Abstract
Lipid abnormalities may have an effect on clinical outcomes of patients on dialysis. Recent studies have indicated that HDL dysfunction is a hallmark of ESRD. In this study, we compared HDL composition and metrics of HDL functionality in patients undergoing hemodialysis (HD) or peritoneal dialysis (PD) with those in healthy controls. We detected a marked suppression of several metrics of HDL functionality in patients on HD or PD. Compositional analysis revealed that HDL from both dialysis groups shifted toward a more proinflammatory phenotype with profound alterations in the lipid moiety and protein composition. With regard to function, cholesterol efflux and anti-inflammatory and antiapoptotic functions seemed to be more severely suppressed in patients on HD, whereas HDL-associated paraoxonase activity was lowest in patients on PD. Quantification of enzyme activities involved in HDL metabolism suggested that HDL particle maturation and remodeling are altered in patients on HD or PD. In summary, our study provides mechanistic insights into the formation of dysfunctional HDL in patients with ESRD who are on HD or PD.
Keywords: hemodialysis, lipids, peritoneal dialysis, cardiovascular disease
ESRD is associated with accelerated cardiovascular disease and high mortality. Mortality rates remain above 20% per year with the use of dialysis, with more than one half of the deaths related to cardiovascular disease.1 The effect of dialysis on metabolism differs between modalities; whereas hemodialysis (HD) is associated with a higher prevalence of hypotension and infectious complications, patients on peritoneal dialysis (PD) face a continuous glucose load.2,3 However, the effect of those metabolic changes on HDL functionality remains largely unknown.
In the general population, HDL cholesterol is associated with reduced cardiovascular events.4 Recent studies provided clear evidence of a lack of association between higher HDL cholesterol levels and lower cardiovascular risk in patients with ESRD.5,6 These studies clearly support the concept of dysfunctional HDL formation in CKD and provide additional evidence that the functionality of HDL might be of crucial importance with regard to cardiovascular protection.
HDL itself is a complex particle with numerous potential atheroprotective activities, including reverse cholesterol transport, inhibition of LDL oxidation, cytokine secretion from macrophages, adhesion molecule expression on endothelial cells, and stimulation of endothelial nitric oxide synthase to promote vasodilation.7 Profound alterations in HDL composition have been reported in patients with ESRD on HD.8–11 We and others have provided evidence that uremic HDL is less effective in promoting cholesterol efflux and shows defective antioxidative and anti-inflammatory capabilities.9–17 The composition of uremic HDL was found to shift toward a proinflammatory phenotype, which was observed to negatively affect cholesterol efflux properties, cytokine production, and adhesion molecule expression on monocytes and endothelial cells.7,9,10,18 Those observations led to the conclusion that specific uremia-induced compositional changes in the lipid and protein moiety render HDL dysfunctional or even proinflammatory. Here, we investigated alterations in HDL composition and metrics of HDL functionality in patients undergoing HD or PD.
Results
Clinical Characteristics of Study Subjects
Patient characteristics were comparable between groups, and all study participants were not patients with diabetes and free of lipid-lowering therapy (Table 1, Supplemental Table 1). The lipid profiles of patients with ESRD displayed increased triglycerides and decreased HDL cholesterol levels, whereas total cholesterol was unaltered in patients on HD and moderately reduced in patients on PD (Table 1). Concomitant treatments were equal between patients on dialysis (Supplemental Table 1). C-reactive protein levels were increased in both dialysis groups and highest in patients on HD (Table 1).
Table 1.
Clinical characteristics of study subjects
| Characteristics | Control | HD | PD |
|---|---|---|---|
| n | 20 | 24 | 14 |
| Age (yr) | 65±7.2 | 59±16.2 | 57±17.5 |
| Men, n (%) | 10 (50) | 12 (50) | 7 (50) |
| Dialysis vintage (yr) | — | 4.4±4.0 | 2.7±2.4 |
| Kt/V | — | 1.51±0.26 | 2.29±0.59 |
| Creatinine (mg/dl) | 0.9±0.2 | 7.6±2.2a | 8.2±2.9a |
| Urea (mg/dl) | 27±8 | 99±27b | 113±38b |
| Urine volume (ml/24 h) | — | 503±596 | 979±658 |
| C-reactive protein (mg/L) | 1.08±2.11 | 10.4±6.5a | 4.5±8.9 |
| Total cholesterol (mg/dl) | 219±33 | 191±72b | 178±38b |
| Free cholesterol (mg/dl) | 63±9 | 63±22 | 56±13 |
| Cholesterylester (mg/dl) | 156±25 | 129±52b | 123±27b |
| Triglycerides (mg/dl) | 120±46 | 196±13b | 170±92 |
| Phospholipids (mg/dl) | 249±30 | 218±52b | 209±32a |
| HDL cholesterol (mg/dl) | 57±15 | 38±13a | 42±15a |
| LDL cholesterol (mg/dl) | 139±32 | 114±55 | 103±38 |
Results are given as means±SDs. Kt/V values represent single pool for HD and weekly values for PD.
Significance from the Kruskal–Wallis with Dunn post hoc test was accepted at the level of 0.01 versus control.
Significance from the Kruskal–Wallis with Dunn post hoc test was accepted at the level of 0.05 versus control.
HDL Composition Is Altered in Patients on HD or PD
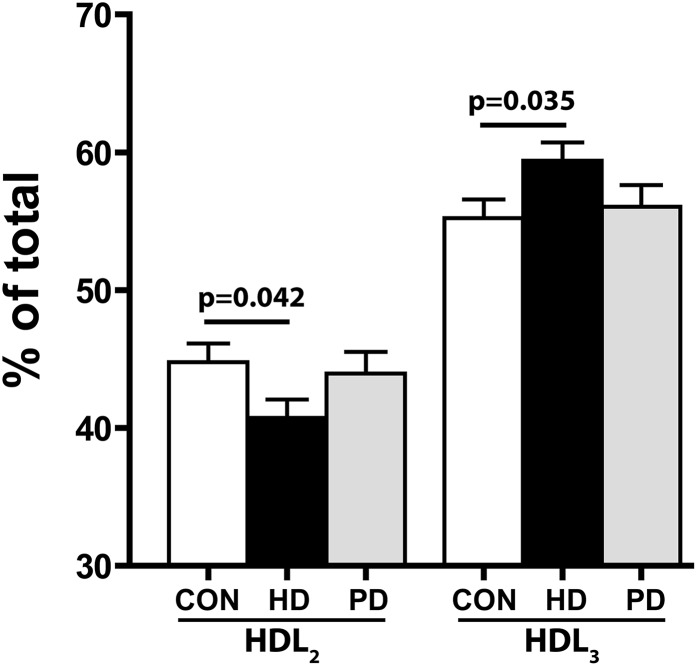
HDL was isolated from study subjects by one-step density ultracentrifugation and subjected to compositional analysis. HDL from patients on HD or PD showed significantly lower levels of cholesterylester and phospholipids and higher levels of triglycerides (Table 2, lipids). The ratio between phospholipid and sphingomyelin, a marker of surface rigidity, was lower in HD HDL (Table 2, lipids). With regard to protein composition, our analysis indicates that HDL from both dialysis groups showed lower amounts of apoA-I, apoA-II, and paraoxonase 1 (Table 2, proteins). ApoC-II, apoC-III, lipoprotein-associated phospholipase A2 (Lp-PLA2), and serum amyloid A (SAA) levels seem to be most profoundly elevated in HD HDL, whereas moderate increases were observed in PD HDL (Table 2, proteins). Our compositional analysis suggested a shift toward a proinflammatory composition in both dialysis groups, with similar alterations in the lipid moiety and more profound alterations in the protein moiety of HD HDL. Moreover, native gel analysis revealed that HD HDL shifts toward the smaller HDL3 subclass, whereas the subclass distribution was unaltered in patients on PD (Figure 1).
