Abstract
Background
Several studies have reported mixed results after carotid endarterectomy (CEA) in patients with chronic renal insufficiency (CRI), and we previously reported the perioperative outcome in patients with CRI by use of serum creatinine (Cr) level and glomerular filtration rate (GFR). However, only a few of these studies used GFR by the Modification of Diet in Renal Disease equation in their analysis of long-term outcome.
Methods
During the study period, 1000 CEAs (926 patients) were analyzed; 940 of these CEAs had Cr levels and 925 had GFR data. Patients were classified into normal (GFR ≥60 mL/min/1.73 m2 or Cr <1.5 mg/dL), moderate CRI (GFR ≥30–59 or Cr ≥1.5–2.9), and severe CRI (GFR <30 or Cr ≥3).
Results
At a mean follow-up of 34.5 months and a median of 34 months (range, 1–53 months), combined stroke and death rates for Cr levels (867 patients) were 9%, 18%, and 44% for Cr <1.5, ≥1.5 to 2.9, and ≥3 (P =.0001) in contrast to 8%, 14%, and 26% for GFR (854 patients) of >60, ≥30 to 59, and <30, respectively (P =.0003). Combined stroke and death rates for asymptomatic patients were 8%, 17%, and 44% (P =.0001) for patients with Cr levels of <1.5, ≥1.5 to 2.9, and ≥3, respectively, vs 7%, 13%, and 24% for a GFR of ≥60, ≥30 to 59, and <30 (P =.0063). By Kaplan-Meier analysis, stroke-free survival rates at 1 year, 2 years, and 3 years were 97%, 94%, and 92% for Cr <1.5; 92%, 85%, and 81% for Cr ≥1.5 to 2.9; and 56%, 56%, and 56% for Cr ≥3 (P < .0001); vs 98%, 95%, and 93% for a GFR ≥60; 93%, 90%, and 86% for a GFR of ≥30 to 59; and 86%, 77%, and 73% for a GFR <30 (P < .0001). These rates for asymptomatic patients at 1 year, 2 years, and 3 years were 97%, 95%, and 93% for Cr <1.5; 94%, 87%, and 82% for Cr ≥1.5 to 2.9; and 56%, 56%, and 56% for Cr ≥3 (P < .0001); vs 98%, 95%, and 94% for a GFR ≥60; 95%, 91%, and 86% for a GFR of ≥30 to 59; and 84%, 80%, and 75% for a GFR <30 (P =.0026). A univariate regression analysis for asymptomatic patients showed that the hazard ratio (HR) of stroke and death was 6.5 (P =.0003) for a Cr ≥3 and 3.1 for a GFR <30 (P =.0089). A multivariate analysis showed that Cr ≥3 had an HR of stroke and death of 4.7 (P =.008), and GFR <30 had an HR of 2.2 (P =.097).
Conclusions
Patients with severe CRI had higher rates of combined stroke/death. Therefore, CEA for these patients (particularly in asymptomatic patients) must be considered with caution.
Carotid interventions performed by vascular surgeons usually have low perioperative complication rates.1 However, in an era of ever-increasing cost containment, with critical evaluations of surgeons and center-specific outcomes, identifying patients at high risk for either perioperative adverse events or poor late survival may potentially change management paradigms. Preoperative parameters that have established negative impacts can and should be used to formulate the optimal treatment in those with poor long-term survival.
Large studies, including one by Hallan et al,2 have clearly shown the effect of chronic kidney disease on long-term survival. Their analysis of the results of more than two million participants that were stratified by age and other adjustments (ie, body mass index, total cholesterol level, diabetes mellitus, and other cardiovascular risk factors) demonstrated statistically increased mean all-cause mortality in every age group stratified with a declining estimated glomerular filtration rate (GFR). The natural history of patients as renal function deteriorates is poor and thus may affect our recommendations for vascular interventions, especially in the asymptomatic cohort.
Data from coronary interventions have pointed to poor outcomes in patients with chronic renal insufficiency (CRI). Three-year clinical outcomes after drug-eluting stent placement in patients with severe renal dysfunction (ie, undergoing hemodialysis) were dramatically inferior to outcomes of those not undergoing hemodialysis. In this study of more than 100 hemodialysis patients, the 3-year risk of both cardiac-related mortality and target vessel revascularization was 16% vs 2% and 19% vs 6%, respectively, compared with patients not requiring hemodialysis.3 Recently, Gallagher et al4 reported on the impact of both diabetes mellitus and renal insufficiency on the 5-year mortality rate after coronary artery bypass graft surgery. The 5-year all-cause mortality of the reference group (absence of diabetes and normal renal function) was reported at 9% compared with 20% in those with renal insufficiency and 28% in diabetics and renal insufficiency patients (P < .0001).
