Abstract
Steroid-dependent nephrotic syndrome (SDNS) carries a high risk of toxicity from steroids or steroid-sparing agents. This open-label, noninferiority, randomized controlled trial at four sites in Italy tested whether rituximab is noninferior to steroids in maintaining remission in juvenile SDNS. We enrolled children age 1–16 years who had developed SDNS in the previous 6–12 months and were maintained in remission with high prednisone doses (≥0.7 mg/kg per day). We randomly assigned participants to continue prednisone alone for 1 month (control) or to add a single intravenous infusion of rituximab (375 mg/m2; intervention). Prednisone was tapered in both groups after 1 month. For noninferiority, rituximab had to permit steroid withdrawal and maintain 3-month proteinuria (mg/m2 per day) within a prespecified noninferiority margin of three times the levels among controls (primary outcome). We followed participants for ≥1 year to compare risk of relapse (secondary outcome). Fifteen children per group (21 boys; mean age, 7 years [range, 2.6–13.5 years]) were enrolled and followed for ≤60 months (median, 22 months). Three-month proteinuria was 42% lower in the rituximab group (geometric mean ratio, 0.58; 95% confidence interval, 0.18 to 1.95 [i.e., within the noninferiority margin of three times the levels in controls]). All but one child in the control group relapsed within 6 months; median time to relapse in the rituximab group was 18 months (95% confidence interval, 9 to 32 months). In the rituximab group, nausea and skin rash during infusion were common; transient acute arthritis occurred in one child. In conclusion, rituximab was noninferior to steroids for the treatment of juvenile SDNS.
Keywords: nephrotic syndrome, randomized controlled trials, primary glomerulonephritis
Idiopathic nephrotic syndrome is characterized by episodes of severe proteinuria and hypoalbuminemia (serum albumin<2.5 g/dl) and is often associated with dyslipidemia and hypercoagulability. It affects 2–10 children per 100,000 per year in Western countries, with a prevalence of 16 cases per 100,000.1
Oral corticosteroids are the cornerstone of therapy and induce disease remission in approximately 90% of patients.2,3 However, up to 85% of these patients relapse within 5 years,4–8 and many will develop steroid dependence. In this situation, the disease relapses within 2 weeks of steroid withdrawal and treatment must be continued indefinitely. Clinical practice guidelines suggest using low-dose prednisone to maintain remission in steroid-dependent forms of the disease (evidence level 2C–D) and corticosteroid-sparing agents (i.e., calcineurin inhibitors) for children who develop steroid-related adverse effects (evidence level 1B).1,9,10 Given the toxicity of these agents, alternative treatment options must be investigated.11,12
Rituximab, a chimeric monoclonal anti-CD20 antibody, is increasingly being used as a steroid-sparing treatment option for children with idiopathic nephrotic syndrome. However, this is based largely on evidence from observational data, which are known to overestimate treatment benefits. One clinical trial in juvenile forms of nephrotic syndrome treated with both steroids and calcineurin inhibitors has reported more modest benefits.13 A recent trial reported more promising results in similarly complicated forms of frequently relapsing and steroid-dependent nephrotic syndrome treated with both steroid and immunosuppressant therapies.14 Overall, all studies have demonstrated that rituximab has temporary effects, although optimal frequency of repeated infusions to optimize benefits and minimize potential risks is unknown. While long-term follow-up data indicate that oral drug–free remission after rituximab injection tends to last longer in children receiving combined therapy who were initially dependent on steroids alone and with shorter disease duration,15 to date no trial has assessed the use of rituximab in early-stage uncomplicated steroid-dependent nephrotic syndrome (SDNS). We conducted a randomized controlled trial in children with SDNS who had normal levels of proteinuria and whose state of complete remission depended on high-dose steroids alone for 6–12 months (i.e., without calcineurin inhibitors) to determine whether rituximab would be noninferior to steroids in maintaining complete disease remission.
