Abstract
The calcium-sensing receptor (CaR) modulates renal calcium reabsorption and parathyroid hormone (PTH) secretion and is involved in the etiology of secondary hyperparathyroidism in CKD. Supraphysiologic changes in extracellular pH (pHo) modulate CaR responsiveness in HEK-293 (CaR-HEK) cells. Therefore, because acidosis and alkalosis are associated with altered PTH secretion in vivo, we examined whether pathophysiologic changes in pHo can significantly alter CaR responsiveness in both heterologous and endogenous expression systems and whether this affects PTH secretion. In both CaR-HEK and isolated bovine parathyroid cells, decreasing pHo from 7.4 to 7.2 rapidly inhibited CaR-induced intracellular calcium (Ca2+i) mobilization, whereas raising pHo to 7.6 potentiated responsiveness to extracellular calcium (Ca2+o). Similar pHo effects were observed for Ca2+o-induced extracellular signal-regulated kinase phosphorylation and actin polymerization and for L-Phe-induced Ca2+i mobilization. Intracellular pH was unaffected by acute 0.4-unit pHo changes, and the presence of physiologic albumin concentrations failed to attenuate the pHo-mediated effects. None of the individual point mutations created at histidine or cysteine residues in the extracellular domain of CaR attenuated pHo sensitivity. Finally, pathophysiologic pHo elevation reversibly suppressed PTH secretion from perifused human parathyroid cells, and acidosis transiently increased PTH secretion. Therefore, pathophysiologic pHo changes can modulate CaR responsiveness in HEK-293 and parathyroid cells independently of extracellular histidine residues. Specifically, pathophysiologic acidification inhibits CaR activity, thus permitting PTH secretion, whereas alkalinization potentiates CaR activity to suppress PTH secretion. These findings suggest that acid-base disturbances may affect the CaR-mediated control of parathyroid function and calcium metabolism in vivo.
Keywords: calcium-sensing receptor, parathyroid hormone, hyperparathyroidism, acidosis., mineral metabolism
Mammalian calcium homeostasis is maintained by the regulated secretion of parathyroid hormone (PTH) and renal calcium reabsorption under the control of the calcium-sensing receptor (CaR).1,2 Hypercalcemia stimulates the CaR to suppress PTH secretion, while chronic underactivation of CaR can contribute to pathologically elevated PTH secretion, such as in secondary hyperparathyroidism of CKD. In a heterologous human embryonic kidney 293 (HEK-293) cell-based expression system, Quinn et al.3 found that the Ca2+o potency of the human CaR is sensitive to supraphysiologic changes in extracellular pH (pHo). By varying the pH of the experimental buffer in 0.5-unit steps from 5.5 to 9, the sensitivity of CaR to Ca2+o (and Mg2+o) was altered; external acidification decreased CaR sensitivity and alkalinization increased CaR sensitivity.3 However, blood pH levels (7.35–7.45 approximately 40 nM H+ concentration) rarely vary beyond ±0.4 pH units in vivo, even under extreme pathologic conditions. Even smaller changes (±0.2) are more generally seen, such as with the acidosis of CKD; however, even this still represents an approximately 58% increase in H+ concentration. Secondary hyperparathyroidism is another complication of CKD. In animal experiments, induction of metabolic acidosis results in increased serum PTH levels and hypercalcemia, whereas induction of alkalosis suppresses serum PTH levels.4–8
Therefore, because acidosis has been associated with increased serum PTH levels in vivo, we examined whether much smaller, pathophysiologically relevant changes in pHo (±0.2) can alter the Ca2+o sensitivity of CaR-mediated signaling pathways in HEK-293 cells and whether such effects can be replicated in parathyroid cells. We also examined whether a specific histidine or free cysteine residue could account for CaR pHo sensitivity and, finally, considered the effect of such pathophysiologic changes in pHo on PTH secretion.
Results
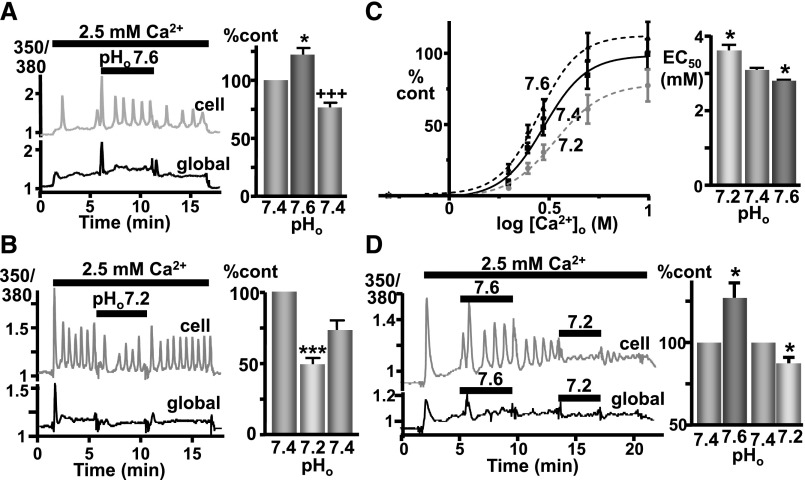
Fura 2–loaded CaR-HEK cells were stimulated with 2.5mM Ca2+o (pH, 7.4) to elicit CaR-induced Ca2+i mobilization and were then switched to the same buffer at pH 7.2 or 7.6 before being returned to pH 7.4. At pH 7.6, CaR responsiveness (i.e., Δ350/380 fura ratio area under the curve) was significantly increased (Figure 1A), an effect that was immediately reversible upon return to pH 7.4. In contrast, lowering pHo to 7.2 significantly inhibited CaR responsiveness (Figure 1B), the effect being immediately reversible. The effect of ±0.2-unit pH changes on Ca2+i mobilization was then tested over a range of Ca2+o concentrations, from 0.5 to 10 mM. Again, CaR responsiveness was significantly inhibited with pH 7.2 (half maximal effective concentration for Ca2+o, 3.6±0.2 mM in 7.2 versus 3.1±0.1 in 7.4; P<0.05) and significantly stimulated with pH 7.6 (2.8±0.0 mM in 7.6; P<0.05). In addition, similar effects of changing pHo (±0.2 unit) were also seen using bicarbonate/CO2 buffers (Figure 1D). See Supplemental Material for the complete methods.
Figure 1.
