Abstract
Validation of current and promising surrogate outcomes for ESRD in randomized controlled trials (RCTs) has been limited. We conducted a systematic review and meta-analysis of RCTs to further inform the ability of surrogate outcomes for ESRD to predict the efficacy of various interventions on ESRD. MEDLINE, EMBASE, and CENTRAL (from inception through September 2013) were searched. All RCTs in adults with proteinuria, diabetes, or CKD stages 1–4 or renal transplant recipients reporting ≥10 ESRD events and a surrogate outcome (change in proteinuria or doubling of serum creatinine [DSCR]) for ESRD during a ≥1-year follow-up were included. Two reviewers abstracted trial characteristics and outcome data independently. To assess the correlation between the surrogate outcomes and ESRD, we determined the treatment effect ratio (TER), defined as the ratio of the treatment effects on ESRD and the effects on the change in surrogate outcomes. TERs close to 1 indicate greater agreement between ESRD and the surrogate, and these ratios were pooled across interventions. We identified 27 trials (97,458 participants; 4187 participants with ESRD). Seven trials reported the effects on change in proteinuria and showed consistent effects for proteinuria and ESRD (TER, 0.82; 95% confidence interval, 0.59 to 1.16), with minimal heterogeneity. Twenty trials reported on DSCR. Treatment effects on DSCR were consistent with the effects on ESRD (TER, 0.98; 95% confidence interval, 0.85 to 1.14), with moderate heterogeneity. In conclusion, DSCR is generally a good surrogate for ESRD, whereas data on proteinuria were limited. Further assessment of the surrogacy of proteinuria using prospective RCTs is warranted.
Keywords: end-stage renal disease, randomized controlled trials, clinical epidemiology
CKD is commonly defined as a GFR<60 ml/min per 1.73 m2 or the presence of markers of kidney damage, including proteinuria.1 The global burden of CKD is increasing, with a prevalence of 10%–16% worldwide.2 The clinical management of people with CKD has been difficult, particularly for those with irreversible kidney failure or ESRD. Preserving kidney function and slowing the progression of kidney disease is thus important. In measuring the efficacy of interventions in clinical trials focusing on this strategy, ESRD (defined as long-term dialysis or kidney transplantation) is the most definitive outcome in kidney disease. However, kidney disease often progresses slowly over many years. This presents logistic challenges in the conduct of randomized controlled trials (RCTs) because long follow-up and extensive resources are required. These considerations may explain the lack of RCTs in nephrology compared with other specialties of medicine.3 The use of appropriate surrogate outcomes may facilitate clinical trial conduct as they reduce samples size requirements and duration of follow-up. Doubling of serum creatinine is frequently used as a surrogate for ESRD in RCTs, but there is ongoing debate regarding its limitations because serum creatinine increases do not necessarily reflect the true rate of renal function decline.4 There is considerable interest in using proteinuria or smaller changes in serum creatinine or eGFR as surrogate outcomes.5–7 However, rigorous validation of a surrogate outcome is needed before it can be implemented in large-scale trials.
We therefore conducted a systematic review and meta-analysis of RCTs, irrespective of intervention or comparator, in adults with proteinuria, diabetes, CKD stages 1–4, or renal transplant recipients to further inform the ability of surrogate outcomes (change in proteinuria and doubling of serum creatinine) for ESRD to predict the efficacy of various interventions on ESRD.
Results
Search Results and Characteristics of Included Studies
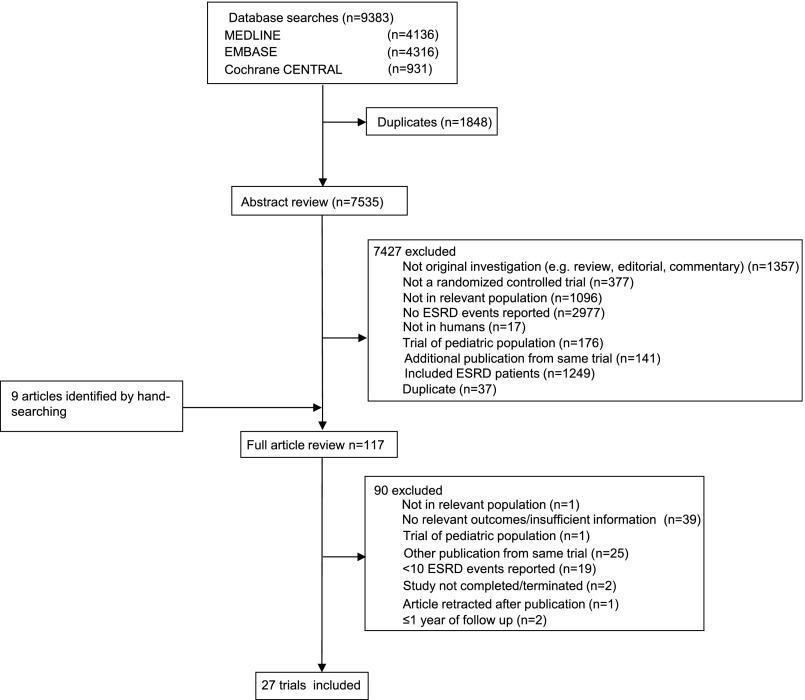
The literature search yielded 9383 articles, of which 117 were reviewed in full text (Figure 1). Of these, 27 RCTs (97,458 participants; 4187 ESRD events) met the inclusion criteria. Most studies identified by our search, but excluded from this review, were not in relevant populations, did not report ESRD events, or were duplicates of reports already identified. Table 1 summarizes the characteristics of the included studies. The trials had a sample size that ranged from 50 to 17,118 participants with follow-up periods from 1.6 to 10.0 years. Of the 27 trials identified, 3 were single-center studies8–10 and 24 were multicenter studies.11–34 Studies were conducted in some or all of the United States, Canada, Europe, Asia, and Oceania, with trial results published between 1992 and 2013. A variety of interventions were assessed, including BP lowering (16 trials12,13,17,19,21–26,28,29,31–34), lipid-lowering therapy (3 trials9,11,15), intensive glucose-lowering therapy (4 trials16,20,27,30), anemia therapy (2 trials14,18), dietary intervention (1 trial10), and chelation therapy (1 trial8).
Figure 1.
Diagram showing the identification process for eligible studies.
Table 1.
