Abstract
Hearing loss is the most common and costly sensory defect in humans, and genetic causes underlie a significant proportion of affected individuals. In mammals, sound is detected by hair cells housed in the cochlea of the inner ear, whose function depends on a highly specialized mechanotransduction organelle, the hair bundle. Understanding the factors that regulate the development and functional maturation of the hair bundle is crucial for understanding the pathophysiology of human deafness. Genetic analysis of deafness genes in animal models, together with complementary forward genetic screens and conditional knock-out mutations in essential genes, have provided great insights into the molecular machinery underpinning hair bundle development and function. In this review, we highlight recent advances in our understanding of hair bundle morphogenesis, with an emphasis on the molecular pathways governing hair bundle polarity and orientation. We next discuss the proteins and structural elements important for hair cell mechanotransduction as well as hair bundle cohesion and maintenance. In addition, developmental signals thought to regulate tonotopic features of hair cells are introduced. Finally, novel approaches that complement classic genetics for studying the molecular etiology of human deafness are presented.
Introduction
Humans have a highly evolved sense of hearing that is critical for spoken communication. Hearing loss is a major public health issue affecting 48 million adults and 2–3 of every 1,000 children in the United States (Hearing Loss Association of America). A vast majority of congenital hearing loss is of sensorineural origin, due to defects in the sound processing machinery of the inner ear. Available treatments for hearing loss are currently very limited, and to develop new therapeutic interventions a fundamental understanding of the molecular physiology of hearing is critical.
The prevalence of congenital hearing loss has both necessitated and facilitated genetic analysis of hearing in humans. Inherited forms of hearing loss can be syndromic, where hearing loss is associated with symptoms in other organs, or nonsyndromic, where hearing loss is the only deficit. Nonsyndromic hearing loss can be categorized based on inheritance patterns: DFNA for autosomal dominant, DFNB for autosomal recessive, DFN for X-linked forms and mitochondrial forms, which are only maternally inherited (see Deafness and Hereditary Hearing Loss Overview http://www.ncbi.nlm.nih.gov/books/NBK1434/ for more details). Over 400 genetic syndromes that include hearing loss have been described and nearly 100 genes responsible for inherited forms of deafness (deafness genes) identified (see Hereditary Hearing loss Homepage, http://hereditaryhearingloss.org/ for an updated deafness gene list). The identification of these genes has provided important entry points into understanding genetic regulation of hearing.
To determine the function of human deafness genes, it is essential to use animal models. The mouse is a particularly attractive model because the anatomy and physiology of the auditory system is similar to that of humans, and tools for genetic manipulation are highly developed. Indeed, mouse knock-out mutations in orthologs of human deafness genes have provided important insights into the normal gene function and likely disease mechanisms. This is complemented by inner ear-specific conditional knock-out (cKO) of otherwise essential genes to further illuminate the genetic network and molecular pathways involved. Moreover, forward genetic screens in mice (and in zebrafish) have identified new genes essential for hearing1–3. Together, these approaches have begun to uncover the molecular underpinnings of auditory development and function.
Here, we will review genes and pathways important for the development of sensory receptor cells in the hearing organ, with a specific focus on the morphogenesis of the stereociliary hair bundle, the mechanotransduction organelle that detects sound. For other critical aspects of sound transduction, readers are referred to a number of other excellent resources listed in Further Reading/Resources.
The machinery for sound transduction
The auditory sensory epithelium
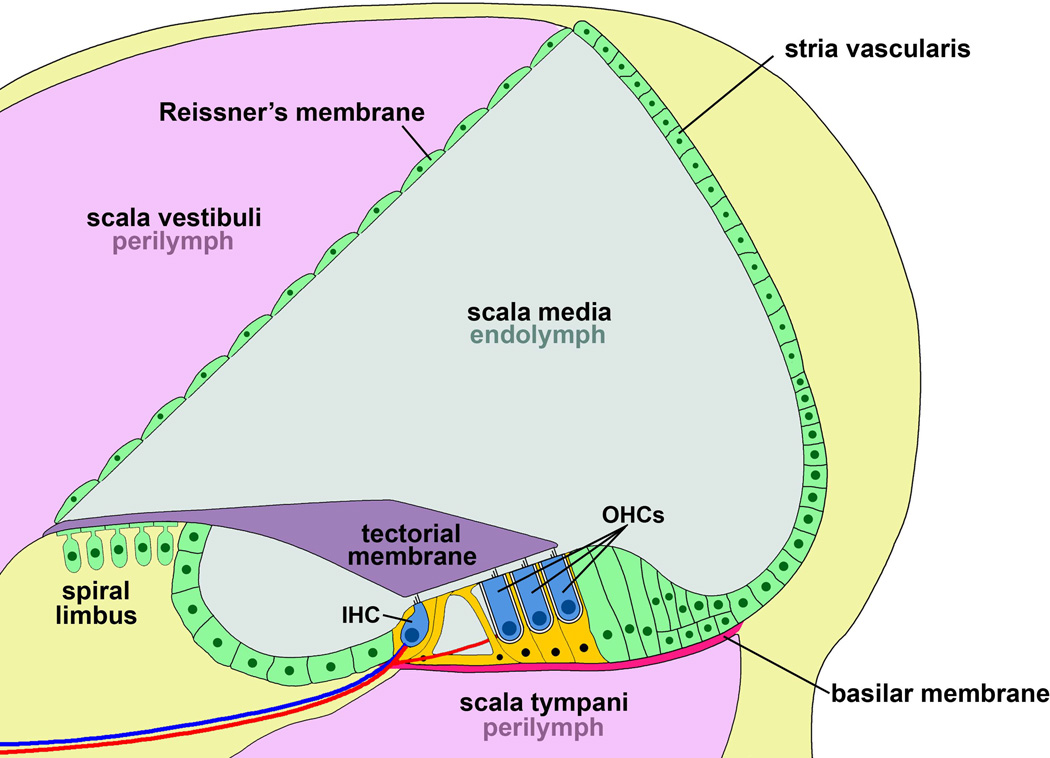
The hearing organ of the inner ear is the spiral-shaped cochlea. It is composed of three fluid-filled chambers that extend along the length of the spiral. The two outer chambers, named the scala vestibuli and scala tympani, are filled with perilymph and sealed off from the centre chamber. The center chamber, the scala media or the cochlear duct, is filled with endolymph that baths the apical surface of the sensory epithelium, called the organ of Corti (OC) (Figure 1). The endolymph is rich in K+ and poor in Na+ and has a positive potential compared to perilymph. The basal surface of the OC is exposed to perilymph and sits on the basilar membrane, an elastic structure that vibrates in response to sound. The OC consists of one row of inner hair cells (IHC) and three rows of outer hair cells (OHC), interdigitated with non-sensory supporting cells (SC) (Figure 2A). Hair cells (HC) are sensory receptors for sound; IHCs transmit information to the brain, while OHCs amplify sound signals. In humans, there are approximately 3,500 IHCs and 12,000 OHCs, and HCs lost by genetic or environmental factors are not replaced by regenerative processes, leading to permanent hearing loss.
Figure 1. Cross-sectional diagram of the cochlear duct.
The scala media, or cochlear duct, is shaded light blue and contains potassium-enriched endolymph secreted from the stria vascularis. The scala vestibuli and scala tympani, separated from the cochlear duct by Reissner’s membrane and the basilar membrane, respectively, are shaded pink and contain perilymph. Auditory hair cells are colored blue, and supporting cells of the organ of Corti are orange. Efferent nerve fibers innervating inner hair cells are colored blue and afferent nerve fibers innervating all rows are red. Other cells types residing outside the organ of Corti are colored green. IHC, inner hair cell; OHCs, outer hair cells.
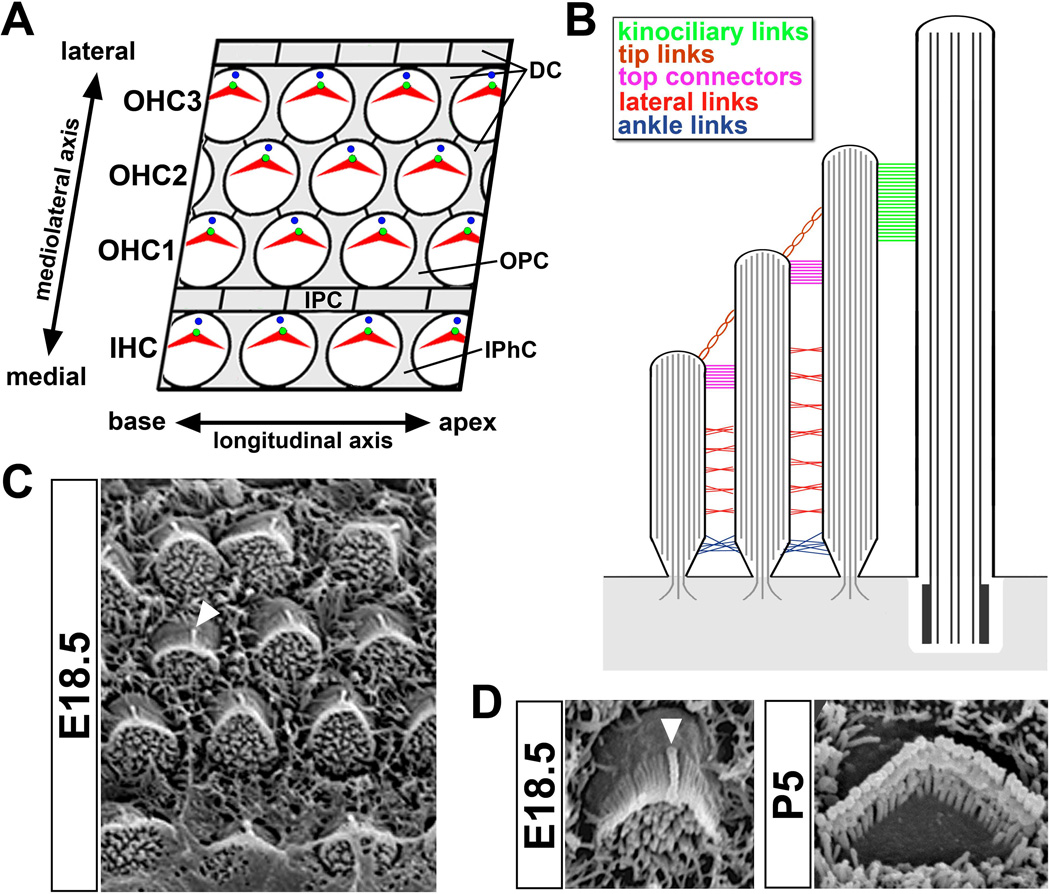
Figure 2. Organization of the organ of Corti and hair bundle.
(A) En face diagram of the mammalian organ of Corti showing the mosaic pattern of hair cells (shaded white) and supporting cells (shaded gray). The mediolateral and longitudinal axes are indicated. Abbreviations indicate distinct cell types: IHC, inner hair cell row; OHC1–3, outer hair cell row; DC, Deiters’ cell; OPC; outer pillar cell; IPC, inner pillar cell; IPhC, inner phalangeal cell. (B) Cross-sectional diagram of the hair bundle depicting five distinct populations of stereociliary links colored according to the accompanying key. Stereocilia rootlets are anchored in the cuticular plate (shaded gray). The kinocilium and underlying basal body are on the right. (C) En face scanning electron micrograph of the mouse organ of Corti at E18.5. White triangle indicates the kinocilium of an individual hair cell. (D) Left, an E18.5 hair bundle showing the kinocilium (white triangle) lying at the vertex of the hair bundle. Right, a P5 bundle showing the mature V-shape and staircase arrangement of the three stereocilia rows in an outer hair cell. Note that the kinocilium has been resorbed.