CaR-induced Ca2+i mobilization is sensitive to pathophysiologic pHo changes in CaR-HEK cells. (A) Representative trace showing Ca2+i changes (Fura-2 ratio) in a single cell (upper trace, “cell”) and “global” (lower trace) cluster of (>10) cells in response to elevated [Ca2+]o (2.5 versus 0.5 mM control) when pHo was changed from 7.4 to 7.6. Changes in [Ca2+]i shown as percentage control of the area under the curve. n=4 coverslips. (B) Identical except testing the effect of decreased pHo (7.2). n=4 coverslips. *P<0.05 and ***P<0.001 versus first pH 7.4 treatment; +++P<0.001 versus pH 7.6 treatment by repeated-measures ANOVA (Tukey post-test) performed on the raw data. (C) Cells were exposed to buffers containing increasing concentrations of Ca2+o (0.5–10 mM) with pHo 7.2, 7.4, or 7.6 with the resulting concentration-effect curves for Ca2+i mobilization shown (left) together with their half maximal effective concentration (EC50) (right). While the EC50 values were significantly different, the maximal responses were not significantly different, despite the apparent trend. *P<0.05 versus pH 7.4 by repeated-measures ANOVA (Dunnett) performed on log EC50 values from four independent experiments (two to five coverslip replicates per data point, >15 cells per coverslip). (D) Similar results were obtained with use of bicarbonate/CO2 buffers, with pH 7.6 increasing Ca2+i mobilization and pH 7.2 decreasing mobilization in a single cell (upper trace) and “global” cluster of (>10) cells (lower trace) in response to 2.5 mM Ca2+o as before. Relative changes in [Ca2+]i are shown in the bar graph. The appropriate pH values were obtained by varying the bicarbonate (and NaCl) concentrations in buffers gassed continuously with 5% CO2/95% O2 (g). For the complete methods, see the Supplemental Material. *P<0.05 versus pH 7.4 treatment by one-tail paired t test; n=7 coverslips.
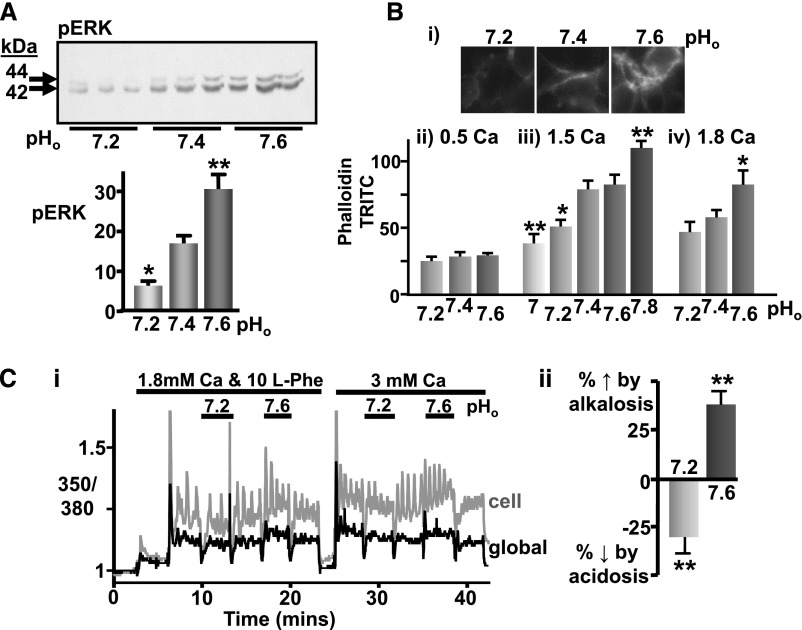
To determine whether this apparent pHo sensitivity of CaR is specific to intracellular Ca2+i mobilization or applies to other effector pathways, two other readouts of CaR activity were used: extracellular signal-regulated kinase (ERK) phosphorylation and actin polymerization.9,10 Indeed, mild acidosis (pHo 7.2) significantly inhibited Ca2+o-induced ERK phosphorylation in CaR-HEK cells (3.5 mM Ca2+o) (Figure 2A), while mild alkalosis (pHo 7.6) increased ERK activation. Similarly, a 0.2-unit reduction in pHo inhibited 1.5 mM Ca2+o-induced actin polymerization while a 0.2-unit increase in pHo potentiated 1.8 mM Ca2+o-induced actin polymerization (Figure 2B). Together these results suggest that the effect of altering pHo affects the Ca2+o sensitivity of the CaR rather than the mechanism of Ca2+i mobilization. Indeed, in the presence of 0.5 mM Ca2+o (which renders the CaR inactive), variations in pHo between 7.2 and 7.6 had no effect on actin polymerization. This finding indicates that changing pHo does not otherwise affect baseline signaling.
Figure 2.
Pathophysiologic pHo changes also affects CaR-induced ERK activation, actin polymerization, and L-Phe responsiveness in CaR-HEK cells. (A) Representative immunoblot showing ERK phosphorylation (pERK) after treatment with 3.5 mM Ca2+o for 10 minutes under different pHo conditions (7.4±0.2). Mean changes in ERK phosphorylation were determined by densitometry and expressed on a bar graph in arbitrary units. n=9 dishes from two independent experiments. *P<0.05 and **P<0.01 versus pHo 7.4 by one-way ANOVA (Dunnet). (B) Representative immunofluoresence images (i) showing phalloidin-TRITC staining following CaR-HEK treatment with 1.5 mM Ca2+o for 3 hours in buffers of pHo ranging from 7.2 to 7.6. Quantification presented on a bar graph as pixels (phalloidin-TRITC) per unit area in five randomly selected regions of the cells is shown below (iii). Also shown are bar graphs from separate experiments where the cells were exposed to 0.5 (ii) or 1.8 (iv) mM Ca2+o. n≥5 coverslips. *P<0.05 and **P<0.01 versus pHo 7.4 by one-way ANOVA (Dunnet). (C) Representative Ca2+i trace (i) showing CaR-HEK cells stimulated first with 10 mM L-Phe (in 1.8 mM Ca2+o) and then with 3 mM Ca2+o under various pHo conditions as shown (0.5 Ca2+o, baseline, pH 7.4 unless otherwise stated). The traces include a typical response in a single cell (gray) as well as the total response in the cell cluster (>10 cells; “global”, black). Quantification (ii) is shown as a bar graph (percentage change in L-Phe responsiveness relative to pHo 7.4 control). n=7 coverslips from two independent experiments. **P<0.01 by repeated-measures ANOVA (Bonferroni).