Characteristics of included studies
| Intervention details | Study, Authors (Year) | Inclusion Criteria | Intervention Types and Dosage | Control Group | Mean Duration of Follow-up (yr) | Participants (n); ESRD Events (n) | Mean Age (yr) | Men (%) | Mean Baseline SCr (mg/dl); mean eGFR (ml/min per 1.73 m2) | Mean Urine Protein (g/d) | Treatment Effect (RR and 95% CIs) on DSCR and ESRD |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BP lowering; RAS blockade versus placebo | Lewis et al. (1993) | Age 18–49 yr; history of T1DM≥7 yr with onset <30 yr ; DR, UPE≥500 mg/d and SCr≤2.5 mg/dl | ACEI (captopril, 25 mg 3 times daily) | Placebo | 3 (median) | 409; 51 | 34.5 | 53 | 1.3; 81.53 | 2.75 | DSCR 0.57 (0.36 to 0.89); ESRD 0.63 (0.37 to 1.07) |
| GISEN-REIN-Stratum 1 (1999) | As above; stratum 1: proteinuria 1–2.9 g/d) | ACEI (ramipril, 1.25 mg) | Placebo | 2.68 | 186; 27 | 49.7 | 74.7 | 1.99; 46.60 | 1.70 | DSCR not assessed; ESRD 0.43 (0.20 to 0.92) | |
| RENAAL (2001) | Age 31–70 yr; T2DM; nephropathy (UACR≥300 mg/L and SCr 1.3–3.0 mg/dl) | ARB (losartan, 100 mg/d) | Placebo | 3.4 | 1513; 341 | 60 | 63.2 | 1.9; NR | NR | DSCR 0.83 (0.69 to 1.00); ESRD 0.77 (0.64 to 0.93) | |
| IDNT (2001)a | Age 30–70 yr; T2DM; HT (SBP>135 mmHg, DBP>85 mmHg, or documented treatment with antihypertensive); and proteinuria (UPE≥900 mg/d); SCr 1.0–3.0 mg/dl for women and 1.2–3.0 mg/dl for men | ARB (irbesartan, 75–300 mg/d); CCB (amlodipine, 2.5–10 mg/d) | Placebo | 2.6 | 1148; 183 | 58.9 | 66.5 | 1.67; NR | 2.9 | DSCR 0.71 (0.57 to 0.90); ESRD 0.80 (0.61 to 1.04) | |
| DIABHYCAR (2004) | Age ≥50 yr, T2DM; UAE≥20 mg/L | ACEI (ramipril, 1.25 mg/d) | Placebo | 4 (median) | 4912; 14 | 65.1 | 69.9 | 1.01; NR | NR | DSCR 0.81 (0.56 to 1.18); ESRD 0.40 (0.13 to 1.29) | |
| TRANSCEND (2009) | Age ≥55 yr with documented CVD or DM with end-organ damage who could not tolerate ACEIs | ARB (telmisartan, 8 mg/d) | Placebo | 4.7 | 5926; 17 | 66.9 | 57 | 1.0; 71.75 | NR | DSCR 1.57 (1.03 to 2.37); ESRD 0.70 (0.27 to 1.85) | |
| ORIENT (2011) | Age 30–70 yr; T2DM; UACR>300 mg/g; SCr 1.0–2.5 mg/dl in women and 1.2–2.5 mg/dl in men | ARB (olmesartan, 10–40 mg/d) | Placebo | 3.2 | 568; 152 | 59.2 | 69.1 | 1.62; NR | UACR 192.2 mg/mmol | DSCR 0.89 (0.73 to 1.09); ESRD 0.95 (0.72 to 1.25) | |
| ALTITUDE (2012) | Age ≥35 yr; T2DM receiving oral antidiabetic agents and/or insulin or fasting plasma glucose ≥126 mg/dl or 2-hour plasma glucose ≥200 mg/dl; ≥1 of following: persistent macroalbuminuria (UACR≥200 mg/g) and eGFR≥30 ml/min per 1.73 m2; persistent microalbuminuria (UACR≥20 mg/g and <200 mg/g) and mean eGFR≥30 and <60 ml/min per 1.73 m2; history of CVD and mean eGFR≥30 and <60 ml/min per 1.73m2 | Direct renin inhibition (aliskiren, 150–300 mg/d) | Placebo | 2.74 (median) | 8561; 234 | 64.5 | 68.6 | NR; 57.0 | UACR 207 mg/g | DSCR 0.97 (0.81 to 1.17); ESRD 1.07 (0.83 to 1.38) | |
| KVT (2013) | Age ≥20 yr; hypertension (BP>130/85 mmHg); SCr ≥2.0 mg/dl | ARB (valsartan, 40–160 mg/d) | Conventional therapy (lifestyle modification, diet therapy; glucose, lipid, anemia, potassium, calcium, phosphate, and BP control) | 1.98 (median) | 293; 106 | 64.1 | 72.4 | 3.2; 17.3 | 1.64 | DSCR 0.47 (0.31 to 0.73); ESRD 0.74 (0.54 to 1.01) | |
| BP lowering; RAS blockade versus CCB | Zucchelli et al. (1992) | Age 18–70 yr with established CKD (SCr 1.8–5.0 mg/dl) | ACEI (captopril, 25–100 mg/d) | CCB (nifedipine, 20–40 mg/d) | 4 | 121; 21 | 55 | 57.9 | 2.95; 30.50 | 1.78 | DSCR not assessed; ESRD 0.50 (0.22 to 1.17) |
| BP lowering: ARB versus ACEI versus ARB+ACEI | ONTARGET (2008)b | Age ≥55 yr; ≥1 of following: CAD, PAD, cerebrovascular disease, or DM | ARB (telmisartan, 80 mg/d); ACEI (ramipril, 10 mg/d) | ARB+ACEI (telmisartan+ramipril) | 4.67 | 17,118; 64 | 66.4 | 73.3 | 1.06; 62.2 | UACR 0.82 mg/mmol | DSCR 1.11 (0.88 to 1.39); ESRD 0.94 (0.57 to 1.53) |
| PRONEDI (2013)c | Age >35 yr; T2DM; CKD diabetic nephropathy stages 2–3; UPCR 300 mg/g | ACEI (lisinopril, 40 mg/d); ARB (irbesartan, 600 mg/d) | ACEI+ARB (lisinopril, 20 mg/d +irbesartan, 300 mg/d) | 2.67 (median) | 63; 11 | 68.3 | 70% in lisinopril; 75% in irbesartan; 78% in dual | 1.51; 48.6 | Albumin 3.9 g/dl | DSCR not assessed; ESRD 1.04 (0.35 to 3.06) | |
| BP lowering; others | Hannadouche et al. (1994) | Age 18–70 yr with CKD (SCr 2.26–4.52 mg/dl) | ACEI (enalapril– target DBP<90 mmHg; starting dose 5–10 mg/d according to SCr) | BB (target DBP <90 mmHg) | 3 | 100; 12 | 51.0 | 53 | 2.99; NR | 2.84 | DSCR not assessed; ESRD 0.46 (0.14 to 1.43) |
| ACCOMPLISH (2010) | Age ≥55 yr, history of coronary events, MI, revascularization, stroke, CKD, peripheral arterial disease, LVH, DM | ACEI+CCB (benazepril, 20 mg/d, + amlodipine, 5 mg/d) | ACEI+diuretic (benazepril, 20 mg/d +hydrochlorothiazide, 12.5 mg/d) | 2.9 | 11506; 55 | 68.3 | 60.5 | 1.00; 78.95 | UACR in CKD patients=28.8 mg/mmol; UACR in non-CKD patients=8.7 | DSCR 0.51 (0.40 to 0.64); ESRD 0.84 (0.49 to 1.42) | |
| BP lowering; intensive versus standard | AASK (2002)d | Self-identified African Americans, age 18–70 yr, GFR 20–65 ml/min per 1.73 m2, no other identified causes of renal insufficiency | Target arterial pressure <92 mmHg | Target arterial pressure 102–107 mmHg | 4 | 1094; 171 | 54.6 | 61.2 | 2.18 for men, 1.77 for women; 45.65 | 0.61 in men; 0.41 in women | DSCR not assessed; ESRD 0.92 (0.70 to 1.21) |
| REIN-2 (2005) | Age 18–70 yr; proteinuria 1–3 g/d and CrCl <45 ml/min per 1.73 m2; included if proteinuria ≥3 g/d and CrCl<70 ml/min per 1.73 m2 | Target SBP<130 mmHg and DBP<80 mmHg | Target DBP<90 mmHg irrespective of SBP | 1.58 (median) | 335; 72 | 53.9 | 74.9 | 2.70; 34.99 | 2.85 | DSCR not assessed; ESRD 1.12 (0.74 to 1.69) | |
| Lipid lowering | Endo et al. (2006) | T2DM with clinical albuminuria (UAE>300 mg/g) | Antihyperlipidemic drug+protein-restricted diet (probucol , 500 mg/d +protein-restricted diet 0.8 g/kg per day) | Protein-restricted diet (0.8 g/kg per day) | 2.38 | 102; 23 | 59.6 | 55.9 | 1.59; NR | 1.75 | DSCR not assessed; ESRD 0.76 (0.37 to 1.59) |
| FIELD (2011) | Age 50–75 yr; T2DM; baseline plasma TC 116–251 mg/dl, plus TC/HDL-C ratio ≥4.0 or plasma TG 89–354 mg/dl | Fibrate (fenofibrate, 200 mg/d) | Placebo | 5 (median) | 9795; 47 | 62.2 | 62.7 | 0.88; 87.70 | UACR 1.12 mg/mmol | DSCR 1.65 (1.27 to 2.13); ESRD 0.81 (0.46 to 1.43) | |
| SHARP (2011) | Age ≥40 yr; CKD with >1 Cr ≥1.7 mg/dl in men or 1.5 mg/dl in women, whether receiving dialysis or not | Combination lipid-lowering drug (simvastatin, 20 mg/d+ezetimibe, 10 mg/d) | Placebo | 4.9 (median) | 6247; 2141 | 62 | 62.6 | NR; 26.6 | UACR 206.5 mg/g | DSCR 0.77 (0.62 to 0.96); ESRD 0.98 (0.91 to 1.05) | |