This study expands data from our previous report5 of early perioperative outcomes of carotid interventions with regard to renal function status and provides long-term data in this cohort.
METHODS
This is a retrospective study of all patients who underwent carotid endarterectomy (CEA) during a 2-year period (2010–2011) at Charleston Area Medical Center, Charleston, WVa. It was approved by our Institutional Review Board, and patient consent was not needed. All patient clinical characteristics/demographics were collected from electronic medical records, and deaths were verified by the Social Security Death Index. Strokes were verified from medical records and, if necessary, by contacting the primary or referring physicians. Indications for CEA were categorized into symptomatic (hemispheric transient ischemic attacks, amaurosis fugax, and hemispheric stroke) or asymptomatic (nonhemispheric transient ischemic attacks and asymptomatic carotid bruit).
Patients with combined coronary artery bypass grafting and CEA, patients undergoing repeated CEA, and patients with acute renal failure were excluded from this analysis. All CEAs were performed under general anesthesia with intravenous heparin and routine shunting.
Patients were classified into the following groups according to either their GFR by the Modification of Diet in Renal Disease (MDRD) equation or their serum creatinine (Cr) level: normal renal function (GFR ≥60 mL/min/1.73 m2 or Cr <1.5 mg/dL), moderate CRI (GFR ≥30–59 mL/min/1.73 m2 or Cr ≥1.5–2.9 mg/dL), and severe CRI (GFR <30 mL/min/1.73 m2 or Cr ≥3 mg/dL). Every effort was made to observe patients postoperatively at regular intervals: 30 days, 6 months, 12 months, and every 12 months thereafter.
The primary end points were all early (30 days perioperative) and late stroke or death. All strokes were confirmed with computed tomography scans or magnetic resonance imaging.
Statistical analysis
The data analysis was performed with SAS 9.2 (SAS Institute, Cary, NC). Categorical variables were compared by a contingency table analysis with a Fisher exact test or χ2 test to determine significant differences. Associations between risk factors and the occurrence of early and late strokes/deaths were tested by logistic regression. Variables with significant associations on univariate analysis were tested with multivariate logistic regression. An α level of ≤.05 was used to determine statistical significance. A Kaplan-Meier analysis was used to determine stroke-free survival rates according to GFR and Cr levels for the whole series and for asymptomatic patients.
RESULTS
Originally, 1000 CEAs were analyzed. This included 926 patients (74 bilateral). Of these 1000 CEAs, 940 had Cr level data and 925 had GFR data. For any survival analysis (stroke/death), only the first CEA was used in bilateral CEAs. Therefore, 926 patients/CEAs were analyzed. Of these 926, 867 of these CEAs had Cr level data and 854 had GFR data. The demographics/clinical characteristics according to renal function status were reported previously.5 Overall, 818 of 1000 CEAs had normal renal function, 113 had moderate CRI, and nine had severe CRI according to their serum Cr levels; vs 614 normal renal function, 274 with moderate CRI, and 37 with severe CRI according to their GFR. As expected, patients with moderate/severe CRI had significantly higher comorbidities, such as hypertension, diabetes mellitus, coronary artery disease, and older age. The perioperative stroke rates for normal renal function, moderate CRI, and severe CRI were 2%, 3.5%, and 11.1% (P =.091) by use of Cr level; vs 1.1%, 7.3%, and 5.4% (P =.018) by use of GFR. Overall, 21 patients had perioperative strokes: 10 were secondary to carotid thrombosis, six were embolic, two had myocardial infarctions followed by stroke, and the cause was unknown in three.
Late clinical outcome/renal status
At a mean follow-up of 34.5 months (range, 1–53 months), the combined stroke and death rates (early and late) were 9%, 18%, and 44% for Cr <1.5, ≥1.5 to 2.9, and ≥3 mg/dL (P =.0001); in contrast to 8%, 14%, and 26% for GFR of >60, 30 to 39, and <30 mL/min/1.73 m2, respectively (P =.0003). The combined stroke and death rates for asymptomatic patients (583 CEAs) were 8%, 17%, and 44% (P =.0001) for patients with Cr of <1.5, ≥1.5 to 2.9, and ≥3 mg/dL, respectively; vs 7%, 13%, and 24% for a GFR of ≥60, 30 to 39, and <30 mL/min/1.73 m2 (574 CEAs), respectively (P =.0063) (Table I).
Table I.
