Abstract
Current guidelines suggest treatment with corticosteroids (CS) in IgA nephropathy (IgAN) when proteinuria is persistently ≥1 g/d despite 3–6 months of supportive care and when eGFR is >50 ml/min per 1.73 m2. Whether the benefits of this treatment extend to patients with an eGFR≤50 ml/min per 1.73 m2, other levels of proteinuria, or different renal pathologic lesions remains unknown. We retrospectively studied 1147 patients with IgAN from the European Validation Study of the Oxford Classification of IgAN (VALIGA) cohort classified according to the Oxford-MEST classification and medication used, with details of duration but not dosing. Overall, 46% of patients received immunosuppression, of which 98% received CS. Treated individuals presented with greater clinical and pathologic risk factors of progression. They also received more antihypertensive medication, and a greater proportion received renin angiotensin system blockade (RASB) compared with individuals without immunosuppressive therapy. Immunosuppression was associated with a significant reduction in proteinuria, a slower rate of renal function decline, and greater renal survival. Using a propensity score, we matched 184 subjects who received CS and RASB to 184 patients with a similar risk profile of progression who received only RASB. Within this group, CS reduced proteinuria and the rate of renal function decline and increased renal survival. These benefits extended to those with an eGFR≤50 ml/min per 1.73 m2, and the benefits increased proportionally with the level of proteinuria. Thus, CS reduced the risk of progression regardless of initial eGFR and in direct proportion to the extent of proteinuria in this cohort.
Keywords: IgA nephropathy, risk factors, progression of chronic renal failure, pathology, immunosuppression, proteinuria
IgA nephropathy (IgAN) is the most common primary glomerulonephritis (GN), and it progresses to ESRD in a considerable proportion of patients.1–3 The control of blood pressure, the use of renin angiotensin system blockade (RASB), and in selected patients, the use of corticosteroids (CS) are the cornerstones of management. The Kidney Disease Improving Global Outcomes (KDIGO) guidelines for the treatment of GN suggest that IgAN patients with a persistent proteinuria ≥1 g/d despite 3–6 months of optimized supportive care, including RASB, and an eGFR>50 ml/min per 1.73 m2 receive a 6-month course of CS.4 Whether the benefits of CS vary, depending on the level proteinuria, eGFR, or differences in pathologic findings, is uncertain because these issues have not yet been addressed by randomized controlled trials (RCTs). Furthermore, the KDIGO guidelines make no recommendations for use of CS in individuals with an initial eGFR≤50 ml/min per 1.73 m2, who are underrepresented in trials. Finally, in some RCTs, only a minority of patients were treated (according to the design of the trials) with RASB.5 Because of these limitations, analysis of large observational cohorts remains a useful tool to refine the management of IgAN.
The Validation Study of the Oxford Classification of IgAN (VALIGA) cohort was assembled from 13 European countries and designed to address the validity of the Oxford classification of IgAN.6,7 Using this large cohort, we were able to address the possible long-term benefits of CS, including in those patients with an initial eGFR≤50 ml/min per 1.73 m2, and investigate whether the level of pretreatment proteinuria or pathologic features have an impact on the effect of CS therapy.
Results
Study Cohort
The VALIGA cohort included 1147 patients of whom 73% were men and 97% were Caucasian. At the time of biopsy, their age was 36±16 years, their eGFR was 73±30 ml/min per 1.73 m2, their proteinuria was 1.3 g/d (interquartile range, 0.6–2.6), and their mean arterial pressure (MAP) was 98±13 mmHg (131/81 mmHg). In regards to the MEST score, mesangial hypercellularity (M1) was present in 28%, endocapillary hypercellularity (E1) was present in 11%, segmental glomerulosclerosis (S1) was present in 70%, and 21% of the patients had >25% tubular atrophy and interstitial fibrosis (T1–2). Eleven percent of biopsies showed crescents, and 7% showed necrosis.
The median follow-up was 4.7 years (2.4–7.9). The rate of renal function decline was 1.8±7.5 ml/min per 1.73 m2/y. Of the patients, 12% developed ESRD, 14% experienced a 50% decrease of renal function, and 16% attained the combined outcome. Proteinuria decreased by a median of 0.5 g/24h during follow-up. Overall, the median number of creatinine, proteinuria, and blood pressure measurements was 5 (3–8), 4 (3–7), and 5 (3–8), respectively.
Therapeutic Interventions and Renal Outcome
Over the course of follow-up, 86% of patients received RASB. Of the patients, 46% (n=523) had immunosuppression. Immunosuppressive regimens included CS (98%, with pulse CS in 34% and oral CS in 93%), azathioprine (17%), cyclophosphamide (16%), mycophenolate mofetil (8%), and calcineurin inhibitor (3%). Immunosuppressants other than CS were used in combination with CS as a first intention in 74%. Otherwise, they were added after an initial course of CS. Only 11 patients received immunosuppression without CS.
Treated and untreated patients differed in almost all clinical and pathologic characteristics (Table 1). Patients receiving immunosuppression had a lower eGFR, higher proteinuria, and more histologic lesions associated with a worse prognosis. In spite of these initial data, their unadjusted outcome was better than that of the untreated subjects. However, they also received more antihypertensive medication, including a higher percentage receiving RASB, and had a greater proportion of the follow-up time while receiving RASB (Table 1).
Table 1.
Characteristics and outcome of the total VALIGA cohort by immunosuppression exposure (n=1147)
| Characteristic | Immunosuppression Treatment Exposure | P Value | |
|---|---|---|---|
| None (n=624; 54%) | Anyb (n=523; 46%) | ||
| Clinical characteristics at biopsy | |||
| Men | 73 | 71 | 0.23 |
| Caucasian/African/Asian/Indian | 98/0/0/1 | 98/1/1/0 | 0.54 |
| Age (y) | 37±16 | 36±17 | 0.16 |
| eGFR (ml/min per 1.73 m2) | 76±29 | 70±32 | 0.002 |
| MAP (mmHg) | 98±13 | 98±13 | 0.99 |
| Prior RASB | 36 | 42 | 0.05 |
| Prior immunosuppression | 1 | 18 | <0.001 |
| Initial proteinuria (g/d) | 0.9 (0.4 to 1.9) | 1.9 (1.0 to 3.5) | <0.001 |
| Biopsy findings | |||
| M1 | 24 | 32 | 0.004 |
| E1 | 9 | 13 | 0.03 |
| S1 | 63 | 78 | <0.001 |
| TA/IF>25% (T1–2) | 18 | 25 | 0.002 |
| Necrosis | 3 | 12 | <0.001 |
| Crescents | 6 | 16 | <0.001 |
| Follow-up | |||
| Length of follow-up (y) | 5.0 (2.4 to 9.1) | 4.2 (2.3 to 6.6) | <0.001 |
| MAP (mmHg) | 96±9 | 95±9 | 0.15 |
| Number of antihypertensive medication | 1.0 (0.6 to 1.9) | 1.0 (0.9 to 2.0) | 0.01 |
| RASB | 81 | 92 | <0.001 |
| Proportion of follow-up under RASB | 0.83 (0.33 to 1.00) | 1.00 (0.70 to 1.00) | <0.001 |
| Time-average proteinuria (g/d) | 0.7 (0.3 to 1.3) | 1.1 (0.6 to 2.0) | <0.001 |
| Fish oil | 13 | 14 | 0.37 |
| Tonsillectomy | 4 | 5 | 0.49 |
| Outcome | |||
| Rate of renal function decline (ml/min per 1.73 m2/y) | −2.2±6.5 | −1.3±8.5 | 0.05 |
| Change in proteinuriaa | −0.3 (−1.1 to 0.2) | −1.0 (−2.4 to −0.2) | <0.001 |
| Reduction in proteinuria<1 g/d | 70 | 77 | 0.04 |
| ESRD | 14 | 10 | 0.008c |
Values are expressed as mean±SD, median (interquartile range), percentage, or as otherwise indicated.