Hair bundle structure and function
HCs are characterized by the presence of a hair bundle (or stereociliary bundle) that projects from their apical surface. The hair bundle consists of three rows of modified microvilli, known as stereocilia, which are graded in height and arranged in a staircase pattern (Figure 2B, D). During development, a single microtubule (MT)-based kinocilium is present at the vertex of the V-shaped hair bundle, adjacent to the tallest row of stereocilia (Figure 2C, D). Stereocilia are finger-like projections filled with a highly cross-linked array of uniformly polarized actin filaments. The base of each stereocilium narrows to a taper near the plasma membrane, where actin filaments form a rootlet that inserts into the cuticular plate, the dense meshwork of actin in the apical domain of the cell. The actin core of each stereocilium allows it to move as a rigid rod and pivot at its base in response to sound vibrations 4.
Stereocilia within a hair bundle are interconnected by distinct types of extracellular filaments called links (Figure 2B). Adjacent rows of stereocilia are connected by tip links, which extend from the tip of a short stereocilium upwards to the side of a taller neighbour. Stereocilia are further interconnected both within and between rows via horizontal top connectors, transient lateral links or shaft connectors, and ankle links. In addition, kinociliary links connect the kinocilium to its neighbouring stereocilia. The cohesion and stiffness of the hair bundle allows the coherent movement of stereocilia essential for maximal sensitivity to deflection 5.
Mechano-electrical transduction in cochlear HCs
The hair bundle is the site of mechano-electrical transduction (MET). In the cochlea, the tips of the tallest row of OHC stereocilia are anchored to the tectorial membrane, an acellular gelatinous matrix that extends over HCs from the spiral limbus (Figure 1). Sound vibrations generate a shearing motion between the basilar membrane and the tectorial membrane, resulting in deflection of the hair bundle. The polarized, staircase structure of the hair bundle renders it directionally sensitive to deflection. Deflections of the hair bundle in the direction of the tallest stereocilia (i.e. along the excitatory axis) are thought to increase tension on the tip links, pulling on the attached membrane and causing mechanically sensitive MET channels to open and depolarization of the HC. This in turn causes neurotransmitter release at the base of the HC, eliciting an action potential in the cochlear nerve afferents. Deflections of the hair bundle toward the shortest stereocilia decrease MET channel open probability and consequently the firing rate of the cochlear nerve 5.
Hair bundle morphogenesis in mice
The mouse has proven to be a powerful model for human hereditary deafness. While the timing of hair bundle morphogenesis is different between humans, who develop functional hair bundles and can hear before birth, and mice, whose hair bundles continue to mature through the first two postnatal weeks, the molecular and cellular processes governing hair bundle morphogenesis are highly similar.
Planar polarization of HCs
The V-shaped hair bundle is a feature of cell-intrinsic planar cell polarity (PCP), which is defined as polarity in the plane perpendicular to the apical-basal axis of the HC. In addition, HC PCP is manifested at the tissue level: all hair bundles are uniformly oriented along the medial-lateral axis of the OC, with their vertices pointing toward the lateral edge of the cochlear duct (Figure 2C, 3A). HC PCP is prominent in both auditory and vestibular sensory epithelia (see ref. 6 for a recent review on PCP regulation in the utricle and saccule).
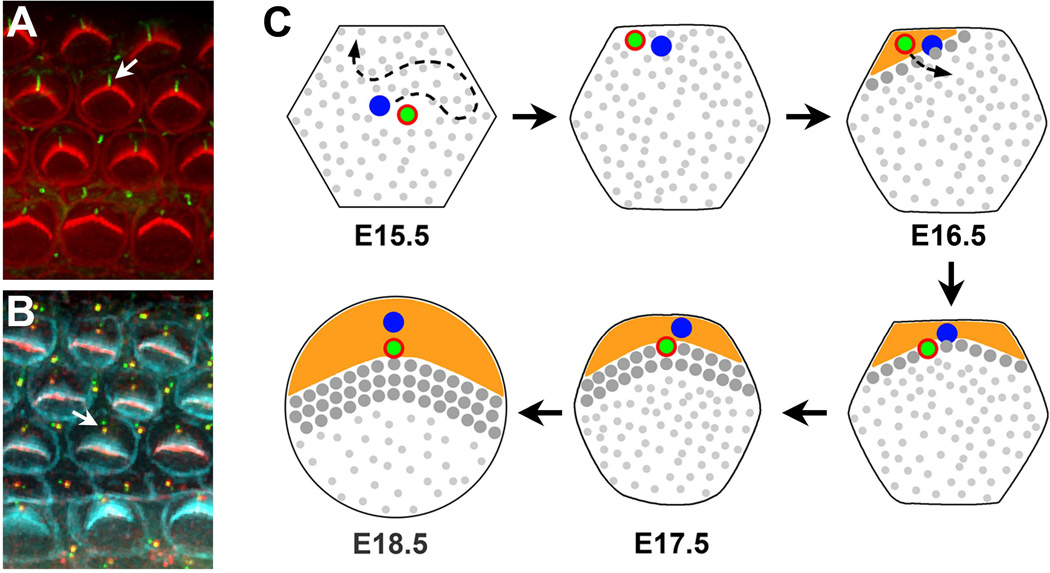
Figure 3. Development of planar cell polarity in the organ of Corti.
(A) En face view of the organ of Corti. Arrow indicates the kinocilium at the hair bundle vertex. Acetylated tubulin, green; actin, red. (B) Planar-polarized arrangement of the basal body and the associated daughter centriole along the medial-lateral axis (arrow). The basal body is labeled by phospho-β-catenin staining (red) and centrioles are labeled by Centrin2-GFP (green). F-actin, cyan. (C) Schematic diagram of kinocilium/basal body movements and early apical morphogenesis in the mouse. At E15.5, the centrally placed kinocilium/basal body (green dot with red annulus) migrates to the cell periphery where it closely associates with the cortex. A bare zone (orange) devoid of microvilli (small gray dots) subsequently develops in the vicinity of the kinocilium. As the bare zone expands, the kinocilium/basal body relocalizes to a more central position where it aligns along the medial-lateral axis with the daughter centriole. Here, the nascent hair bundle forms with the kinocilium positioned at its vertex (large gray dots), abutting the expression domain of bare zone proteins. Daughter centrioles, blue dots.
Following cell cycle exit, HC differentiation proceeds in a gradient along the base-to-apex and medial-to-lateral axes of the cochlea, starting around embryonic day (E) 15.5. One of the earliest events in HC differentiation is the migration of the kinocilium, a specialized primary cilium, from the center of the HC apical surface toward the lateral edge of the HC apical surface (Figure 3C). This is followed by graded elongation of stereocilia around the kinocilium, and nascent V-shaped hair bundles form by E17.5, with the kinocilium at their vertex. The kinocilium is attached to the basal body immediately below the apical surface of HCs. The positioning of the basal body after it migrates towards the hair cell periphery is initially imprecise; though biased toward the lateral pole, it can be positioned across a wider area than its final location fixed at the lateral pole (Figure 3B, C). Consequently, nascent hair bundles exhibit a range of orientations and are continually reoriented until their vertices are perfectly aligned toward the lateral pole by the first postnatal week. The kinocilium then recedes and is absent from the mature hair bundle (Figure 2D). Thus, the kinocilium appears to play a developmental role in regulating the shape and orientation of the hair bundle.
Acquisition of the MET machinery and staircase formation
Evidence from immunolocalization, ultra structural and electrophysiological analysis indicates that HCs acquire their MET machinery in a base-to-apex gradient from postnatal day (P) 0 to P2 5. During the first postnatal week, the graded heights of stereocilia become prominent, forming the staircase pattern of the adult hair bundle (Figure 2D). Growth of stereocilia occurs via addition of new actin monomers to the barbed ends of filaments that are aligned toward the stereocilia tips 7. Moreover, the F-actin core becomes increasingly cross-linked, and rootlets formed by dense actin bundles extend through the ankle region and penetrate into the cuticular plate, increasing the rigidity of the hair bundle. During the early postnatal weeks, the auditory ribbon synapses also form and mature, and the onset of hearing occurs around P12-P14 5.
Molecular pathways for hair bundle morphogenesis
Precise hair bundle organization and orientation is under exquisite genetic control. A reaction–diffusion model of hair bundle morphogenesis suggests that the cell boundary and the kinocilium are two signaling centers that generate morphogen patterns to establish the hair bundle’s shape 8. While necessarily simplified, this model provides a useful framework for understanding the complex interplay between hair-cell intrinsic factors and cell-cell interactions that regulate hair bundle morphogenesis, which is increasingly evident through genetic analysis.
A crucial role of the kinocilium in hair bundle shape and orientation
The role of the kinocilium in hair bundle development has been demonstrated through genetic ablation of the kinocilium. Specifically, cilium extension and maintenance is dependent on the transport of particles along the axoneme mediated by the evolutionarily conserved intraflagellar transport (IFT) machinery 9. Inner ear-specific deletion of three IFT genes, Ift88, Kif3a and Ift20, all result in HCs with stunted or absent kinocilia and flattened hair bundles, indicating a role for the kinocilium in establishing the V-shape of the hair bundle 10–12. Flattened or misshapen/circular bundles that become separated from the kinocilium are also observed in mouse mutants for genes implicated in human ciliopathies, which include Bardet–Biedl syndrome (BBS)13, Meckel–Gruber syndrome (MKS)14 and Alström syndrome (Alms)15. In addition, CEP250 and DCDC2, which may have cilia-related functions, have recently been implicated in inherited human deafness 16, 17. However their putative functions in hair bundle morphogenesis are unknown. The kinocilium is connected to the tallest stereocilia through kinociliary links (Figure 2B). Similar to ciliary mutants, in mice lacking the CD2 isoform of protocadherin-15 (Pcdh15), a component of the kinociliary link, kinocilia are separated from hair bundles, which frequently have a flattened or circular shape 18. Thus, kinociliary links are required for establishing the normal V-shape of the hair bundle and may be regulated by ciliary genes, for example, via transport of kinociliary link components.
Regulation of hair bundle polarity and orientation by Rho family GTPases
Rho family GTPases, including RhoA, Rac and Cdc42, are central regulators of the actin and MT cytoskeleton 19. In fibroblasts, they mediate the formation of stress fibers, lamellipodia and filopodia, respectively. They also regulate MT dynamics and/or orientation of the MT organizing center (MTOC) via distinct effectors. Through coordinated regulation of actin and MT dynamics, Rac and Cdc42 have evolutionarily conserved functions in directed cell migration and cell polarity. Not surprisingly, Rho family GTPases are all involved in hair bundle morphogenesis. Although RhoA itself has not been directly implicated in hair bundle development, gain-of-function mutations in Diaphanous 1 and 3, which encode members of formin-family actin-nucleating proteins activated by RhoA, cause autosomal dominant forms of human hearing loss. As formins interact with the barbed ends of actin filaments to stimulate their growth, Diaphanous genes may play a role in stereocilia growth and maintenance. Interestingly, mouse models overexpressing Diaphanous 3 have elongated and fused stereocilia in IHCs but not OHCs, leading to progressive hearing loss 20.
