number of scents, each related to a different ecological function. The production of these aromas could be regulated by the presence/absence of larvae of other Drosophila species, suggesting the crucial importance of female egg-laying sites selection for larval behavior. Very little is known about Drosophila larva pheromones [44]. On the other hand, recognition of congeners in Drosophila larva keeps them in proximity to individuals of their own species, which may also have consequences for later adult life and mating. Studies on Drosophila larva routines in the wild will be decisive in validating these hypotheses. In summary, Drosophila larvae exhibit sophisticated odor-based behaviors linked with the use of the space available within breeding sites.
Supporting Information
D. simulans and D. melanogaster adults emerged from the fruits.
(JPG)
Larvae of D. immigrans, D. busckii and D. melanogaster were observed eating the microorganisms.
(JPG)
(DOC)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors have no support or funding to report.
References
- 1. Andrewartha HG, Birch LC. The distribution and abundance of animals Chicago: The University of Chicago Press; 1954. [Google Scholar]
- 2. Mayr E. Animal species and evolution The new synthesis. Cambridge, Massachusetts: The Belknap Press of Harvard University Press; 1963. [Google Scholar]
- 3. Wilson EO. Sociobiology The new synthesis. Cambridge, Massachusetts: The Belknap Press of Harvard University Press, 1975. [Google Scholar]
- 4. Yew JY, Dreisewerd K, Luftmann H, Müthing J, Pohlentz G, Kravitz EA. A new male sex pheromone and novel cuticular cues for chemical communication in Drosophila . Curr Biol. 2009; 19: 1245–1254. 10.1016/j.cub.2009.06.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zuleta V, Arizmendi C, Ruiz-Dubreuil G, Godoy-Herrera R. The development and genetics of larval foraging behavior in Drosophila funebris that breed in necrotic tissue of Chilean cactus Echinopsis chilensis . Behav Genet. 2008; 38: 525–530. 10.1007/s10519-008-9217-0 [DOI] [PubMed] [Google Scholar]
- 6. Del Pino F, Jara C, Pino L, Godoy-Herrera R. The neuro-ecology of Drosophila pupation behavior. PLoS ONE. 2014; 9(7): e102159 10.1371/journalpone.0102159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Souza H M L, Brito da Cunha A, Dos Santos E P. Adaptive polymorphism of behavior evolved in laboratory populations of Drosophila willistoni . Amer Nat. 1970; 104: 175–189. [Google Scholar]
- 8. Powell JR. Progress and prospects in evolutionary biology The Drosophila model. New York: Oxford University Press; 1997. [Google Scholar]
- 9. Del Pino F, Salgado E, Godoy-Herrera R. Plasticity and genotype x environment interactions for locomotion in Drosophila melanogaster larvae. Behav Genet. 2012; 42: 162–169. 10.1007/s10519-011-9490-1 [DOI] [PubMed] [Google Scholar]
- 10. Beaver RA. Non-equilibrium “island” communities diptera breeding in dead snails. J Anim Ecol. 1977; 46: 783–798. [Google Scholar]
- 11. Atkinson WD, Shorrocks B. Competition on a divided and ephemeral resource: a simulation model. J Anim Ecol. 1981; 50: 335–344. [Google Scholar]
- 12. Foster JLM, Flogeman JC. Identification and ecology of bacterial communities associated with necrosis of three cactus species. App Environ Microbiol. 1993; 59: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Becher PG, Flick G, Rozpdowska E, Schimth A, Hagman A, Lebreton S, Larsson MC, Hansson BS, Piskur J, Witzgall P, Bengtsson M. Yeast, not fruit volatiles mediate Drosophila melanogaster attraction, oviposition and development. Func Ecol. 2012; 26: 822–828. [Google Scholar]
- 14. Beltramí M, Medina-Muñoz MC, Arce D, Godoy-Herrera R. Drosophila pupation behavior in the wild. Evol Ecol. 2010; 24: 347–358. [Google Scholar]
- 15. Flogeman JC, Abril JR. Ecological and evolutionary importance of host plant chemistry In: Barker JSF, Starmer WT, MacIntyre RJ, editors. Ecological and evolutionary genetics of Drosophila. New York: Plenum Press; 1990. pp 121–143. [Google Scholar]
- 16. Masuda- Nakagawa IM, Gendre N, O’Kane CJ, Stocker RF. Localized olfactory representation in mushroom bodies of Drosophila larvae. Proc Natl Acad Sci USA. 2010; 106: 10314–10319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Larsson MC, Domingos AI, Jones WD, Chiappe ME, Amrein H. et al. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron. 2004; 43: 703–714. [DOI] [PubMed] [Google Scholar]
- 18. Takeshi I, Vosshall LB. Topographic mapping–the olfactory system. Cold Spring Harb Perspect Biol. 2010; 2: a001776 10.1101/cshperspect.a001776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brncic D. Coexistencia de diferentes especies de Drosophila en frutas fermentadas naturalmente. Medio Ambiente. 1987; 8: 3–9. [Google Scholar]
- 20. Barker JSF, Starmer WT, MacIntyre RJ. Ecological and evolutionary genetics of Drosophila New York: Plenum Press; 1990. [Google Scholar]
