Abstract
Objective
Identifying groups of individuals with similar patterns of body mass index (BMI) change during childhood may increase understanding of the relationship between childhood BMI and adult health.
Methods
Discrete classes of BMI z-score change were determined in 1,920 American Indian children with at least four non-diabetic health exams between the ages of 2 and 18 years using latent class trajectory analysis. In subsets of subjects, data were available for melanocortin-4 receptor (MC4R) sequencing; in utero exposure to type 2 diabetes (T2D); or, as adults, oral glucose tolerance tests, onset of T2D, or body composition.
Results
Six separate groups were identified. Individuals with a more modern birth year, an MC4R mutation, or in utero exposure to T2D were clustered in the two groups with high increasing and chronic overweight z-scores (p<0.0001). The z-score classes predicted adult percent fat (p<0.0001, partial r2=0.18 adjusted for covariates). There was a greater risk for T2D, independent from adult BMI, in 3 classes (lean increasing to overweight, high increasing, and chronic overweight z-scores) compared to the two leanest groups (respectively: HRR= 3.2, p=0.01; 6.0, p=0.0003; 11.6, p<0.0001).
Conclusions
Distinct patterns of childhood BMI z-score change associate with adult adiposity, and may impact risk of T2D.
Keywords: childhood obesity, latent class trajectory analysis, type 2 diabetes mellitus, lifecourse physiology
Introduction
Degree of adiposity during childhood often tracks into adulthood, and obesity in childhood contributes to a decreased life expectancy and increased adult morbidity1, 2. Studies have attempted to identify critical time periods in childhood where weight gain may impact development of adult obesity1, 3, 4 with varying results. These critical time periods may differ among studies because more than one distinct childhood growth trajectory5–14 exists that is associated with adult weight. Other studies have determined differing patterns of childhood weight gain5, 6, 8–11, and the influence such trajectories may have on future morbidity including asthma and kidney disease5, 12, 14, 15. However, many studies had a limited number of ages represented, did not have individuals with follow-up data as adults, or included populations with a relatively low prevalence of obesity. Many of these studies also did not have data on known contributors to increased childhood adiposity to test the validity of their models. It is unclear if differing pathways of childhood BMI change have an impact on adult physiology or the risk of type 2 diabetes mellitus (T2D), or if only the weight attained by the end of childhood is important.
The National Institutes of Health (NIH) previously conducted a longitudinal study of health in the Gila River Indian Community (GRIC) of Arizona, a population which is primarily Pima Indians, and has a high prevalence of obesity16. These data are well suited to understanding patterns of childhood change and any effects on adult health. Conventional statistical techniques assume that all members of a population conform to a single pattern of change over time. Latent class trajectory analysis (LCTA), however, is a semiparametric group-based modeling strategy that groups subjects with similar patterns of change into classes, each class with a distinctly different trajectory of change. Factors influencing childhood growth trajectory and the association of these trajectories to adult physiology can then be assessed. We hypothesized that LCTA17 would identify more than one pattern of BMI z-score change during childhood in the GRIC. In addition, we hypothesized that the identified classes would be associated with differences in adult measures such as percent body fat, insulin action or type 2 diabetes risk.
Methods
Subjects
Subjects participated in a longitudinal study of health where, between 1965 and 2007, all residents of the GRIC older than 4 years were invited to participate in exams as frequently as every two years, as previously described18, and the consent allowed for review of medical records. For children under the age of 18 years, written informed consent was obtained from a parent or guardian, and the child assented. Adults provided written informed consent. The study protocol (NCT00339482) was approved by the Institutional Review Board of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK).
Data for birth weight (n=704 with available data, limited to gestational age 36 weeks) as well as height and weight prior to the fifth birthday were obtained from review of well-child visits. Height and weight measures after age 4 years were from study exams, and were measured using a stadiometer and calibrated scale, respectively. All study exams included a 75g, 2 hour oral glucose tolerance test (OGTT). All nondiabetic19, nonpregnant visits with complete data for age, height, weight, fasting and 2h glucose were considered. There were 7528 children with at least one visit before age 18 years. Only subjects with complete data from at least four visits between the ages of 2 and 18 years were included in the LCTA. The final dataset for LCTA included 8619 visits between the ages of 2 and 18 years from 1920 children; 42%, 39%, 16% and 3% of participants had 4, 5, 6, and 7 visits, respectively. The average range of years between the first and last visit was 9.9±2.1 years.
Genetic and in utero variables
Degree of Pima heritage is the self-reported number of great-grandparents of full Pima or related Tohono O’odham heritage. Individuals were considered to have exposure to Type 2 Diabetes Mellitus (T2D) in utero if their mothers were known to have T2D prior to pregnancy or had an OGTT consistent with T2D before the birth date of the individual, as previously described20. Our definition of in utero exposure to T2D included children born to both mothers with gestational diabetes mellitus and those with T2D preceding the pregnancy. Information about the T2D status of the mother prior to the birth date was available for 1334 subjects.
MC4R haploinsufficiency is the most common monogenic form of obesity in humans21 with a greater effect on BMI change in childhood22. Direct sequencing of the entire coding region of the melanocortin 4 receptor (MC4R) was previously done in the majority of subjects in the larger study, and six loss-of-function MC4R mutations were identified in the GRIC22. There were 1514 subjects with data contributing to the LCTA that also had MC4R genotype data available. Of these 1514 subjects, 38 individuals were heterozygous for one of these 6 mutations.
