Abstract
Trigonella foenum graecum commonly known as Fenugreek exerts normoglycemic and insulinotropic effects in humans by compounds from its seed and leaf extracts. Some studies reported that treating pregnant mice with fenugreek seed could cause toxic effects on the nervous system of its pubs during developmental growth, while in some other studies neuroprotective properties were considered for it. Safety of anti-diabetic drugs for nervous system is very important because peripheral neuropathy is a common complication of diabetes and hazardous drugs could worsen it. In this study, the effect of treatment with fenugreek seed extract on the function of sciatic nerves of neuropathic mice was evaluated. Neuropathy was induced in male mice by pyridoxine intoxication. After that, animals were treated with 0.2, 2 and 20 mg/kg of hydro-alcoholic extract of fenugreek seeds for 10 days, tail flick, electrophysiological and histological assays were performed to evaluate the effect of fenugreek seed extract on function of the peripheral nerves. Our data showed that fenugreek has anti neuropathic effect and restores the function of nerve fibers. Results of electrophysiological recordings stated that the highest rate of healing was occurred in 20 mg/kg fenugreek extract treated animals. In conclusion, findings of the present study demonstrate that treatment with fenugreek seed extract can potentially facilitate healing from pyridoxine induced peripheral neuropathy in mice.
Keywords: peripheral neuropathy, pyridoxine, Trigonella foenum graecum
Introduction
Peripheral neuropathy is a common complication of many disorders, such as metabolic, infectious and auto-immune diseases (Gorshtein and Levy, 2005[12]). Because of its high incidence in diabetes, it is one of the major complications in diabetic patients (Gregory et al., 2012[13]). Control of neuropathy in diabetic patients is difficult and even with precise control of blood glucose the risk of neuropathy prevalence still remains (Callaghan et al., 2012[7]). Consequently, among candidate drugs and herbals for treatment of diabetes, some of them that exert both normoglycemic and neuroprotective effects could be more appropriate for control of disease.
Fenugreek (Trigonella foenum graecum) is a plant that belongs to the Leguminosae family (Kaviarasan et al., 2006[17]). It is established that fenugreek seed extract has anti-diabetic effects through several pathways, such as restoring pancreatic β cell function and inhibiting sucrase and alpha-amylase activities (Baquer et al., 2011[1]). It is full of 4-hydroxyisoleucine, which directly induces insulin secretion from pancreatic β cells (Kaviarasan et al., 2006[17]). Furthermore, there are some evidences that its seed extract reduces serum triglycerides, total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C) (Baquer et al., 2011[1]). Beside these properties, some anti-inflammatory and anti-nociceptive actions were also attributed to its seed extract (Kawabata et al., 2011[18]).
Otherwise there are some reports about its hazardous developmental side effects on the nervous system of human infants, which their mothers used fenugreek seed during gestation (Khalki et al., 2012[19]). According to animal studies, exposure of pregnant mice to high dose (more than 500 mg/kg) of fenugreek seed extract could cause neuro-developmental defects in pubs (Khalki et al., 2012[19]). Another study showed that, feeding diabetic rats with fenugreek seed powder (5 % w/w) mixed with standard pellet food reduces their blood glucose to near normal level and protects their sciatic nerves against the structural abnormalities found in diabetes (Preet et al., 2005[24]).
According to above studies, there are controversial judges about the effects of fenugreek on the nervous system. Since people of some African and Asian countries are using fenugreek because of its anti-diabetic effects, and taking into account that diabetes indigenously has neuro-pathological side effects, it may worsen their peripheral neuropathy if they consume neurotoxic supplements.
In this study electrophysiological and behavioral assays were used to investigate the effect of hydro-alcoholic extract of fenugreek seed on functional improvement of peripheral neuropathy in mice. For this purpose pyridoxine induced neuropathic mice were treated with fenugreek seed extract in concentrations near to its therapeutic antidiabetic doses.
Materials and Methods
Induction of peripheral neuropathy in mice
Totally, 40 male BALB/c mice with the average body weight of 40-50 grams, were selected and divided into five groups: 1: normal (non-neuropathic), 2: control (neuropathic + vehicle treated), 3: neuropathic + 0.2 mg/kg fenugreek seed extract treated, 4: neuropathic + 2 mg/kg fenugreek seed extract treated, and 5: neuropathic + 20 mg/kg fenugreek seed extract treated.