Results
Study Participants
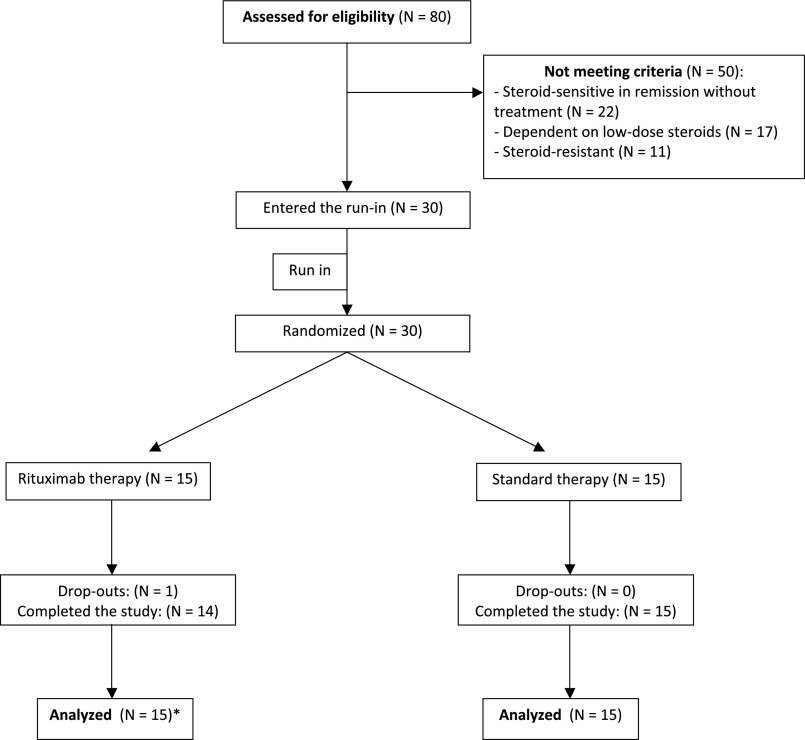
Between April 2009 and December 2012 we screened 80 children. Of these, all 30 eligible children consented to participate. After the run-in period 15 were randomly assigned to each study group (Figures 1 and 2) and followed until April 2014. Reasons for ineligibility included steroid-resistant disease (n=11), use of low-dose steroids (n=17), and treatment-free remission (n=22). Although all participants were receiving high-dose prednisone (>0.7 mg/kg per day) at the beginning of the run-in period, at the end of the run-in period the average prednisone doses were 0.1 mg/kg per day lower (see Concise Methods). Participant characteristics at the end of the 1-month run-in period were similar in both study groups (Table 1). Mean age of the participants was 7 years (range, 2.6–13.5 years), and 70% of patients were male. Average disease duration was 2.5 years. Before developing steroid-dependent disease, participants had experienced a median of 3 relapses (range, 2–8). Proteinuria levels were in the normal range and were similar in both treatment groups, as were average prednisone doses, levels of baseline kidney function, serum albumin, and cholesterol. Three children per group had received antiproliferative agents before the study (five received cyclophosphamide and one received mycophenolate mofetil); all had been steroid dependent for 6–12 months at enrollment, and none had signs of steroid toxicity (Supplemental Table 1) or growth deficit (Supplemental Table 2). All participants remained in the study for at least 1 year except one, whose parents withdrew consent after randomization. Median duration of follow-up was 22 months (range, 1–60 months).
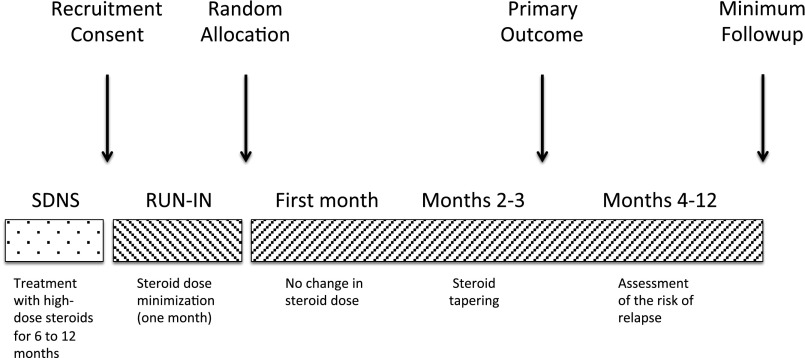
Figure 1.
Study design.
Figure 2.
Flowchart. The parents of one participant in the treatment group withdrew consent immediately after randomization. All remaining participants remained in the study for at least 1 year. *Intention-to-treat principle.
Table 1.