Adult measures
Of these 1920 subjects, 820 (43%) had a study visit without evidence of T2D from the above protocol (NCT00339482) between the ages of 20 and 40 years with complete data available for early adult BMI, blood pressure, and fasting and 2 hour glucose and insulin concentrations. Data from all available adult visits between the age of 20 and 40 years were used to determine onset of T2D (n=873; 68 events). Serum insulin concentrations were measured by radioimmunoassay using the Herbert modification of the Yalow and Berson method23 or one of two automated analyzers (Concept 4 (ICN Radiochemicals, Cost Mesa CA); Access analyzer (Beckman instruments)). Because three insulin assays were used throughout the study, insulin values were converted to z-scores by assay ((individual insulin concentration meanassay)/SDassay) for comparability.
A subset of subjects (n=313) also participated, as young adults, in the ongoing study of the metabolic determinants of type 2 diabetes conducted on our clinical research unit (CRU) as previously described24, which includes measurements of body composition, insulin action, and 24h energy expenditure. Exclusion criteria for this inpatient study were evidence of T2D via a 75g OGTT or other medical or psychiatric disease. All participants provided separate written informed consent for this study, and the study protocol (NCT00340132) was approved by the NIDDK IRB. A subset (n=111) of these individuals had assessment of insulin action using a euglycemic-hyperinsulinemic clamp, as previously described25, where the rate of glucose required to maintain euglycemia during a primed continuous insulin infusion of 40 mU/m2/min was the measure of insulin action (M). The average coefficient of variation for M was 2.3±1.0% for this analysis. There were 93 subjects with measurement of sedentary 24h energy expenditure (EE) from a whole room indirect calorimeter as previously described26. Because of the small number in the EE subset analysis, the differences from expected 24h EE were used as variables after accounting for known adult predictors (fat mass, fat free mass, age, sex, ethnicity) in the larger population of all 529 individuals with EE measures.
Statistics
Alpha was set at 0.05. Subject characteristics, except for degree of Pima heritage which was left skewed, are presented as means±standard deviations. Degree of Pima heritage is reported as median and interquartile range. Corrections for multiple comparisons in individual analyses are described below, but because the study was primarily exploratory in nature, no global correction for the number of analyses was made. All analyses were performed using SAS 9.2 and SAS Enterprise Guide 4.1.
BMI z-scores were calculated from standards published by the Centers for Disease Control27 using the recommended equation for normalized z-scores. BMI was also categorized as a dichotomous variable, indicating if the subject was overweight (OW) or not at the visit, to determine patterns of progression to an unhealthy weight. OW was defined as above the US 95th percentile for age28. LCTA, using PROC TRAJ17, was used to determine distinct classes of BMI and BMI z-score change over time, as well as differing OW trajectories to a BMI greater than the 95th percentile. PROC TRAJ identifies homogeneous clusters of growth patterns, where the degree and direction of change can vary freely. The number of groups (k) in the model and the functional relationship of each group with time (linear v quadratic v cubic) were varied until the best possible model was obtained. Model criteria included an improvement in Bayesian Information Criterion (BIC) compared to a k-1 model, all classes with posterior probabilities >0.8 and with at least 50 people (>3%)29, stability of the trajectories during modeling, parsimony, and theoretical justification from prior literature3, 5, 6, 8–11, 13, 14. Subjects were assigned to a single trajectory using the maximum-probability assignment rule. Posterior probabilities are the average post hoc probability that subjects belong to the assigned trajectory. Random generation of the start values did not alter the selected model. The weighted kappa coefficient was used to test for agreement between assignment to BMI and BMI z-score classes. After comparison of the models created with LCTA using the three variables (BMI, BMI z-score, OW), BMI z-score classes were used in further analyses because they were comprehensible, were relatively representative of the BMI classes (see Results), and provided a greater depth of information than the OW classes.
Clustering of known risk factors for increased adiposity within BMI z-score groups was assessed with the Jonckheere trend test. Differences in continuous variables between groups were compared using 1-way ANOVA followed by Tukey’s post hoc pairwise test. Identified differences in adult variables were assessed further in multivariate regression models accounting for age, sex, birth year, degree of Pima heritage and adult BMI. Concurrent percent body fat and glucose concentration were also included in the models of M and insulin z-scores, respectively. M was log transformed to meet the assumptions of linear regression.
Time to T2D was calculated from the age of 20 years to onset of T2D determined from either the OGTT results19 or chart review if the diagnosis occurred between study visits. The number of events in the 2 leanest BMI z-score groups was small (n<5), and the assumption of proportional hazards was not met between them, so they were combined into a joint comparator. Cox proportional hazards models were used to determine the hazard rate ratio (HRR) associated with the other four BMI z-score groups, adjusting for degree of Pima heritage, birth year, sex, as well as maximum adult BMI.