| Glucose lowering; intensive versus standard | UKPDS (1998) | T2DM; fasting plasma glucose >6 mmol/L on 2 mornings, 1–3 wk apart | Intensive glucose lowering (FPG<6 mmol/L; in insulin-treated patients, premeal glucose 4–7 mm/L) with sulfonylurea (chlorpropamide, 100–500 mg/d; glibenclamide, 2.5–20 mg/d; glipizide, 2.5–40 mg/d) | Conventional therapy with diet (FPG<15 mmol/L without symptoms of hyperglycemia) | 10 (median) | 3867; 25 | 53.3 | 61 | 0.92; NR | NR | DSCR 0.42 (0.15 to 1.18); ESRD 0.74 (0.33 to 1.67) |
| VADT (2009) | T2DM with inadequate response to maximal doses of an oral agent or insulin therapy | Intensive glucose lowering (absolute reduction of 1.5% in HbA1c compared with standard therapy) | Standard glucose control | 5.6 (median) | 1766; 18 | 60.4 | 97.1 | 1.0; NR | NR | DSCR 1.00 (0.74 to 1.35); ESRD 0.64 (0.25 to 1.64) | |
| ACCORD (2010)e | Age 40–79 yr; T2DM; HbA1c≥7.5%; history of CVD or age 55–79 yr with anatomic evidence of significant atherosclerosis, albuminuria, LVH, or at ≥2 risk factors for CVD | Intensive glucose lowering (HbA1c<6.0%); intensive BP lowering (SBP<120 mmHg in 4733 participants); lipid-lowering therapy (in 5518 participants; fenofibrate) | Standard glucose control (HbA1c 7.0%–7.9%); standard BP lowering (SBP<140 mmHg); placebo in lipid-lowering therapy | 3.7 (intensive; median); 5 (standard; median) | 10,234; 289 | 62.2 | 62 | 0.90; 90 | UACR 1.54 mg/mmol | DSCR 1.10 (0.96 to 1.26); ESRD 0.91 (0.73 to 1.15) | |
| ADVANCE (2013)f | Age ≥55 yr; T2DM at ≥30 yr and a history of major macrovascular or microvascular disease or ≥1 other risk factor for vascular disease | Intensive glucose control (HbA1c≤6.5%); BP control (SBP<145 mmHg; perindopril, 4 mg+indapamide, 1.25 mg) | Standard glucose control (with target glycated hemoglobin levels defined on the basis of local guidelines; placebo for BP group) | 5 (median) | 11140; 27 | 65.7 | 57.5 | 0.98; 78 | UACR 15 (median) | DSCR 1.15 (0.81 to 1.62); ESRD 0.35 (0.15 to 0.83) | |
| Anemia treatment: high versus partial | Gouva et al. (2004) | Predialysis patients with renal impairment resulting from any cause other than DM with SCr 2.0–6.0 mg/dl and hemoglobin 9.0–11.6 g/dl | Early initiation of EPO (immediately started on 50 U/kg per wk EPO-α with appropriate titration aiming for hemoglobin ≥13 g/dl) | Deferred treatment (start EPO only when hemoglobin decreased to <9 g/dl) | 1.88 (median) | 88; 28 | 65.5 | 56.8 | 3.32; 24.04 | 0.62 | DSCR 0.48 (0.20 to 1.16); ESRD 0.53 (0.28 to 1.02) |
| CAPRIT (2012) | Age 18–80 yr; primary or secondary kidney allograft performed at ≥12 mo before, estimated CrCl <50 ml/min per 1.73 m2; SCr variation <20% in previous 3 mo; using standard immunosuppressive | Complete correction of anemia (target hemoglobin 13–15 g/dl) | Partial correction of anemia (hemoglobin 10.5–11.5 g/dl) | 2 | 125; 16 | 48.9 | 48.8 | 2.12; 34.1 | Albumin 41 g/L | DSCR 0.20 (0.04 to 0.86); ESRD 0.23 (0.07 to 0.76) | |
| Other interventions | Facchini and Saylor et al. (2003) | T2DM; various degrees of renal failure (GFR 15–75 ml/min per 1.73 m2) and otherwise unexplained proteinuria (350–12,000 mg/d) | Carbohydrate-restricted, low-iron-available, polyphenol-enriched dietg | Standard protein diet (0.8 g/kg of protein) | 3.9 | 191; 27 | 59.5 | 52.9 | 1.85; 63.05 | 2.47 | DSCR 0.56 (0.34 to 0.92); ESRD 0.54 (0.26 to 1.11) |
| Chen et al. (2012) | Age 30–83 yr; high-normal body lead burden (lead 80–600 µg; SCr ≤3.9 mg/dl) | Chelation therapy (2-hr 1 g calcium disodium EDTA intravenous infusions+saline solution 200 ml until body lead burden was 60 g) | Weekly 2-hr infusions 20 ml of 50% glucose+saline solution 200 ml over 5 wk | 2.25 | 50; 15 | 58.1 | 80 | 2.85; 28.6 | 3.9 | DSCR 0.53 (0.29 to 0.95); ESRD 0.36 (0.13 to 0.99) |
SCr, serum creatinine; RR, relative risk; RAS, renin-angiotensin system; T1DM, type 1 diabetes mellitus; DR, diabetic retinopathy; UPE, urinary protein excretion; ACEI, angiotensin-converting enzyme inhibitor; DSCR, doubling of serum creatinine; GISEN-REIN, Gruppo Italiano di Studi Epidemiologici in Nefrologia–REIN; T2DM, type 2 diabetes mellitus; UACR, urinary albumin-to-creatinine ratio; ARB, angiotensin-receptor blocker; HT, hypertension; SBP, systolic BP; DBP, diastolic BP; CCB, calcium-channel blocker; DIABHYCAR, Non-insulin-dependent diabetes, Hypertension, Microalbuminuria or Proteinuria, Cardiovascular Events, and Ramipril study; TRANSCEND, Telmisartan Randomized Assessment Study in ACE Intolerant Subjects with Cardiovascular Disease; CVD, cardiovascular disease; DM, diabetes mellitus; ORIENT, Olmesartan Reducing Incidence of Endstage Renal Disease in Diabetic Nephropathy Trial; ALTITUDE, Aliskiren Trial in Type 2 Diabetes Using Cardiorenal Endpoints; KVT, Kanagawa Valsartan Trial; CAD, coronary artery disease; PAD, peripheral artery disease; PRONEDI, Progresión de Nefropatía Diabética; UPCR, urinary protein-to-creatinine ratio; ACCOMPLISH, Avoiding Cardiovascular Events through Combination Therapy in Patients Living with Systolic Hypertension; MI, myocardial infarction; LVH, left ventricular hypertrophy; AASK, African American Study of Kidney Disease and Hypertension; CrCl, creatinine clearance; FIELD, Fenofibrate Intervention and Event Lowering in Diabetes; TC, total cholesterol; HDL-C, HDL cholesterol; TG, triglyceride; SHARP, Study of Heart and Renal Protection; Cr, creatinine; UKPDS, United Kingdom Prospective Diabetes Study; FPG, fasting plasma glucose; VADT, Veterans Affairs Diabetes Trial; HbA1c, hemoglobin A1c; ACCORD, Action to Control Cardiovascular Disease in Diabetes; ADVANCE, Action in Diabetes and Vascular disease: preterAx and diamicroN-MR Controlled Evaluation; EPO, erythropoietin; CAPRIT, Correction of Anemia and Progression of Renal Insufficiency in Transplant patients.
IDNT had three treatment groups (ARB versus CCB versus placebo). For the purpose of analysis, we used the ARB and placebo groups.
ONTARGET had three treatment groups (ARB versus ACEI versus ARB+ACEI). We used the ARB and ACEI groups.
PRONEDI trial had three treatment groups (ACEI versus ARB versus ACEI+ARB). We used the ARB and ACEI groups.
AASK trial was a 3×2 factorial trial; data presented here represent the BP target intervention.
ACCORD trial was a double 2×2 factorial trial assessing intensive glucose-lowering therapy, intensive BP-lowering therapy, and lipid-lowering therapy;
ADVANCE trial was a 2×2 factorial-design trial assessing intensive glucose-lowering and BP-lowering therapies.
Fifty percent reduction in carbohydrates; substitute iron-enriched red meats with iron-poor white meats and with protein-enriched food items known to inhibit iron absorption; eliminate all beverages other than tea, water, and red wine; milk recommended for breakfast; exclusive use of polyphenol-enriched extra-virgin olive oil.
The mean age of study participants ranged between 34.5 and 68.3 years, with the proportion of men between 48.8% and 97.1%. Eighteen trials specified the presence of diabetes as part of their inclusion criteria.8–10,12,13,15–17,19–27,30 The mean GFR of the study participants ranged from 17.3 to 90.0 ml/min per 1.73 m2.