Using conditional mutants, it has been shown that Rac1, through its effector p21-activated kinase (PAK), and Cdc42, through its effector aPKC, both play key roles in HC PCP and hair bundle morphogenesis 21, 22. In Rac1 cKO cochleae, hair bundles were misoriented and flattened or misshapen with aberrantly positioned kinocilia. Similar defects were observed upon deletion of Cdc42 in OHCs and SCs at the onset of HC differentiation. Interestingly, deletion of Cdc42 in HCs at a later stage using a different Cre driver did not affect hair bundle PCP or staircase formation, and instead caused stereocilia maintenance defects in mature hair bundles 23. These data indicate non-redundant functions of Rac1 and Cdc42 in hair bundle formation and an additional role of Cdc42 in hair bundle maintenance.
A microtubule mediated machinery for kinocilium/basal body positioning
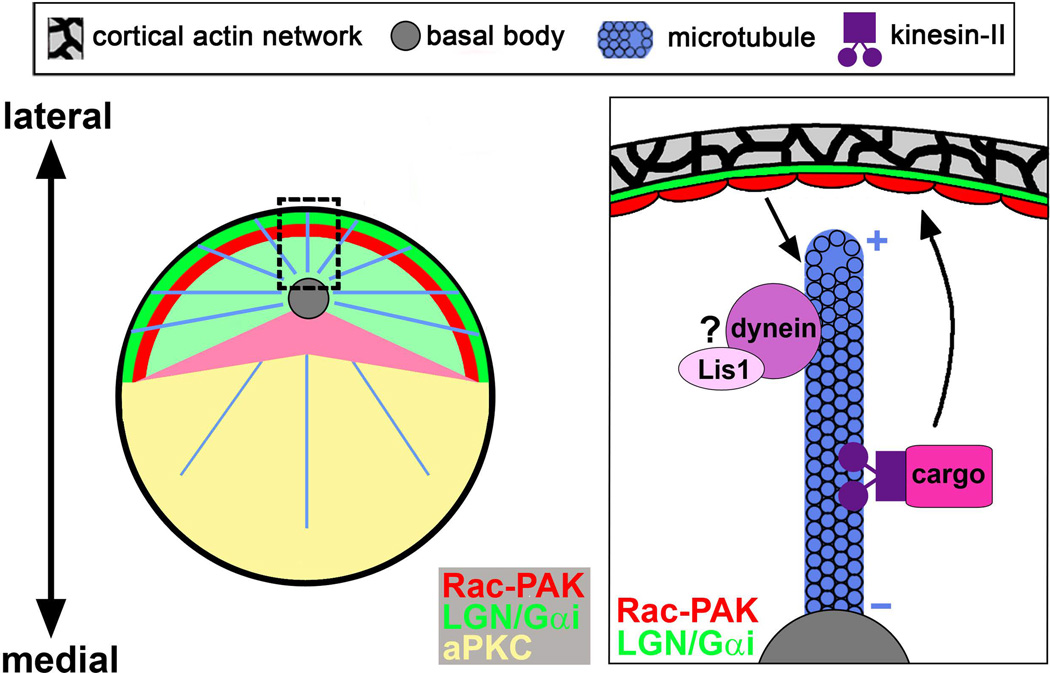
KIF3A is a component of the plus-end directed kinesin-II motor required for ciliogenesis. In Kif3a cKO cochleae, basal bodies are found at an aberrantly basal position in HCs and uncoupled from hair bundle orientation, which has not been reported in other ciliary mutants. Moreover, KIF3A regulates localized Rac-PAK activity on the HC cortex following the migration of the basal body to the lateral pole. Whereas it is asymmetrically enriched along the lateral borders of wild-type HCs, Rac-PAK activity is diffusely localized around HC borders in Kif3a cKO cochleae 11. These unique phenotypes implicate a nonciliary function of KIF3A, specifically MT-mediated intracellular transport, in regulating basal body positioning and Rac-PAK signaling. In addition to templating the kinocilium, the basal body anchors and organizes the cytoplasmic MT array of the HC. This network of MTs is presumed to be dynamic during embryogenesis, and it has been proposed that MT plus ends interact with the HC cortex via a “search and capture” mechanism 24, which anchors the basal body near the lateral pole and induces localized Rac-PAK activation on the HC cortex; kinesin-II/KIF3A likely facilitates Rac activation through cortical delivery of an as yet unidentified cargo (Figure 4).
Figure 4. Model for establishment of hair bundle polarity by microtubule-mediated hair cell-intrinsic machinery.
Left, en face diagram of an individual hair cell showing the distribution of proteins implicated in basal body positioning, colored according to the key. The nascent hair bundle is colored pink with the basal body (gray circle) at its vertex. Centriolar microtubules are depicted as blue lines. Right, magnified view of the boxed area on the left. During development, cortical LGN/Gαi recruits LIS1/dynein to exert force on centriolar microtubules, pulling the kinocilium/basal body toward the lateral hair cell cortex. Interaction of microtubule plus-ends with the hair cell cortex stimulates Rac-PAK signaling, likely through delivery of a kinesin-II cargo. In turn, localized Rac-PAK activity stabilizes microtubule cortical attachment to position the basal body appropriately. Plus and minus signs indicate the plus-and minus-end of the microtubule, respectively.
Recently, a converging set of studies has lent strong support for this model through functional dissection of an evolutionarily conserved pathway for asymmetric cell division (ACD) and mitotic spindle orientation in HC PCP (see Sidebar 1).
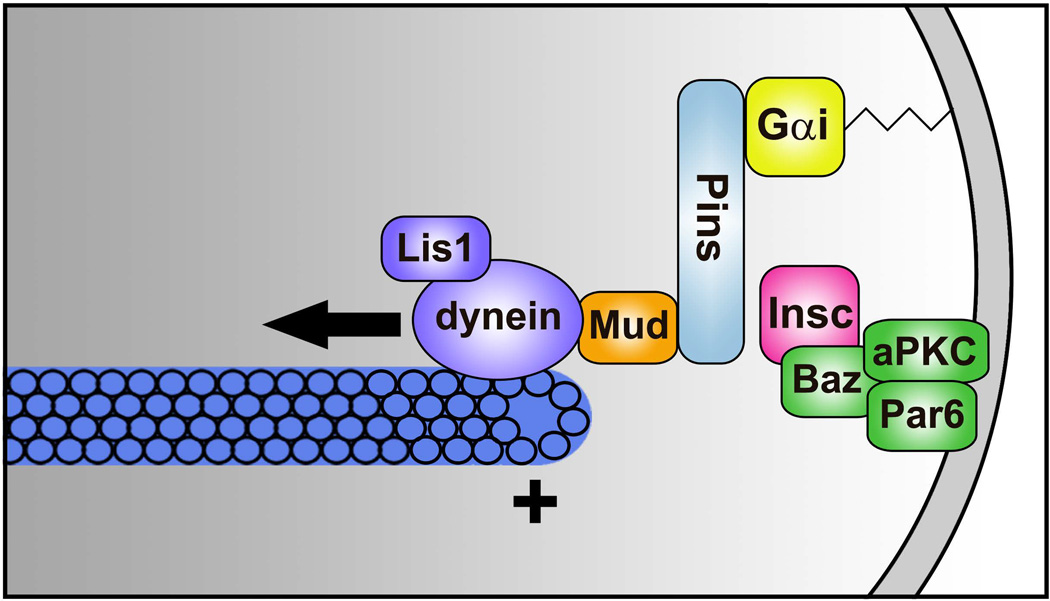
Sidebar 1. Asymmetric cell division in Drosophila neuroblasts.
Recent advances into understanding the molecular machinery for cell-intrinsic polarity have been informed by seminal investigations in the Drosophila neuroblast, where cortical polarity proteins act in concert with cytoplasmic dynein and astral MTs to orchestrate asymmetric cell division 25. Neuroblasts give rise to the fly central nervous system through repeated rounds of asymmetric divisions to produce another neuroblast and a smaller daughter cell with limited proliferative and fate potential. In Drosophila neuroblasts, asymmetric apical enrichment of the Par3/Par6/aPKC complex serves as a cortical polarity cue for spindle orientation (Figure 5). Binding of the adaptor protein, Inscuteable (ortholog of mammalian INSC), links the Par complex to Pins (ortholog of mammalian LGN/GPSM2 and AGS3/GPSM1). Pins, in turn, binds the membrane-anchored heterotrimeric G protein Gα and the MT and dynein-binding protein Mud (ortholog of mammalian NuMA), thereby coupling cell polarity with the mitotic spindle. Mud engages the cytoplasmic dynein motor to generate pulling force on astral MTs which appropriately orients the mitotic spindle for subsequent asymmetric division. The dynein regulator Lis1 is also required for neuroblast spindle orientation and regulates MT dynamics as well as cortical targeting of dynein 25. This spindle orientation machinery is highly conserved in mammals.
Remarkably, perturbations of LIS1, Gαi, LGN, or INSC result in basal body positioning and hair bundle shape and orientation defects, demonstrating a requirement of the conserved spindle orientation pathway for PCP in post-mitotic HCs 26–28. Of note, mutations in GPSM2, the human LGN homolog, cause Chudley-McCullough syndrome, a human hereditary disorder characterized by deafness and brain abnormalities, highlighting the importance of this pathway in brain and HC development 29.
In the cochlea, many of these proteins show polarized distributions at the apical surface of HCs. LGN, INSC, and Gαi are localized to a lateral “bare zone” devoid of microvilli (Figure 3C and 4), which is opposed by a complementary, medial domain of aPKC-Par6b. These domains abut the base of the nascent hair bundle. In one study, Par3 is localized to the lateral bare zone and cell junction, suggesting that Par3 may recruit LGN/Gαi to the lateral cell cortex via binding to INSC, analogous to its role in Drosophila neuroblasts 28. The localization of Par3 to the opposite side of the cell from aPKC-Par6b 28 suggests that regulation of cortical polarity cues in HCs may be different from Drosophila neuroblasts or the C. elegans zygote 25. The asymmetric cortical aPKC localization in HCs is regulated by Gαi and Cdc42 22, 27. Moreover, the LGN/Gαi/dynein module likely forms a positive feedback loop with cortical Rac-PAK signaling to establish basal body position, as LIS1-deficient HCs show reduced Rac-PAK activity that correlates with MT organization defects 26.
