Abstract
Background
There is increasing emphasis on the appropriateness and quality of acute surgical care for patients with serious illness, and at the end-of-life. However, there is a lack of evidence regarding outcomes after emergent major abdominal surgery among patients with advanced cancer to guide treatment decisions. This analysis seeks to characterize adverse outcomes (mortality, complications, institutional discharge) and to identify factors independently associated with 30-day mortality among patients with disseminated cancer who undergo emergent abdominal surgery for intestinal obstruction or perforation.
Methods
This is a retrospective cohort study of 875 disseminated cancer patients undergoing emergency surgery for perforation (n=499) or obstruction (n=376) at hospitals participating in the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) from 2005-2012. Predictors of 30 day mortality were identified using multivariate logistic regression.
Results
Among patients who underwent surgery for perforation, 30-day mortality was 34%, 67% had complications and 52% were discharged to an institution. Renal failure, septic shock, ascites, dyspnea at rest, and dependent functional status were independent preoperative predictors of death at 30 days. When complications were considered, postoperative respiratory complications and age (75-84 years) were also predictors of mortality.
Patients who had surgery for obstruction had a 30-day mortality rate of 18% (n=68), 41% had complications, and 60% were discharged to an institution. Dependent functional status and ascites were independent predictors of death at 30 days. In addition to these predictors, postoperative predictors of mortality included respiratory and cardiac complications. Few patients (4%) had DNR orders prior to surgery.
Conclusions
Emergency abdominal operations in patients with disseminated cancer are highly morbid and many patients die soon after surgery. High rates of complications, and low rates of pre-existing DNR orders highlight the need for targeted interventions to reduce complications and integrate palliative approaches into the care of these patients.
Level of Evidence
III
Study Type
Prognostic
Keywords: Advanced Cancer, Emergency Surgery
Background
There is intensifying interest among clinicians, policy-makers, and the public in the appropriateness and effectiveness of care for patients with life-limiting illness and patients at the end of life (1). Almost one-third of older patients undergo a surgical procedure in their last year of life, and many in their last weeks of life (2). As compared to those who do not have surgery, patients who undergo operative interventions spend more days in the hospital and in intensive care during their last year of life. At the same time, studies show that patients with advanced illness prioritize comfort and time at home with family over longevity if the burdens of treatments are high (3). While palliative procedures in advanced stage cancer patients have been shown to improve patient symptom management (4), the outcomes after emergency operations are less clear.
Data suggest that disease-directed care, rather than palliative care, does not prolong life and may in fact cause worse quality of life in patients with advanced cancer and poor bereavement outcomes for survivors. High-intensity treatments in advanced stage cancer patients have been found to prolong suffering without meeting patients' personal goals at the end of life (5-7). In contrast, less aggressive treatments have been associated with better quality of life, better symptom management and improved outcomes for bereaved survivors (8). Moreover, continued aggressive care and lack of focus on palliation at the end of life is associated with a negative impact on the patients' family (6, 7, 9, 10). The decision to have an operation and the decision to continue intensive treatment after complications ensue depend on the burden of treatment, the potential outcomes, and the likelihood of those outcomes (11). Therefore, it would be immensely helpful for clinicians, patients and surrogates to better understand predictors of 30-day mortality after surgery to guide perioperative decision making and to identify cancer patients least likely to benefit from an operation.
The goals of this study are to 1) improve surgeons' ability to prognosticate patient outcomes and inform perioperative conversations about treatment preferences and decisions for intervention among patients with advanced cancer who undergo emergency surgery for perforation or obstruction and 2) describe a cohort that could benefit from perioperative palliative care. As such, we sought to quantify mortality within 30 days of surgery and to identify preoperative characteristics and postoperative complications that predict short-term mortality. We hypothesize that patients with disseminated cancer will experience a high burden of complications and high postoperative mortality.
Methods
Data source and study cohort
This is a multi-institution cohort study of patients with a preoperative diagnosis of disseminated cancer who underwent emergent major abdominal operations for either intestinal obstruction or perforation. De-identified data was obtained from the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) database that includes patients from hospitals enrolled from 2005 to 2012. ACS NSQIP data is retrosprospectively collected by trained surgical clinical reviewers at each participating center. ACS NSQIP collects information by capturing all cases or by systematic randomization using an 8-day cycle. Preoperative, intraoperative, and postoperative data variables are obtained using concrete data collection with standardized definitions and processes (12). ACS NSQIP data has proven validity and reliability with a reported inter-rater reliability of approximately 98% (12).
Patients included in this study were at least 18 years old, had a preoperative diagnosis of disseminated cancer, and underwent an emergency operation for intestinal obstruction or perforation by the primary International Classification of Diseases, 9th edition (ICD-9) code. Disseminated cancer, as defined by ACS NSQIP, includes any patient who has cancer that has spread to one or more sites from the primary site. In addition, the presence of multiple metastases must indicate that the cancer is widespread, fulminant, or near terminal. Emergency operations are defined by ACS NSQIP as operations that must be performed as soon as possible and no later than 12 hours after the patient has been admitted to the hospital or after the onset of related preoperative symptoms as determined by the surgeon or anesthesiologist.
We identified patients with the primary diagnosis of intestinal perforation or obstruction using ICD-9 codes. Obstruction codes included in this study were: 552.2, 552.8, 560.xx, 560.2, 560.8, 560.9, 562.1, or 569.8. Perforation codes included: 532.10, 532.11, 532.20, 532.21, 532.50, 532.51, 532.60, 532.61 and 562.11, 569.82, 569.83. These two ICD-9 classified groups were analyzed separately.
