Abstract
IL-25 is an important immune regulator that can promote Th2 immune response-dependent immunity, inflammation, and tissue repair in asthma, intestinal infection, and autoimmune diseases. In this study, we examined the effects of IL-25 in renal ischemic/reperfusion injury (IRI). Treating IRI mice with IL-25 significantly improved renal function and reduced renal injury. Furthermore, IL-25 treatment increased the levels of IL-4, IL-5, and IL-13 in serum and kidney and promoted induction of alternatively activated (M2) macrophages in kidney. Notably, IL-25 treatment also increased the frequency of type 2 innate lymphoid cells (ILC2s) and multipotent progenitor type 2 (MPPtype2) cells in kidney. IL-25–responsive ILC2 and MPPtype2 cells produced greater amounts of Th2 cytokines that associated with the induction of M2 macrophages and suppression of classically activated (M1) macrophages in vitro. Finally, adoptive transfer of ILC2s or MPPtype2 cells not only reduced renal functional and histologic injury in IRI mice but also induced M2 macrophages in kidney. In conclusion, our data identify a mechanism whereby IL-25-elicited ILC2 and MPPtype2 cells regulate macrophage phenotype in kidney and prevent renal IRI.
Keywords: IL-25, innate lymphoid cells, multipotent progenitor type 2, ischemia/reperfusion, injury
IL-25 (also known as IL-17E) is a member of the IL-17 cytokine gene family and is produced by several cell types, including T lymphocytes, mast cells, eosinophils, basophils, and epithelial cells in the lung and intestine.1–3 Administration of IL-25 to mice has been shown to induce a Th2 immune response characterized by the overproduction of IL-4, IL-5, and IL-13.4 IL-25 facilitates pathogenic Th2 cell responses, increases serum levels of IgE and blood eosinophilia, and enhances the recruitment of inflammatory cells in asthma and allergic inflammation.1,5,6 However, IL-25 is an important regulator of host defense and promotes immunity to helminth infections.7–9 Moreover, IL-25 is also required to limit chronic intestinal inflammation and experimental autoimmune encephalomyelitis through controlling Th1/Th17 cell responses.3,10 The role of IL-25 in CKD was recently investigated and administration of IL-25 attenuated renal injury in mice with adriamycin nephropathy (AN) via inducing Th2 immune responses.11
Four independent research groups recently identified previously unrecognized innate immune cell populations that were capable of contributing to Th2 cytokine responses in vivo. These cell populations were named natural helper cells, nuocytes, innate type 2 helper (Ih2) cells, or multipotent progenitor type 2 (MPPtype2) cells.12–15 Based on developmental, phenotypic, and functional similarities, natural helper cells, nuocytes, and Ih2 cells have been collectively categorized as group 2 or type 2 innate lymphoid cells (ILC2s).16,17 In response to the epithelial cytokines IL-25 and IL-33, ILC2s expand and produce large amounts of type 2 cytokines, particularly IL-13 and IL-5 through the expression of the receptors of these cytokines, IL-17RB and ST2, respectively. ILC2s play critical roles in promoting immunity to helminth parasites, allergic airway inflammation, and lung epithelial repair.12,14,18,19 In contrast with ILC2s, MPPtype2 cells express unique cell surface markers and exhibit the ability to differentiate into cells of the monocyte and granulocyte lineages, suggesting that MPPtype2 cells may be a distinct population.15,20 Administration of IL-25 promotes the accumulation of ILC2 and MPPtype2 cells at multiple tissue sites, whereas whether IL-25 induces expansion of ILC2 and MPPtype2 cells in kidney is unknown.
Ischemic/reperfusion injury (IRI) is the primary cause of AKI and is also relevant to a number of clinical situations, including kidney transplantation. Macrophages contribute to the initiation of IRI through secretion of cytokines, recruitment of neutrophils, and induction of epithelial cell apoptosis and also play an important role in recovery or regeneration processes from IRI by modulating immune responses against inflammation.21–23 We previously reported that IL-25 induced Th2 immune responses by increasing levels of IL-4, IL-5, and IL-13 in serum, kidney, and kidney draining lymph nodes (KLDNs), and thereby induced alternatively activated (M2) macrophages and protected against renal injury in AN.11 In this study, we evaluated IL-25’s ability to protect against renal injury in mice with IRI, and further examined possible mechanisms underlying its effect on ILC2s, MPPtype2 cells, and macrophages in kidney. Here, we provide evidence that IL-25 is a critical cytokine in both promoting Th2 immune responses and preventing renal injury in murine IRI. Interestingly, ILC2 and MPPtype2 cells were expanded in kidney of mice treated with IL-25, and adoptive transfer of ILC2 or MPPtype2 cells attenuated renal injury in mice with IRI via the induction of M2 macrophages in kidney.
Results
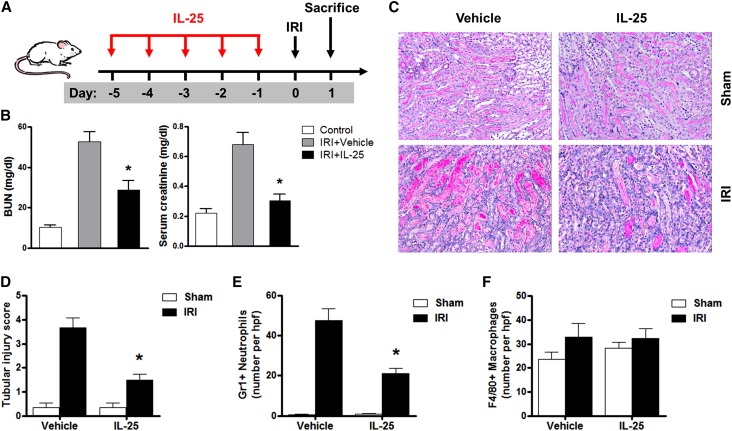
IL-25 Protected against Renal Injury in IRI Mice
BUN and serum creatinine were significantly increased in bilateral IRI mice compared with those of control mice and were significantly improved in bilateral IRI mice treated with IL-25 (Figure 1, A and B). In renal IRI, renal injury was characterized by tubular necrosis, tubular dilation, cast formation, and tubular cell vacuolization. Tubular injury of postischemic kidney was significantly increased compared with that of sham kidney and significantly reduced in IRI mice treated with IL-25 (Figure 1, C and D). Gr-1+ neutrophil infiltration in the outer medulla of postischemic kidney was significantly increased compared with that of sham kidney and significantly reduced in IRI mice treated with IL-25 (Figure 1E). However, interstitial infiltration with F4/80+ macrophages was not reduced in the outer medulla of IRI mice treated with IL-25 compared with that of control IRI mice (Figure 1F). Together, IL-25 attenuated postischemic renal failure and renal IRI.
Figure 1.
IL-25 protects against renal injury in IRI mice. (A) BALB/c mice are administered with mouse recombinant IL-25 daily for 5 consecutive days before unilateral or bilateral IRI operation. Mice are euthanized at day 1 after IRI. (B) BUN and creatinine levels are assessed in control, IRI+vehicle, and IRI+IL-25 groups at day 1 after bilateral IRI. (C) PAS-stained sections of kidney outer medulla from unilateral IRI mice treated with PBS or IL-25. (D) Semiquantitative assessment of tubular injury in the IRI+vehicle and IRI+IL-25 groups at day 1 after unilateral IRI. (E and F) Numbers of Gr1+ neutrophils and F4/80+ macrophages are assessed by immunofluorescence staining in outer medulla of kidney. Values represent the mean±SEM of evaluations from each group (n=6 per group). *P<0.05 versus IRI+vehicle. PAS, periodic acid–Schiff. Original magnification, ×200 in C.