Results
Latent Class Trajectories
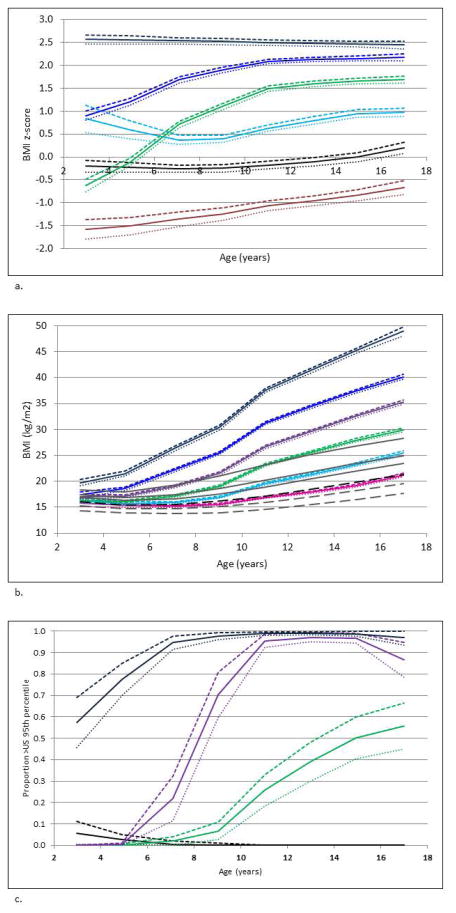
LCTA identified six BMI z-score trajectory classes, which we descriptively called ‘below average’, ‘average’, ‘lean increasing to overweight’, ‘stable above average’, ‘high increasing’ and ‘chronic overweight’ z-score classes (Figure 1a). LCTA also identified six BMI classes (Figure 1b), and, using the dichotomous OW variable, 4 patterns of progression to a weight >95th US percentile (Figure 1c). These 4 classes included a group that was always lean (<95th percentile) and 3 classes that progressed to overweight either early, at prepuberty or at puberty (Figure 1c). The internal validity of the BMI z-score model, as represented by the posterior probabilities, was good (Table 1). In addition, there was reasonable agreement between subject assignment to the 6 BMI z-score classes and the 6 identified BMI classes (weighted kappa = 0.5). Because the BMI z-score trajectory classes were more accessible, representative of the BMI classes, and provided more information than the OW classes, the BMI z-score model was used for all further analyses. Characteristics of the BMI z-score groups, including the average BMI per 2 y age intervals, are shown in Table 1. There was good separation of the average BMI at each time period between the BMI z-score classes (Table 1, Figure 1a).
Figure 1. Classes Identified by Latent Class Trajectory Analysis.
(a) Six Classes of BMI z-score Change Occurring during Childhood Identified. Brown = Below Average BMI (10% of study population), black = Average BMI (15%), green = Lean Increasing to Overweight (23%), light blue = Stable Above Average BMI (22%), blue = High Increasing BMI (26%), navy = Chronic Overweight (4%). (b) Six Classes of BMI Change Occurring during Childhood Identified. Gray dashed lines = 5th and 25th BMI percentiles for the United States population, black dashed line = 50th BMI percentile for the US population, solid gray lines = 75th, 85th, 95th percentiles for the US population. Pink = trending 50th percentile (20%), light blue = increasing to 85th percentile (25%), green = increasing to 95th percentile at puberty (21%), purple = increasing to 95th percentile prepuberty (19%), blue = increasing to 95th percentile early (11%), navy = Chronic Overweight (4%). (c) Four Classes of Progressing to a Weight Greater than the 95th Percentile during Childhood Identified. Black = always lean (45%), green = developing risk of overweight at puberty (19%), purple = developing risk of overweight prepuberty (19%), navy = chronic risk of overweight (17%). Lower 95th percent confidence intervals are shown by dotted lines, higher 95th percent confidence intervals are shown by dashed lines.
Table 1.
Anthropometric characteristics (by 2 year intervals) of the 6 BMI z-score trajectory groups
| BMI z- score Group |
Number | Posterior Probability |
Males (%) |
Number of Visits |
Birth Weight (g)ac (n=704) |
Average BMI (kg/m2) [Number Represented] | Early Adult BMI (kg/m2)a de |
Height (cm)ab |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age 2 to 4 (years)c |
Age 4 to 6 (years) c |
Age 6 to 8 (years)d |
Age 8 to 10 (years)d |
Age 10 to 12 (years)d |
Age 12 to 14 (years)d |
Age 14 to 16 (years)d |
Age 16 to 18 (years)d |
||||||||
| Below Average | 73 | 0.92 | 56 | 4.3±0.6 | 3047±455 | 14.4±0. 9$ [13] | 13.8±0. 6$ [21] | 13.9±0. 6 [35] | 14.5±0. 7[50] | 15.3±1. 1 [54] | 16.6±1. 1[43] | 18.1±1. 8[48] | 19.5±2. 2[37] | 23.2±4.6 (20.9, 25.6) | 169±7# (166, 171) |
| Average | 289 | 0.88 | 49 | 4.4±0.6 | 3285±472$ | 15.7±0. 8$ [39] | 15.2±0. 7# [86] | 15.2±0. 8 [166] | 15.8±1. 2[215] | 16.9±1. 3[214] | 18.5±1. 5[208] | 20.2±1. 8[175] | 22.1±2. 5 [142] | 26.2±4.3 (24.6, 27.9) | 166±8$ (164, 167) |
| Stable Above Average | 418 | 0.86 | 40b | 4.5±0.7 | 3432±436# | 17.2±1. 3# [50] | 16.1±1. 0# [120] | 16.2±1. 0 [243] | 17.4±1. 3[294] | 19.4±1. 7[310] | 21.7±2. 4 [320] | 23.6±2. 5 [247] | 25.2±2. 8[233] | 29.8±5.4 (28.2, 31.4) | 167±8$ (165, 169) |
| Lean Increasing to Overweight | 446 | 0.85 | 43b | 4.5±0.7 | 3365±491$ | 15.3±0. 8$ [87] | 15.6±0. 9# [168] | 17.4±1. 6[253] | 20.1±2. 4[285] | 23.3±2. 4[331] | 26.0±2. 9 [308] | 28.0±3. 4 [262] | 30.0±3. 9 [220] | 34.9±6.2 (33.3, 36.6) | 168±9# (166, 170) |