Non-diabetic neuropathy was induced in mice, for this purpose they were treated with high doses (350 mg/kg, ip) of pyridoxine twice a day for eight days (Callizot et al., 2001[8]). Before fenugreek therapy, tail flick assays were performed to state the incidence of neuropathy in the animals. After establishment of neuropathy incidence, fenugreek treatment was initiated and continued for ten days. For production of injection solution, 100 gr of seeds were powdered finely, soaked in 80 % ethanol and placed in room temperature for five days. Then the extract was cleared, filtered, and evaporated under the laminar hood; the produced dried material was used for treatments. Each group received the specified amount of dried material solved in distilled water intraperitoneally (i.p). Before injections, the injection solutions were filtered through 0.02 μm filters. The final doses for treatments were: 0.2, 2 and 20 mg of dried seed extract per kilogram of the body weight of animal solved in 250 µl of distilled water, same amounts of distilled water (250 µl) were injected to the animals of the control group.
Tail flick test
Tail flick tests were used to obtain the spinal tail flick response to the noxious thermal stimuli (Rani and Gupta, 2012[27]). In this study tail flick tests were performed in three sessions: before any procedure, after neuropathy induction and finally after fenugreek treatment. The tail flick response was elicited by using a tail flick apparatus. Each mouse was located in strainer and the distal half of its tail was situated under the source of radiant heat. The time elapsed until the animal flicked its tail was determined. Fifteen-seconds cut off latency was reserved to prevent damage to the tail.
Electrophysiological assay
After the final tail flick test, animals were killed by cervical dislocation, their sciatic nerves were removed by surgical incision and placed in modified Lock's solution (pH 7.4), which made up of 140 mM NaCl, 1.2 mM MgCl2, 5.6 mM KCl, 2.2 mM CaCl2, 10 mM Tris buffer (tris-(hydroxymethyl)amino-methane), and 10 mM glucose. To record electrical events, the sciatic nerves were placed in humid nerve chamber and for prevention of tissue drying drops of Lock's solution were pulled on them. CEPTU instrument (Complete Electrophysiology Teaching Unit, Washington Bioscience) was used for electrical stimulation of the nerves and their compound action potential acquisition. Isolated nerves were stimulated by a defined stimulus (intensity: 1.5 v, delay: 0, width: 0.05 ms) and their evoked compound action potentials were amplified by the gain of 1000. To monitor the data digitally we used a bench top computer and digital oscilloscope software. The output wire of amplifier were connected to the microphone wire input of the computer, so the computer sound card was used as an analog to digital board. Digital oscilloscope software (Virtins Sound Card Oscilloscope, Multi-Instrument Software, version 2.1) was used to visualize the action potential curve on the screen. Images were captured from electrical events of nerves and they were analyzed by Image-J software to calculate conduction velocity, duration and voltage of action potential in each nerve.
Histochemical staining
After electrophysiological assays sciatic nerves of each animal were placed in 10 % paraformaldehyde solution overnight, next day they were processed by tissue processor, after that all of the nerves belonging to each group were paraffin embedded separately into the different blocks and 5 µm thickened transverse sections were harvested from them with a microtome. Staining procedure was continued by deparaffination, rehydration and incubation of sections in the 1 % Hematoxilin-Eosin solution for eight minutes. Afterward, sections were dehydrated and mounted with xylene based mounting medium. Three separate sections were selected from the beginning, the middle, and the end of each nerve block, and then one image was captured from center of the nerve in each section, totally 48 images were captured from each group and the number of nerve fibers in each image were counted.
Statistical analysis
Data from electrophysiological and histological assays were analyzed with SPSS 17 software using paired T student test and differences were assumed significant when P-values were less than 0.05.