Table 2.
HDL composition
| Composition | Controls | HD | PD |
|---|---|---|---|
| Lipids | |||
| TC (µg/mg protein) | 216±35 | 192±37 | 192±39 |
| CE (µg/mg protein) | 164±27 | 144±24a | 144±26a |
| FC (µg/mg protein) | 52.9±10.1 | 48.5±15.1 | 47.9±13.6 |
| TG (µg/mg protein) | 39.4±5.0 | 65.9±11.8b | 69.2±11.5b |
| PL (µg/mg protein) | 423±58 | 366±50b | 359±51b |
| SM (µg/mg protein) | 52.2±9.6 | 52.9±12.2 | 50.5±13.0 |
| S1P (ng/mg protein) | 249±16 | 247±44 | 265±32 |
| PL/SM ratio | 8.5±2.7 | 7.1±1.2a | 7.5±2.0 |
| Proteins | |||
| ApoA-I (µg/mg protein) | 560±48 | 442±34b | 461±38b |
| ApoA-II (µg/mg protein) | 158±21 | 125±27b | 136±30b |
| ApoC-II (µg/mg protein) | 5.2±2.6 | 9.3±3.5a | 6.5±2.7 |
| ApoC-III (µg/mg protein) | 23.9±8.0 | 41.2±13.6b | 32.7±11.3a |
| ApoE (µg/mg protein) | 12.3±4.7 | 13.6±7.1 | 9.3±5.0 |
| SAA (µg/mg protein) | 6.8±8.4 | 17.6±12.7b | 9.9±11.0 |
| PON (relative index) | 1.00±0.50 | 0.44±0.29b | 0.37±0.22b |
| Lp-PLA2 (relative index) | 1.00±1.16 | 1.95±1.83a | 1.10±1.02 |
Results are given as means±SDs. TC, total cholesterol; CE, cholesterylester; FC, free cholesterol; TG, triglyceride; PL, phospholipid; SM, sphingomyelin; S1P, sphingosine-1-phosphate; PON, paraoxonase.
Significance from one-way ANOVA and the least significant difference post hoc test was accepted at the level of 0.05 versus control.
Significance from one-way ANOVA and the least significant difference post hoc test was accepted at the level of 0.01 versus control.
Figure 1.
HD HDL shifts toward the smaller HDL3 subclass. HDL (5 µg protein/lane) was separated by native gradient gel electrophoresis and stained with Coomassie Brilliant Blue. Intensity blots of individual samples were obtained, and the peak areas of HDL2 and HDL3 were calculated. Total peak area was set to 100% and used to calculate the percentage of HDL2/HDL3. Values shown represent means±SEMs of three individual experiments. CON, control.
Functional Metrics of HDL from Patients on HD or PD
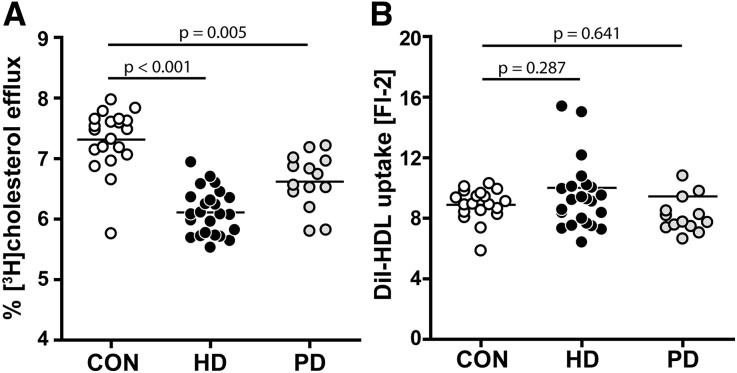
Major determinants of HDL-mediated reverse cholesterol transport are the capabilities of HDL to mediate cholesterol efflux from macrophages and other cells and promote cholesterol delivery to the liver. We observed that HDL from both dialysis groups showed a substantially impaired capability to promote cholesterol efflux from macrophages, with the most profound alterations in patients on HD (Figure 2A). Correlation analysis revealed that compositional variations in HDL of patients on dialysis were associated with impaired cholesterol efflux capacity, with HDL phospholipids being the strongest positive predictor in each dialysis subgroup (Supplemental Table 2). To assess whether HDL lipid delivery toward liver cells is altered in patients on dialysis, we labeled the lipid moiety of HDL with the lipophilic dye 1,1′-Dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine (DiI) which is a reliable surrogate to determine HDL lipid delivery to cells.19,20 Interestingly, we observed that HDL lipids from all studied groups were taken up equally well by liver cells (Figure 2B).
Figure 2.
Cholesterol efflux capability is impaired in HDL from patients on HD or PD. (A) HDL levels from 24 patients on HD, 14 patients on PD, and 20 control subjects (CON) were examined for their ability to efflux [3H]-cholesterol from TO-901317–stimulated, lipid-loaded RAW 264.7 macrophages. [3H]-cholesterol–labeled cells were incubated with HDL (50 µg/ml) for 2 hours at 37°C. Cholesterol efflux is expressed as the radioactivity in the medium relative to total radioactivity in medium and cells. (B) HDL was labeled with DiI and examined for its ability to promote lipid uptake by HepG2 cells. Cellular uptake of the fluorescent lipophilic dye DiI was quantified by flow cytometry. Values shown represent means of three independent experiments.
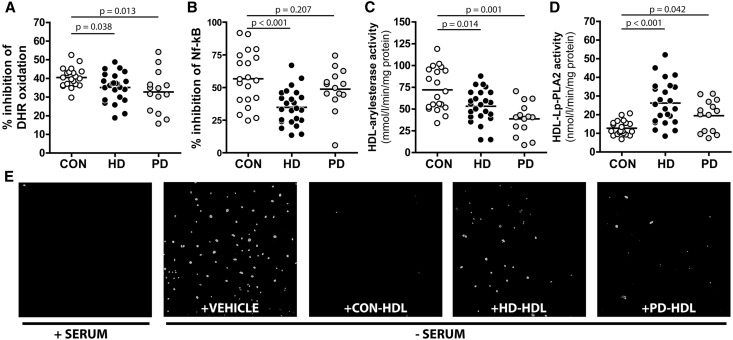
Other than the important role of HDL in reverse cholesterol transport, HDL has additional properties that are thought to be antiatherogenic. The major proteins of HDL, apoA-I, and apoA-II as well as other proteins, such as paraoxonase, that cotransport with HDL in plasma are well known to have antioxidant properties.21,22 As a consequence, HDL has the ability to inhibit the oxidative modification of LDL in a process that reduces the atherogenicity of these lipoproteins. We assessed the antioxidative activity of HDL particles by measuring the inhibition of free radical-induced oxidation of dihydrorhodamine (DHR),23 a dye that fluorescence highly increases on oxidation. We observed that HDL from patients on HD or PD was less potent in inhibiting this process (Figure 3A). HDL also possesses anti-inflammatory properties by its ability to inhibit the activation of monocytes, thereby reducing the recruitment of blood monocytes into the artery wall. To assess the anti-inflammatory capability of HDL, we used a human monocyte cell line with a reporter cassette for the NF-κB, which induces green fluorescent protein (GFP) expression on NF-κB translocation into the nucleus.24 We stimulated NF-κB activation with LPS in the presence of HDL to counteract this effect. Notably, HD HDL was less efficient in reducing NF-κB activation, whereas PD HDL was as efficient as control HDL (Figure 3B).