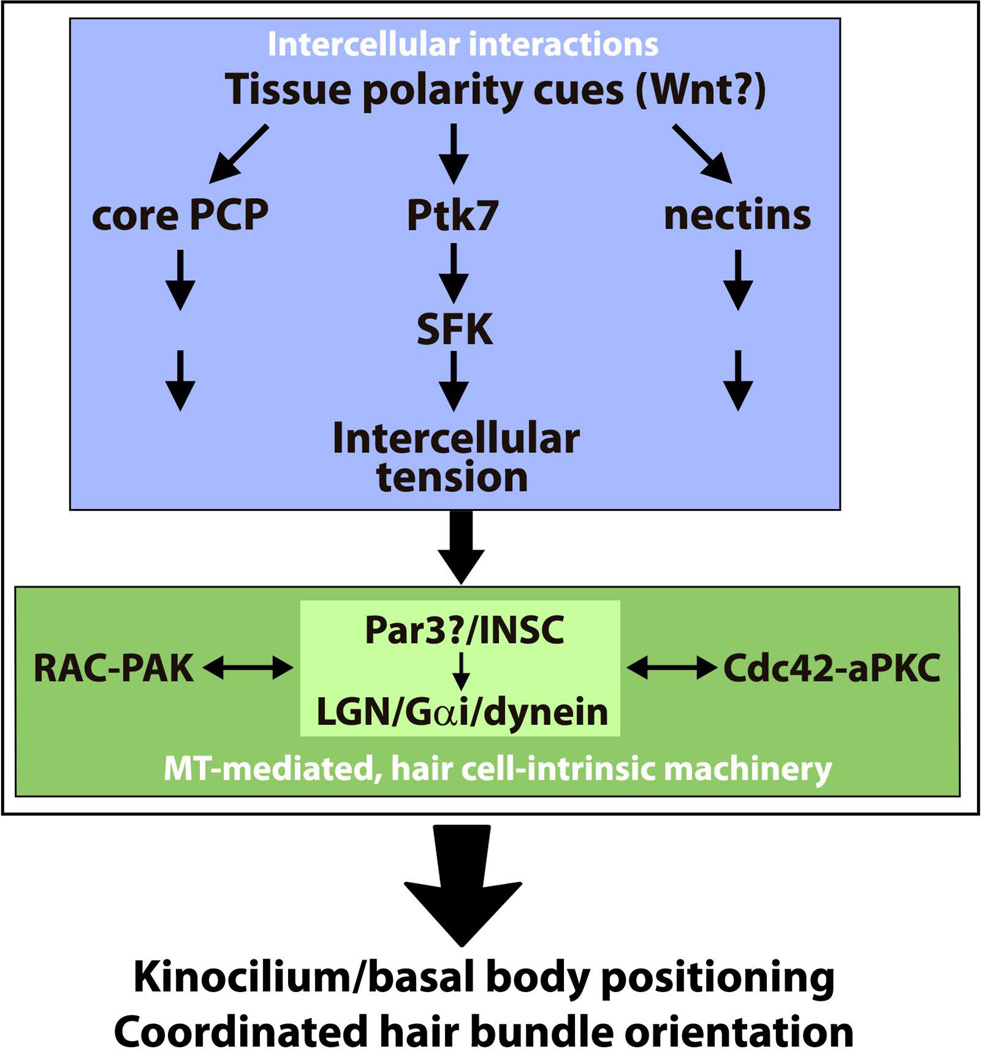
Together, these data suggest that Rac-PAK signaling, Cdc42-aPKC signaling and the LGN/Gαi/dynein pathway coordinately regulate basal body positioning by promoting MT capture at the lateral pole of HCs (Figure 4). Although interactions between these cell-intrinsic pathways are incompletely understood, it has been proposed that they form a positive feedback loop whereby cortical MT capture stimulates localized Rac-PAK activity that in turn stabilizes cortical MT attachment to position the basal body (Figures 4 and 6).
Figure 6. A two-tier model of the establishment of planar cell polarity.
Coordinated kinocilium/basal body positioning and hair bundle orientation is achieved through the integration of intercellular signaling (blue box) with microtubule-mediated, hair-cell intrinsic polarity machinery (green box).
G-protein signaling mediated migration of the kinocilium/basal body
While mechanisms for basal body positioning are emerging, less is known about how the centrifugal migration of the kinocilium/basal body is initiated, as the same genetic mutations do not abolish kinocilium migration significantly. However, treatment of cochlear explants with pertussis toxin, which inhibits Gα signaling, partially disrupts kinocilium migration 27. A small fraction of treated HCs form circular hair bundles with a centrally-placed kinocilium. Therefore, it is likely that in addition to Gαi, other G proteins are involved in kinocilium/basal body migration.
Coordination of hair bundle orientation by intercellular interactions
Because HCs are separated from one another by intervening SCs, the uniform orientation of hair bundles along the medial-lateral axis of the cochlea must involve intercellular interactions between HCs and SCs. In the last decade, three intercellular signaling pathways have been identified to orient PCP in the OC.
The Wnt/PCP pathway
First discovered in Drosophila, the evolutionarily conserved core PCP pathway regulates polarized cell behaviors during tissue morphogenesis, including convergent extension movements during axis elongation and neural tube closure. The core components of the PCP pathway include the transmembrane proteins Frizzled (Fzd), Van Gogh (Vangl) and Flamingo/Celsr as well as the cytoplasmic proteins Dishevelled (Dvl1–3), Prickle and Diego/Ankrd6. In the mammalian inner ear, the core PCP proteins FZD3/6, VANGL1/2, DVL2/3 and Ankrd6 show asymmetric membrane localization in the OC, and disruption of the PCP pathway causes a characteristic misorientation of hair bundles and a shortened cochlear duct 30, 31. Wnt/PCP signaling aligns hair bundle orientation by spatially coordinating asymmetric cortical domains of Rac-PAK signaling and regulates E-cadherin-mediated intercellular adhesion 21, 32. In addition to HC PCP, Vangl2 has been shown to regulate the position and shape of phalangeal processes of Deiters’ cells (see Figure 2A) during postnatal development 33.
A number of genes have been identified as modulators of the core PCP pathway. They include the receptor tyrosine kinases Ryk and Ror1/2, the apical-basal polarity protein Scribbled, the COPII coat protein Sec24b and the E3 ubiquitin ligases Smurf1/2 34, 35. Notably, although most cilia-related mutants do not affect asymmetric localization of core PCP proteins, the ciliary proteins BBS8 and IFT20 have been shown to interact with the core PCP protein VANGL2 and regulate its localization, suggesting a possible nonciliary function in VANGL2 targeting 12.
The PTK7/SFK pathway
Protein tyrosine kinase 7 (Ptk7), which encodes a conserved receptor-tyrosine pseudokinase, was identified by gene-trapping as a novel regulator of PCP in vertebrates 36. While Ptk7 is required for many of the same developmental processes controlled by the Wnt/PCP pathway, including convergent extension, neural tube closure and HC PCP, genetic and molecular analysis indicates that PTK7 acts in parallel to the Wnt/PCP pathway and signals through Src family kinases (SFK) to regulate actomyosin contractility and intercellular tension in the OC 37, 38. The tension-sensitive actin-binding protein vinculin is preferentially recruited to the medial borders of HCs in a PTK7-dependent manner, providing evidence for anisotropic tension in the OC. These data have led to a model whereby “tug-of-war” interactions between HCs and SCs impinge on the MT-mediated machinery for basal body positioning to orient PCP; increased tension on the medial borders of HCs may locally inhibit MT cortical capture, thereby favoring MT capture at the lateral pole.
Nectin-mediated cell-cell adhesions
Nectins are Ca2+-independent IG-like cell-adhesion molecules that interact in-trans to induce intercellular adhesion through activation of Rac and Cdc42 39. Nectin-1 and −3 are differentially expressed in HCs and SCs, respectively, and their heterotypic interaction is required for the cellular mosaic in the OC. In Nectin-3 deficient mice, some HCs are aberrantly in contact with one other, accompanied by hair bundle morphology and kinocilium positioning defects, similar to Rac1 cKO phenotypes 40. These results suggest that nectin-mediated HC-SC adhesion may activate Rac to control HC PCP. Conversely, both Rac activity and nectin-1/3 localization are reduced in Lis1 cKO OC, suggesting that Rac may regulate nectin localization 26.
A two-tier signaling hierarchy mediates HC PCP in the OC
How does tissue-level PCP signaling integrate with the cell-instrinsic MT-mediated machinery? Accumulating evidence strongly supports a two-tier hierarchy of HC planar polarity regulation (Figure 6). PCP of individual HCs, including polarized basal body position and the V-shaped hair bundle, is established by a cell-instrinsic effector machinery consisting of the LGN/Gα/dynein, Rac-PAK and Cdc42-aPKC modules. These cell-intrinsic pathways are capable of operating in the absence of tissue polarity cues to drive planar polarization of individual cells, as core PCP genes or Ptk7 are not required for planar polarity features of individual HCs. Nor is polarized distribution of cell-intrinsic polarity proteins disrupted in core PCP mutants 21, 27, 28. The robustness of the intrinsic polarization process is likely ensured by feedback interactions among the above-mentioned modules. Tissue-level PCP signaling superimposes extrinsic or tissue polarity cues on HCs through intercellular interactions that are in turn interpreted by the cell-intrinsic effector machinery. Although the precise mechanism of this crosstalk is unknown, Rho family GTPases such as Rac and cortical polarity proteins such as Par3 are well positioned to integrate tissue polarity information. Interestingly, there is evidence that tissue-level and cell-intrinsic PCP signaling may have non-overlapping spatial and temporal domains. Specifically, cortical LGN/Gαi and aPKC occupy a more apical domain than Vangl2 along the apical-basal axis of HCs in the P0 OC, indicating a spatial segregation of the two systems at this stage 27. Moreover, the cell-instrinsic pathway is required to maintain HC planar polarity, as revealed by postnatal deletion of Lis1 26. This activity likely contributes to the postnatal refinement of HC PCP that occurs independently of the core PCP pathway 33.
Transient stereociliary links that regulate hair bundle cohesion
Transient lateral links
Hair bundle formation is coincident with the establishment of PCP, during which stereocilia and the kinocilum within the nascent hair bundle must be held together as a cohesive unit via stereociliary links for normal PCP execution. Mouse models of Usher syndrome (USH, see Sidebar 2) have been instrumental in the identification of molecular composition of stereociliary links. In addition to the kinociliary links, stereocilia in the nascent hair bundle are interconnected via tip links and transient lateral links (Figure 2B). All three types of links are formed by heterophilic adhesion complexes composed of homodimers of the USH1 proteins PCDH15 and cadherin-23 (CDH23) (Table 1). Null mutations in Cdh23 and Pcdh15 as well as three other USH1 genes cause fragmentation and misorientation of nascent hair bundles, accompanied by aberrantly positioned kinocilia, indicating the role of USH1 genes in organizing kinociliary and interstereociliary links that maintain hair bundle cohesion during both PCP establishment and maintenance 41.
• Sidebar 2: Usher syndrome.
User syndrome is the leading genetic cause of combined hearing and vision loss in humans 17. It is categorized into three types, based on the degree of hearing loss, vestibular deficits and vision loss. Usher syndrome type 1 (USH1) is characterized by severe-to-profound hearing loss, vestibular deficits and early onset vision loss. Usher syndrome type 2 (USH2) is characterized by mild-to-severe hearing loss and normal vestibular function. Usher syndrome type 3 (USH3) manifests with progressive hearing loss and variable onset of vision loss. USH2 is most common, followed by USH1 and USH3. To date, six genes have been implicated in USH1, which encode PCDH15, CDH23, the scaffold proteins harmonin and SANS, the actin motor protein myosin VIIa, and the calcium and integrin-binding protein CIB2. The three USH2 genes encode the transmembrane protein usherin, the adhesion G protein coupled receptor GPR98 (also known as VLGR1) and PDZ-domain containing scaffold protein whirlin, respectively. One USH2 modifier encodes the scaffold protein PDZD7. The USH3 genes are clarin-1 (CLRN1) and histidyl-tRNA synthetase (HARS). Finally, CEP250, which encodes a centrosome-associated protein, is mutated in atypical USH.
Table 1.
| Link type | Composition | Appearance in mouse |
Associated proteins /regulators |
Associated human deafness loci |
|
|---|---|---|---|---|---|
| Permanent | Tip | CDH23 PCDH15 |
E17.5 | myosin VIIa, SANS, harmonin TMHS/LHFPL5 |
USH1* |
| Horizontal top connectors |
stereocilin | P9 | None reported | DFNB16 | |
| Transient | Tansient lateral links / Shaft connectors |
CDH23 PCDH15 / PTPRQ? |
E17.5 | myosin VIIa, SANS, harmonin |
USH1* |
| Kinociliary | CDH23 PCDH15-CD2 |
E17.5 | myosin VIIa, SANS, harmonin |
USH1* | |
| Ankle | GPR98 VLGR1 usherin |
P0 | PDZD7, whirlin | USH2* |
This link type is also associated with additional forms of nonsydromic hearing loss. See http://hereditaryhearingloss.org for a comprehensive list.
