Abstract
Galectin-3 has been linked to incident renal disease, experimental renal fibrosis, and nephropathy. However, the association among galectin-3, renal function, and adverse outcomes has not been described. We studied this association in two large cohorts of patients over a broad range of renal function. We measured galectin-3 concentrations in baseline samples from the German Diabetes mellitus Dialysis (4D) study (1168 dialysis patients with type 2 diabetes mellitus) and the Ludwigshafen Risk and Cardiovascular Health (LURIC) study (2579 patients with coronary angiograms). Patients were stratified into three groups: eGFR of ≥90 ml/min per 1.73 m2, 60–89 ml/min per 1.73 m2, and <60 ml/min per 1.73 m2. We correlated galectin-3 concentrations with demographic, clinical, and biochemical parameters. The association of galectin-3 with clinical end points was assessed by Cox proportional hazards regression within 10 years (LURIC) or 4 years (4D) of follow-up. Mean±SD galectin-3 concentrations were 12.8±4.0 ng/ml (eGFR≥90 ml/min per 1.73 m2), 15.6±5.4 ng/ml (eGFR 60–89 ml/min per 1.73 m2), 23.1±9.9 ng/ml (eGFR<60 ml/min per 1.73 m2), and 54.1±19.6 ng/ml (dialysis patients of the 4D study). Galectin-3 concentration was significantly associated with clinical end points in participants with impaired kidney function, but not in participants with normal kidney function. Per SD increase in log-transformed galectin-3 concentration, the risks of all-cause mortality, cardiovascular mortality, and fatal infection increased significantly. In dialysis patients, galectin-3 was associated with the combined end point of cardiovascular events. In conclusion, galectin-3 concentrations increased with progressive renal impairment and independently associated with cardiovascular end points, infections, and all-cause death in patients with impaired renal function.
Keywords: cardiovascular disease, CKD, hemodialysis
Galectin-3, also known as 35-kD lectin, IgE-binding protein, laminin-binding protein, and Mac-2, is a 250 amino-acid galactose-specific lectin with a high affinity for β-galactosides and IgE. It is a member of the multifunctional galectin family1,2 and is ubiquitously expressed, for instance in the heart, the kidney, blood vessels, and macrophages.3 Alternative splicing results in multiple transcript variants. Galectin-3 is a so-called chimera-type galectin, with a C-terminal end that contains the carbohydrate recognition domain and an N-terminal domain. When ligands bind the carbohydrate recognition domain of galectin-3, it forms oligodimers consisting of cross-linking oligosaccharides in the interstitial space. This dimerization serves to strengthen cell-cell interactions and has been associated with stiffening of the extracellular matrix and fibrogenesis. Furthermore, like other members of the galectin family, galectin-3 may serve as a pattern-recognition receptor, binding glycans on the surfaces of viruses, bacteria, protista, and fungi.4 As such, galectin-3 plays a role in tissue fibrosis, immunity, and the inflammatory response. Experimental studies in models of cancer, congestive heart failure (CHF), and inflammatory disease have convincingly shown that galectin-3 expression is increased in these pathologic conditions and contributes to the pathophysiology of these diseases.5–7
Similarly, in renal disease, galectin-3 has been shown to play a role in the onset and development of diabetic and nondiabetic nephropathy.8 Recent studies showed that genetic disruption of the gene encoding galectin-3 attenuates renal fibrosis in a model of unilateral ureter obstruction.9 Furthermore, pharmacologic inhibition of galectin-3 with modified citrus pectin attenuated renal fibrosis and preserved kidney function.10 Interestingly, galectin-3 is also secreted by unknown mechanisms into the bloodstream and may be useful as a biomarker. For example, galectin-3 concentrations are associated with impaired outcomes in CHF, with incremental prognostic value over brain natriuretic peptide.11,12
It has been reported that elevated galectin-3 levels precede the development of CKD and are associated with rapid declines in eGFR,13 but no data for galectin-3 concentrations in patients with impaired kidney function (often referred to as CKD, stages 2–5D) have been reported. On the basis of current knowledge from experimental and observational studies, we hypothesized that increasing galectin-3 concentrations would occur in patients over the range of normal kidney function, to mildly and moderately impaired kidney function, to ESRD (dialysis). We also hypothesized that the concentration of circulating galectin-3 would be associated with adverse outcomes in patients with impaired renal function. To that end, we evaluated galectin-3 levels in 2578 patients from the Ludwigshafen Risk and Cardiovascular Health (LURIC) study and 1168 patients participating in the German Diabetes mellitus Dialysis (4D) study.
Results
Study Populations
A total of 1168 patients from the 4D study and 2579 patients from the LURIC study were included. The median follow-up period was 4.0 years in the 4D study and 10 years in the LURIC study. The average age was 66±8 years in the 4D study, and 54% of participants were men. In the LURIC study, the mean age was 62.8±10.5 years, and 68.3% of participants were men.
Of the 1168 4D patients, 575 patients (49.2%) died during follow-up. Of these deaths, 150 (12.8%) were caused by sudden cardiac death (SCD) and 119 (10.2%) were attributable to infection. A total of 432 patients (40.0%) reached the combined cardiovascular end point, with myocardial infarction (MI) and stroke occurring in 183 patients (15.7%) and 97 patients (8.3%), respectively.
Of the 2579 LURIC patients, 759 (29.4%) died during follow-up. SCD occurred in 208 patients (8.1%), 112 (4.3%) died as a result of CHF, 75 (2.9%) died because of fatal MI, and 45 (1.7%) suffered fatal stroke. Cardiovascular disease caused a total of 479 deaths (18.6%), whereas 57 patients (2.2%) died due to infection.