Figure 3.
Functional analyses reveal dysfunctional HDL in HD and PD patients. (A) Antioxidative activity of HDL was measured by inhibition of free radical-induced oxidation of DHR. Increase in fluorescence was monitored over time at 538 nm, and inhibition was calculated from slopes of individual samples. Results are expressed as percentage of inhibition compared with DHR oxidation in the absence of HDL. (B) Anti-inflammatory function of HDL was tested using a human monocyte cell line containing a reporter cassette for the NF-κB, which induces GFP expression on NF-κB translocation into the nucleus. Cells were pretreated for 90 minutes with 50 µg/ml HDL and stimulated for 24 hours with 50 ng/ml LPS. Afterward, GFP expression was assessed by flow cytometry. (C) Arylesterase activity of HDL-associated paraoxonase was measured by using phenylacetate as substrate. (D) Lp-PLA2 activity of HDL was measured using 2-thio platelet activating factor as substrate. The arylesterase and Lp-PLA2 activities of HDL were calculated from the slopes of the kinetic chart of three independent experiments. (E) Human coronary artery endothelial cells were incubated in serum-containing (+SERUM) or serum-free (−SERUM) media in the absence or presence of 150 µg/ml HDL from healthy controls (CON-HDL), patients on HD (HD-HDL), or patients on PD (PD-HDL) overnight. Subsequently, cells were stained for caspase 3/7 and imaged by confocal microscopy. (A–D) All values shown represent means of two independent experiments. CON, control.
Recent evidence has highlighted the importance of associated enzymes on HDL functionalities.25,26 In particular, the activity of paraoxonase has been related to the antioxidative activity of HDL,27 whereas Lp-PLA2 activity has been hypothesized to be involved in atherogenesis through pathways related to inflammation.28,29 We observed that HDL of both dialysis groups showed reduced paraoxonase-mediated arylesterase activity; however, PD HDL exhibited the lowest activity (Figure 3C). This is in good agreement with protein mass measurements (Table 2, proteins) and previous reports on HDL-associated paraoxonase activity in patients on dialysis.13,30 In contrast to paraoxonase activity, Lp-PLA2 activity was increased in HD HDL and PD HDL (Figure 3D).
Apoptotic cell death after injury of the vascular endothelium is assumed to play an important role in the pathogenesis of atherosclerosis. There is clear evidence that HDL effectively suppresses apoptosis of endothelial cells.31 Serum starvation significantly induced caspase 3/7, an early marker of apoptosis in primary human coronary artery endothelial cells (Figure 3E). This activation was almost completely abolished by treatment with control HDL, whereas PD HDL and HD HDL seemed to be less effective. We observed a borderline negative correlation between the time spent on dialysis and cholesterol efflux capability (r=−0.305, P=0.07), whereas it was not associated with other functional parameters of HDL.
Activities of Serum Enzymes Involved in HDL Metabolism
Given that HDL composition and function are significantly altered in patients on dialysis, we assessed key regulators in HDL metabolism, such as phospholipid transfer protein (PLTP), lecithin-cholesterylester transferase (LCAT), and cholesterylester transfer protein (CETP). In agreement with previous reports,32–35 we found that LCAT activity was significantly reduced in patients on HD (Figure 4A). Recent studies have provided conflicting data on PLTP activity in patients on HD,34,36 and no experimental data are available for patients on PD. We observed a marked increase in PLTP activity in patients on HD or PD, almost doubling the activity of control samples (Figure 4B). PLTP activity was associated with a reduction in phospholipids, apoA-I (r=−0.614, P<0.001), and apoA-II (r=−0.485, P<0.001) and an increase in apoC-II (r=0.332, P=0.01) and apoC-III (r=0.473, P<0.001). Moreover, correlation analysis indicated that PLTP activity was negatively correlated with paraoxonase activity of HDL (Supplemental Table 3), suggesting that PLTP plays an important role in remodeling HDL composition in disease. We also assessed CETP activity and found it to be unaltered (Figure 4C).
Figure 4.
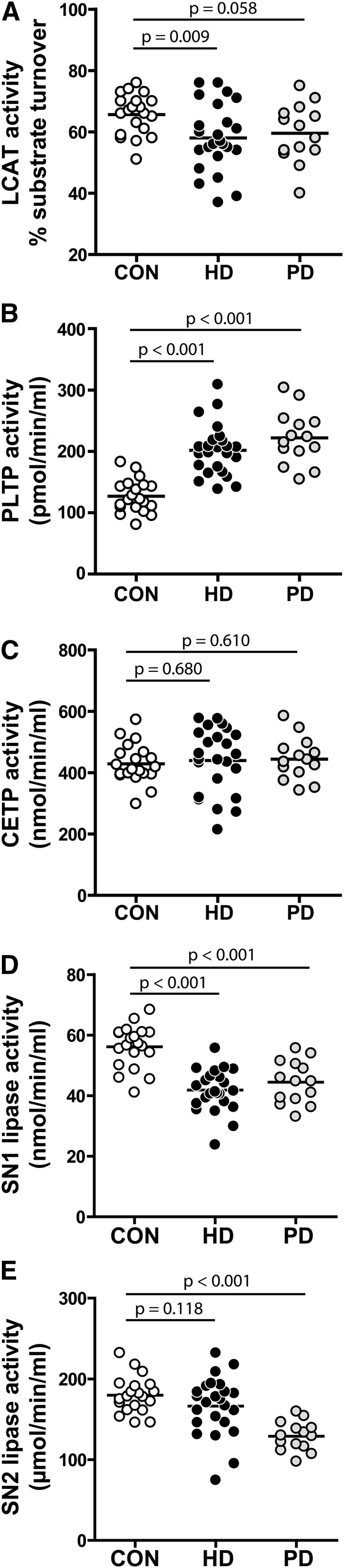
Analysis of serum enzymes indicates that HDL metabolism is impaired in dialysis patients. Serum samples from 24 patients on HD, 14 patients on PD, and 20 control subjects (CON) were examined for activities of (A) LCAT, (B) PLTP, (C) CETP, (D) SN1 lipases, and (E) SN2 lipases. Values shown represent means of two independent experiments.
We further sought to analyze serum lipolytic activities releasing acyl chains in stereospecific numbering 1 (SN1) and stereospecific numbering 2 (SN2) positions, because activities of lipases are altered under inflammatory conditions and can profoundly affect HDL composition.37 We observed that, in both dialysis groups, the SN1 lipolytic activity in serum was significantly reduced (Figure 4D), whereas SN2 lipolytic activity was significantly decreased in patients on PD (Figure 4E).
