Abstract
Tamm-Horsfall protein (THP) is a glycoprotein uniquely expressed in the kidney. We recently showed an important role for THP in mediating tubular cross-talk in the outer medulla and in suppressing neutrophil infiltration after kidney injury. However, it remains unclear whether THP has a broader role in neutrophil homeostasis. In this study, we show that THP deficiency in mice increases the number of neutrophils, not only in the kidney but also in the circulation and in the liver, through enhanced granulopoiesis in the bone marrow. Using multiplex ELISA, we identified IL-17 as a key granulopoietic cytokine specifically upregulated in the kidneys but not in the liver of THP−/− mice. Indeed, neutralization of IL-17 in THP−/− mice completely reversed the systemic neutrophilia. Furthermore, IL-23 was also elevated in THP−/− kidneys. We performed real-time PCR on laser microdissected tubular segments and FACS-sorted renal immune cells and identified the S3 proximal segments, but not renal macrophages, as a major source of increased IL-23 synthesis. In conclusion, we show that THP deficiency stimulates proximal epithelial activation of the IL-23/IL-17 axis and systemic neutrophilia. Our findings provide evidence that the kidney epithelium in the outer medulla can regulate granulopoiesis. When this novel function is added to its known role in erythropoiesis, the kidney emerges as an important regulator of the hematopoietic system.
Keywords: chemokine, cytokines, immunology, pathology, renal injury
Tamm-Horsfall protein (THP, also known as Uromodulin) is a unique glycoprotein because it is exclusively expressed in the kidney, in tubular cells of the thick ascending limbs (TALs).1–3 Within TAL cells, THP is targeted predominantly to the apical membrane domain, cleaved proteolytically, and secreted in the urine. However, basolateral release of THP in the interstitium and circulation is also observed.1,4,5 The association of THP with acute and chronic forms of renal disease, such as familial juvenile hyperuricemic nephropathy, AKI, and CKD, argues for important regulatory functions of this glycoprotein in the pathogenesis of kidney disease.1,4,6–10 Interestingly, interstitial deposits of THP are frequently associated with tubulointerstitial diseases, suggesting a potential link between the interstitial presence of THP and inflammation.8,11–13 We recently provided challenging evidence supporting a role for THP in controlling neutrophil infiltration during kidney injury,2,5,10,14 and promoting recovery.5,14 The presence of THP released from TALs inhibits the production of cytokines and chemokines such as CXCL214 in injured neighboring proximal tubules (PTs), suggesting that THP mediates a regulatory cross-talk between TALs and PTs serving to suppress inflammation and neutrophil infiltration.2
The expression of THP and its level in the circulation are significantly decreased in various forms of kidney disease.2,5,15,16 For example, the expression of THP is significantly reduced at the peak of AKI.5,17,18 Furthermore, several studies have confirmed that advanced CKD is associated with decreased levels of THP in the urine and in the circulation,16,19,20 thereby creating a state of “relative THP deficiency.” Interestingly, in a murine knockout model, THP deficiency in a noninjured state was associated with a systemic increase in proinflammatory cytokines and chemokines.21 Therefore, these observations prompted us to examine the role of THP in regulating inflammation not only in the kidney, but also systemically. The possibility that THP regulates systemic inflammation could explain the generalized inflammatory phenotype and neutrophilia observed in “THP-deficient states,” such as what is reported with advanced CKD.22–25
This study was designed to investigate the effect of THP deficiency on systemic neutrophil homeostasis. We hypothesized that THP deficiency causes systemic neutrophilia through the production of growth factors by the kidney that stimulate granulopoiesis. We show, for the first time, that THP deficiency causes systemic neutrophilia, which is dependent on the renal activation of the IL-23/IL-17 axis. Surprisingly, our results underscore the importance of the proximal renal tubular epithelium in regulating systemic neutrophil homeostasis. Our novel findings expand the cross-talk between the kidney and bone marrow beyond the regulation of erythropoiesis, to also include the regulation of granulopoiesis.
Results
THP Deficiency Causes Renal and Systemic Neutrophilia
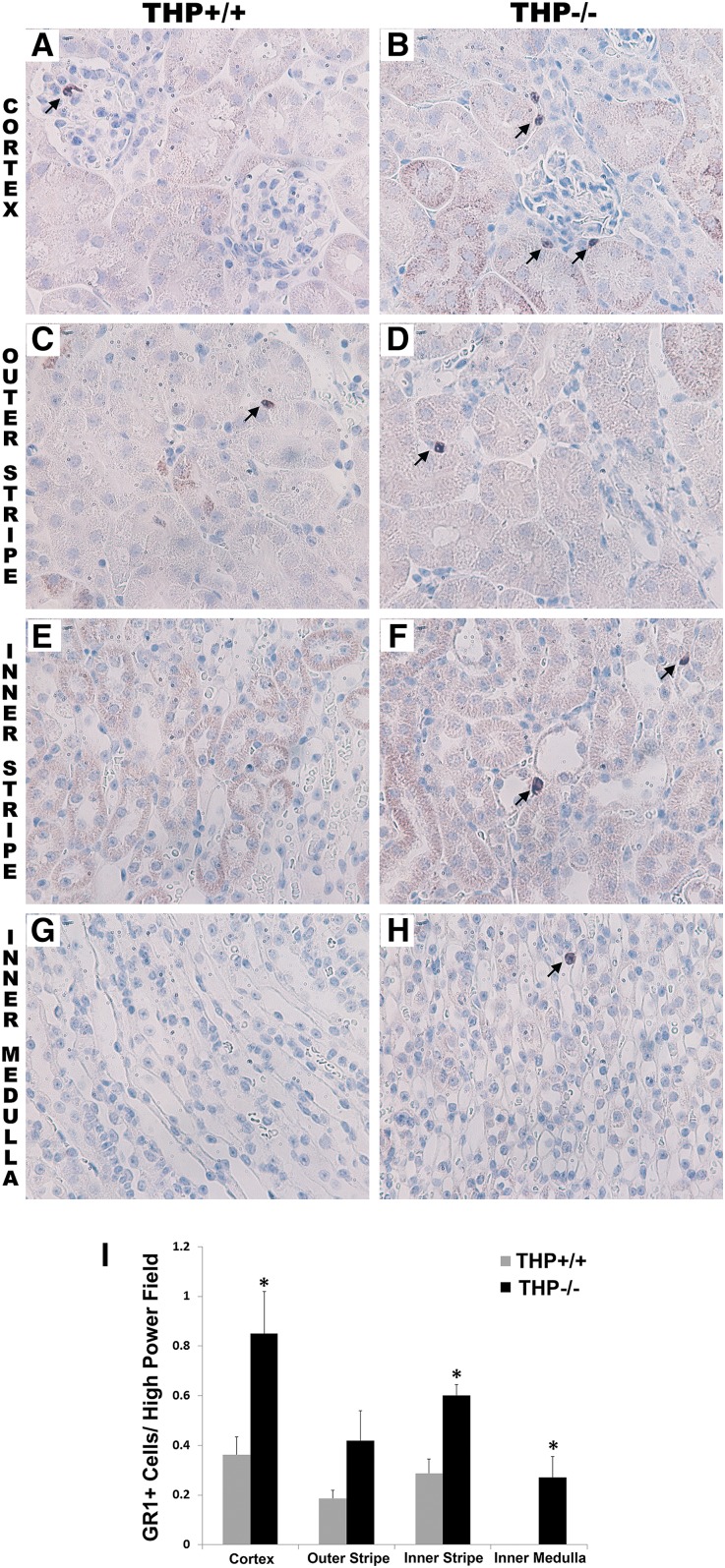
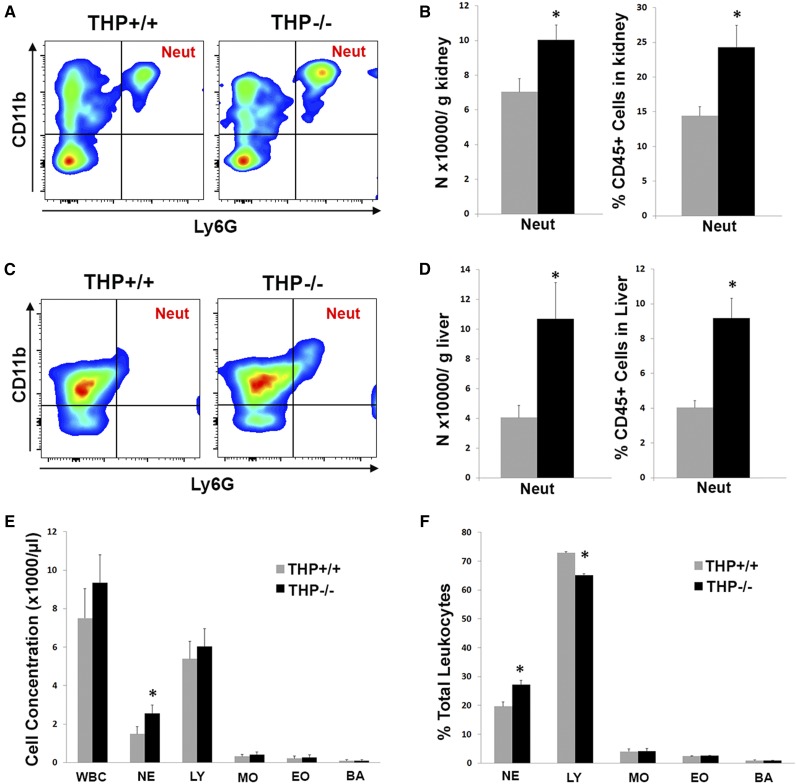
