Abstract
Importance
Short transverse myelitis (STM, <3 vertebral segments) is considered non-characteristic of neuromyelitis optica spectrum disorders (NMOSD). Poor recognition of the potential for STM to occur in NMOSD may lead to increased disability from delay in diagnosis and appropriate treatment.
Objectives
To determine the frequency of short lesions at the initial myelitis manifestation of NMOSD, and to compare the demographic, clinical and radiological characteristics of aquaporin-4-IgG (AQP4-IgG) seropositive and seronegative STM.
Design, Setting, Participants
We reviewed the records and images of Mayo Clinic AQP4-IgG positive NMOSD patients identified from 1996-2014. Inclusion criteria were: 1) first TM episode; 2) MRI performed ≤90 days from symptom onset; 3) Spinal cord T2-hyperintense lesion <3 vertebral segments; 4) AQP4-IgG seropositivity; 5) final diagnosis NMO or NMOSD. Patients with an initial longitudinally extensive transverse myelitis (LETM) were excluded (n=151). Patients with STM, seronegative for AQP4-IgG, among an Olmsted County population-based cohort of inflammatory demyelinating disorders of the central nervous system were used as a control group.
Main Outcomes and Measures
Delay to diagnosis in months, clinical and radiological characteristics and disability measured by ambulatory status.
Results
Twenty-five AQP4-IgG seropositive patients with an initial STM were included, representing 14% of initial myelitis episodes among NMOSD patients. The STM episode was: the first manifestation of NMOSD in 10 patients (40%); preceded by optic neuritis in 13 patients (52%); and preceded by a nausea and vomiting episode in 2 (8%). In comparison to the excluded NMOSD patients with an initial LETM, delay to diagnosis/treatment was greater when initial lesions were short (p=0.016). In AQP4-IgG positive STM cases subsequent myelitis episodes were longitudinally extensive in 92%. Attributes more common in aquaporin-4-IgG-positive STM patients than in 27 population-based aquaporin-4-IgG-negative STM patients (p<0.05) included: non-Caucasian ethnicity; tonic spasms; co-existing autoimmunity; MRI (central cord lesions, T1 hypointensity; brain inconsistent with multiple sclerosis) and CSF (oligoclonal bands lacking).
Conclusions and Relevance
STM is not uncommon in NMOSD and when present delays diagnosis/treatment. Clinical and radiological characteristics identified in this study may help select STM patients at highest risk for an NMOSD. STM does not exclude consideration of aquaporin-4-IgG testing nor NMOSD diagnosis.
Keywords: magnetic resonance imaging, transverse myelitis, Devic’s disease
Longitudinally extensive transverse myelitis (LETM), defined by MRI as extending 3 or more vertebral segments is the most specific radiologic finding supporting neuromyelitis optica (NMO) diagnosis, in adult patients,1, 2 and prompts clinicians to test for aquaporin-4-IgG (AQP4-IgG).3 Seropositivity confirms the diagnosis of an NMO spectrum disorder (NMOSD), predicts recurrent myelitis or optic neuritis and dictates therapeutic options.4 Early and accurate diagnosis of NMO or NMOSD is important to minimize cumulative disability from repeated attacks.5 The goal of early immunosuppression is to prevent attack-related disability.6 Short transverse myelitis (STM; lesions defined by MRI as not extending 3 vertebral segments), is far more common in multiple sclerosis (MS)7 than in NMO.8-13 Although AQP4-IgG-seropositivity is predicted to be infrequent in STM,8 it is not known how frequently cord lesions are short in AQP4-IgG-positive patients. Our study’s goal was to determine the frequency of short lesions in patients with an initial myelitis manifestation of NMOSD, and to compare the demographic, clinical and radiological characteristics of seropositive and seronegative patients with STM.
Methods
Patient Ascertainment and Inclusion Criteria
This study was approved by the Mayo Institutional Review Board (IRB# 07-007453) and all patients included provided written informed for research studies. We reviewed the records and images of 319 AQP4-IgG seropositive NMO and NMOSD patients identified from 1996-2014 through our clinical and serological databases at Mayo Clinic Rochester (MN), Scottsdale (AZ) and Jacksonville (FL). Inclusion criteria: 1) first transverse myelitis episode;14 2) MRI performed ≤90 days from symptom onset and radiological details available for first myelitis attack 3) Spinal cord T2-hyperintense lesion shorter than 3 vertebral segments; 4) AQP4-IgG seropositivity; 5) final diagnosis NMO or NMOSD.1 Twenty six patients met inclusion criteria.