| High Increasing | 514 | 0.84 | 43b | 4.5±0.8 | 3469±471# | 17.2±1. 0# [171] | 17.7±1. 6 [223] | 20.5±2. 3[269] | 24.2±2. 9[336] | 27.8±3. 1 [366] | 31.2±3. 9 [333] | 33.7±3. 9 [249] | 35.4±4. 5 [211] | 39.4±6.6 (37.7, 41.1) | 170±7# (168, 172) |
| Chronic Overweight | 180 | 0.83 | 53 | 4.6±0.8 | 3599±491 | 20.6±2. 3 [63] | 21.9±3. 0 [90] | 25.8±3. 4 [109] | 30.0±3. 6 121] | 34.3±4. 4[117] | 37.9±5. 5 [115] | 39.9±5. 8[97] | 42.0±7. 0 [62] | 44.3±7.7 (42.0, 46.6) | 171±10 # (169, 174) |
| Total | 1920 | 0.86 | 45 | 4.5±0.7 | 3419±503 | [423] | [708] | [1075] | [1301] | [1392] | [1327] | [1078] | [905] | 32.8±7.9 | 165±8 |
BMI data presented as mean±SD;
mean±SD (95% confidence interval) adjusted for age (birth weight is adjusted for gestational age), sex, degree of Pima heritage, Birth year;
p<0.01 for frequency different from 50%;
Symbols indicate values that are similar to one another (p>0.05 for the difference);
p<0.05 between all comparisons;
Early Adult BMI occurred at an average age of 23.7±4.2
To further test the validity of the identified BMI z-score classes, we assessed the association of known risk factors for obesity and these groups. On average, birth year was more recent in the lean increasing to overweight, high increasing and chronic overweight BMI z-score classes (Table 2). However, a greater degree of Pima heritage was only observed in the lean increasing to overweight and the chronic overweight BMI z-score groups. Individuals with in utero exposure to T2D or MC4R haploinsufficiency were more likely to be in the high increasing and chronic overweight groups (p<0.0001) (Table 2). Of the 38 individuals with MC4R haploinsufficiency and the 76 individuals with in utero exposure to T2D, 71% and 72%, respectively, were classified into either the high increasing or chronic overweight classes. LCTA identified similar classes if the study population was divided into birth cohorts born before and after 1975 or into males and females.
Table 2.
Potential Determinants of BMI z-score Group
| BMI z-score Group | Birth Year (n=1920) | Degree of Pima Heritage (n=1920) | ODMa (n=1334) | Functional MC4R Mutationa (n=1514) |
|---|---|---|---|---|
| Below Average | 1974±14$ | 6$ (4, 8) | 0 [0.0%] | 1 [2.0%] |
| Average | 1974±13$ | 7$ (5, 8) | 4 [2.0%] | 0 [0.0%] |
| Stable Above Average | 1973±12$ | 7$ (5, 8) | 5 [1.7%] | 3 [1.0%] |
| Lean Increasing to Overweight | 1977±13 | 7# (6, 8) | 12 [3.8%] | 7 [1.9%] |
| High Increasing | 1983±12# | 6$ (4, 8) | 38 [11.0%] | 19 [4.7%] |
| Chronic Overweight | 1984±11# | 7# (6, 8) | 17 [13.8%] | 8 [5.4%] |
| All | 1977±13 | 7 (5, 8) | 76 [5.7%] | 38 [2.5%] |
Data presented as mean±SD (95% confidence interval) for birth year, median (interquartile range) for degree of Pima heritage, or absolute number [% of group]. Symbols indicate values that are similar to one another (p>0.05 for difference).
Jonckheere trend test for ordered differences between classes p<0.0001
Degree of Pima Heritage = number of great-grandparents of full Pima heritage; ODM = in utero exposure to diabetes mellitus; MC4R = melanocortin 4 receptor
Impact of BMI z-score groups on Adult Characteristics
Final early adult BMI and height for the BMI z-score classes is shown in Table 1. In 313 individuals, childhood BMI z-score group explained an additional 18%, 41%, and 34% of the variance in percent fat, fat mass and fat free mass, respectively, beyond that explained by adult age, sex, birth year and degree of Pima heritage (Table 3). Differences in insulin action (M), glucose, blood pressure and deviations from predicted 24h EE between BMI z-score groups are shown in Table 3. None of these variables differed between BMI z-score class after accounting for adult BMI. In addition, fasting and 2h insulin z-scores did not differ between BMI z-score groups after accounting for adult BMI. However, the risk of developing T2D was elevated in the lean increasing to overweight, high increasing and chronic overweight groups compared to the below average and average BMI z-score groups (Table 3, Figure 2). The HRRs of these 3 groups were robust to sensitivity analyses including addition of maximum lifetime BMI to the model (respectively: HRR (95% CI) = 3.2 (1.3, 7.9), p=0.01; 6.0 (2.3, 16.1), p=0.0003; 11.6 (3.7, 36.5) p<0.0001) or exclusion of individuals with an MC4R mutation or exposure to diabetes in utero.
Table 3.
