Abstract
Increases in glomerular size occur with normal body growth and in many pathologic conditions. In this study, we determined associations between glomerular size and numbers of glomerular resident cells, with a particular focus on podocytes. Kidneys from 16 male Caucasian-Americans without overt renal disease, including 4 children (≤3 years old) to define baseline values of early life and 12 adults (≥18 years old), were collected at autopsy in Jackson, Mississippi. We used a combination of immunohistochemistry, confocal microscopy, and design-based stereology to estimate individual glomerular volume (IGV) and numbers of podocytes, nonepithelial cells (NECs; tuft cells other than podocytes), and parietal epithelial cells (PECs). Podocyte density was calculated. Data are reported as medians and interquartile ranges (IQRs). Glomeruli from children were small and contained 452 podocytes (IQR=335–502), 389 NECs (IQR=265–498), and 146 PECs (IQR=111–206). Adult glomeruli contained significantly more cells than glomeruli from children, including 558 podocytes (IQR=431–746; P<0.01), 1383 NECs (IQR=998–2042; P<0.001), and 367 PECs (IQR=309–673; P<0.001). However, large adult glomeruli showed markedly lower podocyte density (183 podocytes per 106 µm3) than small glomeruli from adults and children (932 podocytes per 106 µm3; P<0.001). In conclusion, large adult glomeruli contained more podocytes than small glomeruli from children and adults, raising questions about the origin of these podocytes. The increased number of podocytes in large glomeruli does not match the increase in glomerular size observed in adults, resulting in relative podocyte depletion. This may render hypertrophic glomeruli susceptible to pathology.
Keywords: podocyte, glomerulus, glomerular hyperfiltration
CKD is a global pandemic.1 There are multiple causes of CKD, and although each cause of CKD shows a particular pathophysiology, most share common features. Arguably, the most significant of these is podocyte injury or dysfunction.2
Podocytes have a highly specialized structure and a very limited capacity to replicate under normal circumstances.3 As major components of the glomerular filtration barrier, they fulfill a number of important functions, including structural support to capillary loops, synthesis of components of the glomerular basement membrane, synthesis and secretion of several cytokines and growth factors, and immunologic functions.4 Consequently, podocyte injury comes at a high price for the glomerulus.
In recent years, the podocyte depletion hypothesis has emerged as a unifying concept in the pathogenesis of glomerular disease.5 On the basis of multiple observations in animal models,4,6–10 podocyte depletion can be defined as absolute when it involves a reduction in the total number of podocytes per glomerulus.11,12 In a landmark study, Wharram et al.5 showed that a 40% reduction in podocyte number is a direct cause of FSGS and therefor, long-standing CKD in rats. To date, a similar threshold has not been defined in humans.
Relative podocyte depletion occurs when the number of podocytes does not keep pace or match an increase in glomerular volume or filtration surface area. Glomerular size increases with normal body growth as well before and during multiple renal pathologies, including both diabetic nephropathy13–17 and FSGS.18–24 It has been suggested that an increase in glomerular size (also known as glomerular hypertrophy) is usually a compensatory mechanism that serves to match physiologic demands and sustain renal function.25 Over the last decade, our group has described multiple factors associated with compensatory glomerular hypertrophy in humans without overt renal disease, including older age, low nephron number (Nglom), obesity, hypertension, and African-American race.26 Interestingly, the boundary between compensatory and pathologic hypertrophy remains unclear and may be associated with relative podocyte depletion.
Despite the current interest in podocyte depletion, few studies to date have reported total numbers of podocytes in normal glomeruli.27–32 This is, in part, because of the difficulty in counting podocytes and the lack of consensus on how to accurately count them. Lemley et al.11 recently compared five methods for estimating podocyte number and recommended that the design–based dissector/optical fractionator method was the optimum technique when sufficient kidney tissue was available. This method provides an estimate of the volume of individual glomeruli as well as the total numbers of each of the resident glomerular cells, including podocytes, endothelial cells, mesangial cells, and parietal epithelial cells (PECs).12 It, thus, provides an ideal tool to analyze both absolute and relative podocyte depletion and associations with numbers of other glomerular resident cell types.
In this study, we used design-based stereology to estimate glomerular volume and the numbers of podocytes and other resident glomerular cells in kidneys collected at autopsy in Caucasian-American boys and men. Our findings provide new insights into possible changes in glomerular cell populations in the context of glomerular hypertrophy in the nondiseased kidney, and they suggest that large adult glomeruli have relative podocyte depletion and therefore, may be susceptible to the development of pathology.
Results
General Demographics
General demographics of four children are provided in Table 1. Briefly, all four children were 3 years of age or younger and presented no complications during pregnancy. Body surface area at the time of death ranged from 0.31 to 0.71 m2. Causes of death for all children were not cardiovascular-related, and there were no signs of hypertension in these subjects. All children had adequate birth weights for gestational age and therefore, nephron number values>0.6 million,33 both of which can serve as good surrogate markers of a normal fetomaternal environment.26 These four subjects provided a baseline to identify changes in glomerular size and cellularity in adulthood.
Table 1.
Demographic data for four male Caucasian-American children without kidney disease
| Ranka | Age (yr) | GA (wk) | BS Area (m2) | Nglom (million) | Nglom/BS Area (million/m2) | Birth Weight (kg) | Hypertensive Status | COD | CVRD |
|---|---|---|---|---|---|---|---|---|---|
| Ch1 | 0.25 | 35 | 0.36 | 1.11 | 3.08 | 2.92 | Normotensive | SIDS | No |
| Ch2 | 0.50 | 39 | 0.31 | 0.84 | 2.71 | 3.54 | Normotensive | Other | No |
| Ch3 | 3.00 | 38 | 0.71 | 1.22 | 1.72 | 2.80 | Normotensive | Accident | No |
| Ch4 | 3.00 | 41 | 0.68 | 0.90 | 1.32 | 3.63 | Normotensive | Accident | No |
| Median | 1.75 | 38.50 | 0.52 | 1.01 | 2.22 | 3.23 | 0% | NA | 0% |
| IQR | 0.3–3.0 | 35.8–40.5 | 0.3–0.7 | 0.9–1.2 | 1.4–2.9 | 2.8–3.6 | NA | NA | NA |
GA, gestational age at birth; BS area, body surface area; Nglom, total nephron number; COD, cause of death; CVRD, cardiovascular-related death; Ch, child; SIDS, sudden infant death syndrome; Other, hematologic, neoplastic, or infectious; NA, not available.
Subjects were ranked on the basis of median IGV.
General demographics for 12 adult subjects are provided in Table 2. The youngest adult was 25 years old, whereas the oldest adult was 49 years of age; 50% of the adult subjects were either overweight or obese (body mass index≥25.0 but <40 kg/m2). Body surface area ranged from 1.45 to 2.64 m2, Nglom ranged from 0.55 to 1.66 million, and Nglom/body surface area ranged from 1.06 to 0.55 million/m2. Birth weight was normal (>2.5 and <4.0 kg34) in those subjects with available data (n=8). Five adults were hypertensive, and five adults had cardiovascular-related deaths. Additional information regarding pathologic analysis of these kidneys, including glomerulosclerosis, cortical fibrosis, and arteriosclerosis, is provided in Supplemental Table 1.
Table 2.
Demographic data for 12 adult Caucasian-American men without kidney disease
| Ranka | Age (yr) | BMI (kg/m2) | BS Area (m2) | Nglom (million) | Nglom/BS Area (million/m2) | Birth Weight (kg) | Hypertensive Status | COD | CVRD |
|---|---|---|---|---|---|---|---|---|---|
| A1 | 43 | 18.19 | 1.57 | 1.66 | 1.06 | 3.38 | Hypertensive | Other | No |
| A2 | 25 | 18.10 | 1.45 | 0.69 | 0.48 | NA | Normotensive | Other | No |
| A3 | 31 | 30.80 | 1.86 | 1.05 | 0.57 | 3.25 | Normotensive | Accident | No |
| A4 | 41 | 34.02 | 2.36 | 1.12 | 0.47 | 3.86 | Normotensive | CAD | Yes |
| A5 | 41 | 23.02 | 1.80 | 0.83 | 0.46 | 3.66 | Normotensive | Other | No |
| A6 | 25 | 23.80 | 1.72 | 0.95 | 0.55 | NA | Normotensive | Cardiac, not CAD | Yes |
| A7 | 47 | 22.75 | 1.81 | 1.15 | 0.64 | NA | Normotensive | Accident | No |
| A8 | 48 | 20.54 | 1.94 | 1.00 | 0.52 | NA | Normotensive | Other | No |
| A9 | 37 | 36.36 | 2.17 | 0.93 | 0.43 | 3.29 | Hypertensive | CAD | Yes |
| A10 | 49 | 35.88 | 2.31 | 0.78 | 0.34 | 3.12 | Hypertensive | CAD | Yes |
| A11 | 37 | 39.42 | 2.64 | 0.76 | 0.29 | 3.23 | Hypertensive | Other | No |
| A12 | 39 | 26.70 | 2.01 | 0.55 | 0.27 | 3.46 | Hypertensive | CAD | Yes |
| Median | 40 | 25.53 | 1.9 | 0.94 | 0.47 | 3.34 | 42% | NA | 42% |
| IQR | 33–46 | 22.8–35.4 | 1.7–2.3 | 0.8–1.1 | 0.4–0.6 | 3.2–3.6 | NA | NA | NA |
BMI, body mass index; BS area, body surface area; Nglom, total nephron number; COD, cause of death; CVRD, cardiovascular-related death; A, adult; Other, infectious, hematologic, neoplastic, or central nervous system-related but not cardiovascular or pulmonary; NA, not available; CAD, coronary artery disease.