We excluded 294 seropositive NMO/NMOSD patients: initial episode LETM (n=151); no MRI spine details at initial myelitis or MRI performed >90 days after onset (n=111); no myelitis episode (n=28); spinal cord lesion asymptomatic (n=3); and dorsal medullary lesion with slight extension to cervical cord (n=1).
Control STM group
We included as controls patients from the Olmsted County population-based cohort of inflammatory demyelinating disease (IDD) who met the following criteria (n=27): 1) STM documented by spine MRI performed within 90 days of myelitis onset; 2) serum sample available; 3) AQP4-IgG negative. Final diagnoses at last follow-up (median, 103 months; range, 21-174): relapsing-remitting MS, 15;7 monophasic STM, 10; and relapsing STM, 2.
Aquaporin-4-IgG assays
AQP4-IgG serostatus was evaluated by one or more assays: enzyme-linked immunosorbent assay (ELISA), tissue-based indirect immunofluorescence3 or transfected cell-based assay (fixed [Euroimmun Inc, Luebeck, Germany] or live [fluorescence-activated cell sorting]).6
Radiological Methodology
A variety of MRI techniques in multiple different scanners were used over 18 years reflecting clinical practice at the time; all available sequences were reviewed. All MRI’s were reviewed by Mayo Clinic neurologists or neuroradiologists.
Statistical Methodology
Descriptive summary statistics were reported as median (range, minimum-maximum) for continuous variables and frequencies and percentages for categorical variables. Comparisons were performed using Wilcoxon rank sum test or Fisher’s exact tests as appropriate using JMP 8.0 software (SAS®).
Definitions
Results
Short transverse myelitis (STM) is not uncommon in the initial myelitis episode of NMOSD
A short lesion (<3 vertebral segments) was the first transverse myelitis event in 25 of 176 patients (14%); 151 (86%) had an initial longitudinally extensive lesion (≥3 vertebral segments).
Comparison of AQP4-IgG-seropositive and AQP4-IgG-seronegative STM
The Table compares demographic, clinical, laboratory and radiological characteristics of the 25 AQP4-IgG-seropositive patients with STM to the population-based control cohort of 27 AQP4-IgG-seronegative patients with STM.
Table.
Clinical, laboratory and radiological findings of short transverse myelitis in aquaporin-4-IgG-positive patients and a population-based cohort of aquaporin-4-IgG-negative patients
| AQP4-IgG (+) (n=25) | AQP4-IgG (-) (n=27) | p value | |
|---|---|---|---|
| Demographics | |||
| Female sex | 18 (72%) | 21 (78%) | 0.63 |
| Median age (range) at myelitis onset | 50 yrs (29-70) | 42 yrs (18-67) | 0.04 |
| Non-Caucasian ethnicitya | 8 of 23 (35%) | 0 (0%) | <0.01 |
| Clinical Features | |||
| Numbness | 24 (96%) | 26 (96%) | 1.0 |
| Weakness | 12 (48%) | 7 (26%) | 0.10 |
| Bowel/bladder | 6 (24%) | 6 (22%) | 0.88 |
| Lhermittes | 4 (16%) | 2 (7%) | 0.41 |
| Tonic spasms | 6 (24%) | 1 (4%) | 0.046 |
| Concomitant nausea and vomiting | 2 (8%) | 0 (0%) | 0.23 |
| Need for gait aid at maximal severity | 4 (16%) | 1 (4%) | 0.18 |
| Personal Hx of autoimmunityb | 10 (40%) | 2 (7%) | <0.01 |
| Family Hx autoimmunity (1st degree relative) | 9 (36%) | 5 (19%) | 0.21 |
| Family Hx MS (1st degree relative) | 0 (0%) | 3 (11%) | 0.24 |
| Laboratory abnormalities | |||
| Antinuclear antibody | 9 of 20 (45%) | 4 of 22 (18%) | 0.1 |
| SSA/double stranded DNA antibodies | 5 of 13 (38%) | 2 of 13 (15%) | 0.38 |
| Cerebrospinal fluidc | |||
| Elevated white cell count (>5/μL)d | 7 of 11 (64%) | 10 of 21 (48%) | 0.47 |
| Elevated protein (>45 mg/dL) | 4 of 9 (44%) | 11 of 21 (52%) | 1.0 |