Immunohistochemistry for GR1 (a commonly used marker for neutrophils) in THP+/+ and THP−/− kidneys shows an increase in the number of GR1+ cells in THP−/− kidneys (Figure 1). This was verified by flow cytometry for neutrophils in the kidney (Figure 2, A and B), in which neutrophils were defined as CD45+, CD11b+, Ly6G+.26 It is important to note that Ly6G is part of the GR1 antigen complex, and is also considered a specific marker for neutrophils.26,27 The increased number of neutrophils was present not only in the kidney, but also in the liver of THP−/− mice (Figure 2, C and D) and in the spleen (Supplemental Figure 1). We subsequently analyzed peripheral blood counts in THP+/+ and THP−/− mice, and demonstrated that THP deficiency is associated with a significant increase in circulating neutrophil counts (Figure 2, E and F). Taken together, these results suggest that THP deficiency causes not only renal but also systemic neutrophilia that extends to other organs such as the liver.
Figure 1.
Immunohistochemistry of GR1 in THP+/+ and THP−/− kidney sections. (A–H) Representative images of sections (two sections/kidney, five kidneys per group) encompassing all areas within the kidney from THP+/+ and THP−/− mice. Arrows show GR1-stained cells in various areas within the kidney. (I) Quantitation of GR1+ cells in each renal zone. Bar graphs are means±SEM. Asterisks represents statistical significance between the two strains (P<0.05). Original magnification, ×60 objective.
Figure 2.
Peripheral and organ neutrophil analysis in THP+/+ and THP−/− mice. (A–D) Flow cytometry analysis of neutrophils in the kidney (A and B) and liver (C and D) from both strains of mice. A and C show representative scatter plots of CD45+ cells in the kidney and liver, respectively, gated for CD11b and Ly6G. Quantitation of neutrophils (defined as CD45+, CD11b+, Ly6G+) in the kidney and liver are shown in B and D, respectively (n=5 per group). Asterisks denote statistical significance between THP+/+ and THP−/− (P<0.05). (E and F) Bar graphs are means±SEM of peripheral white blood cell count and its subtypes from THP+/+ and THP−/− mice (n=8 per group). E depicts cell concentration in blood, whereas F shows the percentage of each cell type within the total white blood cell count. A significant increase in neutrophils is noted in THP−/− versus THP+/+ (*P<0.05). Neut, neutrophil; WBC, white blood cell count; NE, neutrophil; LY, lymphocyte; MO, monocyte; EO, eosinophil; BA, basophil.
THP Deficiency Causes Enhanced Granulopoiesis in the Bone Marrow
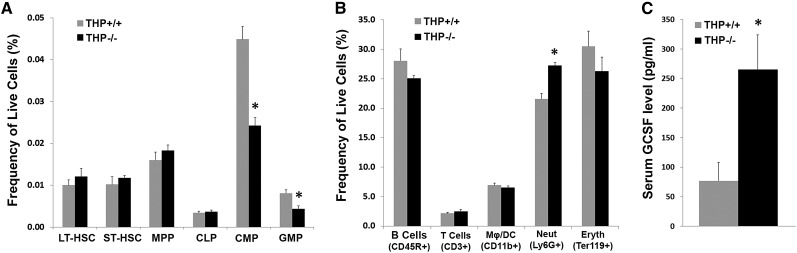
To verify whether the generalized systemic neutrophilia in the periphery and within organs was due to increased granulopoiesis in the bone marrow, we performed detailed analysis of bone marrow from THP+/+ and THP−/− mice using flow cytometry as described28–30 and shown in Figure 3, Supplemental Figure 2. Figure 3A shows a significant decrease in the marrow of THP−/− mice of two classes of granulocyte progenitor cells: common myeloid progenitors and granulocyte-macrophage progenitors. In parallel, there was a significant increase in the number of differentiated neutrophils expressing Ly6G (Figure 3B). The increased neutrophil count in the bone marrow was also verified using an automated hematology analyzer (Supplemental Figure 3). Taken together, our data provide strong evidence of enhanced granulopoiesis (decreased progenitors in the marrow, increased circulating differentiated cells), which explains the generalized neutrophilia observed in THP−/− mice. Because enhanced granulopoiesis is strongly dependent on the presence of granulocyte colony-stimulating factor (G-CSF), we verified that THP−/− mice had an increased level of G-CSF in the serum compared with THP+/+ mice (Figure 3C).
Figure 3.
Bone marrow analysis by flow cytometry. (A and B) Quantitation of progenitor cells within bone marrow (A) and quantitation of bone marrow cells with differentiation markers (B), as described in the Concise Methods, from THP+/+ and THP−/− mice (n=5 each group). The Mϕ/DC population shown is CD11b+ and Ly6G−. The decrease in granulocyte progenitors and increase in neutrophils strongly support enhanced granulopoiesis. (C) ELISA assay for G-CSF, performed on sera from THP+/+ and THP−/− mice (n=5 each group). Asterisk denotes statistical significance between the two strains. LT-HSC, long-term hematopoietic stem cell; ST-HSC, short-term hematopoietic stem cell; MPP, multipotent progenitor; CLP, common lymphoid progenitor; CMP, common myeloid progenitor; GMP, granulocyte-macrophage progenitor.