Next, we examined the effect of 0.2-unit pHo changes on the response to the CaR-positive allosteric modulator L-Phe (10 mM) to determine whether the pHo sensitivity is exclusive to orthosteric agonism with Ca2+o. We observed significant potentiation of L-Phe-induced Ca2+i mobilization in pHo 7.6 and attenuation in pHo 7.2 (Figure 2C), similar to that observed for Ca2+o.
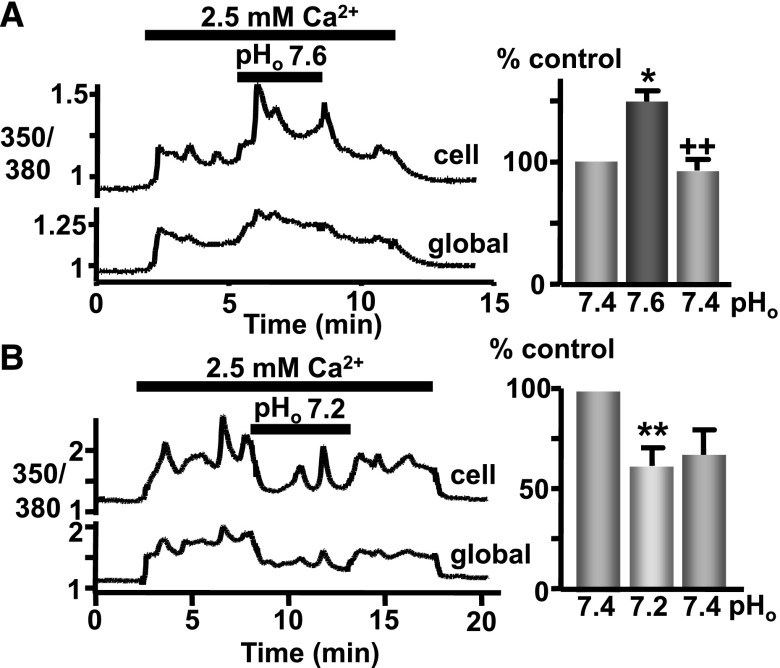
We then assessed whether pathophysiologic changes in pHo also affect CaR-induced Ca2+i mobilization in parathyroid cells, in which the CaR is expressed endogenously. Indeed, raising pHo to 7.6 while stimulating CaR-induced Ca2+i mobilization in bovine parathyroid cells (2.5 mM Ca2+o) significantly potentiated the response, an effect that was fully and immediately reversible upon return to pHo 7.4 (Figure 3A). In addition, lowering pHo to 7.2 promptly inhibited the Ca2+i mobilization elicited in parathyroid cells in response to 2.5mM Ca2+o (Figure 3B).
Figure 3.
CaR-induced Ca2+i mobilization is sensitive to pathophysiologic pHo changes in bovine parathyroid cells. (A) Trace showing Ca2+i changes (quantified as a bar graph, as before) in a single cell (upper trace; “cell”) or the “global” (lower trace) cluster of cells in the field of view, in response to elevated [Ca2+]o (2.5 versus 0.8 mM control) at pHo 7.4 or 7.6 (resulting changes normalized to pH 7.4 control). (B) Identical experiment except examining the effect of lowering pHo (7.2) during CaR stimulation. n=6–8 coverslips. *P<0.05 and **P<0.01 versus initial pH 7.4 response; ++P<0.01 versus pH 7.6 by Kruskal–Wallis (Dunn post-test).
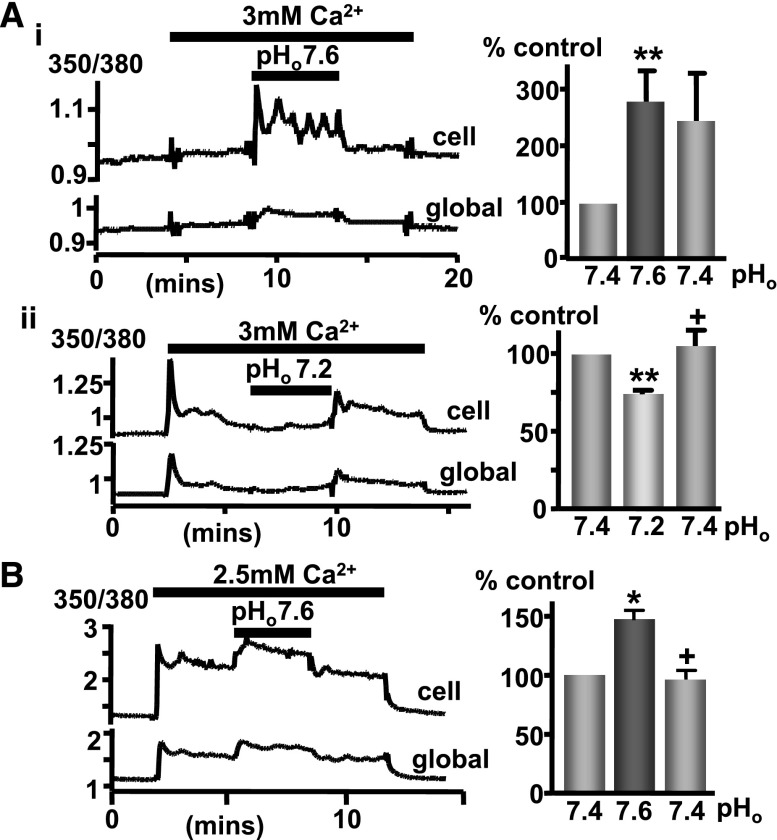
In considering the potential (patho)physiologic relevance of CaR pHo-sensitivity, it should be noted that serum albumin can bind calcium in a pH-dependent manner. Thus, albumin releases calcium under acidic conditions, which might tend to counteract the concomitant CaR inhibition, whereas alkaline conditions promote calcium binding to albumin and thus potentially counteract the CaR stimulation. Thus, we tested the effects of pHo on CaR-induced Ca2+i mobilization in the presence of a physiologic concentration of 5% (w/v) albumin. Exposure of CaR-HEK cells to 3 mM Ca2+o (in 5% [w/v] albumin) at pH 7.4 resulted in an increase in Ca2+i concentration, and as before, lowering pHo to 7.2 resulted in a reversible attenuation of the CaR response (Figure 4Ai). In addition, increasing pHo to 7.6 while stimulating CaR with 3 mM Ca2+o (in 5% albumin) potentiated the response (Figure 4Aii). This was further tested in bovine parathyroid cells; again, raising pHo to 7.6 potentiated CaR activity despite the presence of 5% (w/v) albumin (Figure 4B).
Figure 4.