Participant characteristics at randomization (following the 1-month run-in period)
| Cases | Control (n=15) | Intervention (n=15) |
|---|---|---|
| Age (yr) | 6.9±3.1 | 6.9±3.6 |
| Boys (%) | 11 (73) | 10 (67) |
| Body weight (kg) | 31±14 | 30±16 |
| Disease duration (yr) | 2.0±2.5 | 2.7±2.4 |
| Median no. of relapses (range)a | 2 (2–7) | 3 (2–8) |
| Steroid toxicity (%)b | 0 | 0 |
| Prednisone dose (mg/kg per day) | 0.60±0.47 | 0.57±0.42 |
| ARB/ACEI use, n ( %) | 2 (13%) | 0 |
| Urinary protein (mg/m2 per day) | 76±48 | 84±39 |
| Serum albumin (g/dl) | 4.0±0.4 | 3.8±0.3 |
| Serum cholesterol (mg/dl) | 172±20 | 164±20 |
| Serum creatinine (mg/dl) | 0.41±0.1 | 0.40±0.2 |
Values are reported as means±SDs for quantitative variables (units) and as absolute (n) and relative (%) frequencies for qualitative variables. ARB/ACEi, angiotensin-receptor blockers or angiotensin-converting enzyme inhibitors.
Number of relapses before the development of steroid dependence.
See Supplemental Table 1 for definitions.
Primary Outcome
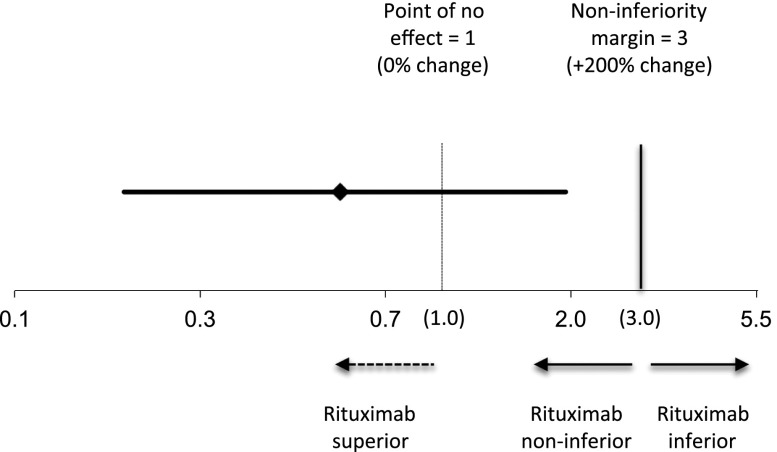
Proteinuria increased at 3 months in the prednisone group (37%; 95% confidence interval [95% CI], 7% to 76%) and decreased in the rituximab group (−37%; 95% CI, −20% to -51%) (Table 2). After we accounted for baseline proteinuria, 3-month proteinuria was 42% lower in the treatment group than the control group, although this did not reach statistical significance (ratio of means, 0.58; 95% CI, 0.18 to 1.95). The upper CI was below the prespecified noninferiority margin of 3 (Figure 3). Results of sensitivity analyses were the same (Supplemental Table 3).
Table 2.
Proteinuria at 3 months (primary endpoint; analysis of covariance model)
| Group | Mean (mg/m2 per day) | Mean Ratio | Change (%) |
|---|---|---|---|
| Prednisone | 49 (2 to 948) | Reference | Reference |
| Rituximab | 28 (2 to 474) | 0.58 (0.18 to 1.95) | −42 (−82 to 95) |
Estimates and 95% CIs of the means of proteinuria at 3 months, their ratio, and percentage change. The model is adjusted for baseline values of proteinuria.
Figure 3.
Graphical representation of the analysis of covariance model. The effect of rituximab is measured as ratio of geometric means of proteinuria at 3 months (0.58; 95% CI, 0.18 to 1.95). The upper limit of the CI (1.95) is smaller than the prespecified noninferiority margin of 3, corresponding to a 200% increment in proteinuria compared with the control group. For results of this analysis see Table 2.