THP-Deficient Kidneys Are an Important Source of Increased IL-17
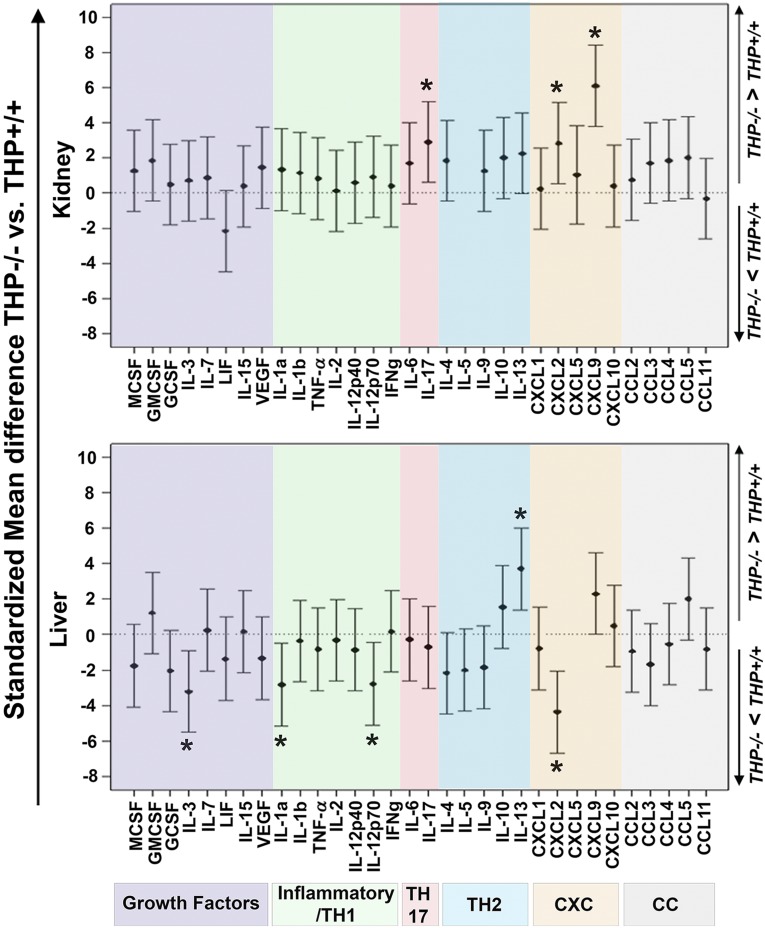
Because THP is uniquely produced in the kidney, we hypothesized that its deficiency causes the kidney to secrete growth factors or cytokines that stimulate granulopoiesis. Therefore, we performed a cytokine/chemokine multiplex ELISA (32 analytes) on kidney extracts from THP−/− and THP+/+ mice. We also performed the same assay on the liver from both mice strains. Figure 4 shows that kidneys from THP−/− mice have a significant increase in the level of IL-17, CXCL-2, and CXCL-9 compared with THP+/+ kidneys. Interestingly, livers extracts from THP−/− mice did not show an increase in any of the progranulocytic factors tested (including IL-17) compared with THP+/+ livers (Figure 4, lower panel). These data underscore the importance of THP in regulating the production of select cytokines/chemokines in the kidney, notably IL-17.
Figure 4.
Cytokine/chemokine multiplex ELISA of the kidneys and livers in THP−/− versus THP+/+. Graphs show standardized mean differences for each analyte between THP−/− and THP+/+ for kidneys (upper, n=5 each group) and liver (lower, n=5 each group). Analytes are grouped based on their biologic function. Asterisk denotes statistical significance between THP−/− and THP+/+. GMCSF, granulocyte macrophage colony stimulating factor; LIF, leukemia inhibitory factor; MCSF, macrophage colony stimulating factor; TNF, tumor necrosis factor-α; VEGF, vascular endothelial growth factor. IL-5 and CXCL5 were undetectable in all the kidneys and livers, respectively.
Increased IL-17 in THP−/− Kidneys Is a Major Determinant of Systemic Neutrophilia
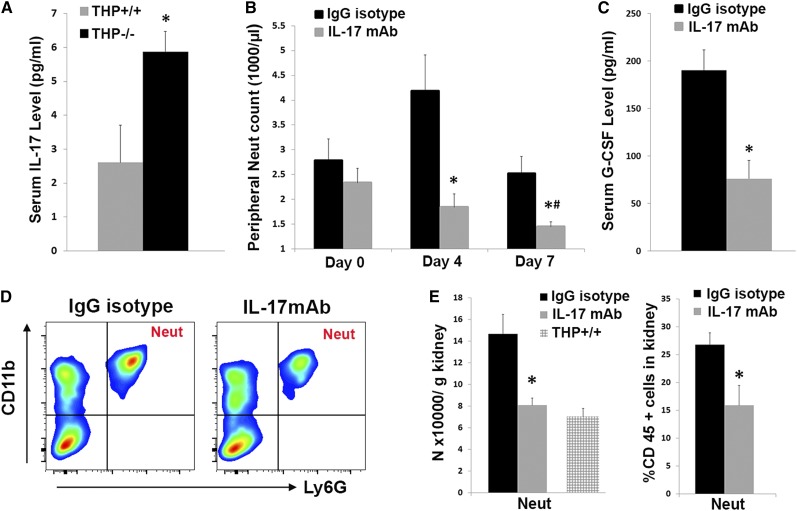
Among the factors differentially increased in THP−/− kidneys, IL-17 is known to be an important activator of granulopoiesis, and it is part of the IL-23/IL-17 axis.31–33 In this well recognized system, the cascade is induced by IL-23, which stimulates specialized T cells to produce IL-17.31,34 In turn, IL-17 can stimulate granulopoiesis by causing a systemic increase in G-CSF.31,35 Using ELISA, we showed that THP−/− mice have increased serum IL-17 levels compared with THP+/+ mice (Figure 5A). To determine the source of increased IL-17 in the kidney, we performed flow cytometry for IL-17 (Supplemental Figure 4). Although the distribution of IL-17+, CD3+ cells (T cells) was comparable between THP+/+ and THP−/− kidneys, we did detect a small increase in IL-17+ neutrophils in THP−/−, which could suggest a potential contribution of these cells to increased IL-17 levels.
Figure 5.
Effect of IL-17 neutralization on systemic neutrophilia in THP−/− mice. (A) Bar graphs are means±SEM of the IL-17 level in sera from THP+/+ and THP−/− mice (n=5 per group) measured with ELISA. (B) Neutrophil counts in THP−/− mice injected with IL-17 neutralizing antibody or IgG isotype at various time points during treatment (n=5 each group). (C) Serum G-CSF on day 7 after IL-17 neutralization or treatment with IgG isotype. (D and E) Flow cytometry of the kidneys from both groups of mice probing specifically for neutrophils. Representative scatter plots from each group are presented in D, whereas E shows the quantitation of neutrophils shown as bar graphs±SEM. The wild-type bar in E is derived from the data presented in Figure 2, and is shown for comparison. IL-17 neutralization in THP−/− significantly reduced serum G-CSF levels, neutrophilia in the periphery and within the kidney. Asterisk denotes statistical significance compared to IgG controls, whereas the symbol # denotes significance compared to Day 0 (P<0.05). Neut, neutrophil.
To verify that increased IL-17 is a major determinant of granulopoiesis and systemic neutrophilia, we neutralized IL-17 in vivo in THP−/− mice with an anti–IL-17 mAb. As shown in Figure 5, IL-17 neutralization significantly reversed the peripheral (Figure 5B) and renal neutrophilia (Figure 5, D and E) in THP−/− mice (neutrophil levels fell to the range seen in THP+/+ mice). Furthermore, serum G-CSF levels were significantly decreased by IL-17 neutralization (Figure 5C). Taken together, these data support the concept that increased IL-17 release from THP−/− kidneys is a major determinant of systemic neutrophilia through enhanced granulopoiesis.