All stroke/death according to serum creatinine (Cr) level and glomerular filtration rate (GFR)
| Serum Cr, mg/dL
|
P value | |||
|---|---|---|---|---|
| Normal (<1.5) | Moderate (≥1.5–2.9) | Severe (>3) | ||
| All stroke/death | 69/757 (9%) | 18/101 (18%) | 4/9 (44%) | .0001 |
| Stroke/death (symptomatic patients, n =284) | 27/247 (11%) | 7/37 (19%) | 0 | .1752 |
| Stroke/death (asymptomatic patients, n =583) | 42/510 (8%) | 11/64 (17%) | 4/8 (44%) | .0001 |
| All death | 61/757 (8%) | 15/101 (15%) | 4/9 (44%) | .0001 |
| All death (symptomatic, n =284) | 26/247 (11%) | 7/37 (19%) | — | .1652 |
| All death (asymptomatic, n =583) | 35/510 (7%) | 8/64 (13%) | 4/9 (44%) | <.0001 |
| Total | 757 | 101 | 9 | |
| GFR, mL/min/1.73 m2
|
P value | |||
|---|---|---|---|---|
| Normal (≥60) | Moderate (≥30–59) | Severe (<30) | ||
| All stroke/death | 45/565 (8%) | 36/254 (14%) | 9/35 (26%) | .0003 |
| Stroke/death (symptomatic patients, n =280) | 17/186 (9%) | 14/84 (17%) | 3/10 (30%) | .0457 |
| Stroke/death (asymptomatic patients, n =574) | 28/379 (7%) | 22/170 (13%) | 6/25 (24%) | .0063 |
| All death | 40/565 (7%) | 32/254 (13%) | 8/35 (23%) | .0009 |
| All death (symptomatic) | 16/186 (9%) | 14/84 (17%) | 3/10 (30%) | .0313 |
| All death (asymptomatic) | 24/379 (6%) | 18/170 (11%) | 5/25 (20%) | .0215 |
| Total | 565 | 254 | 35 | |
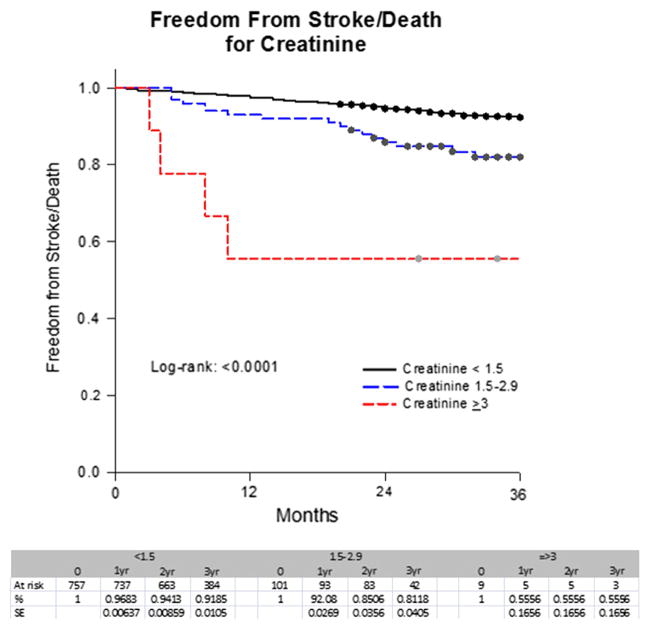