Subjects were ranked on the basis of median IGV.
Glomerular Volumes and Cell Numbers
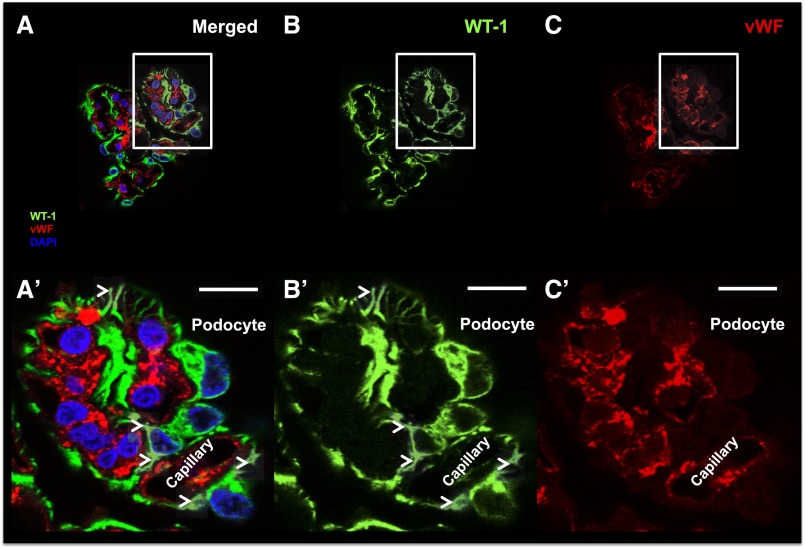
Individual glomerular volume (IGV) was estimated in a total of 480 glomeruli (30 per subject). The three smallest and the three largest glomeruli per subject (10th and 90th percentiles; n=96 glomeruli) were selected for additional cellular analysis. Podocytes were identified according to several criteria (Figure 1, A and A′), namely specific cytoplasmic immunostaining for Wilms’ Tumor 1 (WT-1) (Figure 1, B and B′), presence of major cytoplasmic projections (Figure 1B′), and nuclear location outside capillary loops. When possible, lack of immunostaining for vWF (Figure 1, C and C′) was also used as part of these criteria. Because of the unexpected expression of WT-1 in the cytoplasmic compartment, we provide additional details regarding the specificity of this marker. Although WT-1 and Synaptopodin (SNP) were expressed within the same cells (Supplemental Figure 1), WT-1 was present in the cell body and major processes, whereas SNP was mostly located in foot processes. Furthermore, WT-1 was consistently found in the podocyte cytoplasm and tissue from nephrectomies and biopsies (Supplemental Figure 2).
Figure 1.
Podocyte identification. (A) A representative confocal image of a glomerular tuft is shown (merged) using (B) WT-1 (green; specific podocyte marker), (C) vWF (red; specific endothelial cell marker), and DAPI (blue; nuclear marker). The corresponding insets show our podocyte identification criteria: (A′) expression of WT-1 in podocyte cytoplasm, (B′) lack of expression of vWF, and (C′) their location outside capillaries. Arrowheads show classic podocyte morphology (major projections). Scale bars, 10 μm. DAPI, 4′,6-diamidino-2-phenylindole.
Initially, we intended to use immunostaining for vWF to identify endothelial cells. However, vWF staining was not consistent in all autopsy samples, and we, therefore, classified all cells on the glomerular tuft that were not podocytes as nonepithelial cells (NECs; the majority of these cells were endothelial and mesangial cells). PECs were identified by their location on Bowman’s capsule.
Glomerular Size Differs Significantly between Children and Adults
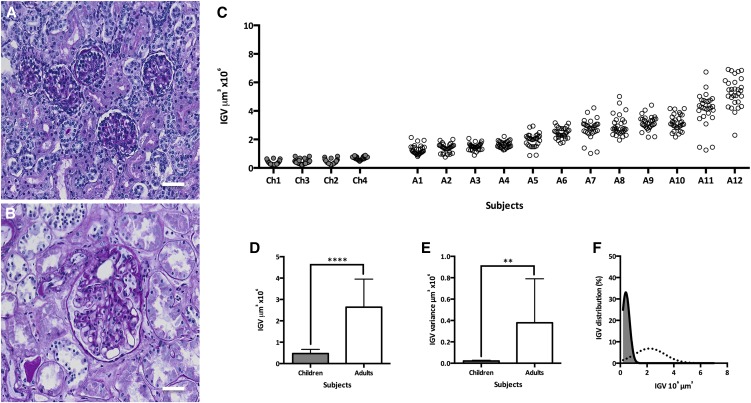
Representative light microscopic images of glomeruli from children and adults are provided in Figure 2, A and B, respectively. IGV estimates for 96 glomeruli in 16 subjects ranked by median IGV are shown in Figure 2C. Considerable variation in glomerular size was present in children, but this was far greater in adults. Adults showed greater aggregated median IGV (Figure 2D) (P<0.001) and IGV variance (Figure 2E) (P<0.01) than children. Median IGV in children was 0.41×106 µm3 (interquartile range [IQR]=0.29×106 µm3–0.64×106 µm3), and in adults, it was 2.42×106 µm3 (IQR=1.54×106 µm3–3.27×106 µm3), a 5.9-fold difference. Figure 2F shows the marked right shift in the adult IGV distribution compared with that in children. The smallest glomerulus observed in children had a volume of 0.21×106 µm3, whereas the largest had a volume of 0.89×106 µm3—a difference of 0.68×106 µm3 or 4.2-fold. In contrast, the smallest glomerulus in adults had a volume of 0.76×106 µm3, whereas the largest had a volume of 6.91×106 µm3, a difference of 6.15×106 µm3 or 9.1-fold.
Figure 2.
Glomeruli from adults are larger and have more variable size than glomeruli from children. A and B show representative images of average glomeruli from children and adults, respectively, using periodic acid–Schiff staining. (C) IGV values in 16 Caucasian-American men and boys. Each circle represents one glomerulus, and each column represents one subject with 30 glomeruli per subject; Ch1–Ch4 represent four young children (gray), and A1–A12 represent 12 adults (white) ranked by median IGV. (D) Aggregated IGV differences between children and adults. (E) Difference in IGV variance per subject between glomeruli from children and adults. (F) IGV distributions in children (solid line with gray area) and adults (dotted line). Bars represent median values with IQR. Scale bars, 50 μm. **P<0.01; ****P<0.001.
Numbers of Glomerular Cells Differ Significantly between Children and Adults
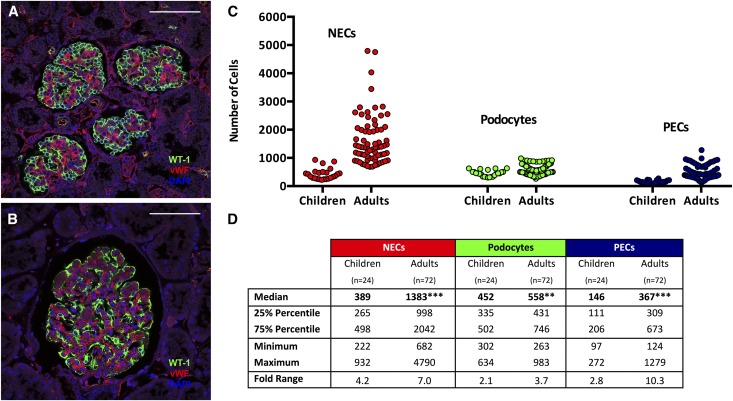
Representative confocal images showing the marked differences in glomerular cellularity between children and adults are shown in Figure 3, A and B. Aggregated data for total numbers of NECs, podocytes, and PECs in children (n=24 glomeruli) and adults (n=72 glomeruli) are reported in Figure 3, C and D, and they clearly show the greater variability in adult cell numbers. In adult glomeruli, the median podocyte count was 558 (IQR=431–746; 3.7-fold), whereas median NEC and PEC counts were 1383 (IQR=998–2042; 7.0-fold) and 367 (IQR=309–673; 10.3-fold), respectively. In children, the median podocyte count was 452 (IQR=335–502; 2.1-fold), whereas median NEC and PEC counts were 389 (IQR=265–498; 4.2-fold) and 146 (IQR=111–206; 2.8-fold), respectively.
