Abstract
Complement activation has a major role in thrombotic microangiopathy (TMA), a disorder that can occur in a variety of clinical conditions. Promising results of recent trials with terminal complement-inhibiting drugs call for biomarkers identifying patients who might benefit from this treatment. The primary aim of this study was to determine the prevalence and localization of complement factor C4d in kidneys of patients with TMA. The secondary aims were to determine which complement pathways lead to C4d deposition and to determine whether complement activation results in deposition of the terminal complement complex. We examined 42 renal sections with histologically confirmed TMA obtained from a heterogeneous patient group. Deposits of C4d, mannose-binding lectin, C1q, IgM, and C5b-9 were scored in the glomeruli, peritubular capillaries, and arterioles. Notably, C4d deposits were present in 88.1% of TMA cases, and the various clinical conditions had distinct staining patterns within the various compartments of the renal vasculature. Classical pathway activation was observed in 90.5% of TMA cases. C5b-9 deposits were present in 78.6% of TMA cases and in 39.6% of controls (n=53), but the staining pattern differed between cases and controls. In conclusion, C4d is a common finding in TMA, regardless of the underlying clinical condition. Moreover, C5b-9 was present in >75% of the TMA samples, suggesting that terminal complement inhibitors may have a beneficial effect in these patients. C4d and C5b-9 should be investigated as possible diagnostic biomarkers in the clinical work-up of patients suspected of having complement-mediated TMA.
Keywords: renal pathology, complement, hemolytic uremic syndrome, thrombosis, renal biopsy, renal transplantation
Thrombotic microangiopathy (TMA) is a devastating disorder characterized by the development of multiple vascular microthrombi, endothelial cell damage, thrombocytopenia, and hemolysis.1 TMA is mostly systemic, with the kidney and brain most commonly affected.2 Renal biopsies can reveal the presence of microthrombi even in the absence of systemic manifestations. TMA can occur in a wide range of diseases.3,4 Given the heterogeneity of TMA-associated diseases, identifying mechanistic pathways common in most cases has diagnostic and therapeutical value.
Since the discovery that defects in complement regulatory genes are the leading cause of atypical hemolytic uremic syndrome (aHUS), evidence has accumulated rapidly that complement activation is important in the pathogenesis of TMA.5 Complement hyperactivation—via excessive stimulation, inadequate regulation, or both—can cause TMA in a variety of underlying clinical conditions: Up to 60% of patients with aHUS have mutations in complement regulatory genes.6 Recent evidence showed that in Shiga toxin–producing Escherichia coli–associated HUS (STEC-HUS), Shiga toxins can directly activate the complement system, causing severe endothelial damage and ultimately leading to TMA.7 An association between complement activation and TMA has also been reported in patients with thrombotic thrombocytopenic purpura, SLE, antiphospholipid syndrome, and antibody-mediated kidney allograft rejection.8–11
The efficacy of eculizumab treatment in patients with TMA in different clinical settings provides further evidence that complement activation is involved in the mechanistic pathway leading to TMA.12–17 Eculizumab is a monoclonal antibody that selectively inhibits the cleavage of complement C5, thereby preventing the generation of C5a and the terminal complement complex, C5b-9. Results from the first systematic trial of eculizumab in patients with aHUS show that eculizumab improved renal function, increased platelet counts, and prevented thrombotic microangiopathic events in most patients.18
Given the heterogeneity of clinical settings under which TMA can occur, biomarkers would be useful to identify which patients would benefit from terminal complement inhibitors. We previously reported that for the diagnosis TMA, glomerular C4d staining is a useful biomarker in patients with SLE with or without antiphospholipid syndrome.9 The aim of this study was to investigate the prevalence and localization of C4d deposits in renal biopsy specimens from patients with TMA in the setting of various underlying clinical conditions, in combination with the prevalence and localization of C5b-9.
RESULTS
Patients and Histopathology
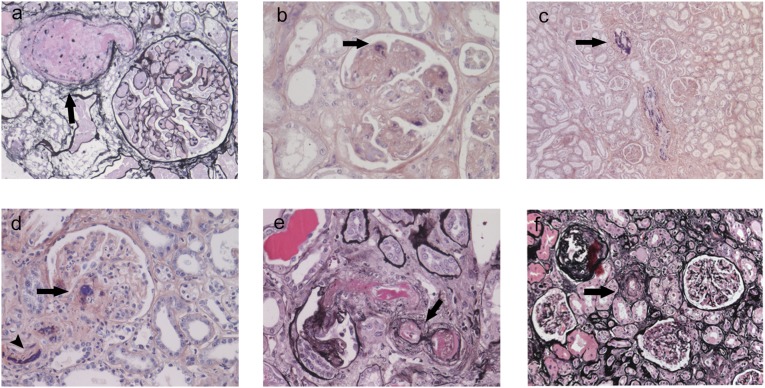
Our search strategy resulted in 145 renal samples with a diagnosis of TMA (110 biopsies, 18 autopsies, 17 nephrectomies). We excluded 103 cases mainly because of missing and/or inadequate clinical data or tissue samples. We eventually included 42 renal tissue samples with histologically confirmed TMA that were obtained from 36 patients. Thirty samples were obtained from 28 patients with native kidney disease, and 12 samples were obtained from 8 kidney transplant recipients. Five patients had follow-up biopsies. Table 1 shows patient characteristics. At the time of renal sampling, 10 patients had neurologic symptoms and 4 had a history of malignant hypertension. All 36 patients had renal dysfunction. Donor-specific antibodies were detected in all 3 kidney transplant recipients with TMA due to rejection. Microthrombi were localized in the glomeruli of 36 TMA samples (85.7%) and in the arterioles of 22 samples (52.4%). Additional histopathologic lesions are summarized in Table 2; typical examples are shown in Figure 1.
Table 1.