- 21. Gerber B, Stocker R. The Drosophila larva as a model for studying sensation and chemosensory learning: a review. Chem Senses. 2007;32: 65–89. [DOI] [PubMed] [Google Scholar]
- 22. Saumweber T, Husse J, Gerber B. Innate attractiveness and associative learnability of odors can be dissociated in larval Drosophila . Chem Senses. 2011; 36: 223–235. 10.1093/chemse/bjq128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Michels B, Chen Y-Ch, Saumweber T, Mishra D, Tanimoto H. et al. Cellular site and molecular mode of synapsin action in associative learning. Learn Mem. 2011; 18: 332–344. 10.1101/lm.2101411 [DOI] [PubMed] [Google Scholar]
- 24. Diegelmann S, Klagges B, Michels B, Schleyer M, Gerber B. Maggot learning and Synapsin function. J Exp Biol. 2013; 216: 939–951. 10.1242/jeb.076208 [DOI] [PubMed] [Google Scholar]
- 25. Duerr JS, Quinn WG. Three Drosophila mutations that block associative learning also affect habituation and sensitization. Proc Nat Acad Sci. 1982; 79: 3646–3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tempel BL, Bonini N, Dawson DR, Quinn WG. Reward and learning in normal and mutant Drosophila . Proc Natl Acad Sci. 1983; 80: 1486–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Godoy-Herrera R. The development and genetics of digging behavior in Drosophila larvae. Heredity. 1986; 56: 33–41. [Google Scholar]
- 28. Brncic D, Koref-Santibañez S. The mesophragmatica group of species of Drosophila . Evolution. 1957; 11: 300–311. [Google Scholar]
- 29. Brncic D. Studies on the evolutionary biology of Chilean species of Drosophila In: Hecht MK, Steere WC, editors. Essays in Evolution and Genetics in Honour of Theodosius Dobzhansky. Amsterdan: North Holland Publishing Company; 1970. pp 401–436. [Google Scholar]
- 30. Markow T, O’Grady P. Drosophila: a guide in species identification and use London: Academic Press (Elsevier); 2006. [Google Scholar]
- 31. Godoy-Herrera R, Burnet B, Connolly K. Hybrid disadvantage in larval foraging behavior of the two neotropical species of Drosophila pavani and Drosophila gaucha . Genetica. 2005; 124: 33–40. [DOI] [PubMed] [Google Scholar]
- 32. Koref-Santibañez S, del Solar E. Courtship and sexual isolation in Drosophila pavani Brncic and Drosophila gaucha Jaeger and Salzano. Evolution. 1961; 15: 401–406. [Google Scholar]
- 33. Ashburner M, Golic KG, Hawley RS. Drosophila: A laboratory Handbook. New York: Cold Spring Harbor Laboratory Press; 2004. [Google Scholar]
- 34. Burdick A.B. New medium of reproductive quality stable at room temperature. Drosoph Inf Ser. 1954; 28: 170. [Google Scholar]
- 35. McCullagh G, Nelder JA. Generalized linear methods London: Chapman and Hall/CRC; 1989. [Google Scholar]
- 36. Medina-Muñoz M.C., Godoy-Herrera R. Dispersal and prepupation behavior of Chilean sympatric Drosophila species that breed in the same site in nature. Behav Ecol. 2004; 16: 316–322. [Google Scholar]
- 37. Gerber B, Stocker RF, Tanimura T, Thum AS. Smelling, tasting, learning: Drosophila as a study case. Results Probl Cell Differ. 2009; 47: 139–185. 10.1007/400_2008_9 [DOI] [PubMed] [Google Scholar]
- 38. Parsons PA. The evolutionary biology of colonizing species Cambridge: Cambridge University Press; 1983. [Google Scholar]
- 39. Lyengar A, Chakraborty TS, Goswami SP, Wu Ch-F, Siddiqui O. Post-eclosion odor experience modifies olfactory receptor neuron coding in Drosophila . Proc Nat Acad Sci USA. 2010; 107: 9855–9860. 10.1073/pnas.1003856107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Swarup S, Huang W, Mackay TFC, Anholt RRH. Analysis of natural variation reveals neurogenetic networks for Drosophila olfactory behavior. Proc Nat Acad Sci USA. 2013; 110: 1017–1022. 10.1073/pnas.1220168110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lavagnino N J, Arya G H, Korovaichuk A, Fanara J J. Genetic architecture of olfactory behavior in Drosophila melanogaster: differences and similarities across development. Behav Genet. 2013;. 43: 348–359. 10.1007/s10519-013-9592-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Suster MI, Bate M. Embryonic assembly of a central pattern generator without sensory input. Nature. 2002; 416: 174–178. [DOI] [PubMed] [Google Scholar]
- 43. Berni J, Pulver SR, Griffith LC, Bates M. Autonomous circuitry for substrate exploration in freely moving Drosophila larvae. Curr Biol. 2012; 22: 1861–1870. 10.1016/j.cub.2012.07.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wyatt TD. Pheromones and animal behavior Chemical signals and signatures. Cambridge: Cambridge University Press; 2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
D. simulans and D. melanogaster adults emerged from the fruits.
(JPG)
Larvae of D. immigrans, D. busckii and D. melanogaster were observed eating the microorganisms.
(JPG)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.