| Oligoclonal bands (>3) | 1 of 11 (9%) | 11 of 21 (52%) | 0.02 |
| Spine MRIe | |||
| Median interval (range), Sx onset to MRI | 15.5 days (2-90) | 24 days (3-90) | 0.81 |
| Single lesion | 18 (72%) | 19 (70%) | 0.9 |
| Median (range) T2 length, vertebral segments | 1 (0.5-2.5) | 1 (0.5-2.5) | 0.09 |
| Spinal cord swellingf | 4 of 15 (27%) | 15 (56%) | 0.11 |
| Gadolinium-enhancing lesion (1 or more) | 14 (56%) | 20 (74%) | 0.17 |
| Central location on axial imagesg | 16 of 29 (55%)f | 12 of 53 (23%)f | <0.01 |
| T1 hypointense foci | 4 of 14 (29%) | 0 of 24 (0%) | 0.01 |
| Subsequent myelitis longitudinally extensiveh | 12 of 13 (92%) | 0 of 8 (0%) | <0.01 |
| Brain lesions meeting MS criteriai | 4 (16%) | 13 of 26 (50%) | 0.02 |
Abbreviations: AQP4-IgG, aquaporin-4-IgG; Hx, history; DNA, deoxyribonucleic acid; MRI, magnetic resonance imaging; MS, multiple sclerosis; NMOSD, neuromyelitis optica spectrum disorder; Sx, symptom; TM, transverse myelitis; yrs, years;
Of those with ethnicity details available. Non-Caucasian ethnicities: Asian, 4; Hispanic, 2; African-American, 1; and Native-American-Indian, 1.
Personal history of autoimmunity (some multiple): thyroid autoimmunity, 4; systemic lupus erythematosus, 2; psoriasis, 2; AChR-IgG-positive myasthenia gravis, 1; rheumatoid arthritis, 1; rheumatic fever, 1; Sjogren syndrome, 1; Autoimmune thrombocytopenia, 1; and Addison disease, 1.
Of those with lumbar puncture performed at time of initial myelitis
Lymphocyte predominant in all four AQP4-IgG (+) patients with details available and in all 10 AQP4-IgG (-) patients.
In 14 patients the MRI’s were not available for re-review and the details were taken from the radiology report by the radiologist or neurologist’s report of the features of the lesion.
- AQP4-IgG (+): cervical, 6 (cervico-medullary junction, 1); thoracic, 17; both, 2.
- AQP4-IgG (-): cervical spine, 13; thoracic spine, 9; both 5;
Of those with details available
Of those with multiple lesions, axial appearance of each counted separately
longitudinally extensive refers to those with T2 signal extending ≥3 vertebral segments
brain lesions by Polman 2011 revised criteria7
Additional details of AQP4-IgG seropositive STM patients
Figures 1 and 2 demonstrate representative images. Myelitis was the first manifestation of NMOSD in 10 patients (40%); in 13 patients (52%) myelitis was preceded by optic neuritis (77% severe or associated with poor recovery; 23% bilateral); in 2 patients (8%) myelitis was preceded by an episode of severe nausea/vomiting. MS was the initial diagnosis in 10 patients (40%) and 6 had received interferon-β treatment for presumed MS. One patient worsened after starting interferon-β and in the remaining five patients no benefit was seen with interferon- β. In 83% of AQP4-IgG-seropositive STM cases the treating physician had questioned the diagnosis of NMOSD because the spinal lesion was short. Three patients (12%) were receiving immunosuppressant therapy at the time of MRI. All patients were seropositive by one or more tests for aquaporin-4-IgG: tissue-based immunofluorescence, 16 (64%: median titer, 7680; range, 480-61440); ELISA, 11 (44%: median 160 units; range 2.2 to >160 [normal, <1.6]); AQP4-transfected cell-based assay, fixed, 8 (32%); live (flow cytometry), 16 (64%). The median period of follow-up from NMOSD onset was 65 months (range, 3-293). The final diagnosis in 17 patients was NMO and in 8 was NMOSD.
Figure 1. MRI features of patients with AQP4-IgG associated short transverse myelitis.