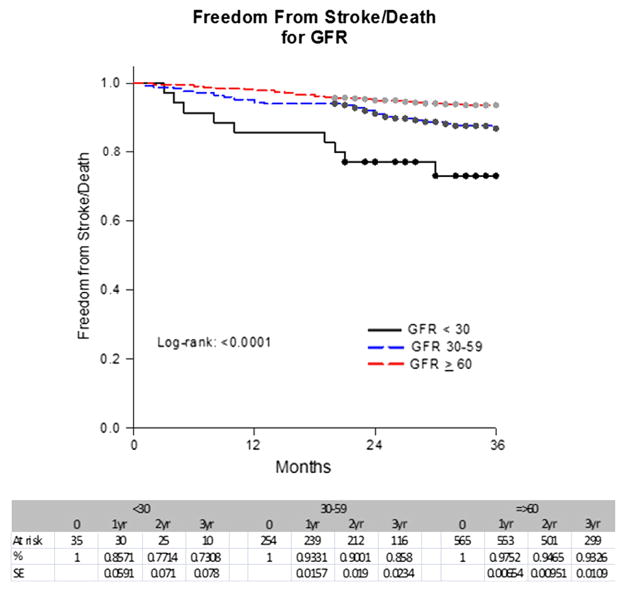
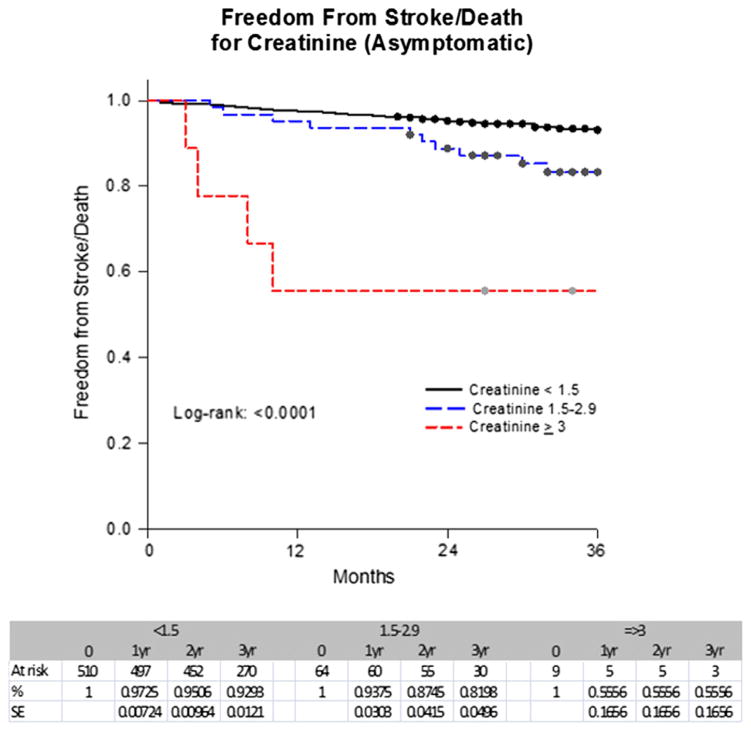
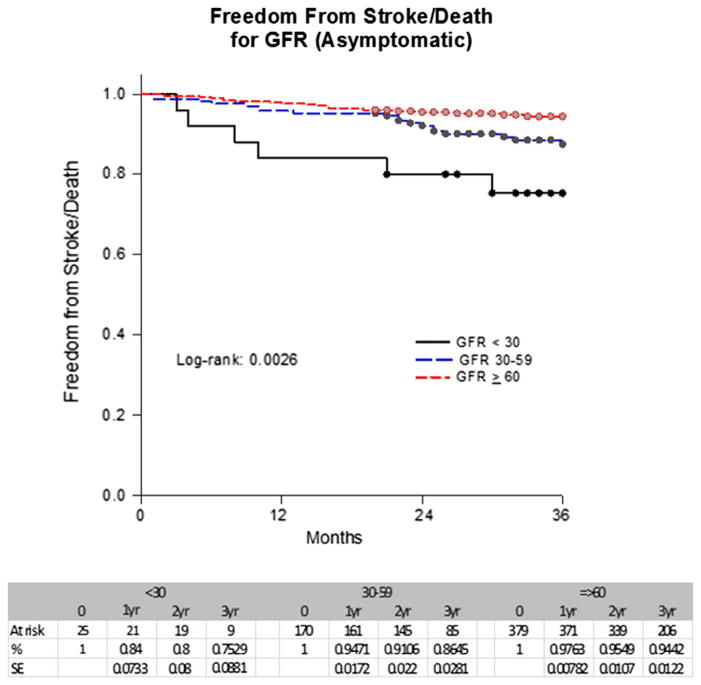
A Kaplan-Meier analysis showed that the stroke-free survival rates at 1 year, 2 years, 3 years, and 4 years were 97%, 94%, 92%, and 88% for Cr <1.5; 92%, 85%, 81%, and 81% for Cr ≥1.5 to 2.9; and 56%, 56%, 56%, and 56% for Cr ≥3 mg/dL (P < .0001) (Fig 1). In contrast, the stroke-free survival rates at 1 year, 2 years, 3 years, and 4 years were 98%, 95%, 93%, and 89% for a GFR ≥60; 93%, 90%, 86%, and 82% for a GFR of ≥30 to 59; and 86%, 77%, 73%, and 73% for a GFR <30 mL/min/1.73 m2 (P < .0001) (Fig 2). These rates for asymptomatic patients for 1 year, 2 years, and 3 years were 97%, 95%, and 93% for a Cr <1.5; 94%, 87%, and 82% for a Cr ≥1.5 to 2.9; and 56%, 56%, and 56% for a Cr ≥3 mg/dL, respectively (P < .0001). In contrast, the rates were 98%, 95%, and 94% for a GFR ≥60; 95%, 91%, and 86% for a GFR ≥30 to 59; and 84%, 80%, and 75% for a GFR <30 mL/min/1.73 m2 (P =.0026) (Figs 3 and 4).
Fig 1.
Freedom from stroke/death by serum creatinine (Cr) level in all patients. SE, Standard error.
Fig 2.
Freedom from stroke/death by glomerular filtration rate (GFR) in all patients. SE, Standard error.
Fig 3.
Freedom from stroke/death by serum creatinine (Cr) level in asymptomatic patients. SE, Standard error.
Fig 4.