Last–first value of proteinuria; a negative value indicates a decrease in proteinuria over the entire follow-up period.
Immunosuppressive treatments are detailed in the text.
Using a time-dependent Cox regression analysis.
Immunosuppression was started at the first assessment in 50% of treated patients, and 81% began treatments within the first 6 months after biopsy. It lasted for a median of 1.3 years (0.5, 4.0). At the end of therapy, eGFR increased (2.4±16.4, P=0.02, paired t test) and proteinuria dropped (-0.8 g/d; -2.1, 0.2). Once treatment was stopped, there was an additional 2.9 years (1.5, 5.5) of available follow-up (Table 2).
Table 2.
Change in eGFR and proteinuria before, during, and after immunosuppressive therapy in all treated patients (n=523)
| Variable | n | P Valued | |
|---|---|---|---|
| Time between initial assessment and start of therapy (y) | 0.0 (0.0 to 0.3) | 523 | |
| eGFR change before start of therapy (ml/min per 1.73 m2)a | −3.2±18.7 | 256 | <0.01 |
| Proteinuria change before start of therapy (g/d)a | −0.1 (−1.0 to 0.4) | 256 | <0.001 |
| Length of therapy (y)b | 1.3 (0.5 to 4.0) | 347 | |
| eGFR change from start to end of therapy (ml/min per 1.73 m2)b | 2.4±16.4 | 347 | 0.02 |
| Proteinuria change from start to end of therapy (g/d)b | −0.8 (−2.1 to 0.2) | 347 | <0.001 |
| Length of follow-up after therapy (y)c | 2.9 (1.5 to 5.5) | 369 | |
| eGFR change from end of therapy to end of follow-up (ml/min per 1.73 m2)c | 2.1±16 | 369 | 0.01 |
| Proteinuria change from end of therapy to end of follow-up (g/d)c | −0.2 (−1.0 to 0.1) | 369 | <0.001 |
Values are expressed as mean±SD or median (interquartile range).
Calculated only in those who did not start therapy at the first available follow-up.
Calculated only in those where a minimal duration of therapy could be determined.
Calculated only in those with follow-up available once therapy ended.
One sample t test compared with 0.
Benefits of CS in Addition to RASB in Propensity-Matched Individuals
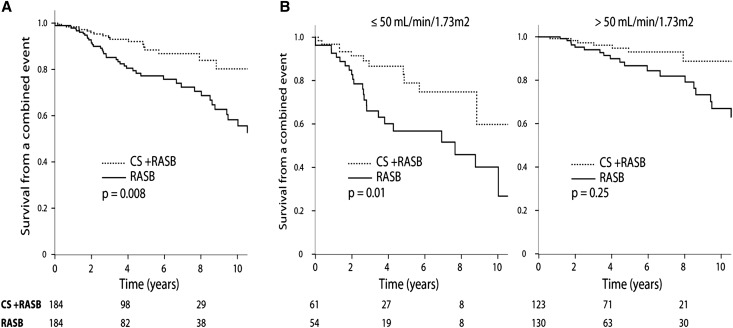
We studied the benefits of CS in a nested case-control study using a propensity score. Within the entire cohort we were able to pair 184 patients treated with CS and RASB to 184 patients using RASB only (Figure 1, Table 3). All of the differences between treated and untreated patient characteristics found in Table 1 were absent once patients were matched to controls. In particular, the proportion of follow-up while receiving RASB, the number of antihypertensive medications, and the use of fish oil were similar. Renal outcomes were better in patients compared with controls (Figure 2A), with a time-dependent hazard ratio of a combined event of 0.48 (0.28–0.82, P=0.008). The CS-treated group experienced a -1.0±7.3 ml/min per 1.73 m2/y rate of renal function decline as opposed to -3.2±8.3 ml/min per 1.73 m2/y in the untreated group (P=.004). Finally, the drop in proteinuria during the entire follow-up period was greater with CS treatment, with 84% achieving a level <1 g/d compared with only 54% with no exposure to CS (P<0.001).
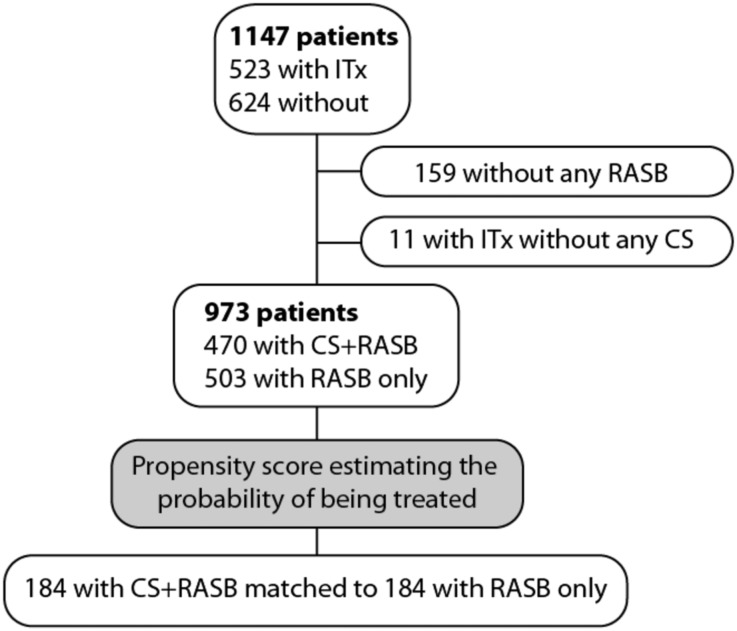
Figure 1.
Patient selection for the nested case control study on corticosteroids (CS). Individuals who did not receive renin-angiotensin system blockade (RASB) or received ITx without CS were excluded. Of the remaining 973 patients, 184 with CS+RASB could be matched to 184 with RASB only. ITx, any immunosuppressive therapy.
Table 3.