Quality assessment of included trials based on key indicators of trial quality showed that earlier, smaller studies provided few details about the process of randomization, allocation concealment, and the use of intention-to-treat analysis techniques (Supplemental Material 1).
Efficacy of Surrogate Outcomes for ESRD
Proteinuria versus ESRD
Overall, seven trials (17,740 participants; 173 ESRD events)—five trials assessing the effects of BP-lowering agents,17,24,28,33,34 one trial in lipid lowering,9 and one trial assessing the effects of chelation therapy8—reported baseline and follow-up data on proteinuria to permit the calculation of the treatment effect ratios and their 95% CIs. In all seven trials, the direction of treatment effect (the point estimate) on proteinuria corresponded with that of the effect on ESRD, and the pooled treatment effect ratio showed minimal heterogeneity across the individual ratios (treatment effect ratio, 0.82; 95% confidence interval [95% CI], 0.59 to 1.16; I2=0%; P for heterogeneity=0.863) (Figure 2). Results were similar in a weighted bubble plot assessing the relationship between the treatment effects on proteinuria and ESRD (Supplemental Material 2).
Figure 2.
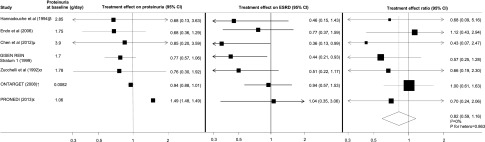
Proteinuria is a promising surrogate for ESRD but data is limited. Comparison of the treatment effects on proteinuria and ESRD. The treatment effect ratio (TER) is the ratio of the relative risks of ESRD and the relative treatment effect on proteinuria; a TER value close to 1 indicates better agreement between the treatment effects. The size of the boxes for the treatment effect ratios (right panel) represent the weight of each study. βauthors have provided final proteinuria data for n=14 in the active (angiotensin-converting enzyme inhibitor [ACEI]) group and n=9 in the control group; µauthors have provided final proteinuria data for n=25 in the active (chelation therapy) group and n=20 in the control group; αauthors have provided final proteinuria data for n=32 in the active (ACEI) group and n=37 in the control group; ↑ONTARGET reported urinary albumin-to-creatinine ratio (UACR; mg/mmol) which has been converted to proteinuria in g/d (UACR mg/mmol was converted to protein excretion mg/d [UACR mg/mmol×10 (10 is the conversion factor)]); εPRONEDI (Progresión de Nefropatía Diabética) reported urinary protein-to-creatinine (UPCR) (g/g), which has been converted to proteinuria in g/d (UPCR g/g was converted to UPCR mg/mmol [UPCR mg/g×0.1131 (0.1131 is the conversion factor)], and UPCR mg/mmol was converted to protein excretion mg/d [UPCR mg/mmol×8.83 (8.83 is the conversion factor)]). Treatment effects on proteinuria are consistent with treatment effects on ESRD. GISEN-REIN, Gruppo Italiano di Studi Epidemiologici in Nefrologia–REIN.
Doubling of Serum Creatinine versus ESRD
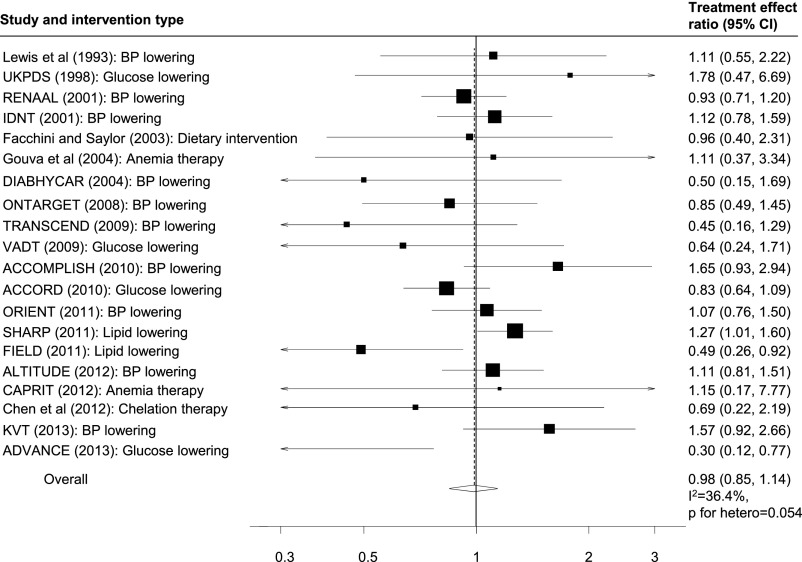
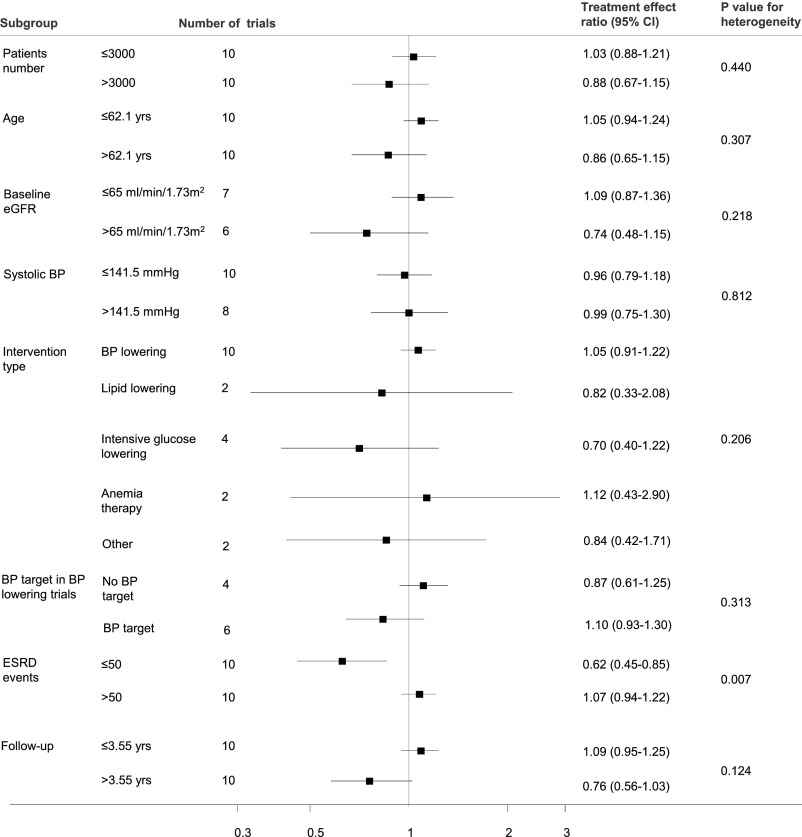
Twenty trials (10 trials assessing the effects of BP lowering,12,13,19,21–26,32 2 trials in lipid lowering,11,15 4 trials comparing the effects of intensive glucose lowering to standard glucose lowering,16,20,27,30 2 trials in anemia therapy,14,18 and 2 trials each assessing the effects of a dietary intervention and chelation therapy8,10) comprising 95,457 participants reported treatment effects on doubling of serum creatinine (3893 events) and ESRD (3850 events) (Figure 3). Overall, the treatment effect on doubling of serum creatinine was consistent with the treatment effect on ESRD (treatment effect ratio, 0.98; 95% CI, 0.85 to 1.14). This consistency was especially observed in trials assessing the effects of a renin-angiotensin system blockade drug against placebo, with a treatment effect ratio between ESRD and doubling of serum creatinine of 1.01 (95% CI, 0.84 to 1.21). However, overall, moderate heterogeneity was observed across the treatment effect ratios (I2=34.9%; P=0.05). A weighted bubble plot assessing for the relationship between the treatment effects on doubling of serum creatinine and ESRD showed similar results (Supplemental Material 3). Subgroup analyses identified the number of ESRD events reported in the trial as a significant source of heterogeneity (P<0.001) (Figure 4).
Figure 3.