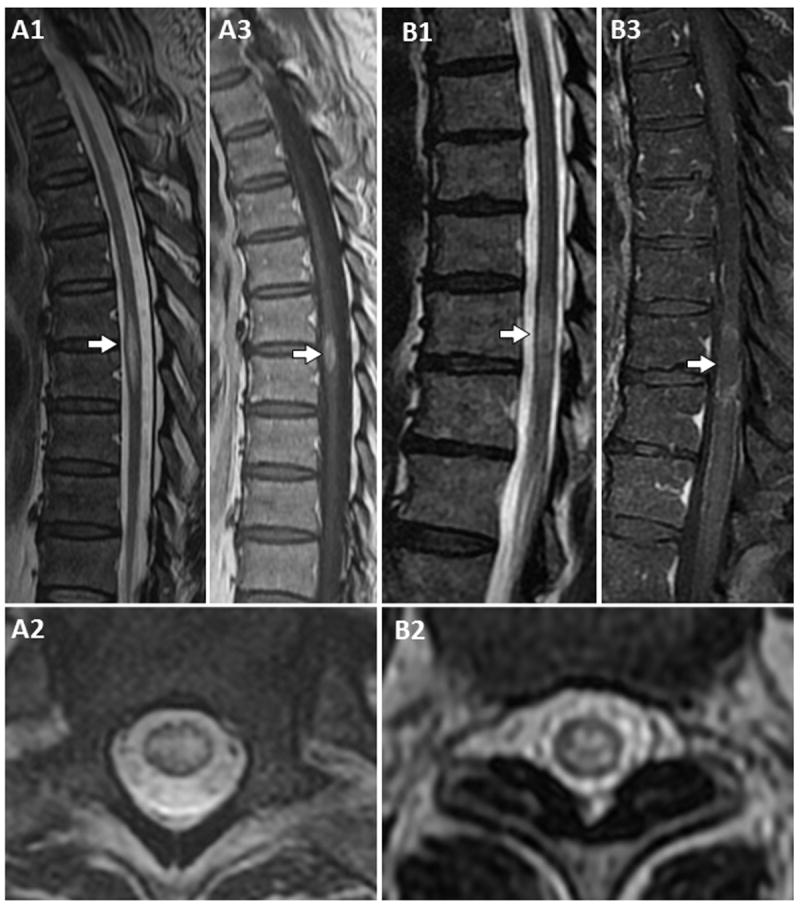
T2-weighted MRI, sagittal images, reveal a short T2-hyperintense lesion (approximately 1.5 vertebral segments long) with spinal cord swelling (A1, arrow), central T2-hyperintensity on axial images (A2) and associated enhancement on T1-weighted post-gadolinium images (A3, arrow). A short T2-hyperintense lesion is shown on T2-weighted sagittal images (B1, arrow: approximately 1 vertebral segment long) with centrally located T2-signal hyperintensity on axial images (B2) and ring-like enhancement on T1-weighted images after gadolinium administration (B3, arrow).
Figure 2. MRI in four patients with an initial short transverse myelitis followed by a subsequent longitudinally extensive myelitis.
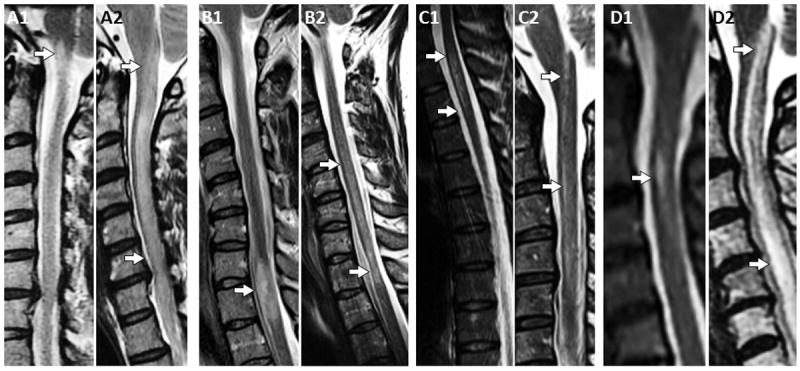
T2-weighted MRI cervical spine revealed a short T2 hyperintense lesion at the left cervicomedullary junction (A1, arrow) with gadolinium enhancement (not shown). This patient relapsed with a cervical longitudinally extensive myelitis (A2, arrows) notable on sagittal T2-weighted images. T2-weighted sagittal images reveal a short spinal cord lesion which extends 1.5 vertebral segments with focal cord enlargement (B1, arrow) and gadolinium enhancement (not shown). This episode was followed by a longitudinally extensive myelitis (B2, arrows [lower arrow showing focal cord atrophy]) notable on sagittal T2-weighted images. A short spinal cord lesion is shown which extends two thoracic vertebral segments (C1, arrows) on sagittal T2-weighted images with gadolinium enhancement (not shown). This patient had a subsequent myelitis relapse with a discontinuous appearing longitudinally extensive lesion in the cervical spinal cord (C2, arrows) on repeat sagittal T2-weighted images. Sagittal T2-weighted MRI reveals a T2-signal hyperintensity extending 1 vertebral segment (D1, arrow) without gadolinium enhancement (post-gadolinium images not shown). This was followed a few months later by a longitudinally extensive myelitis in the cervical spinal cord (D2, arrows) on repeat sagittal T2-weighted images.