Moreover, mice deficient for the USH3 gene Clrn1 have fragmented hair bundles with circular miniclusters of stereocilia and progressive OHC and SC degeneration, leading to early onset hearing loss. Clrn1 encodes a four transmembrane protein that is localized to the hair bundle and may regulate hair bundle cohesion in concert with the USH1 genes 17.
Nherf1, a PDZ domain protein that interacts with ERM (Ezrin, Radixin and Moesin) family cytoskeleton-plasma membrane linkers, has also been implicated in hair bundle shape and cohesion. Nherf1 is localized to stereocilia and interacts with CDH23. Nherf1-deficient mice have dysmorphic OHC hair bundles with mislocalized kinocilia in the basal and middle region of the cochlea, resulting in hearing deficits 42.
Ankle links
Ankle links are located above the basal tapers of stereocilia (Figure 2B). They are present only during the first postnatal week in mammalian cochlear HCs but persist in mature vestibular HCs (Table 1). The functional importance of the ankle links is revealed by mouse models for USH2 (see Sidebar 2). USH2 proteins usherin and GPR98/VLGR1 form the ankle links through their heterotypic interaction. The PDZ-domain containing proteins whirlin and PDZD7 interact with usherin and GPR98 and together form an interdependent ankle link complex, which may also contain the transmembrane protein vezatin and the adenylyl cyclase AC6. Importantly, disruption of the USH2 proteins or PDZD7 leads to disorganized hair bundles and congenital deafness, indicating that ankle links play an essential developmental role in hair bundle maturation and cohesion 17.
Interstereociliary links in the adult hair bundle
The tip links and the MET machinery
In addition to kinociliary and transient lateral links, USH1 proteins mediate formation of tip links, which persist in the adult hair bundle and are involved in the mechanical gating of the MET channels located at their lower insertion point, namely the tips of shorter stereocilia 5. Homodimers of PCDH15, specifically the CD2 splice isoform, and homodimers of CDH23 form the lower and upper parts of tip links, respectively 43. Transmission electron microscopy evidence suggests that the tip links are attached to electron-dense patches underneath the membrane. The USH1 proteins harmonin, SANS, myosin VIIa and CIB2 are localized to these patches and may play a role in anchoring the tip links. The loss of USH1 proteins results in compromised mechanotransduction and hearing loss, demonstrating a critical role of tip links in MET 43.
It is suggested that the MET channel is directly coupled to the tip link and likely forms a complex with PCDH15. Although the molecular composition of the MET channel is still unknown, recent advances have identified several transmembrane proteins critically required for HC mechanotransduction and function in close association with the MET channel 5. Specifically, multispan transmembrane proteins encoded by the deafness genes TMC1, TMC2, TMIE and TMHS/LHFPL5 all interact with PCDH15 and are likely subunits of the MET channel. TMC1/2 have been proposed to be the pore-forming subunits the MET channel, though this model is still under debate and awaits further evidence. TMIE and TMHS/LHFPL5 may tether the MET channel to tip links. While TMIE is dispensable for tip link assembly, TMHS/LHFPL5 has multiple proposed functions, including mediating tip-link assembly, stereocilia targeting of both PCDH15 and TMC1 and serving as an allosteric regulator of the MET channel 44, 45.
While tip links are clearly involved in normal HC mechanotransduction, there is some evidence that HCs can still transduce mechanical stimuli in their absence, which may involve other types of extracellular links. For example, in myosin-XVa-deficient mice, although IHCs have hair bundles with abnormally short stereocilia that lack tip links, they can still generate MET currents in response to deflection by a stiff probe 46. Furthermore, anomalous transducer currents with reversed mechanosensitivity can still be evoked in the absence tip links or putative MET channel subunits, suggesting that these conditions may alter normal properties of the MET channel or unmask other channels that can also contribute to the mechano-responsiveness 5.
Horizontal top connectors
Horizontal top connectors are located near stereocilia tips and maintain the cohesiveness of the mature OHC hair bundle. Stereocilin, encoded by the causative gene for DFNB16, is localized to and required for the formation of horizontal top connectors. Stereocilin also mediates the attachment between the tallest stereocilia and the tectorial membrane. Interestingly, while hair bundle development and function is initially normal, including tip-link formation, stereocilin-deficient mice have progressive hearing loss accompanied by progressive loss of tip links and disconnection of stereocilia. Thus, stereocilin is essential for the cohesion and stiffness of the adult OHC hair bundle and tip-link stability 47.
Hair bundle maintenance
Stereocilia base-apical plasma membrane attachment
Stereocilia membranes partition into distinct domains along their length, each containing specific lipids and proteins 48. Sialic acid-containing glycosphingolipids, also called gangliosides, are enriched in the taper domain at the stereocilia base. Phosphatidylinositol 4,5-bisphosphate, on the other hand, is absent from the basal taper region but localized throughout the tip and shaft of stereocilia.
A protein complex localized at the stereocilia base and HC apical membrane plays a crucial role in tethering actin filaments to the plasma membrane. These include PTPRQ, a receptor-like inositol lipid phosphatase, the ERM family member radixin, CLIC5, a cytoskeletal linker and the novel protein taperin. The localization of this complex to the taper region is mediated by the pointed-end directed motor myosin VI, which acts as both a transporter and cytoskeleton-plasma membrane linker 49. Moreover, the ganglioside GM3 is required for the compartmentalization of PTPRQ and myosin VI at the stereocilia base 50. Mutations affecting any of these proteins, or the GM3 synthase ST3GAL5 cause inherited deafness in humans and/or mice. A phenotype shared among these mutant mice is stereocilia degeneration in OHCs and fusion of IHC stereocilia starting from the second postnatal week, leading to progressive HC death 50. Similar phenotypes were observed in mice with HC-specific deletion of Cdc42 using an Atoh1-Cre driver or deficient for the Nherf1 paralog Nherf2 23, 42. Together, these observations suggest that membrane and cytoskeletal proteins in the taper domain participate in an active process regulated by Cdc42 that stabilizes connections between the plasma membrane and actin cytoskeleton at the stereocilia base.
PTPRQ is also proposed to mediate the formation of shaft connectors, which are functionally similar to transient lateral links. Interestingly, miR-96, the first microRNA linked to inherited deafness, mediates postnatal morphological and functional maturation of cochlear HCs at least in part through upregulation of Ptprq expression 51.
Regulation of hair bundle morphogenesis by GTPase signaling
Emerging evidence implicates additional GTPase signaling pathways in hair bundle morphogenesis. Members of the engulfment and cell motility (ELMO) protein family, ELMOD1 and ELMOD3, have been implicated in inherited deafness in mice and humans 52. ELMOD3 is dynamically expressed in HCs and enriched in stereocilia, the kinocilium and the apical bare zone. Mouse Elmod1 mutations cause stereocilia degeneration in OHCs and fusion of IHC stereocilia during postnatal development, resulting in deafness and balance deficits. ELMOD1–3 possesses GTPase-activating protein (GAP) activity against ARL2, an Arf family small GTPase that regulates MT dynamics. Thus, ELMOD proteins may regulate hair bundle integrity by modulating small GTPase signaling in stereocilia and the kinocilium, though the relevant small GTPases in HCs have not been identified.
TBC1D24 is another putative regulator of GTPase signaling implicated in both the syndromic deafness DOORS (onychodystrophy, osteodystrophy, mental retardation, and seizures) syndrome and the nonsyndromic DFNB86 53. Moreover, missense mutations in TBC1D24 are linked to autosomal-dominant hearing loss 54. TBC1D24 interacts with and may regulate ARF6, a small GTPase crucial for membrane trafficking. TBC1D24 is localized to stereocilia during development and also expressed in spiral ganglion neurons. Therefore, TBC1D24 may have multiple functions in the cochlea that await further investigation.
Recently, mutations in FAM65B, which encodes a cytoplasmic protein that interacts with and inhibits RhoA 55, have been linked to an inherited form of deafness in humans. FAM65B is localized to stereocilia and the HC apical membrane. Knockdown of fam65b in zebrafish resulted in a decreased number of HCs and hearing loss. However, its precise role in hair bundle morphogenesis is unknown 56.
Regulation of stereocilia length and stability
Numerous regulators of actin polymerization and bundling are implicated in inherited deafness, indicating that actin filament length and turnover in stereocilia must be tightly controlled to ensure normal hair bundle function. Stereocilia are comprised of β-actin and γ-actin, and the absence of either results in stereocilia defects and progressive hearing loss 57.
Live imaging and quantitative analysis revealed that stereocilia actin turns over very slowly with the exception of the stereocilia tips, and shorter rows of stereocilia have a higher actin turnover rate 7, 58. Several actin regulatory proteins are transported by myosin motors to the stereocilia tips and regulate length of stereocilia during development. Specifically, espin 1, an isoform of the actin bundling protein espin, is transported by myosin IIIa to promote stereocilia elongation 59. Another tip complex required for stereocilia elongation is composed of myosin XVa and its cargos, the scaffold protein whirlin and the actin capping/bundling protein EPS8 60. Absence of either component of this complex results in shortened stereocilia and deafness. Whereas EPS8 is required for stereocilia elongation during development, its paralog, EPS8L2, is implicated in progressive hearing loss and regulates stereocilia length and maintenance in the adult cochlea 61. The tip-links may have a positive effect on stereocilia length in the short and middle rows since their loss during development causes the shorter rows of stereocilia to shorten. On the other hand, stereocilia length in the mature hair bundle is negatively regulated by myosin VIIa and twinfilin, an actin monomer binding and barbed-end capping protein. Recent evidence suggests that myosin VIIa transports twinfilin-2 preferentially to the tips of shorter stereocilia rows, where it inhibits actin polymerization 62.
In addition, the length and stability of stereocilia are regulated by a number of actin bundling and cross-linking proteins localized throughout stereocilia. Espin, implicated in inherited deafness, is a major actin bundling protein required for stereocilia growth and maintenance during postnatal development 63. TRIOBP, an actin bundling protein localized to the stereocilia rootlets, is required for rootlet formation and stereocilia rigidity, which are essential for mechanotransduction 64. Other actin-crosslinking and bundling proteins that play a role in stereocilia maintenance include fascin-2, plastin 1 and Xin-actin-binding-repeat containing protein 2 (XIRP2). In the absence of these cross-linkers, stereocilia frequently show progressive shortening and degeneration, resulting in hearing loss 65–67.
Stereocilia repair/regeneration following noise induced damage
Exposure to loud noise damages the organ of Corti, leading to noise-induced hearing loss (NIHL). Destruction of cochlear hair cells and damage to their hair bundles are major contributing factors to NIHL. Depending on the level of noise and the duration of exposure, NIHL may be temporary if noise-induced damage is repaired. However, the loss becomes permanent when damaged hair cells or neurons die, and currently there are no existing pharmacological treatments.
Tip links experience tension in response to sound and therefore are prone to noise-induced breakage. Broken tip links typically re-form within 24 hours. A recent study has revealed that tip-link regeneration occurs through a two-step mechanism that recapitulates their formation during development 68. Initially, PCDH15 forms both ends of the new tip link with CDH23 subsequently replacing PCDH15 at the upper end, thereby restoring MET.