Freedom from stroke/death by glomerular filtration rate (GFR) in asymptomatic patients. SE, Standard error.
Univariate Cox regression and multivariate analysis of combined early and late strokes/deaths
Tables II and III summarize the effect of multiple risk factors on the combined early and late strokes/deaths after CEA. By a univariate analysis (Table II), Cr ≥3 mg/dL had a hazard ratio (HR) of 5.9 (95% confidence interval [CI], 2.1–16; P =.0006) and GFR <30 mL/min/1.73 m2 had an HR of 3.2 (95% CI, 1.6–6.3; P =.0011) for all strokes and deaths. By a multivariate analysis (Table III), Cr ≥3 mg/dL had an HR of 3.4 (95% CI, 1.1–9.8; P =.0269) and GFR <30 mL/min/1.73 m2 had an HR of 2.1 (95% CI, 1–4.5; P =.0637) for all strokes and deaths.
Table II.
Univariate analysis of risk factors for all strokes/deathsa
| Effect | Hazard ratio | 95% Wald confidence limits | P valueb | |
|---|---|---|---|---|
| Cr level ≥3 mg/dL | 5.9 | 2.1 | 16 | .0006 |
| GFR <30 mL/min/1.73 m2 | 3.2 | 1.6 | 6.3 | .0011 |
| Symptomatic | 1.3 | 0.8 | 1.9 | .2767 |
| Hypertension | 1.4 | 0.8 | 2.5 | .2718 |
| Renal insufficiency | 2.3 | 1.4 | 3.7 | .0005 |
| Stroke | 1.6 | 0.95 | 2.6 | .077 |
| Diabetes mellitus | 1.5 | 1.0 | 2.3 | .0382 |
| Coronary artery disease | 1.3 | 0.8 | 1.9 | .2783 |
| Hypercholesterolemia | 0.8 | 0.6 | 1.3 | .4368 |
| Chronic obstructive pulmonary disease | 1.2 | 0.7 | 1.9 | .5068 |
| Morbid obesity | 1.0 | 0.2 | 4.1 | .9959 |
| Smoker | 0.9 | 0.6 | 1.3 | .5321 |
Cr, Creatine; GFR, glomerular filtration rate.
Cr level, 867 patients; GFR, 854 patients.
Boldface entries indicate statistical significance.
Table III.
Multivariate analysis of all patients for all strokes/deathsa
| Effectb | Hazard ratio | 95% Wald confidence limits | P valuec | |
|---|---|---|---|---|
| Cr level ≥3 mg/dL | 3.4 | 1.1 | 9.8 | .0269 |
| Renal insufficiency | 1.9 | 1.1 | 3.1 | .0175 |
| Diabetes mellitus | 1.3 | 0.9 | 2.0 | .1714 |
| Effectd | Hazard ratio | 95% Wald confidence limits | P valuec | |
|---|---|---|---|---|
| GFR <30 mL/min/1.73 m2 | 2.1 | 1 | 4.5 | .0637 |
| Renal insufficiency | 1.8 | 1.0 | 3.1 | .0409 |
| Diabetes mellitus | 1.3 | 0.9 | 2.0 | .1934 |
Cr, Creatine; GFR, glomerular filtration rate.
Cr level, 867 patients; GFR, 854 patients.
Including Cr level, renal insufficiency history, and diabetes mellitus.
Boldface entries indicate statistical significance.
Including GFR, renal insufficiency history, and diabetes mellitus.
By a univariate analysis of asymptomatic patients only, Cr ≥3 mg/dL had an HR of 6.5 (95% CI, 2.3–18; P =.0003) and GFR <30 had an HR of 3.1 (95% CI, 1.3–7.2; P =.0089) for all strokes and deaths. By a multivariate analysis of asymptomatic patients only, Cr ≥3 mg/dL had an HR of 4.7 (95% CI, 1.5–14.7; P =.008) and GFR <30 mL/min/1.73 m2 had an HR of 2.2 (95% CI, 0.9–5.8; P =.0969) for all strokes and deaths (Tables IV and V).
Table IV.
Univariate analysis of all strokes/deaths—asymptomatic patients onlya
| Effect | Hazard ratio | 95% Wald confidence limits | P valueb | |
|---|---|---|---|---|
| Cr level ≥3 mg/dL | 6.5 | 2.3 | 18 | .0003 |
| GFR <30 mL/min/1.73 m2 | 3.1 | 1.3 | 7.2 | .0089 |
| Hypertension | 1.3 | 0.6 | 2.6 | .5219 |
| Renal insufficiency | 2.4 | 1.3 | 4.3 | .0039 |
| Stroke | 1.7 | 0.8 | 3.4 | .1481 |
| Diabetes mellitus | 1.1 | 0.7 | 1.9 | .6443 |
| Coronary artery disease | 1.1 | 0.7 | 1.8 | .73 |
| Hypercholesterolemia | 0.9 | 0.5 | 1.5 | .5732 |
| Chronic obstructive pulmonary disease | 1.1 | 0.6 | 2.0 | .7207 |
| Morbid obesity | 0.7 | 0.1 | 5.2 | .7391 |
| Smoker | 0.7 | 0.4 | 1.2 | .1686 |
Cr, Creatine; GFR, glomerular filtration rate.