Physiologic albumin concentration fails to prevent changes in pHo from modulating CaR activity. (A) Changes in CaR-HEK cell Ca2+i concentration (measured as before) in a single cell or the “global” cluster of cells in response to elevated [Ca2+]o (3 versus 0.5 mM control) in buffer supplemented with 5% (w/v) BSA at pHo 7.6 (i) or 7.2 (ii). The resulting changes (area under the curve) are normalized to pH 7.4 control and displayed as a bar graph. (B) Identical experiment except using bovine parathyroid cells (2.5 versus 0.8 mM control at pHo 7.4 or 7.6). n≥5 coverslips. *P<0.05 and **P<0.01 versus initial pH 7.4 response; +P<0.05 versus pH 7.6 (or 7.2) by Kruskal–Wallis (Dunn post-test).
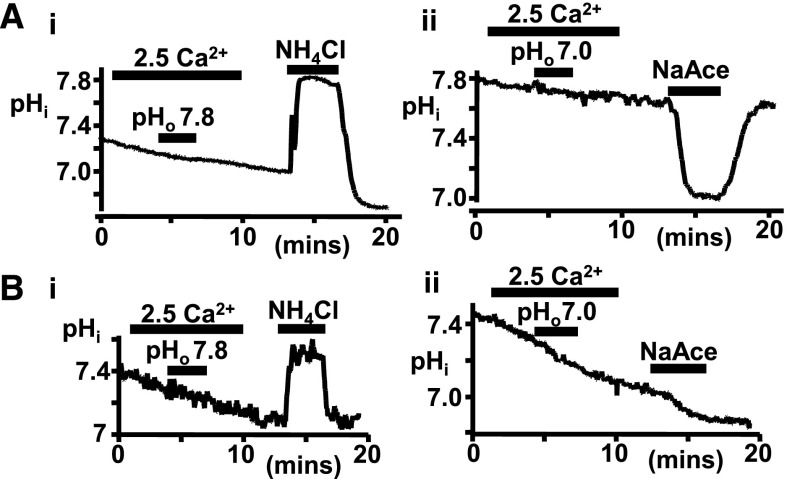
To test whether the changes in pHo might instead be acting by altering intracellular pH (pHi), CaR-HEK and bovine parathyroid cells were loaded with the pH-sensitive dye BCECF and exposed to CaR stimulation in the presence or absence of even greater changes in pHo, namely pH 7.0 for acidosis and pH 7.8 for alkalosis. However, in neither cell type did pHo 7 or 7.8 (or indeed CaR activation itself) affect pHi over the timescales used (Figure 5). Therefore, these data suggest that the consequence of altering pHo on intracellular signaling is via an effect on the CaR per se. Ammonium chloride and sodium acetate were used as positive controls to demonstrate that pHi changes could be detected in the BCECF-loaded cells. Indeed, ammonium chloride elicited intracellular alkalinization in both cell types, whereas sodium acetate induced a marked intracellular acidification in CaR-HEK cells and had a much smaller effect in bovine parathyroid cells.
Figure 5.
Acute pHo changes do not affect pHi levels in CaR-HEK or parathyroid cells. (A, i) Trace showing pHi changes (BCECF ratio) in a cluster of CaR-HEK cells in response to elevated [Ca2+]o (2.5 versus 0.5 mM control) at pHo 7.4 and 7.8. Ammonium chloride (20 mM, NH4Cl2) was used as a positive control. Quantification comparing control (baseline extrapolated) and treated (actual) values for calculated pHi. (A, ii) Identical experiment except examining the effect of lowering pHo (7.0) and with sodium acetate (20 mM, NaAc) used as positive control. (B, i) Trace showing pHi changes (BCECF ratio) in a cluster of bovine parathyroid cells in response to elevated [Ca2+]o (2.5 versus 0.8 mM control) at pHo 7.4 and 7.8 (NH4Cl positive control). (B, ii) Identical experiment except examining the effect of lowering pHo (7.0). n=3–7 coverslips. No significant change (by paired t test) in pHi occurred after acute changes in pHo in either cell type.
Effect of Altered pHo on Parathyroid Hormone Secretion
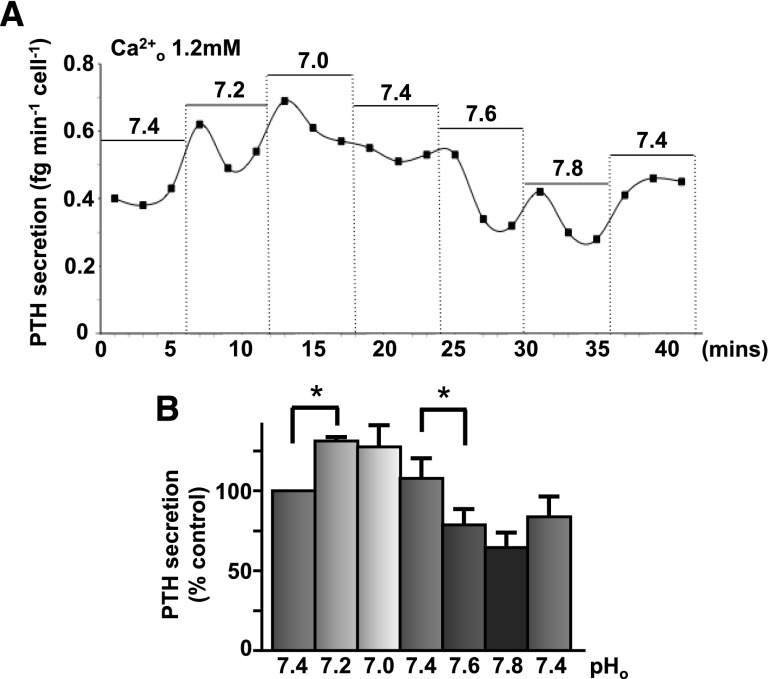
Parathyroid hormone secretion was measured in human parathyroid cells. In the presence of physiologic free Ca2+o concentration (1.2 mM), lowering pHo (7.2 then 7.0) caused an initial increase in PTH secretion whereas raising pH (7.6 then 7.8) suppressed PTH secretion (Figure 6). When pHo was lowered to normal (7.4), PTH secretion then rose again, suggesting that the suppression was fully reversible. Preliminary experiments investigating the effect of 0.4-unit pHo changes on PTH secretion from both bovine and human parathyroid cells (and conducted before those shown in Figure 6) showed a similar pHo sensitivity, with alkalosis suppressing PTH secretion significantly in both cases (not shown).
Figure 6.