Doubling of serum creatinine is generally a good surrogate for ESRD. Comparison of the treatment effects on doubling of serum creatinine and ESRD. Treatment effect ratio close to 1 indicates better agreement between the treatment effects; the size of the boxes for the treatment effect ratios (right panel) represent the weight of each study; the treatment effects on doubling of serum creatinine are consistent with the treatment effects on ESRD. ACCOMPLISH, Avoiding Cardiovascular Events through Combination Therapy in Patients Living with Systolic Hypertension; ACCORD, Action to Control Cardiovascular Disease in Diabetes; ADVANCE, Action in Diabetes and Vascular disease: preterAx and diamicroN-MR Controlled Evaluation; ALTITUDE, Aliskiren Trial in Type 2 Diabetes Using Cardiorenal Endpoints; CAPRIT, Correction of Anemia and Progression of Renal Insufficiency in Transplant patients; DIABHYCAR, Non-insulin-dependent diabetes, Hypertension, Microalbuminuria or Proteinuria, Cardiovascular Events, and Ramipril study; FIELD, Fenofibrate Intervention and Event Lowering in Diabetes; KVT, Kanagawa Valsartan Trial; ONTARGET, ONgoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial; ORIENT, Olmesartan Reducing Incidence of Endstage Renal Disease in Diabetic Nephropathy Trial; SHARP, Study of Heart and Renal Protection; TRANSCEND, Telmisartan Randomised Assessment Study in ACE Intolerant Subjects with Cardiovascular Disease; UKPDS, United Kingdom Prospective Diabetes Study; VADT, Veterans Affairs Diabetes Trial.
Figure 4.
Doubling of serum creatinine may be a less effective surrogate for ESRD in trials of patients with better preserved kidney function. Subgroup analysis to explore sources of heterogeneity in the pooled treatment effect ratio comparing doubling of serum creatinine and ESRD.
Sensitivity Analysis
Only four trials provided follow-up 24-hour urine protein excretion data between baseline and 13 months for the assessment of early change in proteinuria as a surrogate for ESRD.8,17,33,34 Follow-up proteinuria measurements at 3, 4, and 12 months (for two studies) were used. The overall results, compared with proteinuria change at end of study, remained essentially unchanged.
Publication Bias
Formal statistical testing showed evidence of publication bias (Egger test P<0.001) for the outcome of ESRD (Supplemental Material 4).
Discussion
For our large systematic review, we comprehensively searched the literature for RCTs assessing the effects of a broad range of interventions on the risk of ESRD to assess the efficacy of change in proteinuria and doubling of serum creatinine as surrogate outcomes for ESRD. Overall, the available data suggest that treatment effects on proteinuria and doubling of serum creatinine are consistent with the effects on ESRD across most intervention types assessed and that, more specifically, doubling of serum creatinine is generally a good surrogate for ESRD while proteinuria requires further assessment of its validity.
The use of surrogate outcomes, particularly in RCTs, is largely based on trial feasibility, including sample size and study duration. Indeed, the lack of effective surrogate outcomes in nephrology has arguably had a substantial influence on the generation of robust level 1 clinical evidence; the number of published RCTs in nephrology has been reported to be lower than in all other subspecialties of internal medicine.3 Prentice’s definition and operational criteria to assess the validity of a surrogate outcome require a surrogate to be not only closely correlated with the true clinical outcome (“individual-level association”) but also for the treatment effect on the surrogate to predict the treatment effect on the true clinical outcome (“trial-level association”).35 Experimental evidence supports the biologic plausibility of proteinuria as a surrogate for ESRD,36,37 demonstrating the relationship between accumulated protein during heavy proteinuria and progressive nephropathy.38 Clinical evidence also supports a strong graded association between baseline proteinuria and the risk of clinically important outcomes, including cardiovascular disease and ESRD.39–42 The prognostic importance of proteinuria has been supported by evidence showing the effectiveness of BP-lowering therapies, particularly on slowing the renal progression of CKD in people with proteinuria at baseline.43 The importance of proteinuria in CKD and the role it plays in the subsequent development of ESRD has identified proteinuria as a potential surrogate outcome in clinical trials5; indeed, proteinuria change as a surrogate for ESRD has been used in many small-scale, earlier-phase trials.44–46 However, proteinuria is currently not accepted as a surrogate outcome in broader large-scale, multicenter trials.5
Accumulating data from post hoc analyses of RCTs, particularly in BP-lowering trials, do, however, suggest that further assessment of the surrogacy of proteinuria is warranted. In a post hoc analysis of a subgroup (n=810) of the African American Study of Kidney Disease and Hypertension, a doubling of the urine protein-to-creatinine ratio in the first 6 months of BP-lowering treatment was associated with a mean±SD greater decline in GFR of 0.63±0.10 ml/min per 1.73 m2 per year and was predictive of progression to ESRD (relative risk, 2.11; 95% CI, 1.89 to 2.36).47 In a another post hoc analysis of the REIN (Ramipril Efficacy In Nephropathy) trial,48 in which 352 participants with chronic nephropathy (defined as creatinine clearance of 20–70 ml/min per 1.73 m2) and persistent proteinuria were randomly assigned to ramipril and placebo, GFR decline was significantly slower in patients who had an initial 3-month reduction in proteinuria compared with those without a short-term reduction (−0.28±0.04 ml/min per 1.73 m2 per month versus −0.53±0.07 ml/min per 1.73 m2 per month; P=0.04). Similar results were reported in post hoc analyses of the Reduction in End Points in Noninsulin-Dependent Diabetes Mellitus with the Angiotensin II Antagonist Losartan (RENAAL)49 and Irbesartan Diabetic Nephropathy Trial (IDNT),50 suggesting that an early change in proteinuria may predict a longer-term renal benefit.
A recent meta-analysis sought to address this issue by assessing the efficacy of proteinuria as a surrogate for kidney disease progression (clinical outcome defined as a composite of doubling of serum creatinine, ESRD, or death) using individual participant-level data from 32 RCTs. Inker et al. reported that an early reduction in proteinuria was associated with lower risk of the clinical outcome and that the overall direction of the treatment effects on early change in proteinuria was consistent with the direction of the treatment effect on the clinical outcome. However, the study could not assess whether the treatment effects on early change in proteinuria were proportional to the effects on the clinical outcomes. While there are key differences between our study and the study by Inker et al., including the use of published-level versus participant-level data, definition of the clinical outcome, the time point at which proteinuria change was determined, and the assessment of doubling of serum creatinine as a surrogate outcome for ESRD (in our study), the overall conclusions are consistent across the two studies and together add complementary information on the efficacy of change in proteinuria as a surrogate outcome for ESRD.
In our sensitivity analysis comparing the surrogacy of early and late proteinuria change, our overall results remained essentially unchanged; the treatment effects on proteinuria were largely consistent across the early and later time periods of study follow-up. While the overall utility of proteinuria as a surrogate for ESRD requires further assessment, these results support the use of early change in proteinuria, compared with longer-term change, as a surrogate for ESRD. The use of early proteinuria change as a surrogate for ESRD has methodologic appeal, particularly because proteinuria occurs at an early stage of kidney disease and thus has important implications for RCT design and conduct, allowing for shorter durations of follow-up and reductions in costs. However, our results on the surrogacy of proteinuria are limited by the critical lack of studies reporting treatment effects on proteinuria and ESRD. Thus, caution is needed with interpreting these results. Further assessment of this issue is warranted.
Contrary to some evidence supporting proteinuria as a surrogate, data also suggest that treatment effects on proteinuria by itself should not be considered a definitive indicator of the likely effects on ESRD. The ONgoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial (ONTARGET), which randomly assigned participants to telmisartan, ramipril, or a combination of both, reported significant reductions in albumin-to-creatinine ratio with combination therapy (P<0.001 compared with ramipril group) but reported contrasting effects on the composite renal outcome (hazard ratio, 1.09; 95% CI, 1.01 to 1.18; P=0.04).24 Several reasons have been suggested to explain the contrasting results,51 such as the low baseline proteinuria of the participants and the inclusion of acute hemodialysis treatment in the composite outcome; however, the differing results support the need for careful further investigations to assess whether change in proteinuria is a valid surrogate for ESRD.