Galectin-3 in Patients at Different Strata of Impaired Kidney Function
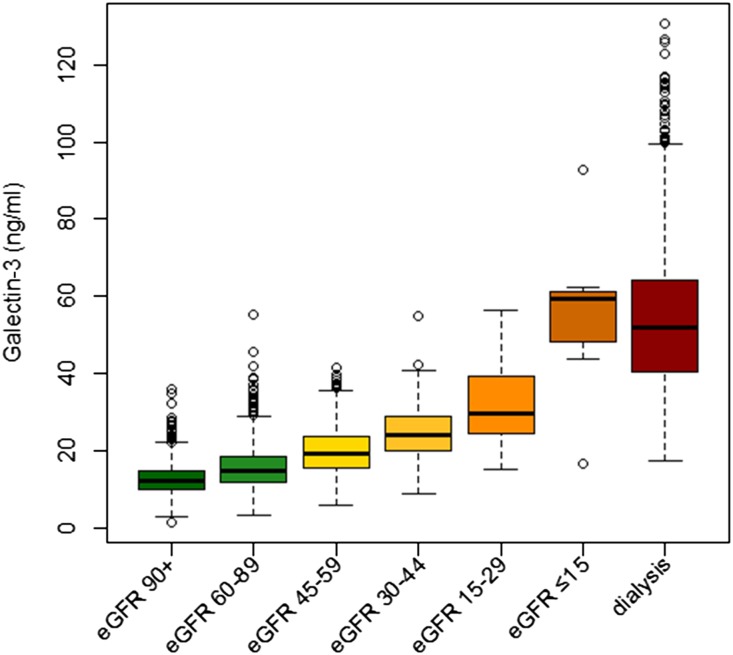
Galectin-3 concentrations increased in parallel with decreasing kidney function. Mean galectin-3 concentrations were 12.8±4.0 ng/ml, 15.6±5.4 ng/ml, 23.1±9.9 ng/ml, and 54.1±19.6 ng/ml in patients with normal kidney function (eGFR≥90 ml/min per 1.73 m2), mildly impaired kidney function (eGFR 60–89 ml/min per 1.73 m2), and moderately impaired kidney function (eGFR<60 ml/min per 1.73 m2), as well as in dialysis patients, respectively (Figure 1, Table 1). We further divided LURIC and 4D study participants according to quartiles of galectin-3 concentration; baseline clinical and biochemical characteristics appear in Supplemental Tables 1 and 2.
Figure 1.
Boxplots showing the distribution of galectin-3 concentration in different stages of kidney function. Patients with eGFR (range of eGFR given in ml/min per 1.73m2) are from the LURIC study; patients on dialysis are from the 4D study. The boxes represent medians with interquartile ranges; whiskers extend to the data points that are closest to the value of the median plus 1.5 times the interquartile range.
Table 1.
Patient baseline characteristics
| Characteristic | LURIC Study | 4D Study | ||
|---|---|---|---|---|
| eGFR≥90 | eGFR=60–89 | eGFR<60a | Hemodialysis | |
| Participants, n (%) | 1209 (36.6) | 1642 (49.7) | 456 (13.8) | 1168 |
| eGFR (ml/min per 1.73 m2) | 101±8 | 77±9 | 46±12 | — |
| Galectin-3 (ng/ml) | 12.8±4.0 | 15.6±5.4 | 23.1±9.9 | 54.1±19.6 |
| Sex (% men) | 78.2 | 66.5 | 58.1 | 54.4 |
| Smoker/ex-smoker (%) | 71.2 | 61.4 | 57.7 | 40.3 |
| Time on dialysis (mo) | — | — | — | 8.3±6.8 |
| Duration of diabetes mellitus (yr) | — | — | — | 18.1±8.8 |
| Systolic BP (mmHg) | 136±22 | 143±24 | 145±26 | 146±22 |
| Diastolic BP (mmHg) | 80±11 | 82±12 | 79±12 | 76±11 |
| BMI (kg/m2) | 27.2±4.0 | 27.7±4.0 | 27.5±4.4 | 27.5±4.8 |
| CAD (%) | 72.9 | 79.9 | 83.8 | 29.8 |
| CHF (%) | 13.0 | 19.0 | 34.5 | 35.8 |
| PVD (%) | 6.8 | 10.3 | 14.7 | 44.9 |
| Hypertension (%) | 61.9 | 77.6 | 83.8 | 88.7 |
| LVH (%) | 6.0 | 8.5 | 12.7 | 12.5 |
| Arrhythmia (%) | 12.6 | 15.6 | 16.2 | 18.5 |
| Laboratory parameters | ||||
| Albumin (g/dl) | 4.4±0.5 | 4.4±0.5 | 4.3±0.6 | 3.8±0.3 |
| hsCRP (mg/L) | 2.4 (1.0–6.7) | 3.5 (1.4–8.7) | 6.5 (2.5–14.9) | 10.6±17.2 |
| Total cholesterol (mg/dl) | 194±41 | 192±37 | 187±40 | 220±42 |
| LDL cholesterol (mg/dl) | 117±35 | 117±34 | 111±34 | 126±29 |
| HDL cholesterol (mg/dl) | 39±11 | 39±11 | 37±11 | 36±13 |
| Triglycerides (mg/dl) | 176±138 | 169±102 | 182±110 | 262±165 |
| Hemoglobin (g/dl) | 14.1±1.3 | 13.8±1.5 | 13.1±1.7 | 10.9±1.4 |
| HbA1c (%) | 6.1±1.2 | 6.4±1.2 | 6.7±1.4 | 6.7±1.3 |
| Potassium (mmol/L) | 4.2±0.3 | 4.2±0.3 | 4.2±0.4 | 5.2±0.8 |
| Calcium (mmol/L) | 2.3±0.1 | 2.3±0.1 | 2.3±0.1 | 2.3±0.2 |
| Phosphate (mg/dL) | 3.5±0.5 | 3.5±0.5 | 3.7±0.7 | 6.0±1.6 |
| Creatinine (mg/dl) | 0.8±0.1 | 1.0±0.1 | 1.4±0.8 | 6.9±2.3 |
| NTpro-BNP (pg/ml) | 143 (63–385) | 329 (138–935) | 1157 (450–2795) | 8139±13,615 |
eGFR values are given in ml/min per 1.73 m2. Data are presented as the mean±SD or the median (interquartile range) unless otherwise specified.
eGFR 45–59 ml/min per 1.73 m2, n=285 (8.6%); eGFR 30–44 ml/min per 1.73 m2, n=122 (3.7%); eGFR 15–29 ml/min per 1.73m2, n=39 (1.2%); and eGFR<15 ml/min per 1.73 m2, n=10 (0.3%).
In LURIC patients, galectin-3 concentrations and eGFR were strongly correlated (Spearman correlation coefficient rs=−0.54; P<0.001). Other variables with moderate to strong correlations with galectin-3 concentration in LURIC participants were age (rs=0.25), C-reactive protein levels measured with high sensitivity (hsCRP) (rs=0.22), glycated hemoglobin (rs=0.19), and N-terminal pro-brain natriuretic peptide (NT-proBNP) (rs=0.28; all P<0.001 (Supplemental Tables 1 and 3). The 4D patients with higher galectin-3 concentrations more often were women, showed a higher prevalence of left ventricular hypertrophy and peripheral vascular disease, and had higher circulating concentrations of potassium, phosphate, hsCRP, and NT-proBNP. Correlation coefficients were highest for the correlation of galectin-3 with sex (rs=0.11), hsCRP (rs=0.15), and NT-proBNP (rs=0.19; all P<0.001 (Supplemental Tables 2 and 4).