Another early sign of acoustic damage to the hair bundle is the appearance of breaks in the stereocilia actin core. Interestingly, γ-actin, but not β-actin, is localized to the break points and mediates repair of actin bundles. γ-actin is also required for routine stereocilia maintenance and repair not induced by noise 69.
In the mammalian OC, damaged hair cells can survive for well over a week after acoustic trauma, with limited hair bundle repair 70. Thus, stimulating hair cell repair through genetic or chemical interventions can tip the balance between hair cell death and functional recovery, thereby ameliorating permanent hearing loss. In addition to its intensely studied role in hair cell differentiation and regeneration, the bHLH transcription factor ATOH1 has been shown to promote hair bundle repair in noise-damaged adult cochlea 70. Importantly, Atoh1 transfection before detectable hair cell death significantly stimulated hair bundle repair and restored hearing thresholds. Dissecting the mechanisms of ATOH1-mediated hair bundle repair, which may be different from ATOH1 function in hair cell regeneration, will likely identify new therapeutic targets for hair bundle repair.
Development of tonotopy in the OC
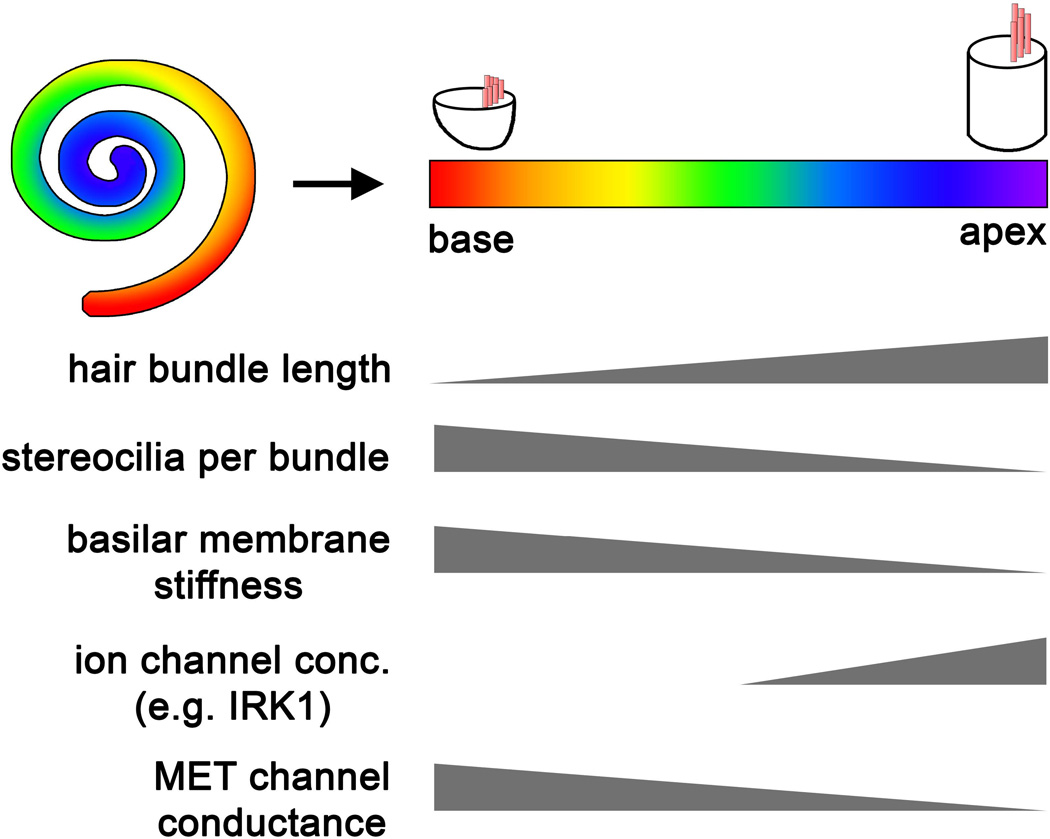
A remarkable feature of the mammalian auditory system is the topological representation of different frequency components of sound signals, or tonotopy. Tonotopic organization begins at the cochlea and is maintained along both the peripheral and central auditory pathways. The tonotopic layout of the OC is such that high frequency sounds stimulate HCs at the base of the cochlea, whereas low frequency sounds stimulate the apex, like the arrangement of a piano keyboard. Along the tonotopic or longitudinal axis of the cochlea, HCs display systematic morphological and physiological differences to allow precise frequency tuning. These include the stereocilia length and number per bundle, the expression levels and isoforms of various ion channels as well as MET channel conductance (Figure 7). Interestingly, this tonotopic gradient in conductance has recently been shown to be dependent on the TMHS/LHFPL5 and TMC1, providing evidence for tonotopic variation of MET channel composition 45. The mechanisms by which the tonotopic gradient is established and maintained are poorly understood, and to date there are no reports of congenital disease attributable to tonotopic defects. Several studies in the chick hearing organ (basilar papilla or BP), which also develops tonotopy, have provided some clues about the underlying molecular pathways. For example, Delta/Notch-like EGF-related receptor (DNER), a novel Notch ligand, is expressed in HCs in a proximal-to-distal increasing gradient and its perturbation results in hair bundle morphology and orientation defects 71. A pair of parallel studies show that a proximal-to-distal increasing gradient of Bmp7 synergizes with a gradient of retinoic acid (RA) signaling to promote distal identity 72, 73. Recently, Sonic Hedgehog (Shh) signaling has been shown to establish positional identity in both the chick BP and mouse cochlea, albeit through non-conserved downstream targets. While Shh signaling upregulates Bmp7 expression in the BP, a tonotopic gradient of Bmp7 is not observed and Bmp pathways are down-regulated by Shh in the developing mouse cochlea 74. Together, these studies suggest that multiple signaling gradients cooperate early in development to establish initial regional identities that prefigure the tonotopic organization of the mature cochlea.
Figure 7. Schematic representation of tonotopic gradients along the basal-apical axis of the mammalian cochlea.
Graded differences in morphological and molecular features underlie regional differences in the mechanical and electrical properties of hair cells.
New approaches for studying HC development and function
Next-generation sequencing technologies have allowed rapid identification of novel human and mouse deafness genes. To complement and extend gene discovery efforts, several emerging technologies hold great promise in aiding functional analysis and dissection of the disease mechanisms of deafness genes. For example, a recent study using single cell gene expression profiling has provided a fine-resolution reconstruction of spatial and temporal dynamics of signaling pathways in developing neuroblasts in the inner ear anlage 75. Application of single-cell transcriptome analysis will be particularly advantageous for profiling rare or heterogeneous populations of inner ear cells to address important outstanding questions such as cell-fate commitment and acquisition of tonotopy. A complementary approach is the development of immortalized multipotent otic progenitor (iMOP) cells that can self-renew and retain the ability to differentiate into functional HCs and neurons 76. These cells are a useful in vitro tool for identification of differentiation factors required for specific inner ear cell lineages as well as high throughput screening for agents that promote HC repair and regeneration.
For in vivo analysis of gene function in hearing, tissue-specific knockdown of genes of interest has been achieved via direct injection of siRNAs into the inner ear 77, 78. However, concerns about efficiency, specificity and duration of target knockdown complicate this approach. A transformative new technology for targeted genome engineering in eukaryotic cells and embryos, the bacterial CRISPR/Cas system 79, has enormous potential for studying human hearing and balance disorders. This system exploits the RNA-guided endonuclease activity of Cas9 to introduce site-specific double-strand breaks that can be repaired via either non-homologous end joining or homology-directed repair. Thus, this technology will allow rapid generation of targeted mutations in deafness genes in popular animal models such as the mouse, zebrafish and even the guinea pig, in which no transgenic methods are currently available. Indeed, CRISPR technology has been successfully applied to reveal the function of the hair bundle protein XIRP2 in stereocilia maintenance and hearing in mice 67. Recently, direct modification of mammalian HCs has been achieved by in vivo co-delivery of Cas9 and a guide RNA, suggesting the exciting therapeutic potential of this approach for human hearing loss 80.
Conclusion
[To transduce sound signals faithfully, HCs develop a suite of precise features, including the planar polarized hair bundle that harbors the MET channel. Genetic analysis in humans and model organisms has greatly advanced our understanding of this complex developmental process. In particular, intercellular PCP signaling impinges on a MT-mediated cell-intrinsic machinery to establish the polarized shape and orientation of the hair bundle. Moreover, USH proteins are required for different types of stereociliary links critical for hair bundle cohesion and HC mechanotransduciton. Several subunits of the MET channel have been identified and shown to interact with the tip-link component PCDH15. Finally, BMP, RA and SHH gradients in the developing OC regulate aspects of tonotopy.
In the future, a multidisciplinary approach combining genetics with modern molecular and cell biology tools will continue to shed light on unresolved questions in hair bundle morphogenesis, including the nature and origin of tissue polarity signals, integration of PCP signaling and staircase formation, the molecular composition of the MET channel, and the development and maintenance of tonotopy.
Figure 5. Mechanisms for asymmetric cell division of Drosophila neuroblasts.
Several protein complexes localize to the apical neuroblast cortex during mitosis to regulate asymmetric cell division. Insc links the Baz/Par6/aPKC complex to Pins, which in turn forms a complex with Gαi and Mud, thereby coupling cell polarity with mitotic spindle orientation. Mud engages the cytoplasmic dynein motor to exert force on astral microtubules to orient the spindle.
Acknowledgements
This work was supported by the National Institutes of Health (NIH) [grant R01 DC DC013773 to X.L]. We apologize to researchers whose original work was not cited due to space constraints.
Footnotes
The authors declare no conflict of interests.
Contributor Information
Xiaowei Lu, Department of Cell Biology, University of Virginia, xl6f@virginia.edu.
Conor W. Sipe, Department of Biology, University of Virginia.