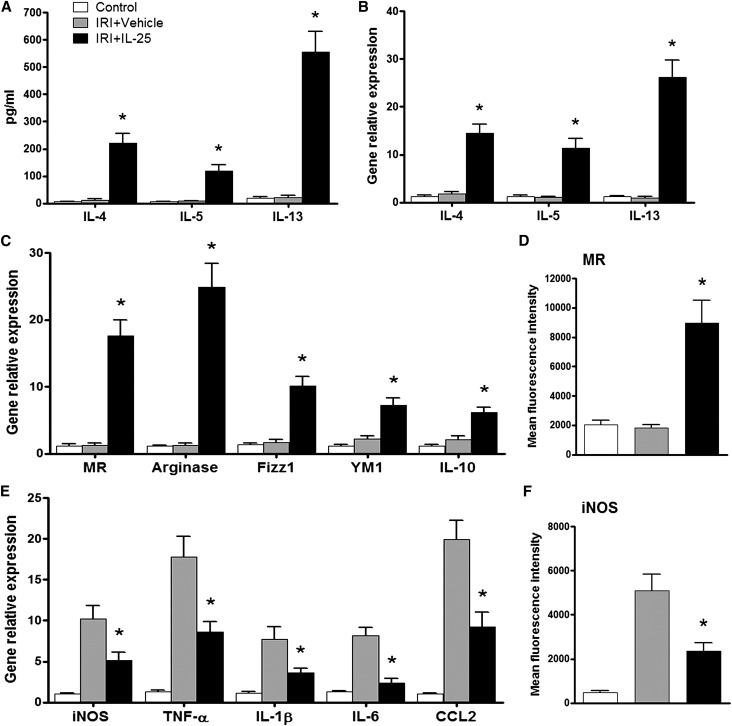
IL-25 Induced Th2 Responses and Alternatively Activated Macrophages In Vivo
To define the mechanisms underlying the protective effect of IL-25 against renal injury, we examined Th2 responses in the periphery and in kidney. In IRI mice treated with IL-25, serum levels of the Th2 cytokines IL-4, IL-5, and IL-13 were significantly increased compared with those of control and IRI mice (Figure 2A). In addition, the mRNA expression of IL-4, IL-5, and IL-13 in kidney was significantly increased in IRI mice given IL-25 compared with that of control and IRI mice (Figure 2B). To further investigate the mechanisms of IL-25’s protective effects, we examined the activation status of endogenous macrophages in the kidney. Interestingly, the kidney macrophages from IRI mice treated with IL-25 had elevated mRNA expression of M2 macrophage markers, including mannose receptor (MR), arginase, FIZZ1, YM1, and IL-10 (Figure 2C). Similarly, FACS analysis demonstrated that MR expression was significantly increased in the kidney macrophages from IRI mice treated with IL-25 compared with those from control IRI mice (Figure 2D). In addition, the expression of M2 macrophage markers, such as MR and arginase, was significantly increased in macrophages isolated from spleen, liver, and lung of IRI mice treated with IL-25 (Supplemental Figure 1), indicating the influence of IL-25 in systemic immune responses rather than a specific effect on renal macrophage function. By contrast, the expression of M1 macrophage markers, including inducible nitric oxide synthase (iNOS), TNF-α, IL-1β, IL-6, and CCL2, was significantly lower in the kidney macrophages from IRI mice treated with IL-25 than that of control IRI mice (Figure 2, E and F). Thus, IL-25 induced Th2 responses in the periphery and kidney, thereby inducing M2 macrophages in kidney, which are known to trigger immunoregulation and tissue repair.
Figure 2.
IL-25 induces Th2 response and alternatively activates macrophages in kidney. (A) IL-4, IL-5, and IL-13 levels in serum are assessed in control, IRI+vehicle, and IRI+IL-25 groups at day 1 after bilateral IRI. (B) The mRNA expression of IL-4, IL-5, and IL-13 in kidney is examined by real-time PCR, and expressed relative to the control of each experiment. F4/80+ kidney macrophages are sorted by flow cytometry in control, IRI+vehicle, and IRI+IL-25 groups at day 1 after bilateral IRI. (C and E) The mRNA expression of MR, arginase, FIZZ1, YM1, IL-10, iNOS, TNF-α, IL-1β, IL-6, and CCL2 is quantified by real-time PCR in F4/80+ kidney macrophages. (D and F) The expression of MR and iNOS (mean fluorescence intensity) in F4/80+ kidney macrophages is assessed by flow cytometry. Values represent the mean±SEM of evaluations from each group (n=6 per group). *P<0.05 versus IRI+vehicle.
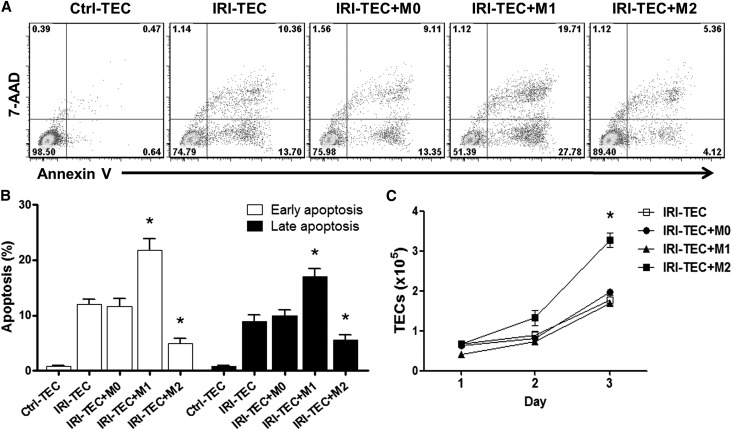
Alternatively Activated Macrophages Promoted Tubular Cell Survival In Vitro
To determine the effects of M1 and M2 macrophages on renal tubular cells in vivo, an in vitro coculture model was established to mimic the in vivo macrophage interaction with injured tubular cells. Simulated ischemic renal tubular epithelial cells (TECs) were induced by immersing the cellular monolayer in mineral oil, and were then cocultured with M0, M1, or M2 macrophages for 1–3 days. The apoptosis of ischemic TECs was significantly increased compared with control TECs, and was further enhanced by coculture with M1 macrophages, whereas coculture with M2 macrophages resulted in a reduction of apoptosis in ischemic TECs (Figure 3, A and B). Furthermore, the number of TECs was significantly increased after coculture with M2 macrophages for 3 days compared with that of ischemic TECs in the absence of macrophages or coculture with M0 or M1 macrophages (Figure 3C). These data indicate that M2 macrophages promoted ischemic tubular cell survival in vitro that may partially mirror the in vivo function of M2 macrophage in renal repair and regeneration.
Figure 3.
Alternatively activated macrophages reduce TEC apoptosis and promote TEC proliferation in vitro. Renal TEC ischemia is induced in vitro by immersing the cellular monolayer in mineral oil for 60 minutes at 37°C. The M0, M1, or M2 macrophages are cocultured with ischemic renal TECs (IRI-TECs) for 1–3 days. TECs are exposed to serum-free K1 medium alone as the nonischemic control (Ctrl-TEC). (A) Representative FACS analysis of apoptosis in TECs after 1-day coculture. (B) Frequency of early apoptosis (Annexin V+7AAD− cells) and late apoptosis (Annexin V+7AAD+ cells) in TECs after 1-day coculture. Values represent the mean±SEM of evaluations from each group (n=5 per group). *P<0.05 versus IRI-TEC and IRI-TEC+M0. (C) Numbers of renal TECs were counted at each time point after coculture. Values represent the mean±SEM of evaluations from each group (n=5 per group). *P<0.05 versus other three groups. 7AAD, 7-aminoactinomycin D.