Association of Galectin-3 with Clinical Outcomes in Different Strata of Impaired Kidney Function
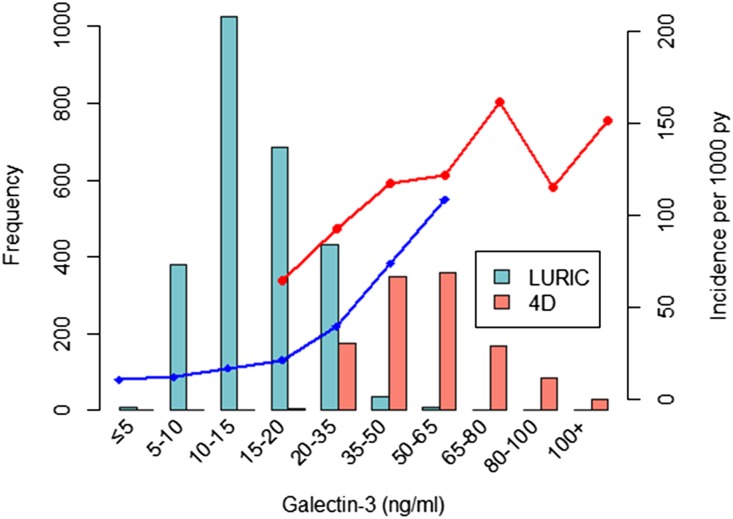
The incidence rate of cardiovascular outcomes was higher in 4D patients than in LURIC participants (Figure 2). This rate increased with rising galectin-3 concentrations in both cohorts, with the exception of a decrease in the group of 4D patients with galectin-3 concentrations of 80–100 ng/ml (Figure 2).
Figure 2.
Relationship between the incidence rate of fatal cardiovascular events (lines) and galectin-3 concentration (columns). py, person-years.
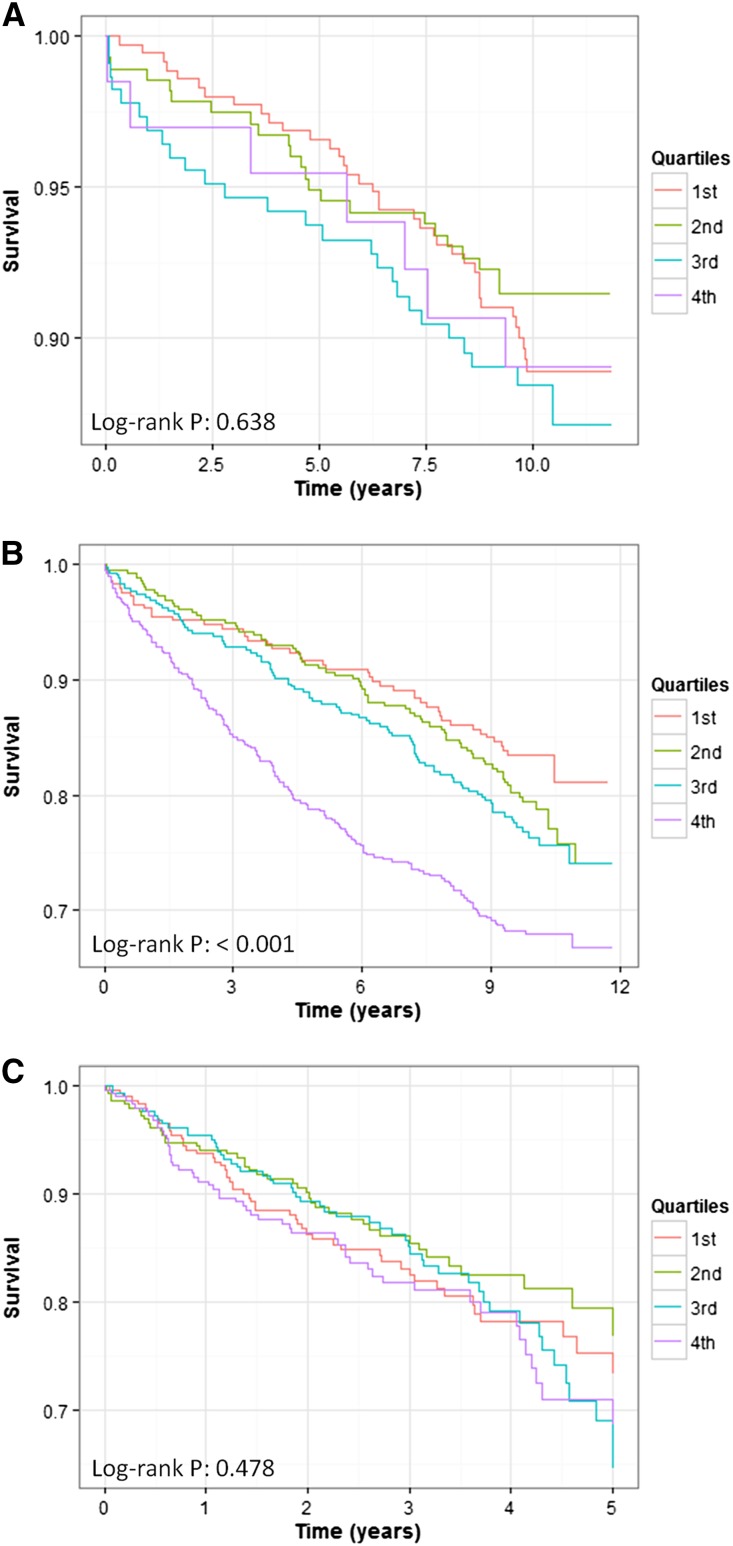
Galectin-3 concentrations at baseline were not significantly associated with any of the investigated end points in patients with normal kidney function (eGFR≥90 ml/min per 1.73 m2; Figure 3A, Supplemental Table 5). In patients with mildly reduced kidney function (eGFR 60–89 ml/min per 1.73 m2), galectin-3 concentrations were associated with all-cause mortality, cardiovascular mortality, SCD, and death due to infection in age- and sex-adjusted models (Figure 3B, Table 2). All-cause mortality and death due to infection remained significant after adjustment for additional risk factors, with hazard ratios (HRs) of 1.15 (95% confidence interval [95% CI], 1.04 to 1.28) and 1.49 (95% CI, 1.03 to 2.16), respectively, per SD increase in log-transformed galectin-3. In patients with eGFR<60 ml/min per 1.73 m2, galectin-3 concentrations were significantly associated with all-cause mortality, cardiovascular mortality, and death due to infection, with HRs of 1.22 (95% CI, 1.06 to 1.41), 1.21 (95% CI, 1.01 to 1.44), and 1.71 (95% CI, 1.08 to 2.70), respectively, in fully adjusted models. In 4D patients, log-transformed galectin-3 concentration was associated with cardiovascular events, stroke, and all-cause mortality; after adjustment for confounders, only the association with cardiovascular events remained significant, with a HR of 1.12 (95% CI, 1.01 to 1.24) (Figure 3C, Table 2).
Figure 3.