Patient characteristics
| Characteristic | Patients with TMA in Kidney Allograft (n=8) | Patients with TMA in Diseased Native Kidney (n=28) | All TMA (n=36) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Recurrent aHUS (n=3) | Drug Toxicity (n=2) | Rejection (n=3) | aHUS (n=11) | STEC-HUS (n=1) | SLE/APS (n=8) | HSCT-TMA (n=6) | IgAN (n=1) | AAV (n=1) | ||
| Age at time of first renal sampling (yr) | 34.8 (6.4–37.2) | 43.4 (40.0–46.9) | 66.4 (31.6–72.0) | 46.0 (22.4–77.2) | 13.5 (NA) | 29.5 (16.6–49.4) | 37.7 (17.5–54.2) | 32.1 (NA) | 35.3 (NA) | 37.7 (6.4–77.2) |
| Women | 1 (33.3) | 2 (100.0) | 2 (66.7) | 9 (81.8) | 0 (0.0) | 8 (100.0) | 3 (50.0) | 0 (0.0) | 0 (0.0) | 25 (69.4) |
| Neurologic symptoms | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (27.3) | 0 (0.0) | 3 (37.5) | 3 (50.0) | 0 (0.0) | 1 (100.0) | 10 (27.8) |
| History of malignant hypertension | 1 (33.3) | 0 (0.0) | 0 (0.0) | 1 (9.1) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 0 (0.0) | 1 (100.0) | 4 (9.5) |
| Renal tissue samples | 6 (100.0) | 2 (100.0) | 4 (100.0) | 11 (100.0) | 1 (100.0) | 10 (100.0) | 6 (100.0) | 1 (100.0) | 1 (100.0) | 42 (100.0) |
| Biopsy | 5 (83.3) | 2 (100.0) | 4 (100.0) | 6 (54.5) | 1 (100.0) | 8 (80.0) | 2 (33.3) | 1 (100.0) | 1 (100.0) | 30 (71.4) |
| Autopsy | 0 (0.0) | 0 (0.0) | 0 (0.0) | 5 (45.5) | 0 (0.0) | 2 (20.0) | 4 (66.7) | 0 (0.0) | 0 (0.0) | 11 (26.2) |
| Nephrectomy | 1 (16.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (2.4) |
Age is expressed as median (range); all other values are expressed as number (percentage). Drug toxicity refers to TMA in renal allograft in cases caused by toxicity of immunosuppressive therapeutics. Rejection refers to TMA in renal allograft cases during a rejection episode. APS, antiphospholipid syndrome; IgAN, IgA nephropathy; AAV, ANCA-associated vasculitis; NA, not applicable.
Table 2.
Histopathologic features of kidney sections from patients with TMA
| Histopathological parameter | All TMA (n=42) |
|---|---|
| Microthrombi | 42 (100.0) |
| Glomerular microthrombi | 36 (85.7) |
| Arteriolar microthrombi | 22 (52.4) |
| Glomerular fibrin deposits | 13 (31.0) |
| Glomerular basement membrane thickening | 6 (14.3) |
| Glomerular basement membrane duplication | 8 (19.0) |
| Arteriolar swelling | 17 (40.5) |
| Endothelial changes | |
| No proliferation, no foamy changes | 12 (28.6) |
| Proliferation, no foamy changes | 8 (19.0) |
| Foamy changes, no proliferation | 5 (11.9) |
| Foamy changes and fibrosis | 17 (40.5) |
| Microaneurysms | 18 (42.9) |
| Fibrinoid necrosis | 2 (4.8) |
Values are expressed as number (percentage).
Figure 1.
Typical examples of renal histopathology in patients with TMA. (A) Microaneurysm (arrow), silver stain (original magnification, ×100). (B) Fibrin microthrombi in glomerular capillaries (arrow), phosphotungstic acid-hematoxylin (PTAH) stain (original magnification, ×100). (C) Fibrin microthrombi in an artery (arrow), PTAH stain (original magnification, ×40). (D) Fibrin microthrombi in an arteriole (arrowhead) and the vascular pole of the glomerulus (arrow), PTAH stain (original magnification, ×100). (E) Organizing microthrombi with recanalization (arrow), silver-stained sample (original magnification, ×100). (F) Arteriolar onion ring (arrow), silver stain (original magnification, ×60).
Prevalence and Localization of C4d and C5b-9 Deposits
C4d deposits were present in 37 of the 42 TMA samples (88.1%) (Table 3). C4d deposits were localized in the glomeruli (76.2% of the samples), peritubular capillaries (9.5%), and arterioles (59.5%) (Supplemental Table 1). C4d staining in glomeruli was focal in 50.0% of the samples and diffuse in 26.2%. C4d staining in arterioles was focal in 33.3% of the samples and diffuse in 26.2%. C4d deposits were present in the same renal vascular structure as the microthrombi in 78.6% of the TMA samples. C5b-9 deposits were present in 78.6% of the samples (Table 3) and were colocalized with C4d deposits in 59.5%. In glomeruli, C5b-9 deposits were mainly focally distributed (Supplemental Table 2). In peritubular capillaries, C5b-9 deposits were not observed in any sample. In arterioles, C5b-9 deposits were present in 69.0% of the samples. C5b-9 deposits were present in the same renal vascular structure as the microthrombi in 61.9% of the TMA samples.
Table 3.
Immunohistochemistry results of TMA kidney sections from patients with various underlying clinical conditions
| Immunohistochemical Stain | Kidney Allografts (n=12) | Native Kidneys(n=30) | All TMA (n=42) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Recurrent aHUS (n=6) | Drug Toxicity (n=2) | Rejection (n=4) | aHUS (n=11) | STEC-HUS (n=1) | SLE/APS (n=10) | HSCT-TMA (n=6) | IgAN (n=1) | AAV (n=1) | ||
| C4d | 6 (100.0) | 2 (100.0) | 4 (100.0) | 8 (72.7) | 1 (100.0) | 9 (90.0) | 6 (100.0) | 1 (100.0) | 0 (0.0) | 37 (88.1) |
| C5b-9 | 5 (83.3) | 2 (100.0) | 3 (75.0) | 9 (81.8) | 0 (0.0) | 8 (80.0) | 6 (100.0) | 0 (0.0) | 0 (0.0) | 33 (78.6) |
| MBL | 5 (83.3) | 0 (0.0) | 1 (25.0) | 3 (27.3) | 0 (0.0) | 2 (20.0) | 1 (16.7) | 0 (0.0) | 0 (0.0) | 12 (28.6) |
| C1q | 6 (100.0) | 2 (100.0) | 4 (100.0) | 9/10 (90.0)a | 1 (100.0) | 8 (80.0) | 6 (100.0) | 1 (100.0) | NAa | 37/40 (92.5)a |
| IgM | 6 (100.0) | 2 (100.0) | 2 (50.0) | 6/10 (60)a | 1 (100.0) | 7 (70.0) | 6 (100.0) | 1 (100.0) | NAa | 31/40 (77.5)a |
Overall staining in biopsy specimens, defined as the presence of staining along the glomeruli, peritubular capillaries, and/or arterioles. Values are expressed as number (percentage). Drug toxicity refers to TMA in renal allograft in cases caused by toxicity of immunosuppressive therapeutics. Rejection refers to TMA in renal allograft cases during a rejection episode. APS, antiphospholipid syndrome; IgAN, IgA nephropathy; AAV, ANCA-associated vasculitis; NA, not applicable.