Characteristics and outcome of propensity-matched individuals
| Characteristic | RASB (n=184) | RASB+CS (n=184) | P Value |
|---|---|---|---|
| Clinical characteristics at biopsy | |||
| Men | 77 | 76 | 0.90 |
| Caucasian | 100 | 99 | 0.38 |
| Age (y) | 38±14 | 39±16 | 0.44 |
| eGFR (ml/min per 1.73 m2) | 69±29 | 68±31 | 0.88 |
| MAP (mmHg) | 101±13 | 99±12 | 0.19 |
| Prior RASB | 53 | 46 | 0.15 |
| Prior immunosuppression | 1.1 | 1.1 | 1.00 |
| Number of antihypertensive medication | 1 (0 to 2) | 1 (0 to 2) | 0.67 |
| Initial proteinuria (g/d) | 1.1 (0.5 to 2.5) | 1.3 (0.8 to 2.4) | 0.12 |
| Pathology findings | |||
| M1 | 30 | 31 | 0.82 |
| E1 | 9 | 12 | 0.73 |
| S1 | 76 | 76 | 0.90 |
| T1–2 | 27 | 28 | 0.73 |
| Necrosis | 7.1 | 9.2 | 0.45 |
| Crescents | 9.2 | 9.2 | 1.00 |
| Follow-up (prior to immunosuppression in the treated group) | |||
| MAP (mmHg) | 99±9 | 99±11 | 0.89 |
| Time-average proteinuria (g/d) | 1.1 (0.5 to 2.3) | 1.2 (0.8 to 2.3) | 0.10 |
| Treatments over entire follow-up | |||
| Length of follow-up (y) | 3.7 (1.9 to 6.7) | 4.4 (2.3 to 6.5) | 0.36 |
| RASB | 100 | 100 | By design |
| Proportion of follow-up time using RASB | 1.00 (0.88 to 1.00) | 1.00 (0.83 to 1.00) | 0.52 |
| Time-average number of antihypertensive medication | 1.3 (1.0 to 2.2) | 1.4 (0.9 to 2.1) | 0.67 |
| CS/pulse CS/oral CS | — | 100/32/93 | By design |
| Azathioprine/cyclophosphamide/MMF/calcineurin inhibitors | 14/10/6/2 | By design | |
| Fish oil | 19 | 16 | 0.50 |
| Tonsillectomy | 1.8 | 4.0 | 0.21 |
| Outcomes | |||
| Rate of renal function decline (ml/min per 1.73 m2/y) | −3.2±8.3 | −1.0±7.3 | 0.004 |
| Change in proteinuria during entire follow-up (g/d)a | −0.3 (−1.1 to 0.3) | −0.8 (−1.6 to −0.2) | <0.001 |
| Reduction proteinuria to <1 g/d | 54 | 84 | <0.001 |
| ESRD | 20 | 7 | 0.003b |
| Combined end point | 27 | 12 | <0.01 |
Values are expressed as mean±SD, median (interquartile range), percentage, or as otherwise indicated.
Last – first available proteinuria; a negative value indicates a reduction in proteinuria over time.
By time-dependent Cox regression analysis.
Figure 2.
Response to CS and RASB compared with RASB alone in propensity-matched individuals. (A) Entire propensity-matched cohort. (B) Stratified by initial eGFR. P values obtained using time-dependent Cox regression.
When excluding individuals with exposure to RASB prior to the first available assessment and comparing those initiated on RASB alone (n=86) with those initiated on RASB and CS (n=100), the CS group still experienced a slower rate of renal function decline (-0.5±4.8 versus -3.1±9.1 ml/min per 1.73 m2/y, P=0.02) and a greater survival from a combined event (hazard ratio of 0.46; 95% confidence interval, 0.24–0.88; P=0.02).
Benefits of CS in Addition to RASB in Subgroups Defined by the Initial eGFR
We then studied whether the benefits of CS on outcome applied to clinically relevant subgroups within the propensity-matched populations. We first addressed the influence of the initial eGFR. We found strong evidence of CS benefits in individuals with an initial eGFR≤50 ml/min per 1.73 m2, with a time-dependent hazard ratio of a combined outcome with the use of CS of 0.38 (95% confidence interval, 0.18–0.82; P=0.01) (Figure 2B). The CS-treated group also had a slower rate of renal function decline, and a higher percentage of patients reached a proteinuria level <1 g/d (Table 4). Although we found a nonsignificant trend toward a greater survival and slower rate of renal function decline in the group with an eGFR>50 ml/min per 1.73 m2, 46% of individuals within this group had a time-average proteinuria <1 g/d, as opposed to 23% in the ≤50 ml/min per 1.73 m2 group. A reduction in proteinuria <1 g/d in those that started with >1 g/d was seen in 90% of CS and RASB compared with 66% with RASB (P=0.001).
Table 4.
Propensity-adjusted effect of corticosteroids on the rate of renal function decline and proteinuria stratified by clinical and biopsy findings
| Variable | n | Slope (ml/min per 1.73 m2/y) | P Value | na (with Initial Proteinuria>1 g/d) | Reaching Proteinuria<1 g/d (%) | P Value |
|---|---|---|---|---|---|---|
| eGFR at time of biopsy | ||||||
| ≤50 ml/min per 1.73 m2 | ||||||
| RASB | 54 | −4.8±7.4 | 0.001 | 43 | 37 | <0.001 |
| CS and RASB | 61 | −0.3±6.2 | 46 | 74 | ||
| >50 ml/min per 1.73 m2 | ||||||
| RASB | 130 | −2.7±8.6 | 0.17 | 59 | 66 | 0.001 |
| CS and RASB | 123 | −1.3±7.7 | 80 | 90 | ||
| Time-average proteinuria (prior to CS in the treated group) | ||||||
| <1 g/d | ||||||
| RASB | 84 | −1.0±8.6 | 0.25 | — | ||
| CS and RASB | 60 | 0.4±4.5 | ||||
| 1 to <3 g/d | ||||||
| RASB | 69 | −3.0±4.8 | 0.03 | 54 | 63 | 0.001 |
| CS and RASB | 95 | −0.9±6.6 | 84 | 88 | ||
| ≥3g/d | ||||||
| RASB | 31 | −10.3±9.8 | 0.03 | 28 | 4 | <0.001 |
| CS and RASB | 29 | −4.1±11.8 | 28 | 64 | ||
| Pathology findings | ||||||
| M0 | ||||||
| RASB | 129 | −2.1±8.0 | 0.08 | 60 | 53 | <0.001 |
| CS and RASB | 127 | −0.6±5.8 | 78 | 85 | ||
| M1 | ||||||
| RASB | 55 | −6.1±8.5 | 0.01 | 42 | 57 | 0.003 |
| CS and RASB | 57 | −1.8±9.7 | 48 | 80 | ||
| E0 | ||||||
| RASB | 167 | −3.2±8.5 | 0.01 | 90 | 52 | <0.001 |
| CS and RASB | 165 | −1.0±7.1 | 112 | 84 | ||
| E1 | ||||||
| RASB | 17 | −4.7±5.5 | 0.10 | 12 | 67 | 0.28 |
| CS and RASB | 19 | −0.6±8.5 | 14 | 86 | ||
| S0 | ||||||
| RASB | 45 | −2.3±7.3 | 0.21 | 14 | 63 | 0.14 |
| CS and RASB | 44 | −0.6±4.7 | 24 | 83 | ||
| S1 | ||||||
| RASB | 139 | −3.7±8.6 | 0.009 | 86 | 52 | <0.001 |
| CS and RASB | 140 | −1.1±7.9 | 102 | 84 | ||
| T0 | ||||||
| RASB | 135 | −2.7±9.0 | 0.09 | 62 | 60 | <0.001 |
| CS and RASB | 132 | −1.0±7.4 | 85 | 88 | ||
| T1 | ||||||
| RASB | 49 | −5.0±5.9 | 0.002 | 40 | 45 | 0.004 |
| CS and RASB | 52 | −0.9±6.9 | 41 | 76 |
Not all patients had initial proteinuria >1 g/d.