Results
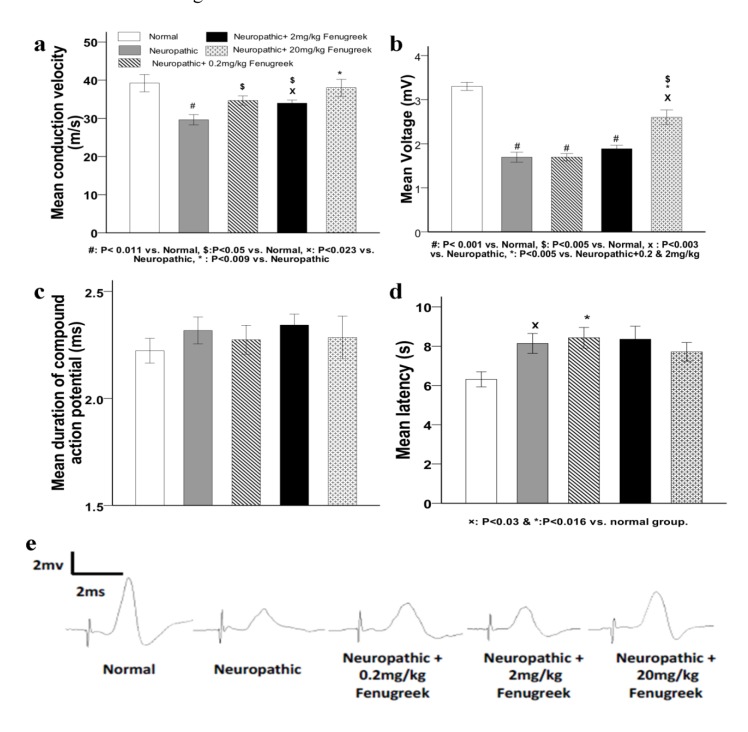
As seen in Figure 1a(Fig. 1), the mean nerve conduction velocities (NCV) in the control (vehicle-treated neuropathic) and 0.2 mg/kg treated groups were significantly lower than that in the normal group (P<0.011), which confirmed the incidence of neuropathy and the absence of healing in these groups. Fenugreek treatment with 2 and 20 mg/kg doses, significantly increased the NCV compared to the vehicle treatment (P<0.023 and P< 0.009, respectively). In comparisons between the treated groups with the normal group, the NCV was not changed significantly when comparing the 20 mg/kg treated group with the normal one, which indicated that recovery was occurred in the 20 mg/kg treated group, while in the other treated doses the healing process was not complete and the NCV was lower significantly compared with the normal group (P<0.05).
Figure 1. The effect of fenugreek treatment on electrophysiological parameters of sciatic nerves. a: mean conduction velocity, b: mean voltage of compound action potential, c: mean duration of compound action potential (ms). d: mean delay in tail flick test. Groups are as same as those represented in a. e: example graphs of nerve compound action potentials recorded from the different groups, at each graph the first sharp wave is the stimulus and the second one is the response of the nerve. Error bars representing the standard error of mean.
Treatment with pyridoxine strongly reduced the voltage of action potential (Figure 1b(Fig. 1)) in all of the neuropathic groups compared with the normal group (P<0.001). In comparisons among the fenugreek treated groups with the non treated group, the reduction of the voltage in the 0.2 and 2 mg/kg treated groups was as similar as non-treated neuropathic group, but it was significantly greater in the 20 mg/kg treated group than that in the other treated animals and the control group (P<0.005 and P<0.003, respectively). These results indicated that the treatment with 20 mg/kg of fenugreek extract was more effective than the other doses on restoring the NCV and the voltage intensity. Comparisons among different groups in the mean duration of action potential showed that there were not any significant differences (Figure 1c(Fig. 1)).
Data of the tail flick tests (Figure 1d(Fig. 1)) represented that there were significant increases in the mean latencies in the control and the 0.2 mg/kg treated groups compared with the normal group (P<0.03 and p<0.016, respectively). Differences in the mean latency of the tail flick were not significant when comparing the 2 mg/kg and the 20 mg/kg fenugreek treated animals with the normal group (P<0.09 and P<0.061, respectively). Otherwise, in comparisons among the treated groups with the control animals, despite, the mean latency was lower in the 20 mg/kg treated group, but the differences were not statistically significant as well as the other treated groups. Taking all together, results of tail flick tests showed controversy; on the one hand, there was no significant difference when comparing the normal and control groups with the 20 mg/kg treated group, while on the other hand, the difference between the control group and the normal group was significant. This controversy could be as a result of the diversities in the pain threshold among animals, which is always a distorting parameter in this kind of behavioral assays.