THP Regulates the Renal IL-23/IL-17 Axis and the Production of IL-23 in S3 Epithelial Segments
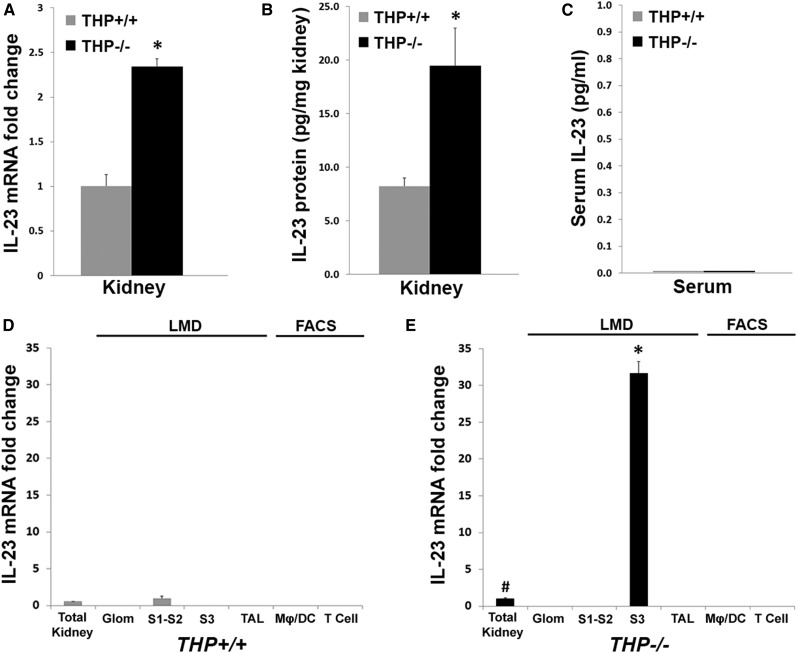
To determine whether the IL-17 surge in THP−/− kidneys is due to increased production of IL-23, we measured IL-23 mRNA and protein in THP−/− and THP+/+ kidneys using real-time PCR and ELISA, respectively. Figure 6, A and B, shows a significant increase in IL-23 mRNA and protein in THP−/− versus THP+/+ kidneys, respectively. These findings suggest that activation of the IL-23/IL-17 axis in the kidney is regulated by THP. Interestingly, we could not detect IL-23 in the serum in either strains of mice (Figure 6C), which could imply that induction of IL-23 is limited to the kidney and does not extend systemically.
Figure 6.
Identification of the source of IL-23 synthesis in kidney using LMD and FACS. (A) IL-23mRNA measurements using real-time PCR in THP+/+ (reference set as 1) and THP−/− total kidney extracts (n=5 per group). (B and C) IL-23 protein in the kidney and in the serum using ELISA, respectively. Asterisks in A and B denote statistical significance between THP−/− and THP+/+ (P<0.05). IL-23 could not be detected in the serum of either mice strain. (D and E) IL-23 mRNA in specific cell types derived from THP+/+ and THP−/− kidneys. Glomeruli, S1–S2, S3, and TAL segments were dissected using LMD. Mϕs/DCs and T cells were obtained using FACS. Total kidney from THP−/− was used as reference sample (set as 1) in all experiments shown in D and E. The # symbol denotes statistical significance versus THP+/+ total kidney, whereas the asterisk in E denotes significance compared with THP−/− total kidney (P<0.05).
We next sought to determine the source of increased IL-23 in THP−/− kidneys. Studies in nonkidney tissues suggested that inflammatory cells such as activated macrophages and possibly T cells are important producers of IL-23.36,37 Therefore, we performed real-time PCR probing for IL-23 on RNA extracted from glomeruli, S1–S2 and S3 proximal tubular segments, TAL cells, macrophages/dendritic cells (Mϕs/DCs), and T cells as shown in Figure 6, D and E. Glomeruli, S1–S2, S3, and TAL segments were isolated using laser microdissection (LMD), shown in Supplemental Figures 5 and 6. FACS analysis was used to isolate Mϕs/DCs and T cells using the schema described in Supplemental Figure 7.
Figure 6D shows that in THP+/+ kidneys, IL-23 mRNA was only detected in S1–S2 segments, albeit at a very low level. In THP−/− kidneys (Figure 6E), IL-23 mRNA was significantly upregulated, and was uniquely detected in S3 segments but not in Mϕs/DCs, T cells, glomeruli, S1–S2 segments, or TAL segments. Therefore, our data show that the proximal tubular epithelium is a major source of IL-23 synthesis in the kidney. In addition, S3 segments appear to be the key activator of the IL-23/IL-17 axis during THP deficiency.
Discussion
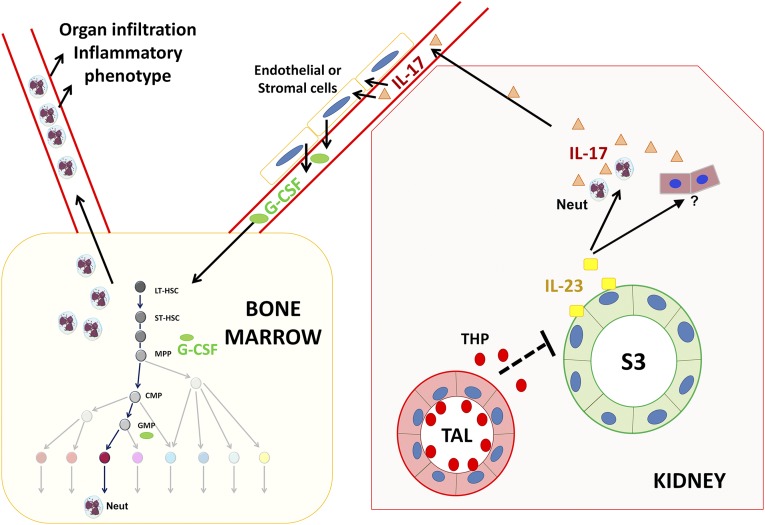
In this study, we investigated the role of THP on systemic neutrophil homeostasis. We show that THP deficiency causes systemic neutrophilia, which is most likely the result of a dysregulated increase in renal IL-23/IL-17. We subsequently show that the S3 tubular epithelium is the source of increased IL-23. To our knowledge, this is the first study showing that the kidney regulates granulopoiesis and neutrophil homeostasis. Therefore, our study expands the key role of the kidney in regulating the hematopoietic system to include not only erythropoiesis through the production of erythropoietin,38 but also granulopoiesis through THP-dependent regulation of the IL-23/IL-17 axis. Figure 7 is a summary illustration outlying the mechanism of THP-regulated granulopoiesis, on the basis of the current data.
Figure 7.
Summary diagram of THP-regulated granulopoiesis. In the kidney, interstitial THP released by the TAL exerts an inhibitory effect on the synthesis of IL-23 by S3 proximal tubular segments in the outer medulla. In the context on THP deficiency, IL-23 is upregulated by S3 segments and released within the kidney to act on neutrophils (Neut) and possibly other cells within the kidney to induce IL-17. IL-17 is subsequently released into the systemic circulation, where it can stimulate the production of G-CSF. G-CSF acts within the bone marrow to stimulate granulopoiesis and systemic release of excess neutrophils. LT-HSC, long-term hematopoietic stem cell; ST-HSC, short-term hematopoietic stem cell; MPP, multipotent progenitor; CMP, common myeloid progenitor; GMP, granulocyte-macrophage progenitor.