Benefits of CS in Addition to RASB in Subgroups Defined by the Proteinuria before Therapy
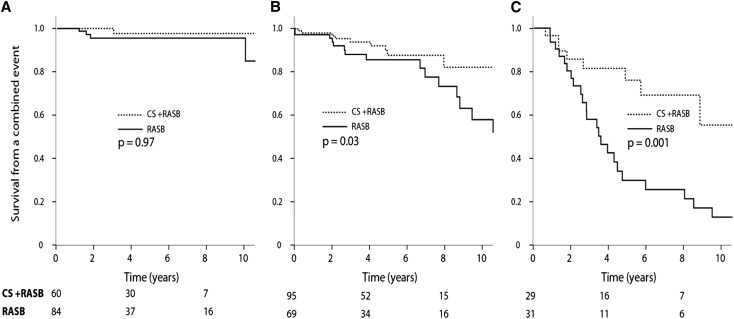
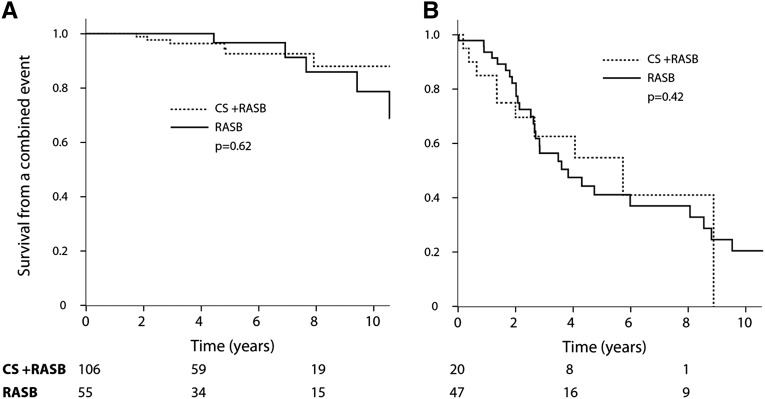
We categorized patients into groups with a time-average proteinuria of <1, 1–3, and ≥3 g/d within the propensity-matched population (Figure 3, Table 4). For the treated group, we used the time-average proteinuria prior to CS exposure. We demonstrated that the benefits of CS increased considerably as proteinuria increased. The absolute difference in the rate of renal function decline favored CS and RASB over RASB alone and was 1.4, 2.1, and 6.2 ml/min per 1.73 m2/y in the groups with a time-average proteinuria of <1, 1 to <3, and ≥3/d, respectively (Table 4). Furthermore, among those with proteinuria of ≥3 g/d, 64% of those receiving CS reached a level <1 g/d compared with only 4% of individuals with no exposure to CS (P<0.001) (Table 4). Achieving proteinuria of <1 g/d closely paralleled a greater renal survival and slower rate of renal function decline. Those who reached this surrogate outcome experienced a favorable outcome regardless of therapy (Figure 4). In the group that did not achieve proteinuria <1 g/d, the rate of renal function decline was -6.5±14.9 ml/min per 1.73 m2/y with CS and RASB compared with -7.6±9.7 ml/min per 1.73 m2/y with RASB only (P=0.72).
Figure 3.
Response to CS and RASB compared with RASB alone in propensity-matched individuals stratified by proteinuria during follow-up, prior to CS in the CS-RASB group. (A) Proteinuria <1 g/d. (B) Proteinuria 1 to <3 g/d. (C) proteinuria ≥3 g/d. Time-average proteinuria was prior to CS in the treated group. P values obtained using time-dependent Cox regression. There was no evident benefit of CS in those with a proteinuria <1 g/d.
Figure 4.
Response to CS and RASB compared with RASB alone in propensity-matched individuals having achieved a reduction in proteinuria to <1g/d (A) or not (B). P values obtained using time-dependent Cox regression. Of the propensity-matched patients, 140 did not have an initial proteinuria ≥1 g/d and were excluded from this analysis.
Benefits of CS in Addition to RASB in Subgroups Defined by Pathology Findings
We then looked at the influence of pathology on the effect of CS and RASB compared with RASB alone. A lower rate of renal function decline and greater reduction in proteinuria could be seen with CS in almost all subgroups of the MEST score (Table 4). Using interaction analyses, pathology findings did not statistically influence the response to CS (P>0.1 for interaction). However, the absolute benefit in the rate of renal function decline between CS and RASB and RASB alone tended to be greater in those when M1, E1, S1, and T1–2 was present (+4.3, +4.1, +2.6, and +4.1 ml/min per 1.73 m2/y, respectively) compared with M0, E0, S0, and T0 (+1.5, +2.2, +1.7, and +1.7 ml/min per 1.73 m2/y, respectively) (Table 4).
Finally, to address possible unforeseen biases using our propensity score, we repeated propensity matching on the basis of slightly different variables. Other paired populations were obtained (Supplemental Table 1). The main comparisons and subgroup analyses on the basis of the initial eGFR and proteinuria prior to therapy were identical to the propensity-matched cohort presented. With regard to the influence of pathology on the benefits of CS, one propensity-derived cohort showed a greater benefit in the presence of S1 over S0, whereas another showed a greater benefit in the presence of M1 over M0.