Adult Characteristics by BMI z-score Trajectory Group
| BMI z-score Group |
Age (years) |
Body Fat (%)a |
Fat mass (kg)a |
Fat Free Mass (kg)a |
Difference in 24h EE from expected (kcal/day)b |
M (mg glucose / kg EMBS / min)c |
Age (years) |
Blood Pressure (mmHg)a |
Fasting Glucose (mmol/l)a |
2h Glucose (mmol/l)a |
Type 2 Diabetes Risk (HRR)d |
|---|---|---|---|---|---|---|---|---|---|---|---|
| n=313 | n=313 | n=313 | n=313 | n=93 | n=111 | n=820 | n=820 | n=820 | n=820 | n=873 (68 events) |
|
| Below Average | 24.4±3.9 | 22.8±10. 6$ (19.2, 26.5) | 18.4±9.4$ (13.4, 23.5) | 48.0±7.7$ (43.9, 52.1) | −70±161 (−206, 66) | 4.3±1.6 (3.2, 5.7) | 25.0±5.9 | 112±9$/65±10$ (106/61 , 117/69) | 5.2±2.1$# (4.4, 6.0) | 6.0±5.0$# (4.5, 7.6) | comparator |
| Average | 25.4±4.7 | 27.0±9.1 $ (24.3, 29.7) | 22.2±10.2$ (19.9, 24.9) | 51.1±9.0$ (49.1, 53.2) | −47±161 (−115, 21) | 3.3±1.5 (2.7, 3.9) | 24.3±4.7 | 117±13$#/69±9 $# (113/66 , 121/72) | 4.9±0.5$ (4.3, 5.5) | 5.6±1.6$ (4.4, 6.7) | comparator |
| Stable Above Average | 25.7±4.6 | 31.0±7.5 (28.7, 33.3) | 29.4±8.8 (27.2, 31.1) | 57.3±10.1 (55.8, 58.9) | 11±155 (−41, 64) | 3.2±0.9 (2.8, 3.6) | 23.6±4.0 | 118±10$#/69±9 $# (114/67 , 121/72) | 5.2±1.4$ (4.6, 5.7) | 6.3±3.2 (5.2, 7.4) | 1.2 (0.5, 3.2) |
| Lean Increasing to Overweight | 25.4±5.0 | 34.3±6.9 # (31.8, 36.8) | 37.7±10.2 (34.9, 38.9) | 62.1±8.8 (60.5, 63.8) | 59±127 (−14, 133) | 2.8±0.6 (2.3, 3.3) | 23.7±4.1 | 120±13#%/70±1 1#% (116/67 , 123/73) | 5.7±2.5# (5.2, 6.3) | 7.1±4.4# (6.0, 8.2) | 2.5 (1.1, 6.0) |
| High Increasing | 23.7±3.6 | 36.5±6.1 # (33.7, 39.3) | 44.8±11.6# (42.4, 47.9) | 69.8±10.5 (67.5, 72.0) | 48±160 (48, 144) | 2.9±0.9 (2.4, 3.6) | 22.9±3.3 | 122±12%/72±1 0# (118/69 , 126/75) | 5.8±2.1# (5.2, 6.4) | 7.3±4.1# (6.1, 8.4) | 4.4 (1.9, 10.6) |
| Chronic Overweight | 24.0±4.0 | 37.3±7.7 # (34.0, 40.7) | 49.2±15.4# (46.6, 54.9) | 76.6±17.0 (73.2, 80.0) | −122±161 (−237, 8) | 3.2±0.5 (2.6, 4.1) | 23.4±4.0 | 123±12%/74±1 0% (119/70 , 128/78) | 6.2±2.9# (5.4, 6.9) | 8.0±5.3# (6.6, 9.5) | 7.1 (2.7, 18.8) |
| All | 25.1±4.6 | 34.5±8.7 | 33.3±13.7 | 60.2±12.4 | −3±8 | 2.9±1.1 | 23.7±4.2 | 116±12/68±10 | 5.5±1.9 | 6.9±3.8 |
Data presented as mean±SD (95% confidence interval); EE = energy expenditure, M = Insulin Action. Symbols indicate values that are similar to one another (p>0.05 for difference).
Adjusted for age, sex, birth year and degree of Pima heritage;
after accounting for age, sex, fat mass, fat free mass, degree of Pima heritage (p=0.06 for 1-way ANOVA);
adjusted for age, sex, birth year, degree of Pima heritage, percent body fat;
adjusted for sex, birth year, degree of Pima heritage
Figure 2. Survival Curve for Time to Type 2 Diabetes in Early Adulthood.
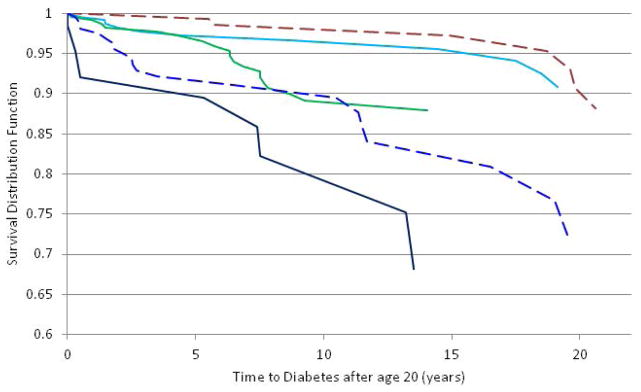
The chronic overweight (navy), high increasing (blue), and lean increasing to overweight (green) BMI z-score trajectory classes were associated with an increased risk for type 2 diabetes compared to the below average and average BMI z-score classes (represented together as the brown line). The light blue line is the stable above average BMI z-score group. The Cox proportional hazards analysis included the following covariates: degree of Pima heritage, birth year and sex (p<0.0001). The following sensitivity analyses did not change the results: including maximum, non-diabetic lifetime BMI in the model; excluding individuals with in utero exposure to T2D or an MC4R mutation.
Discussion
Latent class trajectory analysis using either BMI or BMI z-scores identified six pathways of childhood weight gain. Individuals in the three classes with a BMI z-score greater than 1.5 at the end of childhood were more likely to have had a more recent birth year, have in utero exposure to T2D, or have an MC4R mutation. These BMI z-score classes associated with greater adult adiposity, but were not independent contributors to adult insulin action or cardiovascular risk factors. Nevertheless, the three groups that ended with a BMI z-score >1.5 had an increased risk of T2D in early adulthood, which was independent from maximum adult BMI, and proportional to the number of years with a z-score greater than one.