Variables and outcomes
We collected data on patient characteristics, preoperative clinical characteristics, and postoperative variables. Patient characteristics include age (categories: < 65, 65-74, 75-84, and ≥ 85 years old), gender, race, comorbidities (hypertension requiring medication, diabetes, chronic obstructive pulmonary disease, myocardial infarction, congestive heart failure, peripheral vascular disease, renal failure, and dialysis), and clinical characteristics (prehospital location of home, dependent functional status, do not resuscitate (DNR) status, chemotherapy use, steroid use, preoperative transfusion, smoking status, American Society of Anesthesiology (ASA) classification > 3, body mass index (BMI) category, ascites, bleeding disorder, dyspnea at rest, impaired sensorium, preoperative pneumonia, preoperative sepsis, and preoperative septic shock). The ASA classification system categorizes patients into 6 groups based on physical status from 1 (normal healthy patient) to 6 (brain-dead patient). Categories 3 or more indicates that a patient has at least severe systemic disease. BMI categories were categorized into groups of < 18.5, 18.5-25, and greater than 25.
Postoperative variables include postoperative complications, unplanned reoperation, unplanned readmission, discharge location, hospital length of stay, in hospital mortality, and 30-day mortality. Postoperative complications include respiratory, hematologic, wound, urologic, deep venous thrombosis, pulmonary embolus, cardiac, and neurologic. Discharge locations included home, skilled nursing facility including rehab, or expired. In-hospital mortality was calculated from the date of surgery to 30 days after the operation. The primary outcome of interest was 30-day overall mortality calculated from the date of surgery.
Statistical Analysis
Patients with intestinal perforation or obstruction were considered separately. Data was assessed for missing values and normality. Descriptive statistics are reported as percentages for categorical variables; median and interquartile range (IQR) were used to describe continuous variables that were not normally distributed. Univariate analysis using Chi square and Wilcoxon Rank-Sum was performed to determine differences between survivors and non-survivors at thirty days. After performing the univariate analysis, forward stepwise multivariate logistic regression was used to select variables from all of the preoperative variables for the first model and then preoperative and complication variables for the second model with the outcome of 30-day mortality. The model ‘entry’ threshold was set to p<0.10 level and model ‘stay’ threshold was p<0.05, including adjustment for age. Two separate analyses were performed for the perforation and obstruction cohorts. In the primary analysis, we only included baseline patient and clinical factors available to the clinician before surgery. In secondary analysis, we included patient factors, clinical factors, and postoperative complications in our model as these would be available to the clinician during postoperative hospitalization. To ensure there was not overfitting of our model, the number of variables was limited to 17 and 7 for perforation and obstruction cases, respectively, based on the number of deaths in each cohort (13, 14). A sensitivity analysis including all significant variables was performed and revealed very similar results to the stepwise regression. In addition, a survival analysis was performed comparing patients who survived versus those who died at 30-days who were found to have wound complications using the Mann-Whitney Test to address possible survivor bias. Multiple imputation chained equations (MICE) method was used to account for missing data (15, 16). Hosmer and Lemeshow Goodness-of-Fit Test was performed for each of the four final logistic regression models and each of the models were found to adequately fit the data (p > 0.05). Analysis was performed using SAS 9.3 (SAS Institute Inc., Cary, NC, USA). α <0.05.
Results
Intestinal Perforation
There were 499 patients with disseminated cancer who underwent an emergency operation due to intestinal perforation. Patient characteristics of this cohort are shown in Table 1. Fifty percent of patients were less than 65 years old and 52% were male. Most were functionally independent (61%). Few (4%) patients had a DNR recorded preoperatively. Over two-thirds (69%) of patients experienced one or more post-operative complications. Thirteen percent of patients underwent an unplanned reoperation and 13% had an unplanned readmission within 30 days of their original operation. Twelve percent of patients were discharged home. More than a quarter (26%) died in the hospital and more than one-third (34%) died within thirty days of the operation.
Table 1. Characteristics of Patients with Disseminated Cancer undergoing Emergency General Surgery Operations.