Cr level, 583 asymptomatic patients; GFR, 574 asymptomatic patients.
Boldface entries indicate statistical significance.
Table V.
Multivariate analysis of all strokes/deaths—asymptomatic patients onlya
| Effectb | Hazard ratio | 95% Wald confidence limits | P valuec | |
|---|---|---|---|---|
| Cr level ≥3 mg/dL | 4.7 | 1.5 | 14.7 | .008 |
| Renal insufficiency | 1.7 | 0.8 | 3.3 | .1367 |
| Diabetes mellitus | 0.9 | 0.5 | 1.6 | .8497 |
| Effectd | Hazard ratio | 95% Wald confidence limits | P valuec | |
|---|---|---|---|---|
| GFR <30 mL/min/1.73 m2 | 2.2 | 0.9 | 5.8 | .0969 |
| Renal insufficiency | 1.8 | 0.9 | 3.5 | .1171 |
| Diabetes mellitus | 0.97 | 0.6 | 1.7 | .9014 |
Cr, Creatine; GFR, glomerular filtration rate.
Cr level, 583 asymptomatic patients; GFR, 574 asymptomatic patients.
Including Cr level, renal insufficiency, and diabetes mellitus.
Boldface entries indicate statistical significance.
Including GFR, renal insufficiency, and diabetes mellitus.
DISCUSSION
Recently, there have been several series bringing to light certain subsets of CEA patients that may have more morbidity and mortality than was first realized. CRI is a major comorbidity that has been examined in the last two decades; however, most of the reported studies have analyzed the impact of CRI on perioperative CEA outcomes,5–10 whereas only a few have analyzed late outcomes.11–14
The majority of recent studies also examined outcomes based on serum Cr level alone, with only a few studies5,10–12,14 using the Cockroft-Gault or MDRD formula to assess Cr clearance; and only two of these studies analyzed late outcomes after CEA.12,14 Two of the larger original series shed some light on CRI and CEA but found conflicting results. In 1997 and 1999, Rigdon et al6 and Sternbergh et al13 both examined the subset of CRI patients undergoing CEA. The limitations in both of these series as well as in many others that have been published were that the severe CRI population, defined by a Cr >3.0 mg/dL, was small. Sternberg et al found that there was a slight difference in the long-term survival but no difference in perioperative outcomes; whereas Rigdon et al found a significant difference in both stroke and death rates in patients with moderate and severe CRI. Several authorities have shown that Cr levels alone are suboptimal markers of renal function, which was thought to be secondary to several factors that may affect Cr metabolism/clearance and discrepancies in reported laboratory values.5,10,11
Between the years of 2008 and 2011, three large series were published that offered more data regarding risk with use of the MDRD formula.10–12 In 2008, Sidawy et al10 reported that patients with moderate CRI were found to have higher comorbidity and mortality rates, with higher rates of stroke and death in patients who were symptomatic. In 2010, Kretz et al11 also reported that a significant increase in perioperative death was found in patients with severe CRI based on serum Cr level alone (Cr >3.0 mg/dL). However, based on the Cockroft-Gault and MDRD formulas, there was an increase in perioperative neurologic events and combined stroke and death rates in patients with severe CRI.11 In 2011, Protack et al12 examined 921 carotid interventions, including both CEA and carotid stenting. No significant difference was found in perioperative strokes; however, there were significantly higher rates of perioperative deaths when the mild and moderate CRI groups were compared with those with normal renal function, and there was also a lower rate of freedom from stroke after carotid artery stenting but no significant difference in freedom from stroke based on renal function after CEA.12 Recently, van Lammeren et al14 reported that patients with a GFR of ≥30 to 59 mL/min/1.73 m2 had a 2.2-fold increased risk of cardiovascular death and a 1.9-fold increased risk of myocardial infarction during the 3 years after CEA compared with patients with a normal GFR, regardless of other cardiovascular risk factors.