Figure 3.
Large glomeruli from adults contained more podocytes, NECs and PECs than glomeruli from children. A and B show representative confocal images of glomeruli from children and adults, respectively, with WT-1 (green), vWF (red), and DAPI (blue). C and D show the numbers of NECs, PECs, and podocytes per glomerulus from children and adults. C illustrates the variability of each cell type between glomeruli from adults and children; each circle represents the value for a single glomerulus,. D provides details of the medians, IQRs (25% and 75% percentiles), minimum and maximum values, and fold ranges per group. Scale bars, 100 μm. DAPI, 4′,6-diamidino-2-phenylindole. **P<0.01; ***P<0.001.
Numbers of Glomerular Cells in the Context of Glomerular Hypertrophy
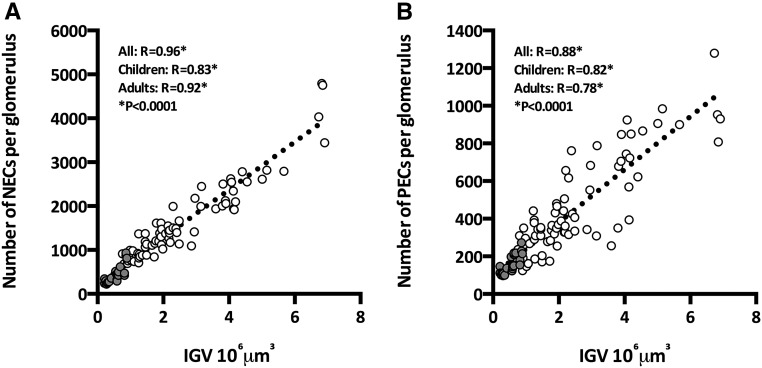
The estimation of absolute numbers of specific cell types in glomeruli of known volume allowed us to examine relationships between glomerular size and numbers of each cell type. As seen in Figure 4A, IGV was directly and strongly associated with the numbers of NECs (aggregated R=0.96, children R=0.83, adult R=0.92; P<0.001 in each case). In a linear regression analysis of aggregated glomeruli (from children and adults), 92% of IGV variability was explained by numbers of NECs (F=1084; P<0.001), predicting an increase of 544 NECs per 106 µm3 glomerular tuft volume. Similarly, IGV was closely directly associated with PEC number (aggregated R=0.88, children R=0.78, adults R=0.86; P<0.001 in each case) (Figure 4B). A linear regression model predicted an increase of 141 PECs per 106 µm3 glomerular tuft volume (R2=0.79; F=357; P<0.001).
Figure 4.
Glomerular size is closely associated with numbers of NECs and PECs. (A) Association between IGV and numbers of NECs in glomeruli from children (gray) and adults (white). (B) Association between IGV and PECs in glomeruli from children (gray) and adults (white).
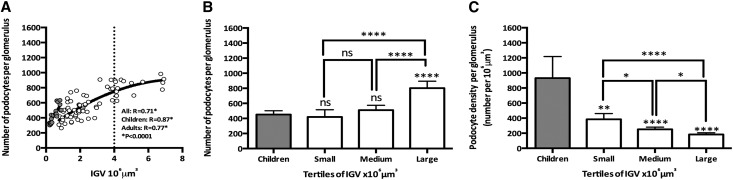
Unlike the situation with PECs and NECs, the association between IGV and podocyte number (Figure 5A) was weaker in adults than in children (aggregated R=0.70, children R=0.87, adults R=0.76; P<0.001 in each case). The adult relationship between number of podocytes and IGV was better fitted in a quadratic model (Figure 5A), with podocyte number plateauing with IGV values approximately >4×106 µm3. Because of the great variability in IGV in adults, we also examined the relationships between podocyte number and glomerular size in children and between tertiles of glomerular size in adults. As shown by Figure 5B, glomeruli from children contained the same number of podocytes as small and medium adult glomeruli (P>0.05). However, large adult glomeruli (tertile 3) contained significantly more podocytes than glomeruli from children and small and medium adult glomeruli (P<0.01). Figure 5C shows that adult glomeruli (all three tertiles) had lower podocyte densities (podocyte number per 106 µm3 glomerular tuft volume) than glomeruli from children (P<0.01 for small glomeruli; P<0.001 for medium and large glomeruli). Large adult glomeruli had a significantly lower podocyte density than small and medium adult glomeruli (P<0.05 for medium glomeruli; P<0.001 for small glomeruli).
Figure 5.
Large adult glomeruli have more podocytes and lower podocyte density than smaller adult glomeruli and glomeruli from children. (A) IGV and numbers of podocytes in children (gray circles) and adults (white circles). The solid line represents the line of best fit in children (linear) and adults (quadratic), and the dotted line shows the beginning of the curve plateau at 4×106 μm3. (B) Number of podocytes in children and adult IGV tertiles (small, medium, and large glomeruli). (C) Podocyte density in children and adult IGV tertiles (small, medium, and large glomeruli). Children: aggregated data from four infants (6 glomeruli per subject; n=24 glomeruli); adult IGV tertiles were tertile 1 (small glomeruli), between 0.76 and 1.72×106 μm3; tertile 2 (medium glomeruli), between 1.74 and 2.86×106 μm3; and tertile 3 (large glomeruli), between 2.94 and 6.91×106 μm3. P values directly over the bars represent comparisons between children (gray bars) and each adult tertile. Bars represent median values with IQR. *P<0.05; **P<0.01; ****P<0.001.
Cell Ratios in the Context of Glomerular Hypertrophy
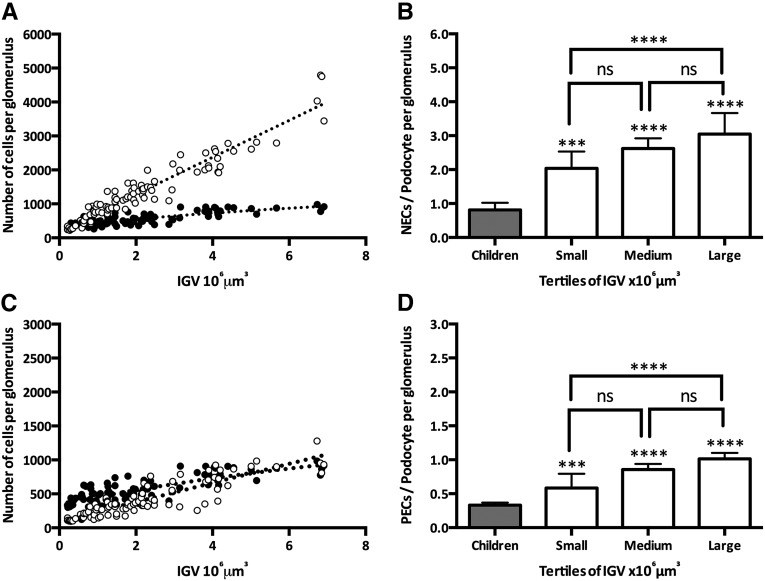
Figure 6A illustrates differences in the trajectories of numbers of NECs and podocytes in the context of IGV (F=550; P<0.001). Although small glomeruli had similar numbers of NECs and podocytes (indeed, podocytes slightly outnumbered NECs), in adult glomeruli, NECs outnumbered podocytes (Figure 6B), and this was most pronounced in large adult glomeruli, in which the NEC-to-podocyte ratio was close to 3:1. In contrast, the trajectories of both PEC number and podocyte number with IGV were not statistically different (P>0.05) (Figure 6C). However, the ratio of PECs/podocyte was significantly higher in adult glomeruli than children glomeruli (1:3; P<0.001 for all tertiles) and large (1:1) compared with small (1:2) adult glomeruli (Figure 6D).