Comparison of NMOSD patients with an initial STM or an initial LETM
In those with details of the severity of their initial myelitis available, patients with STM were less likely to need a gait aid at nadir (4/25 [16%]) than those with LETM (71/126 [56%]; p<0.001). The median interval from symptom onset to MRI spine was similar for patients with STM and LETM (15.5 days [range, 2-90] vs 14 days [range, 1-90] p=0.20). The median delay to diagnosis was greater for patients with an initial STM (5 months [range, 0-20]) than for patients with initial LETM (0 months [range, 0-21]; p=0.016).
Discussion
This study demonstrates that it is not uncommon for the first myelitis episode of NMOSD to be less than 3 vertebral segments long. Neurologists customarily consider STM incompatible with a diagnosis of NMOSD. In the present study, this misconception delayed the diagnosis and initiation of appropriate treatment, both being critical to minimize recurrent attack-related disability.5, 6 Another practical consideration is that therapies favored for MS may exacerbate NMO.15 Of 10 patients initially misdiagnosed as MS, 6 were treated with interferon-β; one patient reported increased frequency and severity of attacks and no benefit was seen with the other 5 patients.
Apart from AQP4-IgG-seropositivity, several clinical and radiological clues helped identify patients at risk for NMOSD amongst those with STM. Non-Caucasian ethnicity was a significant predictor of NMOSD risk; although the Olmsted County population (from which the study controls were ascertained) has a relatively low proportion of non-caucasians (11.1%) by comparison with other regions of the USA, overrepresentation of non-Caucasians in NMOSD is well recognized.16 Indeed, in a previously reported African-American patient an initial STM attack delayed both NMOSD diagnosis and NMO-appropriate treatment.9 Other clinical predictors of AQP4-IgG seropositivity in patients with STM included older age, personal history of autoimmunity (e.g., myasthenia gravis) and tonic spasms (recognized to be more frequent in NMO than MS17). Centrally located axial T2-hyperintensities (MS lesions are typically peripheral)7 and T1-hypointensity were also significant predictors of AQP4-IgG-positivity. Patients without typical MS brain lesions and lacking CSF oligoclonal bands also were significantly more likely to be AQP4-IgG-positive. In a previously reported NMOSD study, 4 patients had STM, three with central spinal cord MRI lesions; two had antinuclear antibodies, and head MRI findings in 3 of the 4 patients did not meet McDonald 2010 criteria for MS.13 Other findings raising the likelihood of NMOSD diagnosis rather than MS in a patient with STM are antecedent severe or bilateral optic neuritis with poor recovery (frequent in our study patients) or an earlier or concomitant episode of protracted nausea or vomiting. We excluded one patient with a short lesion extending from the dorsal medulla to the upper cervical cord (Figure 3) as this was predominantly a brainstem lesion. It is important to recognize this type of lesion (which may be associated with a short or longitudinally-extensive cord lesion) as it is suggestive of NMOSD,11, 18 although in our experience it is not pathognomonic (Dr’s Flanagan and Pittock, unpublished observations).
Figure 3. MRI of dorsal medullary lesion extending to upper cervical spinal cord.