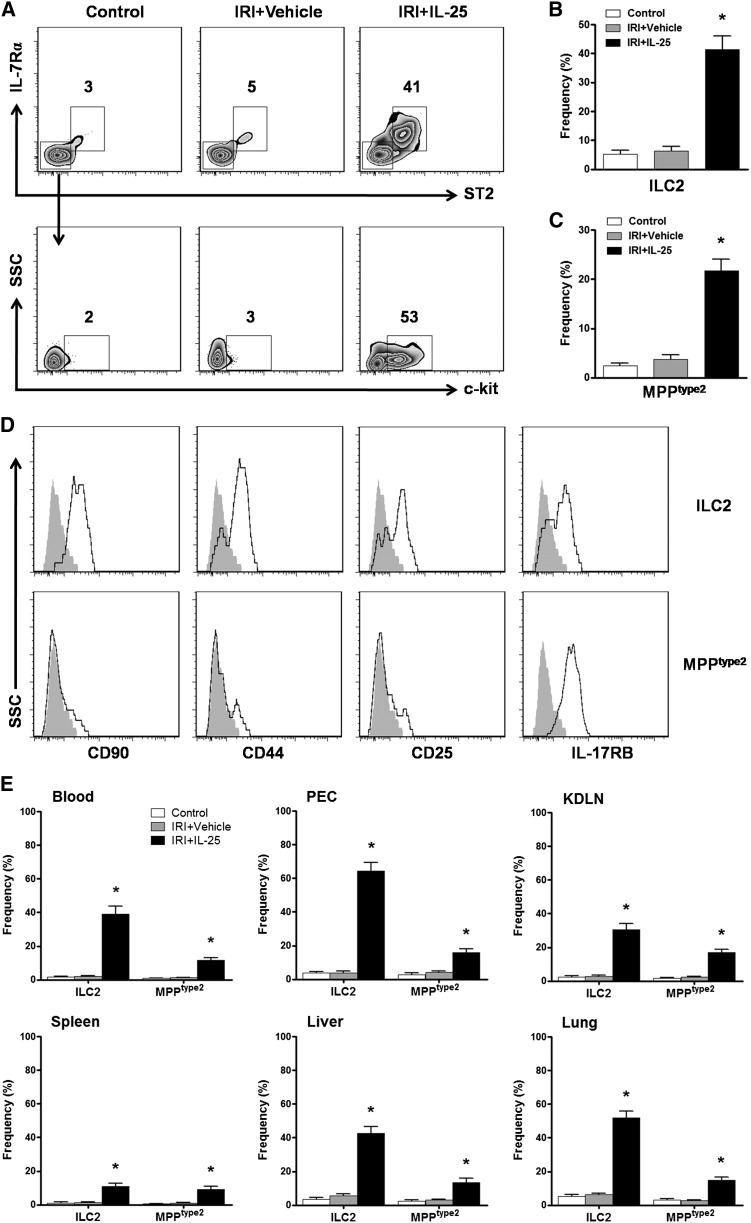
IL-25 Elicited ILC2 and MPPtype2 In Vivo
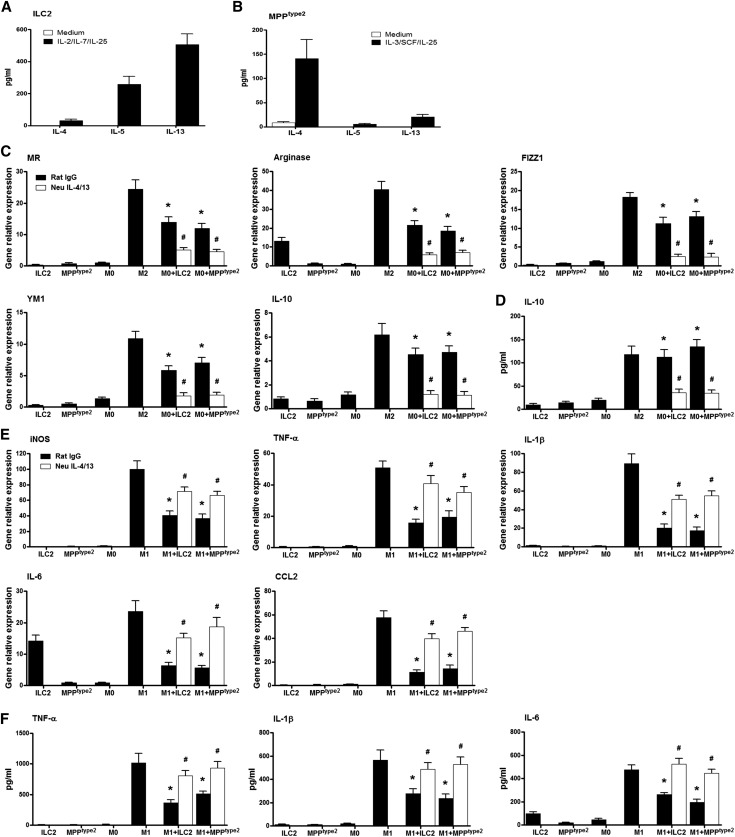
IL-25 was recently shown to elicit previously unrecognized innate immune cells including ILC2 and MPPtype2 in multiple anatomic sites where they contribute to Th2 immune responses and regulate inflammation. To test whether IL-25 elicits similar or distinct innate cell populations in kidney of IRI mice, we used a combination of CD45, lineage (Lin) markers (CD3, CD4, CD8α, TCRβ, TCRγδ, CD19, B220, CD49b, CD11b, CD11c, FcεRIα, Gr-1, and Ter-119), ST2, IL-7Rα, c-kit, CD90, CD44, CD25, and IL-17RB. After pregating on CD45+ cells to exclude any contaminating kidney epithelial cells, Lin− cells in kidney were analyzed for expression of surface markers that define Lin−ST2+IL-7Rα+ ILC2 versus Lin−ST2−IL-7Rα−c-kit+ MPPtype2 cells (Figure 4A).12,20 Compared with control and IRI mice, IL-25–treated IRI mice exhibited significant increases in the frequencies of Lin−ST2+IL-7Rα+ ILC2 and Lin−ST2−IL-7Rα−c-kit+ MPPtype2 in kidney (Figure 4, B and C). In addition, Lin−ST2+IL-7Rα+ cells in kidney expressed CD90, CD44, CD25, and IL-17RB, a phenotype consistent with ILC2.12,13 Lin−ST2−IL-7Rα−c-kit+ cells in kidney expressed IL-17RB but lacked expression of CD90, CD44, and CD25, a phenotype consistent with that of MPPtype2 cells but distinct from ILC2s (Figure 4D).15,20 Moreover, IL-25–elicited ILC2 and MPPtype2 cells were observed in multiple anatomic sites including the blood, peritoneal cavity, KLDNs, spleen, liver, and lung, which is consistent with previous studies (Figure 4E). Therefore, these data indicate that IL-25 elicits both ILC2 and MPPtype2 response in kidney, which may be associated with the induction by IL-25 of Th2 responses in kidney.
Figure 4.
IL-25 elicits ILC2 and MPPtype2 in kidney. BALB/c mice are administered mouse recombinant IL-25 daily for 5 consecutive days before IRI operation. Indicated tissues are harvested at day 1 after IRI, and the frequency of ILC2 and MPPtype2 cells ire assessed by flow cytometry. (A) Representative FACS analysis showing the gating strategy to identify ST2+IL-7Rα+ ILC2 and ST2−IL-7Rα−c-kit+ MPPtype2 in the CD45+Lin− cells from kidney of control, IRI+vehicle, or IRI+IL-25 mice. (B and C) Frequency of ST2+IL-7Rα+ ILC2 and ST2−IL-7Rα−c-kit+ MPPtype2 in the CD45+Lin− cell compartment from the kidneys of control, IRI+vehicle, or IRI+IL-25 mice. (D) Histogram showing expression of CD90, CD44, CD25, and IL-17RB on ILC2 and MPPtype2 cells of kidney from IL-25–treated mice. Specific markers (black lines) and isotype controls (gray-filled areas) are shown. (E) Frequency of ST2+IL-7Rα+ ILC2 and ST2−IL-7Rα−c-kit+ MPPtype2 in the CD45+Lin− cell compartment from blood, PEC, KDLN, spleen, liver, and lung of control, IRI+vehicle, or IRI+IL-25 mice. Values represent the mean±SEM of evaluations from each group (n=6 per group). *P<0.05 versus control (IRI+vehicle). PEC, peritoneal exudate cells.
ILC2 and MPPtype2 Induced M2 Macrophages and Suppressed M1 Macrophages In Vitro
ILC2 and MPPtype2 were isolated from BALB/c mice treated with IL-25. ILC2s produced greater amounts of IL-5 and IL-13, but low amounts of IL-4, in culture supernatant with IL-2/IL-7/IL-25 stimulation (Figure 5A).12,24 By contrast, after culture with IL-3/stem cell factor/IL-25, MPPtype2 produced elevated levels of IL-4 but little IL-13 (Figure 5B).15,20 To test whether ILC2 and MPPtype2 regulate macrophage activation, bone marrow–derived macrophages were cultured with activated ILC2 and MPPtype2 in vitro. Both ILC2 and MPPtype2 increased the mRNA expression of M2 macrophage markers, including MR, arginase, FIZZ1, YM1, and IL-10. IL-10 levels were significantly increased in the supernatant of macrophages cocultured with ILC2 or MPPtype2 in comparison to macrophages alone. Critically, induction of the M2 phenotype of macrophages by ILC2 and MPPtype2 was blocked by IL-4/IL-13 neutralizing antibodies (Figure 5, C and D). By contrast, M1 macrophages cocultured with ILC2 or MPPtype2 significantly reduced their proinflammatory phenotype, including iNOS, TNF-α, IL-1β, IL-6, and CCL2. TNF-α, IL-1β, and IL-6 levels were significantly decreased in the supernatant of M1 macrophages cocultured with ILC2 or MPPtype2 compared with M1 macrophages alone. The effect on M1 macrophages of ILC2 or MPPtype2 was blocked by IL-4/IL-13 neutralizing antibodies (Figure 5, E and F). Thus, these data suggest that ILC2 and MPPtype2 induce M2 macrophages and suppress M1 macrophages through producing Th2 cytokines in vitro.