Secondary Outcomes
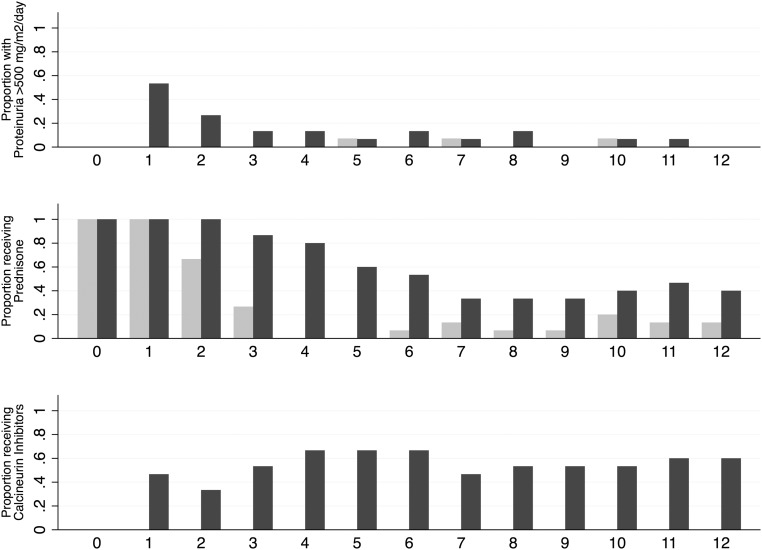
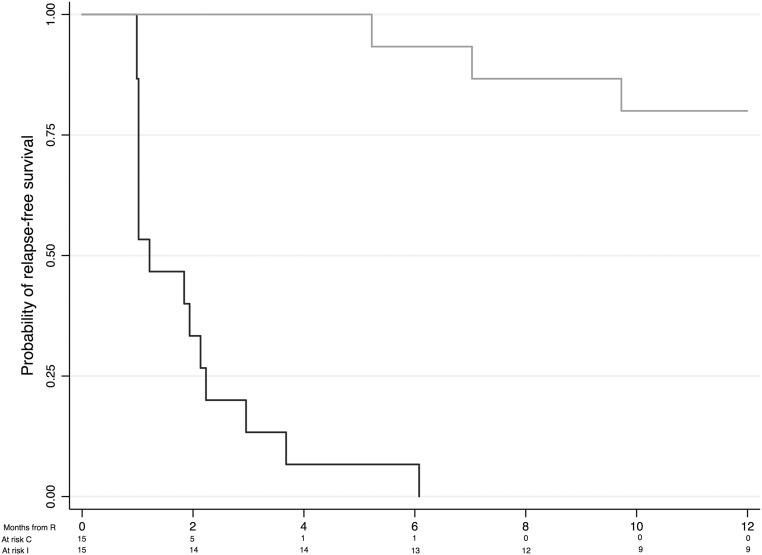
Figure 4 shows the proportion of children with proteinuria >500 mg/m2 per day and those using prednisone or calcineurin inhibitors. Although levels of proteinuria remained similar in the two groups, children treated with rituximab were less likely to require steroids or steroid-sparing agents to maintain remission. The mean±SD prednisone dose at 3 months was lower in the rituximab group than in the control group (0.09±0.21 versus 0.54±0.39 mg/kg per day; P<0.001). Figure 5 summarizes 1-year relapse-free survival by treatment group. Fourteen of 15 children in the control group relapsed during tapering of the prednisone dose, whereas only 1 child in the rituximab group relapsed within 6 months of randomization, resulting in a significant difference between the two survival curves (P<0.01). Of the participants assigned to the rituximab group, relapse-free survival was 66% (95% CI, 38% to 85%) at 1 year and 34% (95% CI, 10% to 59%) at 2 years (Supplemental Figure 1). By study end (follow-up range, 1–60 months), 14 relapses had occurred in 9 children randomly assigned to rituximab (relapse rate, 0.39 per patient-year; 95% CI, 0.26 to 0.61 per patient-year), and 7 children had received one to three additional rituximab courses. Six children in the rituximab group did not experience a relapse. The median relapse-free time between rituximab treatments was 18 months (95% CI, 9 to 32 months) (Supplemental Figure 2).
Figure 4.
Proportion of children with proteinuria >500 mg/m2 per day (top), receiving prednisone (middle) or steroid-sparing agents (bottom) by treatment group. Light gray indicates rituximab group; dark gray indicates control group.
Figure 5.