Discussion
The latest studies provide evidence that higher HDL cholesterol levels are not associated with lower mortality risk and coronary artery disease severity in patients with reduced kidney function.5,38 Dysfunctional HDL might confound the outcome of HDL-targeted therapies in these patients.9–11,13,30,39 In this study, we observed that HDL from patients on HD or PD showed a shift toward a more inflammatory phenotype, with alterations in the lipid moiety and distinct alterations in the protein composition. Our comprehensive analysis revealed a profound suppression of several metrics of HDL functionality in patients on HD or PD, which is shown in the graphic overview in Figure 5.
Figure 5.
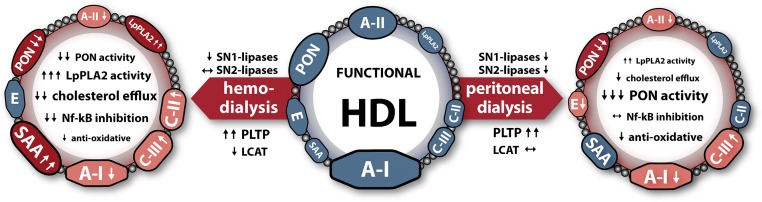
Dialysis modalities and HDL composition and function. Shown are alterations in protein content on the outer circle, where dark red-colored proteins indicate proteins that are highly remodeled (>50%) and light red-colored proteins indicate moderately altered proteins (<50%). Functional alterations of HDL as well as modulation of serum enzyme activities are indicated with arrows. ↓↓ and ↑↑ indicate highly altered functions, and ↓ and ↑ indicate moderately altered functions. E, apolipoprotein E; PON, paraoxonase.
A finding of particular interest was that HDL from both dialysis groups showed a remarkable impaired capability to promote cholesterol efflux. Correlation analysis revealed that compositional alterations in patients on dialysis were associated with impaired cholesterol efflux capability. In line with our previous findings,9 HDL phospholipids and apoA-I were the strongest positive predictors (Supplemental Table 2), whereas apoC-III and SAA levels were negatively associated with HDL cholesterol efflux capability. We observed that HDL from patients on HD showed a clear trend toward the smaller HDL3 subclass, suggesting that particle maturation is impaired. A crucial enzyme involved in this maturation step is LCAT.40 In agreement with previous reports, we observed a reduction in LCAT activity in patients on HD.32–35 The importance of LCAT in HDL maturation was underlined by an inverse correlation with the phospholipid content, which was strongest in patients on HD (r=−0.704, P<0.001) and absent in patients on PD (r=−0.328, P=0.27). These data suggest that reduced LCAT activity contributes to delayed HDL maturation in patients on HD, which might explain the observed shift in HDL2/HDL3 distribution. Our finding agrees with previous reports showing that the conversion of pre-β-HDL into spherical HDL is severely delayed in patients on HD.33 Importantly, the ratio of HDL2 to HDL3 significantly correlated with cholesterol efflux capability (r=0.550, P=0.005). Therefore, both increased levels of SAA and apoC-III and low LCAT activity might explain differences in cholesterol efflux properties of HDL from patients on HD or PD. A finding of particular interest was that PLTP activity was highly increased and that SN1 lipolytic activity was significantly decreased in both dialysis groups. A major source for SN1 lipolytic activity is lipoprotein lipase, with activity that has been found to be low in patients on HD.41 Lipoprotein lipase activity alters HDL composition, leading to higher phospholipid and lower triglycerides contents.42 Therefore, a reduction in lipoprotein lipase activity in patients on HD or PD is expected to reduce HDL phospholipid content associated with a decreased cholesterol efflux capability. PLTP transfers phospholipids from triglyceride-rich lipoproteins to HDL, regulating the size of HDL particles.43 Given that, in healthy adults, a significant negative relationship between HDL size and PLTP serum activity was observed,44 PLTP seems to be another important uremia-associated factor altering HDL composition and cholesterol efflux capability.
A previous study has reported that elevation of PLTP in mice is associated with augmented atherosclerosis, despite the lowering effect on apoB-containing lipoproteins.45–47 PLTP overexpression in mice reduced HDL-associated paraoxonase activity. This might be of particular relevancy, given that there is evidence of a mechanistic link between HDL-associated paraoxonase activity, systemic oxidative stress, and prospective cardiovascular risk.27 In contrast to PLTP-overexpressing mice, PLTP-deficient mice are less susceptible to atherosclerosis. Surprisingly, PLTP-deficient mice also show lower serum paraoxonase activity. However, functional analysis of HDL from these animals showed that PLTP deficiency increased the anti-inflammatory potential of HDL and that, on the basis of protein weight, HDL had approximately the same paraoxonase activity as control animals.48 Moreover, reduced HDL-associated paraoxonase was shown to directly associate with impaired endothelial nitric oxide production and loss of the endothelial anti-inflammatory and endothelial repair-stimulating effects of HDL.49 Another interesting finding of this study was that PD HDL exhibited particularly low paraoxonase activity. The substantial reduction of HDL paraoxonase activity might be explained by increased glycation of HDL in patients on PD.50,51 Depending on their peritoneal membrane transporter characteristics, patients on PD can absorb from approximately 100 to 200 g glucose per day.52
HDL has been found to have potent anti-inflammatory activities on myeloid cells, including monocytes, macrophages, and monocyte-derived dendritic cells, resulting in suppression of cytokine and chemokine production, downregulation of costimulatory molecules, and inhibition of antigen presentation.21,53,54 We measured the anti-inflammatory potential of HDL directly by its ability to inhibit the activation of NF-κB, an important regulator of inflammatory responses.55 An impaired activity was seen in HDL from patients on HD, whereas PD HDL showed no reduced capability to inhibit the activation of NF-κB (Figure 3B). A recent study provided evidence that SAA mediates the proinflammatory effects of HDL isolated from patients with CKD, affecting cytokine production and adhesion molecule expression on monocytes and myeloid dendritic cells.10 In agreement with those previous reports,9–11 we found that HD HDL contains significantly increased SAA levels that might impair the anti-inflammatory activity of HDL (Table 2, proteins).
In our study cohort, patients on HD had different types of vascular access (Supplemental Table 1). We performed a correlation analysis to evaluate the effect of vascular access on HDL function and composition. Our analysis indicates, within the small sample size of the subgroups, that functional and compositional alterations of HDL showed very similar trends for the different types of vascular access (Supplemental Table 4).
Furthermore, we observed that the time spent on dialysis tended to correlate with the cholesterol efflux capability of HDL, whereas it was not associated with other functional parameters of HDL. Subgroup analysis showed that this trend was only present in patients on HD (r=−0.382, P=0.07). However, it has to be noted that average dialysis vintage was 4.4 years for patients on HD and <3 years for patients on PD, precluding a statement on cause-and-effect relationship.
Limitations of this explorative study are the cross-sectional design and the small sizes of the patient cohorts, which were from a single institution. Therefore, our study is limited by the correlative nature, not permitting causal inference.