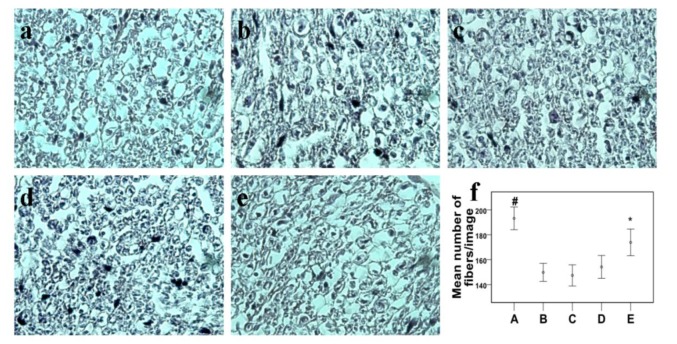
As it was predicted, there were significant differences in the mean number of axons existed in the nerves when comparing the normal group with the other groups (P<0.001), which states that there was a significant loss in the number of nerve fibers in all of the neuropathic groups. In comparisons among the neuropathic subjects, the highest number of nerve fibers were counted in the 20 mg/kg treated animals as compared with the other neuropathic groups (p<0.01), which confirmed the incidence of rapid healing in this group (Figure 2f(Fig. 2)).
Figure 2. Cross sections of mice sciatic nerves stained with H&E (with 40X magnification) from different groups, a: Normal, b: Neuropathic, c: Neuropathic + 0.2 mg/kg fenugreek, d: Neuropathic + 2 mg/kg fenugreek, e: Neuropathic + 20 mg/kg fenugreek. f: The mean number of nerve fibers counted in the nerve sections of the different groups. Error bars in f representing the standard error of mean.
Discussion
Damage to the cell membrane due to the high exposure to free radicals, as it happens in the diabetes, is one of the major reasons for peripheral neuropathy. Therefore diabetic patients beside difficulties in the control of their blood glucose are always under the risk of neuropathy incidence. There are several herbal drugs with known anti-diabetic effects, but there is not enough data about their safety for the nervous system. Usage of these kinds of herbals could help to decrease the blood glucose but may affect the neuropathy and worsen it. Among these drugs fenugreek and its derivatives such as trigonelline like to be neuroprotective, but there are some reports about their adverse effects on the nervous system during developmental growth (Khalki et al., 2010[20], 2012[19]).
Results of the present study indicated that in the low to moderate doses fenugreek is safe for the nervous system and could help to improve the function of the nerve fibers. Electrophysiological data indicated that, NCV and voltage of action potential were improved in the 20 mg/kg fenugreek treated neuropathic mice. However, behavioral data was not completely in accordance with the electrophysiological assays, which could be due to differences among animals in the pain threshold, and the differences in compatibility with the conditions of the test, which could strongly affect the results of behavioral assays.
Results of histological staining were in accordance with the results of electrical assays since the higher rate of axon recovery was seen in the 20 mg/kg treated group. Considering the overall results of this study, fenugreek seed extract remedy exerts some improving effects on the function of neuropathic nerves. Our study confirmed the results of previous studies that treatment with Trigonella foenum graecum seed powder restored the histopathological alterations in the sciatic nerves and ocular tissues of diabetic rats (Preet et al., 2005[24], 2006[25]).