| Preoperative Variable: | Perforation (N=499) % | Obstruction (N=376) % |
|---|---|---|
| Age | ||
| <65 | 50.1 | 45.2 |
| 65-74 | 29.5 | 29.0 |
| 75-84 | 17.6 | 22.1 |
| 85+ | 2.8 | 3.7 |
| Race, white | 58.7 | 54.3 |
| Comorbid Disease: | ||
| Hypertension (requiring medication) | 50.9 | 52.9 |
| Diabetes | 16.2 | 14.1 |
| Chronic Obstructive Pulmonary Disease | 9.8 | 13.8 |
| Myocardial Infarction | 1.2 | 0 |
| Congestive Heart Failure | 1.0 | 1.86 |
| Peripheral Vascular Disease | 1.2 | 1.1 |
| Renal failure | 5.6 | 3.7 |
| Dialysis | 2.8 | 0.8 |
| Preoperative Clinical Characteristics: | ||
| Pre-hospital Location (Home) | 80.2 | 87.2 |
| Independent Functional Status | 60.7 | 71.5 |
| DNR | 3.8 | 3.7 |
| Chemotherapy Use | 36.7 | 30.3 |
| Steroid Use | 26.5 | 8.0 |
| Transfusions | 5.6 | 2.7 |
| Smoking status | 24.0 | 17.6 |
| ASA Class >3 | 57.3 | 31.9 |
| BMI (categories) | ||
| <18.5 | 14.6 | 13.0 |
| 18.5-25 | 34.9 | 46.3 |
| 25+ | 50.5 | 40.7 |
| Ascites | 16.8 | 16.8 |
| Bleeding disorder | 16.4 | 18.9 |
| Dyspnea at rest | 13.6 | 4.8 |
| Impaired Sensorium | 6.0 | 3.5 |
| Pneumonia | 4.8 | 3.7 |
| Sepsis | 35.3 | 7.7 |
| Septic Shock | 21.0 | 4.3 |
| Postoperative Events: | ||
| Any Complication | 68.7 | 46.5 |
| Respiratory Complication | 34.1 | 19.7 |
| Hematologic Complication | 26.9 | 14.1 |
| Wound Complication | 23.4 | 13.0 |
| On Ventilator >48 hours | 9.2 | 4.0 |
| Urologic Complication | 6.8 | 6.4 |
| Deep Venous Thrombosis | 5.4 | 5.1 |
| Pulmonary Embolus | 1.0 | 1.6 |
| Cardiac Complication | 4.0 | 2.7 |
| Neurologic Complication | 1.8 | 0.5 |
| Fail to Rescue | 24.6 | 13.0 |
| Unplanned Reoperation | 12.6 | 7.3 |
| Unplanned Readmission | 13.0 | 13.5 |
| Length of stay days (IQR) | 13(8-23) | 12(8-19) |
| Discharge Location | ||
| Home | 12.0 | 21.4 |
| Other (rehab, nursing facility, continued hospitalization) | 52.7 | 60.3 |
| In hospital Mortality | 25.5 | 11.2 |
| Overall 30-day mortality | 34.1 | 18.1 |
IQR=Interquartile Range
SD=Standard Deviation
Results of univariate analysis comparing characteristics of patients who survived to those who died within 30 days of surgery are shown in Table 2. Patients who died were significantly more likely to have comorbidities, functional dependence, and preoperative septic shock. Those who died were more likely to experience respiratory complications (48% versus 27%, p <0.001), and mechanical ventilation > 48 hours (18% versus 5%, p <0.001).
Table 2. Characteristics Associated with Survival and Overall Mortality at 30 Days After Surgery for Intestinal Perforation.
| Preoperative Variable | Alive at 30 Days (N=329) % | Dead at 30 Days (N=170) % | P-value |
|---|---|---|---|
| Age | |||
| <65 | 52.6 | 45.3 | 0.07 |
| 65-74 | 30.4 | 27.7 | |
| 75-84 | 14.6 | 23.5 | |
| 85+ | 2.4 | 3.5 | |
| Sex, male | 52.9 | 49.4 | 0.51 |
| Race, white | 81.9 | 78.4 | 0.48 |
| Comorbid Disease: | |||
| HTN (requiring medication) | 48.3 | 55.9 | 0.13 |
| Diabetes | 14.3 | 20.0 | 0.12 |
| Chronic Obstructive Pulmonary Disease | 7.3 | 14.7 | 0.01 |
| Myocardial Infarction | 0.9 | 1.8 | 0.41 |
| Congestive Heart Failure | 0.9 | 1.2 | 1.0 |
| Peripheral Vascular Disease | 1.2 | 2.2 | 0.67 |
| Renal failure | 3.0 | 10.6 | <0.001 |
| Dialysis | 2.1 | 4.1 | 0.25 |
| Preoperative Clinical Characteristics: | |||
| Prehospital Location (Home) | 81.8 | 79.9 | 0.63 |
| Independent Functional Status | 69.3 | 44.6 | <0.001 |
| Do Not Resuscitate Order | 2.0 | 10.1 | <0.001 |
| Chemotherapy | 50.0 | 42.8 | 0.20 |
| Steroid use | 24.0 | 31.2 | 0.09 |
| Transfusions | 5.5 | 5.9 | 0.84 |
| Smoking status | 23.7 | 24.7 | 0.83 |
| ASA Class >3 | 51.1 | 69.4 | <0.001 |
| Body Mass Index | 0.07 | ||
| <18.5 | 15.5 | 12.9 | |
| 18.5-25 | 31.3 | 41.8 | |
| 25+ | 53.2 | 45.3 | |
| Ascites | 12.5 | 25.3 | <0.001 |
| Bleeding disorder | 15.5 | 18.2 | 0.45 |
| Dyspnea at rest | 7.6 | 25.3 | <0.001 |
| Impaired Sensorium | 4.4 | 13.8 | <0.01 |
| Pneumonia | 4.0 | 10.1 | 0.03 |
| Sepsis | 39.5 | 27.1 | 0.01 |
| Septic Shock | 11.9 | 38.8 | <0.001 |
| Postoperative Events: | |||
| Any Complication | 67% | 72% | 0.22 |
| Respiratory Complication | 27.1 | 47.6 | <0.001 |
| Hematologic Complication | 26.1 | 28.2 | 0.67 |
| Wound Complication | 29.8 | 11.2 | <0.001 |
| On Ventilator >48 hours | 4.9 | 17.6 | <0.001 |
| Urologic Complication | 6.4 | 7.6 | 0.58 |
| Deep Venous Thrombosis | 6.1 | 4.1 | 0.41 |
| Pulmonary Embolus | 1.2 | 0.6 | 0.67 |
| Cardiac Complication | 2.4 | 7.1 | 0.02 |
| Neurologic Complication | 1.2 | 2.9 | 0.29 |
| Fail to Rescue | 0 | 72.4 | <0.001 |
| Unplanned Reoperation | 16.2 | 3.7 | 0.17 |
| Unplanned Readmission | 16.9 | 3.7 | 0.10 |
| Length of stay days (IQR) | 14(9-27) | 10.5(5-18) | <.001* |
Wilcoxon Rank-Sum test
IQR=Interquartile Range
In the multivariate logistic regression analysis including clinical factors available before surgery, predictors of 30-day overall mortality were renal failure (OR 2.7 with 95% CI: 1.1-6.8), septic shock (OR 3.2 with 95% CI: 1.9-5.3), dyspnea at rest (OR 2.5 with 95% CI: 1.4-4.7), ascites (OR 2.2 with 95% CI: 1.3-3.7), and dependent functional status (OR 2.0 with 95% CI: 1.3-3.1). When postoperative complications were included in the model, respiratory complications (OR 2.1 with 95% CI: 1.3-3.3) also predicted mortality (Figure 1). Patients found to have wound complications had a lower odds of 30-day mortality (OR 0.29 with 95% CI: 0.16-0.53); however, a survival analysis revealed that this finding was due to survival bias. After accounting for death in the survival analysis there was no difference in wound complications between those who died or survived at 30 days (p = 0.34).