Survival analysis for patients with varying severity of renal impairment according to galectin-3 levels. Kaplan–Meier curves for fatal cardiovascular events according to quartiles of galectin-3 in LURIC patients with eGFR≥90 ml/min per 1.73 m2 (A), LURIC patients with an eGFR<90 ml/min per 1.73 m2 (B), and dialysis patients from the 4D study (C).
Table 2.
Association of galectin-3 concentration with clinical end points in different stages of renal disease
| Outcome | LURIC Study | 4D Study | |||||||
|---|---|---|---|---|---|---|---|---|---|
| eGFR≥90 | eGFR=60–89 | eGFR<60 | P Value for Trendc | Hemodialysis | |||||
| HR (95% CI) | P Valueb | HR (95% CI) | P Valueb | HR (95% CI) | P Valueb | HR (95% CI) | P Valueb | ||
| MIa | |||||||||
| Unadjusted | 1.09 (0.68 to 1.74) | 0.73 | 1.29 (0.94 to 1.76) | 0.11 | 1.64 (1.01 to 2.68) | 0.05 | 0.02 | 1.12 (0.97 to 1.30) | 0.12 |
| Model 1 | 1.05 (0.64 to 1.71) | 0.85 | 1.33 (0.96 to 1.84) | 0.09 | 1.71 (1.03 to 2.83) | 0.04 | 0.01 | 1.16 (0.99 to 1.35) | 0.06 |
| Model 2 | 0.93 (0.56 to 1.59) | 0.80 | 1.20 (0.86 to 1.67) | 0.28 | 1.61 (0.97 to 2.68) | 0.07 | 0.09 | 1.16 (0.99 to 1.35) | 0.06 |
| SCD | |||||||||
| Unadjusted | 0.97 (0.71 to 1.31) | 0.82 | 1.25 (1.03 to 1.51) | 0.03 | 1.05 (0.81 to 1.35) | 0.74 | 0.10 | 1.01 (0.82 to 1.20) | 0.87 |
| Model 1 | 0.92 (0.68 to 1.26) | 0.62 | 1.24 (1.02 to 1.51) | 0.04 | 1.10 (0.84 to 1.43) | 0.49 | 0.07 | 0.99 (0.83 to 1.17) | 0.99 |
| Model 2 | 0.88 (0.63 to 1.23) | 0.47 | 1.15 (0.94 to 1.40) | 0.17 | 1.05 (0.80 to 1.39) | 0.73 | 0.26 | 0.99 (0.83 to 1.18) | 0.89 |
| Death due to heart failure | |||||||||
| Unadjusted | 1.03 (0.63 to 1.68) | 0.91 | 1.15 (0.89 to 1.49) | 0.28 | 1.46 (1.05 to 2.04) | 0.02 | 0.03 | 1.26 (0.91 to 1.75) | 0.16 |
| Model 1 | 0.98 (0.59 to 1.62) | 0.92 | 1.10 (0.84 to 1.42) | 0.49 | 1.49 (1.06 to 2.09) | 0.02 | 0.03 | 1.28 (0.92 to 1.77) | 0.15 |
| Model 2 | 0.95 (0.58 to 1.54) | 0.83 | 1.01 (0.78 to 1.31) | 0.95 | 1.31 (0.91 to 1.88) | 0.15 | 0.19 | 1.25 (0.89 to 1.75) | 0.20 |
| Strokea | |||||||||
| Unadjusted | 1.29 (0.69 to 2.38) | 0.43 | 1.21 (0.78 to 1.83) | 0.37 | 1.42 (0.77 to 2.59) | 0.26 | 0.11 | 1.36 (1.11 to 1.67) | 0.003 |
| Model 1 | 1.20 (0.57 to 2.56) | 0.63 | 1.14 (0.75 to 1.75) | 0.54 | 1.47 (0.81 to 2.67) | 0.20 | 0.18 | 1.30 (1.04 to 1.61) | 0.02 |
| Model 2 | 1.26 (0.53 to 2.99) | 0.61 | 1.39 (0.74 to 1.73) | 0.58 | 1.37 (0.72 to 2.59) | 0.34 | 0.26 | 1.25 (0.99 to 1.57) | 0.06 |
| All-cause mortality | |||||||||
| Unadjusted | 1.12 (0.96 to 1.31) | 0.16 | 1.25 (1.13 to 1.38) | <0.001 | 1.23 (1.07 to 1.42) | 0.003 | <0.001 | 1.09 (1.00 to 1.18) | 0.05 |
| Model 1 | 1.05 (0.89 to 1.23) | 0.58 | 1.22 (1.10 to 1.36) | <0.001 | 1.30 (1.12 to 1.50) | <0.001 | <0.001 | 1.10 (1.00 to 1.20) | 0.04 |
| Model 2 | 0.99 (0.84 to 1.18) | 0.95 | 1.15 (1.04 to 1.28) | 0.01 | 1.22 (1.05 to 1.41) | 0.01 | <0.001 | 1.07 (0.98 to 1.17) | 0.13 |
| Cardiovascular eventsa | |||||||||
| Unadjusted | 1.07 (0.87 to 1.32) | 0.52 | 1.21 (1.06 to 1.37) | 0.004 | 1.21 (1.02 to 1.43) | 0.03 | <0.001 | 1.13 (1.03 to 1.24) | 0.01 |
| Model 1 | 1.01 (0.81 to 1.25) | 0.95 | 1.19 (1.04 to 1.35) | 0.01 | 1.25 (1.05 to 1.48) | 0.01 | <0.001 | 1.12 (1.01 to 1.23) | 0.03 |
| Model 2 | 0.96 (0.76 to 1.20) | 0.70 | 1.10 (0.97 to 1.25) | 0.16 | 1.21 (1.01 to 1.44) | 0.04 | 0.02 | 1.12 (1.01 to 1.24) | 0.03 |
| Death due to infection | |||||||||
| Unadjusted | 2.14 (0.83 to 5.51) | 0.12 | 1.65 (1.15 to 2.36) | 0.01 | 1.63 (1.06 to 2.48) | 0.03 | <0.001 | 1.19 (0.99 to 1.44) | 0.06 |
| Model 1 | 1.79 (0.64 to 5.00) | 0.27 | 1.67 (1.14 to 2.44) | 0.01 | 1.75 (1.13 to 2.73) | 0.01 | <0.001 | 1.20 (0.99 to 1.46) | 0.06 |
| Model 2 | 2.27 (0.70 to 7.36) | 0.17 | 1.49 (1.03 to 2.16) | 0.04 | 1.71 (1.08 to 2.70) | 0.02 | <0.001 | 1.14 (0.93 to 1.39) | 0.20 |
eGFR values are given in ml/min per 1.73 m2. Model 1 was adjusted for age and sex. Model 2 was adjusted for age and sex, with additional adjustments for smoking status, systolic BP, body mass index, LDL cholesterol, lipid-lowering therapy (LURIC) or atorvastatin treatment (4D), diabetes mellitus (LURIC) or duration of diabetes mellitus (4D), triglycerides, and C-reactive protein.