Figure 6.
Numbers of NECs, PECs, and podocytes in the context of glomerular volume. (A) Numbers of podocytes (black circles) and NECs (white circles) in the context of IGV. (B) NEC-to-podocyte ratio in glomeruli from children and adults by adult IGV tertiles. (C) Numbers of PECs (black circles) and podocytes (white circles) in the context of IGV. (D) PEC-to-podocyte ratio in glomeruli from children and adults by adult IGV tertiles. P values directly over the bars represent comparisons between children (gray bars) and each adult tertile. Bars represent median values with IQR. ***P<0.001; ****P<0.001.
Histologic Evidence of Possible Sources of Podocytes
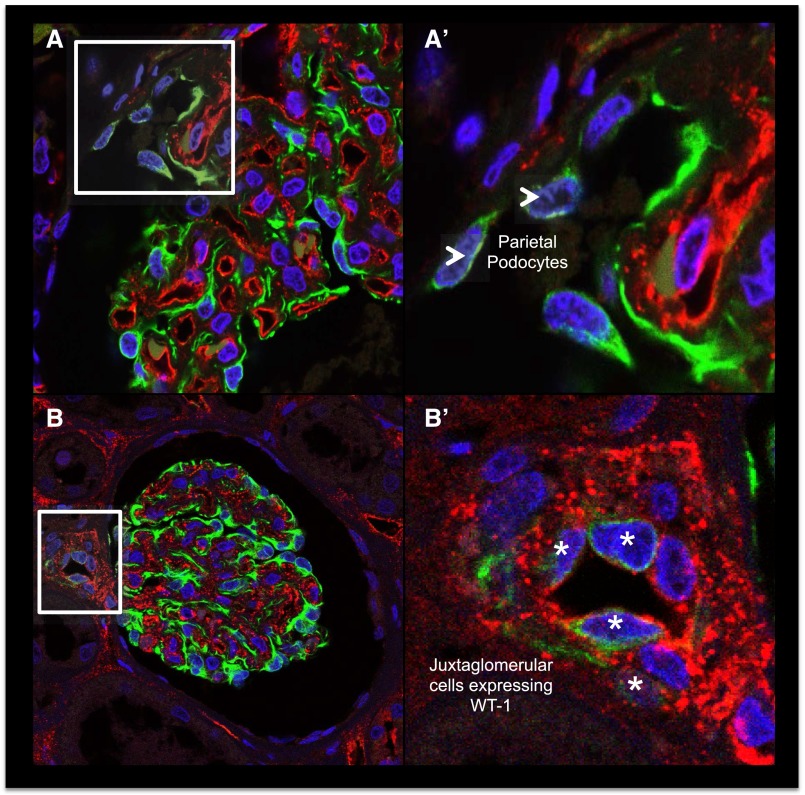
WT-1+PECs (also known as parietal podocytes) were found in approximately 90% of glomeruli in both children and adults. These cells were typically found close to the vascular pole and never observed near the tubular pole (Figure 7, A and A′). WT-1+cells were also occasionally observed in the tunica intima and the tunica media of arterioles in the juxtaglomerular apparatus (JGA) (Figures 7, B and B′).
Figure 7.
Possible podocyte sources in the human kidney. (A and A′) Parietal podocytes: PECs expressing WT-1 are located close to the vascular pole of a glomerulus (arrowheads). (B and B′) WT-1+cells were also occasionally observed in the tunica intima and the tunica media of arterioles in the JGA (asterisks).
Zonal Analyses in Adult Glomeruli
From 72 sampled adult glomeruli, 31 glomeruli were located in the outer cortex (superficial), 24 glomeruli were located in the middle cortex, and 17 glomeruli were located in the inner cortex (juxtamedullary). There were no zonal differences in IGV, podocyte number, or podocyte density (Supplemental Figure 3).
Discussion
The three major findings of this study were (1) glomerular size and numbers of glomerular cells vary widely in children and adults without overt kidney disease, with greater variation seen in adults; (2) large adult glomeruli contain more podocytes than glomeruli from four young children and small adult glomeruli; and (3) these large adult glomeruli have a lower podocyte density, which may leave them in a setting of relative podocyte depletion.
Glomerular hypertrophy plays pivotal roles in the development of FSGS2,18,19 and the progression of diabetic nephropathy,35–38 two of the most common causes of CKD. According to the hyperfiltration theory,25 an imbalance between glomerular mass and physiologic requirements drives glomerular hypertrophy as an appropriate compensatory response to sustain renal function. We hypothesize that human glomerular growth is initially a healthy compensatory step, which if exaggerated, can result in pathology.
Human glomeruli have been reported to increase in size up to 7-fold from infancy to adulthood.39,40 These findings confirm this significant increase in glomerular size from childhood to adulthood, although it is important to note that kidneys from only four children were analyzed in this study. We report a 5.9-fold increase in glomerular volume between childhood and adulthood and show a much greater variability in glomerular sizes in adults than in children. This latter finding suggests that the great variability in IGV found within and between adults may be established later in life.41 We have previously reported that increased IGV median and variance in adult American men are closely associated with low nephron number,33 older age,42 obesity,43 African-American race,44 and hypertension.45 This study suggests that glomerular hypertrophy is initially associated with body growth from childhood to adulthood. Additional adult hypertrophy occurs in association with aging, nephron loss, hypertension, and obesity—all variables that can be present individually or combined, reflecting the multifactorial nature of glomerular hypertrophy.41,46
To date, only a handful of studies have estimated total podocyte number in adult human glomeruli, and these studies focused on pathology. Studies of type 2 diabetes identified absolute podocyte depletion in the early stages of diabetic nephropathy, which was closely related to disease progression.27–30 Absolute podocyte depletion was also reported in studies of IgA nephropathy and hypertensive nephrosclerosis.31,32 Although these studies provided valuable insights into the association between podocyte number and the development and progression of renal disease, our study shows, for the first time, that podocyte number varies within and between subjects without overt renal disease.
In this study, median podocyte number per adult glomerulus was 558, with the lowest count being 263 podocytes and the highest count being 983 podocytes (3.7-fold range). This finding raises two important questions. (1) When is podocyte number determined? (2) What is the optimal number of podocytes required in an adult glomerulus? These questions are considered in turn.
These findings provide several novel insights into the establishment of adult podocyte number. Glomeruli in four young children contained fewer podocytes than large adult glomeruli; >30 years ago, Olivetti et al.47 described a similar pattern between young, adult, and uninephrectomized rats. Taken together, these findings suggest that additional podocytes may be acquired by certain glomeruli after early childhood. The origin of these additional podocytes remains unknown, but in recent years, there has been great interest in the topic of podocyte replacement in the postnatal period.
Mature podocytes exit the cell cycle to take on a terminally differentiated phenotype and express cyclin–dependent kinase inhibitors, such as p16INK4a, P21Cip1, P27Kip1, and P57Kip2.48–52 After this point, podocytes are considered incapable of undergoing mitosis under normal conditions, although podocyte proliferation occurs in certain pathologic conditions, such as HIV-associated nephropathy.53,54 Podocytes can be induced to proliferate in vitro when cultured from freshly isolated glomeruli,55 but these cells express low levels of many of the podocyte–specific differentiation markers, suggesting that podocytes are only capable of proliferating after they have dedifferentiated to a certain degree. Recent evidence suggests that progenitor cells residing in the JGA56 and Bowman’s capsule (PECs)57–61 may give rise to podocytes. Other lines of evidence suggest that new podocytes may arise from bone marrow.62–64 In this study, WT-1+PECs (parietal podocytes) were observed near the vascular pole in most glomeruli in children and adults, and some WT-1+cells were occasionally observed in the tunica intima and the tunica media of arterioles in the JGA. Whether any of these cells give rise to new podocytes deserves additional attention.
There is much interest in whether PECs can give rise to podocytes.59,65 Wanner et al.66 recently showed that podocyte generation is mainly active during glomerular development and may occur after acute glomerular injury but was not observed in aging kidneys or in response to nephron loss. Interestingly, a recent study by Berger et al.67 showed that parietal podocytes (PECs expressing podocyte markers) disappeared from Bowman’s capsule as glomeruli gradually underwent physiologic hypertrophy, suggesting that there is a functional podocyte reserve that directly differentiates into podocytes on Bowman’s capsule. Berger et al.68 proposed that this is explained by the lack of space to accommodate podocytes on the small glomerular tuft of a young child. This hypothesis is supported by our findings that show that glomeruli from young children are small and replete with podocytes (high podocyte numerical density) but have a lower podocyte number than large glomeruli from adults.