Figure 5.
ILC2 and MPPtype2 induce M2 macrophages and suppress M1 macrophages in vitro. ILC2 and MPPtype2 are isolated from BALB/c mice treated with IL-25 by flow sorting. (A and B) Sorted ILC2s are cultured with medium only or with IL-2 (10 ng/ml), IL-7 (10 ng/ml), and IL-25 (100 ng/ml) for 2 days. MPPtype2 cells are incubated with medium only or with IL-3 (10 ng/ml), SCF (50 ng/ml), and IL-25 (100 ng/ml) for 2 days. IL-4, IL-5, and IL-13 levels in culture supernatants are measured by ELISA. Data represent the mean±SEM of four independent experiments. (C and D) Bone marrow macrophages are incubated with complete medium (M0), recombinant IL-4/IL-13 (M2), or ILC2 or MPPtype2 in the presence of IL-4/IL-13 neutralizing antibodies or control rat IgG for 24 hours. The mRNA expression of MR, arginase, FIZZ1, YM1, and IL-10 is assessed by real-time PCR in ILC2s, MPPtype2 cells, and bone marrow macrophages. The levels of IL-10 in culture supernatants are measured by ELISA. Data represent the mean±SEM of five independent experiments. *P<0.05 versus M0; #P<0.05 versus rat IgG. (E and F) Bone marrow macrophages are stimulated with LPS (M1) for 24 hours, and then cocultured with ILC2 or MPPtype2 in the presence of IL-4/IL-13 neutralizing antibodies or control rat IgG for 24 hours. The mRNA expression of iNOS, TNF-α, IL-1β, IL-6, and CCL2 is assessed by real-time PCR in ILC2s, MPPtype2 cells, and bone marrow macrophages. The levels of TNF-α, IL-1β, and IL-6 in culture supernatants are measured by ELISA. Data represent the mean±SEM of five independent experiments. SCF, stem cell factor. $P<0.05 versus M1; #P<0.05 versus rat IgG.
Adoptive Transfer of ILC2 and MPPtype2 Attenuated Renal Injury in IRI
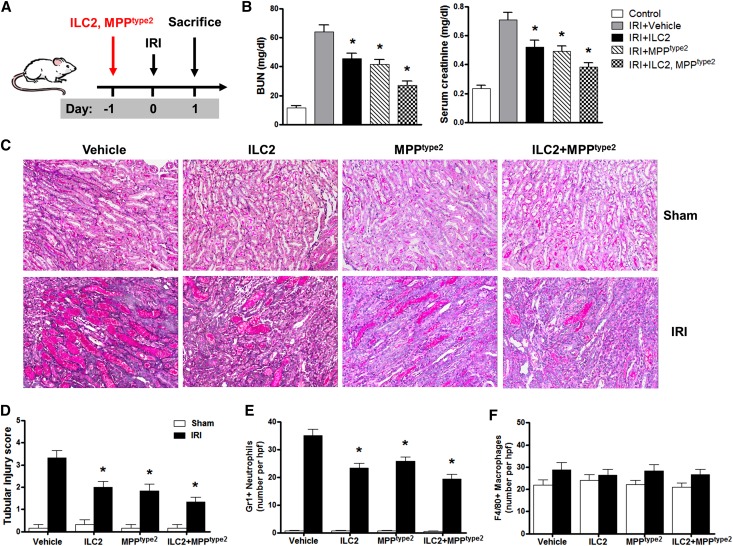
We next investigated the in vivo function of ILC2 and MPPtype2 by adoptive transfer study. The ILC2 and MPPtype2 were separated from BALB/c mice treated with IL-25, and adoptively transferred into IRI BALB/c mice 1 day before IRI (Figure 6A). Transfused ILC2, MPPtype2, or both ILC2 and MPPtype2 cells significantly improved renal function in bilateral IRI mice, including reduction of BUN and serum creatinine (Figure 6B). Examination of renal histology (Figure 6C) and tubular injury scoring (Figure 6D) 1 day after IRI confirmed that tubule damage was improved in IRI mice treated with ILC2, MPPtype2, or both ILC2 and MPPtype2 cells compared with that of control IRI mice. Gr-1+ neutrophil infiltration in the outer medulla of postischemic kidney was significantly increased compared with that of sham kidney and was significantly reduced in IRI mice treated with ILC2, MPPtype2, or both ILC2 and MPPtype2 cells (Figure 6E). However, interstitial infiltration with F4/80+ macrophages was not reduced in outer medulla of IRI mice treated with ILC2, MPPtype2, or both ILC2 and MPPtype2 cells compared with that of control IRI mice (Figure 6F). These data indicate that both ILC2 and MPPtype2 have protective effects on renal function and injury in mice with renal IRI.
Figure 6.
Adoptive transfer of ILC2 and MPPtype2 attenuates renal injury in IRI. (A) ILC2 and MPPtype2 are isolated from BALB/c mice treated with IL-25 by flow sorting, and are adoptively transferred into BALB/c mice 1 day before unilateral or bilateral IRI. Mice are euthanized at day 1 after IRI. (B) BUN and creatinine levels are assessed in control, IRI+vehicle, IRI+ILC2, IRI+MPPtype2, and IRI+ILC2 and MPPtype2 groups at day 1 after bilateral IRI. (C) PAS-stained sections of kidney outer medulla from unilateral IRI mice treated with PBS, ILC2, MPPtype2, or both ILC2 and MPPtype2 cells. (D) Semiquantitative assessment of tubular injury in IRI+vehicle, IRI+ILC2, IRI+MPPtype2, and IRI+ILC2 and MPPtype2 groups at day 1 after unilateral IRI. (E and F) Numbers of Gr1+ neutrophils and F4/80+ macrophages are assessed by immunofluorescence staining in outer medulla of kidney. Values represent the mean±SEM of evaluations from each group (n=6 per group). *P<0.05 versus IRI+vehicle. PAS, periodic acid–Schiff. Original magnification, ×200 in C.