Fatal and nonfatal events in 4D, and only fatal events in LURIC.
After Bonferroni correction for 4×7=28 tests, a P value of <0.002 would be regarded as significant.
P for trend represents the comparison across the three eGFR categories (≥90 ml/min per 1.73 m2, 60–89 ml/min 1.73 m2, and <60 ml/min per 1.73 m2).
In an exploratory analysis, we further subdivided LURIC subjects into four eGFR strata (Supplemental Tables 6 and 7) in an effort to better define an eGFR range in which galectin-3 has maximal predictive power. The P value for the trend revealed that galectin-3 was more predictive when eGFR decreased for all-cause mortality, cardiovascular events, and death due to infection (Supplementary Table 7). Furthermore, we created hazard plots to better visualize the relationship between galectin-3 and outcomes (Supplemental Figure 1). In the LURIC study, the lower 95% CI bound passed the reference log hazard value of zero when galectin-3 concentrations exceeded 20.4–21.9 ng/ml (Supplemental Figure 1A); the CI bound remained larger than zero for higher concentrations (P=0.02). Furthermore, the log hazard was nearly constant between 12.0 and 17.2 ng/ml and between 24.7 and 32.2 ng/ml. In the 4D study, galectin-3 concentrations above approximately 32 ng/ml appeared to have no additional significant predictive value for cardiovascular mortality (Supplemental Figure 1B).
Notably, 4D patients all had diabetes mellitus and were at extreme risk for cardiovascular events; the LURIC study consisted of patients with considerably lower risk. We conducted additional sensitivity analyses and stratified patients by the prevalence of coronary artery disease (CAD) and diabetes mellitus in the LURIC study. The presence or absence of these conditions did not substantially affect the predictive value of galectin-3 concentration, although infection-related mortality was strongly predicted in patients with diabetes mellitus, in both the LURIC and 4D studies (Supplemental Tables 8 and 9).
Finally, we performed sensitivity analyses using an alternative approach without stratification. In these analyses, we investigated the association of galectin-3 concentrations with clinical outcomes in the LURIC cohort, adjusting for potential confounders as well as for eGFR. In the fully adjusted model, galectin-3 was significantly associated with all-cause mortality (HR, 1.15; 95% CI, 1.05 to 1.25) and death due to infection (HR, 1.46; 95% CI, 1.06 to 2.01). There was a borderline association with cardiovascular mortality (HR, 1.10; 95% CI, 0.98 to 1.22) (Supplemental Table 10).
Discussion
This investigation is an analysis of two large studies of the relationship among galectin-3 concentration, renal function, and outcomes in patients over the entire spectrum of kidney function, ranging from normal in the LURIC study to patients with ESRD in the 4D study. The main findings of this study are (1) that circulating galectin-3 concentrations are inversely related to kidney function, exceeding the reference range by as much as 4- to 5-fold in dialysis patients, and (2) that circulating galectin-3 concentrations are associated with clinical outcomes only in patients with impaired kidney function.
Galectin-3, Kidney (Dys)Function, and Outcomes
Although there have been studies describing the strong relation of galectin-3 levels with kidney function,14,15 galectin-3 concentrations in renal disease have not been reported. We observed that galectin-3 concentrations were strongly and inversely correlated with eGFR (Supplemental Tables 1 and 3), and were further elevated in patients on dialysis: the mean galectin-3 concentration was 54 ng/ml, whereas the mean concentrations in the general population are approximately 11–14 ng/ml,13,14,28 rising to approximately 15–25 ng/ml in CHF.11,12,15,16,29 Various reasons may underlie this marked elevation. In previous analyses of CHF, adding renal function to regression models substantially compromised the prognostic power of galectin-3.11,12,15,16 It is possible that galectin-3 is, at least in part, handled or cleared by the kidney.
Within the kidney, galectin-3 is found in the ureteric bud, in the collecting ducts,17,18 and in tubules.19 In models of renal damage, galectin-3 expression is rapidly upregulated.9,20 Renal galectin-3 is mainly produced and secreted by inflammatory cells and macrophages. Galectin-3’s binding affinity is also modulated by pH,21 which is known to vary substantially within the kidney. Galectin-3 exerts multiple and sometimes even opposite effects. For example, galectin-3 disruption accelerated renal fibrosis in a murine model of diabetic nephropathy,8 whereas it reduced renal fibrosis in a murine model of unilateral ureter obstruction.9 It is therefore currently not clear whether renal galectin-3 participates in tissue repair or promotes kidney damage. On the other hand, elevated circulating levels of galectin-3 have unequivocally been associated with poor outcome in various disease states, leading us to hypothesize that increased galectin-3 levels would be associated with adverse clinical profiles and adverse outcomes.
Our most striking finding was that galectin-3 concentrations seemed to confer variable prognostic information depending on the severity of the patient’s renal impairment. In the LURIC study, galectin-3 was not associated with long-term outcomes as long as the eGFR was ≥90 ml/min per 1.73 m2. However, galectin-3 concentration was significantly associated with the clinical end points of all-cause mortality, cardiovascular mortality, death due to infection, and SCD in patients with a lower eGFR of 60–89 ml/min per 1.73 m2. In patients with an eGFR<60 ml/min per 1.73 m2, galectin-3 concentration was furthermore associated with MI and death due to CHF. It has been reported that the prognostic value of galectin-3 is attenuated when corrected for eGFR,15 one of the main motivations for this study. In our analyses, we did not include eGFR as a covariate in the models of the different eGFR strata, but instead we divided the entire LURIC cohort into subgroups according to eGFR, which in our opinion provides better insight in the relationship among eGFR, galectin-3 levels, and outcomes (Figure 2).
Galectin-3 in Dialysis: Cardiovascular and Infectious Adverse Events
In the 4D study, galectin-3 concentrations predicted all-cause mortality, cardiovascular events, stroke, and death due to infection. By contrast, the incidence rates of MI and SCD were not significantly related to galectin-3 concentrations, although SCD was frequent.22 We hypothesized that galectin-3 would be associated with MI, because galectin-3 expression has been detected in atherosclerotic plaques.23 However, the role for galectin-3 in atherosclerosis may be ambiguous; some authors have suggested that galectin-3 attenuates the progression of atherosclerosis,24 whereas others have suggested the opposite.25
Interestingly, we observed a relevant increase in deaths due to infectious diseases at increasing galectin-3 levels, both in 4D patients and in the LURIC study (most strikingly in LURIC participants with diabetes mellitus). There is ample evidence that galectin-3 is important in infection and in the inflammatory response,26 involving mast cells, neutrophils, monocytes, and T cells. Mice that lack the gene encoding galectin-3 have an impaired inflammatory response.7 In a rat model of experimental GN, galectin-3 has been shown to modulate mesangial cell proliferation and matrix synthesis.27 On the basis of these experimental observations, galectin-3 may promote inflammation and infection; our current observations support this hypothesis.