A novel method on the basis of flow cytometry was used recently to report a 7% increase in podocyte number after acute podocyte injury.66 This is an interesting approach that has many advantages (i.e., does not rely on time-consuming techniques). However, we believe that stereologic methods may also provide an important perspective. For example, stereology-based approaches consider the context of glomerular size and cortical location, both of which have significant value. We believe that this highlights how histologic/stereologic approaches, even if laborious and time consuming, are still valuable tools for the study of podocyte biology. Additional studies, especially combining lineage-tracing models and unbiased stereology, are urgently needed to quantify the efficiency of glomerular podocyte gain and possible modifiable mechanisms.
To our knowledge, we present for the first time the concept of human podocyte endowment, a term that refers to the number of podocytes with which we are born. Although the sample size of children in this study is small, we believe that this concept is important and follows a series of contributions by our group to understand the role of nephron endowment in the risk, development, and progression of renal disease.26,33,69–75 On the basis of the variation in podocyte number in children and adults, we postulate that some glomeruli at birth may be better equipped to deal with glomerular stress than others. It is evident that a more comprehensive analysis of the numbers of podocytes in newborns and young children is warranted, especially to find associations with low birth weight, prematurity, and adverse fetomaternal environments. We have commenced studies to answer this critical question.
With the recent interest in the role of podocyte depletion in glomerular pathology, the question arises as to the optimum number of podocytes required in a glomerulus to maintain glomerular health. Wharram et al.5 defined 40% podocyte depletion as the threshold for established glomerular pathology in a rat model of podocyte injury. Earlier, Lemley et al.31 reported that a similar degree of podocyte loss (approximately one third) marked the deterioration of glomerular structure and function in patients with IgA nephropathy. However, there is still no consensus on a reliable cutoff value for absolute podocyte depletion in the human kidney. Although this study has revealed a 3.7-fold variation in podocyte number per adult glomerulus, at the moment, we cannot provide a set value for an optimal podocyte number.
Despite containing more podocytes, hypertrophic adult glomeruli showed a substantial decrease in podocyte density, which may already mark an increased risk of glomerulosclerosis. The aim of glomerular hypertrophy is to increase filtration surface area to sustain renal function.76 Previous studies in animal models and human tissue have shown that glomerular hypertrophy is closely associated with endothelial or mesangial cell hyperplasia.77–79 Therefore, it is not surprising to find a strong association between glomerular volume and numbers of NECs, which included endothelial80 and mesangial81 cells. A recent study by Fukuda et al.82 showed the critical importance of podocyte hypertrophy when compensating during glomerular hypertrophy. This study provided evidence that a possible pathway for pathologic glomerular hypertrophy was a mismatch between the volume of podocytes and glomerular volume.82 Our study also indicates that, in hypertrophic human glomeruli, there is an increase in the NEC-to-podocyte ratio, providing another piece of evidence to support clear differences in replication potential of each cell population in the context of glomerular hypertrophy. Taken together, we propose that there is evidence of relative podocyte depletion in hypertrophic glomeruli and that additional studies in pathologic tissue will provide critical evidence to define a threshold of podocyte density marking the development of glomerulosclerosis.
Interestingly, PECs have gained much attention in recent years because of their role in the development of glomerulosclerosis.57,59,83,84 Activated PECs can avidly proliferate, migrate, and produce extracellular matrix.85 To our knowledge, this study provides the first estimates of total PEC numbers in human glomeruli. The numbers of PECs were highly variable in adult glomeruli (up to a 10.3-fold range) and closely associated with glomerular size. The biologic significance of higher numbers of PECs in hypertrophic glomeruli remains unclear, but multiple hypotheses can be proposed, including (1) preservation of a physical barrier during glomerular growth, (2) limited podocyte gain to cope with increases in the filtration surface area, and (3) early PEC activation.
Although subjects included in this study come from the largest and most comprehensive kidney autopsy series in the world, this study has several limitations. We acknowledge the inherent limitation of a cross-sectional study design. We emphasize that, although the number of young children included in this study is small, we used all available subjects that met our inclusion criteria: men, Caucasian Americans, younger than 3 years of age, adequate birth weight for gestational age, and no intercurrences during pregnancy. Another limitation was the inability to present data for endothelial and mesangial cells separately because of the suboptimal vWF immunostaining in some of the autopsy samples. We would advise caution before extrapolating these findings to different cohorts (i.e., women and African Americans). Finally, our selection of glomeruli for cell counting allowed us to compare small and large glomeruli. This sampling strategy assumes that the relationship between glomerular size and cell numbers is continuous across the range of glomerular volumes.
In conclusion, this study provides evidence of (1) glomerular hypertrophy in adult glomeruli and a strong direct association between glomerular size and numbers of NECs; (2) higher numbers of podocytes in large adult glomeruli that may reflect both podocyte endowment at birth and podocyte gain in the postnatal period; and (3) relative podocyte depletion in large adult glomeruli that may increase their risk to develop glomerulosclerosis.
Concise Methods
Subject Selection
Sixteen Caucasian-American men and boys were selected for study: four children (≤3 years old) and 12 adults (≥18 years old) without renal disease. The four children presented no complications during pregnancy and had adequate birth weights for gestational age. Adult subjects presented different CKD risk factors (older age, low Nglom, high body surface area, hypertension, or cardiovascular-related deaths). Kidneys were obtained from autopsies performed at the University of Mississippi Medical Center (Jackson, Mississippi). Ethics approval was obtained in advance from the Institutional Review Board of the University of Mississippi Medical Center and the Monash University Human Research Ethics Committee. On collection, kidneys were perfusion fixed with 10% buffered formalin, bisected, and then immersed in 10% formalin for 10 days. Representative kidney blocks from the upper pole and midportion of the kidney were embedded in paraffin as previously described.40
Measurement of Pathologic Parameters
The methodology to assess pathology parameters has been described in previous publications.73,86 Briefly, representative kidney blocks from the upper pole and the midportion of each kidney were paraffin embedded. Sections were cut at 4 μm and stained with periodic acid–Schiff-hematoxylin and picrosirius red stains for fibrillar collagen. Measurement of the percentage of glomerulosclerosis and arterial intimal thickening (arteriosclerosis) was performed on periodic acid–Schiff-hematoxylin–stained sections. The percentage of sclerotic glomeruli was estimated by counting sclerosed and nonsclerosed glomerular cross-sections in nonoverlapping ×100 microscopic fields moving from the superficial to the inner cortex, with at least 400 glomerular cross-sections being sampled per subject. The severity of arteriosclerosis was measured as a ratio of the thickness of the intima to the outer wall diameter at a magnification of ×400 in interlobular arteries 90–250 μm in diameter using the linear measurement function of Image-Pro Plus morphometric software (Media Cybernetics Inc., Bethesda, MD). With the same software, cortical fibrosis was measured in ×200 images as the proportion of cortex staining red with the picrosirius stain.
Estimation of Glomerular Volume and Cell Numbers
We estimated IGVs and the absolute numbers of podocytes, NECs, and PECs per glomerulus using a previously described design–based stereologic approach.12 This method is considered the gold standard for quantification of podocyte number and glomerular volume when sufficient tissue is available.11 In brief, 50 serial paraffin sections at 14-µm thickness were obtained for each kidney. Using these sections, 30 glomeruli (10 glomeruli each from the outer, middle, and inner cortexes) per subject were sampled using physical disectors.41,42 Section profiles of these 30 sampled glomeruli were then imaged using an Olympus DotSlide system, generally providing between 8 and 16 profiles per glomerulus. Glomerular profiles were labeled with a flag and a unique identifier. These virtual images served as maps to find all profiles of each of these glomeruli during confocal microscopy. The volumes of all 30 sampled individual glomeruli per kidney were estimated using the Cavalieri estimator.87
Every second section (in adults) and every section (in children) were then immunostained to facilitate cell identification and counting. Sections were immunostained using an antibody against WT-1 antigen (monoclonal mouse anti-human WT-1; M356101, clone 6F-H2 [1:50]; for podocyte identification; DAKO) and an antibody against vWF (1:200), which in this case, was a polyclonal rabbit anti-human vWF (A008202; for endothelial cell identification; DAKO). In this study, WT-1 immunostaining was found exclusively in podocyte cytoplasm. Although immunostaining for WT-1 is most often reported to be nuclear, it is well known that WT-1 isoforms are present in the nuclei and cytoplasm of many cell types, including mouse mesonephros, mouse mesothelioma, and differentiated embryonic stem cells.88 This localization of WT-1 immunostaining in podocyte cytoplasm confirms the findings of the work by Su et al.,30 which used this same antibody and reported immunostaining of human podocyte cytoplasm, and agrees with the manufacturer’s (DAKO) specifications regarding this antibody. To further validate this WT-1 antibody as a specific marker of podocyte cytoplasm, we performed double-labeled immunostaining for WT-1 and SNP, a well characterized podocyte marker. Both WT-1 and SNP were localized in the cytoplasm of the same cells (Supplemental Figure 1). To ensure that the WT-1 immunostaining of podocyte cytoplasm in our autopsy samples was not an artifact of postmortem autolysis, we tested nephrectomy and biopsy samples from our human tissue bank. In every case, specific immunostaining of podocyte cytoplasm was observed (Supplemental Figure 2).