Our findings indicate that for many variables, it is only the final weight achieved that matters rather than the pathway to that weight. However, the longer children remain lean, the less likely they are to develop T2D. Other studies have tried to determine if early life experiences such as childhood obesity or scarcity lead to programming of future body composition and adult disease1–4, 12, 24, 30–32. However, assuming that BMI change occurs in a homogenous manner can lead to loss of information. In a population with a high prevalence of childhood obesity33, we found six separate BMI z-score trajectories of change. We also found 4 classes using the dichotomous overweight variable including an always-lean group plus 3 classes with differing periods for progression to a weight greater than the 95th percentile, i.e. at an early age, at a prepubertal age, and at puberty. Our results are consistent with findings by others6, 8, 9, 11, 12. In particular, one similar study, which assessed patterns of progression to overweight from age 2 to 20 years, found the same four groups12 as we did. This supports the concept that there is 3 separate time periods where childhood obesity is more likely to develop. Because of the magnitude of increased BMI in some individuals in our study, the BMI z-score groups provided additional information beyond the dichotomous overweight classes. Most studies that assessed for distinct patterns of change in childhood using BMI or BMI z-scores have found between 4 and 7 groups5–7, 13, depending on the age range included and propensity for excess weight within the study population. We did not find a very low BMI z-score group as others have5, 6, 13, but did find a chronic overweight group. In the studies that used data extending throughout childhood, as we did, similar groups were found5,13. However, we noted differing proportions within some classes than other studies, with a greater number of individuals assigned to groups with higher BMI z-scores. Identifying varying patterns of change may help determine differing etiologic pathways to obesity. For example, future research might focus on the underlying reasons why some children’s growth follow the lean increasing to overweight versus the average BMI z-score trajectory even though both groups have similar BMIs at age 2. This may eventually lead to preventative strategies to help children maintain a stable z-score throughout childhood.
To validate the model selection procedure, we assessed whether known risk factors for increased childhood adiposity were associated with the BMI z-score classes. On average, the lean increasing to overweight, high increasing and chronic overweight classes had a more recent birth year consistent with the known increase in prevalence of obesity in the GRIC after World War II34. We also assessed for clustering of in utero exposure to T2D35 and MC4R haploinsufficiency22 in the determined trajectories. Greater than 70% of individuals with these risk factors were in the high increasing and chronic overweight groups. In future studies, it may be useful to categorize individuals into trajectory classes to assist with identifying new genetic risk targets. It is highly likely that environmental factors also contribute to which pathway of weight change an individual follows. In support of this hypothesis, it is known that maternal behavior has a stronger influence on food intake than in utero exposure to T2D36. Other studies have found that sedentary behavior11, maternal weight gain during pregnancy5, psychological distress7, and poverty9 are all risk factors for childhood weight trajectory classes.
BMI z-score trajectory explained a large proportion of the variance in adult body composition, consistent with prior studies37. A prior study demonstrated that BMI change after the age of 2 was a better predictor of adult fat mass than BMI change in infancy37. Although it is known that a lower than expected energy expenditure in the Pima Indian population is associated with future weight gain in adults38, individuals with relatively low energy expenditure did not group into a distinct trajectory. Most notably, the pathway of childhood BMI change was a risk factor for T2D during early adulthood that was independent from maximum adult BMI. This increased risk was not reflected in the measure of insulin action, which implicates impaired insulin release as the etiology behind the increased risk. Unfortunately, the number of individuals from this analysis who also had evaluation of beta cell function on our CRU was too small to assess for differences in insulin release (n<50). Alternatively, we may not have observed an effect of childhood trajectory on insulin action due to a survivor bias, as T2D was an exclusion criterion for participation in the study of adult phenotyping. In our study, the degree of increased risk was proportional to the duration of time with a BMI z-score >1 in childhood. This is consistent with other studies demonstrating that early onset of overweight and duration of obesity, as an adult, was associated with an increased risk of T2D39, 40.
One of the limitations of any secondary analysis is that the data was not originally collected to evaluate the current research question so, in this case, no exact measures of puberty were collected. Another limitation is the differing number of participants in our subset analyses, although we assumed that those in each subset were representative of the larger group. However, the power for the proportional hazards model was based on 68 cases of T2D, and in a larger dataset, the slightly increased risk of T2D in the ‘stable above average’ group may reach significance. In addition, we did not have available data for social and environmental contributors including maternal data, food intake assessments or physical activity measures in either of the two studies from which data were used. In general, findings relating to the risk of obesity and T2D in the Pima Indian population have been prototypic of other populations.
Conclusion
In this study, we identified distinct patterns of BMI z-score change during childhood. Individuals in the three trajectory groups that maintained a consistent z-score <1, on average, had a non-obese adult BMI and a lower risk of T2D than the other three groups. The duration of time with a BMI z-score >1 in childhood was associated with increasing risk of adult onset T2D that was independent from adult BMI. Although known risk factors for increased adiposity clustered in trajectories with greater late adolescent BMI z-scores, these risk factors were present in only a small number of individuals in these trajectories indicating that many of the contributors to childhood obesity may be modifiable, and that identification and intervention to maintain a stable BMI z-score <1 will result in health benefits throughout the entire life course.
What is already known about this subject?
Adiposity during childhood may influence adult body composition and physiology.
More than one pathway of weight gain may exist during childhood within a population.
Latent class trajectory analysis, or latent class growth modeling, can be used to identify unique groups in a population that follow distinct patterns of change over time.
What does this study add?
Six different pathways of childhood weight gain exist within this American Indian population, and known risk factors for childhood adiposity cluster within pathways with higher BMI US z-scores.
The identified childhood BMI z-score classes are associated with differences in adult adiposity.
Three BMI z-score classes had an increased risk of type 2 diabetes mellitus in early adulthood (< 40 years) that was independent from adult BMI and proportional to the number of years with a US z-score greater than one.
Acknowledgments
Funding agency: This research was supported by the Intramural Research Program of the NIDDK, NIH.