The finding that THP deficiency causes systemic neutrophilia, even without injury is remarkable, and agrees with previous findings by Liu et al. that THP deficiency causes a systemic proinflammatory phenotype and splenomegaly.21 To determine whether the kidney is the source of a progranulopoetic factor in the setting of THP deficiency, we used an unbiased approach with multiplex ELISA for 32 preset cytokines/chemokines. Comparing the kidney to the liver enabled us to determine that the kidney specifically has an increased level of IL-17, which is a known activator of granulopoiesis.31,32 The fact that IL-17 is increased in the kidney and the serum, but not in the liver, in THP−/− mice strongly supports that the kidney itself is an important source of IL-17. The key role of IL-17 in stimulating granulopoiesis and neutrophilia was then confirmed by in vivo neutralization. Although IL-1β in conjunction with IL-23 have been reported to stimulate IL-17 production,39 there was no differential increase in IL-1β in THP−/− kidneys, suggesting that it does not play a significant role in inducing IL-17 and granulopoiesis. The increase in CXCL9 (in conjunction with IL-17) observed in THP−/− kidneys is consistent with recent findings by Paust and colleagues that IL-17 stimulates the expression of CXCL9.40 The fact that a few proinflammatory cytokines/chemokines were decreased in THP−/− liver (e.g., CXCL2, IL-3, IL-1α, and IL-12p70) could be reactive to the observed neutrophilia, and the systemic inflammatory environment induced by THP−/− kidneys.
We attempted to verify the source of IL-17 in THP−/− kidneys. Unfortunately, we could not reliably amplify IL-17 mRNA in the kidney (using two different primers; Supplemental Table 1) despite consistent detection of the protein using ELISA. This could be due to the extremely short t1/2 and/or low abundance of IL-17 mRNA. Previous studies showed that few populations of T cells are major sources of IL-17 in experimental GN.40,41 This is consistent with findings from other tissues with chronic inflammation.33 Li et al. also previously showed that neutrophils are a significant source of IL-17 during kidney injury.42 Our flow cytometry studies suggest that increased IL-17+ neutrophils could be a potential source for increased IL-17 in THP−/− kidneys. At this time, we cannot completely rule out additional sources of increased IL-17 in THP−/− kidneys such as parenchymal or stromal cells,43 and this is currently the topic ongoing investigations in the laboratory.
IL-17 is downstream from IL-23 in the well defined IL-23/IL-17 axis.31–34,41,42 We showed an increased level of IL-23 mRNA and protein in the THP−/− kidney, which supports that activation of renal IL-23/IL-17 is an important determinant of the observed neutrophilia in THP−/− mice. Interestingly, it is thought that kidney Mϕs/DCs are the major source of IL-23.41 Furthermore, the site of IL-23 production could depend on the disease model.41 Using the combination of LMD and FACS to isolate specific epithelial and immune cells in the kidney, we show that PTs are a major source of IL-23 compared with renal Mϕs/DCs, T cells, glomeruli, or TALs. To our knowledge, this is the first study to clearly define a major source of IL-23 production in the kidney using this powerful approach. Our findings underscore the importance of proximal tubular epithelium in regulating the IL-23/IL-17 axis in the kidney. The fact that THP deficiency markedly induces IL-23 in S3 segments emphasizes the role of THP as a major modulator of IL-23 synthesis and fits with our previous findings that THP is an important mediator of tubular cross-talk in the outer medulla.2,5,14 Our current data expand the importance of THP-S3 interaction beyond the kidney, and suggest that the kidney outer medulla is an important regulator of systemic neutrophil homeostasis.
The current data also support our previous conclusions that THP−/− kidneys are more prone to upregulation of CXCL2 (MIP-2 chemokine, neutrophil chemoattractant) during AKI.10,14 In fact, we report in this work that THP−/− kidneys have increased CXCL2 even at baseline. Collectively, results from this and previous studies will advance our understanding of the pathogenesis of neutrophil infiltration during AKI, especially because a state of relative THP deficiency in wild-type kidneys is consistently observed in the early stages of AKI.5,17,18 We propose that this relative THP deficiency at the onset of AKI exquisitely sensitizes the kidney to neutrophil infiltration by enhancing granulopoiesis, systemic neutrophilia and increasing renal CXCL2. The increased expression of THP that we reported during kidney recovery5 could therefore be essential to halt the influx of neutrophils to the kidney by a dual action on the bone marrow (decreasing production) and on the kidney (decreased chemotaxis). It is worth mentioning that previous reports have described a proinflammatory role for THP.44,45 These studies were based predominantly on in vitro or ex vivo data with highly aggregated, and potentially highly immunogenic,46 urinary THP.2 To this date, there is no evidence to suggest that interstitial THP exists in the same highly aggregated form.3 Our in vivo studies with THP−/− mice demonstrate that THP has an anti-inflammatory role, which could reflect the net outcome of the complex interactions of THP with various cells types within the kidney interstitium, especially a counterinflammatory effect on epithelial cells.2,5,14
This study may have clinical implications well beyond AKI. Several studies showed that THP levels in the urine and in the serum decrease with advanced CKD and tubular atrophy,16,19,20 which is most likely due to decreased expression by the kidney, as was shown recently by Ledo et al.47 Interestingly, recent data from clinical studies also demonstrate that CKD is associated with development of neutrophilia, an increased neutrophil to lymphocyte ratio, and other markers of systemic inflammation.22,24,25 We propose that the relative THP deficiency occurring in kidneys with CKD activates the IL-23/IL-17 axis, which in turn stimulates granulopoiesis and a systemic inflammatory phenotype. Our study also underscores the need for large-scale clinical studies to better define the dynamic changes in THP levels during the progression of kidney disease.
In conclusion, we show that THP deficiency stimulates proximal epithelial activation of the IL-23/IL-17 axis and systemic neutrophilia. Our findings provide novel insights on how the kidney, through uniquely producing THP, can regulate granulopoiesis in the bone marrow and systemic neutrophil homeostasis.
Concise Methods
Mice
Animal experiments and protocols were approved by the Indianapolis Veterans Affairs Animal Care and Use Committee. Age-matched 8- to 12-week-old THP knockout animals (129/SvEv THP−/−) and wild-type background strain were used as previously described,5,10,48,49 and maintained in a specific pathogen-free facility. Neutralization of IL-17 was done using a rat mAb (MAB421; R&D Systems) injected daily at 50 µg/mouse for 6 days. Isotype IgG2a (14-4321-85; eBioscience) was used as control. Complete blood count analysis was performed on 50 µl of heparinized blood using a Hemavet 950FS analyzer.
Immunohistochemical Analyses
For GR-1 staining (ab2557; Abcam, Inc.), 4% paraformaldehyde perfusion–fixed tissues were subsequently embedded in paraffin and processed for standard histochemistry. Negative control without primary antibody was used. Cells were counted on high (×60 objective) power fields, using five fields from each kidney area per slide (two slides per kidney, and five kidneys each group).
Flow Cytometry on Kidneys, Livers, and Spleens
Flow cytometry was performed on homogenized, digested tissues as previously described.50 In brief, kidneys or livers were homogenized and digested with collagenase, and subsequently strained using 70-μm filters. Spleens were minced between two frosted slides and strained. Cell counts and viability were done using a Countess (Life Technologies) automated system. Then, 5×106 cells from each sample were used for staining after blocking nonspecific binding with anti-CD16/CD32 (14-0161-82; eBioscience). Flow cytometry was done using a BD LSRII flow cytometer. For absolute cell counting, we used a dual platform approach in which the percentage of cells of interest, out of total live cells, was determined by flow cytometry. Live cell concentration per specimen was determined with the Countess automated counter as described above. To account for tissue weight variability, we normalized by the weight of the corresponding tissue.