Discussion
IgAN is the most common cause of ESRD as a result of primary GN in young adults. Evidence from RCTs suggests that CS may reduce the risk of progression in high-risk individuals with IgAN with >50 ml/min per 1.73 m2 and proteinuria of at least 1 g/d8 despite 6 months of optimal supportive therapy.9,10 However, the design limitations of these available RCTs explain the assignment of a low level of evidence (2C) to support the suggestion for the use of CS made in the KDIGO guidelines. Using a propensity score methodology to normalize comparisons between treated and untreated individuals, CS slowed the rate of renal function decline and increased the survival without ESRD or a 50% decline in renal function. The absolute benefits of CS increased in parallel to proteinuria and were also present in those with an initial eGFR<50 ml/min per 1.73 m2. There was no evident benefit of CS in those with proteinuria <1 g/d. We could not convincingly demonstrate that pathology findings from the Oxford classification influenced the benefits of CS; however, a trend existed toward greater advantages in patients with more severe histologic findings. Our findings also support the use of reduction in proteinuria (spontaneous or after CS) as a valid surrogate outcome because both treated and untreated groups experienced a similar poor outcome if they did not achieve a reduction in proteinuria to <1 g/d.11,12
Recent KDIGO recommendations provide no guidance for immunosuppression in IgAN patients presenting with eGFR between 30 and 50 ml/min per 1.73 m2 because RCTs have seldom recruited this high-risk population.4 There are, however, case reports13 suggesting the efficacy of CS even in patients with advanced chronic kidney disease.14 A small RCT in only 38 patients with serum creatinine of 130–250 µmol/l and high-grade proteinuria15 showed an increased renal survival with the combination of CS and cyclophosphamide. However, use of RASB was not universal. In a retrospective analysis, the outcome of patients with histologically advanced IgAN treated with the combination of CS and cyclophosphamide was also significantly better than supportive therapy, but again not all patients received RASB.16 Recently, two studies demonstrated a reduction in proteinuria and stabilization of renal function in patients presenting with an eGFR<30 ml/min per 1.73 m2 treated with either CS alone or with a combination of CS and azathioprine. Both groups decreased their proteinuria and possibly slowed the rate of renal function decline.17,18 Unfortunately, there was no control arm of patients not receiving CS in either study. RCTs in high-risk populations are ongoing.19
Our analyses excluded patients receiving immunosuppression but not CS because only 2% of the VALIGA cohort was treated in this way. However, we included individuals receiving CS and other immunosuppressants because excluding them might have eliminated higher-risk patients. We also excluded patients not receiving RASB during follow-up because this is now usually regarded as the standard of care. Current guidelines do not take into consideration the putative greater benefit of CS treatment with higher proteinuria, as suggested by our analysis. Weighing the benefits and risks of CS interventions, we favor the treatment of patients with high-grade proteinuria and favor avoiding it in those with <1 g/d because achieving this threshold is associated with a more favorable outcome regardless of therapy. Interestingly, in treated patients, proteinuria further decreased and eGFR increased during follow-up, even after immunosuppression was withdrawn (Table 2), suggesting a legacy effect.20 It has been demonstrated that in patients with IgAN treated with CS for 6 months, the effect of treatment on the stabilization of eGFR may persist over years.5 In a study using a similar regimen of CS, the proportion of those who achieved a remission in proteinuria increased up to 6 years after treatment.21
The degree of mesangial hypercellularity, segmental glomerulosclerosis, and tubular atrophy/interstitial fibrosis as defined by the Oxford Classification of IgAN22 negatively predicted outcome in the VALIGA cohort,6 but this ability was reduced by immunosuppressive treatment. Histologic lesions may be influenced by treatments. In a limited number of repeat biopsy studies, CS were shown to reduce mesangial23 and endocapillary proliferation, tuft necrosis and glomerular crescents, interstitial monocyte infiltration and interstitial edema, and also lesions once thought to be irreversible, such as the percentage of segmentally sclerotic glomeruli and extent of interstitial fibrosis.24 In this analysis of the VALIGA study, there was a nonsignificant trend for higher efficacy of CS in more advanced lesions.
Our study has several limitations. The VALIGA dataset was obtained retrospectively. Almost all participants were Caucasian, and caution is warranted before extrapolating these findings to other ethnic populations. Nothing is known about the compliance with treatment, the occurrence of adverse events with either therapy, and the doses of CS and RASB. These results were obtained in patients who received treatment soon after presentation. It is impossible to know whether these findings extend to patients who received treatment later in their time course. Errors or missing data are inevitable; however, they would tend to bias toward the null hypothesis. Despite the use of a rigorous statistical approach,25 there are no ways to adjust for unmeasured variables. Also, individuals receiving immunosuppression are followed closely, whereas those doing well may have been excluded from the study or prematurely lost to follow-up. In addition, we do not know if the centers reported patients that are representative of all of their IgAN patients, and we did not have access to data on drug dosing. Finally, findings regarding pathology may be influenced by the time elapsed between biopsy and the start of therapy when the MEST score may have changed. However, treatment was begun by 6 months in most patients.
In conclusion, this study supports the use of CS in addition to RASB with a proteinuria>1 g/d, even with an initial eGFR≤50 ml/min per 1.73 m2. Although this retrospective analysis cannot provide an explicit recommendation for the use of CS within this group until randomized trials confirm our findings, clinicians should consider this option, especially in those with an elevated proteinuria despite optimal conservative therapy.
Concise Methods
Study Design
The VALIGA cohort was assembled retrospectively in 2011.6 Briefly, it included children and adults with IgAN and a follow-up of at least 1 year, or shorter if they had progressed to ESRD. Individuals with Henoch-Schönlein nephritis, diabetes, or cancer were excluded. There were no restrictive eGFR or proteinuria entry criteria. Demographics, use of immunosuppression, or use of RASB, with either angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, prior to biopsy were assessed. At the time of biopsy and during follow-up, blood pressure, serum creatinine, and proteinuria were recorded; the number of antihypertensive medications and the use of RASB, CS, other immunosuppressive agents (cyclophosphamide, mycophenolate mofetil, calcineurin inhibitors), or fish oil were also recorded. Immunosuppressive therapy was reported as intention to treat. From available follow-up data, we estimated the duration of therapy. When multiple immunosuppressants were used, we recorded whether they were started simultaneously or sequentially. No information on the dosage of medication was available.
The original renal biopsies were reviewed in Oxford, United Kingdom, by one of two pathologists and scored using the Oxford Classification of IgAN (MEST score).22 Pathologists were blinded to clinical data and local pathologists’ scores. The MEST score consisted of mesangial hypercellularity (M0 <50% of glomeruli showing hypercellularity; M1 >50% of glomeruli showing hypercellularity), endocapillary hypercellularity (E0 absent; E1 present), segmental glomerulosclerosis (S0 absent; S1 present), and tubular atrophy/interstitial fibrosis (T0 <25%; T1 25%–50%; T2 >50%). The presence of any crescents or necrosis was also noted because these findings often influence treatment decisions.26 Physicians from each center reported the clinical data to the central database in Turin, Italy.
Definitions
eGFR was estimated using the Modification of Diet in Renal Disease formula in adults (≥18 years of age)27 and the Schwartz formula in children.28 The maximum eGFR was set at 120 ml/min per 1.73 m2 because the accuracy of eGFR above this level is imprecise using these formulas and can bias estimation of the rate of renal function decline. For each child, the SD score for MAP was calculated and used to normalize MAP to adult values.29,30 MAP was calculated as 1/3 of the pulse pressure (systolic blood pressure–diastolic blood pressure) plus diastolic blood pressure. MAP was adjusted in children to >130/80 mmHg in case of an MAP SD score >1.29 The outcomes were the rate of renal function decline (slope of eGFR) and the combined outcome of a 50% reduction in renal function over time or ESRD, defined as eGFR<15 ml/min per 1.73 m2. We also addressed, as a surrogate outcome, the proportion of individuals with an initial proteinuria>1 g/d that achieved proteinuria<1 g/d during follow-up.11 In individuals receiving immunosuppression, we also calculated the eGFR and proteinuria changes before, during, and after therapy.