Two cases had insufficient tissue remaining for IgM and C1q staining. The first case had AAV, and the second case had aHUS.
Complement Pathways
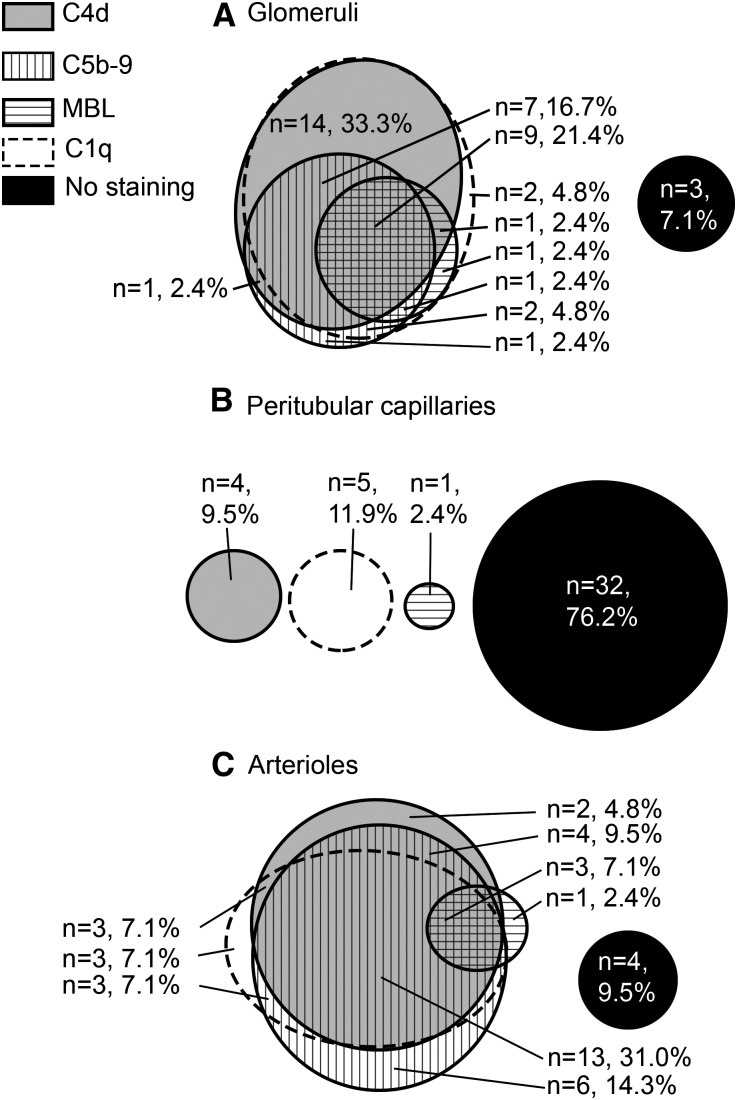
Classical pathway activation was observed solely (61.9%) or in combination with the lectin pathway (28.6%), in total representing 90.5% of TMA cases (Table 4). The complement pathways are summarized per renal vascular structure in Supplemental Table 3. In glomeruli, classical pathway activation was observed in 90.5% of cases. In peritubular capillaries, complement deposits were predominantly absent (76.2%). In arterioles, classical pathway activation was observed in 69.0% of the samples. IgM deposits were present in 77.5% of samples. The presence of glomerular IgM deposits was significantly associated with the presence of glomerular C4d deposits (P<0.05). Figure 2 summarizes colocalization patterns of C4d, C5b-9, C1q, and mannose-binding lectin (MBL) deposits in glomeruli, peritubular capillaries, and arterioles.
Table 4.
Complement pathways in TMA kidney sections from patients with various underlying clinical conditions
| Complement Pathway | Kidney Allografts (n=12) | Native Kidneys (n=30) | All TMA (n=42) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Recurrent aHUS (n=6) | Drug Toxicity (n=2) | Rejection (n=4) | aHUS (n=11) | STEC-HUS (n=1) | SLE/APS (n=10) | HSCT-TMA (n=6) | IgAN (n=1) | AAV (n=1) | ||
| Complement negative | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.0) | 1 (2.4) |
| Classical pathway | 1 (16.7) | 2 (100.0) | 3 (75.0) | 6 (54.5) | 1 (100.0) | 7 (70.0) | 5 (83.3) | 1 (100.0) | 0 (0.0) | 26 (61.9) |
| Both classical and lectin pathway | 5 (83.3) | 0 (0.0) | 1 (25.0) | 3 (27.3) | 0 (0.0) | 2 (20.0) | 1 (16.7) | 0 (0.0) | 0 (0.0) | 12 (28.6) |
| Unknown pathway | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (18.2) | 0 (0.0) | 1 (10.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (7.1) |
Values are expressed as number (percentage). Drug toxicity refers to TMA in renal allograft in cases caused by toxicity of immunosuppressive therapeutics. Rejection refers to TMA in renal allograft cases during a rejection episode. APS, antiphospholipid syndrome; IgAN, IgA nephropathy; AAV, ANCA-associated vasculitis; NA, not applicable.
Figure 2.
Evidence for classical pathway activation is frequently observed in glomeruli and arterioles. Values represent the number of renal sections stained as indicated. Deposition frequency is shown for the (A) glomeruli, (B) peritubular capillaries, and (C) arterioles. In both glomeruli and arterioles, C4d deposits are frequently colocalized with C1q deposits, reflecting activation of the classical pathway. If present, MBL deposits are frequently colocalized with C4d and/or C1q deposits, reflecting that the lectin pathway was occasionally activated, but often together with the classical pathway.