We thank the volunteers who participated in our studies. We also thank the clinical staff of the Phoenix Epidemiology and Clinical Research Branch for conducting the examinations.
Footnotes
Disclosure Statement: The authors have nothing to disclose.
References
- 1.Dietz WH. Childhood weight affects adult morbidity and mortality. The Journal of nutrition. 1998;128(2 Suppl):411S–414S. doi: 10.1093/jn/128.2.411S. [DOI] [PubMed] [Google Scholar]
- 2.Must A, Strauss RS. Risks and consequences of childhood and adolescent obesity. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 1999;23 (Suppl 2):S2–11. doi: 10.1038/sj.ijo.0800852. [DOI] [PubMed] [Google Scholar]
- 3.De Kroon ML, Renders CM, Van Wouwe JP, Van Buuren S, Hirasing RA. The Terneuzen birth cohort: BMI changes between 2 and 6 years correlate strongest with adult overweight. PloS one. 2010;5(2):e9155. doi: 10.1371/journal.pone.0009155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams SM, Goulding A. Early adiposity rebound is an important predictor of later obesity. Obesity (Silver Spring) 2009;17(7):1310. doi: 10.1038/oby.2009.104. [DOI] [PubMed] [Google Scholar]
- 5.Huang RC, de Klerk NH, Smith A, Kendall GE, Landau LI, Mori TA, et al. Lifecourse childhood adiposity trajectories associated with adolescent insulin resistance. Diabetes Care. 2011;34(4):1019–25. doi: 10.2337/dc10-1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jansen PW, Mensah FK, Nicholson JM, Wake M. Family and neighbourhood socioeconomic inequalities in childhood trajectories of BMI and overweight: longitudinal study of Australian children. PloS one. 2013;8(7):e69676. doi: 10.1371/journal.pone.0069676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kubzansky LD, Gilthorpe MS, Goodman E. A prospective study of psychological distress and weight status in adolescents/young adults. Annals of behavioral medicine : a publication of the Society of Behavioral Medicine. 2012;43(2):219–28. doi: 10.1007/s12160-011-9323-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li C, Goran MI, Kaur H, Nollen N, Ahluwalia JS. Developmental trajectories of overweight during childhood: role of early life factors. Obesity (Silver Spring) 2007;15 (3):760–71. doi: 10.1038/oby.2007.585. [DOI] [PubMed] [Google Scholar]
- 9.Mustillo S, Worthman C, Erkanli A, Keeler G, Angold A, Costello EJ. Obesity and psychiatric disorder: developmental trajectories. Pediatrics. 2003;111(4 Pt 1):851–9. doi: 10.1542/peds.111.4.851. [DOI] [PubMed] [Google Scholar]
- 10.Nonnemaker JM, Morgan-Lopez AA, Pais JM, Finkelstein EA. Youth BMI trajectories: evidence from the NLSY97. Obesity (Silver Spring) 2009;17(6):1274–80. doi: 10.1038/oby.2009.5. [DOI] [PubMed] [Google Scholar]
- 11.O'Brien M, Nader PR, Houts RM, Bradley R, Friedman SL, Belsky J, et al. The ecology of childhood overweight: a 12-year longitudinal analysis. International journal of obesity. 2007;31(9):1469–78. doi: 10.1038/sj.ijo.0803611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silverwood RJ, Pierce M, Hardy R, Thomas C, Ferro C, Savage C, et al. Early-life overweight trajectory and CKD in the 1946 British birth cohort study. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2013;62(2):276–84. doi: 10.1053/j.ajkd.2013.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith AJ, O'Sullivan PB, Beales DJ, de Klerk N, Straker LM. Trajectories of childhood body mass index are associated with adolescent sagittal standing posture. International journal of pediatric obesity : IJPO : an official journal of the International Association for the Study of Obesity. 2011;6(2–2):e97–106. doi: 10.3109/17477166.2010.530664. [DOI] [PubMed] [Google Scholar]
- 14.Ventura AK, Loken E, Birch LL. Developmental trajectories of girls' BMI across childhood and adolescence. Obesity (Silver Spring) 2009;17(11):2067–74. doi: 10.1038/oby.2009.123. [DOI] [PubMed] [Google Scholar]
- 15.Rzehak P, Wijga AH, Keil T, Eller E, Bindslev-Jensen C, Smit HA, et al. Body mass index trajectory classes and incident asthma in childhood: results from 8 European Birth Cohorts--a Global Allergy and Asthma European Network initiative. The Journal of allergy and clinical immunology. 2013;131(6):1528–36. doi: 10.1016/j.jaci.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Knowler WC, Pettitt DJ, Savage PJ, Bennett PH. Diabetes incidence in Pima indians: contributions of obesity and parental diabetes. Am J Epidemiol. 1981;113(2):144–56. doi: 10.1093/oxfordjournals.aje.a113079. [DOI] [PubMed] [Google Scholar]
- 17.Jones BL, Nagin DS. Advances in Group-Based Trajectory Modeling and an SAS Procedure for Estimating Them. Sociological Methods and Research. 2007;35:542– 572. [Google Scholar]
- 18.Pavkov ME, Hanson RL, Knowler WC, Bennett PH, Krakoff J, Nelson RG. Changing patterns of type 2 diabetes incidence among Pima Indians. Diabetes Care. 2007;30 (7):1758–63. doi: 10.2337/dc06-2010. [DOI] [PubMed] [Google Scholar]