The healing effects of fenugreek seed extract could be assigned to its nutraceuticals such as trigonelline and 4-hydroxy isoleuscine (Moorthy et al., 2010[21]; Morani et al., 2012[22]; Zhao et al., 2002[32]; Zhou et al., 2012[33]). Trigonelline exists abundantly in fenugreek seed, and previous reports confirmed that it exerts a healing effect on the pyridoxine induced auditory neuropathy in mice (Hong et al., 2009[15]). Furthermore, it has been reported that trigonelline regenerates neurite outgrowth and reconstructs synapses in the neuroblastoma cell lines (Tohda et al., 1999[31], 2005[30]). Since trigonelline is a nicotinic acid derivative and nicotinic acid elevates the mRNA expression of nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF), if trigonelline exerts similar effects, this could be considered as a possible mechanism involved in the neuroprotective effects of fenugreek seed extract (Brailoiu et al., 2005[2]; French et al., 1999[10]; Qiao et al., 2003[26]; Rosato-Siri et al., 2006[28]; Serres and Carney, 2006[29]). Based on the reported data, the insulinotropic effect of fenugreek seeds could restore the structural alterations of sciatic nerves in alloxan induced diabetic rats (Preet et al., 2005[24]). This insulinotropic property was attributed to the presence of high concentration of 4-hydroxyisoleucine in its extract, which directly stimulates insulin secretion from pancreatic beta cells (Broca et al.,1999[4], 2000[5], 2004[3]; Gupta et al., 2001[14]). Since there are some developmental similarities between neurons and beta cells, so 4-hydroxyisoleucine could exert similar effect on the neurons (Furuzawa et al., 1996[11]). On the other hand, as well as stimulating insulin secretion, 4-hydroxyisoleucine decreases the insulin resistance in peripheral tissues and liver by stimulating insulin receptor substrate-associated phosphatidylinositol (PI) 3-kinase activity (Jaiswal et al., 2012[16]). Furthermore, NGF and BDNF receptors need PI3 kinase pathway to exert their anti-apoptotic and nerve growth stimulating effects (Bui et al., 2002[6]; Culmsee et al., 2002[9]; Nguyen et al., 2010[23]). Overall, trigonelline increases NGF and BDNF, while 4-hydroxyisoleucine increases insulin and activates insulin dependent PI3-kinase pathway which is necessary for neuroprotective function of NGF and BDNF, so these interactions between trigonelline, nerve growth factor, 4-hydroxyisoleucine and insulin could bring reasonable explanation for its neuroprotective effects.
In conclusion, our findings represented that fenugreek seed extract is neuro-regenerative and its consumption as an antidiabetic remedy, beside elevating the insulin secretion and lowering the blood glucose, could be beneficial for the control of peripheral neuropathy.
References
- 1.Baquer NZ, Kumar P, Taha A, Kale RK, Cowsik SM, McLean P. Metabolic and molecular action of Trigonella foenum-graecum (fenugreek) and trace metals in experimental diabetic tissues. J Biosci. 2011;36:383–396. doi: 10.1007/s12038-011-9042-0. [DOI] [PubMed] [Google Scholar]
- 2.Brailoiu E, Hoard JL, Filipeanu CM, Brailoiu GC, Dun SL, Patel S, et al. Nicotinic acid adenine dinucleotide phosphate potentiates neurite outgrowth. J Biol Chem. 2005;280:5646–5650. doi: 10.1074/jbc.M408746200. [DOI] [PubMed] [Google Scholar]
- 3.Broca C, Breil V, Cruciani-Guglielmacci C, Manteghetti M, Rouault C, Derouet M, et al. Insulinotropic agent ID-1101 (4-hydroxyisoleucine) activates insulin signaling in rat. Am J Physiol. 2004;287:E463–E471. doi: 10.1152/ajpendo.00163.2003. [DOI] [PubMed] [Google Scholar]
- 4.Broca C, Gross R, Petit P, Sauvaire Y, Manteghetti M, Tournier M, et al. 4-Hydroxyisoleucine: experimental evidence of its insulinotropic and antidiabetic properties. Am J Physiol. 1999;277:E617–E623. doi: 10.1152/ajpendo.1999.277.4.E617. [DOI] [PubMed] [Google Scholar]