Adoptive Transfer of ILC2 and MPPtype2 Induced Th2 Response and Alternatively Activated Macrophages in Kidney
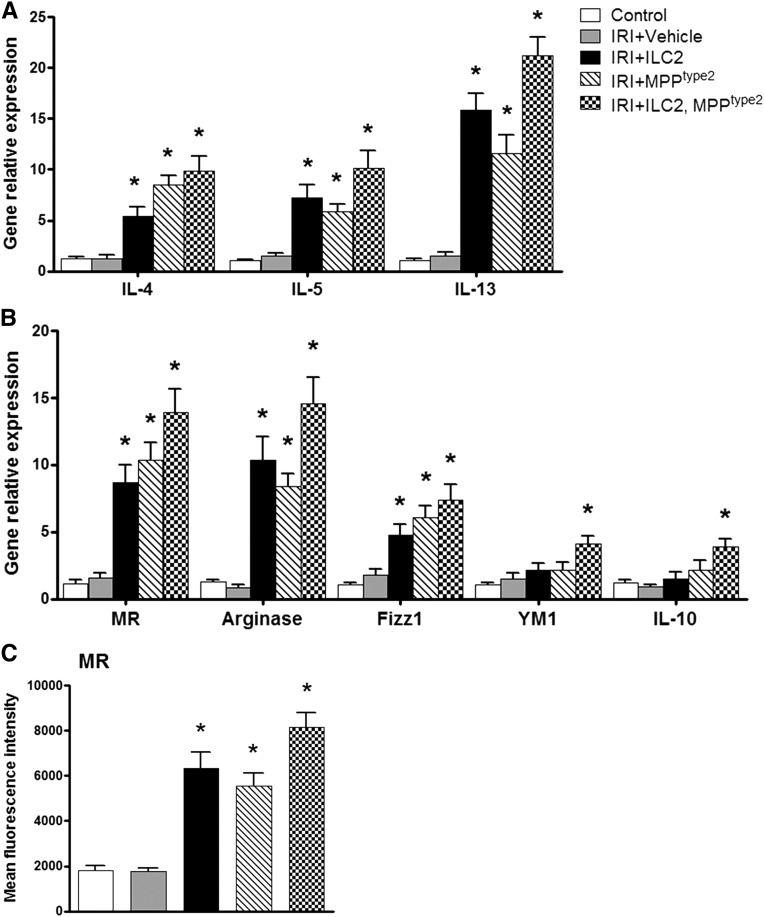
To further investigate the mechanisms of the protective effects of ILC2 and MPPtype2 in IRI mice, we first examined whether ILC2 and MPPtype2 cells distribute into kidney. As expected, fluorescently labeled ILC2 and MPPtype2 cells were observed in both sham and IRI kidney, whereas the number of ILC2 and MPPtype2 cells in IRI kidney was significantly higher than that in sham kidney (Supplemental Figure 2, A and B). In addition, fluorescently labeled ILC2 and MPPtype2 cells were also observed in spleen, liver, and lung (Supplemental Figure 2C). Next, we assessed Th2 cytokine expression in kidney and phenotype of endogenous kidney macrophages. The mRNA expression of IL-4, IL-5, and IL-13 in kidney was significantly increased in IRI mice given ILC2, MPPtype2, or both ILC2 and MPPtype2 cells compared with control and IRI mice (Figure 7A). Meanwhile, the endogenous kidney macrophages of IRI mice treated with ILC2 or MPPtype2 expressed high levels of MR, arginase, and FIZZ1, but not YM1 and IL-10 (Figure 7, B and C). In addition, adoptive transfer of both ILC2 and MPPtype2 cells elevated the expression of M2 macrophage markers in endogenous kidney macrophages, including MR, arginase, FIZZ1, YM1, and IL-10 (Figure 7, B and C). These data suggest that ILC2 and MPPtype2 prevent IRI through inducing Th2 response and M2 macrophages in kidney.
Figure 7.
Adoptive transfer of ILC2 and MPPtype2 induces Th2 response and alternatively activated macrophages in kidney. (A) mRNA expression of IL-4, IL-5, and IL-13 in kidney from control, IRI+vehicle, IRI+ILC2, IRI+MPPtype2, and IRI+ILC2 and MPPtype2 groups is examined by real-time PCR, and is expressed relative to the control of each experiment. F4/80+ kidney macrophages are sorted by flow cytometry in control, IRI+vehicle, IRI+ILC2, IRI+MPPtype2, and IRI+ILC2 and MPPtype2 groups at day 1 after IRI. (B) The mRNA expression of MR, arginase, FIZZ1, YM1, and IL-10 is quantified by real-time PCR in F4/80+ kidney macrophages. (C) The expression of MR (mean fluorescence intensity) in F4/80+ kidney macrophages is assessed by flow cytometry. Values represent the mean±SEM of evaluations from each group (n=6 per group). *P<0.05 versus IRI+vehicle.
Discussion
In this study, IL-25 induced a Th2 immune response and significantly prevented renal structural and functional injury in mice with IRI. The mechanisms underlying the protective effect of IL-25 might be associated with its initiation of Th2 immune responses through expansion of innate immune cells, ILC2 and MPPtype2. IL-25–responsive ILC2 and MPPtype2 cells produced a great amount of Th2 cytokines and were shown to induce M2 macrophages and suppress activation of M1 macrophages in vitro. Furthermore, adoptive transfer of ILC2 or MPPtype2 cells prevented renal injury in mice with IRI via activation of Th2 response and induction of M2 macrophages in kidney, which reduced renal inflammation and promoted tissue repair. These data demonstrate that IL-25 may be a useful therapeutic strategy for AKI.
IL-25 has been shown to promote Th2 immune responses, thereby inducing allergic airway inflammation.1,5 However, IL-25 also plays a critical role in suppressing intestinal chronic inflammation, regulating adipose tissue inflammation and protecting against LPS-induced lethal endotoxemia.3,25,26 We have demonstrated that IL-25 attenuated renal injury in AN, a model of CKD, whereas the potential role of IL-25 in acute kidney disease is unclear. AKI induced by IRI is associated with an acute reduction of blood flow that produces hypoxia-induced vascular and tubular dysfunction. Innate immunity appears to be the dominant pathway of renal injury induction and progression in this model.27 Administration of IL-25 before IRI significantly improved renal function and reduced renal injury in IRI mice. This is the first report to show that IL-25 can prevent renal injury in acute kidney disease. IL-25 induced Th2 immune response in both the periphery and within the kidneys of IRI mice characterized by the overproduction of IL-4, IL-5, and IL-13, which is consistent with previous studies in intestinal chronic inflammation and autoimmune disease.3,10 Enhancing Th2 immunity with exogenous IL-4 or IL-13 was also shown to reduced renal inflammation and injury in experimental immune-mediated GN.28–31 By contrast, IL-4–deficient or STAT6-deficient mice had markedly worse renal functional and tubular injury after ischemia compared with the wild type.32 IL-4 neutralizing antibody reversed the protective effect of Sphingosine 1-phosphate 3–deficient dendritic cells in IRI mice.33 Moreover, the kidney M2 macrophages were significantly increased in IRI mice treated with IL-25. Our group first reported that IL-25 induced M2 macrophages within the kidney of AN mice and discovered that induced M2 macrophages may be an important mechanism underlying IL-25’s protective role in AN.11 Hams et al. recently reported that treating obese mice with IL-25 induced weight loss and improved glucose tolerance, and was associated with increased infiltration of M2 macrophages into the visceral adipose tissue (VAT).26 Similarly, Yang and colleagues demonstrated that IL-25–responsive M2 macrophages play an important role in the induction of type 2 immunity to Heligmosomoides bakeri infection.34 M2 macrophages, which regulate inflammation and promote tissue repair, have been applied as a treatment of experimental kidney diseases.22,35–38 For instance, Lee et al. found that adoptive transfer of M2 macrophages early after injury reduced renal injury in mice with IRI.22 We found that M2 macrophages protected against apoptosis of ischemic TECs and promoted proliferation of ischemic TECs in vitro. Therefore, the mechanisms underlying the renoprotective effects of IL-25 could involve its initiation of Th2 immune responses and induction of M2 macrophages.
A most interesting finding in this study was the induction by IL-25 of ILC2 and MPPtype2 in kidney, demonstrated here for the first time. Treating IRI mice with IL-25 increased the frequency of ILC2 and MPPtype2 cells in multiple tissue sites, including the blood, peritoneal cavity, spleen, liver, and lung, which is consistent with previous studies.15,20 Moreover, IL-25–elicited ILC2 and MPPtype2 cells were observed in kidney and KDLN of IRI mice. ILC2 and MPPtype2 cells have been shown to contribute to Th2 cytokine responses in helminth infections and allergic airway inflammation. Thus, it is likely that ILC2 and MPPtype2 cells elicited by IL-25 are involved in the induction of Th2 response in the local environment of the kidney.
Next, we examined whether ILC2 and MPPtype2 cells could regulate activation status of macrophage in vitro and in vivo. As we previously demonstrated, IL-25 treatment reduced renal injury of AN via induction of Th2 response and M2 macrophages in vivo. However, IL-25 did not promote induction of M2 macrophage directly and IL-25–modulated Th2 cells induced M2 macrophages in vitro. Here, we found that IL-25–responsive ILC2 and MPPtype2 cells produced greater amounts of Th2 cytokines and induced M2 macrophages in vitro. Moreover, IL-25–responsive ILC2 and MPPtype2 cells are able to suppress LPS-activated macrophages, leading to reduced proinflammatory mediator production in vitro. However, the effects on macrophages of ILC2 and MPPtype2 cells were dependent on their secretion of Th2 cytokines. Molofsky et al. reported that ILC2s are the major source of IL-5 and IL-13 in VAT and deletion of ILC2s causes significant reduction of M2 macrophages in VAT.39 To determine whether ILC2 and MPPtype2 cells could induce M2 macrophages in kidney, the effect of ILC2 and MPPtype2 cells was further examined in IRI mice. Indeed, administration of ILC2 or MPPtype2 cells not only attenuated renal functional and histologic injury in IRI mice but also induced M2 macrophages in kidney. Therefore, this study has discovered a new mechanism underlying the renoprotective effects of IL-25—that IL-25–responsive ILC2 and MPPtype2 cells directly modulate macrophage phenotype, thereby contributing to recovery of renal injury in IRI mice. In addition to the effects of IL-25 on T cells, macrophages, ILC2s, and MPPtype2 cells, IL-25 may act on other immune cells including mast cells, natural killer cells, and dendritic cells, which play a critical role in progression of acute kidney disease. However, the IL-25 receptor is also expressed on renal TECs, so whether IL-25 regulates function of TECs needs further investigation.