One-year relapse-free survival by treatment group in the prednisone (control; dark gray) and rituximab (intervention; light gray) groups. The risk of relapse was reduced by 98% in children treated with rituximab (hazard ratio, 0.02; 95% CI, 0.01 to 0.15). C, comparator group; I, intervention group; R, randomization.
Hematologic Data
CD20 counts were reduced to <1% at 1 month in all participants treated with rituximab. After 3 months, CD20 counts remained undetectable in all these children. Mean time to CD20 reconstitution was 5.8 months (median, 5.5; range, 4–12 months). None of the participants had lymphocytopenia or neutropenia. Serum levels of IgG 3 months after rituximab infusion were the same as in the comparator group (Supplemental Table 4).
Adverse Events
During rituximab infusion, all participants reported mild nausea and/or skin rash, which were successfully treated in all cases by slowing the infusion rate and increasing the dose of chlorfenamine. One participant had fever with migrating skin rash and acute arthritis at the hip joint 32 days after the rituximab infusion. Resolution was rapidly and completely achieved in 48 hours with nonsteroidal anti-inflammatory medications. No adverse events occurred in the control group.
Discussion
This trial used a parallel-arm, noninferiority design to test whether rituximab was noninferior to standard care (prednisone) in maintaining proteinuria levels within the normal range. We chose this design because the study population included children successfully maintained in complete remission by prednisone therapy. We found that a single infusion of rituximab allowed steroid withdrawal in children with early-stage uncomplicated SDNS and was noninferior to steroids in maintaining remission. At 1 year, 66% of the children assigned to rituximab were still in steroid-free remission. Median relapse-free time following each infusion was 18 months, and the short-term adverse event profile of rituximab included only reversible side effects during the drug infusion. Steroid withdrawal was not possible in the control group, and all children required a steroid-sparing agent within a month of attempting the steroid taper.
Our findings in uncomplicated forms of SDND treated with a single rituximab infusion complement those from a recent Japanese trial. That trial reported a 73% reduction in the risk of relapse (versus 98% in our study) and a median relapse-free survival duration of 9 months (versus 18 months in our study) in children with complicated forms of frequently relapsing and steroid-dependent nephrotic syndrome treated with four weekly doses of rituximab.14 Important reasons might explain this discrepancy. First, our study included only children with uncomplicated disease responsive to and dependent on steroids alone, as well as substantially shorter disease duration at enrollment (2.5 versus 8 years). Second, our study was more susceptible to bias because it was open label, whereas the Japanese study was a double-blind, placebo-controlled trial.
Our study findings also echo those from two recent observational studies15,16 examining the role of rituximab as a steroid-sparing agent in SDNS and involving a total of 70 children and 20 adults. In both studies, rituximab reduced the rate of relapse and improved kidney function in children with SDNS. The present randomized controlled trial provides longer-term data consistent with these findings.
The limited toxicity of rituximab and the potential benefits of maintaining disease remission while avoiding steroids and calcineurin inhibitors support the use of rituximab as a steroid-sparing agent in juvenile SDNS, but the effects of rituximab in other forms of nephrotic syndrome remain uncertain. Data from a previous trial, for example, do not support the use of rituximab in children with nephrotic syndrome that is resistant to steroids and calcineurin inhibitors.17 In forms of nephrotic syndrome that depend on both steroids and calcineurin inhibitors, rituximab may be used to attain oral drug–free remission,13 but a single rituximab infusion may be required as frequently as every 6 months.15 While more doses may prolong drug-free remission, this may increase the risk of adverse events, such as neutropenia.18 In the Japanese trial, for example, lymphocytopenia and neutropenia occurred in 17% and 8% of the children, respectively.14 Results from our study suggest that oral drug–free remission may be up to three times as long in children with a relatively recent diagnosis of SDNS who are maintained in complete remission with steroids alone.