Figure 1.
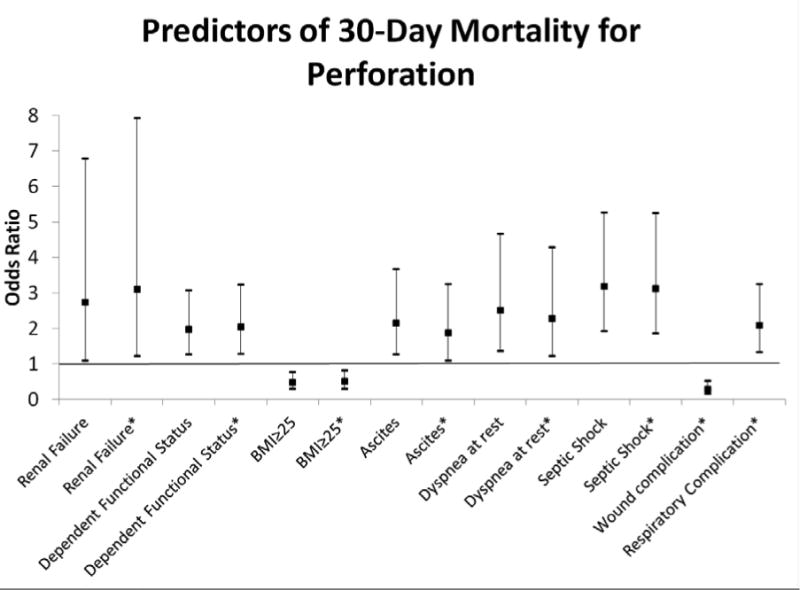
Predictors of 30-Day mortality for patients undergoing emergent operations for perforation. All predictors were statistically significant with p<0.05.
Model including preoperative variables alone: AUC 0.7754 (95% CI: 0.7563-0.7946)
Model including pre- and postoperative variables: AUC 0.8111 (95% CI: 0.7934-0.8289)
* Indicates variables from the model including postoperative predictors.
Obstruction
There were 376 patients with disseminated cancer who underwent an emergency operation for intestinal obstruction. Patient characteristics are depicted in Table 1. Most patients were less than 65 years old (45%) and male (51%); 72% were functionally independent. Four percent of patients had a DNR order recorded preoperatively. Almost half (47%) experienced one or more post-operative complications. Seven percent of patients underwent a reoperation and 14% of patients had a readmission after their initial operation. Most (60%) patients were discharged to a nursing facility. Eleven percent of patients died in the hospital and 18% of patients died within 30-days of the operation.
Results of univariate analysis comparing characteristics of patients who survived to patients who died within 30 days of surgery are shown in Table 3. As in patients with perforation, patients who died within 30 days of surgery for obstruction were significantly more likely to have comorbidities, functional dependence, and complications than survivors.
Table 3. Characteristics Associated with Survival and Overall Mortality at 30 Days After Surgery for Intestinal Obstruction.