- 5.Broca C, Manteghetti M, Gross R, Baissac Y, Jacob M, Petit P, et al. 4-Hydroxyisoleucine: effects of synthetic and natural analogues on insulin secretion. Eur J Pharmacol. 2000;390:339–345. doi: 10.1016/s0014-2999(00)00030-3. [DOI] [PubMed] [Google Scholar]
- 6.Bui NT, König HG, Culmsee C, Bauerbach E, Poppe M, Krieglstein J, et al. p75 neurotrophin receptor is required for constitutive and NGF-induced survival signalling in PC12 cells and rat hippocampal neurones. J Neurochem. 2002;81:594–605. doi: 10.1046/j.1471-4159.2002.00841.x. [DOI] [PubMed] [Google Scholar]
- 7.Callaghan BC, Little AA, Feldman EL, Hughes RA. Enhanced glucose control for preventing and treating diabetic neuropathy. Cochrane Database Syst Rev. 2012;6:CD007543. doi: 10.1002/14651858.CD007543.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Callizot N, Warter JM, Poindron P. Pyridoxine-induced neuropathy in rats: a sensory neuropathy that responds to 4-methylcatechol. Neurobiol Dis. 2001;8:626–635. doi: 10.1006/nbdi.2001.0408. [DOI] [PubMed] [Google Scholar]
- 9.Culmsee C, Gerling N, Lehmann M, Nikolova-Karakashian M, Prehn JH, Mattson MP, et al. Nerve growth factor survival signaling in cultured hippocampal neurons is mediated through TrkA and requires the common neurotrophin receptor P75. Neuroscience. 2002;115:1089–1108. doi: 10.1016/s0306-4522(02)00539-0. [DOI] [PubMed] [Google Scholar]
- 10.French SJ, Humby T, Horner CH, Sofroniew MV, Rattray M. Hippocampal neurotrophin and trk receptor mRNA levels are altered by local administration of nicotine, carbachol and pilocarpine. Brain Res Mol Brain Res. 1999;67:124–136. doi: 10.1016/s0169-328x(99)00048-0. [DOI] [PubMed] [Google Scholar]
- 11.Furuzawa Y, Ohmori Y, Watanabe T. Immunohistochemical studies of neural elements in pancreatic islets of the cat. J Vet Med Sci. 1996;58:641–646. doi: 10.1292/jvms.58.641. [DOI] [PubMed] [Google Scholar]
- 12.Gorshtein A, Levy Y. Intravenous immunoglobulin in therapy of peripheral neuropathy. Clin Rev Allergy Immunol. 2005;29:271–279. doi: 10.1385/CRIAI:29:3:271. [DOI] [PubMed] [Google Scholar]
- 13.Gregory JA, Jolivalt CG, Goor J, Mizisin AP, Calcutt NA. Hypertension-induced peripheral neuropathy and the combined effects of hypertension and diabetes on nerve structure and function in rats. Acta Neuropathol. 2012;124:561–573. doi: 10.1007/s00401-012-1012-6. [DOI] [PubMed] [Google Scholar]
- 14.Gupta A, Gupta R, Lal B. Effect of Trigonella foenum-graecum (fenugreek) seeds on glycaemic control and insulin resistance in type 2 diabetes mellitus: a double blind placebo controlled study. J Assoc Physicians India. 2001;49:1057–1061. [PubMed] [Google Scholar]
- 15.Hong BN, Yi TH, Kim SY, Kang TH. High-dosage pyridoxine-induced auditory neuropathy and protection with coffee in mice. Biol Pharm Bull. 2009;32:597–603. doi: 10.1248/bpb.32.597. [DOI] [PubMed] [Google Scholar]
- 16.Jaiswal N, Maurya CK, Venkateswarlu K, Sukanya P, Srivastava AK, Narender T, et al. 4-Hydroxyisoleucine stimulates glucose uptake by increasing surface GLUT4 level in skeletal muscle cells via phosphatidylinositol-3-kinase-dependent pathway. Eur J Nutr. 2012;51:893–898. doi: 10.1007/s00394-012-0374-9. [DOI] [PubMed] [Google Scholar]
- 17.Kaviarasan S, Ramamurty N, Gunasekaran P, Varalakshmi E, Anuradha CV. Fenugreek (Trigonella foenum graecum) seed extract prevents ethanol-induced toxicity and apoptosis in Chang liver cells. Alcohol Alcohol. 2006;41:267–273. doi: 10.1093/alcalc/agl020. [DOI] [PubMed] [Google Scholar]
- 18.Kawabata T, Cui MY, Hasegawa T, Takano F, Ohta T. Anti-inflammatory and anti-melanogenic steroidal saponin glycosides from Fenugreek (Trigonella foenum-graecum L.) seeds. Planta Med. 2011;77:705–710. doi: 10.1055/s-0030-1250477. [DOI] [PubMed] [Google Scholar]