Statistical Analyses
Normally distributed variables are presented as mean and SD and compared using an independent or paired t test as appropriate. Nonparametric continuous variables are presented as a median with interquartile range (25th and 75th percentile) and compared using an independent or paired Mann–Whitney U test as appropriate. Categorical variables are summarized using proportions and compared using the Pearson chi-squared test. Time-dependent Cox proportional hazard analyses were used to investigate if the use of CS therapy was associated with a greater survival from renal failure or a 50% decrease in renal function (combined event). It takes into consideration the survival time prior to the initiation of therapy to address lead-time bias and any eGFR change from the initial assessment to the start of therapy.
The purpose of this study was to assess the benefits of CS overall and in subgroups of interest defined by the initial eGFR, proteinuria, and pathology findings. Because this study was retrospective and the therapy choices were unstandardized, patients with and without therapy were likely to differ in risk factors of progression. To be able to compare outcomes in patients with CS therapy with controls of similar risk, we used the propensity score.31 Multiple methodologic choices were made in performing this analysis (Figure 1). They are as follows:
Only individuals receiving RASB were considered, and RASB had to be given prior to or at the start of CS.
Because the recommendation of CS for high-risk IgAN is the prime question we were addressing, subjects receiving immunosuppression without CS were excluded from the propensity-matched analyses. However, we did include individuals who had received both CS and another immunosuppressive agent to avoid a selection bias because these may have received the additional agent on the basis of a higher-risk profile or after failure of CS.
-
Using a logistic regression model, we calculated a predicted probability (propensity score) to receive CS for each patient. The variables in the model to estimate the propensity scores were as follows:
Initial assessment: age, sex, eGFR, proteinuria, any immunosuppression prior to the biopsy.
Pathology findings: MEST score and the presence of crescents and necrosis (because these findings are generally considered risk factors in spite of negative results from retrospective database analyses).
Follow-up assessment: time-average proteinuria and time-average blood pressure before the start of immunosuppression for the treated group (because this may change after immunosuppression).
Other treatments: time-average number of blood pressure medications, proportion of the follow-up under RASB, and any use of fish oil.
We matched each patient treated with CS to a control with the closest propensity score using a maximum difference of ±0.05.31
Matched treated and untreated groups were compared. The benefits of therapy were studied in subgroups defined by the initial eGFR, proteinuria findings, and pathology findings. We categorized individuals by an initial eGFR≤50 and >50 ml/min per 1.73 m2. We considered groups with a time-average proteinuria (before therapy in the treated group) <1, 1 to <3, and ≥3 g/d. Finally, we studied the benefits of therapy in the presence or absence of M, E, S, and T lesions. Interactions studies were performed using general linear models.
We also report findings of the basis of different models of propensity score matching from the one previously detailed (e.g., using maximal proteinuria prior to treatment instead of initial proteinuria) to address whether our findings were robust. We used SPSS 19 statistical software (IBM Corporation, New York), and the statistical significance level was set at 0.05.
Disclosures
None.
Acknowledgments
The study was granted by the first research call of the European Renal Association-European Dialysis and Transplant Association (ERA-EDTA) in 2009.
S.T., H.T.C., J.F., I.S.D.R., D.C., and R.C. are steering committee members.
The VALIGA centers’ list of nephrologists includes the following: V. Tesar, D. Maixnerova (Nephrology, First Faculty of Medicine and General University Hospital, Prague, Czech Republic); S. Lundberg (Nephrology, Karolinska University Hospital, Karolinska Institutet, Stockholm, Sweden); L. Gesualdo (Nephrology, Emergency and Organ Transplantation, University of Bari “Aldo Moro”, Foggia-Bari, Italy); F. Emma, L. Fuiano (Nephrology, Pediatrico Bambino Gesu Hospital, Rome, Italy); G. Beltrame, C. Rollino (Nephrology, San Giovanni Bosco Hospital, Turin, Italy); R.Coppo, A. Amore, R. Camilla, L. Peruzzi (Nephrology, Regina Margherita Children's Hospital,Turin, Italy); M. Praga (Nephrology, Hospital 12 de Octubre, Madrid, Spain); S. Feriozzi, R. Polci, (Nephrology, Belcolle Hospital,Viterbo, Italy); G. Segoloni, L.Colla (Nephrology, S. Giovanni Battista University Hospital, Turin, Italy); A. Pani, A. Angioi, L. Piras (Nephrology, G. Brotzu Hospital, Cagliari, Italy); J. Feehally (John Walls Renal Unit, Leicester General Hospital, Leicester, United Kingdom); G. Cancarini, S. Ravera (Nephrology, Spedali Civili University Hospital, Brescia, Italy); M. Durlik (Transplantation Medicine and Nephrology, Warsaw Medical University, Warsaw, Poland); E. Moggia (Nephrology, Santa Croce Hospital, Cuneo, Italy); J. Ballarin (Nephrology, Fundacion Puigvert, Barcelona, Spain); S. Di Giulio (Nephrology, San Camillo Forlanini Hospital, Rome, Italy); F. Pugliese, I. Serriello (Nephrology, Policlinico Umberto I University Hospital, Rome, Italy); Y. Caliskan, I. Kilicaslan (Nephrology, Internal Medicine, Istanbul Faculty of Medicine, Istanbul, Turkey); F. Locatelli, L. Del Vecchio (Nephrology, A. Manzoni Hospital, Lecco, Italy); J.F.M. Wetzels, H. Peters (Nephrology and Patholog, Radboud University Nijmegen Medical Center, Nijmegen, The Netherlands); U. Berg (Pediatrics, Department of Clinical Science, Intervention and Technology, Huddinge, Sweden); F. Carvalho, A.C. da Costa Ferreira (Nephrology, Hospital de Curry Cabral, Lisbon, Portugal); M. Maggio (Nephrology, Hospital Maggiore di Lodi, Lodi, Italy); A. Wiecek (Nephrology, Endocrinology and Metabolic Diseases, Silesian University of Medicine, Katowice, Poland); M. Ots-Rosenberg(Nephrology, Tartu University Clinics, Tartu, Estonia); R. Magistroni (Nephrology, Policlinic of Modena and Reggio Emilia; Modena, Italy); R. Topaloglu, Y. Bilginer (Pediatric