References
- 1.Nicolson T. The genetics of hearing and balance in zebrafish. Annu Rev Genet. 2005;39:9–22. doi: 10.1146/annurev.genet.39.073003.105049. [DOI] [PubMed] [Google Scholar]
- 2.Schwander M, Sczaniecka A, Grillet N, Bailey JS, Avenarius M, Najmabadi H, Steffy BM, Federe GC, Lagler EA, Banan R, et al. A forward genetics screen in mice identifies recessive deafness traits and reveals that pejvakin is essential for outer hair cell function. J Neurosci. 2007;27:2163–2175. doi: 10.1523/JNEUROSCI.4975-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.White JK, Gerdin AK, Karp NA, Ryder E, Buljan M, Bussell JN, Salisbury J, Clare S, Ingham NJ, Podrini C, et al. Genome-wide generation and systematic phenotyping of knockout mice reveals new roles for many genes. Cell. 2013;154:452–464. doi: 10.1016/j.cell.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Furness D, Hackney C. The Structure and Composition of the Stereociliary Bundle of Vertebrate Hair Cells. In: Eatock R, Fay R, Popper A, editors. Vertebrate Hair Cells. Vol. 27. New York: Springer; 2006. pp. 95–153. [Google Scholar]
- 5.Fettiplace R, Kim KX. The physiology of mechanoelectrical transduction channels in hearing. Physiol Rev. 2014;94:951–986. doi: 10.1152/physrev.00038.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deans MR. A balance of form and function: planar polarity and development of the vestibular maculae. Semin Cell Dev Biol. 2013;24:490–498. doi: 10.1016/j.semcdb.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang DS, Piazza V, Perrin BJ, Rzadzinska AK, Poczatek JC, Wang M, Prosser HM, Ervasti JM, Corey DP, Lechene CP. Multi-isotope imaging mass spectrometry reveals slow protein turnover in hair-cell stereocilia. Nature. 2012;481:520–524. doi: 10.1038/nature10745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobo A, Hudspeth AJ. Reaction-diffusion model of hair-bundle morphogenesis. Proc Natl Acad Sci U S A. 2014;111:15444–15449. doi: 10.1073/pnas.1417420111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sung CH, Leroux MR. The roles of evolutionarily conserved functional modules in cilia-related trafficking. Nat Cell Biol. 2013;15:1387–1397. doi: 10.1038/ncb2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones C, Roper VC, Foucher I, Qian D, Banizs B, Petit C, Yoder BK, Chen P. Ciliary proteins link basal body polarization to planar cell polarity regulation. Nat Genet. 2008;40:69–77. doi: 10.1038/ng.2007.54. [DOI] [PubMed] [Google Scholar]
- 11.Sipe CW, Lu X. Kif3a regulates planar polarization of auditory hair cells through both ciliary and non-ciliary mechanisms. Development. 2011;138:3441–3449. doi: 10.1242/dev.065961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.May-Simera HL, Petralia RS, Montcouquiol M, Wang YX, Szarama KB, Liu Y, Lin W, Deans MR, Pazour GJ, Kelley MW. Ciliary proteins Bbs8 and Ift20 promote planar cell polarity in the cochlea. Development. 2015;142:555–566. doi: 10.1242/dev.113696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zaghloul NA, Katsanis N. Mechanistic insights into Bardet-Biedl syndrome, a model ciliopathy. The Journal of Clinical Investigation. 2009;119:428–437. doi: 10.1172/JCI37041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barker AR, Thomas R, Dawe HR. Meckel-Gruber syndrome and the role of primary cilia in kidney, skeleton, and central nervous system development. Organogenesis. 2014;10:96–107. doi: 10.4161/org.27375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jagger D, Collin G, Kelly J, Towers E, Nevill G, Longo-Guess C, Benson J, Halsey K, Dolan D, Marshall J, et al. Alstrom Syndrome protein ALMS1 localizes to basal bodies of cochlear hair cells and regulates cilium-dependent planar cell polarity. Hum Mol Genet. 2011;20:466–481. doi: 10.1093/hmg/ddq493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grati M, Chakchouk I, Ma Q, Bensaid M, Desmidt A, Turki N, Yan D, Baanannou A, Mittal R, Driss N, et al. A missense mutation in DCDC2 causes human recessive deafness DFNB66, likely by interfering with sensory hair cell and supporting cell cilia length regulation. Hum Mol Genet. 2015;24:2482–2491. doi: 10.1093/hmg/ddv009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mathur P, Yang J. Usher syndrome: Hearing loss, retinal degeneration and associated abnormalities. Biochim Biophys Acta. 2015;1852:406–420. doi: 10.1016/j.bbadis.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Webb SW, Grillet N, Andrade LR, Xiong W, Swarthout L, Della Santina CC, Kachar B, Muller U. Regulation of PCDH15 function in mechanosensory hair cells by alternative splicing of the cytoplasmic domain. Development. 2011;138:1607–1617. doi: 10.1242/dev.060061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 20.Schoen CJ, Burmeister M, Lesperance MM. Diaphanous homolog 3 (Diap3) overexpression causes progressive hearing loss and inner hair cell defects in a transgenic mouse model of human deafness. PLoS One. 2013;8:56520. doi: 10.1371/journal.pone.0056520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grimsley-Myers CM, Sipe CW, Geleoc GS, Lu X. The small GTPase Rac1 regulates auditory hair cell morphogenesis. J Neurosci. 2009;29:15859–15869. doi: 10.1523/JNEUROSCI.3998-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirjavainen A, Laos M, Anttonen T, Pirvola U. The Rho GTPase Cdc42 regulates hair cell planar polarity and cellular patterning in the developing cochlea. Biol Open. 2015;4:516–526. doi: 10.1242/bio.20149753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ueyama T, Sakaguchi H, Nakamura T, Goto A, Morioka S, Shimizu A, Nakao K, Hishikawa Y, Ninoyu Y, Kassai H, et al. Maintenance of stereocilia and apical junctional complexes by Cdc42 in cochlear hair cells. J Cell Sci. 2014;127:2040–2052. doi: 10.1242/jcs.143602. [DOI] [PubMed] [Google Scholar]
- 24.Kirschner M, Mitchison T. Beyond self-assembly: From microtubules to morphogenesis. Cell. 45:329–342. doi: 10.1016/0092-8674(86)90318-1. [DOI] [PubMed] [Google Scholar]
- 25.Lu MS, Johnston CA. Molecular pathways regulating mitotic spindle orientation in animal cells. Development. 2013;140:1843–1856. doi: 10.1242/dev.087627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sipe CW, Liu L, Lee J, Grimsley-Myers C, Lu X. Lis1 mediates planar polarity of auditory hair cells through regulation of microtubule organization. Development. 2013;140:1785–1795. doi: 10.1242/dev.089763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ezan J, Lasvaux L, Gezer A, Novakovic A, May-Simera H, Belotti E, Lhoumeau AC, Birnbaumer L, Beer-Hammer S, Borg JP, et al. Primary cilium migration depends on G-protein signalling control of subapical cytoskeleton. Nat Cell Biol. 2013;15:1107–1115. doi: 10.1038/ncb2819. [DOI] [PubMed] [Google Scholar]
- 28.Tarchini B, Jolicoeur C, Cayouette M. A molecular blueprint at the apical surface establishes planar asymmetry in cochlear hair cells. Dev Cell. 2013;27:88–102. doi: 10.1016/j.devcel.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 29.Doherty D, Chudley AE, Coghlan G, Ishak GE, Innes AM, Lemire EG, Rogers RC, Mhanni AA, Phelps IG, Jones SJ, et al. GPSM2 mutations cause the brain malformations and hearing loss in Chudley-McCullough syndrome. Am J Hum Genet. 2012;90:1088–1093. doi: 10.1016/j.ajhg.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones C, Qian D, Kim SM, Li S, Ren D, Knapp L, Sprinzak D, Avraham KB, Matsuzaki F, Chi F, et al. Ankrd6 is a mammalian functional homolog of Drosophila planar cell polarity gene diego and regulates coordinated cellular orientation in the mouse inner ear. Developmental Biology. 2014;395:62–72. doi: 10.1016/j.ydbio.2014.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goodrich LV, Strutt D. Principles of planar polarity in animal development. Development. 2011;138:1877–1892. doi: 10.1242/dev.054080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chacon-Heszele MF, Ren D, Reynolds AB, Chi F, Chen P. Regulation of cochlear convergent extension by the vertebrate planar cell polarity pathway is dependent on p120-catenin. Development. 2012;139:968–978. doi: 10.1242/dev.065326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Copley CO, Duncan JS, Liu C, Cheng H, Deans MR. Postnatal refinement of auditory hair cell planar polarity deficits occurs in the absence of Vangl2. J Neurosci. 2013;33:14001–14016. doi: 10.1523/JNEUROSCI.1307-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Green J, Nusse R, van Amerongen R. The role of Ryk and Ror receptor tyrosine kinases in Wnt signal transduction. hCold Spring Harb Perspect Biol. 2014:6. doi: 10.1101/cshperspect.a009175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ezan J, Montcouquiol M. Revisiting planar cell polarity in the inner ear. Semin Cell Dev Biol. 2013;24:499–506. doi: 10.1016/j.semcdb.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 36.Lu X, Borchers AG, Jolicoeur C, Rayburn H, Baker JC, Tessier-Lavigne M. PTK7/CCK-4 is a novel regulator of planar cell polarity in vertebrates. Nature. 2004;430:93–98. doi: 10.1038/nature02677. [DOI] [PubMed] [Google Scholar]
- 37.Lee J, Andreeva A, Sipe CW, Liu L, Cheng A, Lu X. PTK7 regulates myosin II activity to orient planar polarity in the mammalian auditory epithelium. Curr Biol. 2012;22:956–966. doi: 10.1016/j.cub.2012.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Andreeva A, Lee J, Lohia M, Wu X, Macara IG, Lu X. PTK7-Src signaling at epithelial cell contacts mediates spatial organization of actomyosin and planar cell polarity. Dev Cell. 2014;29:20–33. doi: 10.1016/j.devcel.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takai Y, Miyoshi J, Ikeda W, Ogita H. Nectins and nectin-like molecules: roles in contact inhibition of cell movement and proliferation. Nat Rev Mol Cell Biol. 2008;9:603–615. doi: 10.1038/nrm2457. [DOI] [PubMed] [Google Scholar]
- 40.Fukuda T, Kominami K, Wang S, Togashi H, Hirata K, Mizoguchi A, Rikitake Y, Takai Y. Aberrant cochlear hair cell attachments caused by Nectin-3 deficiency result in hair bundle abnormalities. Development. 2014;141:399–409. doi: 10.1242/dev.094995. [DOI] [PubMed] [Google Scholar]
- 41.Lefevre G, Michel V, Weil D, Lepelletier L, Bizard E, Wolfrum U, Hardelin JP, Petit C. A core cochlear phenotype in USH1 mouse mutants implicates fibrous links of the hair bundle in its cohesion, orientation and differential growth. Development. 2008;135:1427–1437. doi: 10.1242/dev.012922. [DOI] [PubMed] [Google Scholar]