- 19.Khalki L, Bennis M, Sokar Z, Ba-M'hamed S. The developmental neurobehavioral effects of fenugreek seeds on prenatally exposed mice. J Ethnopharmacol. 2012;139:672–677. doi: 10.1016/j.jep.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 20.Khalki L, M'hamed SB, Bennis M, Chait A, Sokar Z. Evaluation of the developmental toxicity of the aqueous extract from Trigonella foenum-graecum (L.) in mice. J Ethnopharmacol. 2010;131:321–325. doi: 10.1016/j.jep.2010.06.033. [DOI] [PubMed] [Google Scholar]
- 21.Moorthy R, Prabhu KM, Murthy PS. Anti-hyperglycemic compound (GII) from fenugreek (Trigonella foenum-graecum Linn.) seeds, its purification and effect in diabetes mellitus. Indian J Exp Biol. 2010;48:1111–1118. [PubMed] [Google Scholar]
- 22.Morani AS, Bodhankar SL, Mohan V, Thakurdesai PA. Ameliorative effects of standardized extract from Trigonella foenum-graecum L. seeds on painful peripheral neuropathy in rats. Asian Pac J Trop Med. 2012;5:385–390. doi: 10.1016/S1995-7645(12)60064-9. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen TL, Kim CK, Cho JH, Lee KH, Ahn JY. Neuroprotection signaling pathway of nerve growth factor and brain-derived neurotrophic factor against staurosporine induced apoptosis in hippocampal H19-7/IGF-IR. [corrected]. Exp Mol Med. 2010;42:583–595. doi: 10.3858/emm.2010.42.8.060. (Ger). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Preet A, Gupta BL, Siddiqui MR, Yadava PK, Baquer NZ. Restoration of ultrastructural and biochemical changes in alloxan-induced diabetic rat sciatic nerve on treatment with Na3VO4 and Trigonella a promising antidiabetic agent. Mol Cell Biochem. 2005;278:21–31. doi: 10.1007/s11010-005-7815-1. [DOI] [PubMed] [Google Scholar]
- 25.Preet A, Siddiqui MR, Taha A, Badhai J, Hussain ME, Yadava PK, et al. Long-term effect of Trigonella foenum graecum and its combination with sodium orthovanadate in preventing histopathological and biochemical abnormalities in diabetic rat ocular tissues. Mol Cell Biochem. 2006;289:137–147. doi: 10.1007/s11010-006-9156-0. [DOI] [PubMed] [Google Scholar]
- 26.Qiao D, Seidler FJ, Violin JD, Slotkin TA. Nicotine is a developmental neurotoxicant and neuroprotectant: stage-selective inhibition of DNA synthesis coincident with shielding from effects of chlorpyrifos. Brain Res Dev Brain Res. 2003;147:183–190. doi: 10.1016/s0165-3806(03)00222-0. [DOI] [PubMed] [Google Scholar]
- 27.Rani S, Gupta MC. Evaluation and comparison of antinociceptive activity of aspartame with sucrose. Pharmacol Rep. 2012;64:293–298. doi: 10.1016/s1734-1140(12)70767-3. [DOI] [PubMed] [Google Scholar]
- 28.Rosato-Siri M, Cattaneo A, Cherubini E. Nicotine-induced enhancement of synaptic plasticity at CA3-CA1 synapses requires GABAergic interneurons in adult anti-NGF mice. J Physiol. 2006;576:361–377. doi: 10.1113/jphysiol.2006.114587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Serres F, Carney SL. Nicotine regulates SH-SY5Y neuroblastoma cell proliferation through the release of brain-derived neurotrophic factor. Brain Res. 2006;1101:36–42. doi: 10.1016/j.brainres.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 30.Tohda C, Kuboyama T, Komatsu K. Search for natural products related to regeneration of the neuronal network. Neurosignals. 2005;14:34–45. doi: 10.1159/000085384. [DOI] [PubMed] [Google Scholar]
- 31.Tohda C, Nakamura N, Komatsu K, Hattori M. Trigonelline-induced neurite outgrowth in human neuroblastoma SK-N-SH cells. Biol Pharm Bull. 1999;22:679–682. doi: 10.1248/bpb.22.679. [DOI] [PubMed] [Google Scholar]
- 32.Zhao HQ, Qu Y, Wang XY, Zhang HJ, Li FM, Masao H. Determination of trigonelline in Trigonella foenum-graecum by HPLC. Zhongguo Zhong Yao Za Zhi. 2002;27:194–196. [PubMed] [Google Scholar]
- 33.Zhou J, Chan L, Zhou S. Trigonelline: A plant alkaloid with therapeutic potential for diabetes and central nervous system disease. Curr Med Chem. 2012;19:3523–3531. doi: 10.2174/092986712801323171. [DOI] [PubMed] [Google Scholar]