Extracellular pH modulates CaR-induced suppression of PTH secretion. (A) Human parathyroid cells were perifused in 1.2 mM calcium-containing buffers at pHo 7.0–7.8 (at 5-minute intervals) and PTH secretion quantified as fg per minute per cell. See Supplemental Material for the complete methods. (B) Quantification of these changes is shown as percentage control. n=3 independent experiments. *P<0.05 by unpaired t test (one-tail) on PTH values following preliminary experiments investigating the effect of 0.4-unit pHo changes on both human and bovine parathyroid cells (not shown).
Investigation of Extracellular Histidine/Cysteine Residues as Possible Sites of CaR pHo Sensitivity
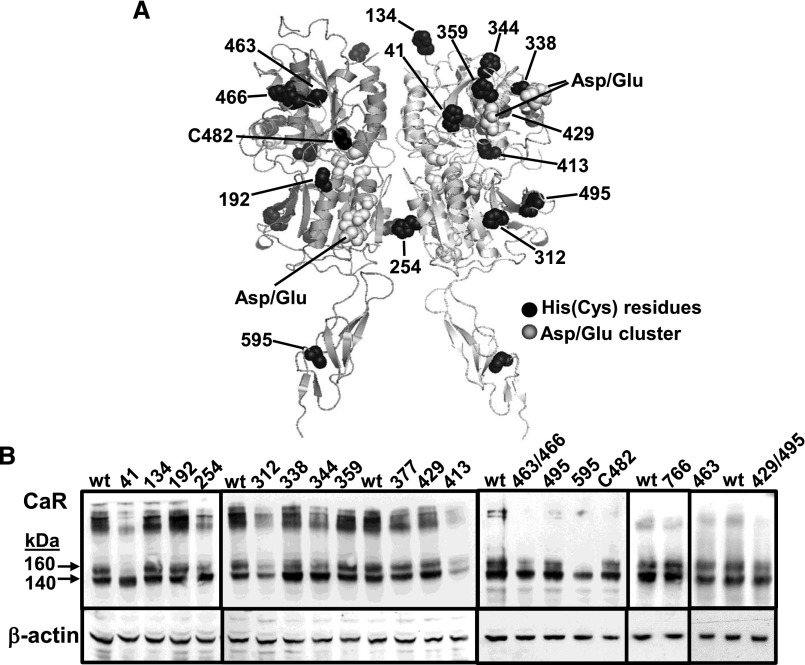
Finally, modeling the homodimeric CaR extracellular domain using sequence alignment with the metabotropic glutamate receptor (mGlu) structure 2e4u11,12 revealed the proximity of certain extracellular histidine sites (in black) to clusters of aspartate and glutamate residues (in gray) previously proposed to be sites of Ca2+o binding (Figure 7A).13,14 The amino acids whose side chain pK values are closest to the physiologic pHo and, thus that might account for pHo sensitivity in CaR, are histidine (approximately 6.5) and free cysteine (approximately 8.5). Indeed, the human CaR contains 16 extracellular histidines plus 1 free cysteine residue (Cys-482). Therefore, each of these residues was mutated individually, histidines to valines and the cysteine to serine, with the resulting 17 CaR mutants expressed transiently in HEK-293 cells. The effects of ±0.2-unit pHo changes on CaR-induced Ca2+i mobilization were then tested in the mutant CaR-expressing cells and compared with their effects on wild-type CaR-expressing cells. For this, concentration-effect curves for Ca2+o-induced Ca2+i mobilization were generated to determine whether the mutations elicit substantial changes in Ca2+o potency/CaR responsiveness per se. All but two of the mutants exhibited significant, sigmoidal Ca2+o sensitivity (at concentrations <10 mM), the exceptions being CaRH41V and CaRH595V (Table 1). See Supplemental Material for the complete methods.
Figure 7.
CaR extracellular domain model showing histidine and free cysteine residue locations relative to aspartate and glutamate clusters. (A) Histidines and the free cysteine are shown in black with the three aspartate/glutamate clusters in light gray.21,22 Not shown are residues CaRH377 and CaRH766 (located in a transmembrane extracellular loop). (B) Immunoblots showing the mutant CaR proteins lacking each of the 17 extracellular histidine or free cysteine residues CaR immunoblots compared with wild-type (wt) CaR and β-actin loading controls.
Table 1.
Characterization of the Ca2+o-sensitivity, relative maximal responses, and pHo sensitivity of the CaR histidine and free cysteine mutants versus wild-type
| Mutant | EC50 (mM) | Emax (%) | (n) | Inhibition by Acidosis (%) | Stimulation by Alkalosis (%) | (n) |
|---|---|---|---|---|---|---|
| WT-CaR | 4.3±0.3 | 100±19 | 16 | −20±2 | 14±3 | 20 |
| H134V | 4.3±0.3 | 93±19 | 6 | −22±6 | 9±4 | 9 |
| H192V | 7.6±1.0a | 118±16 | 5 | −22±9 | 34±13 | 7 |
| H254V | 3.9±0.6 | 90±22 | 6 | −10±5 | 35±9 | 6 |
| H312V | 3.3±0.5 | 80±8 | 5 | −26±5 | 32±10 | 6 |
| H338V | 5.7±0.1 | 162±17 | 4 | −22±7 | 16±4 | 6 |
| H344V | 6.1±0.5b | 82±6 | 8 | −38±10 | 55±26 | 6 |
| H359V | 5.2±0.5 | 152±22 | 8 | −24±13 | 11±5 | 6 |
| H377V | 5.8±0.7 | 143±11 | 8 | −43±6 | 19±12 | 7 |
| H413V | 3.9±0.4 | 104±20 | 5 | −16±9 | 13±3 | 9 |
| H429V | 5.7±0.7 | 91±11 | 3 | −13±13 | 9±3 | 7 |
| H463V | 5.9±1.1 | 90±13 | 6 | −40±5 | 23±6 | 6 |
| H463V/H466V | 4.7±0.3 | 70±9 | 9 | −17±8 | 21±5 | 7 |
| H495V | 4.8±0.6 | 180±52 | 3 | −11±7 | 11±6 | 8 |
| H766V | 3.3±0.2 | 131±9 | 9 | −22±4 | 20±8 | 9 |
| C482S | 3.7±0.7 | 81±39 | 3 | −36±5 | 26±8 | 7 |
| H429V/H495V | 5.0±0.4 | 53±7 | 7 | −35±4 | 19±5 | 7 |
| H41V | >10 | – | 9 | −43±6 | 22±11 | 8 |
| H595V | >10 | – | 9 | −22±7 | 13±5 | 9 |
Two independent series of experiments tested (1) the Ca2+o senstivity and maximal response for each CaR mutant (extracellular histidine or free cysteine residues replaced with valine or serine respectively) versus wild-type and (2) the effect of decreasing or increasing pHo by 0.2 unit on receptor responsiveness. All but four of the CaR mutants exhibited Ca2+o sensitivity not significantly different from wild-type CaR. For CaRH192V and CaRH344V the EC50 values for Ca2+o (mM) were increased significantly. Regarding maximal responsiveness to Ca2+o, none of these CaR mutants responded differently to wild-type (Kruskal–Wallis, Dunn multiple comparison test) with the exception of CaRH41V and CaRH595V that required additional cotreatment with a positive allosteric modulator (1 mM R-467) to achieve robust Ca2+i mobilization (not shown). Next, none of the mutants exhibited significantly altered CaR pHo sensitivity (one-way ANOVA with Dunnett) in response to pathophysiologic alkalosis (pHo 7.6) or acidosis (pHo 7.2) in the presence of 3.5 mM Ca2+o. Because CaRH41V and CaRH595V lacked responsiveness to 3.5 mM Ca2+o, the percentage change values quoted for them are not directly relative to wild-type CaR, but merely represent the percentage change of their CaR response from the immediately prior response in pH 7.4. EC50 half maximal effective concentration; Emax, maximal responsiveness. n=coverslips, from a minimum of two independent transfections in the case of the pHo experiments.