The mechanisms mediating the effects of rituximab in SDNS are not clear. Most participants in the present trial remained in remission for several months after reconstitution of CD20+ lymphocytes, suggesting that the clinical effect of rituximab may continue beyond its biologic activity on CD20 and that, therefore, CD20 levels alone are not helpful in decision-making about timing of repeated infusions. Rituximab depletes the B cell population, yet this disease has been historically considered a T cell disorder19,20 that is potentially mediated by a circulating factor.21,22 Published investigations offer some insight as to potential explanations for this apparent paradox.23–25 First, podocytes express the B cell costimulatory molecule B7–1 in response to various pathologic stimuli,26,27 and rituximab may delay the appearance of B7–1 on the podocytes, as in B cells of patients with non-Hodgkin lymphoma.28 In support of this theory, abatacept, a blocker of costimulatory molecules, has been used with success in five cases of otherwise treatment-resistant FSGS.27 Second, depletion of B cells may restore the regulatory T-cell population,29,30 which is deficient in nephrotic syndrome.19,31 Rituximab may also deplete the IL-17–producing CD4+ T cells (Th17), which express CD20 on their surface and have been implicated in the pathogenesis of the disease.32,33 The anti-Th17 effect of rituximab formed the theoretical basis for its use in rheumatoid arthritis34–37 and in small vessel vasculitis.38 Finally, rituximab exerts direct and nonimmunologic effects on the podocyte. Rituximab binds to the sphingomyelin phosphodiesterase acid–like 3b protein (SMPDL3b) expressed in the podocyte lipid raft microdomains, the critical plasma membrane signaling platforms for the regulation of the cell cytoskeleton.39 Binding of rituximab to SMPDL3b may prevent the downregulation of the acid sphingomyelinase activity induced by FSGS sera and preserve podocyte structure and function.39
Our study has limitations, including failure to use placebo among controls, lack of blinding, and small sample size. However, we worked to mitigate the consequences of these sources of bias by treating all participants with the same steroid-tapering schedule, using an objective laboratory measure as the outcome, minimizing type I and type II errors in our sample size calculation, and following patients for up to 5 years.
Several questions need to be addressed before rituximab use on a large scale can be recommended. Data are not available on long-term benefits and harms or on the optimal frequency of repeated rituximab infusions, particularly in children relapsing within 6 months of the first rituximab infusion (1 of 15 children in this study). We do not know whether SDNS is a progressive disease or a disease that affects some children more severely than others, and therefore we do not know whether the strategy to maximize the benefits of rituximab therapy should be based on the stage of the disease or the patient characteristics. Preliminary data from this study indicate that most children can be maintained in remission using rituximab treatment alone with repeated infusions every 9–30 months. Second, the long-term safety of rituximab remains uncertain, including the risk of malignancy and progressive multifocal leukoencephalopathy. According to a recent systematic review, these longer-term adverse events that have been reported in the literature occurred in individuals who had received other immunosuppressive medications before rituximab and in nonrenal patients.40 Finally, most recent reports, including the present trial, have included only white patients, and further studies of patients from different ethnic groups are needed.
In summary, data from the present clinical trial indicate that rituximab allows the complete withdrawal of steroids in juvenile, steroid-dependent forms of nephrotic syndrome without adversely affecting clinical outcomes and has an acceptable short-term adverse event profile.
Concise Methods
Trial Design
This multicenter, open-label, randomized, noninferiority trial was conducted at four sites in Italy between April 2009 and April 2014, with a planned minimum follow-up of 1 year. Participants underwent a 1-month run-in period during which instruction on urine collection and dipstick readings were carefully reviewed, proteinuria was monitored, adherence assessed, and oral prednisone dose reduced to the minimum dose required in the previous 6 months. Following randomization, steroid treatment was continued for 1 month and then tapered as tolerated in both groups. Steroids were tapered in an attempt to determine whether a single pulse of rituximab would allow complete steroid withdrawal and maintain steroid-free remission. A parallel-arm, noninferiority design was used to test whether rituximab was noninferior to standard care (prednisone) in maintaining proteinuria levels within a prespecified margin. An independent data and safety monitoring board reviewed safety data once half the participants had been enrolled and again at the end of the study. The protocol was approved by the ethics committee of the coordinating and data collection center and registered in the European Clinical Trials Database (EudraCT: 2008–004486–26).
Participants
Eligible participants were 1–16 years of age with an eGFR>60 ml/min per 1.73 m241,42 who had idiopathic, steroid-dependent nephrotic syndrome for a minimum of 6 to a maximum of 12 months at the time of study entry, regardless of disease duration. Participants must have been receiving high-dose steroids (≥0.7 mg/kg per day)1 continuously to maintain remission for the 3 months preceding enrollment. Participants were excluded if they had received calcineurin inhibitors at any time or had received cyclophosphamide or mycophenolate in the 6 months before enrollment.