Cells were counted in 96 glomeruli, which were comprised of 6 glomeruli from each of 16 subjects. The 6 glomeruli analyzed per subject were the 3 smallest and the 3 largest (representing the 10th and 90th percentiles, respectively) from 30 glomeruli per subject used for IGV estimation. Every immunostained section from each of six subsampled glomeruli per subject was imaged with a Leica SP5 laser confocal microscope (Leica MicroSystems, Manheim, Germany). Optical dissectors were used to sample and therefore, count cells in 8 of 14 µm available for each glomerular profile (we did not count cells in a 3-µm guard region at the top and bottom of each section as detailed in ref. 12). Cells were sampled and counted using optical dissectors on the series of 1-µm confocal optical images, stacked as a virtual slide, and opened using an ImageJ89 macro. After all newly appearing nuclei had been identified, we defined podocytes (tuft cells WT-1+ and vWF−), NECs (tuft cells WT-1−), and PECs (cells located on Bowman’s capsule). Podocyte density was calculated by dividing absolute podocyte number per glomerulus by IGV. Cell number ratios were calculated by dividing total numbers of NECs by total numbers of podocytes (NEC-to-podocyte ratio) and total numbers of PECs by total numbers of podocytes (PEC-to-podocyte ratio).
Subjects with the diagnosis of FSGS were excluded from any stereologic analysis in this autopsy cohort. Importantly, this method allowed the identification of glomeruli with signs of segmental sclerosis,12 which were excluded from this study.
Statistical Analyses
Data were analyzed using GraphPad Prism, version 5.04 for Windows (La Jolla, CA) and StataCorp. (Statistical Software: Release 8; StataCorp., College Station, TX). Values are expressed as medians±IQRs unless otherwise stated. Mann–Whitney U test was used to compare variables with skewed distributions, and Spearman rank coefficient was used as a measurement of associations. F tests were applied to assess if lines of best fit were different. Linear regression analysis was performed using IGV as an independent variable and numbers of NECs, PECs, and podocytes as dependent variables. In all instances, a P value<0.05 was considered statistically significant.
Disclosures
None.
Acknowledgments
Parts of this work were presented in poster format during the American Society of Nephrology Kidney Week on October 30–November 4, 2012 in San Diego, CA and on November, 11–16, 2014 in Philadelphia, PA.
The authors acknowledge the facilities, scientific and technical assistance from members of the Monash Micro Imaging Platform and members of the Monash Histology Platform. V.G.P. received a Monash Research Graduate School Scholarship and a Faculty of Medicine International Postgraduate Scholarship to support his PhD candidature. This research was funded by the National Health and Medical Research Council (grant number: 606619).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014070641/-/DCSupplemental.
References
- 1.Saran R, Shahinian V: CKD: A pandemic calling for concerted public health action. Adv Chronic Kidney Dis 17: 213–214, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Wiggins RC: The spectrum of podocytopathies: A unifying view of glomerular diseases. Kidney Int 71: 1205–1214, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Reiser J, Sever S: Podocyte biology and pathogenesis of kidney disease. Annu Rev Med 64: 357–366, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jefferson JA, Alpers CE, Shankland SJ: Podocyte biology for the bedside. Am J Kidney Dis 58: 835–845, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wharram BL, Goyal M, Wiggins JE, Sanden SK, Hussain S, Filipiak WE, Saunders TL, Dysko RC, Kohno K, Holzman LB, Wiggins RC: Podocyte depletion causes glomerulosclerosis: Diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J Am Soc Nephrol 16: 2941–2952, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Kriz W, Elger M, Nagata M, Kretzler M, Uiker S, Koeppen-Hageman I, Tenschert S, Lemley KV: The role of podocytes in the development of glomerular sclerosis. Kidney Int Suppl 45: S64–S72, 1994 [PubMed] [Google Scholar]
- 7.Mundel P, Shankland SJ: Podocyte biology and response to injury. J Am Soc Nephrol 13: 3005–3015, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Durvasula RV, Shankland SJ: Podocyte injury and targeting therapy: An update. Curr Opin Nephrol Hypertens 15: 1–7, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Shankland SJ: The podocyte’s response to injury: Role in proteinuria and glomerulosclerosis. Kidney Int 69: 2131–2147, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Jefferson JA, Nelson PJ, Najafian B, Shankland SJ: Podocyte disorders: Core Curriculum 2011. Am J Kidney Dis 58: 666–677, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lemley KV, Bertram JF, Nicholas SB, White K: Estimation of glomerular podocyte number: A selection of valid methods. J Am Soc Nephrol 24: 1193–1202, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Puelles VG, Douglas-Denton RN, Cullen-McEwen L, McNamara BJ, Salih F, Li J, Hughson MD, Hoy WE, Nyengaard JR, Bertram JF: Design-based stereological methods for estimating numbers of glomerular podocytes. Ann Anat 196: 48–56, 2014 [DOI] [PubMed] [Google Scholar]
- 13.Guo M, Ricardo SD, Deane JA, Shi M, Cullen-McEwen L, Bertram JF: A stereological study of the renal glomerular vasculature in the db/db mouse model of diabetic nephropathy. J Anat 207: 813–821, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei P, Lane PH, Lane JT, Padanilam BJ, Sansom SC: Glomerular structural and functional changes in a high-fat diet mouse model of early-stage Type 2 diabetes. Diabetologia 47: 1541–1549, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Cusumano AM, Bodkin NL, Hansen BC, Iotti R, Owens J, Klotman PE, Kopp JB: Glomerular hypertrophy is associated with hyperinsulinemia and precedes overt diabetes in aging rhesus monkeys. Am J Kidney Dis 40: 1075–1085, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Osterby R, Parving HH, Hommel E, Jørgensen HE, Løkkegaard H: Glomerular structure and function in diabetic nephropathy. Early to advanced stages. Diabetes 39: 1057–1063, 1990 [DOI] [PubMed] [Google Scholar]
- 17.Osterby R, Gundersen HJ, Nyberg G, Aurell M: Advanced diabetic glomerulopathy. Quantitative structural characterization of nonoccluded glomeruli. Diabetes 36: 612–619, 1987 [DOI] [PubMed] [Google Scholar]
- 18.Ijpelaar DH, Schulz A, Koop K, Schlesener M, Bruijn JA, Kerjaschki D, Kreutz R, de Heer E: Glomerular hypertrophy precedes albuminuria and segmental loss of podoplanin in podocytes in Munich-Wistar-Frömter rats. Am J Physiol Renal Physiol 294: F758–F767, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Young RJ, Hoy WE, Kincaid-Smith P, Seymour AE, Bertram JF: Glomerular size and glomerulosclerosis in Australian aborigines. Am J Kidney Dis 36: 481–489, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Noël LH: Morphological features of primary focal and segmental glomerulosclerosis. Nephrol Dial Transplant 14[Suppl 3]: 53–57, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Cahill MM, Ryan GB, Bertram JF: Biphasic glomerular hypertrophy in rats administered puromycin aminonucleoside. Kidney Int 50: 768–775, 1996 [DOI] [PubMed] [Google Scholar]
- 22.Lee HS, Lim SD: The significance of glomerular hypertrophy in focal segmental glomerulosclerosis. Clin Nephrol 44: 349–355, 1995 [PubMed] [Google Scholar]