P<0.05 by one-way ANOVA, Dunnett post hoc test.
P<0.001 by one-way ANOVA, Dunnett post hoc test.
For the 15 CaR mutants that exhibited robust Ca2+o sensitivity, the effect of ±0.2-unit pHo changes on CaR-induced Ca2+i mobilization was then tested after exposure to 3.5 mM Ca2+o. Acidosis-elicited attenuation and alkalosis-mediated potentiation of CaR activity did not significantly decrease in any of the CaR mutants (relative to wild-type CaR), suggesting that none of them contribute to pHo sensitivity in CaR (Table 1). Having failed to identify a sole histidine residue as being responsible for eliciting CaR pHo sensitivity, the two histidine mutants that produced the largest trend reductions in pHo sensitivity, namely CaRH429V and CaRH495V, were then comutated to determine whether together they might inhibit CaR pHo sensitivity. However, the pHo sensitivity of CaRH429V/H495V was not significantly different from that of wild-type CaR.
For the two remaining mutants that exhibited reduced Ca2+o sensitivity (and lower protein abundance of the mature 160-kDa CaR protein), more robust stimulation was required, namely 5 mM Ca2+o plus 1 μM R467 (positive allosteric modulator) for CaRH595V and 30 mM Ca2+o plus 1 μM R467 for CaRH41V (Figure 7B, Table 1). Because these treatments represent supramaximal stimuli for wild-type CaR and thus are unsuitable for testing the effects of altering pHo on the wild-type receptor, the resulting data for CaRH41V and CaRH595V cannot be compared directly to wild-type CaR but are appropriate for qualitative comparison by examining their signaling before and after the change in pHo. In the case of both mutations, lowering pHo significantly inhibited the CaR response while raising pHo significantly increased activity in the mutant CaRs as before. Therefore, taken together, there was no evidence that any of the 17 histidine residues or single free cysteine (Cys-482) contributed to the pHo sensitivity of CaR.
Discussion
Ca2+o potency for CaR is sensitive to large changes in ambient pHo.3,15 The pH of human plasma is maintained between 7.35 and 7.45, representing a 12.5% deviation above and below the normal H+ concentration of 4×10−8 M (i.e., pH 7.40). Indeed, a 1-unit pH change would represent a lethal 10-fold change in H+ concentration,16 and even a smaller drop to 7.1 and below represents a medical emergency. This led us to investigate whether the CaR can also respond to smaller, pathophysiologically relevant changes in pHo. Because metabolic acidosis and secondary hyperparathyroidism are both common consequences of CKD, the current data may even provide a mechanistic link between the two.
Here we have demonstrated that pathophysiologically relevant (0.2-unit) changes in pHo significantly modulate Ca2+o-induced Ca2+i mobilization in CaR-HEK cells and elicit similar effects on two other readouts of CaR-mediated signaling: ERK phosphorylation and actin polymerization. Thus, the effect of the pHo change is not specific for one particular signaling pathway and thus likely occurs at the level of the CaR. Indeed, no acute changes in pHi were detected in CaR-HEK or bovine parathyroid cells after exposure to larger (0.4-unit) changes in pHo over the timescale tested, supporting the idea that the change in pHo elicits an extracellular, as opposed to nonspecific intracellular, effect on CaR activity. Quinn et al.3 reported slow cytoplasmic acidification/alkalinization in CaR-HEKs in response to much larger 1- to 2-unit pHo decreases/increases, respectively; however, the slow rate of change failed to account for the much faster pHo-mediated change in CaR sensitivity.
Regarding the CaR agonist selectivity of the pHo effect, Quinn et al.3 found that Mg2+o was similarly affected by pHo as for Ca2+o, both of these cations being orthosteric CaR agonists. Here we found that L-Phe–induced Ca2+i mobilization was also sensitive to pathophysiologic changes in pHo, suggesting that allosteric CaR modulation is similarly sensitive to pHo and thus unlikely to counteract the effect in vivo.
Of note, the enhancement of CaR signaling with pHo 7.6 and inhibition with pHo 7.2 was also observed in bovine parathyroid cells, in which the CaR is expressed endogenously. Consistent with this observation was the clear suppression of PTH secretion from bovine and human parathyroid cells after perifusion in alkaline buffer (pHo 7.8; preliminary experiments not shown). Repeating these experiments with use of 0.2-unit pHo changes, we observed a transient rise in PTH secretion from human parathyroid cells that was not sustained when pHo was lowered to 7.2; we also noted sustained suppression of PTH secretion when pHo was increased to 7.6. These in vitro data are consistent with observations that metabolic acidosis is associated with increased serum PTH and calcium levels and that alkalosis suppresses serum PTH in vivo.4,6–8 The current findings suggest a CaR-based mechanism for these previous data from animal studies. It will be interesting therefore to determine therefore whether acidosis and/or alkalosis elicit chronic changes in PTH secretion in vivo. Interestingly, at age 80 years, human blood H+ concentration is 6%–7% higher than at age 20,17 and there is a concomitant rise in serum PTH levels.18
With regard to metabolic acidosis and secondary hyperparathyroidism in CKD, while secondary hyperparathyroidism is known to be due partly to hyperphosphatemia and partly to decreased calcitriol levels (resulting in part to lowered plasma free calcium concentration), it is interesting to speculate that acidosis may also contribute to elevated PTH secretion rates by suppressing CaR sensitivity, as observed here. In addition, the current data suggest that raising pHo promotes CaR-mediated suppression of PTH secretion and thus may provide the basis for a useful adjuvant therapy for secondary hyperparathyroidism in CKD. Clinically, low bicarbonate concentration in predialysis patients predicts subsequent coronary artery calcification.19 Because oral sodium bicarbonate supplementation slows the rate of decline of renal function in patients with CKD and low plasma bicarbonate concentrations,20 the effect of this co-therapy on the development of secondary hyperparathyroidism and vascular calcification might be considered for investigation. Of note, acidosis also increases fibroblast growth factor-23 expression in osteoblasts, raising the question of whether treating acidosis in CKD may lower fibroblast growth factor 2321 and, indeed, PTH secretion.