Nephrology and Rheumatology, Hacettepe University, Ankara, Turkey); M. D'Amico (Nephrology, S. Anna Hospital, Como, Italy); M. Stangou (Nephrology, Hippokration General Hospital, Aristotle University of Thessaloniki, Thessaloniki, Greece); F. Giacchino (Nephrology, Ivrea Hospital, Ivrea, Italy); D. Goumenos, P. Kalliakmani, M. Gerolymos (Nephrology, University Hospital of Patras, Patras, Greece); K. Galesic (Nephrology, University Hospital Dubrava, Zagreb, Croatia); C. Geddes (Renal Unit, Western Infirmary Glasgow, Glasgow, United Kingdom); K. Siamopoulos, O. Balafa (Nephrology, Medical School University of Ioanina, Ioannina, Greece); M. Galliani (Nephrology, S.Pertini Hospital, Rome, Italy); P. Stratta, M. Quaglia (Nephrology, Maggiore della Carità Hospital, Piemonte Orientale University, Novara, Italy); R. Bergia, R. Cravero (Nephrology, Degli Infermi Hospital, Biella, Italy); M. Salvadori, L. Cirami ( Nephrology, Careggi Hospital, Florence, Italy); B. Fellstrom, H. Kloster Smerud (Renal Department, University of Uppsala, Uppsala, Sweden); F. Ferrario, T. Stellato (Nephropathology, San Gerardo Hospital Monza, Italy); J. Egido, C. Martin (Nephrology, Fundacion Jimenez Diaz, Madrid, Spain); J. Floege, F. Eitner (Nephrology and Immunology, Medizinische Klinik II, University of Aachen, Aachen, Germany); A. Lupo, P. Bernich (Nephrology, University of Verona, Verona, Italy); P. Menè (Nephrology, S. Andrea Hospital, Rome, Italy); M. Morosetti (Nephrology, Grassi Hospital, Ostia, Italy); C. van Kooten, T. Rabelink, M.E.J. Reinders (Nephrology, Leiden University Medical Centre, Leiden, The Netherlands); J.M. Boria Grinyo (Nephrology, Hospital Bellvitge, Barcelona, Spain); S. Cusinato, L. Benozzi (Nephrology, Borgomanero Hospital, Borgomanero, Italy); S. Savoldi, C. Licata (Nephrology, Civile Hospital, Ciriè, Italy); M. Mizerska-Wasiak (Pediatrics, Medical University of Warsaw, Warsaw, Poland); G. Martina, A. Messuerotti (Nephrology, Chivasso Hospital, Chivasso, Italy); A. Dal Canton, C. Esposito, C. Migotto (Nephrology Units, S. Matteo Hospital and Maugeri Foundation, Pavia, Italy); G. Triolo, F. Mariano (Nephrology CTO, Turin, Italy); C. Pozzi (Nephrology, Bassini Hospital, Cinisello Balsamo, Italy); and R. Boero (Nephrology, Martini Hospital, Turin, Italy).
The VALIGA centers’ list of pathologists includes the following: G. Mazzucco (Turin, Italy); C. Giannakakis (Rome, Italy); E. Honsova (Prague, Czech Republic); B. Sundelin (Stockholm, Sweden); A.M. Di Palma (Foggia-Bari, Italy); F. Ferrario (Monza, Italy); E. Gutiérrez (Madrid, Spain); A.M. Asunis (Cagliari, Italy); J. Barratt (Leicester, United Kingdom); R. Tardanico (Brescia, Italy); A. Perkowska-Ptasinska (Warsaw, Poland); J. Arce Terroba (Barcelona, Spain); M. Fortunato (Cuneo, Italy); A. Pantzaki (Thessaloniki, Greece); Y. Ozluk (Istanbul, Turkey); E. Steenbergen (Nijmegen, The Netherlands); M. Soderberg (Huddinge, Sweden); Z. Riispere (Tartu, Estonia); L. Furci (Modena, Italy); D. Orhan (Ankara, Turkey); D. Kipgen (Glasgow, United Kingdom); D. Casartelli (Lecco, Italy); D. Galesic Ljubanovic (Zagreb, Croatia); E. Bertoni (Florence, Italy); P. Cannata Ortiz (Madrid, Spain); H.J. Groene (Heidelberg, Germany); A. Stoppacciaro (Rome, Italy); I. Bajema, J. Bruijn (Leiden, The Netherlands); X. Fulladosa Oliveras (Barcelona, Spain); J. Maldyk (Warsaw, Poland); and E. Ioachim (Ioannina, Greece).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “IgA Nephritis with Declining Renal Function: Treatment with Corticosteroids May Be Worthwhile,” on pages 2071–2073.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014070697/-/DCSupplemental.
References
- 1.Radford MG, Jr, Donadio JV, Jr, Bergstralh EJ, Grande JP: Predicting renal outcome in IgA nephropathy. J Am Soc Nephrol 8: 199–207, 1997 [DOI] [PubMed] [Google Scholar]
- 2.Berthoux FC, Mohey H, Afiani A: Natural history of primary IgA nephropathy. Semin Nephrol 28: 4–9, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Moriyama T, Tanaka K, Iwasaki C, Oshima Y, Ochi A, Kataoka H, Itabashi M, Takei T, Uchida K, Nitta K: Prognosis in IgA nephropathy: 30-year analysis of 1,012 patients at a single center in Japan. PLoS One 9: e91756, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Radhakrishnan J, Cattran DC: The KDIGO practice guideline on glomerulonephritis: reading between the (guide)lines—Application to the individual patient. Kidney Int 82: 840–856, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Pozzi C, Andrulli S, Del Vecchio L, Melis P, Fogazzi GB, Altieri P, Ponticelli C, Locatelli F: Corticosteroid effectiveness in IgA nephropathy: Long-term results of a randomized, controlled trial. J Am Soc Nephrol 15: 157–163, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Coppo R, Troyanov S, Bellur S, Cattran D, Cook HT, Feehally J, Roberts IS, Morando L, Camilla R, Tesar V, Lunberg S, Gesualdo L, Emma F, Rollino C, Amore A, Praga M, Feriozzi S, Segoloni G, Pani A, Cancarini G, Durlik M, Moggia E, Mazzucco G, Giannakakis C, Honsova E, Sundelin BB, Di Palma AM, Ferrario F, Gutierrez E, Asunis AM, Barratt J, Tardanico R, Perkowska-Ptasinska A, VALIGA study of the ERA-EDTA Immunonephrology Working Group : Validation of the Oxford classification of IgA nephropathy in cohorts with different presentations and treatments. Kidney Int 86: 828–836, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cattran DC, Coppo R, Cook HT, Feehally J, Roberts IS, Troyanov S, Alpers CE, Amore A, Barratt J, Berthoux F, Bonsib S, Bruijn JA, D’Agati V, D’Amico G, Emancipator S, Emma F, Ferrario F, Fervenza FC, Florquin S, Fogo A, Geddes CC, Groene HJ, Haas M, Herzenberg AM, Hill PA, Hogg RJ, Hsu SI, Jennette JC, Joh K, Julian BA, Kawamura T, Lai FM, Leung CB, Li LS, Li PK, Liu ZH, Mackinnon B, Mezzano S, Schena FP, Tomino Y, Walker PD, Wang H, Weening JJ, Yoshikawa N, Zhang H, Working Group of the International IgA Nephropathy Network and the Renal Pathology Society : The Oxford classification of IgA nephropathy: Rationale, clinicopathological correlations, and classification. Kidney Int 76: 534–545, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Pozzi C, Bolasco PG, Fogazzi GB, Andrulli S, Altieri P, Ponticelli C, Locatelli F: Corticosteroids in IgA nephropathy: A randomised controlled trial. Lancet 353: 883–887, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Manno C, Torres DD, Rossini M, Pesce F, Schena FP: Randomized controlled clinical trial of