In summary, we provide novel mechanistic insights into the formation of dysfunctional HDL in patients with ESRD on HD and PD. Given that both the ability of HDL to promote cholesterol efflux and HDL-associated paraoxonase activity predict cardiovascular outcome,27,56 our results might provide valuable information to develop rational HDL-raising therapeutic strategies.
Concise Methods
Study Subjects and Blood Collection
Stable patients with ESRD undergoing PD or HD/hemodiafiltration three times per week could be included in the study when they passed the exclusion criteria of being under 18 years of age, having diabetes, being on lipid-lowering therapy, having active malignancies, having liver cirrhosis, having coagulopathy, and having acute inflammation or systemic infection. Demographic data, medical history, concomitant medication, current dialysis treatment parameters, such as Kt/V, and residual renal function were recorded at the time of enrollment. Blood sampling was performed before a mid-week dialysis session (patients on HD/hemodiafiltration) or during the monthly visit (patients on PD) using standard serum tubes (Greiner Bio-one, Kremsmünster, Austria). Healthy blood donors were recruited and included in the study when they passed the same exclusion criteria as patients on dialysis and were free of any sign of kidney disease. The control group was matched for age and sex. Patients on HD had different types of vascular access (Supplemental Table 1). We performed correlation analysis to examine the composition and several metrics of HDL function of patients on HD or PD. Functional and compositional alterations of HDL showed similar trends in patients on HD or PD (Supplemental Table 4). Furthermore, iron indices and intravenous iron administration were not significantly associated with HDL function in our study cohort (Supplemental Tables 5 and 6). The study protocol and all study procedures were reviewed and approved by the local ethics committee (21–523 ex 09/10). All participants gave written informed consent before being enrolled into the study.
Materials
Radiochemicals were purchased from Hartman Analytic (Braunschweig, Germany). All other reagents were obtained from Sigma-Aldrich (Vienna, Austria).
Preparation of Lipoproteins
Serum density was adjusted with potassium bromide (Sigma-Aldrich) to 1.24 g/ml, and a two-step density gradient was generated in centrifuge tubes (16×76 mm; Beckman Instruments, Krefeld, Germany) by layering the density-adjusted plasma underneath an NaCl density solution (1.063 g/ml) as described with minor modifications.57 Tubes were sealed and centrifuged at 90,000 rpm for 4 hours in a 90Ti fixed angle rotor (Beckman). After centrifugation, the HDL- and LDL-containing bands were separately collected, desalted through PD10 columns (GE Healthcare, Vienna, Austria), and immediately used for experiments or stored at −70°C. Carbamylated LDL was generated by incubation with 20 mg/ml potassium cyanate for 4 hours. Afterward, LDL was desalted through PD10 columns to remove excess potassium cyanate. To evaluate the effect of the isolation method on HDL function, we compared the arylesterase activity of HDL-containing fractions derived from polyethylene glycol precipitation58 with one-step density gradient centrifugation as described above. Our analysis suggests that both isolation methods are comparable in terms of HDL-associated arylesterase activity (Supplemental Figure 1).
Determination of Plasma and HDL Lipid Composition
Levels of total cholesterol, nonesterified cholesterol, triglycerides, and phospholipids (Diasys, Holzheim, Germany) were measured enzymatically. Sphingomyelin and sphingosine-1-phosphate were measured with commercially available ELISA (Cayman Europe, Talinn, Estonia). LDL cholesterol was calculated according to the Friedewald equation using HDL cholesterol values measured in the supernatant of the phosphotungstic precipitation.
Apo Determination by Immunoturbidimetry
ApoA-I, apoA-II, apoB, apoC-II, apoC-III, and apoE (Greiner, Flacht, Germany) were determined by immunoturbidimetry. All lipoprotein analyses were performed on an Olympus AU640 analyzer (Olympus Diagnostika, Hamburg, Germany).
Cellular Cholesterol Efflux Assays
Cholesterol efflux capacity was quantified as described previously.9 RAW 264.7 macrophages were maintained in DMEM plus 10% FBS and 1× penicillin-streptomycin. Cells were plated at 3×106 on 48-well plates and grown overnight. Then, cells were labeled with 1 μCi/ml [3H]-cholesterol in medium supplemented with 5% FBS and 50 µg/ml carbamylated LDL for 24 hours. For the last 14 hours of incubation, cells were additionally stimulated with the LXR agonist TO-901317 (2 µmol/L). Cells were washed two times and equilibrated with serum-free medium containing 0.2% BSA for 2 hours at 37°C. Afterward, cells were washed two times, and 200 µl cholesterol acceptor (50 µg/ml HDL) was added in serum-free media for 2 hours at 37°C. Supernatants and lysed cells (lysed with 0.3 M sodium hydroxide containing 0.1% SDS) were separately collected, and radioactivity was measured by liquid scintillations counting. Cholesterol efflux to the acceptors is expressed as the radioactivity in the medium relative to total radioactivity in medium and cells. Specific cholesterol efflux was calculated by subtracting cholesterol efflux in the absence of HDL from efflux in the presence of HDL.
DiI HDL Uptake
HDL was labeled with DiI as described previously.60 Briefly, DiI was added to HDL from a stock solution (30 mg/ml in DMSO) to yield a final ratio of 300 µg DiI per 1 mg HDL protein. The mixture was incubated for 20 hours at 37°C, and excess dye was removed by gel filtration with the PD Spin-Trap G-25 (GE Healthcare). The specific fluorescence at 565 nm for each individual HDL preparation was determined, expressed as fluorescence per 1 mg protein, and included in the uptake calculation.
HepG2 cells were cultivated in DMEM containing 10% FBS and 1× penicillin-streptomycin. Cells were seeded at 2.5×106 on 48-well plates, grown overnight, and incubated with 20 µg/ml DiI HDL in serum-free DMEM with 2 mg/ml BSA for 3 hours. Cells were washed two times with 2 mg/ml BSA in PBS and one time in PBS. Cells were detached with accutase (Sigma-Aldrich), washed with PBS, and fixed with BD CellFIX solution (BD Biosciences). Uptake of the fluorescence lipid tracer DiI was analyzed by flow cytometry in the FL-2 channel, and 5,000 cells were counted for each condition.
Arylesterase and Lp-PLA2 Activity
Ca2+-dependent arylesterase activity was determined with a photometric assay using phenylacetate as previously described.61 HDL (0.5 µg protein) was added to 200 µl buffer containing 100 mmol/L Tris, 2 mmol/L CaCl2 (pH 8.0), and 1 mmol/L phenylacetate in a 96-well quartz glass plate (Hellma, Baden, Germany). The rate of hydrolysis of phenylacetate was monitored by the increase of absorbance at 270 nm, and readings were taken every 15 seconds at room temperature to generate a kinetic plot. The slope from the kinetic chart was used to determine the increase in fluorescence per minute. Enzymatic activity was calculated with the Beer–Lambert Law from the molar extinction coefficient of 1310 mol×L−1×cm−1 for phenylacetate. Lp-PLA2 activity was measured with a commercially available photometric assay (Cayman Europe) with 2-thio platelet activating factor as substrate.