The interaction between the innate immune system and renal injury is a prominent area of research because of the current progress of innate immune studies in kidney disease. Although previous studies from our group and others have outlined the importance of M2 macrophages in reducing renal inflammation and promoting tissue repair, this study suggests a future potential role for modulating cytokine activity in kidney disease, either to upregulate IL-25 or other Th2 cytokines directly, thereby artificially promoting ILC2 and MPPtype2 expansion and localized M2 macrophage polarization. This study raises the potential for IL-25 and IL-25–elicited innate immune cells as therapeutics for attenuating tubular injury of postischemic kidney.
Concise Methods
IRI Murine Model and IL-25 Administration
Six- to 8-week-old male BALB/c mice obtained from the Shanghai Laboratory Animal Center of Chinese Academy of Sciences were used in this study. The Animal Ethics Committee of Xinxiang Medical University approved all procedures. Unilateral or bilateral renal ischemia was imposed in BALB/C mice under isoflurane anesthesia. The kidneys were exposed via a midline abdominal excision; the left renal pedicle or both renal pedicles was clamped for 30 minutes using a nontraumatic sterile clamp (Roboz). After clamp removal, the kidney was inspected for restoration of blood flow as evidenced by returning to its original color before closing the wound with standard sutures. After the procedure, mice were given 1 ml of warm normal saline intraperitoneally to prevent dehydration. The animals were kept at a constant temperature (37°C) during the procedure and allowed to recover. Animals subjected to sham operation were used as controls.
BALB/c mice were divided into three groups: control, IRI with vehicle, and IRI with IL-25 treatment. For IL-25 treatment, mice were administered 0.5 μg mouse recombinant IL-25 (R&D Systems) intraperitoneally daily for 5 consecutive days before IRI operation. The dose and duration were selected according to previously published studies.3,7,20,26 Control animals received PBS only. All mice were euthanized at day 1 after IRI operation. Blood, peritoneal exudate cells, KDLN, spleen, liver, lung, and kidney were harvested for analysis. ILC2 and MPPtype2 cells were examined in multiple tissues by flow cytometry. For functional assessment, mice were injected with IL-25 or vehicle (PBS) intraperitoneally daily for 5 consecutive days, and then underwent bilateral ischemia-reperfusion for 30 minutes on both kidneys by midline abdominal incision. BUN and creatinine levels were measured using a Hitachi 747 automatic analyzer.
ILC2 and MPPtype2 Cell Labeling and Adoptive Transfer to IRI Mice
BALB/c mice were divided into five groups: control, IRI with vehicle, IRI with ILC2 treatment, IRI with MPPtype2 treatment, and IRI with ILC2 and MPPtype2 treatment. For ILC2 and MPPtype2 treatment, ILC2 and MPPtype2 were isolated from BALB/c mice treated with IL-25 daily for 5 consecutive days, and then CFSE-labeled 2×105 ILC2, 1×105 MPPtype2 or 2×105 ILC2, and 1×105 MPPtype2 cells were transferred into BALB/c mice by a single tail-vein injection 1 day before IRI operation. All mice were euthanized at day 1 after IRI operation. Blood, peritoneal exudate cells, KDLN, spleen, liver, lung, and kidney were harvested for analysis. The distribution of CFSE-labeled ILC2 and MPPtype2 was analyzed in kidney, spleen, liver, and lung sections by inverted fluorescence microscopy. The number of transfused ILC2 and MPPtype2 was quantitated in 8–10 nonoverlapping high-power fields (×400). For functional assessment, mice were injected with ILC2, MPPtype2, or vehicle (PBS) by a single tail-vein injection 1 day before IRI operation, and then underwent bilateral ischemia-reperfusion for 30 minutes on both kidneys by midline abdominal incision. BUN and creatinine levels were measured using a Hitachi 747 automatic analyzer.
Cell Suspension Preparation
Spleen and KDLNs were isolated, minced, and digested for 30 minutes at 37°C in RPMI 1640 medium containing 1 mg/ml collagenase D (Roche) and 100 μg/ml DNase I (Roche). The digested cell suspension was then passed through a 40-μm cell strainer. Kidney, liver, and lung were perfused with saline before removal and digested with collagenase and DNase as previously described. Kidney, liver, and lung were cut into 1- to 2-mm3 pieces, placed in DMEM containing 1 mg/ml collagenase IV (Sigma-Aldrich), and 100 μg/ml DNase I (Roche) for 40 minutes at 37°C with intermittent agitation. The digested cell suspension was then passed through 40-μm cell strainer. The cell suspensions were allowed to settle for 10 minutes, after which the upper three-fourths (lower density cells) was removed for subsequent assays. F4/80+ macrophages were sorted from kidney, spleen, liver, and lung by FACS. Sorted macrophages were used for real-time PCR analyses to detect phenotypes of these macrophages. Some sorted macrophages were stained with anti-mouse mannose receptor (BioLegend) or iNOS (BD Biosciences) and analyzed by flow cytometry.
FACS Analyses
For FACS analysis, single-cell suspensions were stained with Fc block/anti-CD16/32 (2.4G2) and antibodies to CD45.2 (104), ST2 (RMST2-2), IL-7Rα (A7R34), c-kit (2B8), CD90.2 (30-H12), CD44 (IM7), CD25 (PC61), and IL-17RB (9B10), as well as antibodies to the following T cell, B cell, natural killer cell, monocyte/macrophage, dendritic cell, eosinophil, neutrophil, and erythroid cell lineages (referred hereafter as Lin): CD3 (145-2C11), CD4 (GK1.5), CD8α (53-6.7), TCRβ (H57-597), TCRγδ (eBioGL3), CD19 (1D3), B220 (RA3-6B2), CD49b (DX5), CD11b (M1/70), CD11c (N418), FcεRIα (MAR-1), Gr-1 (RB6-8C5), and Ter-119 (all purchased from eBioscience or BioLegend). Cells were analyzed on an LSR II flow cytometer (BD Biosciences). For FACS sorting, single-cell suspensions were pregated on hematopoietic cells using anti-CD45.2 antibody, and then lineage (CD3/CD4/CD8α/TCRβ/TCRγδ/CD19/B220/CD49b/CD11b/CD11c/FcεRIα/Gr-1/Ter-119) was used to exclude immune cells and 4′,6-diamidino-2-phenylindole was used to exclude dead cells. ILC2 cell populations (Lin−ST2+IL-7Rα+) and MPPtype2 cells (Lin−ST2−IL-7Rα−c-kit+) were sorted using a FACSAria II (BD Biosciences). After sorting, cells were used for phenotypic and functional assays.
ILC2 and MPPtype2 Coculture with Macrophages
ILC2 and MPPtype2 were isolated from BALB/c mice treated with IL-25. ILC2s were cultured in RPMI 1640 medium, supplemented with 10% FBS, penicillin (100 U/ml), and streptomycin (100 μg/ml), plus IL-2 (10 ng/ml), IL-7 (10 ng/ml), and IL-25 (100 ng/ml) for 2 days. MPPtype2 cells were incubated in the presence of IL-3 (10 ng/ml), stem cell factor (50 ng/ml), and IL-25 (100 ng/ml) for 2 days. Cell-free supernatants were assessed for IL-4, IL-5, and IL-13 cytokine production by ELISA (eBioscience). Primary cultures of murine macrophages were obtained from bone marrow of BALB/c mice by a previously described technique.11,40 Macrophages derived from bone marrow were cultured in RPMI 1640 medium, supplemented with 10% FBS, penicillin (100 U/ml), and streptomycin (100 μg/ml), plus 10 ng/ml macrophage colony-stimulating factor for 6 days, and F4/80+ macrophages were sorted by flow cytometry. Cell viability was >95%. Macrophages were seeded onto 12-well culture plates (2×105 cells/well) and incubated with complete medium (referred to as M0), mouse recombinant IL-4/IL-13 (10 ng/ml each; R&D Systems) (referred to as M2), ILC2 (2×104 cells/well) or MPPtype2 (2×104 cells/well) in the presence of IL-4/IL-13 neutralizing antibodies (eBioscience), or control rat IgG1 (eBioscience) for 24 hours. In parallel, macrophages (2×105 cells/well) were stimulated with LPS (100 ng/ml; Sigma-Aldrich) (referred to as M1) for 24 hours, and then cocultured with ILC2 (2×104 cells/well) or MPPtype2 (2×104 cells/well) in the presence of IL-4/IL-13 neutralizing antibodies (eBioscience) or control rat IgG1 (eBioscience) for 24 hours. Macrophages were then used for real-time PCR and ELISA analyses to detect macrophage phenotype.