Low pH displaces bound calcium from serum albumin and could compensate for the acid-mediated decline in CaR responsiveness by increasing the free calcium concentration.22 However, in the current study, inclusion of 5% (w/v) albumin in the physiologic saline solution (i.e., at a physiologically relevant concentration) had little or no effect on the pHo sensitivity of the CaR. Indeed, albumin was present in both sets of experiments measuring human and bovine PTH secretion, and this did not prevent detection of an alkalosis-induced suppression of PTH secretion. Thus, over the pathophysiologic pHo range, the effect of changing pHo on CaR activity appears greater than any potential effect on calcium buffering/displacement. Indeed, one study found that between pH 6.8 and 7.4, the calcium-binding affinity of albumin is not altered significantly, whereas at pH 8 the association constant increases 4-fold.23
Extracellular histidine residues account for proton sensitivity in several membrane proteins, including the anion exchange protein 2,24 ovarian cancer G protein–coupled receptor-125 and purinergic P2×4 receptor.26,27 Despite this, we found no evidence that any of the 16 extracellular histidine residues or the one free-cysteine residue (Cys-482) are individually responsible for CaR pHo sensitivity, at least over the pathophysiologic pHo range tested. Among members of the homologous family C GPCRs, mGluR4 is also inhibited by acidity and activated by alkalinity whereas mGluRs 1, 5 and 8 are pHo insensitive.28 However, no histamines are shared exclusively between CaR and mGluR4. Therefore, we conclude, by exclusion, that the most likely molecular mediators of CaR pHo sensitivity are extracellular clusters of aspartate and glutamate residues.3 That is, despite the low pKa values of Glu and Asp side chains in their free amino acid forms (around 4), when clustered, their pKa values may lie closer to the physiologic pH range (approximately 7).29,30 Finally, while we cannot rule out the possible contribution of other pHo-sensitive membrane proteins to these data, the pHo changes had no effect in cells lacking the CaR (not shown) or incubated in low Ca2+o concentration (Figure 2Bii).
In conclusion, the human CaR exhibits pHo sensitivity over the pathophysiologic range of pHo witnessed in vivo. This pHo sensitivity of the CaR exerts functionally significant effects on PTH secretion and thus might have wider relevance for whole-body calcium homeostasis and indeed for the secondary hyperparathyroidism of CKD.
Concise Methods
Cell Culture and Calcium-Sensing Receptor Functional Assays
HEK-293 cells, stably transfected with human parathyroid CaR, were cultured in DMEM (supplemented with 10% [v/v] FBS), loaded with Fura-2/AM and assayed for Ca2+i by dual-excitation wavelength microfluorometry in experimental buffer (20 mM HEPES, pH 7.4, 125 mM NaCl, 4 mM KCl, 1.2 mM CaCl2, 0.5 mM MgCl2, 5.5 mM glucose) as described previously.10 Intracellular pH was quantified similarly using the pH-sensitive fluorescent dye BCECF. CaR-induced ERK phosphorylation was detected by semi-quantitative immunoblotting using a phospho-specific polyclonal antibody9 against the lysates of cells solubilized in RIPA buffer supplemented with protease and phosphatase inhibitors. To assess actin stress fiber assembly, paraformaldehyde-fixed cells were stained with Phalloidin-TRITC and imaged by fluorescence microscopy.10
Site-Directed Mutagenesis
CaR mutations were introduced into the human CaR by QuikChange (Stratagene) site-directed mutagenesis, then transiently transfected into HEK-293 cells using FuGENE-6.
Parathyroid Gland Preparation and PTH Secretion Assay
Bovine parathyroid cells (abattoir-sourced) were obtained by collagenase-digestion9 and cultured on collagen-coated coverslips. Normal human parathyroid cells were obtained by collagenase-digestion after neck surgery9 (procedures performed under institutional ethical guidelines and with patients’ written informed consent). PTH levels were quantified by ELISA after perifusion with buffer containing 125 mM NaCl, 4 mM KCl, 1.25 mM CaCl2, 1 mM MgCl2, 0.8 mM Na2HPO4, 20 mM HEPES (pH 7.4, NaOH) supplemented with 0.1% D-glucose, 2.8 mM basal amino acid mixture (defined previously9), and 1 mg/ml BSA.
Statistical Analyses
Data are presented as means±SEM, and statistical significance was determined using GraphPad Prism software. For the complete methods, see the Supplemental Material.
Disclosures
None.
Acknowledgments
The authors would like to thank J&B Fitton and Dr. Richard Murray (University of Liverpool) for their help in obtaining bovine parathyroid glands and Professor Geoffrey Hendy (McGill University, Montreal, QC, Canada) for helpful discussions.
This work was supported by grants from the Australian National Health and Medical Research Council (APP1011922) and Kidney Research UK (IN4/2008). K.C. and W.M. were supported by Quota Studentships from the Biotechnology and Biological Sciences Research Council.
Portions of this work were presented in Abstract form at the World Congress of Nephrology in Milan and at the International Congress of Endocrinology in Florence.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014070653/-/DCSupplemental.