Potential Clinical Implications
Increased galectin-3 levels have been associated with adverse clinical outcomes in the general population (the Prevention of Renal and Vascular Endstage Disease study14 and the Framingham Heart Study13,28) and in patients with heart failure (the ProBNP Investigation of Dyspnea in the Emergency Department study29 and the Coordinating Study Evaluating Outcomes of Advising and Counseling in Heart Failure11), and we now extend this association to patients with renal disease. Our study shows that galectin-3 may be used to risk-stratify patients with renal disease across the spectrum of renal impairment severity. However, in view of the lack of a “gold standard” for risk stratification in renal disease, it is difficult to judge whether galectin-3 is the best option for risk stratification in renal patients.
Whether patients with elevated galectin-3 levels would benefit more from specific therapy should be studied. Interestingly, the Framingham study demonstrated that elevated galectin-3 levels precede the development of CKD,13 and several other risk factors that are amenable to intervention were correlated with galectin-3. Finally, galectin-3 may not just be a marker of disease, like troponin or NT-proBNP, but may also be a target for therapy, as substantiated in experimental studies.10 Importantly, very recent results of a phase II study comparing treatment with the galectin-3 inhibitor GCS-100 versus placebo in patients with CKD stage 3b suggested that treatment significantly improved eGFR compared with placebo (http://ljpc.com/la-jolla-pharmaceutical-company-reports-positive-top-line-results-from-phase-2-clinical-trial-of-gcs-100-in-chronic-kidney-disease). Galectin-3 may be helpful in assessing prognosis, guiding existing therapy, and perhaps even prompting the initiation of a specific anti–galectin-3 therapy. Prospective studies are underway to address these questions.
Limitations and Strengths
Potential limitations of the study need to be acknowledged. We utilized a post hoc analysis within two selected cohorts of German patients: the LURIC study (with a large percentage of patients with angiographically diagnosed CAD) and the 4D study (including patients suffering from type 2 diabetes mellitus undergoing dialysis). Therefore, the relationship between high galectin-3 levels and adverse outcome may not be generalizable to other patient populations. In our study, residual confounding factors cannot be excluded. Proteinuria was not measured; therefore, the presented data on renal impairment were confined to eGFR. However, the literature suggests no meaningful role of proteinuria in the association between galectin-3 and mortality risk, indicating that residual confounding as a result of proteinuria is likely to be small in this study.14 We did not have urine samples to assess residual renal function in 4D patients, and no other measurements of fibrosis were available. We thus cannot determine the proportions of circulating galectin-3 originating from the kidney, heart, or elsewhere. Furthermore, in this study, we obviously had no insight into tissue or cellular concentrations of galectin-3, and we used circulating galectin-3 concentration as a proxy for total galectin-3 concentration.
The main strengths of this study were the specific outcomes analyzed in two independent cohorts. In this context, the long-term follow-up and high incidence of prespecified and centrally adjudicated end points should be highlighted.
Circulating galectin-3 concentrations increase in parallel with decreasing kidney function and are markedly elevated in dialysis patients with type 2 diabetes mellitus. Galectin-3 is significantly associated with clinical end points, but only in patients with reduced kidney function. These observations provide novel insight into the relationships among galectin-3 levels, impaired kidney function, and renal disease. The precise role of galectin-3 in the pathophysiology of kidney disease and its complications, however, warrant further study.
Concise Methods
Study Design and Participants
The methodology and results of the LURIC and 4D studies were previously reported in detail.30–32 Briefly, the LURIC study consists of 3316 Caucasian patients hospitalized for coronary angiography between 1997 and 2000 at a tertiary care center in Southwestern Germany (Heart Center Ludwigshafen). Clinical indications for angiography were chest pain or a positive noninvasive stress test suggestive of myocardial ischemia. Mean follow-up for overall mortality and cause-specific mortality was 10 years. Galectin-3 values were available for 2578 study participants. The LURIC study was approved by the ethics committee at the Ärztekammer Rheinland-Pfalz and was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participants.
The 4D study was a prospective, randomized controlled trial investigating atorvastatin in 1255 patients with type 2 diabetes mellitus, aged 18–80 years and on dialysis for <2 years. Between March 1998 and October 2002, patients were recruited at 178 dialysis centers in Germany. Mean follow-up was 4 years, during which clinical information, including adverse events, was collected. Blood samples were taken before the start of dialysis sessions and the administration of drugs. Serum was available for the current analysis from 1168 of 1255 patients. The 4D study was approved by the medical ethics committees, and all patients gave written informed consent before inclusion.
Clinical End Points
For the LURIC study, information on vital status was obtained from local registries. Death certificates, medical records from local hospitals, and autopsy data were reviewed independently by two experienced clinicians who were blinded to patient characteristics and who classified the causes of death. In the case of disagreement, the cause of death was decided by a principal investigator of the study. Cardiovascular death included the following categories: SCD, fatal MI, death due to CHF, death immediately after intervention to treat CAD, fatal stroke, and other causes of death due to CAD.
The primary end point of the 4D study was cardiovascular events (cardiovascular end point) defined as a composite of cardiac death including SCD, nonfatal MI, or stroke, whichever occurred first. The 4D study end points were centrally adjudicated according to predefined criteria. CHF was defined according to the New York Heart Association classification system. Adjudication was performed by three members of the end point committee, who were blinded to study treatment. For this analysis, SCD, stroke, MI, cardiovascular end point, death due to infection, death due to CHF, and all-cause mortality were chosen to be separate outcome measures and were based on the primary judgment of the end point committee during the 4D study.
Clinical Definitions
In the LURIC study, CAD was defined angiographically using the maximum luminal narrowing estimated by visual analysis. CAD was defined as the presence of a visible luminal narrowing (>20% stenosis) in at least 1 of 15 coronary segments according to the classification of the American Heart Association. Diabetes mellitus was defined according to American Diabetes Association 2010 guidelines as increased fasting glucose concentrations (>126 mg/dl) and/or postchallenge glucose concentrations (2 hours after the 75 g glucose load >200 mg/dl) and/or elevated glycated hemoglobin concentrations (>6.5%) and/or history of diabetes mellitus. Hypertension was defined as systolic and/or diastolic BP>140 mmHg and/or >90 mmHg or a significant history of hypertension. eGFR was estimated using the 2012 Chronic Kidney Disease Epidemiology Collaboration eGFRcreat-cys equation, as previously described.33 Patients were stratified into categories by eGFR according to recent Kidney Disease Improving Global Outcomes guidelines.34 In the 4D study, CAD was defined by a history of MI, coronary artery bypass grafting surgery, percutaneous coronary intervention, and the presence of CAD, as documented by angiography. BP was measured in sitting position. Body mass index was calculated as weight (in kilograms) divided by height (in meters) squared.