Our results support the utility of doubling of serum creatinine, an already frequently used surrogate for ESRD, particularly in BP-lowering interventions. Doubling of serum creatinine as a surrogate for ESRD is based on the rationale that patients experiencing a halving of kidney function (which is approximately equivalent to a doubling of baseline serum creatinine) would most likely progress to kidney failure requiring long-term or renal transplantation. Because it is presumed that doubling of serum creatinine will occur more frequently compared with ESRD, it has been incorporated into the commonly used composite renal outcome of dialysis, renal transplantation, and death. Despite its broad use in renal trials, there have been ongoing debates regarding its limitations, particularly because doubling of serum creatinine does not precisely reflect the rate of renal function decline.4,52 On the basis of this, it is plausible that smaller proportional increases in serum creatinine (or decreases in eGFR) could also predict risk of ESRD. Indeed, lesser changes in GFR or the rate of GFR decline have been considered as potential surrogates for ESRD.7 A recent post hoc analysis of the RENAAL study and IDNT to assess whether smaller declines in GFR (eGFR declines of 40%, 30%, and 20%) would improve statistical power showed that these lesser eGFR declines resulted in more participants reaching the surrogate outcome but did not consistently improve statistical power of the clinical trials.6 Furthermore, the recent data also suggest that additional considerations, such as the presence of an acute effect on eGFR from the trial therapy, need to be made in considering the use of lesser eGFR reductions as surrogate endpoints for ESRD.6,53 Further studies are needed to assess the potential of lesser changes in serum creatinine or eGFR as surrogates for ESRD.
Our results also suggest that doubling of serum creatinine is generally a good surrogate for ESRD but may not be adequate in some circumstances. In subgroup analysis, we identified the number of ESRD events reported in the trials as a source of heterogeneity, showing that in trials reporting ≤50 ESRD events, ESRD was underestimated by doubling of serum creatinine. The number of ESRD events in trials is largely influenced by the severity of baseline kidney disease, suggesting that doubling of serum creatinine is less effective as a surrogate in trials that include patients with better-preserved kidney function.
Subgroup analysis did not show intervention type as an important source of heterogeneity. However, these results need to be interpreted with caution because most included trials were of BP-lowering interventions. Indeed, when assessed individually, the treatment effect ratios of lipid- and glucose-lowering interventions differed substantially from the point of null (i.e., ideal surrogate) compared with the BP-lowering interventions.
Our systematic review benefits from the many identified RCTs to explore the utility of surrogate outcomes for ESRD across a broad spectrum of intervention types. However, our study has limitations that should be considered in interpreting the results. Our study is primarily limited by the assessment of published trial-level data and the lack of RCTs specifically designed to assess the efficacy of surrogate outcomes for ESRD. Only seven trials reported sufficient information to allow comparison of treatment effects on proteinuria and ESRD. In describing the correlation between surrogate outcomes and ESRD, we have attempted to assess the validity of surrogate outcomes for ESRD by addressing only one of Prentice’s criteria for the validation of surrogates. In addition, our analysis did not account for the possible correlation between the variance in the estimated treatment effects on the surrogate outcomes and ESRD. In this review, we have assessed the efficacy of surrogate outcomes for treated ESRD. A substantial number of events with doubling of serum creatinine may not result in treated kidney failure. This review has not determined the surrogacy of lesser changes in serum creatinine or GFR. Use of individual participant-level data have great potential to address these questions. Finally, we detected significant publication bias for the outcome of ESRD, and it may thus be possible that the association between the treatment effects on proteinuria and ESRD in these studies differ.
We found that across a broad range of interventions, the treatment effect on surrogate outcomes of ESRD, including change in proteinuria and doubling of serum creatinine, is generally consistent with the effect on ESRD. Our study suggests that further assessment to explore the validity of proteinuria as a surrogate for ESRD through prospective trials is needed.
Concise Methods
Data Sources and Search Strategy
We did a systematic review of the literature according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement for the conduct of meta-analyses of RCTs. Relevant studies were identified by searching MEDLINE via Ovid (1950 through September 2013), EMBASE (1966 through September 2013), and the Cochrane Library database (CENTRAL; no date restriction), using relevant text words and medical subject headings (Supplemental Material 5). We limited the search to RCTs but placed no restrictions on the type of intervention or language. Reference lists from identified trials and review articles were manually scanned to identify other relevant studies. Complete methods are available in the Supplemental Material.
Study Selection
Two authors performed (M.J. and T.C.T.) independently performed the literature search, data extraction, and quality assessment using a standardized approach. All completed RCTs, irrespective of intervention or comparator, conducted in adults (age ≥18 years) with proteinuria, diabetes, CKD stages 1–4 (defined as per Kidney Disease Improving Global Outcomes CKD stages),1 or renal transplant recipients and reporting the outcome of ≥10 ESRD events (defined as the need for long-term or renal transplantation) and any one of the following surrogate outcomes during a follow-up of ≥1 year were eligible for inclusion in the systematic review: (1) change in proteinuria or (2) doubling of serum creatinine from baseline.
Data Extraction and Quality Assessment
We obtained published reports for each trial and extracted selected information, including study and baseline patient characteristics, the type of intervention, follow-up duration, and outcome events. Study quality was judged by reporting of randomization method, concealment of treatment allocation, similarity of treatment groups at baseline, description of the eligibility criteria, completeness of follow-up, and use of intention-to-treat analysis. A third reviewer (B.H.) adjudicated disagreements over abstracted data.
Outcomes
We collected data on ESRD, change in proteinuria (from baseline to end of study; 24-hour proteinuria, urinary albumin-to-creatinine ratio, urinary protein-to-creatinine ratio; absolute and proportional; or as defined by authors), and doubling of serum creatinine.
Data Synthesis and Analyses
Individual-study relative risks and 95% CIs were calculated from event numbers (for binary outcomes, including doubling of creatinine and ESRD) extracted from each trial before data pooling. On the basis of the rationale that for an ideal surrogate outcome, the treatment effect on the change of the surrogate outcome would be proportional to that of the treatment effect on the change of the clinical outcome,7,54 we assessed the validity of proteinuria and doubling of serum creatinine as surrogate outcomes for ESRD based on treatment effect ratios. The treatment effect ratio was defined as the relative risk for the treatment effect on ESRD divided by the relative treatment effect on the surrogate outcome (i.e., treatment effects on surrogate outcomes also presented as ratios). A treatment effect ratio value close to 1 indicates greater agreement between ESRD and the surrogate outcomes. Consistent with prior studies,55 the treatment effect on change in proteinuria was expressed as a ratio of the end-of-study proteinuria values (urine protein excretion in g/d) and the geometric baseline proteinuria values between the two study treatment groups. For doubling of serum creatinine, the relative risk was calculated on the basis of the event numbers and treatment group sizes. These ratios were pooled across interventions. A summary estimate of the treatment effect ratio was obtained using a random-effects model.
The percentage of variability across studies attributable to heterogeneity beyond chance was estimated using the I2 statistic.56 We also assessed the relationship between the treatment effects on ESRD and surrogate outcomes using linear regression weighted by the inverse of the variance for the estimate of the treatment effect on ESRD. We explored potential heterogeneity in estimates of the treatment effect ratio by comparing summary results obtained from subsets of studies grouped by number of patients and mean values for age, baseline eGFR, systolic BP, intervention types, number of ESRD events reported, and follow-up duration. We assessed potential publication bias with the Egger test, represented graphically with the Begg funnel plot of the natural log of the relative risk versus its SEM.57 A two-sided P value <0.05 was considered to represent statistically significant differences. Statistical analyses were performed with Stata software, version 9.2 (Stata Corp., College Station, TX).
Sensitivity Analyses
We performed sensitivity analyses to assess whether earlier change in proteinuria, compared with change at the end of study (i.e., late change), better predicted ESRD. As was done in prior studies, we defined early change in proteinuria as the change in 24-hour urine protein excretion from baseline to the first follow-up measurement between 2.5 and 13 months.
Disclosures
None.
Acknowledgments
M.J. is supported by postdoctoral fellowships from the Canadian Institutes of Health Research, Alberta Innovates Health Solutions, and the National Health and Medical Research Council (NHMRC) of Australia. M.W. is supported by the NHMRC. B.H. is supported by the Roy and Vi Baay Chair in Kidney Disease. M.T. and B.M. are supported by salary awards from Alberta Innovates–Health Solutions.
The corresponding author had full access to all data in the study and takes responsibility for the integrity of the data and accuracy of the analysis.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014040396/-/DCSupplemental.