Native Kidneys
Thirty of 42 TMA samples were derived from diseased native kidneys. C4d deposits were present in 25 of these samples (83.3%) (Table 3). Specifically, C4d deposits were present in the glomeruli, peritubular capillaries, and arterioles of 23 (76.7%), 1 (3.3%), and 15 (50.0%) of these samples, respectively (Supplemental Table 1). C5b-9 deposits were present in 76.7% of the native kidneys.
SLE and Antiphospholipid Syndrome
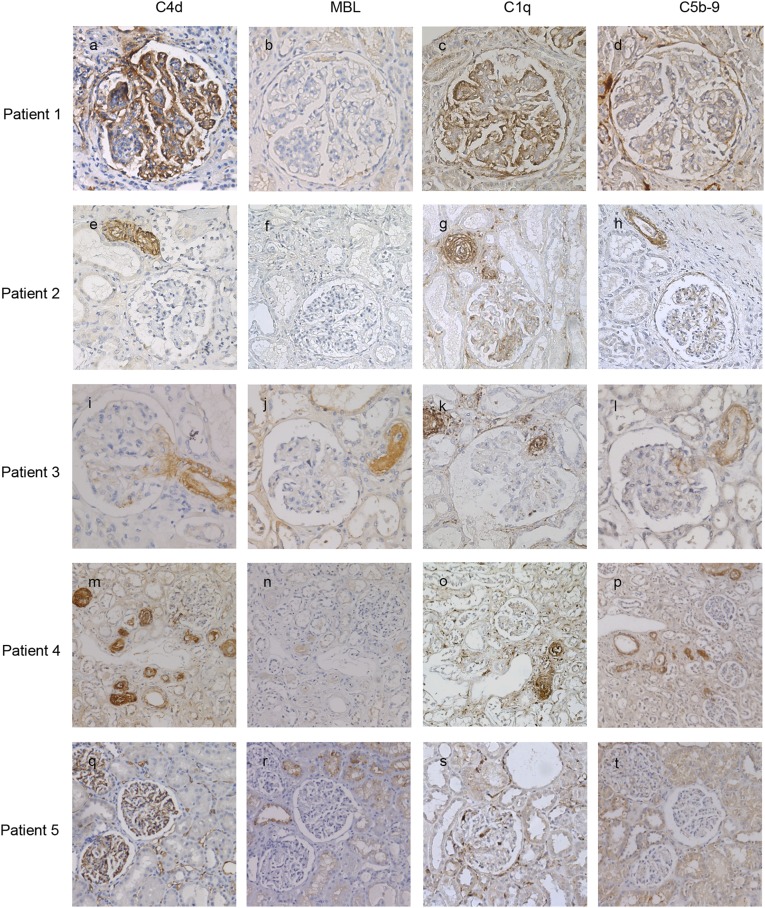
TMA cases associated with SLE with or without antiphospholipid syndrome were characterized by glomerular C4d staining (90.0%), which was diffuse in 50% of the samples. C4d deposits were less prevalent in arterioles (50.0%). C5b-9 deposits were less prevalent than C4d deposits (80.0%) and were localized in glomeruli (60.0%) and in arterioles (60.0%). Figure 3, A–D, shows typical examples of these stains.
Figure 3.
Typical examples of complement deposits in TMA patients with different underlying clinical conditions. The sections were stained for C4d (A, E, I, M, Q), MBL (B, F, J, N, R), C1q (C, G, K, O, S), or C5b-9 (D, H, L, P, T). Patient 1 developed TMA after systemic lupus erythematosus and had C4d (A) and C1q (C) deposits in the glomeruli without MBL deposition (B) or colocalization with C5b-9 deposits (C). Original magnification, ×100 in A–D. Patient 2 had aHUS and had C4d (E) and C1q (G) deposits in the arterioles without MBL deposits (F) but with colocalization of C5b-9 deposits (H). Original magnification, ×60 in E–H. Patients 3 and 4 developed TMA following hematopoietic stem cell transplantation. Patient 3 had C4d deposits in an arteriole at the vascular pool of a glomerulus (I) with colocalization of MBL (J), C1q (K), and C5b-9 (L). Patient 4 had C4d (M) and C1q (O) deposits in the arterioles without MBL deposits (N) but with colocalization of C5b-9 deposits (P). Original magnification, ×100 in I–L; ×40 in M–P. Patient 5 developed TMA following kidney transplantation and had C4d (Q) and C1q (S) deposits in the peritubular capillaries without colocalization of MBL (R) or C5b-9 deposits (T). Original magnification, ×60 in Q–T.
aHUS and STEC-HUS
Cases with aHUS had C4d deposits in glomeruli (54.5%) and arterioles (54.5%). If present, arteriolar C4d stained predominantly in a diffuse pattern. C5b-9 deposits were observed in most aHUS samples (81.8%) and predominantly localized in arterioles. Figure 3, E–H, shows typical examples of these stains. One patient had STEC-HUS: Classical pathway activation was observed, C4d deposits were focally present in glomeruli, and C5b-9 deposits were absent.
TMA Associated with Hematopoietic Stem Cell Transplantation
The complement staining pattern in TMA associated with hematopoietic stem cell transplantation (HSCT-TMA) resembled the staining pattern of aHUS cases. C4d deposits were present in all six samples and were predominantly localized in the glomeruli (100.0%) and in the arterioles (66.7%). C5b-9 deposits were observed in all samples. C5b-9 deposits were localized in the arterioles of all samples and stained predominantly in a diffuse pattern. Furthermore, classical pathway activation was observed in all cases. Four patients had a history of graft-versus-host disease; three of these patients had both C4d and C5b-9 deposits in the arterioles. Figure 3, I–P, shows typical examples of these stains.
IgA Nephropathy and ANCA-Associated Vasculitis
One patient had TMA associated with IgA nephropathy. C4d deposits were present only in glomeruli and stained in a diffuse pattern; C1q deposits were present in the glomeruli and arterioles, whereas MBL and C5b-9 deposits were absent. Another patient had TMA associated with granulomatosis with polyangiitis. Complement deposits were not observed in this patient.
Transplanted Kidneys
Twelve of the 42 renal TMA samples were obtained from transplanted kidneys with TMA (Table 3). C4d deposits were present in all 12 transplanted kidney samples; specifically, C4d deposits were present in the glomeruli, peritubular capillaries, and arterioles of 8 (66.7%), 3 (25.0%), and 10 (83.3%) of these samples, respectively. C5b-9 deposits were present in 83.3% of the transplanted kidneys.