Galectin-3 Measurement
In the 4D study, galectin-3 concentrations were measured in serum samples taken at baseline and stored at −80°C until analysis. Galectin-3 concentration was determined using an ELISA developed by BG Medicine (Waltham, MA).35 This assay quantitatively measures the concentration of human galectin-3 in ethylenediaminetetraacetic acid–stabilized plasma or in serum; it has a high sensitivity (lower limit of detection 1.13 ng/ml; intra-assay variability 3.2%; interassay variability 5.6%) and exhibits no cross-reactivity with collagens or other members of the galectin family of proteins. Commonly used cardiovascular medications such as angiotensin-converting enzyme inhibitors, β-blockers, spironolactone, furosemide, acetylsalicylic acid, warfarin, coumarin, and digoxin do not interfere with the assay.35
In the LURIC study, galectin-3 concentration was measured in plasma samples taken at baseline and stored at −80°C until analysis on an ARCHITECT analyzer (Abbott Diagnostics, Abbott Park, IL) using the same antibodies and the same conjugate as used in the manual ELISA, with a lower limit of detection of 1.01 ng/ml and intra-assay and interassay variabilities of 3.2% and 0.8%, respectively.36 This assay yields identical results with serum or plasma, when either the manual or the automated procedure is used.35,36
Statistical Analyses
Patient characteristics are presented according to decreasing eGFR in the LURIC study and for ESRD patients in the 4D study (Table 1). Normally distributed variables are expressed as means±SD. Non-normally distributed variables are presented as medians with interquartile ranges.
The association of galectin-3 with clinical outcomes was assessed via Cox regression analyses. HRs and corresponding 95% CIs were determined. Sequential models were fitted, first including no covariates (unadjusted) and then stepwise adjusting for multiple variables. The first model was adjusted for age and sex. The second model was additionally adjusted for the established cardiovascular risk factors of smoking status, systolic BP, body mass index, LDL cholesterol, lipid-lowering therapy (LURIC) or atorvastatin treatment (4D), diabetes mellitus (LURIC) or duration of diabetes mellitus (4D), triglycerides, and hsCRP. P values for trends were calculated via stratified Cox regression analysis. To visualize the relation between galectin-3 levels and fatal cardiovascular events, we created hazard plots after correction for cardiovascular risk factors.
All reported probability values are two tailed, and P<0.05 was considered statistically significant. Analyses were performed using SPSS (version 20.0) and R (version 3.0.2; http://www.R-project.org) software.
Disclosures
BG Medicine and Abbott Laboratories provided kits for these studies. The University Medical Center Groningen, which employs W.H.v.G., P.v.d.H., and R.A.d.B., has received research support from BG Medicine and Abbott Laboratories. R.A.d.B. consulted for Abbott Laboratories and BG Medicine.
Acknowledgments
We thank Martin Dokter for expert technical assistance.
This work was also supported by grants from the German Federal Ministry for Education and Research (BMBF01EO1004). The LURIC study has received funding from the German ministry for education and research, AtheroSysMed project (systems medicine of coronary herat disease and stroke), grant 01ZX1313AKroed; the European Union Sixth Framework Program (Bloodomics integrated project, grant LSHM-CT-2004-503485) and Seventh Framework Program (Atheroremo, grant agreement 201668; and RiskyCAD, grant agreement 305739), as well as from the INTERREG IV Oberrhein Program (Project A28, Genetic Mechanisms of Cardiovascular Diseases), with support from the European Regional Development Fund and the Wissenschaftsoffensive TMO. C.D. was supported by a research fellowship (Habilitationsstipendium) from the Medical Faculty of the University of Würzburg. R.A.d.B. is supported by the Netherlands Heart Foundation (grant 2007T046) and the Netherlands Organization for Scientific Research (NWO VIDI, grant 917.13.350).
An abstract of the work was presented at the 2014 European Renal Association-European Dialysis and Transplant Association Congress in Amsterdam, The Netherlands, on May 31–June 3, 2014.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014010093/-/DCSupplemental.
References
- 1.Liu FT, Hsu DK, Zuberi RI, Kuwabara I, Chi EY, Henderson WR, Jr: Expression and function of galectin-3, a beta-galactoside-binding lectin, in human monocytes and macrophages. Am J Pathol 147: 1016–1028, 1995 [PMC free article] [PubMed] [Google Scholar]
- 2.Yang RY, Rabinovich GA, Liu FT: Galectins: Structure, function and therapeutic potential. Expert Rev Mol Med 10: e17, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Kim H, Lee J, Hyun JW, Park JW, Joo HG, Shin T: Expression and immunohistochemical localization of galectin-3 in various mouse tissues. Cell Biol Int 31: 655–662, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Vasta GR: Galectins as pattern recognition receptors: Structure, function, and evolution. Adv Exp Med Biol 946: 21–36, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Boer RA, Voors AA, Muntendam P, van Gilst WH, van Veldhuisen DJ: Galectin-3: A novel mediator of heart failure development and progression. Eur J Heart Fail 11: 811–817, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Liu FT, Rabinovich GA: Galectins as modulators of tumour progression. Nat Rev Cancer 5: 29–41, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Rabinovich GA, Liu FT, Hirashima M, Anderson A: An emerging role for galectins in tuning the immune response: Lessons from experimental models of inflammatory disease, autoimmunity and cancer. Scand J Immunol 66: 143–158, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Iacobini C, Amadio L, Oddi G, Ricci C, Barsotti P, Missori S, Sorcini M, Di Mario U, Pricci F, Pugliese G: Role of galectin-3 in diabetic nephropathy. J Am Soc Nephrol 14[Suppl 3]: S264–S270, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Henderson NC, Mackinnon AC, Farnworth SL, Kipari T, Haslett C, Iredale JP, Liu FT, Hughes J, Sethi T: Galectin-3 expression and secretion links macrophages to the promotion of renal fibrosis. Am J Pathol 172: 288–298, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kolatsi-Joannou M, Price KL, Winyard PJ, Long DA: Modified citrus pectin reduces galectin-3 expression and disease severity in experimental acute kidney injury. PLoS ONE 6: e18683, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Boer RA, Lok DJ, Jaarsma T, van der Meer P, Voors AA, Hillege