References
- 1.Brown EM: Role of the calcium-sensing receptor in extracellular calcium homeostasis. Best Pract Res Clin Endocrinol Metab 27: 333–343, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Tyler Miller R: Control of renal calcium, phosphate, electrolyte, and water excretion by the calcium-sensing receptor. Best Pract Res Clin Endocrinol Metab 27: 345–358, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Quinn SJ, Bai M, Brown EM: pH Sensing by the calcium-sensing receptor. J Biol Chem 279: 37241–37249, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Bichara M, Mercier O, Borensztein P, Paillard M: Acute metabolic acidosis enhances circulating parathyroid hormone, which contributes to the renal response against acidosis in the rat. J Clin Invest 86: 430–443, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Felsenfeld AJ, Rodríguez M, Aguilera-Tejero E: Dynamics of parathyroid hormone secretion in health and secondary hyperparathyroidism. Clin J Am Soc Nephrol 2: 1283–1305, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Lopez I, Aguilera-Tejero E, Felsenfeld AJ, Estepa JC, Rodriguez M: Direct effect of acute metabolic and respiratory acidosis on parathyroid hormone secretion in the dog. J Bone Miner Res 17: 1691–1700, 2002 [DOI] [PubMed] [Google Scholar]
- 7.López I, Aguilera-Tejero E, Estepa JC, Rodríguez M, Felsenfeld AJ: Role of acidosis-induced increases in calcium on PTH secretion in acute metabolic and respiratory acidosis in the dog. Am J Physiol Endocrinol Metab 286: E780–E785, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Lopez I, Rodriguez M, Felsenfeld AJ, Estepa JC, Aguilera-Tejero E: Direct suppressive effect of acute metabolic and respiratory alkalosis on parathyroid hormone secretion in the dog. J Bone Miner Res 18: 1478–1485, 2003 [DOI] [PubMed] [Google Scholar]
- 9.McCormick WD, Atkinson-Dell R, Campion KL, Mun HC, Conigrave AD, Ward DT: Increased receptor stimulation elicits differential calcium-sensing receptor(T888) dephosphorylation. J Biol Chem 285: 14170–14177, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies SL, Gibbons CE, Vizard T, Ward DT: Ca2+-sensing receptor induces Rho kinase-mediated actin stress fiber assembly and altered cell morphology, but not in response to aromatic amino acids. Am J Physiol Cell Physiol 290: C1543–C1551, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Kunishima N, Shimada Y, Tsuji Y, Sato T, Yamamoto M, Kumasaka T, Nakanishi S, Jingami H, Morikawa K: Structural basis of glutamate recognition by a dimeric metabotropic glutamate receptor. Nature 407: 971–977, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Muto T, Tsuchiya D, Morikawa K, Jingami H: Structures of the extracellular regions of the group II/III metabotropic glutamate receptors. Proc Natl Acad Sci U S A 104: 3759–3764, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang Y, Zhou Y, Castiblanco A, Yang W, Brown EM, Yang JJ: Multiple Ca(2+)-binding sites in the extracellular domain of the Ca(2+)-sensing receptor corresponding to cooperative Ca(2+) response. Biochemistry 48: 388–398, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang Y, Zhou Y, Yang W, Butters R, Lee HW, Li S, Castiblanco A, Brown EM, Yang JJ: Identification and dissection of Ca(2+)-binding sites in the extracellular domain of Ca(2+)-sensing receptor. J Biol Chem 282: 19000–19010, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doroszewicz J, Waldegger P, Jeck N, Seyberth H, Waldegger S: pH dependence of extracellular calcium sensing receptor activity determined by a novel technique. Kidney Int 67: 187–192, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Edwards SL: Pathophysiology of acid base balance: The theory practice relationship. Intensive Crit Care Nurs 24: 28–38, quiz 38–40, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Frassetto L, Sebastian A: Age and systemic acid-base equilibrium: Analysis of published data. J Gerontol A Biol Sci Med Sci 51: B91–B99, 1996 [DOI] [PubMed] [Google Scholar]
- 18.Sherman SS, Hollis BW, Tobin JD: Vitamin D status and related parameters in a healthy population: The effects of age, sex, and season. J Clin Endocrinol Metab 71: 405–413, 1990 [DOI] [PubMed] [Google Scholar]
- 19.Oka M, Ohtake T, Mochida Y, Ishioka K, Maesato K, Moriya H, Hidaka S, Kobayashi S: Correlation of coronary artery calcification with pre-hemodialysis bicarbonate levels in patients on hemodialysis. Ther Apher Dial 16: 267–271, 2012 [DOI] [PubMed] [Google Scholar]
- 20.de Brito-Ashurst I, Varagunam M, Raftery MJ, Yaqoob MM: Bicarbonate supplementation slows progression of CKD and improves nutritional status. J Am Soc Nephrol 20: 2075–2084, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krieger NS, Culbertson CD, Kyker-Snowman K, Bushinsky DA: Metabolic acidosis increases fibroblast growth factor 23 in neonatal mouse bone. Am J Physiol Renal Physiol 303: F431–F436, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang S, McDonnell EH, Sedor FA, Toffaletti JG: pH effects on measurements of ionized calcium and ionized magnesium in blood. Arch Pathol Lab Med 126: 947–950, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Kragh-Hansen U, Vorum H: Quantitative analyses of the interaction between calcium ions and human serum albumin. Clin Chem 39: 202–208, 1993 [PubMed] [Google Scholar]
- 24.Stewart AK, Kurschat CE, Burns D, Banger N, Vaughan-Jones RD, Alper SL: Transmembrane domain histidines contribute to regulation of AE2-mediated anion exchange by pH. Am J Physiol Cell Physiol 292: C909–C918, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Ludwig MG, Vanek M, Guerini D, Gasser JA, Jones CE, Junker U, Hofstetter H, Wolf RM, Seuwen K: Proton-sensing G-protein-coupled receptors. Nature 425: 93–98, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Clarke CE, Benham CD, Bridges A, George AR, Meadows HJ: Mutation of histidine 286 of the human P2X4 purinoceptor removes extracellular pH sensitivity. J Physiol 523: 697–703, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan P, Warwicker J: Evidence for the adaptation of protein pH-dependence to subcellular pH. BMC Biol 7: 69, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levinthal C, Barkdull L, Jacobson P, Storjohann L, Van Wagenen BC, Stormann TM, Hammerland LG: Modulation of group III metabotropic glutamate receptors by hydrogen ions. Pharmacology 83: 88–94, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Prod’hom B, Pietrobon D, Hess P: Interactions of protons with single open L-type calcium channels. Location of protonation site and dependence of proton-induced current fluctuations on concentration and species of permeant ion. J Gen Physiol 94: 23–42, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen XH, Tsien RW: Aspartate substitutions establish the concerted action of P-region glutamates in repeats I and III in forming the protonation site of L-type Ca2+ channels. J Biol Chem 272: 30002–30008, 1997 [DOI] [PubMed] [Google Scholar]