- 42.Kamiya K, Michel V, Giraudet F, Riederer B, Foucher I, Papal S, Perfettini I, Le Gal S, Verpy E, Xia W, et al. An unusually powerful mode of low-frequency sound interference due to defective hair bundles of the auditory outer hair cells. Proc Natl Acad Sci U S A. 2014;111:9307–9312. doi: 10.1073/pnas.1405322111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pepermans E, Petit C. The tip-link molecular complex of the auditory mechano-electrical transduction machinery. Hearing Research. Jun 3; doi: 10.1016/j.heares.2015.05.005. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 44.Zhao B, Wu Z, Grillet N, Yan L, Xiong W, Harkins-Perry S, Muller U. TMIE is an essential component of the mechanotransduction machinery of cochlear hair cells. Neuron. 2014;84:954–967. doi: 10.1016/j.neuron.2014.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beurg M, Xiong W, Zhao B, Muller U, Fettiplace R. Subunit determination of the conductance of hair-cell mechanotransducer channels. Proc Natl Acad Sci U S A. 2015;112:1589–1594. doi: 10.1073/pnas.1420906112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stepanyan R, Belyantseva IA, Griffith AJ, Friedman TB, Frolenkov GI. Auditory mechanotransduction in the absence of functional myosin-XVa. J Physiol. 2006;576:801–808. doi: 10.1113/jphysiol.2006.118547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Verpy E, Leibovici M, Michalski N, Goodyear RJ, Houdon C, Weil D, Richardson GP, Petit C. Stereocilin connects outer hair cell stereocilia to one another and to the tectorial membrane. J Comp Neurol. 2011;519:194–210. doi: 10.1002/cne.22509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao H, Williams DE, Shin JB, Brugger B, Gillespie PG. Large membrane domains in hair bundles specify spatially constricted radixin activation. J Neurosci. 2012;32:4600–4609. doi: 10.1523/JNEUROSCI.6184-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salles FT, Andrade LR, Tanda S, Grati M, Plona KL, Gagnon LH, Johnson KR, Kachar B, Berryman MA. CLIC5 stabilizes membrane-actin filament linkages at the base of hair cell stereocilia in a molecular complex with radixin, taperin, and myosin VI. Cytoskeleton (Hoboken) 2014;71:61–78. doi: 10.1002/cm.21159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoshikawa M, Go S, Suzuki S-i, Suzuki A, Katori Y, Morlet T, Gottlieb SM, Fujuwara M, Iwasaki K, Strauss KA, et al. Ganglioside GM3 is essential for the structural integrity and function of cochlear hair cells. Human Molecular Genetics. 2015 doi: 10.1093/hmg/ddv041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen J, Johnson SL, Lewis MA, Hilton JM, Huma A, Marcotti W, Steel KP. A reduction in Ptprq associated with specific features of the deafness phenotype of the miR-96 mutant mouse diminuendo. Eur J Neurosci. 2014;39:744–756. doi: 10.1111/ejn.12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jaworek TJ, Richard EM, Ivanova AA, Giese AP, Choo DI, Khan SN, Riazuddin S, Kahn RA, Riazuddin S. An alteration in ELMOD3, an Arl2 GTPase-activating protein, is associated with hearing impairment in humans. PLoS Genet. 2013;9:1003774. doi: 10.1371/journal.pgen.1003774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rehman AU, Santos-Cortez RL, Morell RJ, Drummond MC, Ito T, Lee K, Khan AA, Basra MA, Wasif N, Ayub M, et al. Mutations in TBC1D24, a gene associated with epilepsy, also cause nonsyndromic deafness DFNB86. Am J Hum Genet. 2014;94:144–152. doi: 10.1016/j.ajhg.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Azaiez H, Booth KT, Bu F, Huygen P, Shibata SB, Shearer AE, Kolbe D, Meyer N, Black-Ziegelbein EA, Smith RJ. TBC1D24 mutation causes autosomal-dominant nonsyndromic hearing loss. Hum Mutat. 2014;35:819–823. doi: 10.1002/humu.22557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gao K, Tang W, Li Y, Zhang P, Wang D, Yu L, Wang C, Wu D. Front-signal-dependent accumulation of the RHOA inhibitor FAM65B at leading edges polarizes neutrophils. J Cell Sci. 2015;128:992–1000. doi: 10.1242/jcs.161497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Diaz-Horta O, Subasioglu-Uzak A, Grati M, DeSmidt A, Foster J, 2nd, Cao L, Bademci G, Tokgoz-Yilmaz S, Duman D, Cengiz FB, et al. FAM65B is a membrane-associated protein of hair cell stereocilia required for hearing. Proc Natl Acad Sci U S A. 2014;111:9864–9868. doi: 10.1073/pnas.1401950111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perrin BJ, Sonnemann KJ, Ervasti JM. beta-actin and gamma-actin are each dispensable for auditory hair cell development but required for Stereocilia maintenance. PLoS Genet. 2010;6:1001158. doi: 10.1371/journal.pgen.1001158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Narayanan P, Chatterton P, Ikeda A, Ikeda S, Corey DP, Ervasti JM, Perrin BJ. Length regulation of mechanosensitive stereocilia depends on very slow actin dynamics and filament-severing proteins. Nat Commun. 2015:6. doi: 10.1038/ncomms7855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Salles FT, Merritt RC, Jr, Manor U, Dougherty GW, Sousa AD, Moore JE, Yengo CM, Dose AC, Kachar B. Myosin IIIa boosts elongation of stereocilia by transporting espin 1 to the plus ends of actin filaments. Nat Cell Biol. 2009;11:443–450. doi: 10.1038/ncb1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Manor U, Disanza A, Grati M, Andrade L, Lin H, Di Fiore PP, Scita G, Kachar B. Regulation of stereocilia length by myosin XVa and whirlin depends on the actin-regulatory protein Eps8. Curr Biol. 2011;21:167–172. doi: 10.1016/j.cub.2010.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Furness DN, Johnson SL, Manor U, Ruttiger L, Tocchetti A, Offenhauser N, Olt J, Goodyear RJ, Vijayakumar S, Dai Y, et al. Progressive hearing loss and gradual deterioration of sensory hair bundles in the ears of mice lacking the actin-binding protein Eps8L2. Proc Natl Acad Sci U S A. 2013;110:13898–13903. doi: 10.1073/pnas.1304644110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rzadzinska AK, Nevalainen EM, Prosser HM, Lappalainen P, Steel KP. MyosinVIIa Interacts with Twinfilin-2 at the Tips of Mechanosensory Stereocilia in the Inner Ear. PLoS ONE. 2009;4:7097. doi: 10.1371/journal.pone.0007097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sekerková G, Richter C-P, Bartles JR. Roles of the Espin Actin-Bundling Proteins in the Morphogenesis and Stabilization of Hair Cell Stereocilia Revealed in CBA/CaJ Congenic Jerker Mice. PLoS Genet. 2011;7:1002032. doi: 10.1371/journal.pgen.1002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kitajiri S, Sakamoto T, Belyantseva IA, Goodyear RJ, Stepanyan R, Fujiwara I, Bird JE, Riazuddin S, Riazuddin S, Ahmed ZM, et al. Actin-bundling protein TRIOBP forms resilient rootlets of hair cell stereocilia essential for hearing. Cell. 2010;141:786–798. doi: 10.1016/j.cell.2010.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Taylor R, Bullen A, Johnson SL, Grimm-Gunter EM, Rivero F, Marcotti W, Forge A, Daudet N. Absence of plastin 1 causes abnormal maintenance of hair cell stereocilia and a moderate form of hearing loss in mice. Hum Mol Genet. 2015;24:37–49. doi: 10.1093/hmg/ddu417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Scheffer DI, Zhang DS, Shen J, Indzhykulian A, Karavitaki KD, Xu YJ, Wang Q, Lin JJ, Chen ZY, Corey DP. XIRP2, an Actin-Binding Protein Essential for Inner Ear Hair-Cell Stereocilia. Cell Rep. 2015;10:1811–1818. doi: 10.1016/j.celrep.2015.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Francis SP, Krey JF, Krystofiak ES, Cui R, Nanda S, Xu W, Kachar B, Barr-Gillespie PG, Shin JB. A short splice form of Xin-actin binding repeat containing 2 (XIRP2) lacking the Xin repeats is required for maintenance of stereocilia morphology and hearing function. J Neurosci. 2015;35:1999–2014. doi: 10.1523/JNEUROSCI.3449-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Indzhykulian AA, Stepanyan R, Nelina A, Spinelli KJ, Ahmed ZM, Belyantseva IA, Friedman TB, Barr-Gillespie PG, Frolenkov GI. Molecular Remodeling of Tip Links Underlies Mechanosensory Regeneration in Auditory Hair Cells. PLoS Biol. 2013;11:1001583. doi: 10.1371/journal.pbio.1001583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Belyantseva IA, Perrin BJ, Sonnemann KJ, Zhu M, Stepanyan R, McGee J, Frolenkov GI, Walsh EJ, Friderici KH, Friedman TB, et al. Gamma-actin is required for cytoskeletal maintenance but not development. Proc Natl Acad Sci U S A. 2009;106:9703–9708. doi: 10.1073/pnas.0900221106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang S-M, Chen W, Guo W-W, Jia S, Sun J-H, Liu H-Z, Young W-Y, He DZZ. Regeneration of Stereocilia of Hair Cells by Forced Atoh1 Expression in the Adult Mammalian Cochlea. PLoS ONE. 2012;7:46355. doi: 10.1371/journal.pone.0046355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kowalik L, Hudspeth AJ. A search for factors specifying tonotopy implicates DNER in hair-cell development in the chick’s cochlea. Dev Biol. 2011;354:221–231. doi: 10.1016/j.ydbio.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mann ZF, Thiede BR, Chang W, Shin JB, May-Simera HL, Lovett M, Corwin JT, Kelley MW. A gradient of Bmp7 specifies the tonotopic axis in the developing inner ear. Nat Commun. 2014;5:3839. doi: 10.1038/ncomms4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thiede BR, Mann ZF, Chang W, Ku YC, Son YK, Lovett M, Kelley MW, Corwin JT. Retinoic acid signalling regulates the development of tonotopically patterned hair cells in the chicken cochlea. Nat Commun. 2014;5:3840. doi: 10.1038/ncomms4840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Son EJ, Ma J-H, Ankamreddy H, Shin J-O, Choi JY, Wu DK, Bok J. Conserved role of Sonic Hedgehog in tonotopic organization of the avian basilar papilla and mammalian cochlea. Proceedings of the National Academy of Sciences. 2015;112:3746–3751. doi: 10.1073/pnas.1417856112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Durruthy-Durruthy R, Gottlieb A, Hartman BH, Waldhaus J, Laske RD, Altman R, Heller S. Reconstruction of the mouse otocyst and early neuroblast lineage at single-cell resolution. Cell. 2014;157:964–978. doi: 10.1016/j.cell.2014.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kwan KY, Shen J, Corey DP. C-MYC transcriptionally amplifies SOX2 target genes to regulate self-renewal in multipotent otic progenitor cells. Stem Cell Reports. 2015;4:47–60. doi: 10.1016/j.stemcr.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jung JY, Avenarius MR, Adamsky S, Alpert E, Feinstein E, Raphael Y. siRNA Targeting Hes5 Augments Hair Cell Regeneration in Aminoglycoside-damaged Mouse Utricle. Mol Ther. 2013;21:834–841. doi: 10.1038/mt.2013.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Oishi N, Chen FQ, Zheng HW, Sha SH. Intra-tympanic delivery of short interfering RNA into the adult mouse cochlea. Hear Res. 2013;296:36–41. doi: 10.1016/j.heares.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hsu Patrick D, Lander Eric S, Zhang F. Development and Applications of CRISPR-Cas9 for Genome Engineering. Cell. 2014;157:1262–1278. doi: 10.1016/j.cell.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zuris JA, Thompson DB, Shu Y, Guilinger JP, Bessen JL, Hu JH, Maeder ML, Joung JK, Chen Z-Y, Liu DR. Cationic lipid-mediated delivery of proteins enables efficient protein-based genome editing in vitro and in vivo. Nat Biotech. 2015;33:73–80. doi: 10.1038/nbt.3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
Further Reading/Resources
- For a further discussion on genetic dissection of hearing, see Michalski N, Petit C. Genetics of auditory mechano-electrical transduction. Pflugers Arch. 2015;467:49–72. doi: 10.1007/s00424-014-1552-9.
- To learn more about patterning and cell fate regulation in the inner ear, see Groves AK, Fekete DM. Shaping sound in space: the regulation of inner ear patterning. Development. 2012;139:245–257. doi: 10.1242/dev.067074.
- To learn more about hair cell development and regeneration, see Jansson L, Kim GS, Cheng AG. Making sense of Wnt signaling-linking hair cell regeneration to development. Front Cell Neurosci. 2015;9:66. doi: 10.3389/fncel.2015.00066.
- To learn more about auditory synapse development, see Wichmann C, Moser T. Relating structure and function of inner hair cell ribbon synapses. Cell and Tissue Research. 2015:1–20. doi: 10.1007/s00441-014-2102-7.
- To learn more about emerging strategies for treating hearing loss, see Muller U, Barr-Gillespie PG. New treatment options for hearing loss. Nat Rev Drug Discov. 2015;14:346–365. doi: 10.1038/nrd4533.