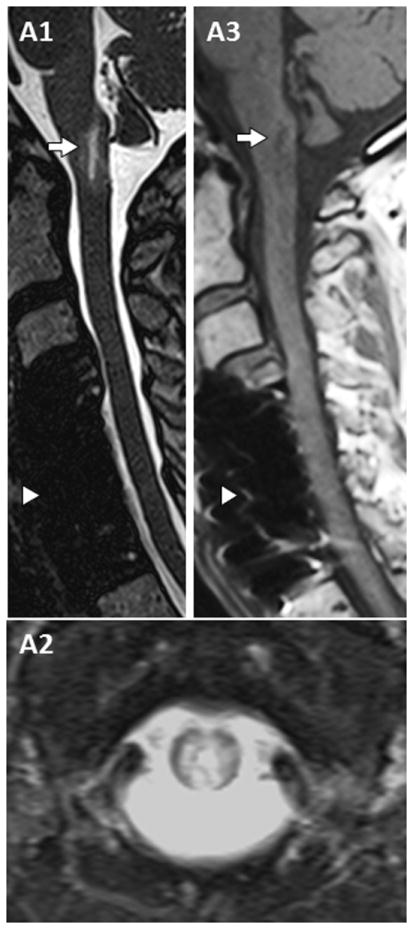
T2-weighted sagittal images reveal a short T2 hyperintense lesion in the dorsal medulla and area postrema region extending towards the upper cervical cord (C1, arrow). Axial images show heterogeneous T2-hyperintensity (C2), some of similar intensity to cerebrospinal fluid, with associated T1-hypointensity (C3, arrow) on T1 weighted images prior to gadolinium administration. Post-operative changes from cervical decompressive surgery are also noted (C1 and C3, arrowhead).
By excluding seronegative NMO cases from our study we ensured that the 14% frequency of STM at first myelitis episode of NMOSD was not an overestimate. By restricting the study to the initial myelitis episode we avoided problems of distinguishing acute non-enhancing lesions from chronic lesions and differentiating acute attacks from pseudo-exacerbations. Also, we excluded patients whose STM occurred after one or more LETM attack and patients with initial concurrent long and short lesions or discontinuous but long-appearing lesions (categorized as longitudinally extensive) because they present less diagnostic dilemma. In 76% of the patients seropositivity was confirmed by AQP4-transfected cell-based assay (M1 isoform) to minimize the low likelihood of false-positive AQP4-IgG results (more frequent with ELISA assays6, 19). Our conclusion that NMOSD was the correct diagnosis in seropositive STM patients is supported by the fact that in 92% of patients lesions were longitudinally extensive lesions at subsequent myelitis attacks. It is therefore apparent that recurrent myelitis lesions in NMOSD are rarely exclusively short.
The imaged cord lesion in NMOSD may be shorter in patients receiving maintenance immunosuppressant therapy at the time of attack (authors’ unpublished observations). In this study only 12% of patients were receiving immunosuppressant therapies when the STM attack occurred. The timing of MRI in the evolution of NMOSD also may influence the length of the imaged lesion;18 early imaging may miss a long lesion11 and late imaging may reveal a discontinuous or short lesion, or no lesion.18, 20 The interval from symptom onset to MRI did not differ significantly for patients with LETM or STM; imaging was performed within 90 days of symptom onset in all patients, and lesional activity was confirmed by gadolinium enhancement in 56% of seropositive STM episodes. In AQP4-IgG positive NMOSD patients evaluated at the time of STM attack, the severity of myelitis at nadir was milder than those with LETM. These observations further support our contention that short spinal cord lesions in NMOSD in this study did not simply reflect the timing of MRI. Furthermore, encountering a short lesion due to imperfect MRI timing may occur in patients with NMOSD as varied timing is a fact of clinical practice and sequential imaging of a single attack is rarely performed.11 Clinicians therefore must be mindful that a short spinal cord lesion does not exclude the diagnosis of NMOSD.
Acknowledgments
Funding provided by the Guthy-Jackson Charitable Foundation and the National Institutes of Health (NS065829).
Footnotes
Author contributions
Dr Flanagan was involved in drafting and revising the manuscript for content, including medical writing for content, study concept and design, analysis and interpretation of data, and acquisition of data.
Dr Weinshenker was involved in revising the manuscript for content and analysis and interpretation of data.
Dr Krecke was involved in revising the manuscript for content and analysis and interpretation of data.
Dr Lennon was involved in revising the manuscript for content and analysis and interpretation of data.
Dr Lucchinetti was involved in revising the manuscript for content and analysis and interpretation of data.
Dr McKeon was involved in revising the manuscript for content and analysis and interpretation of data.
Dr Wingerchuk was involved in revising the manuscript for content and analysis and interpretation of data.