| Preoperative Variable | Alive at 30 Days (N= 308) % | Dead at 30 Days (N=68) % | P-value |
|---|---|---|---|
| Age | |||
| <65 | 46.1 | 41.2 | 0.51 |
| 65-74 | 27.9 | 33.8 | |
| 75-84 | 22.7 | 19.1 | |
| 85+ | 3.3 | 5.9 | |
| Sex, male | 48.1 | 63.2 | 0.03 |
| Race, white | 76.9 | 91.1 | 0.04 |
| Comorbid Disease: | |||
| HTN (requiring medication) | 53.9 | 48.5 | 0.43 |
| Diabetes | 14.0 | 14.7 | 0.85 |
| Chronic Obstructive Pulmonary Disease | 12.0 | 22.1 | 0.05 |
| Myocardial Infarction | 0 | 0 | 1.0 |
| Congestive Heart Failure | 1.0 | 5.9 | 0.02 |
| Peripheral Vascular Disease | 1.2 | 1.7 | 0.58 |
| Renal failure | 3.2 | 5.9 | 0.29 |
| Dialysis | 0.6 | 1.5 | 0.45 |
| Preoperative Clinical Characteristics: | |||
| Prehospital Location (Home) | 90.5 | 75.0 | <0.01 |
| Independent Functional Status | 76.9 | 47.8 | <0.001 |
| Do Not Resuscitate Order | 4.0 | 6.8 | 0.32 |
| Chemotherapy use | 38.6 | 30.5 | 0.30 |
| Steroid use | 8.1 | 7.4 | 1.0 |
| Transfusions | 2.3 | 4.4 | 0.40 |
| Smoking status | 15.9 | 25.0 | 0.08 |
| ASA Class >3 | 28.2 | 48.5 | <0.01 |
| Body Mass Index | 0.24 | ||
| <18.5 | 14.3 | 7.4 | |
| 18.5-<25 | 46.4 | 45.6 | |
| 25+ | 39.3 | 47.1 | |
| Ascites | 14.0 | 29.4 | <0.01 |
| Bleeding disorder | 18.2 | 22.1 | 0.50 |
| Dyspnea at rest | 2.3 | 16.2 | <0.001 |
| Impaired Sensorium | 2.0 | 13.6 | <0.001 |
| Pneumonia | 2.8 | 11.9 | <0.01 |
| Sepsis | 5.8 | 16.2 | <0.01 |
| Septic Shock | 3.6 | 7.4 | 0.18 |
| Postoperative Events: | |||
| Any Complication | 40.9 | 72.1 | <0.001 |
| Respiratory Complication | 14.3 | 44.1 | <0.001 |
| Hematologic Complication | 13.0 | 19.1 | 0.183 |
| Wound Complication | 14.0 | 8.8 | 0.32 |
| On Ventilator >48 hours | 2.3 | 11.8 | 0.002 |
| Urologic Complication | 5.2 | 11.8 | 0.06 |
| Deep Venous Thrombosis | 5.2 | 4.4 | 1.0 |
| Pulmonary Embolus | 1.3 | 2.9 | 0.30 |
| Cardiac Complication | 0.6 | 11.8 | <0.001 |
| Neurologic Complication | 0 | 2.9 | 0.03 |
| Fail to Rescue | 0 | 72.1 | <0.001 |
| Unplanned Reoperation | 8.9 | 0 | 0.07 |
| Unplanned Readmission | 9.3 | 33.3 | 0.900 |
| Length of stay days (Median IQR) | 12(8-18) | 12.5(7.5-19.0) | 0.92* |
Wilcoxon Rank-Sum test
IQR=Interquartile Range
In the multivariate logistic regression analysis including clinical factors available before surgery, predictors of 30-day overall mortality were preoperative functional dependence (OR 2.7 with 95% CI: 1.4-5.1) and ascites (OR 2.3 with 95% CI: 1.2-4.5). When postoperative complications were included in the model, cardiac complications (OR 10.7 with 95% CI: 2.36-55.9) and respiratory complications (OR 3.5 with 95% CI: 1.9-6.6) also predicted mortality (Figure 2).
Figure 2.

Predictors of 30-Day mortality for patients undergoing emergent operations for obstruction. All predictors were statistically significant with p<0.05.
Model including preoperative variables alone: AUC 0.7998 (95% CI: 0.7737-0.8258)
Model including pre- and postoperative variables: AUC 0.7789 (95% CI: 0.7520-0.8058)
* Indicates variables from the model including postoperative predictors.
Discussion
This study shows that one in three patients with disseminated cancer who undergo surgery for perforation and one in six who undergo surgery for obstruction will die within 30-days of their operation. Most who have surgery for perforation and almost half who have surgery for obstruction will experience complications, few patients are discharged home, and over 10% of patients in both groups were readmitted to the hospital. Not surprisingly, patients with more comorbidities, functional dependence, ascites, dyspnea, and preoperative sepsis had higher odds of death. Postoperative cardiopulmonary complications were independently associated with death. These data confirm our hypothesis that patients with disseminated cancer experience a high burden of complications and high postoperative mortality after these operations, and can be used to formulate prognosis and identify patients who could benefit from inpatient palliative care. This information should be useful to patients, clinicians and caregivers in setting expectations for the post-operative hospitalization, discharge location, and overall survival both at the time when the decision is made for surgery, and for establishing realistic expectations if complications ensue.
Patients with acute, life-threatening surgical conditions are often offered an operation as a life saving measure and our study finds that the majority of patients survive their hospitalization and the month after surgery. However, a substantial number die soon after surgery, and many others experience postoperative complications, reoperations, stays in nursing facilities, and readmissions. These postoperative events have previously been shown to adversely affect patients' quality of life (17, 18). In addition, the mortality rates found in our patient population are higher than previously reported emergency laparotomy rates in the general population of around 17% (19). Previous studies have shown that patients who understand their poor prognosis near the end of life are unlikely to choose invasive treatments that can prolong suffering and time away from home (8, 20, 21). Communication with patients and their families about expected surgical outcomes, the impact of surgery on the patient's overall survival, and the patient's goals and values around life-sustaining treatment and prolonged institutionalization is important in this moment of crisis to avoid overly-burdensome treatments where the outcomes are most likely unacceptable to the patient (3).