Nephrotic syndrome was defined as proteinuria in the nephrotic range (>1000 mg/m2 per day or protein-to-creatinine ratio>4 mg/mg in a single urine specimen) or proteinuria ranging from 250 to 1000 mg/m2 per day in association with hypoalbuminemia (serum albumin<2.5 g/dl) or dyslipidemia (total cholesterol>250 mg/dl). Remission was defined as stable proteinuria<150 mg/m2 per day. Genetic testing and renal biopsy were not required because juvenile nephrotic syndrome is diagnosed largely on the basis of a response to prednisone, as per Kidney Disease Improving Global Outcomes guidelines.1 Cases were considered steroid dependent if patients responded to full doses of prednisone (2 mg/kg) but experienced two consecutive relapses during prednisone tapering or within 2 weeks of prednisone withdrawal.1 Angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers were used at the discretion of the investigators and were kept constant during the study.
We excluded children with previous episodes of macrohematuria, a history of hepatitis B or C, HIV infection, positivity of any marker of autoimmunity (antinuclear antibody, nuclear DNA, ANCA), and low complement C3 levels. Children requiring diuretics, albumin, or anticoagulants were also excluded.
Randomization and Masking
Participants were randomly assigned in a 1:1 ratio to continue standard therapy alone (oral prednisone) or standard therapy plus a single infusion of rituximab. Randomization was done following a permuted-block design with blocks of variable sizes (4–6). A distant site with no clinical involvement in the trial generated the randomization list and kept the allocation concealed. Assignments were notified electronically after signed consent was obtained (and assent for participants who were capable of assenting). Because of the nature of the intervention, clinical investigators and study nurses were not blinded to group assignment, nor were participants.
Study Treatments
In the intervention group, rituximab (MabThera/RITUXAN; 375 mg/m2) was diluted in normal saline (1 mg/ml) and administered at a rate of 0.5–1.5 ml/min over approximately 6 hours following the infusion of 2.5–5 mg of intravenous chlorfenamine maleate (based on the local protocol and patient tolerance), methylprednisolone (2 mg/kg) in normal saline, and oral paracetamol (8 mg/kg). In both groups, prednisone was continued at the same doses as during the run-in, then tapered off by 0.3 mg/kg per week starting at 30 days and withdrawn if proteinuria levels were still <1 g/m2 per day at the end of the taper. In both groups treatment was restarted if proteinuria was ≥1 g/m2 per day during follow-up. In the control group, relapses were treated with prednisone (maximum dose, 2 mg/kg per day) or steroid-sparing agents, including cyclosporine, tacrolimus, or cyclophosphamide, at the discretion of local investigators. Rituximab could be used only after failure of other steroid-sparing agents. In the treatment group relapses were treated with rituximab.
Follow-up
Study visits occurred at baseline and every 3 months thereafter, unless complications or relapses occurred. Study coordinators maintained ongoing contact with the children, their families, and the family physician to collect clinical data, including BP and potential adverse events (Supplemental Table 5). Proteinuria was evaluated daily using a simple dipstick test and monthly using a 24-hour urine collection. Because of the frequent false-positivity of dipsticks,43 we planned a 24-hour urine collection for readings of ≥1+. Kidney function, plasma proteins, and cholesterol values were measured monthly. White blood cell and lymphocyte population counts were monitored monthly in the rituximab group.
Outcomes
Participants randomly assigned to rituximab therapy were compared with those receiving standard care for the primary outcome of the percentage change in daily proteinuria at 3 months. The primary outcome was assessed at 3 months because most SDNS relapses occur within 2 weeks of prednisone withdrawal. We assessed the risk of relapse during the year following randomization as a secondary endpoint and collected data on repeated relapses beyond the initial year. Relapse was defined as proteinuria of 1000 mg/m2 per day (protein-to-creatinine ratio>4 mg/mg) or proteinuria>500 mg/m2 per day (protein-to-creatinine ratio, 2–4 mg/mg) that was associated with hypoalbuminemia.