More recently, in 2013, we reported on the perioperative results of 940 CEAs, discerning the effect of CRI on perioperative outcomes.5 Using serum Cr level alone, we found that there was no significant difference in perioperative major adverse event (MAE) rates between normal renal function, moderate CRI, and severe CRI patients (2.4%, 4.4%, and 11.1%; P =.089), which can, in part, be related to the small number of severe CRI patients. However, with use of the GFR (MDRD), significant differences in perioperative stroke rates (1.1%, 3.7%, and 5.4% for normal renal function, moderate CRI, and severe CRI; P =.018) and all cardiac complications (4.8% vs 1.8% for moderate or severe CRI vs normal; P =.008) were noted, along with a trend toward higher rates of all MAEs (stroke, myocardial infarction, and death [1.8%, 4%, and 5.4%, for normal renal function, moderate CRI, and severe CRI, respectively; P =.086]).
This study is an updated report of the late clinical outcomes of these patients with CRI. The mean follow-up period of 34.5 months in this study has resulted in long-term data that have provided more insight into the outcomes of CEA in these patients over time. Our present series is, to the best of our knowledge, one of a few series in the literature to effectively examine long-term MAEs after CEA in this population of patients. Statistical significance was attained in nearly all clinical outcomes that were examined.
With use of Cr level alone, the early and late mortality rates were higher in the moderate and severe CRI groups compared with those with normal renal function (P =.0001), as were the combined stroke and death rates (P =.0001). With use of GFR, the early and late mortality rates were also higher in patients with moderate and severe CRI than in normal patients (P =.0009), as were combined stroke and death rates (P =.0003). The stroke-free survival rates at 1 year, 2 years, 3 years, and 4 years, likewise, also favored those with normal renal function (P < .0001).
By a univariate analysis of risk factors on the outcome of all (early and late) strokes and deaths, Cr >3.0 mg/dL (HR, 5.9; P =.0006), GFR <30 mL/min/1.73 m2 (HR, 3.2; P =.0011), and diabetes mellitus (HR, 1.5; P =.0382) were found to be significant. A multivariate analysis showed that Cr ≥3.0 mg/dL had an HR of 3.4 (P =.0269), whereas GFR <30 mL had an HR of 2.1 (P =.0637) for all strokes and deaths.
Our present study also examined differences in outcomes based on symptomatic status, and results strongly correlated with what was found perioperatively. With both serum Cr level and GFR, overall mortality rates in asymptomatic patients reached significance (P < .0001, P =.0215, respectively), as did combined all stroke and death rates (P =.0001, P =.0063, respectively), with higher rates being found in those with moderate and severe CRI compared with patients with normal renal function. This was not necessarily true for symptomatic patients. With serum Cr level alone, both death rates and combined stroke and death rates did not reach statistical significance (P =.1652, P =.1752, respectively); but when GFR was used, significantly higher rates of both death and combined stroke and death (P =.0313, P =.0457) were seen. Univariate analysis also showed significance with both Cr > 3 mg/dL (HR, 6.5; P =.0003) and GFR<30 mL (HR, 3.1; P =.0089) in asymptomatic patients for combined all strokes and deaths.
Our study does have its limitations. Similar to other series before it, it is retrospective in nature and has only a small number of patients with severe renal insufficiency (by use of serum Cr level); however, because it is one of the few larger series to separate patients into subgroups on the basis of GFR, a larger number of CRI patients was attained (311 patients, including 37 patients with severe CRI). We found that in addition to higher rates of perioperative MAEs in looking at GFR, there also appears to be a higher rate of MAEs in the long term as well. In particular, it did not matter whether we used the serum Cr level or GFR; all deaths (early and late) and combined stroke and deaths were found to be significantly higher in patients with moderate and severe CRI. We found that both asymptomatic and symptomatic patients had significantly higher long-term stroke and death rates with use of the GFR and that asymptomatic patients, in particular, also had higher long-term stroke and death rates with use of serum Cr level alone.
CONCLUSIONS
Our long-term results not only support our original perioperative findings for CRI patients undergoing CEA, compared with those with normal renal function, but also elucidate possibly higher long-term stroke and death rates. Although it raises the question as to whether intervention is beneficial in this population of patients, more data may be needed to reach a definitive conclusion. Our series emphasizes, however, that all patients with moderate to severe CRI, based on serum Cr level and even more so on GFR, should have a thorough preoperative assessment, including a cardiac evaluation, before deciding to undergo CEA. This is even more important in asymptomatic patients with severe CRI, who should understand that they carry a potentially high risk of adverse outcomes, both perioperatively and long term. Therefore, especially in these asymptomatic patients, careful consideration should be given to conservative medical therapy and not to undergo carotid intervention.
Footnotes
Author conflict of interest: none.