Recurrence of aHUS
We found that aHUS recurred in six renal allograft samples of three patients. Complement deposition patterns resembled that of the native aHUS cases. Both C4d and C5b-9 deposits were predominantly present in glomeruli (83.3%) and arterioles (83.3%).
Drug Toxicity and Rejection
TMA was attributed to drug toxicity of cyclosporine and tacrolimus in two cases. In three cases (four biopsy samples), TMA was attributed to a rejection episode. Clinical overlap was observed in both groups. Two case-patients with rejection received concomitant high dose-immunosuppressive therapy, but changing the therapeutic regimen did not improve renal function. Similarly, one case attributed to drug toxicity had diffuse staining of C4d in peritubular capillaries, suggesting a humoral rejection episode. Both groups were characterized by C4d deposits in all scored renal vascular structures. C5b-9 deposits were present in all samples of drug toxicity cases and in three of four cases with rejection. Examples of these stains are shown in Figure 3, Q–T.
Controls
C4d deposits were significantly more prevalent in cases than in controls (P<0.001); this difference remained significant when the deposits were subcategorized for glomeruli (P<0.001), peritubular capillaries (P<0.05), and arterioles (P<0.001). All samples obtained from healthy controls, Alport syndrome controls, and kidney transplant controls were C4d negative, but C4d deposits were present in three control samples of biopsy samples with FSGS (5.6% of all control samples). C5b-9 deposits were also significantly more prevalent in cases than in controls (P<0.001), and this difference remained significant when the deposits were subcategorized for glomeruli (P<0.001) and arterioles (P<0.05). C5b-9 deposits were not observed in the peritubular capillaries of any sample. C5b-9 deposits were present in 21 control samples (39.6%), including 7 healthy control samples, 5 FSGS control samples, and 9 transplant control samples; all 5 Alport syndrome controls were negative for C5b-9 deposits. In the control samples, C5b-9 deposits were almost exclusively localized in arterioles; only 1 sample had glomerular C5b-9 deposits, which were focal.
DISCUSSION
Our study demonstrates that complement activation in the kidney is a common denominator of TMA in a heterogeneous group of patients. Our findings provide insight into the etiology of TMA and may have therapeutic consequences. The relatively widespread presence of C4d deposits indicates that complement is activated—and may be causally involved—in most histologically confirmed cases of TMA. Moreover, our observation that terminal complement complex C5b-9 deposits were present and were colocalized with C4d deposits suggests that at least 75% of all TMA cases—both with and without confirmed mutations in complement regulatory genes—might benefit from complement-inhibiting therapeutics. This is consistent with results from recent clinical trials and case reports describing the effects of eculizumab, a terminal complement inhibitor.12–18
Different C4d staining patterns were observed. Cases with SLE and IgA nephropathy were characterized by diffuse glomerular C4d staining. Kidney transplant cases attributed to drug toxicity or a rejection episode had varied staining patterns. Native cases with aHUS and transplant cases in which aHUS recurred were characterized by arteriolar C4d staining. Interestingly, cases with HSCT-TMA also showed arteriolar C4d staining. Arteriolar C4d staining has been described previously in native kidneys, transplanted kidneys, and other transplanted organs.19–22 The etiology of arteriolar C4d deposition is unknown. In light of our data, arteriolar C4d deposition in biopsy samples from patients with TMA may reflect a deficit in complement regulation (such as in aHUS), whereas C4d staining in glomeruli and peritubular capillaries occurs mainly in association with antibody-mediated complement activation (such as in SLE or antiphospholipid syndrome).
C4d is a widely used biomarker for complement activation that remains covalently bound, long after the complement pathway–initiating factors have dissociated.23 To distinguish between the lectin and classical pathways, we stained samples for MBL and C1q. Lectin pathway activation was observed in patients with aHUS, either in native kidney biopsy specimens or renal transplant biopsy specimens in which aHUS recurred. The origin of a microthrombotic lesion as a result of complement activation in the lectin pathway is unknown.
The classical pathway was activated in most patients. A large subgroup had underlying clinical conditions in which antibodies could have mediated the renal endothelial injury. Classical pathway activation was observed in 90.0% of patients with SLE with or without antiphospholipid syndrome, either solely (70.0% of cases) or in combination with the lectin pathway (20%). These findings are consistent with previous data reporting an association between glomerular C4d staining and microthrombi in SLE and antiphospholipid syndrome.9,24 In these patients, accumulation of antibodies in the glomeruli probably leads to classical pathway activation, endothelial injury, and the subsequent formation of microthrombi. In addition, all samples obtained from six patients who developed TMA after hematopoietic stem cell transplantation contained C4d deposits, predominantly in glomeruli but also in arterioles. Four patients had graft-versus-host disease, and three of these patients had both C4d and C5b-9 deposits in arterioles. C4d represented the classical pathway in all cases, suggesting that the classical complement pathway led to activation of the terminal complement complex in these patients with graft-versus-host disease. Similar findings were reported by others, suggesting that an antibody-mediated component of graft-versus-host disease may cause TMA as a result of severe endothelial damage, possibly in combination with drug toxicity.22,25
Classical pathway activation was also observed in cases without apparent antibody-mediated injury, such as cases with drug toxicity–associated TMA, native aHUS, recurrent aHUS, and the STEC-HUS. Here, the presence of complement deposits along the renal vasculature may reflect a consequence of renal damage, rather than—or perhaps in addition to—reflecting an underlying cause of the damage. Two hypotheses may explain the presence of C4d as a consequence of damage. First, chronic endothelial cell injury may cause the endothelial cells to produce components of the glomerular basement membrane. This can result in the formation of a duplicate glomerular basement membrane, entrapment of aspecific IgM and C3, and thus mimicry of immune complex deposition.26 In our cohort, the presence of glomerular C4d and IgM were associated with each other. Furthermore, all eight TMA cases with a duplicated glomerular basement membrane had classical pathway activation, and four had both glomerular IgM and C4d deposits. Second, complement activation may be induced by intravascular cellular debris and hypoxic or injured endothelium. Experimental studies in humans, C3 and C4 knockout mice, and mice treated with C5a receptor antagonists show that complement activation is involved in ischemia-reperfusion injury in the context of transplantation or stroke.27–30 In TMA, ischemia-reperfusion injury could lead to complement activation, possibly amplifying complement-mediated injury.