HL, van Veldhuisen DJ: Predictive value of plasma galectin-3 levels in heart failure with reduced and preserved ejection fraction. Ann Med 43: 60–68, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lok DJ, Van Der Meer P, de la Porte PW, Lipsic E, Van Wijngaarden J, Hillege HL, van Veldhuisen DJ: Prognostic value of galectin-3, a novel marker of fibrosis, in patients with chronic heart failure: Data from the DEAL-HF study. Clin Res Cardiol 99: 323–328, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Seaghdha CM, Hwang SJ, Ho JE, Vasan RS, Levy D, Fox CS: Elevated galectin-3 precedes the development of CKD. J Am Soc Nephrol 24: 1470–1477, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Boer RA, van Veldhuisen DJ, Gansevoort RT, Muller Kobold AC, van Gilst WH, Hillege HL, Bakker SJ, van der Harst P: The fibrosis marker galectin-3 and outcome in the general population. J Intern Med 272: 55–64, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Gopal DM, Kommineni M, Ayalon N, Koelbl C, Ayalon R, Biolo A, Dember LM, Downing J, Siwik DA, Liang CS, Colucci WS: Relationship of plasma galectin-3 to renal function in patients with heart failure: Effects of clinical status, pathophysiology of heart failure, and presence or absence of heart failure. J Am Heart Assoc 1: e000760, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang WH, Shrestha K, Shao Z, Borowski AG, Troughton RW, Thomas JD, Klein AL: Usefulness of plasma galectin-3 levels in systolic heart failure to predict renal insufficiency and survival. Am J Cardiol 108: 385–390, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bullock SL, Johnson TM, Bao Q, Hughes RC, Winyard PJ, Woolf AS: Galectin-3 modulates ureteric bud branching in organ culture of the developing mouse kidney. J Am Soc Nephrol 12: 515–523, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Winyard PJ, Bao Q, Hughes RC, Woolf AS: Epithelial galectin-3 during human nephrogenesis and childhood cystic diseases. J Am Soc Nephrol 8: 1647–1657, 1997 [DOI] [PubMed] [Google Scholar]
- 19.Okamura DM, Pasichnyk K, Lopez-Guisa JM, Collins S, Hsu DK, Liu FT, Eddy AA: Galectin-3 preserves renal tubules and modulates extracellular matrix remodeling in progressive fibrosis. Am J Physiol Renal Physiol 300: F245–F253, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishiyama J, Kobayashi S, Ishida A, Nakabayashi I, Tajima O, Miura S, Katayama M, Nogami H: Up-regulation of galectin-3 in acute renal failure of the rat. Am J Pathol 157: 815–823, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.von Mach T, Carlsson MC, Straube T, Nilsson U, Leffler H, Jacob R: Ligand binding and complex formation of galectin-3 is modulated by pH variations. Biochem J 457: 107–115, 2014 [DOI] [PubMed] [Google Scholar]
- 22.Shamseddin MK, Parfrey PS: Sudden cardiac death in chronic kidney disease: Epidemiology and prevention. Nat Rev Nephrol 7: 145–154, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Nachtigal M, Al-Assaad Z, Mayer EP, Kim K, Monsigny M: Galectin-3 expression in human atherosclerotic lesions. Am J Pathol 152: 1199–1208, 1998 [PMC free article] [PubMed] [Google Scholar]
- 24.Iacobini C, Menini S, Ricci C, Scipioni A, Sansoni V, Cordone S, Taurino M, Serino M, Marano G, Federici M, Pricci F, Pugliese G: Accelerated lipid-induced atherogenesis in galectin-3-deficient mice: Role of lipoxidation via receptor-mediated mechanisms. Arterioscler Thromb Vasc Biol 29: 831–836, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Nachtigal M, Ghaffar A, Mayer EP: Galectin-3 gene inactivation reduces atherosclerotic lesions and adventitial inflammation in ApoE-deficient mice. Am J Pathol 172: 247–255, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeon SB, Yoon HJ, Chang CY, Koh HS, Jeon SH, Park EJ: Galectin-3 exerts cytokine-like regulatory actions through the JAK-STAT pathway. J Immunol 185: 7037–7046, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Sasaki S, Bao Q, Hughes RC: Galectin-3 modulates rat mesangial cell proliferation and matrix synthesis during experimental glomerulonephritis induced by anti-Thy1.1 antibodies. J Pathol 187: 481–489, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Ho JE, Liu C, Lyass A, Courchesne P, Pencina MJ, Vasan RS, Larson MG, Levy D: Galectin-3, a marker of cardiac fibrosis, predicts incident heart failure in the community. J Am Coll Cardiol 60: 1249–1256, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Kimmenade RR, Januzzi JL, Jr, Ellinor PT, Sharma UC, Bakker JA, Low AF, Martinez A, Crijns HJ, MacRae CA, Menheere PP, Pinto YM: Utility of amino-terminal pro-brain natriuretic peptide, galectin-3, and apelin for the evaluation of patients with acute heart failure. J Am Coll Cardiol 48: 1217–1224, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Wanner C, Krane V, März W, Olschewski M, Asmus HG, Krämer W, Kühn KW, Kütemeyer H, Mann JF, Ruf G, Ritz E, Deutsche Diabetes-Dialyse-Studie (4D) Study Group : Randomized controlled trial on the efficacy and safety of atorvastatin in patients with type 2 diabetes on hemodialysis (4D study): Demographic and baseline characteristics. Kidney Blood Press Res 27: 259–266, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Wanner C, Krane V, März W, Olschewski M, Mann JF, Ruf G, Ritz E, German Diabetes and Dialysis Study Investigators : Atorvastatin in patients with type 2 diabetes undergoing hemodialysis. N Engl J Med 353: 238–248, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Winkelmann BR, März W, Boehm BO, Zotz R, Hager J, Hellstern P, Senges J, LURIC Study Group (LUdwigshafen RIsk and Cardiovascular Health) : Rationale and design of the LURIC study—a resource for functional genomics, pharmacogenomics and long-term prognosis of cardiovascular disease. Pharmacogenomics 2[Suppl 1]: S1–S73, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS, CKD-EPI Investigators : Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 367: 20–29, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.National Kidney Foundation : KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl 3: 1–150, 2013 [DOI] [PubMed] [Google Scholar]
- 35.Christenson RH, Duh SH, Wu AH, Smith A, Abel G, deFilippi CR, Wang S, Adourian A, Adiletto C, Gardiner P: Multi-center determination of galectin-3 assay performance characteristics: Anatomy of a novel assay for use in heart failure. Clin Biochem 43: 683–690, 2010 [DOI] [PubMed] [Google Scholar]
- 36.La’ulu SL, Apple FS, Murakami MM, Ler R, Roberts WL, Straseski JA: Performance characteristics of the ARCHITECT Galectin-3 assay. Clin Biochem 46: 119–122, 2013 [DOI] [PubMed] [Google Scholar]