Presented at the Twenty-eighth Annual Meeting of the New England Society for Vascular Surgery and Eastern Vascular Society, Boston, Mass, September 11–14, 2014.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
AUTHOR CONTRIBUTIONS
Conception and design: AA, MS, PS, LD, AM
Analysis and interpretation: AA, LD
Data collection: MS, PS, BC, WJ, AM
Writing the article: AA, MS, PS
Critical revision of the article: AA, LD
Final approval of the article: AA, MS, PS, BC, WJ, LD, AM
Statistical analysis: AA, LD
Obtained funding: Not applicable
Overall responsibility: AA
References
- 1.AbuRahma AF, Stone PA, Srivastava M, Hass SM, Mousa AY, Dean LS, et al. The effect of surgeon’s specialty and volume on the perioperative outcome of carotid endarterectomy. J Vasc Surg. 2013;58:666–72. doi: 10.1016/j.jvs.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 2.Hallan SI, Matsushita K, Sang Y, Mahmoodi BK, Black C, Ishani A, et al. Chronic Kidney Disease Prognosis Consortium. Age and association of kidney measures with mortality and end-stage renal disease. JAMA. 2012;308:2349–60. doi: 10.1001/jama.2012.16817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Otsuka Y, Ishiwata S, Inada T, Kanno H, Eyo E, Hayashi Y, et al. Comparison of haemodialysis patients with respect to clinical characteristics and 3-year clinical outcomes after sirolimus-eluting stent implantatation: insights for the Japan muilt-centre post-marketing surveillance registry. Eur Heart J. 2011;32:829–37. doi: 10.1093/eurheartj/ehq480. [DOI] [PubMed] [Google Scholar]
- 4.Gallagher S, Kapur A, Lovell MJ, Jones DA, Kirkwood A, Hassan S, et al. Impact of diabetes mellitus and renal insufficiency on 5-year mortality following coronary artery bypass graft surgery: a cohort study of 4869 UK patients. Eur J Cardiothorac Surg. 2014;45:1075–81. doi: 10.1093/ejcts/ezt630. [DOI] [PubMed] [Google Scholar]
- 5.AbuRahma AF, Srivastava M, Chong B, Dean LS, Stone PA, Koszewski A. Impact of chronic renal insufficiency using serum creatinine vs. glomerular filtration rate on perioperative clinical outcomes of carotid endarterectomy. J Am Coll Surg. 2013;216:525–33. doi: 10.1016/j.jamcollsurg.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 6.Rigdon EE, Monajjem N, Rhodes RS. Is carotid endarterectomy justified in patients with severe chronic renal insufficiency? Ann Vasc Surg. 1997;11:115–9. doi: 10.1007/s100169900020. [DOI] [PubMed] [Google Scholar]
- 7.Debing E, van den Brande P. Chronic renal insufficiency and risk of early mortality in patients undergoing carotid endarterectomy. Ann Vasc Surg. 2006;20:609–13. doi: 10.1007/s10016-006-9080-5. [DOI] [PubMed] [Google Scholar]
- 8.Tarakji A, McConaughy A, Nicholas GG. The risk of carotid endarterectomy in patients with chronic renal insufficiency. Curr Surg. 2006;63:326–9. doi: 10.1016/j.cursur.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Ascher E, Marks NA, Schutzer RW, Hingorani AP. Carotid endarterectomy in patients with chronic renal insufficiency: a recent series of 184 cases. J Vasc Surg. 2005;41:24–9. doi: 10.1016/j.jvs.2004.10.047. [DOI] [PubMed] [Google Scholar]
- 10.Sidawy AN, Aidinian G, Johnson ON, III, White PW, DeZee KJ, Henderson WG. Effect of chronic renal insufficiency on outcomes of carotid endarterectomy. J Vasc Surg. 2008;48:1423–30. doi: 10.1016/j.jvs.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 11.Kretz B, Abello N, Brenot R, Steinmetz E. The impact of renal insufficiency on the outcome of carotid surgery is influenced by the definition used. J Vasc Surg. 2010;51:43–50. doi: 10.1016/j.jvs.2009.08.070. [DOI] [PubMed] [Google Scholar]
- 12.Protack CD, Bakken AM, Saad WE, Davies MG. Influence of chronic renal insufficiency on outcomes following carotid revascularization. Arch Surg. 2011;146:1135–41. doi: 10.1001/archsurg.2011.142. [DOI] [PubMed] [Google Scholar]
- 13.Sternbergh WC, III, Garrard CL, Gonze MD, Manord JD, Bowen JC, Money SR. Carotid endarterectomy in patients with significant renal dysfunction. J Vasc Surg. 1999;29:672–7. doi: 10.1016/s0741-5214(99)70313-7. [DOI] [PubMed] [Google Scholar]
- 14.van Lammeren GW, Moll FL, Blankestign PJ, de Kleijn DPV, Bots ML, Verhaar MC, et al. Decreased kidney function: an unrecognized and often untreated risk factor for secondary cardiovascular events after carotid surgery. Stroke. 2011;42:307–12. doi: 10.1161/STROKEAHA.110.597559. [DOI] [PubMed] [Google Scholar]