- 23.Suzuki J, Yoshikawa N, Nakamura H: A quantitative analysis of the glomeruli in focal segmental glomerulosclerosis. Pediatr Nephrol 8: 416–419, 1994 [DOI] [PubMed] [Google Scholar]
- 24.Nyberg E, Bohman SO, Berg U: Glomerular volume and renal function in children with different types of the nephrotic syndrome. Pediatr Nephrol 8: 285–289, 1994 [DOI] [PubMed] [Google Scholar]
- 25.Brenner BM: Nephron adaptation to renal injury or ablation. Am J Physiol 249: F324–F337, 1985 [DOI] [PubMed] [Google Scholar]
- 26.Puelles VG, Hoy WE, Hughson MD, Diouf B, Douglas-Denton RN, Bertram JF: Glomerular number and size variability and risk for kidney disease. Curr Opin Nephrol Hypertens 20: 7–15, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Pagtalunan ME, Miller PL, Jumping-Eagle S, Nelson RG, Myers BD, Rennke HG, Coplon NS, Sun L, Meyer TW: Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest 99: 342–348, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meyer TW, Bennett PH, Nelson RG: Podocyte number predicts long-term urinary albumin excretion in Pima Indians with Type II diabetes and microalbuminuria. Diabetologia 42: 1341–1344, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Dalla Vestra M, Masiero A, Roiter AM, Saller A, Crepaldi G, Fioretto P: Is podocyte injury relevant in diabetic nephropathy? Studies in patients with type 2 diabetes. Diabetes 52: 1031–1035, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Su J, Li SJ, Chen ZH, Zeng CH, Zhou H, Li LS, Liu ZH: Evaluation of podocyte lesion in patients with diabetic nephropathy: Wilms’ tumor-1 protein used as a podocyte marker. Diabetes Res Clin Pract 87: 167–175, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Lemley KV, Lafayette RA, Safai M, Derby G, Blouch K, Squarer A, Myers BD: Podocytopenia and disease severity in IgA nephropathy. Kidney Int 61: 1475–1485, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Wang G, Lai FM, Kwan BC, Lai KB, Chow KM, Li PK, Szeto CC: Podocyte loss in human hypertensive nephrosclerosis. Am J Hypertens 22: 300–306, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Zimanyi MA, Hoy WE, Douglas-Denton RN, Hughson MD, Holden LM, Bertram JF: Nephron number and individual glomerular volumes in male Caucasian and African American subjects. Nephrol Dial Transplant 24: 2428–2433, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boulet SL, Schieve LA, Boyle CA: Birth weight and health and developmental outcomes in US children, 1997-2005. Matern Child Health J 15: 836–844, 2011 [DOI] [PubMed] [Google Scholar]
- 35.Stackhouse S, Miller PL, Park SK, Meyer TW: Reversal of glomerular hyperfiltration and renal hypertrophy by blood glucose normalization in diabetic rats. Diabetes 39: 989–995, 1990 [DOI] [PubMed] [Google Scholar]
- 36.Fabris B, Candido R, Armini L, Fischetti F, Calci M, Bardelli M, Fazio M, Campanacci L, Carretta R: Control of glomerular hyperfiltration and renal hypertrophy by an angiotensin converting enzyme inhibitor prevents the progression of renal damage in hypertensive diabetic rats. J Hypertens 17: 1925–1931, 1999 [DOI] [PubMed] [Google Scholar]
- 37.Herbach N, Schairer I, Blutke A, Kautz S, Siebert A, Göke B, Wolf E, Wanke R: Diabetic kidney lesions of GIPRdn transgenic mice: Podocyte hypertrophy and thickening of the GBM precede glomerular hypertrophy and glomerulosclerosis. Am J Physiol Renal Physiol 296: F819–F829, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Lemley KV: A basis for accelerated progression of diabetic nephropathy in Pima Indians. Kidney Int Suppl 83: S38–S42, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Cortes P, Zhao X, Dumler F, Tilley BC, Atherton J: Age-related changes in glomerular volume and hydroxyproline content in rat and human. J Am Soc Nephrol 2: 1716–1725, 1992 [DOI] [PubMed] [Google Scholar]
- 40.Hughson M, Farris AB, 3rd, Douglas-Denton R, Hoy WE, Bertram JF: Glomerular number and size in autopsy kidneys: The relationship to birth weight. Kidney Int 63: 2113–2122, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Puelles VG, Zimanyi MA, Samuel T, Hughson MD, Douglas-Denton RN, Bertram JF, Armitage JA: Estimating individual glomerular volume in the human kidney: Clinical perspectives. Nephrol Dial Transplant 27: 1880–1888, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Samuel T, Hoy WE, Douglas-Denton R, Hughson MD, Bertram JF: Determinants of glomerular volume in different cortical zones of the human kidney. J Am Soc Nephrol 16: 3102–3109, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Hoy WE, Hughson MD, Zimanyi M, Samuel T, Douglas-Denton R, Holden L, Mott S, Bertram JF: Distribution of volumes of individual glomeruli in kidneys at autopsy: Association with age, nephron number, birth weight and body mass index. Clin Nephrol 74[Suppl 1]: S105–S112, 2010 [DOI] [PubMed] [Google Scholar]
- 44.Hoy WE, Hughson MD, Diouf B, Zimanyi M, Samuel T, McNamara BJ, Douglas-Denton RN, Holden L, Mott SA, Bertram JF: Distribution of volumes of individual glomeruli in kidneys at autopsy: Association with physical and clinical characteristics and with ethnic group. Am J Nephrol 33[Suppl 1]: 15–20, 2011 [DOI] [PubMed] [Google Scholar]
- 45.Hughson MD, Puelles VG, Hoy WE, Douglas-Denton RN, Mott SA, Bertram JF: Hypertension, glomerular hypertrophy and nephrosclerosis: The effect of race. Nephrol Dial Transplant 29: 1399–1409, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Puelles VG, Douglas-Denton RN, Zimanyi MA, Armitage JA, Hughson MD, Kerr PG, Bertram JF: Glomerular hypertrophy in subjects with low nephron number: Contributions of sex, body size and race. Nephrol Dial Transplant 29: 1686–1695, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Olivetti G, Anversa P, Melissari M, Loud AV: Morphometry of the renal corpuscle during postnatal growth and compensatory hypertrophy. Kidney Int 17: 438–454, 1980 [DOI] [PubMed] [Google Scholar]
- 48.Marshall CB, Shankland SJ: Cell cycle regulatory proteins in podocyte health and disease. Nephron, Exp Nephrol 106: e51–e59, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Marshall CB, Shankland SJ: Cell cycle and glomerular disease: A minireview. Nephron, Exp Nephrol 102: e39–e48, 2006 [DOI] [PubMed] [Google Scholar]
- 50.Griffin SV, Hiromura K, Pippin J, Petermann AT, Blonski MJ, Krofft R, Takahashi S, Kulkarni AB, Shankland SJ: Cyclin-dependent kinase 5 is a regulator of podocyte differentiation, proliferation, and morphology. Am J Pathol 165: 1175–1185, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hiromura K, Haseley LA, Zhang P, Monkawa T, Durvasula R, Petermann AT, Alpers CE, Mundel P, Shankland SJ: Podocyte expression of the CDK-inhibitor p57 during development and disease. Kidney Int 60: 2235–2246, 2001 [DOI] [PubMed] [Google Scholar]
- 52.Combs HL, Shankland SJ, Setzer SV, Hudkins KL, Alpers CE: Expression of the cyclin kinase inhibitor, p27kip1, in developing and mature human kidney. Kidney Int 53: 892–896, 1998 [DOI] [PubMed] [Google Scholar]
- 53.Conaldi PG, Bottelli A, Baj A, Serra C, Fiore L, Federico G, Bussolati B, Camussi G: Human immunodeficiency virus-1 that induces hyperproliferation and dysregulation of renal glomerular epithelial cells. Am J Pathol 161: 53–61, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lasagni L, Lazzeri E, Shankland SJ, Anders HJ, Romagnani P: Podocyte mitosis - a catastrophe. Curr Mol Med 13: 13–23, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mundel P, Reiser J, Kriz W: Induction of differentiation in cultured rat and human podocytes. J Am Soc Nephrol 8: 697–705, 1997 [DOI] [PubMed] [Google Scholar]