We used the following anti-mouse fluorochrome-conjugated mAbs: CD45 (130-091-610; Miltenyi Biotech), Ly6G (560600; BD Bioscience), CD11b (48-0112-80; eBioscience), and propidium iodide for viability. Of CD45+ cells within the kidney and liver, neutrophils were defined as CD45+, CD11b+, Ly6G+. FlowJo software (version 10; FlowJo LLC) was used for flow analysis and plotting.
Flow cytometry for IL-17 was done on kidney cells that underwent ex vivo stimulation for 4 hours with Cell Stimulation Cocktail (plus protein transport inhibitors) (00-4975; eBioscience), as described by others.41,42 Experiments without cell stimulation did not give a reliable IL-17 signal (data not shown). Cells were stained for fixable viability (65-0864-14; eBioscience), CD45, CD11b, Ly6G, and CD3 (48-0032; eBioscience) before undergoing intracellular permeabilization/fixation (88-8832; eBioscience) and staining for IL-17 (12-7177-81; eBioscience).
Flow Cytometry on Bone Marrow
Low-density bone marrow cells from both THP+/+ and THP−/− mice were washed once with stain wash (PBS, 1% bovine calf serum, and 1% penicillin-streptomycin), followed by antibody staining for 15 minutes on ice with fluorochrome-conjugated mAbs against the following markers: (1) c-Kit (CD117), Sca-1 (CD34), Flk2 (CD135), IL7Ra (CD127), and Fc-γR (CD16/32) for progenitor cells; and (2) CD3 (T cells), CD11b (myeloid), CD45R (B cells), Ter119 (erythroid), and Ly6G (neutrophil) for differentiated cells. All mAbs were obtained from BD Biosciences, except that CD127 was from eBioscience and anti–Fc-γR was from Biolegend. Cells were washed one more time with stain wash buffer, and data were acquired on BD LSRII (BD Biosciences). Progenitor cell content was gated and analyzed (Supplemental Figure 2) as previously described,29,30 according to the following definitions: LSK (Lin− Sca1+ c-Kit+), long-term hematopoietic stem cell (Lin− Sca1+ c-Kit+ CD34− CD135−), multipotent progenitor (Lin− Sca1+ c-Kit+ CD34+ CD135+), short-term hematopoietic stem cell (Lin− Sca1+ c-Kit+ CD34+ CD135−), common lymphoid progenitor (Lin− Sca1lo CD117lo CD135hi CD127+), common myeloid progenitor (Lin− Sca1− CD117+ CD34+ CD16/32lo), and granulocyte-macrophage progenitor (Lin− Sca1− CD117+ CD34+ CD16/32hi).
LMD
Sections from each kidney were snap frozen in optimum cutting temperature medium using dry ice and kept at −80°C until use. They were subsequently cut using a microtome at 8-µm sections on Leica polyphenylene sulfate membrane slides (catalog no. 11505268). Histochemical staining was performed immediately before dissection and consisted of a series of rapid sequences of the following: (1) 100% EtOH for 1 minute; (2) 95% EtOH for 30 seconds; (3) 75% EtOH for 30 seconds; (4) 50% EtOH for 30 seconds; (5) water 1, 30 seconds; (6) water 2, 30 seconds; (7) Histogene Staining Solution (12241-05; Life Technologies) 100 μl for 40 seconds; (8) water 3, 30 seconds; (9) 75% EtOH for 30 seconds; (10) 95% EtOH for 30 seconds; (11) 100% EtOH for 30 seconds; and (12) Xylene 90 seconds. Sections were air dried and immediately taken to a Leica LMD6000 microscope. Dissection was performed at ×40 magnification. We dissected 40–60 S3 segments and 80–100 TAL tubules from each section. Three sections were dissected from each kidney/mouse.
For dissection of glomeruli and S1–S2 segments, we used immunofluorescence LMD, using the following sequence of staining: (1) 100% EtOH for 30 seconds×2; (2) 95% EtOH for 20 seconds×2; (3) 75% EtOH for 20 seconds×2; (4) 50% EtOH for 20 seconds×2; (5) water 1, 30 seconds×2; (6) water 2, 30 seconds×2; (7) staining with FITC-phalloidin plus 4′,6-diamidino-2-phenylindole (molecular probes) in PBS plus 2%BSA for 3–5 minutes; (8) phosphate buffered water wash for 30 seconds×3; and (9) air dry for 5 minutes.
RNA was extracted using PicoPure RNA kit (12204-01; Life Technologies). Because of the finite RNA yield, RNA was pooled for each group (n=3 mice per group). An additional concentration step was performed using standard isopropanol precipitation, before RT and real-time PCR. The purity of RNA was verified using established markers for each tubular segment within the nephron (Supplemental Figures 5 and 6).51,52 RNA from total kidney extracts was used as a positive control for all markers. To validate our methodology with additional controls (Supplemental Figure 8), we verified that IL-1β was exclusively expressed in Mϕ/DCs,44 whereas macrophage colony stimulating factor (known to be expressed in myeloid and renal epithelium26,53) was detected in S3 segments, TALs, and Mϕs/DCs.
FACS Analyses
FACS was performed using BD FACSAria using the schema shown in Supplemental Figure 7. Cells were isolated from kidneys similar to what was described in the flow cytometry section, and were subsequently enriched in leukocytes using Lympholyte M (Cedarlane) gradient followed by magnetic beads directed enrichment of CD45+ cells using MACS Separation Columns (130-042-201; Miltenyi Biotech). To maximize yield, kidneys were pooled from eight mice. Mϕs/DCs were defined as CD45+, CD11b+, Ly6G−. T cells were defined as CD45+, CD11b−, CD3+, B220−. The following additional anti-mouse–conjugated mAbs were used: CD3 (48-0032; eBioscience) and B220 (17-0452; eBioscience). mRNA extraction from recovered cells was performed using the PicoPure RNA kit from Life Technologies.
ELISA Assays
A multiplex cytokine/chemokine assay containing 32 prespecified analytes (MCYTOMAG-70K-PX32; EMD Millipore) was performed on kidney and liver lysates from THP+/+ and THP−/− kidneys (n=5 per group). Values were initially reported in picograms per milligram protein/tissue after normalization to the protein concentration for each tissue. Statistical analysis is described below. In separate experiments, ELISA for IL-17 and G-CSF (MCYTOMAG-70K; EMD Millipore) on sera from the two strains of mice (n=5 per group) were also performed. We also performed IL-23 ELISA (MCYTOMAG-74K-01) on kidney tissue extracts and sera from the two strains.
Real-Time PCR
Real-time PCR was performed as previously described5 in an Applied Biosystems ViiA7 system using TaqMan Gene Expression Assays (all from Applied Biosystems; Supplemental Table 1). All expression was normalized to gluconate dehydrogenase endogenous control and reported as fold change compared with control using the ΔΔ threshold cycle method, according to the manufacturer’s instructions.
Statistical Analyses
Values of each experimental group are reported as means±SEM. For most experiments, a two tailed t test was used to examine the difference in means for continuous data. A nested ANOVA design was used for quantitation of GR1+ cells in immunohistochemistry. Statistical significance was determined at the 0.05 significance level. For ELISA multiplex analysis, two-sample t tests were used to compare the value for each test between the two experimental groups. The mean difference for each test value between THP−/− versus THP+/+ and its 95% confidence interval were then calculated. Because each test was measured in different metrics, the values were standardized by using the raw test value divided by its SEM. The standardized test difference and 95% confidence interval between the two strains for all the tests are presented in Figure 4 for kidneys and liver separately.