Determination of the Antioxidative Capacity of HDL
The antioxidative activity of HDL was determined as previously described with modifications.23 Briefly, 15 µg HDL protein was placed in a 384-well plate, and 100 µl 7 µmol/L DHR reagent containing 1 mmol/L 2,2′-azobis-2-methyl-propanimidamide-dihydrochloride was added. The increase in fluorescence was monitored using an xMark plate reader (Biorad, Vienna, Austria) over time at 538 nm. After an initial lag phase of about 20 minutes, the rate of oxidation was linear. The increase in fluorescence was calculated from the linear range and used for comparison. The increase in fluorescence per minute of DHR in the absence of HDL was set to 100%, and individual HDL samples were calculated as percentage of inhibition of DHR oxidation.
Determination of the Anti-Inflammatory Capacity of HDL
U937 cells, a monocyte cell line containing a 5× NF-κB–GFP reporter cassette24 (obtained from Herbert Strobl, Medical University of Graz, Graz, Austria), were cultivated in RPMI 1640 containing 10% FBS and 1× penicillin-streptomycin. For experiments, 25,000 cells were placed in 1.1-ml microtubes (Bioquote) in 100 µl RPMI 1640 with 7.5% FBS. Cells were pretreated for 90 minutes with 50 µg/ml HDL and stimulated for 24 hours with 50 ng/ml LPS. After the stimulation, cells were centrifuged at 400×g for 7 minutes, the supernatant was discarded, and cells were fixed in 100 µl BD CellFIX solution (BD Biosciences). The expression of NF-κB GFP was assessed by flow cytometry.
Caspase 3/7 Activation Assay
Human coronary artery endothelial cells (Lonza, Basel, Switzerland) were cultured in EBM-2 Bullet Kit Media (Lonza) and used between passages 4 and 8. Cells were seeded on culture dishes with glass bottoms and grown overnight. Then, cells were starved in serum-free media in the absence or presence of 100 µg/ml HDL for 24 hours. Cells were stained with CellEvent Caspase 3/7 Green Detection Reagent (Life Technologies, Vienna, Austria) and imaged by confocal microscopy.
HDL Particle Size Analysis
HDL (5 µg protein per lane) was separated by native gradient gel electrophoresis (4%–16% NativePage; Life Technologies). Gels were run for 120 minutes at a constant voltage of 150 V in NativePage running buffer (Life Technologies). Afterward, gels were fixed with 25% isopropanol/10% acetic acid for 10 minutes and stained overnight with Coomassie Brilliant Blue G-250 (Thermo Fisher Scientific). To determine the size distribution of isolated HDL, an image of the gel was analyzed with ImageJ software. Intensity blots of individual samples were obtained, and the peak areas for HDL2 and HDL3 separately were calculated with the help of the standard proteins (NativeMark; Life Technologies) containing BSA (7.1 nm), lactate dehydrogenase (8.2 nm), B-phycoerythrin (10.5 nm), apoferritin band 1 (12.2 nm), and apoferritin band 2 (18.0 nm). To compare individual samples, the percentage of HDL2 and HDL3 peak area on the total peak area was calculated and used for analysis.
PLTP and CETP Activity Assays
PLTP and CETP were measured in serum with assay kits from Abnova (Eubio, Vienna, Austria). Both activity assays use a specific donor molecule containing a fluorescent self-quenched neutral lipid (for CETP) or phospholipid (for PLTP) that is transferred to an acceptor molecule in the presence of CETP or PLTP. Enzyme-mediated transfer of the fluorescent neutral lipid to the acceptor molecule results in an increase in fluorescence (excitation=465 nm; emission=535 nm).
SN1 and SN2 Lipase Activity Assays
SN1 and SN2 lipase activity was measured in serum with commercially available kits (Cayman Europe). In the SN1 lipase assay, lipases hydrolyze arachidonoyl-1-thioglycerol to AA and thioglycerol, which react with a thiol fluorometric detector to yield a highly fluorescent product that results in an increase in fluorescence (excitation=385 nm; emission=515 nm).
The SN2 lipase assay uses the 1,2-dithio analog of diheptanoyl-phosphatidylcholine as a substrate. On hydrolyses of the thio ester bond at the SN2 position, free thiols are detected using 5,5-dithio-bis-(2-nitrobenzoic acid), which results in an increase in absorbance at 414 nm.
LCAT Activity Assay
LCAT was measured in serum with a commercially available kit from Merck (Darmstadt, Germany). Samples were incubated with LCAT substrate for 5 hours at 37°C. The fluorescent substrate emits fluorescence at 470 nm. When the substrate is hydrolyzed by LCAT, a monomer is released that emits fluorescence at 390 nm. The LCAT activity is assessed over time and expressed in change of 470/390-nm emission intensity.
SDS-PAGE and Western Blotting
To assess HDL-associated Lp-PLA2 and paraoxonase mass, 10 µg HDL was separated by SDS-PAGE, blotted, and probed with Lp-PLA2 antibody (10279; Cayman Europe) and paraoxonase 1 antibody (clone 17A12; Abcam, Inc., Cambridge, UK) as described previously. Western blots were densitometrically analyzed, and the Lp-PLA2 and paraoxonase content was calculated as the relative index to the average of the control population.
Statistical Analyses
Results are expressed as means and SDs or numbers and percentages of the total study group. Study groups were compared using ANOVA with the least significant difference post hoc test. For non-Gaussian data, the Mann–Whitney U test was used for two-group comparisons, and the Kruskal–Wallis test with Dunn’s post hoc test was used for three-group comparisons. The association between HDL functionalities and enzymatic activities of plasma enzymes with clinical and compositional data was assessed with the use of Pearson correlation coefficients, with partial correlation coefficients after adjustment for C-reactive protein levels and dialysis vintage. All analyses were performed with GraphPad Prism, version 4 or SPSS, version 21.
Disclosures
None.
Acknowledgments
This work was supported by the Austrian National Bank Grants 15883 (to M.H.), 14263 (to A.H.), and 14853 (to G.M.) and Austrian Science Fund (FWF) Grants P22521-B18 (to A.H.) and P22976-B18 (to G.M.). S.C. and M.T. were funded by the Medical University of Graz within the framework of Program DK-MOLIN FWF-W1241.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014030309/-/DCSupplemental.