Preoperative dependent functional status was a consistent predictor of mortality in our study. Functional status is emerging as a predictor for poor outcomes in a number of surgical populations (22, 23). In cancer patients, functional status is used to determine fitness for chemotherapy (24). The Eastern Cooperative Oncology Group functional status assessment tool (25) is routinely used to determine fitness for chemotherapy, and can be helpful in determining fitness to withstand surgery as well. In another study using data from the ACS NSQIP, Farhat et al. showed that among elderly general surgery patients, frailty increased the odds of death 11 times (26). Thus, it is no surprise that functional dependency was a strong predictor of mortality in this study. Functional status, measured using instrumental activities of daily living, has previously been found to predict extended hospital stay and increased complication rates in elderly cancer patients undergoing elective operations (27). Together, these findings highlight the need for surgeons to use functional impairment as a tool for preoperative risk assessment.
This study corroborates others in highlighting the importance of complications in increasing overall mortality in surgery patients (28). Khuri et al. showed that in a cohort of non-cardiac, general surgery patients in ACS NSQIP, complications, independent of pre-operative patient risk, reduced median patient survival by 69% (29). Surgical complications also lead to worse long term quality of life after cancer operations (17, 29). In situations where a patient's overall life expectancy is weeks or months due to their underlying cancer, new prognostic information may change a patient's goals from a focus on life extending therapy to treatment focused on quality of life and reducing suffering (20). Experts in communicating with seriously ill patients have advised increased use of time limited trials when the expectations for treatments are uncertain (30). In these scenarios, patients/surrogates and clinicians agree to use invasive treatments for a defined period of time to meet clear therapeutic goals (days to weeks) (31). If these goals are not met, treatments are discontinued to avoid indefinite use of burdensome, non-beneficial treatments. Findings from this study and others suggest that the development postoperative complications may serve as a pause point to reconsider the overall goals of care and likelihood of treatment success.
Intestinal perforation or obstruction may be side effects of cancer treatment or from disease progression itself. Although surgery may be immediately life-saving, intestinal perforation or obstruction in cancer patients frequently signals a critical downward inflection point in the patient's overall trajectory. A study by Pameijer and colleagues revealed that patients with metastatic cancer who present with obstructive symptoms were found to have a median survival of three months regardless of operative or conservative treatment (32). In these cases, it is important for acute care surgeons to recognize opportunities to include palliative approaches, alongside life prolonging care, to reduce the overall symptom burden, facilitate advance care planning and to support caregivers (33). Palliative care is an approach to treatment that focuses on quality of life and relief of suffering and should not be reserved for the final stages of terminal illness. The high morbidity and short term mortality in this study demonstrates the need to clarify treatment goals and engage palliative care early in the patient's hospital course. It is important to note that palliative care focuses on intensive symptom management, which may still necessitate operative interventions in this patient population. Early initiation of palliative care in patients with metastatic cancer has been found to improve patient quality of life, mood, and extend survival (34). The very low preoperative DNR rates found in this study suggests that patients may not have conversations regarding end-of-life goals until acute situations arise. By providing structured communication and palliative care training to practicing surgeons and trainees, non-beneficial treatments could be avoided in seriously ill patients who present with surgical emergencies (3).
The findings in this study must be viewed with respect to important limitations. First, ACS NSQIP only includes patients who received surgery biasing these results towards patients fit enough for surgery or who elect aggressive care. It is important to note that the outcomes of patients who do not undergo an operation are not captured in this study. Due to limitations of the ACS NSQIP, this study does not include measures of postoperative quality of life, functional status outcomes, and symptom burden that are meaningful to patients. In addition, the ACS NSQIP database only allows measurement of mortality outcomes to 30-days. Thirty day mortality is an incomplete measure of the postoperative experience and has been criticized as a patient-centered outcome measure (35). Previous studies have described that perioperative mortality may be better evaluated if patient deaths out to 90-days are assessed (36). A future prospective study of these patients assessing survival to 90-days would shed further light on the true perioperative mortality rates. Also, mortality outcomes vary in disseminated cancer patients by site of primary tumor (37). While all of these patients fit the ACS NSQIP definition of “disseminated cancer”, this is a heterogeneous population with varying survival expectations based on primary tumor type. However, the rigor of data collection in the ACS NSQIP should increase selection of patients who have very advanced disease. Finally, limitations of the ACS NSQIP prevent us from measuring other outcomes important to patients and their families including patient and family satisfaction, quality of life, symptom burden, quality of death and dying, and bereavement outcomes among survivors. It is likely that there were positive outcomes among decedents and survivors that this data did not allow us to measure.
Emergency surgery in patients with disseminated cancer is associated with high rates of adverse outcomes and patients who survive require long hospital stays after which they are unlikely to be discharged home. Communicating effectively about expectations for recovery and outcomes that are most relevant for individual patients assists patients and their families to make decisions aligned with their goals of care. Reducing complications, particularly cardiopulmonary complications will be critical to improving survival after surgery in these highly vulnerable patients. Findings from this study should help clinicians to identify patients most likely to benefit from surgery, and identify opportunities to integrate palliative approaches to improve comfort.
Acknowledgments
Christy Cauley is currently receiving a grant (R25CA092203) from the National Cancer Institute at the National Institutes of Health
Footnotes
Conflict of interest: No conflicts exist for any of the authors.
Author Contribution Statement: Christy Cauley contributed to the literature search, study design, data analysis, data interpretation, writing, and revisions. M Panizales contributed to the study design, data interpretation and critical revisions. G Reznor contributed to the data analysis, data interpretation, and revision. A Haynes contributed to the study design, data interpretation, and revisions, J Havens, E Kelley and A Mosenthal contributed to the data interpretation and critical revisions. Z Cooper contributed to the literature search, study design, data interpretation, writing, and revisions.