- 56.Pippin JW, Sparks MA, Glenn ST, Buitrago S, Coffman TM, Duffield JS, Gross KW, Shankland SJ: Cells of renin lineage are progenitors of podocytes and parietal epithelial cells in experimental glomerular disease. Am J Pathol 183: 542–557, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smeets B, Moeller MJ: Parietal epithelial cells and podocytes in glomerular diseases. Semin Nephrol 32: 357–367, 2012 [DOI] [PubMed] [Google Scholar]
- 58.Macconi D, Sangalli F, Bonomelli M, Conti S, Condorelli L, Gagliardini E, Remuzzi G, Remuzzi A: Podocyte repopulation contributes to regression of glomerular injury induced by ACE inhibition. Am J Pathol 174: 797–807, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shankland SJ, Anders HJ, Romagnani P: Glomerular parietal epithelial cells in kidney physiology, pathology, and repair. Curr Opin Nephrol Hypertens 22: 302–309, 2013 [DOI] [PubMed] [Google Scholar]
- 60.Zhang J, Hansen KM, Pippin JW, Chang AM, Taniguchi Y, Krofft RD, Pickering SG, Liu ZH, Abrass CK, Shankland SJ: De novo expression of podocyte proteins in parietal epithelial cells in experimental aging nephropathy. Am J Physiol Renal Physiol 302: F571–F580, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pichaiwong W, Hudkins KL, Wietecha T, Nguyen TQ, Tachaudomdach C, Li W, Askari B, Kobayashi T, O’Brien KD, Pippin JW, Shankland SJ, Alpers CE: Reversibility of structural and functional damage in a model of advanced diabetic nephropathy. J Am Soc Nephrol 24: 1088–1102, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Poulsom R, Forbes SJ, Hodivala-Dilke K, Ryan E, Wyles S, Navaratnarasah S, Jeffery R, Hunt T, Alison M, Cook T, Pusey C, Wright NA: Bone marrow contributes to renal parenchymal turnover and regeneration. J Pathol 195: 229–235, 2001 [DOI] [PubMed] [Google Scholar]
- 63.Perry J, Tam S, Zheng K, Sado Y, Dobson H, Jefferson B, Jacobs R, Thorner PS: Type IV collagen induces podocytic features in bone marrow stromal stem cells in vitro. J Am Soc Nephrol 17: 66–76, 2006 [DOI] [PubMed] [Google Scholar]
- 64.Prodromidi EI, Poulsom R, Jeffery R, Roufosse CA, Pollard PJ, Pusey CD, Cook HT: Bone marrow-derived cells contribute to podocyte regeneration and amelioration of renal disease in a mouse model of Alport syndrome. Stem Cells 24: 2448–2455, 2006 [DOI] [PubMed] [Google Scholar]
- 65.Shankland SJ, Smeets B, Pippin JW, Moeller MJ: The emergence of the glomerular parietal epithelial cell. Nat Rev Nephrol 10: 158–173, 2014 [DOI] [PubMed] [Google Scholar]
- 66.Wanner N, Hartleben B, Herbach N, Goedel M, Stickel N, Zeiser R, Walz G, Moeller MJ, Grahammer F, Huber TB: Unraveling the role of podocyte turnover in glomerular aging and injury. J Am Soc Nephrol 25: 707–716, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Berger K, Schulte K, Boor P, Kuppe C, van Kuppevelt TH, Floege J, Smeets B, Moeller MJ: The regenerative potential of parietal epithelial cells in adult mice. J Am Soc Nephrol 25: 693–705, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Berger K, Moeller MJ: Podocytopenia, parietal epithelial cells and glomerulosclerosis. Nephrol Dial Transplant 29: 948–950, 2014 [DOI] [PubMed] [Google Scholar]
- 69.Walker KA, Bertram JF: Kidney development: Core curriculum 2011. Am J Kidney Dis 57: 948–958, 2011 [DOI] [PubMed] [Google Scholar]
- 70.Bertram JF, Douglas-Denton RN, Diouf B, Hughson MD, Hoy WE: Human nephron number: Implications for health and disease. Pediatr Nephrol 26: 1529–1533, 2011 [DOI] [PubMed] [Google Scholar]
- 71.Hughson MD, Gobe GC, Hoy WE, Manning RD, Jr., Douglas-Denton R, Bertram JF: Associations of glomerular number and birth weight with clinicopathological features of African Americans and whites. Am J Kidney Dis 52: 18–28, 2008 [DOI] [PubMed] [Google Scholar]
- 72.Hoy WE, Bertram JF, Denton RD, Zimanyi M, Samuel T, Hughson MD: Nephron number, glomerular volume, renal disease and hypertension. Curr Opin Nephrol Hypertens 17: 258–265, 2008 [DOI] [PubMed] [Google Scholar]
- 73.Hughson MD, Douglas-Denton R, Bertram JF, Hoy WE: Hypertension, glomerular number, and birth weight in African Americans and white subjects in the southeastern United States. Kidney Int 69: 671–678, 2006 [DOI] [PubMed] [Google Scholar]
- 74.Hoy WE, Hughson MD, Singh GR, Douglas-Denton R, Bertram JF: Reduced nephron number and glomerulomegaly in Australian Aborigines: A group at high risk for renal disease and hypertension. Kidney Int 70: 104–110, 2006 [DOI] [PubMed] [Google Scholar]
- 75.Douglas-Denton RN, McNamara BJ, Hoy WE, Hughson MD, Bertram JF: Does nephron number matter in the development of kidney disease? Ethn Dis 16: S2-40-5, 2006 [PubMed]
- 76.Mauer SM, Lane P, Zhu D, Fioretto P, Steffes MW: Renal structure and function in insulin-dependent diabetes mellitus in man. J Hypertens Suppl 10: S17–S20, 1992 [DOI] [PubMed] [Google Scholar]
- 77.Steffes MW, Schmidt D, McCrery R, Basgen JM, International Diabetic Nephropathy Study Group : Glomerular cell number in normal subjects and in type 1 diabetic patients. Kidney Int 59: 2104–2113, 2001 [DOI] [PubMed] [Google Scholar]
- 78.White KE, Bilous RW, Marshall SM, El Nahas M, Remuzzi G, Piras G, De Cosmo S, Viberti G: Podocyte number in normotensive type 1 diabetic patients with albuminuria. Diabetes 51: 3083–3089, 2002 [DOI] [PubMed] [Google Scholar]
- 79.Nadasdy T, Laszik Z, Blick KE, Johnson LD, Silva FG: Proliferative activity of intrinsic cell populations in the normal human kidney. J Am Soc Nephrol 4: 2032–2039, 1994 [DOI] [PubMed] [Google Scholar]
- 80.Dane MJ, van den Berg BM, Avramut MC, Faas FG, van der Vlag J, Rops AL, Ravelli RB, Koster BJ, van Zonneveld AJ, Vink H, Rabelink TJ: Glomerular endothelial surface layer acts as a barrier against albumin filtration. Am J Pathol 182: 1532–1540, 2013 [DOI] [PubMed] [Google Scholar]
- 81.Abboud HE: Mesangial cell biology. Exp Cell Res 318: 979–985, 2012 [DOI] [PubMed] [Google Scholar]
- 82.Fukuda A, Chowdhury MA, Venkatareddy MP, Wang SQ, Nishizono R, Suzuki T, Wickman LT, Wiggins JE, Muchayi T, Fingar D, Shedden KA, Inoki K, Wiggins RC: Growth-dependent podocyte failure causes glomerulosclerosis. J Am Soc Nephrol 23: 1351–1363, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Moeller MJ, Kuppe C: Glomerular disease: The role of parietal epithelial cells in hyperplastic lesions. Nat Rev Nephrol 10: 5–6, 2014 [DOI] [PubMed] [Google Scholar]
- 84.Romagnani P: Parietal epithelial cells: Their role in health and disease. Contrib Nephrol 169: 23–36, 2011 [DOI] [PubMed] [Google Scholar]
- 85.Smeets B, Kuppe C, Sicking EM, Fuss A, Jirak P, van Kuppevelt TH, Endlich K, Wetzels JF, Gröne HJ, Floege J, Moeller MJ: Parietal epithelial cells participate in the formation of sclerotic lesions in focal segmental glomerulosclerosis. J Am Soc Nephrol 22: 1262–1274, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hoy WE, Douglas-Denton RN, Hughson MD, Cass A, Johnson K, Bertram JF: A stereological study of glomerular number and volume: Preliminary findings in a multiracial study of kidneys at autopsy. Kidney Int Suppl 83: S31–S37, 2003 [DOI] [PubMed] [Google Scholar]
- 87.Gundersen HJ, Jensen EB: The efficiency of systematic sampling in stereology and its prediction. J Microsc 147: 229–263, 1987 [DOI] [PubMed] [Google Scholar]
- 88.Niksic M, Slight J, Sanford JR, Caceres JF, Hastie ND: The Wilms’ tumour protein (WT1) shuttles between nucleus and cytoplasm and is present in functional polysomes. Hum Mol Genet 13: 463–471, 2004 [DOI] [PubMed] [Google Scholar]
- 89.Collins TJ: ImageJ for microscopy. Biotechniques 43[Suppl]: 25–30, 2007 [DOI] [PubMed] [Google Scholar]