References
- 1.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Burkart J: Metabolic consequences of peritoneal dialysis. Semin Dial 17: 498–504, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Garibotto G, Bonanni A, Verzola D: Effect of kidney failure and hemodialysis on protein and amino acid metabolism. Curr Opin Clin Nutr Metab Care 15: 78–84, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR: High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med 62: 707–714, 1977 [DOI] [PubMed] [Google Scholar]
- 5.Zewinger S, Speer T, Kleber ME, Scharnagl H, Woitas R, Lepper PM, Pfahler K, Seiler S, Heine GH, März W, Silbernagel G, Fliser D: HDL cholesterol is not associated with lower mortality in patients with kidney dysfunction. J Am Soc Nephrol 25: 1073–1082, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silbernagel G, Genser B, Drechsler C, Scharnagl H, Grammer TB, Stojakovic T, Krane V, Ritz E, Wanner C, März W: HDL cholesterol, apolipoproteins, and cardiovascular risk in hemodialysis patients [published online ahead of print July 10, 2014]. J Am Soc Nephrol doi:ASN.2013080816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marsche G, Saemann MD, Heinemann A, Holzer M: Inflammation alters HDL composition and function: Implications for HDL-raising therapies. Pharmacol Ther 137: 341–351, 2013 [DOI] [PubMed] [Google Scholar]
- 8.Moberly JB, Attman PO, Samuelsson O, Johansson AC, Knight-Gibson C, Alaupovic P: Alterations in lipoprotein composition in peritoneal dialysis patients. Perit Dial Int 22: 220–228, 2002 [PubMed] [Google Scholar]
- 9.Holzer M, Birner-Gruenberger R, Stojakovic T, El-Gamal D, Binder V, Wadsack C, Heinemann A, Marsche G: Uremia alters HDL composition and function. J Am Soc Nephrol 22: 1631–1641, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weichhart T, Kopecky C, Kubicek M, Haidinger M, Döller D, Katholnig K, Suarna C, Eller P, Tölle M, Gerner C, Zlabinger GJ, van der Giet M, Hörl WH, Stocker R, Säemann MD: Serum amyloid A in uremic HDL promotes inflammation. J Am Soc Nephrol 23: 934–947, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tölle M, Huang T, Schuchardt M, Jankowski V, Prüfer N, Jankowski J, Tietge UJ, Zidek W, van der Giet M: High-density lipoprotein loses its anti-inflammatory capacity by accumulation of pro-inflammatory-serum amyloid A. Cardiovasc Res 94: 154–162, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Kennedy DJ, Tang WH, Fan Y, Wu Y, Mann S, Pepoy M, Hazen SL: Diminished antioxidant activity of high-density lipoprotein-associated proteins in chronic kidney disease. J Am Heart Assoc 2: e000104, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moradi H, Pahl MV, Elahimehr R, Vaziri ND: Impaired antioxidant activity of high-density lipoprotein in chronic kidney disease. Transl Res 153: 77–85, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto S, Yancey PG, Ikizler TA, Jerome WG, Kaseda R, Cox B, Bian A, Shintani A, Fogo AB, Linton MF, Fazio S, Kon V: Dysfunctional high-density lipoprotein in patients on chronic hemodialysis. J Am Coll Cardiol 60: 2372–2379, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Speer T, Rohrer L, Blyszczuk P, Shroff R, Kuschnerus K, Krüankel N, Kania G, Zeqinger S, Akhmedov A, Shi Y, Martin T, Perisa D, Winnik S, Müller MF, Sester U, Wernicke G, Jung A, Guttleck U, Eriksson U, Geisel J, Deanfield J, von Eckardstein A, Lüuscher TF, Fliser D, Bahlmann FH, Landmesser U: Abnormal high-density lipoprotein induces endothelial dysfunction via activation of Toll-like receptor-2. Immunity 38(4), 754–768, 2013 [DOI] [PubMed] [Google Scholar]
- 16.Kotani K, Kimura S, Gugliucci A: Paraoxonase-1 and ischemia-modified albumin in patients with end-stage renal disease. J Physiol Biochem 67: 437–441, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Shroff R, Speer T, Colin S, Charakida M, Zewinger S, Staels B, Chinetti-Gbaguidi G, Hettrich I, Rohrer L, O’Neill F, McLoughlin E, Long D, Shanahan CM, Landmesser U, Fliser D, Deanfield JE: HDL in children with CKD promotes endothelial dysfunction and an abnormal vascular phenotype [published online ahead of print May 22, 2014]. J Am Soc Nephrol doi:ASN.2013111212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jurek A, Turyna B, Kubit P, Klein A: The ability of HDL to inhibit VCAM-1 expression and oxidized LDL uptake is impaired in renal patients. Clin Biochem 41: 1015–1018, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Gaidukov L, Nager AR, Xu S, Penman M, Krieger M: Glycine dimerization motif in the N-terminal transmembrane domain of the high density lipoprotein receptor SR-BI required for normal receptor oligomerization and lipid transport. J Biol Chem 286: 18452–18464, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holzer M, Trieb M, Konya V, Wadsack C, Heinemann A, Marsche G: Aging affects high-density lipoprotein composition and function. Biochim Biophys Acta 1831: 1442–1448, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barter PJ, Nicholls S, Rye KA, Anantharamaiah GM, Navab M, Fogelman AM: Antiinflammatory properties of HDL. Circ Res 95: 764–772, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Kontush A, Chapman MJ: Antiatherogenic function of HDL particle subpopulations: Focus on antioxidative activities. Curr Opin Lipidol 21: 312–318, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Kelesidis T, Currier JS, Huynh D, Meriwether D, Charles-Schoeman C, Reddy ST, Fogelman AM, Navab M, Yang OO: A biochemical fluorometric method for assessing the oxidative properties of HDL. J Lipid Res 52: 2341–2351, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jörgl A, Platzer B, Taschner S, Heinz LX, Höcher B, Reisner PM, Göbel F, Strobl H: Human Langerhans-cell activation triggered in vitro by conditionally expressed MKK6 is counterregulated by the downstream effector RelB. Blood 109: 185–193, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Aviram M, Rosenblat M: Paraoxonases (PON1, PON2, PON3) analyses in vitro and in vivo in relation to cardiovascular diseases. Methods Mol Biol 477: 259–276, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Mackness B, Turkie W, Mackness M: Paraoxonase-1 (PON1) promoter region polymorphisms, serum PON1 status and coronary heart disease. Arch Med Sci 9: 8–13, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhattacharyya T, Nicholls SJ, Topol EJ, Zhang R, Yang X, Schmitt D, Fu X, Shao M, Brennan DM, Ellis SG, Brennan ML, Allayee H, Lusis AJ, Hazen SL: Relationship of paraoxonase 1 (PON1) gene polymorphisms and functional activity with systemic oxidative stress and cardiovascular risk. JAMA 299: 1265–1276, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu MM, Zhang J, Wang WY, Wu WY, Ma YL, Chen WH, Wang YP: The inhibition of lipoprotein-associated phospholipase A2 exerts beneficial effects against atherosclerosis in LDLR-deficient mice. Acta Pharmacol Sin 32: 1253–1258, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zalewski A, Macphee C: Role of lipoprotein-associated phospholipase A2 in atherosclerosis: Biology, epidemiology, and possible therapeutic target. Arterioscler Thromb Vasc Biol 25: 923–931, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Vaziri ND, Navab K, Gollapudi P, Moradi H, Pahl MV, Barton CH, Fogelman AM, Navab M: Salutary effects of hemodialysis on low-density lipoprotein proinflammatory and high-density lipoprotein anti-inflammatory properties in patient with end-stage renal