Contributor Information
M. T. Panizales, Email: mpanizales@partners.org.
G. Reznor, Email: greznor@partners.org.
A. B. Haynes, Email: abhaynes@mgh.harvard.edu.
J. M. Havens, Email: jhavens@partners.org.
E. Kelley, Email: ekelly1@partners.org.
A.C. Mosenthal, Email: mosentac@njms.rutgers.edu.
Z. Cooper, Email: zcooper@partners.org.
References
- 1.Medicine Io. Washington, DC: The National Academies Press; 2014. Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life; p. 630. [PubMed] [Google Scholar]
- 2.Kwok AC, Semel ME, Lipsitz SR, Bader AM, Barnato AE, Gawande AA, et al. The intensity and variation of surgical care at the end of life: a retrospective cohort study. Lancet. 2011 Oct 15;378(9800):1408–13. doi: 10.1016/S0140-6736(11)61268-3. [DOI] [PubMed] [Google Scholar]
- 3.Cooper Z, Courtwright A, Karlage A, Gawande A, Block S. Pitfalls in Communication That Lead to Nonbeneficial Emergency Surgery in Elderly Patients With Serious Illness: Description of the Problem and Elements of a Solution. Ann Surg. 2014 May 23; doi: 10.1097/SLA.0000000000000721. [DOI] [PubMed] [Google Scholar]
- 4.Miner TJ, Brennan MF, Jaques DP. A prospective, symptom related, outcomes analysis of 1022 palliative procedures for advanced cancer. Annals of surgery. 2004 Oct;240(4):719–26. doi: 10.1097/01.sla.0000141707.09312.dd. discussion 26-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wallston KA, Burger C, Smith RA, Baugher RJ. Comparing the quality of death for hospice and non-hospice cancer patients. Medical care. 1988 Feb;26(2):177–82. doi: 10.1097/00005650-198802000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Wright AA, Keating NL, Balboni TA, Matulonis UA, Block SD, Prigerson HG. Place of death: correlations with quality of life of patients with cancer and predictors of bereaved caregivers' mental health. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010 Oct 10;28(29):4457–64. doi: 10.1200/JCO.2009.26.3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wright AA, Zhang B, Ray A, Mack JW, Trice E, Balboni T, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA : the journal of the American Medical Association. 2008 Oct 8;300(14):1665–73. doi: 10.1001/jama.300.14.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mack JW, Weeks JC, Wright AA, Block SD, Prigerson HG. End-of-life discussions, goal attainment, and distress at the end of life: predictors and outcomes of receipt of care consistent with preferences. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010 Mar 1;28(7):1203–8. doi: 10.1200/JCO.2009.25.4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siegel MD, Hayes E, Vanderwerker LC, Loseth DB, Prigerson HG. Psychiatric illness in the next of kin of patients who die in the intensive care unit. Critical care medicine. 2008 Jun;36(6):1722–8. doi: 10.1097/CCM.0b013e318174da72. [DOI] [PubMed] [Google Scholar]
- 10.Azoulay E, Pochard F, Kentish-Barnes N, Chevret S, Aboab J, Adrie C, et al. Risk of post-traumatic stress symptoms in family members of intensive care unit patients. American journal of respiratory and critical care medicine. 2005 May 1;171(9):987–94. doi: 10.1164/rccm.200409-1295OC. [DOI] [PubMed] [Google Scholar]
- 11.Fried TR, Bradley EH, Towle VR, Allore H. Understanding the treatment preferences of seriously ill patients. The New England journal of medicine. 2002 Apr 4;346(14):1061–6. doi: 10.1056/NEJMsa012528. [DOI] [PubMed] [Google Scholar]
- 12.Hall BL, Hamilton BH, Richards K, Bilimoria KY, Cohen ME, Ko CY. Does surgical quality improve in the American College of Surgeons National Surgical Quality Improvement Program: an evaluation of all participating hospitals. Annals of surgery. 2009 Sep;250(3):363–76. doi: 10.1097/SLA.0b013e3181b4148f. [DOI] [PubMed] [Google Scholar]
- 13.Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. Journal of clinical epidemiology. 1996 Dec;49(12):1373–9. doi: 10.1016/s0895-4356(96)00236-3. [DOI] [PubMed] [Google Scholar]
- 14.Concato J, Feinstein AR, Holford TR. The risk of determining risk with multivariable models. Annals of internal medicine. 1993 Feb 1;118(3):201–10. doi: 10.7326/0003-4819-118-3-199302010-00009. [DOI] [PubMed] [Google Scholar]
- 15.White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Statistics in Medicine. 2011;30(4):377–99. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- 16.Horton NJ, K K. Much ado about nothing: A comparison of missing data methods and software to fit incomplete data regression models. American Statistician. 2007;61:79–90. doi: 10.1198/000313007X172556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown SR, Mathew R, Keding A, Marshall HC, Brown JM, Jayne DG. The impact of postoperative complications on long-term quality of life after curative colorectal cancer surgery. Annals of surgery. 2014 May;259(5):916–23. doi: 10.1097/SLA.0000000000000407. [DOI] [PubMed] [Google Scholar]