Dr Shuster was involved in revising the manuscript for content and analysis and interpretation of data.
Dr Jiao was involved in revising the manuscript for content and analysis and interpretation of data.
Dr Horta was involved in revising the manuscript for content and analysis and interpretation of data.
Dr Pittock was involved in drafting and revising the manuscript for content, including medical writing for content, study concept and design, analysis and interpretation of data, acquisition of data and study supervision.
Financial Disclosure Statements
Dr. Flanagan has no disclosures.
Dr Krecke has no disclosures
Dr Weinshenker has received a research grant from the Guthy Jackson Foundation. He receives royalties from RSR for a technology license related to a test for aquaporin-4 autoantibodies for diagnosis of neuromyelitis optica. He serves on data safety monitoring committees for Novartis, Biogen-Idec and Mitsubishi pharmaceutical companies, and serves on an adjudication panel for Medimmune Pharmaceuticals. He served as a consultant for GlaxoSmithKline, Elan, Ono, Chugai and Alexion pharmaceutical companies. He serves on editorial boards for Neurology, the Canadian Journal of Neurological Sciences, and Turkish Journal of Neurology.
Dr Lennon receives royalties for technology relating to aquaporin 4 (AQP4) antibodies for diagnosis of neuromyelitis optica (NMO), is a named inventor on filed patents that relate to functional AQP4/NMO-IgG assays and NMO-IgG as a cancer marker, and receives research support from the National Institutes of Health (grant NS065829-01).
Dr McKeon has no disclosures.
Dr Lucchinetti shares in royalties from marketing of kits for detecting AQP4 autoantibody and from the sale of Blue Books of Neurology: Multiple Sclerosis 3 (Saunders Elsevier, 2010); she receives research support from the NIH (NS49577-R01 [PI]), the Guthy-Jackson Charitable Foundation (PI), and the National Multiple Sclerosis Society (RG 3185B3 [PI]).
Dr. Wingerchuk has received research support from Alexion and TerumoBCT, has served as a consultant to Alexion, MedImmune, and Chugai Pharmaceuticals, and has received financial compensation for service on an adjudication committee for a MedImmune clinical trial.
Dr Shuster has no disclosures.
Dr Jiao has no disclosures.
Dr Horta has no disclosures.
Dr. Pittock is a named inventor on patents (#12/678,350 filed 2010 and #12/573,942 filed 2008) that relate to functional AQP4/NMO-IgG assays and NMO-IgG as a cancer marker; receives research support from Alexion Pharmaceuticals, Inc., the Guthy-Jackson Charitable Foundation, and the National Institutes of Health (NS065829). Dr. Pittock has provided consultation to Alexion Pharmaceuticals, MedImmune LLC, and Chugai Pharma but has received no personal fees or personal compensation for these consulting activities. All compensation for consulting activities is paid directly to Mayo Clinic.
References
- 1.Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG. Revised diagnostic criteria for neuromyelitis optica. Neurology. 2006 May 23;66(10):1485–1489. doi: 10.1212/01.wnl.0000216139.44259.74. [DOI] [PubMed] [Google Scholar]
- 2.Banwell B, Tenembaum S, Lennon VA, et al. Neuromyelitis optica-IgG in childhood inflammatory demyelinating CNS disorders. Neurology. 2008 Jan 29;70(5):344–352. doi: 10.1212/01.wnl.0000284600.80782.d5. [DOI] [PubMed] [Google Scholar]
- 3.Lennon VA, Wingerchuk DM, Kryzer TJ, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004 Dec 11-17;364(9451):2106–2112. doi: 10.1016/S0140-6736(04)17551-X. [DOI] [PubMed] [Google Scholar]
- 4.Weinshenker BG, Wingerchuk DM, Vukusic S, et al. Neuromyelitis optica IgG predicts relapse after longitudinally extensive transverse myelitis. Annals of neurology. 2006 Mar;59(3):566–569. doi: 10.1002/ana.20770. [DOI] [PubMed] [Google Scholar]