- 19.Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26 (Suppl 1):S5–20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 20.Pettitt DJ, Baird HR, Aleck KA, Bennett PH, Knowler WC. Excessive obesity in offspring of Pima Indian women with diabetes during pregnancy. N Engl J Med. 1983;308(5):242–5. doi: 10.1056/NEJM198302033080502. [DOI] [PubMed] [Google Scholar]
- 21.Hinney A, Bettecken T, Tarnow P, Brumm H, Reichwald K, Lichtner P, et al. Prevalence, spectrum, and functional characterization of melanocortin-4 receptor gene mutations in a representative population-based sample and obese adults from Germany. J Clin Endocrinol Metab. 2006;91(5):1761–9. doi: 10.1210/jc.2005-2056. [DOI] [PubMed] [Google Scholar]
- 22.Thearle MS, Muller YL, Hanson RL, Mullins M, Abdussamad M, Tran J, et al. Greater impact of melanocortin-4 receptor deficiency on rates of growth and risk of type 2 diabetes during childhood compared with adulthood in Pima Indians. Diabetes. 2012;61(1):250–7. doi: 10.2337/db11-0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herbert V, Lau KS, Gottlieb CW, Bleicher SJ. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965;25(10):1375–84. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- 24.Thearle MS, Bunt JC, Knowler WC, Krakoff J. Childhood predictors of adult acute insulin response and insulin action. Diabetes Care. 2009;32(5):938–43. doi: 10.2337/dc08-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lillioja S, Bogardus C. Obesity and insulin resistance: lessons learned from the Pima Indians. Diabetes Metab Rev. 1988;4(5):517–40. doi: 10.1002/dmr.5610040508. [DOI] [PubMed] [Google Scholar]
- 26.Ravussin E, Lillioja S, Anderson TE, Christin L, Bogardus C. Determinants of 24-hour energy expenditure in man. Methods and results using a respiratory chamber. The Journal of clinical investigation. 1986;78(6):1568–78. doi: 10.1172/JCI112749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, et al. CDC growth charts: United States. Advance data. 2000;(314):1–27. [PubMed] [Google Scholar]
- 28.Barlow SE, Expert C. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120 (Suppl 4):S164–92. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 29.Andruff H, Carraro N, Thompson A, Gaudreau P. Latent Class Growth Modelling: A Tutorial. Tutorials in Quantitative Methods for Psychology. 2009;5(1):11–24. [Google Scholar]
- 30.Gamborg M, Andersen PK, Baker JL, Budtz-Jorgensen E, Jorgensen T, Jensen G, et al. Life course path analysis of birth weight, childhood growth, and adult systolic blood pressure. Am J Epidemiol. 2009;169(10):1167–78. doi: 10.1093/aje/kwp047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hitze B, Bosy-Westphal A, Plachta-Danielzik S, Bielfeldt F, Hermanussen M, Muller MJ. Long-term effects of rapid weight gain in children, adolescents and young adults with appropriate birth weight for gestational age: the Kiel Obesity Prevention Study. Acta paediatrica. 2010;99(2):256–62. doi: 10.1111/j.1651-2227.2009.01573.x. [DOI] [PubMed] [Google Scholar]
- 32.Jeffreys M, Lawlor DA, Galobardes B, McCarron P, Kinra S, Ebrahim S, et al. Lifecourse weight patterns and adult-onset diabetes: the Glasgow Alumni and British Women's Heart and Health studies. International journal of obesity. 2006;30(3):507–12. doi: 10.1038/sj.ijo.0803161. [DOI] [PubMed] [Google Scholar]
- 33.Lindsay RS, Cook V, Hanson RL, Salbe AD, Tataranni A, Knowler WC. Early excess weight gain of children in the Pima Indian population. Pediatrics. 2002;109(2):E33. doi: 10.1542/peds.109.2.e33. [DOI] [PubMed] [Google Scholar]
- 34.Price RA, Charles MA, Pettitt DJ, Knowler WC. Obesity in Pima Indians: large increases among post-World War II birth cohorts. American journal of physical anthropology. 1993;92(4):473–9. doi: 10.1002/ajpa.1330920406. [DOI] [PubMed] [Google Scholar]
- 35.Touger L, Looker HC, Krakoff J, Lindsay RS, Cook V, Knowler WC. Early growth in offspring of diabetic mothers. Diabetes Care. 2005;28(3):585–9. doi: 10.2337/diacare.28.3.585. [DOI] [PubMed] [Google Scholar]
- 36.Gluck ME, Venti CA, Lindsay RS, Knowler WC, Salbe AD, Krakoff J. Maternal influence, not diabetic intrauterine environment, predicts children's energy intake. Obesity (Silver Spring) 2009;17(4):772–7. doi: 10.1038/oby.2008.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yliharsila H, Kajantie E, Osmond C, Forsen T, Barker DJ, Eriksson JG. Body mass index during childhood and adult body composition in men and women aged 56–70 y. Am J Clin Nutr. 2008;87(6):1769–75. doi: 10.1093/ajcn/87.6.1769. [DOI] [PubMed] [Google Scholar]
- 38.Piaggi P, Thearle MS, Bogardus C, Krakoff J. Lower energy expenditure predicts long-term increases in weight and fat mass. J Clin Endocrinol Metab. 2013;98(4):E703–7. doi: 10.1210/jc.2012-3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Everhart JE, Pettitt DJ, Bennett PH, Knowler WC. Duration of obesity increases the incidence of NIDDM. Diabetes. 1992;41(2):235–40. doi: 10.2337/diab.41.2.235. [DOI] [PubMed] [Google Scholar]
- 40.Power C, Thomas C. Changes in BMI, duration of overweight and obesity, and glucose metabolism: 45 years of follow-up of a birth cohort. Diabetes Care. 2011;34 (9):1986–91. doi: 10.2337/dc10-1482. [DOI] [PMC free article] [PubMed] [Google Scholar]