References
- 1.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group : KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl 3: 1–150, 2013 [Google Scholar]
- 2.Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, Saran R, Wang AY, Yang CW: Chronic kidney disease: Global dimension and perspectives. Lancet 382: 260–272, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Strippoli GF, Craig JC, Schena FP: The number, quality, and coverage of randomized controlled trials in nephrology. J Am Soc Nephrol 15: 411–419, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Lambers Heerspink HJ, Perkovic V, de Zeeuw D: Is doubling of serum creatinine a valid clinical 'hard' endpoint in clinical nephrology trials? Nephron 119: c195–c199; discussion c9, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Levey AS, Cattran D, Friedman A, Miller WG, Sedor J, Tuttle K, Kasiske B, Hostetter T: Proteinuria as a surrogate outcome in CKD: Report of a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis 54: 205–226, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Lambers Heerspink HJ, Weldegiorgis M, Inker LA, Gansevoort R, Parving HH, Dwyer JP, Mondal H, Coresh J, Greene T, Levey AS, de Zeeuw D: Estimated GFR decline as a surrogate end point for kidney failure: A post hoc analysis from the Reduction of End Points in Non-Insulin-Dependent Diabetes With the Angiotensin II Antagonist Losartan (RENAAL) study and Irbesartan Diabetic Nephropathy Trial (IDNT). Am J Kidney Dis 63: 244–250, 2014 [DOI] [PubMed] [Google Scholar]
- 7.Stevens LA, Greene T, Levey AS: Surrogate end points for clinical trials of kidney disease progression. Clin J Am Soc Nephrol 1: 874–884, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Chen KH, Lin JL, Lin-Tan DT, Hsu HH, Hsu CW, Hsu KH, Yen TH: Effect of chelation therapy on progressive diabetic nephropathy in patients with type 2 diabetes and high-normal body lead burdens. Am J Kidney Dis 60: 530–538, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Endo K, Miyashita Y, Sasaki H, Ohira M, Saiki A, Koide N, Otsuka M, Oyama T, Takeyoshi M, Ito Y, Shirai K: Probucol delays progression of diabetic nephropathy. Diabetes Res Clin Pract 71: 156–163, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Facchini FS, Saylor KL: A low-iron-available, polyphenol-enriched, carbohydrate-restricted diet to slow progression of diabetic nephropathy. Diabetes 52: 1204–1209, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tomson C, Wanner C, Krane V, Cass A, Craig J, Neal B, Jiang L, Hooi LS, Levin A, Agodoa L, Gaziano M, Kasiske B, Walker R, Massy ZA, Feldt-Rasmussen B, Krairittichai U, Ophascharoensuk V, Fellström B, Holdaas H, Tesar V, Wiecek A, Grobbee D, de Zeeuw D, Grönhagen-Riska C, Dasgupta T, Lewis D, Herrington W, Mafham M, Majoni W, Wallendszus K, Grimm R, Pedersen T, Tobert J, Armitage J, Baxter A, Bray C, Chen Y, Chen Z, Hill M, Knott C, Parish S, Simpson D, Sleight P, Young A, Collins R, SHARP Investigators : The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): A randomised placebo-controlled trial. Lancet 377: 2181–2192, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bakris GL, Sarafidis PA, Weir MR, Dahlöf B, Pitt B, Jamerson K, Velazquez EJ, Staikos-Byrne L, Kelly RY, Shi V, Chiang YT, Weber MA, ACCOMPLISH Trial investigators : Renal outcomes with different fixed-dose combination therapies in patients with hypertension at high risk for cardiovascular events (ACCOMPLISH): A prespecified secondary analysis of a randomised controlled trial. Lancet 375: 1173–1181, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S, RENAAL Study Investigators : Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 345: 861–869, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Choukroun G, Kamar N, Dussol B, Etienne I, Cassuto-Viguier E, Toupance O, Glowacki F, Moulin B, Lebranchu Y, Touchard G, Jaureguy M, Pallet N, Le Meur Y, Rostaing L, Martinez F, CAPRIT study Investigators : Correction of postkidney transplant anemia reduces progression of allograft nephropathy. J Am Soc Nephrol 23: 360–368, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis TM, Ting R, Best JD, Donoghoe MW, Drury PL, Sullivan DR, Jenkins AJ, O’Connell RL, Whiting MJ, Glasziou PP, Simes RJ, Kesäniemi YA, Gebski VJ, Scott RS, Keech AC, Fenofibrate Intervention and Event Lowering in Diabetes Study investigators : Effects of fenofibrate on renal function in patients with type 2 diabetes mellitus: The Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) Study. Diabetologia 54: 280–290, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD, VADT Investigators : Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 360: 129–139, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Fernandez Juarez G, Luño J, Barrio V, de Vinuesa SG, Praga M, Goicoechea M, Cachofeiro V, Nieto J, Fernández Vega F, Tato A, Gutierrez E, PRONEDI Study Group : Effect of dual blockade of the renin-angiotensin system on the progression of type 2 diabetic nephropathy: a randomized trial. Am J Kidney Dis 61: 211–218, 2013 [DOI] [PubMed] [Google Scholar]
- 18.Gouva C, Nikolopoulos P, Ioannidis JP, Siamopoulos KC: Treating anemia early in renal failure patients slows the decline of renal function: A randomized controlled trial. Kidney Int 66: 753–760, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Imai E, Chan JC, Ito S, Yamasaki T, Kobayashi F, Haneda M, Makino H, ORIENT study investigators : Effects of olmesartan on renal and cardiovascular outcomes in type 2 diabetes with overt nephropathy: A multicentre, randomised, placebo-controlled study. Diabetologia 54: 2978–2986, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ismail-Beigi F, Craven T, Banerji MA, Basile J, Calles J, Cohen RM, Cuddihy R, Cushman WC, Genuth S, Grimm RH, Jr, Hamilton BP, Hoogwerf B, Karl D, Katz L, Krikorian A, O’Connor P, Pop-Busui R, Schubart U, Simmons D, Taylor H, Thomas A, Weiss D, Hramiak I, ACCORD trial group : Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: An analysis of the ACCORD randomised trial. Lancet 376: 419–430, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD, The Collaborative Study Group : The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. N Engl J Med 329: 1456–1462, 1993 [DOI] [PubMed] [Google Scholar]
- 22.Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I, Collaborative Study Group : Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 345: 851–860, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Mann JF, Schmieder RE, Dyal L, McQueen MJ, Schumacher H, Pogue J, Wang X, Probstfield JL, Avezum A, Cardona-Munoz E, Dagenais GR, Diaz R, Fodor G, Maillon JM, Rydén L, Yu CM, Teo KK, Yusuf S, TRANSCEND (Telmisartan Randomised Assessment Study in ACE Intolerant Subjects with Cardiovascular Disease) Investigators : Effect of telmisartan on renal outcomes: A randomized trial. Ann Intern Med 151: 1–10, W1-2, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Mann JF, Schmieder RE, McQueen M, Dyal L, Schumacher H, Pogue J, Wang X, Maggioni A, Budaj A, Chaithiraphan S, Dickstein K, Keltai M, Metsärinne K, Oto A, Parkhomenko A, Piegas LS, Svendsen TL, Teo KK, Yusuf S, ONTARGET investigators : Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): A multicentre, randomised, double-blind, controlled trial. Lancet 372: 547–553, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Marre M, Lievre M, Chatellier G, Mann JF, Passa P, Ménard J, DIABHYCAR Study Investigators : Effects of low dose ramipril on cardiovascular and renal outcomes in patients with type 2 diabetes and raised excretion of urinary albumin: Randomised, double blind, placebo controlled trial (the DIABHYCAR study). BMJ 328: 495, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parving HH, Brenner BM, McMurray JJ, de Zeeuw D, Haffner SM, Solomon SD, Chaturvedi N, Persson F, Desai AS, Nicolaides M, Richard A, Xiang Z, Brunel P, Pfeffer MA, ALTITUDE Investigators : Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med 367: 2204–2213, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Perkovic V, Heerspink HL, Chalmers J, Woodward M, Jun M, Li Q, MacMahon S, Cooper ME, Hamet P, Marre M, Mogensen CE, Poulter N, Mancia G, Cass A, Patel A, Zoungas S, ADVANCE Collaborative Group : Intensive glucose control improves kidney outcomes in patients with type 2 diabetes. Kidney Int 83: 517–523, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Ruggenenti P, Perna A, Gherardi G, Garini G, Zoccali C, Salvadori M, Scolari F, Schena FP, Remuzzi G: Renoprotective properties of ACE-inhibition in non-diabetic nephropathies with non-nephrotic proteinuria. Lancet 354: 359–364, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Ruggenenti P, Perna A, Loriga G, Ganeva M, Ene-Iordache B, Turturro M, Lesti M, Perticucci E, Chakarski IN, Leonardis D, Garini G, Sessa A, Basile C, Alpa M, Scanziani R, Sorba G, Zoccali C, Remuzzi G, REIN-2 Study Group : Blood-pressure control for renoprotection in patients with non-diabetic chronic renal disease (REIN-2): Multicentre, randomised controlled trial. Lancet 365: 939–946, 2005 [DOI] [PubMed] [Google Scholar]