Although C4d deposits were present in 88.1% of the TMA samples, C4d deposits were colocalized with C5b-9 deposits in only 59.5%. It would be interesting to investigate whether terminal complement inhibitors would have therapeutic benefits in particular in patients showing a combination of C4d and C5b-9 deposits. For future studies evaluating the effect of complement-inhibiting therapeutics in various clinical settings of TMA, we would like to make the following recommendations: The main benefit of C4d as a biomarker lies in identifying cases in which tissue samples may not reveal the TMA lesion due to sampling error, as our previous study showed.9 C5b-9 might seem the most interesting biomarker to predict an effect of its direct agent in the form of a terminal complement inhibitor, but our data show that C5b-9 deposits are present in a considerable percentage of controls, in particular in arterioles, and are sometimes colocalized with hyalinosis (data not shown). Therefore, the combination of C4d and C5b-9 may eventually be the most useful to evaluate a likely benefit of complement-inhibiting therapeutics for patients with TMA.
Our study has several limitations. Because of technical limitations, we could not use the paraffin-embedded tissue samples to retrospectively test cases for the most prevalent mutations in genes that encode complement regulatory proteins. Future studies should investigate whether distinct complement-staining patterns in the kidney reflect specific genotypes in the background of TMA, as well as possible differences in the expression of complement regulatory proteins in the kidneys of patients with TMA. Furthermore, our sample size is relatively small, and selection bias may have occurred because patients with straightforward clinical presentation or contraindications such as severe bleeding risk often do not undergo biopsy. Because TMA is a rare complication of many diseases, a large multicenter and prospective study would be necessary to overcome these issues.
In conclusion, our study shows that C4d is a common denominator in TMA, regardless of the underlying clinical condition. That C5b-9 is present in >75% of renal biopsy specimens from patients with TMA suggests that terminal complement inhibitors may have a beneficial effect in these patients. Finally, C4d and C5b-9 should be investigated as possible diagnostic biomarkers in the clinical work-up of patients suspected of having complement-mediated TMA.
CONCISE METHODS
Patients and Controls
We retrospectively searched the database of our hospital’s Department of Pathology for patients with a diagnosis of TMA who underwent renal biopsy, nephrectomy, and/or autopsy from 1991 through 2010 at the Leiden University Medical Center. Search terms included HUS, microthrombi, thrombi, TMA, TTP, microangiopathy, and thrombocytopenia. Cases were reviewed by an experienced nephropathologist (I.M.B.). Histologically confirmed TMA was defined as the presence of one or more platelet thrombi obstructing vessel lumens on renal biopsy, with or without thickening of the arterioles and capillaries, endothelial swelling and detachment, or widening of the subendothelial space.
Healthy control samples without TMA (n=9) were obtained from Eurotransplant donor kidneys that were unsuitable for transplantation because of technical deficits. Diseased control samples without TMA (n=44) included the following three groups: native renal biopsy specimens from patients with FSGS (n=19), native renal biopsy specimens from patients with Alport syndrome (n=5), and kidney transplant biopsy specimens showing a variety of lesions other than antibody-mediated rejection (n=20). This kidney transplant control group included interstitial fibrosis and tubular atrophy (n=6), T cell–mediated rejection (n=7), no apparent lesions (n=1), calcineurin inhibitor toxicity and/or hyalinosis (n=4), and recurrent disease (n=2).
Two independent investigators (J.C. and L.vE.) analyzed the clinical data and correspondence, which were retrospectively obtained from the medical records and included the underlying clinical diagnosis; serum LDH; hemoglobin; thrombocytes; ADAMTS13; urea and creatinine; and the presence of schistocytes, neurologic symptoms, and/or renal dysfunction at the time of tissue sampling.
Native and Transplanted Kidney Case Groups
Renal samples were obtained from 28 patients with native kidney disease and 8 kidney transplant recipients. Patients with native kidney disease were classified into one of six groups: aHUS (n=11), including patients with and without demonstrated mutations in complement regulatory genes; diarrhea-associated HUS (n=1), defined as HUS following gastroenteritis caused by E. coli; HSCT-TMA (n=6); SLE with or without antiphospholipid syndrome (n=8); IgA nephropathy (n=1); and ANCA-associated vasculitis (n=1). Kidney transplant recipients were classified into one of three groups: recurrent aHUS (n=3), defined as cases with recurrence of microthrombi in the renal allograft, with or without known mutations in complement regulatory genes; drug toxicity (n=2), defined as cases with high-dose cyclosporine and/or tacrolimus that improved clinically after a change in therapeutic regimen; and rejection (n=3), defined as cases with TMA that developed in association with a rejection episode.
Histopathology
Renal tissue was fixed in 10% buffered formalin and embedded in paraffin. Paraffin-embedded sections were stained with hematoxylin and eosin, periodic acid–Schiff, silver-stain, and phosphotungstic acid-hematoxylin.
Immunohistochemistry
To investigate complement activation, immunohistochemical staining was performed for various components of the complement system. Paraffin-embedded sections were cut at 4-µm thickness, deparaffinized, and subjected to antigen retrieval. After blocking endogenous peroxidases, the sections were incubated in the relevant primary antibody for 1 hour. Binding of the primary antibody was visualized using the appropriate horseradish peroxidase–labeled secondary antibodies and diaminobenzidine as the chromogen. Finally, the sections were counterstained with hematoxylin. The primary antibodies included antibodies against C4d (BI-RC4d; Biomedica Gruppe, Vienna, Austria; 1:50), C1q (A0136; Dako, Glostrup, Denmark; 1:800), MBL (HPA002027; Sigma-Aldrich, St. Louis, MO; 1:500), and sC5b-9 (A239; Quidel, San Diego, CA; 1:150). C4d is a cleavage product of C4 that binds covalently to the target tissue. The deposition of C4d can result from activation of the classical pathway (represented by C1q) or from activation of the lectin pathway (represented by MBL). Activation of any of the complement pathways can lead to deposition of the terminal complement complex, C5b-9. The optimum antibody dilution and incubation time were determined empirically for each antibody by performing a titration experiment on positive control sections.