Primary Culture of Mouse Renal TECs
Primary mouse TECs were generated following established methods adapted from Doctor et al.41 In brief, kidneys were harvested after cardiac perfusion with saline to remove blood cells. The tissue from the outer cortex was cut into pieces of approximately 1 mm3 and then digested in DMEM containing 1 mg/ml collagenase IV (Sigma-Aldrich), and 100 μg/ml DNase I (Roche) for 40 minutes at 37°C with intermittent agitation. Renal TECs were separated by centrifugation using Percoll solution and cultured in defined K1 medium: DMEM/F12 medium supplemented with 10 ng/ml EGF, 1 ng/ml PGE1, 5 μg/ml insulin, 5 μg/ml transferrin, 5 ng/ml sodium selenite, 5×10−11 M triiodothyronine, 5×10−8 M hydrocortisone, 100 U/ml penicillin, 100 μg/ml streptomycin, 25 mM HEPES, and 5% FBS.
Simulated Ischemic Renal TECs Coculture with Macrophages
Ischemia in renal TECs was simulated by immersing the cellular monolayer in mineral oil according to the protocol of Meldrum et al.42 This immersion simulated ischemia by restricting cellular exposure to oxygen and nutrients as well as by limiting metabolite washout. Briefly, renal TECs (1×105) were placed in six-well tissue culture plates in serum-free K1 medium for 24 hours, washed twice with PBS, and immersed in mineral oil for 60 minutes at 37°C. After extensive washing with PBS, TECs were incubated in K1 medium. Bone marrow–derived macrophages were polarized to M0 (medium), M1 (LPS), or M2 (IL-4/IL-13) in vitro. The M0, M1, or M2 macrophages were seeded on a 0.4-µm Transwell insert (Falcon) and cocultured with ischemic renal TECs for 1–3 days. TECs were exposed to serum-free K1 medium alone as the nonischemic control. Numbers of renal TECs were determined at each time point by trypsinizing and counting the cells. Apoptosis of TECs at 1 day after the coculture were measured using FACS with 7-aminoactinomycin D and Annexin V staining following the manufacturer’s protocol (BD Biosciences).
ELISA of Cytokines
IL-4, IL-5, IL-13, IL-10, TNF-α, IL-1β, and IL-6 levels in sera and culture supernatants were assayed using an ELISA kit purchased from eBioscience. ELISA was performed according to the manufacturer’s protocol.
Quantitative RT-PCR
F4/80+ endogenous renal macrophages were sorted by FACS. Cell viability was >95%. Total RNA was isolated from kidney or endogenous renal macrophages by the RNeasy Mini Kit (Qiagen), and was reverse-transcribed with the First-Strand cDNA Synthesis Kit (Fermantas). Real-time PCR was performed on the Rotogene-6000 Real-Time Thermo cycler (Corbett Research) using the SYBR Master Mix (Invitrogen). The analysis method was as previously described43 and the PCR primer sequences are presented in Supplemental Table 1.
Histology and Immunofluorescence
Coronal sections of kidney tissue were stained with periodic acid–Schiff. Histologic changes in the corticomedullary junction and outer medulla were evaluated semiquantitatively.22,44 Briefly, tubular damage was estimated in 8–10 high-power fields (×200) per section by using a scoring system based on the percentage of damaged tubules per field (0, normal; 1, <10%; 2, 10%–25%; 3, 25%–50%; 4, 50%–75%; and 5, >75%). The mean score of each animal was compared. To avoid selection bias, the areas to be viewed for morphometric analysis were anatomically identical for each section and positioned before microscopic visualization.
For immunofluorescence staining, rat anti-mouse Gr-1 (Ly-6G, 1/100; BioLegend) or F4/80 (1/100; eBioscience) was used as the primary antibody and AF488 goat anti-rat IgG (1/1000; Invitrogen) as the secondary antibody. Control rat IgG to primary antibodies was included in staining. The number of interstitial Gr-1+ and F4/80+ cells was quantitated in 8–10 nonoverlapping outer medulla fields (×400).
Statistical Analyses
Renal functional data were log-transformed before analysis to stabilize the variance. Statistical tests included the unpaired, two-tailed t test using Welch’s correction for unequal variances and one-way ANOVA with Tukey’s multiple comparison test. Statistical analyses were performed using Prism software (version 5; GraphPad). Results are expressed as the mean±SEM. P<0.05 was considered statistically significant.
Disclosures
None.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (no. 81200506) and the Henan Province Education Department (no. 14A310015).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014050479/-/DCSupplemental.
References
- 1.Angkasekwinai P, Park H, Wang YH, Wang YH, Chang SH, Corry DB, Liu YJ, Zhu Z, Dong C: Interleukin 25 promotes the initiation of proallergic type 2 responses. J Exp Med 204: 1509–1517, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ikeda K, Nakajima H, Suzuki K, Kagami S, Hirose K, Suto A, Saito Y, Iwamoto I: Mast cells produce interleukin-25 upon Fc epsilon RI-mediated activation. Blood 101: 3594–3596, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Zaph C, Du Y, Saenz SA, Nair MG, Perrigoue JG, Taylor BC, Troy AE, Kobuley DE, Kastelein RA, Cua DJ, Yu Y, Artis D: Commensal-dependent expression of IL-25 regulates the IL-23-IL-17 axis in the intestine. J Exp Med 205: 2191–2198, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, Menon S, Clifford T, Hunte B, Lesley R, Muchamuel T, Hurst SD, Zurawski G, Leach MW, Gorman DM, Rennick DM: IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity 15: 985–995, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Tamachi T, Maezawa Y, Ikeda K, Kagami S, Hatano M, Seto Y, Suto A, Suzuki K, Watanabe N, Saito Y, Tokuhisa T, Iwamoto I, Nakajima H: IL-25 enhances allergic airway inflammation by amplifying a TH2 cell-dependent pathway in mice. J Allergy Clin Immunol 118: 606–614, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Corrigan CJ, Wang W, Meng Q, Fang C, Eid G, Caballero MR, Lv Z, An Y, Wang YH, Liu YJ, Kay AB, Lee TH, Ying S: Allergen-induced expression of IL-25 and IL-25 receptor in atopic asthmatic airways and late-phase cutaneous responses. J Allergy Clin Immunol 128: 116–124, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Fallon PG, Ballantyne SJ, Mangan NE, Barlow JL, Dasvarma A, Hewett DR, McIlgorm A, Jolin HE, McKenzie AN: Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J Exp Med 203: 1105–1116, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao A, Urban JF, Jr, Sun R, Stiltz J, Morimoto M, Notari L, Madden KB, Yang Z, Grinchuk V, Ramalingam TR, Wynn TA, Shea-Donohue T: Critical role of IL-25 in nematode infection-induced alterations in intestinal function. J Immunol 185: 6921–6929, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zaiss MM, Maslowski KM, Mosconi I, Guenat N, Marsland BJ, Harris NL: IL-1β suppresses innate IL-25 and IL-33 production and maintains helminth chronicity. PLoS Pathog 9: e1003531, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kleinschek MA, Owyang AM, Joyce-Shaikh B, Langrish CL, Chen Y, Gorman DM, Blumenschein WM, McClanahan T, Brombacher F, Hurst SD, Kastelein RA, Cua DJ: IL-25 regulates Th17 function in autoimmune inflammation. J Exp Med 204: 161–170, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao Q, Wang C, Zheng D, Wang Y, Lee VW, Wang YM, Zheng G, Tan TK, Yu D, Alexander SI, Harris DC, Wang Y: IL-25 induces M2 macrophages and reduces renal injury in proteinuric kidney disease. J Am Soc Nephrol 22: 1229–1239, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa J, Ohtani M, Fujii H, Koyasu S: Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature 463: 540–544, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, Bucks C, Kane CM, Fallon PG, Pannell R, Jolin HE, McKenzie AN: Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature 464: 1367–1370, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Price AE, Liang HE, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, Locksley RM: Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci U S A 107: 11489–11494, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saenz SA, Siracusa MC, Perrigoue JG, Spencer SP, Urban JF, Jr, Tocker JE, Budelsky AL, Kleinschek MA, Kastelein RA, Kambayashi T, Bhandoola A, Artis D: IL25 elicits a multipotent progenitor cell population that promotes T(H)2 cytokine responses. Nature 464: 1362–1366, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie AN, Mebius RE, Powrie F, Vivier E: Innate lymphoid cells—a proposal for uniform nomenclature. Nat Rev Immunol 13: 145–149, 2013 [DOI] [PubMed] [Google Scholar]