Disclosures
None.
Acknowledgments
We acknowledge the Bioplex, Pathology, and Flow Cytometry cores at Indiana University School of Medicine for assistance with this project, and we thank the Koman Tissue Bank for assistance with LMD. We also acknowledge Dr. Timothy Sutton for his critical review of this article.
This work was supported by a US Department of Veterans Affairs merit award to T.M.E.-A.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014070664/-/DCSupplemental.
References
- 1.Bachmann S, Koeppen-Hagemann I, Kriz W: Ultrastructural localization of Tamm-Horsfall glycoprotein (THP) in rat kidney as revealed by protein A-gold immunocytochemistry. Histochemistry 83: 531–538, 1985 [DOI] [PubMed] [Google Scholar]
- 2.El-Achkar TM, Wu XR: Uromodulin in kidney injury: An instigator, bystander, or protector? Am J Kidney Dis 59: 452–461, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rampoldi L, Scolari F, Amoroso A, Ghiggeri G, Devuyst O: The rediscovery of uromodulin (Tamm-Horsfall protein): From tubulointerstitial nephropathy to chronic kidney disease. Kidney Int 80: 338–347, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Jennings P, Aydin S, Kotanko P, Lechner J, Lhotta K, Williams S, Thakker RV, Pfaller W: Membrane targeting and secretion of mutant uromodulin in familial juvenile hyperuricemic nephropathy. J Am Soc Nephrol 18: 264–273, 2007 [DOI] [PubMed] [Google Scholar]
- 5.El-Achkar TM, McCracken R, Liu Y, Heitmeier MR, Bourgeois S, Ryerse J, Wu XR: Tamm-Horsfall protein translocates to the basolateral domain of thick ascending limbs, interstitium, and circulation during recovery from acute kidney injury. Am J Physiol Renal Physiol 304: F1066–F1075, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Howie AJ, Brewer DB: Extra-tubular deposits of Tamm-Horsfall protein in renal allografts. J Pathol 139: 193–206, 1983 [PubMed] [Google Scholar]
- 7.Serafini-Cessi F, Malagolini N, Cavallone D: Tamm-Horsfall glycoprotein: Biology and clinical relevance. Am J Kidney Dis 42: 658–676, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Zager RA, Cotran RS, Hoyer JR: Pathologic localization of Tamm-Horsfall protein in interstitial deposits in renal disease. Lab Invest 38: 52–57, 1978 [PubMed] [Google Scholar]
- 9.Köttgen A, Glazer NL, Dehghan A, Hwang SJ, Katz R, Li M, Yang Q, Gudnason V, Launer LJ, Harris TB, Smith AV, Arking DE, Astor BC, Boerwinkle E, Ehret GB, Ruczinski I, Scharpf RB, Chen YD, de Boer IH, Haritunians T, Lumley T, Sarnak M, Siscovick D, Benjamin EJ, Levy D, Upadhyay A, Aulchenko YS, Hofman A, Rivadeneira F, Uitterlinden AG, van Duijn CM, Chasman DI, Paré G, Ridker PM, Kao WH, Witteman JC, Coresh J, Shlipak MG, Fox CS: Multiple loci associated with indices of renal function and chronic kidney disease. Nat Genet 41: 712–717, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El-Achkar TM, Wu XR, Rauchman M, McCracken R, Kiefer S, Dagher PC: Tamm-Horsfall protein protects the kidney from ischemic injury by decreasing inflammation and altering TLR4 expression. Am J Physiol Renal Physiol 295: F534–F544, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel R, McKenzie JK, McQueen EG: Tamm-Horsfall urinary mucoprotein and tubular obstruction by casts in acute renal failure. Lancet 1: 457–461, 1964 [DOI] [PubMed] [Google Scholar]
- 12.Resnick JS, Sisson S, Vernier RL: Tamm-Horsfall protein. Abnormal localization in renal disease. Lab Invest 38: 550–555, 1978 [PubMed] [Google Scholar]
- 13.Cotran RS, Galvanek E: Immunopathology of human tubulo-interstitial diseases: Localization of immunoglobulins complement and Tamm-Horsfall protein. Contrib Nephrol 16: 126–131, 1979 [DOI] [PubMed] [Google Scholar]
- 14.El-Achkar TM, McCracken R, Rauchman M, Heitmeier MR, Al-Aly Z, Dagher PC, Wu XR: Tamm-Horsfall protein-deficient thick ascending limbs promote injury to neighboring S3 segments in an MIP-2-dependent mechanism. Am J Physiol Renal Physiol 300: F999–F1007, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Köttgen A, Hwang SJ, Larson MG, Van Eyk JE, Fu Q, Benjamin EJ, Dehghan A, Glazer NL, Kao WH, Harris TB, Gudnason V, Shlipak MG, Yang Q, Coresh J, Levy D, Fox CS: Uromodulin levels associate with a common UMOD variant and risk for incident CKD. J Am Soc Nephrol 21: 337–344, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thornley C, Dawnay A, Cattell WR: Human Tamm-Horsfall glycoprotein: Urinary and plasma levels in normal subjects and patients with renal disease determined by a fully validated radioimmunoassay. Clin Sci (Lond) 68: 529–535, 1985 [DOI] [PubMed] [Google Scholar]
- 17.Safirstein R: Gene expression in nephrotoxic and ischemic acute renal failure. J Am Soc Nephrol 4: 1387–1395, 1994 [DOI] [PubMed] [Google Scholar]
- 18.Safirstein R, Megyesi J, Saggi SJ, Price PM, Poon M, Rollins BJ, Taubman MB: Expression of cytokine-like genes JE and KC is increased during renal ischemia. Am J Physiol 261: F1095–F1101, 1991 [DOI] [PubMed] [Google Scholar]
- 19.Risch L, Lhotta K, Meier D, Medina-Escobar P, Nydegger UE, Risch M: The serum uromodulin level is associated with kidney function. Clin Chem Lab Med 52: 1755–1761, 2014 [DOI] [PubMed] [Google Scholar]
- 20.Dawnay AB, Cattell WR: Serum Tamm-Horsfall glycoprotein levels in health and in renal disease. Clin Nephrol 15: 5–8, 1981 [PubMed] [Google Scholar]
- 21.Liu Y, El-Achkar TM, Wu XR: Tamm-Horsfall protein regulates circulating and renal cytokines by affecting glomerular filtration rate and acting as a urinary cytokine trap. J Biol Chem 287: 16365–16378, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Solak Y, Yilmaz MI, Sonmez A, Saglam M, Cakir E, Unal HU, Gok M, Caglar K, Oguz Y, Yenicesu M, Karaman M, Ay SA, Gaipov A, Turk S, Vural A, Carrero JJ: Neutrophil to lymphocyte ratio independently predicts cardiovascular events in patients with chronic kidney disease. Clin Exp Nephrol 17: 532–540, 2013 [DOI] [PubMed] [Google Scholar]
- 23.Okyay GU, Inal S, Oneç K, Er RE, Paşaoğlu O, Paşaoğlu H, Derici U, Erten Y: Neutrophil to lymphocyte ratio in evaluation of inflammation in patients with chronic kidney disease. Ren Fail 35: 29–36, 2013 [DOI] [PubMed] [Google Scholar]