For C4d staining, a renal specimen obtained from a patient with antibody-mediated allograft rejection and C4d-positive peritubular capillary staining (confirmed by immunofluorescence) was used as a positive control. Negative controls were obtained by incubating the positive control samples and TMA cases in negative control rabbit immunoglobulin fraction (X0936; Dako). For MBL staining, a section of healthy liver was used as the positive control, and omitting the primary antibody served as the negative control. For C1q staining, a section of healthy tonsil served as a positive control and negative control was obtained by adding the negative control rabbit immunoglobulin fraction (X0936) to the positive control section. For C5b-9 staining, a renal specimen obtained from a patient with antibody-mediated allograft rejection and confirmed C5b-9–positive staining served as the positive control, and the negative control was obtained by incubating the positive control section in the negative control mouse IgG2b antibody (X0944; Dako).
To investigate the presence of IgM deposits, direct immunofluorescence staining was performed on renal paraffin sections of 4-µm thickness. After deparaffinization, the sections were incubated in Protease 24 for 1 hour at 37°C. The sections were then incubated for 1 hour with an FITC-conjugated Fc-specific F(ab’)2 mouse anti-human IgM antibody (1:40; Protos Immunoresearch, Burlingame, CA).
Evaluation of Histopathologic and Immunohistochemistry Results
Stained sections were evaluated by two investigators (I.M.B. and D.C.) who were blinded with respect to the clinical data. The sections were scored according to the presence of microthrombi in the glomeruli and arterioles, the presence of fibrin in the glomeruli, thickening and double contours of the glomerular basement membrane, glomerular fibrinoid necrosis, arterial and arteriolar endothelial swelling, and microaneurysms. Endothelial changes were scored on the basis of proliferation and foamy changes. Immunohistochemical staining patterns in peritubular capillaries was scored in accordance with the Banff 2007 criteria for C4d staining.31 Immunohistochemical staining patterns in nonsclerotic segments of glomeruli were scored as absent, focal, or diffuse. Positivity in glomeruli typically followed a global staining pattern along the glomerular capillary walls. For immunohistochemical staining patterns of arterioles, positive staining was defined as circumferential staining along the vessel lumina. Positivity in the proportion of the total number of arterioles in the tissue specimen resulted in a scoring of absent, focal, or diffuse. Finally, an overall staining score comprising the scorings of peritubular capillaries, glomeruli and arterioles was given.
On the basis of absence or presence of the various complement components, the cases were categorized into five groups of complement activation. “Complement-negative” was defined as the absence of C1q, MBL, C4d, and C5b-9 deposits; “classical pathway” was defined as the presence of C1q deposits and the absence of MBL deposits or the presence of both IgM and C4d deposits and the absence of MBL deposits; “lectin pathway” was defined as the presence of MBL deposits and the absence of C1q deposits; “both classical and lectin pathways” was defined as the presence of both C1q and MBL deposits; and “unknown complement pathway” was defined as the presence of C4d and/or C5b-9 deposits in the absence of C1q, IgM and MBL deposits.
Two cases had insufficient tissue samples remaining for IgM and C1q staining. The first case had ANCA-associated vasculitis, and the renal biopsy was negative for C4d, MBL, and C5b-9 deposits in every scored location (i.e., the glomeruli, peritubular capillaries, and arterioles); this case was therefore classified as complement-negative. The second case with insufficient tissue sample remaining had aHUS, and the native renal biopsy specimen contained C5b-9 deposits in the arterioles. Overall, this case was classified as unknown complement pathway; when we subcategorized for each location, this case was considered complement negative in the glomeruli and peritubular capillaries and as unknown complement pathway in the arterioles.
Statistical Analyses
Categorical variables were compared using the chi-squared test (or the Fisher exact test if the sample size was small). SPSS software, version 20.0 (IBM, Armonk, NY), was used to perform all analyses. Differences with a P value<0.05 were considered statistically significant.
Ethics
All tissue samples were coded and then handled and analyzed anonymously in accordance with the Dutch National Ethics Guidelines (Code for Proper Secondary Use of Human Tissue, Dutch Federation of Medical Scientific Societies). This national code enables researchers to use human material that became available within the framework of patient care; this human material can be used for research purposes if properly coded and handled anonymously.
Disclosures
None.
Acknowledgments
The results presented in this paper have not been published previously except in abstract form at the 44th Annual American Society of Nephrology Conference.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014050429/-/DCSupplemental.
References
- 1.Moake JL: Thrombotic microangiopathies. N Engl J Med 347: 589–600, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Tsai HM: The molecular biology of thrombotic microangiopathy. Kidney Int 70: 16–23, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Besbas N, Karpman D, Landau D, Loirat C, Proesmans W, Remuzzi G, Rizzoni G, Taylor CM, Van de Kar N, Zimmerhackl LB, European Paediatric Research Group for HUS : A classification of hemolytic uremic syndrome and thrombotic thrombocytopenic purpura and related disorders. Kidney Int 70: 423–431, 2006 [DOI] [PubMed] [Google Scholar]
- 4.George JN, Nester CM: Syndromes of thrombotic microangiopathy. N Engl J Med 371: 654–666, 2014 [DOI] [PubMed] [Google Scholar]