- 17.Licona-Limón P, Kim LK, Palm NW, Flavell RA: TH2, allergy and group 2 innate lymphoid cells. Nat Immunol 14: 536–542, 2013 [DOI] [PubMed] [Google Scholar]
- 18.Barlow JL, Bellosi A, Hardman CS, Drynan LF, Wong SH, Cruickshank JP, McKenzie AN: Innate IL-13-producing nuocytes arise during allergic lung inflammation and contribute to airways hyperreactivity. J Allergy Clin Immunol 129: 191.e4–198.e4, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CG, Doering TA, Angelosanto JM, Laidlaw BJ, Yang CY, Sathaliyawala T, Kubota M, Turner D, Diamond JM, Goldrath AW, Farber DL, Collman RG, Wherry EJ, Artis D: Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol 12: 1045–1054, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saenz SA, Siracusa MC, Monticelli LA, Ziegler CG, Kim BS, Brestoff JR, Peterson LW, Wherry EJ, Goldrath AW, Bhandoola A, Artis D: IL-25 simultaneously elicits distinct populations of innate lymphoid cells and multipotent progenitor type 2 (MPPtype2) cells. J Exp Med 210: 1823–1837, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li L, Okusa MD: Macrophages, dendritic cells, and kidney ischemia-reperfusion injury. Semin Nephrol 30: 268–277, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee S, Huen S, Nishio H, Nishio S, Lee HK, Choi BS, Ruhrberg C, Cantley LG: Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol 22: 317–326, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin SL, Li B, Rao S, Yeo EJ, Hudson TE, Nowlin BT, Pei H, Chen L, Zheng JJ, Carroll TJ, Pollard JW, McMahon AP, Lang RA, Duffield JS: Macrophage Wnt7b is critical for kidney repair and regeneration. Proc Natl Acad Sci U S A 107: 4194–4199, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Halim TY, Krauss RH, Sun AC, Takei F: Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity 36: 451–463, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Caruso R, Stolfi C, Sarra M, Rizzo A, Fantini MC, Pallone F, MacDonald TT, Monteleone G: Inhibition of monocyte-derived inflammatory cytokines by IL-25 occurs via p38 Map kinase-dependent induction of Socs-3. Blood 113: 3512–3519, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Hams E, Locksley RM, McKenzie AN, Fallon PG: Cutting edge: IL-25 elicits innate lymphoid type 2 and type II NKT cells that regulate obesity in mice. J Immunol 191: 5349–5353, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jang HR, Ko GJ, Wasowska BA, Rabb H: The interaction between ischemia-reperfusion and immune responses in the kidney. J Mol Med (Berl) 87: 859–864, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Tam FW, Smith J, Karkar AM, Pusey CD, Rees AJ: Interleukin-4 ameliorates experimental glomerulonephritis and up-regulates glomerular gene expression of IL-1 decoy receptor. Kidney Int 52: 1224–1231, 1997 [DOI] [PubMed] [Google Scholar]
- 29.Tipping PG, Kitching AR, Huang XR, Mutch DA, Holdsworth SR: Immune modulation with interleukin-4 and interleukin-10 prevents crescent formation and glomerular injury in experimental glomerulonephritis. Eur J Immunol 27: 530–537, 1997 [DOI] [PubMed] [Google Scholar]
- 30.Kitching AR, Turner AL, Wilson GR, Edgtton KL, Tipping PG, Holdsworth SR: Endogenous IL-13 limits humoral responses and injury in experimental glomerulonephritis but does not regulate Th1 cell-mediated crescentic glomerulonephritis. J Am Soc Nephrol 15: 2373–2382, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Sandovici M, Henning RH, van Goor H, Helfrich W, de Zeeuw D, Deelman LE: Systemic gene therapy with interleukin-13 attenuates renal ischemia-reperfusion injury. Kidney Int 73: 1364–1373, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Yokota N, Burne-Taney M, Racusen L, Rabb H: Contrasting roles for STAT4 and STAT6 signal transduction pathways in murine renal ischemia-reperfusion injury. Am J Physiol Renal Physiol 285: F319–F325, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Bajwa A, Huang L, Ye H, Dondeti K, Song S, Rosin DL, Lynch KR, Lobo PI, Li L, Okusa MD: Dendritic cell sphingosine 1-phosphate receptor-3 regulates Th1-Th2 polarity in kidney ischemia-reperfusion injury. J Immunol 189: 2584–2596, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang Z, Grinchuk V, Urban JF, Jr, Bohl J, Sun R, Notari L, Yan S, Ramalingam T, Keegan AD, Wynn TA, Shea-Donohue T, Zhao A: Macrophages as IL-25/IL-33-responsive cells play an important role in the induction of type 2 immunity. PLoS ONE 8: e59441, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y, Wang YP, Zheng G, Lee VW, Ouyang L, Chang DH, Mahajan D, Coombs J, Wang YM, Alexander SI, Harris DC: Ex vivo programmed macrophages ameliorate experimental chronic inflammatory renal disease. Kidney Int 72: 290–299, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Cao Q, Wang Y, Zheng D, Sun Y, Wang Y, Lee VW, Zheng G, Tan TK, Ince J, Alexander SI, Harris DC: IL-10/TGF-beta-modified macrophages induce regulatory T cells and protect against adriamycin nephrosis. J Am Soc Nephrol 21: 933–942, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferenbach DA, Ramdas V, Spencer N, Marson L, Anegon I, Hughes J, Kluth DC: Macrophages expressing heme oxygenase-1 improve renal function in ischemia/reperfusion injury. Mol Ther 18: 1706–1713, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jung M, Sola A, Hughes J, Kluth DC, Vinuesa E, Viñas JL, Pérez-Ladaga A, Hotter G: Infusion of IL-10-expressing cells protects against renal ischemia through induction of lipocalin-2. Kidney Int 81: 969–982, 2012 [DOI] [PubMed] [Google Scholar]
- 39.Molofsky AB, Nussbaum JC, Liang HE, Van Dyken SJ, Cheng LE, Mohapatra A, Chawla A, Locksley RM: Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J Exp Med 210: 535–549, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson CR, Kitz D, Little JR: A method for the derivation and continuous propagation of cloned murine bone marrow macrophages. J Immunol Methods 65: 319–332, 1983 [DOI] [PubMed] [Google Scholar]
- 41.Doctor RB, Chen J, Peters LL, Lux SE, Mandel LJ: Distribution of epithelial ankyrin (Ank3) spliceoforms in renal proximal and distal tubules. Am J Physiol 274: F129–F138, 1998 [DOI] [PubMed] [Google Scholar]
- 42.Meldrum KK, Meldrum DR, Hile KL, Burnett AL, Harken AH: A novel model of ischemia in renal tubular cells which closely parallels in vivo injury. J Surg Res 99: 288–293, 2001 [DOI] [PubMed] [Google Scholar]
- 43.Cao Q, Wang L, Du F, Sheng H, Zhang Y, Wu J, Shen B, Shen T, Zhang J, Li D, Li N: Downregulation of CD4+CD25+ regulatory T cells may underlie enhanced Th1 immunity caused by immunization with activated autologous T cells. Cell Res 17: 627–637, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Alikhan MA, Jones CV, Williams TM, Beckhouse AG, Fletcher AL, Kett MM, Sakkal S, Samuel CS, Ramsay RG, Deane JA, Wells CA, Little MH, Hume DA, Ricardo SD: Colony-stimulating factor-1 promotes kidney growth and repair via alteration of macrophage responses. Am J Pathol 179: 1243–1256, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]