- 5.Wingerchuk DM, Hogancamp WF, O’Brien PC, Weinshenker BG. The clinical course of neuromyelitis optica (Devic’s syndrome) Neurology. 1999 Sep 22;53(5):1107–1114. doi: 10.1212/wnl.53.5.1107. [DOI] [PubMed] [Google Scholar]
- 6.Jiao Y, Fryer JP, Lennon VA, et al. Aquaporin 4 IgG Serostatus and Outcome in Recurrent Longitudinally Extensive Transverse Myelitis. JAMA neurology. 2014 Jan 1;71(1):48–54. doi: 10.1001/jamaneurol.2013.5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Annals of neurology. 2011 Feb;69(2):292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scott TF, Kassab SL, Pittock SJ. Neuromyelitis optica IgG status in acute partial transverse myelitis. Archives of neurology. 2006 Oct;63(10):1398–1400. doi: 10.1001/archneur.63.10.1398. [DOI] [PubMed] [Google Scholar]
- 9.Ortega MR, Tornes L, Rammohan KW. NMO spectrum presenting as partial myelitis. Multiple sclerosis. 2013 Feb;19(2):252–253. doi: 10.1177/1352458512445303. [DOI] [PubMed] [Google Scholar]
- 10.Chan KH, Ramsden DB, Yu YL, et al. Neuromyelitis optica-IgG in idiopathic inflammatory demyelinating disorders amongst Hong Kong Chinese. European journal of neurology : the official journal of the European Federation of Neurological Societies. 2009 Mar;16(3):310–316. doi: 10.1111/j.1468-1331.2008.02376.x. [DOI] [PubMed] [Google Scholar]
- 11.Asgari N, Skejoe HP, Lennon VA. Evolution of longitudinally extensive transverse myelitis in an aquaporin-4 IgG-positive patient. Neurology. 2013 Jul 2;81(1):95–96. doi: 10.1212/WNL.0b013e318297ef07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siritho S, Nakashima I, Takahashi T, Fujihara K, Prayoonwiwat N. AQP4 antibody-positive Thai cases: clinical features and diagnostic problems. Neurology. 2011 Aug 30;77(9):827–834. doi: 10.1212/WNL.0b013e31822c61b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sato DK, Nakashima I, Takahashi T, et al. Aquaporin-4 antibody-positive cases beyond current diagnostic criteria for NMO spectrum disorders. Neurology. 2013 Jun 11;80(24):2210–2216. doi: 10.1212/WNL.0b013e318296ea08. [DOI] [PubMed] [Google Scholar]
- 14.Group. TMCW. Proposed diagnostic criteria and nosology of acute transverse myelitis. Neurology. 2002 Aug 27;59(4):499–505. doi: 10.1212/wnl.59.4.499. [DOI] [PubMed] [Google Scholar]
- 15.Kim SH, Kim W, Li XF, Jung IJ, Kim HJ. Does interferon beta treatment exacerbate neuromyelitis optica spectrum disorder? Multiple sclerosis. 2012 Oct;18(10):1480–1483. doi: 10.1177/1352458512439439. [DOI] [PubMed] [Google Scholar]
- 16.Mealy MA, Wingerchuk DM, Greenberg BM, Levy M. Epidemiology of neuromyelitis optica in the United States: a multicenter analysis. Archives of neurology. 2012 Sep;69(9):1176–1180. doi: 10.1001/archneurol.2012.314. [DOI] [PubMed] [Google Scholar]
- 17.Kim SM, Go MJ, Sung JJ, Park KS, Lee KW. Painful tonic spasm in neuromyelitis optica: incidence, diagnostic utility, and clinical characteristics. Archives of neurology. 2012 Aug;69(8):1026–1031. doi: 10.1001/archneurol.2012.112. [DOI] [PubMed] [Google Scholar]
- 18.Asgari N, Skejoe HP, Lillevang ST, Steenstrup T, Stenager E, Kyvik KO. Modifications of longitudinally extensive transverse myelitis and brainstem lesions in the course of neuromyelitis optica (NMO): a population-based, descriptive study. BMC neurology. 2013;13:33. doi: 10.1186/1471-2377-13-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waters PJ, McKeon A, Leite MI, et al. Serologic diagnosis of NMO: a multicenter comparison of aquaporin-4-IgG assays. Neurology. 2012 Feb 28;78(9):665–671. doi: 10.1212/WNL.0b013e318248dec1. discussion 669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collongues N, Cabre P, Marignier R, et al. A benign form of neuromyelitis optica: does it exist? Archives of neurology. 2011 Jul;68(7):918–924. doi: 10.1001/archneurol.2011.127. [DOI] [PubMed] [Google Scholar]