- 24.Tian N, Penman AD, Manning RD, Jr, Flessner MF, Mawson AR: Association between circulating specific leukocyte types and incident chronic kidney disease: The Atherosclerosis Risk in Communities (ARIC) study. J Am Soc Hypertens 6: 100–108, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fried L, Solomon C, Shlipak M, Seliger S, Stehman-Breen C, Bleyer AJ, Chaves P, Furberg C, Kuller L, Newman A: Inflammatory and prothrombotic markers and the progression of renal disease in elderly individuals. J Am Soc Nephrol 15: 3184–3191, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Heng TS, Painter MW, Immunological Genome Project Consortium : The Immunological Genome Project: Networks of gene expression in immune cells. Nat Immunol 9: 1091–1094, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Wojtasiak M, Pickett DL, Tate MD, Londrigan SL, Bedoui S, Brooks AG, Reading PC: Depletion of Gr-1+, but not Ly6G+, immune cells exacerbates virus replication and disease in an intranasal model of herpes simplex virus type 1 infection. J Gen Virol 91: 2158–2166, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Seita J, Weissman IL: Hematopoietic stem cell: Self-renewal versus differentiation. Wiley Interdiscip Rev Syst Biol Med 2: 640–653, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bryder D, Rossi DJ, Weissman IL: Hematopoietic stem cells: The paradigmatic tissue-specific stem cell. Am J Pathol 169: 338–346, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chitteti BR, Kobayashi M, Cheng Y, Zhang H, Poteat BA, Broxmeyer HE, Pelus LM, Hanenberg H, Zollman A, Kamocka MM, Carlesso N, Cardoso AA, Kacena MA, Srour EF: CD166 regulates human and murine hematopoietic stem cells and the hematopoietic niche. Blood 124: 519–529, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stark MA, Huo Y, Burcin TL, Morris MA, Olson TS, Ley K: Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity 22: 285–294, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Iwakura Y, Ishigame H: The IL-23/IL-17 axis in inflammation. J Clin Invest 116: 1218–1222, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Di Cesare A, Di Meglio P, Nestle FO: The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J Invest Dermatol 129: 1339–1350, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL: Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem 278: 1910–1914, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Schwarzenberger P, Huang W, Ye P, Oliver P, Manuel M, Zhang Z, Bagby G, Nelson S, Kolls JK: Requirement of endogenous stem cell factor and granulocyte-colony-stimulating factor for IL-17-mediated granulopoiesis. J Immunol 164: 4783–4789, 2000 [DOI] [PubMed] [Google Scholar]
- 36.Pirhonen J, Matikainen S, Julkunen I: Regulation of virus-induced IL-12 and IL-23 expression in human macrophages. J Immunol 169: 5673–5678, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, Vega F, Yu N, Wang J, Singh K, Zonin F, Vaisberg E, Churakova T, Liu M, Gorman D, Wagner J, Zurawski S, Liu Y, Abrams JS, Moore KW, Rennick D, de Waal-Malefyt R, Hannum C, Bazan JF, Kastelein RA: Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity 13: 715–725, 2000 [DOI] [PubMed] [Google Scholar]
- 38.Fried W: Erythropoietin and erythropoiesis. Exp Hematol 37: 1007–1015, 2009 [DOI] [PubMed] [Google Scholar]
- 39.Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH: Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity 31: 331–341, 2009 [DOI] [PubMed] [Google Scholar]
- 40.Paust HJ, Turner JE, Riedel JH, Disteldorf E, Peters A, Schmidt T, Krebs C, Velden J, Mittrücker HW, Steinmetz OM, Stahl RA, Panzer U: Chemokines play a critical role in the cross-regulation of Th1 and Th17 immune responses in murine crescentic glomerulonephritis. Kidney Int 82: 72–83, 2012 [DOI] [PubMed] [Google Scholar]
- 41.Turner JE, Krebs C, Tittel AP, Paust HJ, Meyer-Schwesinger C, Bennstein SB, Steinmetz OM, Prinz I, Magnus T, Korn T, Stahl RA, Kurts C, Panzer U: IL-17A production by renal γδ T cells promotes kidney injury in crescentic GN. J Am Soc Nephrol 23: 1486–1495, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li L, Huang L, Vergis AL, Ye H, Bajwa A, Narayan V, Strieter RM, Rosin DL, Okusa MD: IL-17 produced by neutrophils regulates IFN-gamma-mediated neutrophil migration in mouse kidney ischemia-reperfusion injury. J Clin Invest 120: 331–342, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin FJ, Jiang GR, Shan JP, Zhu C, Zou J, Wu XR: Imbalance of regulatory T cells to Th17 cells in IgA nephropathy. Scand J Clin Lab Invest 72: 221–229, 2012 [DOI] [PubMed] [Google Scholar]
- 44.Darisipudi MN, Thomasova D, Mulay SR, Brech D, Noessner E, Liapis H, Anders HJ: Uromodulin triggers IL-1β-dependent innate immunity via the NLRP3 inflammasome. J Am Soc Nephrol 23: 1783–1789, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Säemann MD, Weichhart T, Zeyda M, Staffler G, Schunn M, Stuhlmeier KM, Sobanov Y, Stulnig TM, Akira S, von Gabain A, von Ahsen U, Hörl WH, Zlabinger GJ: Tamm-Horsfall glycoprotein links innate immune cell activation with adaptive immunity via a Toll-like receptor-4-dependent mechanism. J Clin Invest 115: 468–475, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Borras-Cuesta F, Fedon Y, Petit-Camurdan A: Enhancement of peptide immunogenicity by linear polymerization. Eur J Immunol 18: 199–202, 1988 [DOI] [PubMed] [Google Scholar]
- 47.Ledo N, Ko YA, Park AS, Kang HM, Han SY, Choi P, Susztak K: Functional genomic annotation of genetic risk loci highlights inflammation and epithelial biology networks in CKD [published online ahead of print September 17, 2014]. J Am Soc Nephrol doi:10.1681/ASN.2014010028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mo L, Huang HY, Zhu XH, Shapiro E, Hasty DL, Wu XR: Tamm-Horsfall protein is a critical renal defense factor protecting against calcium oxalate crystal formation. Kidney Int 66: 1159–1166, 2004 [DOI] [PubMed] [Google Scholar]
- 49.Mo L, Zhu XH, Huang HY, Shapiro E, Hasty DL, Wu XR: Ablation of the Tamm-Horsfall protein gene increases susceptibility of mice to bladder colonization by type 1-fimbriated Escherichia coli. Am J Physiol Renal Physiol 286: F795–F802, 2004 [DOI] [PubMed] [Google Scholar]
- 50.Sutton TA, Hato T, Mai E, Yoshimoto M, Kuehl S, Anderson M, Mang H, Plotkin Z, Chan RJ, Dagher PC: p53 is renoprotective after ischemic kidney injury by reducing inflammation. J Am Soc Nephrol 24: 113–124, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kohda Y, Murakami H, Moe OW, Star RA: Analysis of segmental renal gene expression by laser capture microdissection. Kidney Int 57: 321–331, 2000 [DOI] [PubMed] [Google Scholar]
- 52.Murakami H, Liotta L, Star RA: IF-LCM: Laser capture microdissection of immunofluorescently defined cells for mRNA analysis rapid communication. Kidney Int 58: 1346–1353, 2000 [DOI] [PubMed] [Google Scholar]
- 53.Zhang MZ, Yao B, Yang S, Jiang L, Wang S, Fan X, Yin H, Wong K, Miyazawa T, Chen J, Chang I, Singh A, Harris RC: CSF-1 signaling mediates recovery from acute kidney injury. J Clin Invest 122: 4519–4532, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]