- 18.Paul Olson TJ, Pinkerton C, Brasel KJ, Schwarze ML. Palliative surgery for malignant bowel obstruction from carcinomatosis: A systematic review. JAMA Surgery. 2014;149(4):383–92. doi: 10.1001/jamasurg.2013.4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clarke A, Murdoch H, Thomas MJ, Cook TM, Peden CJ. Mortality and postoperative care after emergency laparotomy. European journal of anaesthesiology. 2011 Jan;28(1):16–9. doi: 10.1097/EJA.0b013e32833f5389. [DOI] [PubMed] [Google Scholar]
- 20.Weeks JC, Cook EF, O'Day SJ, Peterson LM, Wenger N, Reding D, et al. Relationship between cancer patients' predictions of prognosis and their treatment preferences. JAMA : the journal of the American Medical Association. 1998 Jun 3;279(21):1709–14. doi: 10.1001/jama.279.21.1709. [DOI] [PubMed] [Google Scholar]
- 21.Zhang B, Nilsson ME, Prigerson HG. Factors important to patients' quality of life at the end of life. Archives of internal medicine. 2012 Aug 13;172(15):1133–42. doi: 10.1001/archinternmed.2012.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al-Temimi MH, Griffee M, Enniss TM, Preston R, Vargo D, Overton S, et al. When Is Death Inevitable after Emergency Laparotomy? Analysis of the American College of Surgeons National Surgical Quality Improvement Program Database. J Am Coll Surg. 2012 Oct;215(4):503–11. doi: 10.1016/j.jamcollsurg.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 23.Robinson TN, Wallace JI, Wu DS, Wiktor A, Pointer LF, Pfister SM, et al. Accumulated frailty characteristics predict postoperative discharge institutionalization in the geriatric patient. Journal of the American College of Surgeons. 2011 Jul;213(1):37–42. doi: 10.1016/j.jamcollsurg.2011.01.056. discussion -4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Isik O, Okkabaz N, Hammel J, Remzi FH, Gorgun E. Preoperative functional health status may predict outcomes after elective colorectal surgery for malignancy. Surg Endosc. 2015;29(5):1051–6. doi: 10.1007/s00464-014-3777-2. [DOI] [PubMed] [Google Scholar]
- 25.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. American journal of clinical oncology. 1982 Dec;5(6):649–55. [PubMed] [Google Scholar]
- 26.Farhat JS, Velanovich V, Falvo AJ, Horst HM, Swartz A, Patton JH, Jr et al. Are the frail destined to fail? Frailty index as predictor of surgical morbidity and mortality in the elderly. The journal of trauma and acute care surgery. 2012 Jun;72(6):1526–30. doi: 10.1097/TA.0b013e3182542fab. discussion 30-1. [DOI] [PubMed] [Google Scholar]
- 27.Audisio RA, Pope D, Ramesh HS, Gennari R, van Leeuwen BL, West C, et al. Shall we operate? Preoperative assessment in elderly cancer patients (PACE) can help. A SIOG surgical task force prospective study. Crit Rev Oncol Hematol. 2008 Feb;65(2):156–63. doi: 10.1016/j.critrevonc.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 28.Scarborough JE, Pappas TN, Bennett KM, Lagoo-Deenadayalan S. Failure-to-pursue rescue: explaining excess mortality in elderly emergency general surgical patients with preexisting “do-not-resuscitate” orders. Annals of surgery. 2012 Sep;256(3):453–61. doi: 10.1097/SLA.0b013e31826578fb. [DOI] [PubMed] [Google Scholar]
- 29.Khuri SF, Henderson WG, DePalma RG, Mosca C, Healey NA, Kumbhani DJ, et al. Determinants of long-term survival after major surgery and the adverse effect of postoperative complications. Ann Surg. 2005 Sep;242(3):326–41. doi: 10.1097/01.sla.0000179621.33268.83. discussion 41-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neuman MD, Allen S, Schwarze ML, Uy J. Using Time-Limited Trials to Improve Surgical Care for Frail Older Adults. Ann Surg. 2014 doi: 10.1097/SLA.0000000000000939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quill TE, Holloway R. Time-limited trials near the end of life. JAMA. 2011 Oct 5;306(13):1483–4. doi: 10.1001/jama.2011.1413. [DOI] [PubMed] [Google Scholar]
- 32.Pameijer CR, Mahvi DM, Stewart JA, Weber SM. Bowel obstruction in patients with metastatic cancer: does intervention influence outcome? International journal of gastrointestinal cancer. 2005;35(2):127–33. doi: 10.1385/IJGC:35:2:127. [DOI] [PubMed] [Google Scholar]
- 33.Surgeons ACo. ST-50 Statement of Principles of Palliative Care. 2005 [PubMed] [Google Scholar]
- 34.Temel JS, Greer JA, Muzikansky A, Gallagher ER, Admane S, Jackson VA, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. The New England journal of medicine. 2010 Aug 19;363(8):733–42. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 35.Schwarze ML, Brasel KJ, Mosenthal AC. Beyond 30-day mortality: aligning surgical quality with outcomes that patients value. JAMA Surg. 2014 Jul;149(7):631–2. doi: 10.1001/jamasurg.2013.5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Visser BCMD, Keegan HBS, Martin MBSN, Wren SMMD. Death After Colectomy: It's Later Than We Think. [Article]: Archives of Surgery. 2009 Nov;144(11):1021–1027. doi: 10.1001/archsurg.2009.197. [DOI] [PubMed] [Google Scholar]
- 37.Ries LAG YJ, Keel GE, Eisner MP, Lin YD, Horner MJ. SEER Survival Monograph: Cancer Survival Among Adults: U.S. SEER Program, 1988-2001, Patient and Tumor Characteristics. Bethesda, MD: NIH; 2007. 2007. Report No. [Google Scholar]