corticosteroids plus ACE-inhibitors with long-term follow-up in proteinuric IgA nephropathy. Nephrol Dial Transplant 24: 3694–3701, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Lv J, Zhang H, Chen Y, Li G, Jiang L, Singh AK, Wang H: Combination therapy of prednisone and ACE inhibitor versus ACE-inhibitor therapy alone in patients with IgA nephropathy: A randomized controlled trial. Am J Kidney Dis 53: 26–32, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Reich HN, Troyanov S, Scholey JW, Cattran DC, Toronto Glomerulonephritis Registry : Remission of proteinuria improves prognosis in IgA nephropathy. J Am Soc Nephrol 18: 3177–3183, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Hirano K, Kawamura T, Tsuboi N, Okonogi H, Miyazaki Y, Ikeda M, Matsushima M, Hanaoka K, Ogura M, Utsunomiya Y, Hosoya T: The predictive value of attenuated proteinuria at 1 year after steroid therapy for renal survival in patients with IgA nephropathy. Clin Exp Nephrol 17: 555–562, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pozzi C, Ferrario F, Visciano B, Del Vecchio L: Corticosteroids in patients with IgA nephropathy and severe chronic renal damage. Case Rep Nephrol 2012: 180691, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pozzi C, Del Vecchio L, Locatelli F: Can immunosuppressive therapy be useful in IgA nephropathy when the ‘Point of No Return’ has already been exceeded? Nephron 92: 699–701, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Ballardie FW, Roberts IS: Controlled prospective trial of prednisolone and cytotoxics in progressive IgA nephropathy. J Am Soc Nephrol 13: 142–148, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Mitsuiki K, Harada A, Okura T, Higaki J: Histologically advanced IgA nephropathy treated successfully with prednisolone and cyclophosphamide. Clin Exp Nephrol 11: 297–303, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Stangou M, Ekonomidou D, Giamalis P, Liakou H, Tsiantoulas A, Pantzaki A, Papagianni A, Efstratiadis G, Alexopoulos E, Memmos D: Steroids and azathioprine in the treatment of IgA nephropathy. Clin Exp Nephrol 15: 373–380, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Pozzi C, Andrulli S, Pani A, Scaini P, Roccatello D, Fogazzi G, Pecchini P, Rustichelli R, Finocchiaro P, Del Vecchio L, Locatelli F: IgA nephropathy with severe chronic renal failure: A randomized controlled trial of corticosteroids and azathioprine. J Nephrol 26: 86–93, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Eitner F, Ackermann D, Hilgers RD, Floege J: Supportive Versus Immunosuppressive Therapy of Progressive IgA nephropathy (STOP) IgAN trial: Rationale and study protocol. J Nephrol 21: 284–289, 2008 [PubMed] [Google Scholar]
- 20.Coppo R: Is a legacy effect possible in IgA nephropathy? Nephrol Dial Transplant 28: 1657–1662, 2013 [DOI] [PubMed] [Google Scholar]
- 21.Tatematsu M, Yasuda Y, Morita Y, Sakamoto I, Kurata K, Naruse T, Yamamoto R, Tsuboi N, Sato W, Imai E, Matsuo S, Maruyama S: Complete remission within 2 years predicts a good prognosis after methylprednisolone pulse therapy in patients with IgA nephropathy. Clin Exp Nephrol 16: 883–891, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Roberts IS, Cook HT, Troyanov S, Alpers CE, Amore A, Barratt J, Berthoux F, Bonsib S, Bruijn JA, Cattran DC, Coppo R, D’Agati V, D’Amico G, Emancipator S, Emma F, Feehally J, Ferrario F, Fervenza FC, Florquin S, Fogo A, Geddes CC, Groene HJ, Haas M, Herzenberg AM, Hill PA, Hogg RJ, Hsu SI, Jennette JC, Joh K, Julian BA, Kawamura T, Lai FM, Li LS, Li PK, Liu ZH, Mackinnon B, Mezzano S, Schena FP, Tomino Y, Walker PD, Wang H, Weening JJ, Yoshikawa N, Zhang H, Working Group of the International IgA Nephropathy Network and the Renal Pathology Society : The Oxford classification of IgA nephropathy: Pathology definitions, correlations, and reproducibility. Kidney Int 76: 546–556, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Shoji T, Nakanishi I, Suzuki A, Hayashi T, Togawa M, Okada N, Imai E, Hori M, Tsubakihara Y: Early treatment with corticosteroids ameliorates proteinuria, proliferative lesions, and mesangial phenotypic modulation in adult diffuse proliferative IgA nephropathy. Am J Kidney Dis 35: 194–201, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Hotta O, Furuta T, Chiba S, Tomioka S, Taguma Y: Regression of IgA nephropathy: A repeat biopsy study. Am J Kidney Dis 39: 493–502, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Stuart EA, Lee BK, Leacy FP: Prognostic score-based balance measures can be a useful diagnostic for propensity score methods in comparative effectiveness research. J Clin Epidemiol 66[Suppl]: S84–S90.e1, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tumlin JA, Hennigar RA: Clinical presentation, natural history, and treatment of crescentic proliferative IgA nephropathy. Semin Nephrol 24: 256–268, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D, Modification of Diet in Renal Disease Study Group : A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Miller WG: Estimating equations for glomerular filtration rate in children: Laboratory considerations. Clin Chem 55: 402–403, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Coppo R, Troyanov S, Camilla R, Hogg RJ, Cattran DC, Cook HT, Feehally J, Roberts IS, Amore A, Alpers CE, Barratt J, Berthoux F, Bonsib S, Bruijn JA, D’Agati V, D’Amico G, Emancipator SN, Emma F, Ferrario F, Fervenza FC, Florquin S, Fogo AB, Geddes CC, Groene HJ, Haas M, Herzenberg AM, Hill PA, Hsu SI, Jennette JC, Joh K, Julian BA, Kawamura T, Lai FM, Li LS, Li PK, Liu ZH, Mezzano S, Schena FP, Tomino Y, Walker PD, Wang H, Weening JJ, Yoshikawa N, Zhang H, Working Group of the International IgA Nephropathy Network and the Renal Pathology Society : The Oxford IgA nephropathy clinicopathological classification is valid for children as well as adults. Kidney Int 77: 921–927, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Wühl E, Witte K, Soergel M, Mehls O, Schaefer F, German Working Group on Pediatric Hypertension : Distribution of 24-h ambulatory blood pressure in children: Normalized reference values and role of body dimensions. J Hypertens 20: 1995–2007, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Weitzen S, Lapane KL, Toledano AY, Hume AL, Mor V: Principles for modeling propensity scores in medical research: A systematic literature review. Pharmacoepidemiol Drug Saf 13: 841–853, 2004 [DOI] [PubMed] [Google Scholar]