- 30.UK Prospective Diabetes Study (UKPDS) Group : Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 352: 837–853, 1998 [PubMed] [Google Scholar]
- 31.Wright JT, Jr, Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J, Cheek D, Douglas-Baltimore JG, Gassman J, Glassock R, Hebert L, Jamerson K, Lewis J, Phillips RA, Toto RD, Middleton JP, Rostand SG, African American Study of Kidney Disease and Hypertension Study Group : Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: Results from the AASK trial. JAMA 288: 2421–2431, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Yasuda T, Endoh M, Suzuki D, Yoshimura A, Ideura T, Tamura K, Kamata K, Toya Y, Umemura S, Kimura K, KVT Study Group : Effects of valsartan on progression of kidney disease in Japanese hypertensive patients with advanced, predialysis, chronic kidney disease: Kanagawa Valsartan Trial (KVT). Hypertens Res 36: 240–246, 2013 [DOI] [PubMed] [Google Scholar]
- 33.Zucchelli P, Zuccalà A, Borghi M, Fusaroli M, Sasdelli M, Stallone C, Sanna G, Gaggi R: Long-term comparison between captopril and nifedipine in the progression of renal insufficiency. Kidney Int 42: 452–458, 1992 [DOI] [PubMed] [Google Scholar]
- 34.Hannedouche T, Landais P, Goldfarb B, el Esper N, Fournier A, Godin M, Durand D, Chanard J, Mignon F, Suo JM, et al. : Randomised controlled trial of enalapril and beta blockers in non-diabetic chronic renal failure. BMJ 309: 833–837, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prentice RL: Surrogate endpoints in clinical trials: Definition and operational criteria. Stat Med 8: 431–440, 1989 [DOI] [PubMed] [Google Scholar]
- 36.Abbate M, Benigni A, Bertani T, Remuzzi G: Nephrotoxicity of increased glomerular protein traffic. Nephrol Dial Transplant 14: 304–312, 1999 [DOI] [PubMed] [Google Scholar]
- 37.Remuzzi G, Bertani T: Pathophysiology of progressive nephropathies. N Engl J Med 339: 1448–1456, 1998 [DOI] [PubMed] [Google Scholar]
- 38.Abbate M, Zoja C, Morigi M, Rottoli D, Angioletti S, Tomasoni S, Zanchi C, Longaretti L, Donadelli R, Remuzzi G: Transforming growth factor-beta1 is up-regulated by podocytes in response to excess intraglomerular passage of proteins: A central pathway in progressive glomerulosclerosis. Am J Pathol 161: 2179–2193, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hemmelgarn BR, Manns BJ, Lloyd A, James MT, Klarenbach S, Quinn RR, Wiebe N, Tonelli M, Alberta Kidney Disease Network : Relation between kidney function, proteinuria, and adverse outcomes. JAMA 303: 423–429, 2010 [DOI] [PubMed] [Google Scholar]
- 40.Iseki K, Ikemiya Y, Iseki C, Takishita S: Proteinuria and the risk of developing end-stage renal disease. Kidney Int 63: 1468–1474, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Ninomiya T, Perkovic V, de Galan BE, Zoungas S, Pillai A, Jardine M, Patel A, Cass A, Neal B, Poulter N, Mogensen CE, Cooper M, Marre M, Williams B, Hamet P, Mancia G, Woodward M, Macmahon S, Chalmers J, ADVANCE Collaborative Group : Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol 20: 1813–1821, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perkovic V, Verdon C, Ninomiya T, Barzi F, Cass A, Patel A, Jardine M, Gallagher M, Turnbull F, Chalmers J, Craig J, Huxley R: The relationship between proteinuria and coronary risk: A systematic review and meta-analysis. PLoS Med 5: e207, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lv J, Ehteshami P, Sarnak MJ, Tighiouart H, Jun M, Ninomiya T, Foote C, Rodgers A, Zhang H, Wang H, Strippoli GF, Perkovic V: Effects of intensive blood pressure lowering on the progression of chronic kidney disease: A systematic review and meta-analysis. CMAJ 185: 949–957, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fallahzadeh MK, Dormanesh B, Sagheb MM, Roozbeh J, Vessal G, Pakfetrat M, Daneshbod Y, Kamali-Sarvestani E, Lankarani KB: Effect of addition of silymarin to renin-angiotensin system inhibitors on proteinuria in type 2 diabetic patients with overt nephropathy: a randomized, double-blind, placebo-controlled trial. Am J Kidney Dis 60: 896–903, 2012 [DOI] [PubMed] [Google Scholar]
- 45.Parving HH, Persson F, Lewis JB, Lewis EJ, Hollenberg NK, AVOID Study Investigators : Aliskiren combined with losartan in type 2 diabetes and nephropathy. N Engl J Med 358: 2433–2446, 2008 [DOI] [PubMed] [Google Scholar]
- 46.de Zeeuw D, Agarwal R, Amdahl M, Audhya P, Coyne D, Garimella T, Parving HH, Pritchett Y, Remuzzi G, Ritz E, Andress D: Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): a randomised controlled trial. Lancet 376: 1543–1551, 2010 [DOI] [PubMed] [Google Scholar]
- 47.Lea J, Greene T, Hebert L, Lipkowitz M, Massry S, Middleton J, Rostand SG, Miller E, Smith W, Bakris GL: The relationship between magnitude of proteinuria reduction and risk of end-stage renal disease: Results of the African American study of kidney disease and hypertension. Arch Intern Med 165: 947–953, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Ruggenenti P, Perna A, Remuzzi G, GISEN Group Investigators : Retarding progression of chronic renal disease: The neglected issue of residual proteinuria. Kidney Int 63: 2254–2261, 2003 [DOI] [PubMed] [Google Scholar]
- 49.de Zeeuw D, Remuzzi G, Parving HH, Keane WF, Zhang Z, Shahinfar S, Snapinn S, Cooper ME, Mitch WE, Brenner BM: Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: Lessons from RENAAL. Kidney Int 65: 2309–2320, 2004 [DOI] [PubMed] [Google Scholar]
- 50.Atkins RC, Briganti EM, Lewis JB, Hunsicker LG, Braden G, Champion de Crespigny PJ, DeFerrari G, Drury P, Locatelli F, Wiegmann TB, Lewis EJ: Proteinuria reduction and progression to renal failure in patients with type 2 diabetes mellitus and overt nephropathy. Am J Kidney Dis 45: 281–287, 2005 [DOI] [PubMed] [Google Scholar]
- 51.Ruggenenti P, Remuzzi G: Proteinuria: Is the ONTARGET renal substudy actually off target? Nat Rev Nephrol 5: 436–437, 2009 [DOI] [PubMed] [Google Scholar]
- 52.Takeuchi N, Takenoshita E, Kato F, Terajima T, Ogawa M, Suzuki S, Fujii T, Kobayashi E, Sakurada T, Satoh N, Ueda S: Doubling of serum creatinine: is it appropriate as the endpoint for CKD? Proposal of a new surrogate endpoint based on the reciprocal of serum creatinine. Clin Exp Nephrol 15: 100–107, 2011 [DOI] [PubMed] [Google Scholar]
- 53.Greene T, Teng C-C, Ying J: Validity and statistical power of alternative eGFR-based endpoints: A report from an NKF FDA Workshop. Presented at Kidney Week 2013, Atlanta, GA, November 5–10, 2013 p. TH-PO211. [Google Scholar]
- 54.Buyse M, Molenberghs G, Burzykowski T, Renard D, Geys H: The validation of surrogate endpoints in meta-analyses of randomized experiments. Biostatistics 1: 49–67, 2000 [DOI] [PubMed] [Google Scholar]
- 55.Inker LA, Levey AS, Pandya K, Stoycheff N, Okparavero A, Greene T, Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) : Early change in proteinuria as a surrogate end point for kidney disease progression: An individual patient meta-analysis. Am J Kidney Dis 64: 74–85, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Woodward M: Epidemiology: Design and Data Analysis, 2nd Ed., Boca Raton, FL, Chapman and Hall/CRC Press, 2005 [Google Scholar]
- 57.Egger M, Davey Smith G, Schneider M, Minder C: Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629–634, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]