- 5.Noris M, Mescia F, Remuzzi G: STEC-HUS, atypical HUS and TTP are all diseases of complement activation. Nat Rev Nephrol 8: 622–633, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Noris M, Remuzzi G: Atypical hemolytic-uremic syndrome. N Engl J Med 361: 1676–1687, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Morigi M, Galbusera M, Gastoldi S, Locatelli M, Buelli S, Pezzotta A, Pagani C, Noris M, Gobbi M, Stravalaci M, Rottoli D, Tedesco F, Remuzzi G, Zoja C: Alternative pathway activation of complement by Shiga toxin promotes exuberant C3a formation that triggers microvascular thrombosis. J Immunol 187: 172–180, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Ruiz-Torres MP, Casiraghi F, Galbusera M, Macconi D, Gastoldi S, Todeschini M, Porrati F, Belotti D, Pogliani EM, Noris M, Remuzzi G: Complement activation: The missing link between ADAMTS-13 deficiency and microvascular thrombosis of thrombotic microangiopathies. Thromb Haemost 93: 443–452, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Cohen D, Koopmans M, Kremer Hovinga IC, Berger SP, Roos van Groningen M, Steup-Beekman GM, de Heer E, Bruijn JA, Bajema IM: Potential for glomerular C4d as an indicator of thrombotic microangiopathy in lupus nephritis. Arthritis Rheum 58: 2460–2469, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Seshan SV, Franzke CW, Redecha P, Monestier M, Mackman N, Girardi G: Role of tissue factor in a mouse model of thrombotic microangiopathy induced by antiphospholipid antibodies. Blood 114: 1675–1683, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Satoskar AA, Pelletier R, Adams P, Nadasdy GM, Brodsky S, Pesavento T, Henry M, Nadasdy T: De novo thrombotic microangiopathy in renal allograft biopsies—role of antibody-mediated rejection. Am J Transplant 10: 1804–1811, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Chapin J, Weksler B, Magro C, Laurence J: Eculizumab in the treatment of refractory idiopathic thrombotic thrombocytopenic purpura. Br J Haematol 157: 772–774, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Barnett AN, Asgari E, Chowdhury P, Sacks SH, Dorling A, Mamode N: The use of eculizumab in renal transplantation. Clin Transplant 27: E216–E229, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Wilson CH, Brown AL, White SA, Goodship TH, Sheerin NS, Manas DM: Successful treatment of de novo posttransplant thrombotic microangiopathy with eculizumab. Transplantation 92: e42–e43, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Hadaya K, Ferrari-Lacraz S, Fumeaux D, Boehlen F, Toso C, Moll S, Martin PY, Villard J: Eculizumab in acute recurrence of thrombotic microangiopathy after renal transplantation. Am J Transplant 11: 2523–2527, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Lapeyraque AL, Malina M, Fremeaux-Bacchi V, Boppel T, Kirschfink M, Oualha M, Proulx F, Clermont MJ, Le Deist F, Niaudet P, Schaefer F: Eculizumab in severe Shiga-toxin-associated HUS. N Engl J Med 364: 2561–2563, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Nester CM, Brophy PD: Eculizumab in the treatment of atypical haemolytic uraemic syndrome and other complement-mediated renal diseases. Curr Opin Pediatr 25: 225–231, 2013 [DOI] [PubMed] [Google Scholar]
- 18.Legendre CM, Licht C, Muus P, Greenbaum LA, Babu S, Bedrosian C, Bingham C, Cohen DJ, Delmas Y, Douglas K, Eitner F, Feldkamp T, Fouque D, Furman RR, Gaber O, Herthelius M, Hourmant M, Karpman D, Lebranchu Y, Mariat C, Menne J, Moulin B, Nürnberger J, Ogawa M, Remuzzi G, Richard T, Sberro-Soussan R, Severino B, Sheerin NS, Trivelli A, Zimmerhackl LB, Goodship T, Loirat C: Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med 368: 2169–2181, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Colvin RB: Antibody-mediated renal allograft rejection: Diagnosis and pathogenesis. J Am Soc Nephrol 18: 1046–1056, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Batal I, Girnita A, Zeevi A, Saab BA, Stockhausen S, Shapiro R, Basu A, Tan H, Morgan C, Randhawa P: Clinical significance of the distribution of C4d deposits in different anatomic compartments of the allograft kidney. Mod Pathol 21: 1490–1498, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Kikić Z, Regele H, Nordmeyer V, Wahrmann M, Kletzmayr J, Bartel G, Böhmig GA: Significance of peritubular capillary, glomerular, and arteriolar C4d staining patterns in paraffin sections of early kidney transplant biopsies. Transplantation 91: 440–446, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Laskin BL, Maisel J, Goebel J, Yin HJ, Luo G, Khoury JC, Davies SM, Jodele S: Renal arteriolar C4d deposition: A novel characteristic of hematopoietic stem cell transplantation-associated thrombotic microangiopathy. Transplantation 96: 217–223, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen D, Colvin RB, Daha MR, Drachenberg CB, Haas M, Nickeleit V, Salmon JE, Sis B, Zhao MH, Bruijn JA, Bajema IM: Pros and cons for C4d as a biomarker. Kidney Int 81: 628–639, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen Y, Chen XW, Sun CY, Dai M, Yan YC, Yang CD: Association between anti-beta2 glycoprotein I antibodies and renal glomerular C4d deposition in lupus nephritis patients with glomerular microthrombosis: A prospective study of 155 cases. Lupus 19: 1195–1203, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Mii A, Shimizu A, Kaneko T, Fujita E, Fukui M, Fujino T, Utsumi K, Yamaguchi H, Tajika K, Tsuchiya S, Iino Y, Katayama Y, Fukuda Y: Renal thrombotic microangiopathy associated with chronic graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Pathol Int 61: 518–527, 2011 [DOI] [PubMed] [Google Scholar]
- 26.Chang A: Thrombotic microangiopathy and the kidney: A nephropathologist's perspective. Diagn Histopathol 19: 158–165, 2013 [Google Scholar]
- 27.de Vries DK, van der Pol P, van Anken GE, van Gijlswijk DJ, Damman J, Lindeman JH, Reinders ME, Schaapherder AF, Kooten C: Acute but transient release of terminal complement complex after reperfusion in clinical kidney transplantation. Transplantation 95: 816–820, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Weiser MR, Williams JP, Moore FD, Jr, Kobzik L, Ma M, Hechtman HB, Carroll MC: Reperfusion injury of ischemic skeletal muscle is mediated by natural antibody and complement. J Exp Med 183: 2343–2348, 1996. 8642343 [Google Scholar]
- 29.Williams JP, Pechet TT, Weiser MR, Reid R, Kobzik L, Moore FD, Jr, Carroll MC, Hechtman HB: Intestinal reperfusion injury is mediated by IgM and complement. J Appl Physiol (1985) 86: 938–942, 1999 [DOI] [PubMed] [Google Scholar]
- 30.Arumugam TV, Shiels IA, Strachan AJ, Abbenante G, Fairlie DP, Taylor SM: A small molecule C5a receptor antagonist protects kidneys from ischemia/reperfusion injury in rats. Kidney Int 63: 134–142, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M, Halloran PF, Baldwin W, Banfi G, Collins AB, Cosio F, David DS, Drachenberg C, Einecke G, Fogo AB, Gibson IW, Glotz D, Iskandar SS, Kraus E, Lerut E, Mannon RB, Mihatsch M, Nankivell BJ, Nickeleit V, Papadimitriou JC, Randhawa P, Regele H, Renaudin K, Roberts I, Seron D, Smith RN, Valente M: Banff 07 classification of renal allograft pathology: Updates and future directions. Am J Transplant 8: 753–760, 2008 [DOI] [PubMed] [Google Scholar]