disease. J Natl Med Assoc 103: 524–533, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mineo C, Shaul PW: Novel biological functions of high-density lipoprotein cholesterol. Circ Res 111: 1079–1090, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dieplinger H, Schoenfeld PY, Fielding CJ: Plasma cholesterol metabolism in end-stage renal disease. Difference between treatment by hemodialysis or peritoneal dialysis. J Clin Invest 77: 1071–1083, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miida T, Miyazaki O, Hanyu O, Nakamura Y, Hirayama S, Narita I, Gejyo F, Ei I, Tasaki K, Kohda Y, Ohta T, Yata S, Fukamachi I, Okada M: LCAT-dependent conversion of prebeta1-HDL into alpha-migrating HDL is severely delayed in hemodialysis patients. J Am Soc Nephrol 14: 732–738, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Pahl MV, Ni Z, Sepassi L, Moradi H, Vaziri ND: Plasma phospholipid transfer protein, cholesteryl ester transfer protein and lecithin:cholesterol acyltransferase in end-stage renal disease (ESRD). Nephrol Dial Transplant 24: 2541–2546, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan MK, Ramdial L, Varghese Z, Persaud JW, Baillod RA, Moorhead JF: Plasma lecithin-cholesterol acyltransferase activities in uraemic patients. Clin Chim Acta 119: 65–72, 1982 [DOI] [PubMed] [Google Scholar]
- 36.Schlitt A, Heine GH, Jiang XC, Messow M, Blankenberg S, Rupprecht HJ, Ulrich C, Buerke M, Werdan K, Lackner KJ, Köhler H, Girndt M: Phospholipid transfer protein in hemodialysis patients. Am J Nephrol 27: 138–143, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Hasham SN, Pillarisetti S: Vascular lipases, inflammation and atherosclerosis. Clin Chim Acta 372: 179–183, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Moradi H, Streja E, Kashyap ML, Vaziri ND, Fonarow GC, Kalantar-Zadeh K: Elevated high-density lipoprotein cholesterol and cardiovascular mortality in maintenance hemodialysis patients. Nephrol Dial Transplant 29: 1554–1562, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Birner-Gruenberger R, Schittmayer M, Holzer M, Marsche G: Understanding high-density lipoprotein function in disease: Recent advances in proteomics unravel the complexity of its composition and biology. Prog Lipid Res 56C: 36–46, 2014 [DOI] [PubMed] [Google Scholar]
- 40.Vaziri ND, Navab M, Fogelman AM: HDL metabolism and activity in chronic kidney disease. Nat Rev Nephrol 6: 287–296, 2010 [DOI] [PubMed] [Google Scholar]
- 41.Näsström B, Olivecrona G, Olivecrona T, Stegmayr BG: Lipoprotein lipase during heparin infusion: Lower activity in hemodialysis patients. Scand J Clin Lab Invest 63: 45–53, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Murdoch SJ, Breckenridge WC: Influence of lipoprotein lipase and hepatic lipase on the transformation of VLDL and HDL during lipolysis of VLDL. Atherosclerosis 118: 193–212, 1995 [DOI] [PubMed] [Google Scholar]
- 43.van Tol A: Phospholipid transfer protein. Curr Opin Lipidol 13: 135–139, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Cheung MC, Wolfbauer G, Deguchi H, Fernández JA, Griffin JH, Albers JJ: Human plasma phospholipid transfer protein specific activity is correlated with HDL size: Implications for lipoprotein physiology. Biochim Biophys Acta 1791: 206–211, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schlitt A, Yan D, Von Gyzicki H, Norin A, Jiang XC: Phospholipid transfer protein has proinflammatory effects. Eur Heart J 25: 630, 2004 [Google Scholar]
- 46.Robins SJ, Lyass A, Brocia RW, Massaro JM, Vasan RS: Plasma lipid transfer proteins and cardiovascular disease. The Framingham Heart Study. Atherosclerosis 228: 230–236, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lie J, de Crom R, van Gent T, van Haperen R, Scheek L, Sadeghi-Niaraki F, van Tol A: Elevation of plasma phospholipid transfer protein increases the risk of atherosclerosis despite lower apolipoprotein B-containing lipoproteins. J Lipid Res 45: 805–811, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Yan D, Navab M, Bruce C, Fogelman AM, Jiang XC: PLTP deficiency improves the anti-inflammatory properties of HDL and reduces the ability of LDL to induce monocyte chemotactic activity. J Lipid Res 45: 1852–1858, 2004 [DOI] [PubMed] [Google Scholar]
- 49.Besler C, Heinrich K, Rohrer L, Doerries C, Riwanto M, Shih DM, Chroni A, Yonekawa K, Stein S, Schaefer N, Mueller M, Akhmedov A, Daniil G, Manes C, Templin C, Wyss C, Maier W, Tanner FC, Matter CM, Corti R, Furlong C, Lusis AJ, von Eckardstein A, Fogelman AM, Lüscher TF, Landmesser U: Mechanisms underlying adverse effects of HDL on eNOS-activating pathways in patients with coronary artery disease. J Clin Invest 121: 2693–2708, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ferretti G, Bacchetti T, Marchionni C, Caldarelli L, Curatola G: Effect of glycation of high density lipoproteins on their physicochemical properties and on paraoxonase activity. Acta Diabetol 38: 163–169, 2001 [DOI] [PubMed] [Google Scholar]
- 51.Boemi M, Leviev I, Sirolla C, Pieri C, Marra M, James RW: Serum paraoxonase is reduced in type 1 diabetic patients compared to non-diabetic, first degree relatives; influence on the ability of HDL to protect LDL from oxidation. Atherosclerosis 155: 229–235, 2001 [DOI] [PubMed] [Google Scholar]
- 52.Holmes CJ: Glucotoxicity in peritoneal dialysis—solutions for the solution! Adv Chronic Kidney Dis 14: 269–278, 2007 [DOI] [PubMed] [Google Scholar]
- 53.Norata GD, Pirillo A, Ammirati E, Catapano AL: Emerging role of high density lipoproteins as a player in the immune system. Atherosclerosis 220: 11–21, 2012 [DOI] [PubMed] [Google Scholar]
- 54.Säemann MD, Poglitsch M, Kopecky C, Haidinger M, Hörl WH, Weichhart T: The versatility of HDL: A crucial anti-inflammatory regulator. Eur J Clin Invest 40: 1131–1143, 2010 [DOI] [PubMed] [Google Scholar]
- 55.Tak PP, Firestein GS: NF-kappaB: A key role in inflammatory diseases. J Clin Invest 107: 7–11, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, Mohler ER, Rothblat GH, Rader DJ: Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med 364: 127–135, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sattler W, Mohr D, Stocker R: Rapid isolation of lipoproteins and assessment of their peroxidation by high-performance liquid chromatography postcolumn chemiluminescence. Methods Enzymol 233: 469–489, 1994 [DOI] [PubMed] [Google Scholar]
- 58.Asztalos BF, de la Llera-Moya M, Dallal GE, Horvath KV, Schaefer EJ, Rothblat GH: Differential effects of HDL subpopulations on cellular ABCA1- and SR-BI-mediated cholesterol efflux. J Lipid Res 46: 2246–2253, 2005 [DOI] [PubMed] [Google Scholar]
- 59.Holzer M, Gauster M, Pfeifer T, Wadsack C, Fauler G, Stiegler P, Koefeler H, Beubler E, Schuligoi R, Heinemann A, Marsche G: Protein carbamylation renders high-density lipoprotein dysfunctional. Antioxid Redox Signal 14: 2337–2346, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stephan ZF, Yurachek EC: Rapid fluorometric assay of LDL receptor activity by DiI-labeled LDL. J Lipid Res 34: 325–330, 1993 [PubMed] [Google Scholar]
- 61.Holzer M, Zangger K, El-Gamal D, Binder V, Curcic S, Konya V, Schuligoi R, Heinemann A, Marsche G: Myeloperoxidase-derived chlorinating species induce protein carbamylation through decomposition of thiocyanate and urea: Novel pathways generating dysfunctional high-density lipoprotein. Antioxid Redox Signal 17: 1043–1052, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]