Statistical Analyses
This study was designed to evaluate whether rituximab was noninferior to standard care in maintaining proteinuria levels in remission with a noninferiority margin of 3 for the geometric mean ratio. This margin was based on previous studies, which reported average proteinuria in children in remission as <300 mg/m2 per day1; a level three times this value remains below the 1000 mg/m2 per day threshold at which prednisone treatment is usually started to treat relapses. We assumed a log-normal distribution of proteinuria and used information from a prior study on its SD.13 With a coefficient of variation of 0.85 (coefficient of variation=exp[SD2]−1) and a type I error rate of 0.01, we estimated that 30 participants would provide a power of 90% to detect a ratio of the geometric means of proteinuria between treatment and control groups <3 (i.e., lower than the prespecified margin), after accounting for a 5% risk of withdrawals.44 We analyzed outcome data according to the intention-to-treat principle, with no interim or subgroup analyses. We modeled 3-month log-transformed proteinuria using an analysis of covariance model with treatment as factor and log-transformed baseline proteinuria as covariate (primary analysis). Missing values at 3 months were replaced using the last-observation-carried-forward method. We conducted sensitivity analyses replacing missing data at 3 months alternately with the highest and the lowest proteinuria value in the study group and using a per-protocol approach. We used the Kaplan-Meier method to describe 1-year relapse-free survival and Cox regression to estimate the effect of treatment. We censored participants at the study end date if they were event free or at the time they left the study (main analyses). In sensitivity analyses we assumed that the event occurred at the last observation time for participants who left the study before the planned 1-year follow-up. We used two-sided tests with a significance level of 0.05 for all analyses. We used Stata software, version 13.1 (http://www.stata.com), and R software, version 3.0.2 (http://www.R-project.org) for all analyses.
Disclosures
All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author). J.R. has received fees from Genetech and Roche.
Acknowledgments
The authors thank Ms. Adriane Lewin for reviewing the reporting material before submission and the research assistants, nurses, and patients and families at all participating centers (Genoa, Milan, Foggia, and Padua) who made this study possible.
The present study was investigator initiated and driven. The Institute Giannina Gaslini provided financial and logistic support to the trial and was in turn supported by funds deriving from Cinque per mille of IRPEF-Finanziamento della ricerca sanitaria, the Italian Ministry of Health, The Renal Child Foundation, and the Fondazione La Nuova Speranza (Progetto integrato per la definizione dei meccanismi implicati nella glomerulo sclerosi focale).
The data safety and monitoring board members included Antonella Trivelli, Giovanni Candiano, Giorgio Piaggio, and Gianluca Caridi. G.M.G. and P.M. declare that all persons who contributed significantly to the work have been here acknowledged. They confirm that they had full access to all the study data and had final responsibility for the decision to submit the study results for publication. In addition, they affirm that this manuscript is an honest, accurate, and transparent account of the study, that no important aspects of the study have been omitted, and that any discrepancies from the study as registered have been explained.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014080799/-/DCSupplemental.
References
- 1.Radhakrishnan J, Cattran DC: The KDIGO practice guideline on glomerulonephritis: reading between the (guide)lines—application to the individual patient. Kidney Int 82: 840–856, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Korbet SM, Schwartz MM, Lewis EJ: Primary focal segmental glomerulosclerosis: Clinical course and response to therapy. Am J Kidney Dis 23: 773–783, 1994 [DOI] [PubMed] [Google Scholar]
- 3.McEnery PT, Strife CF: Nephrotic syndrome in childhood. Management and treatment in patients with minimal change disease, mesangial proliferation, or focal glomerulosclerosis. Pediatr Clin North Am 29: 875–894, 1982 [PubMed] [Google Scholar]
- 4.Ghiggeri GM, Carraro M, Vincenti F: Recurrent focal glomerulosclerosis in the era of genetics of podocyte proteins: Theory and therapy. Nephrol Dial Transplant 19: 1036–1040, 2004 [DOI] [PubMed] [Google Scholar]
- 5.ISKDC : Primary nephrotic syndrome in children: Clinical significance of histopathologic variants of minimal change and of diffuse mesangial hypercellularity. A report of the International Study of Kidney Disease in Children. Kidney Int 20: 765–771, 1981 [DOI] [PubMed] [Google Scholar]
- 6.Kyrieleis HA, Löwik MM, Pronk I, Cruysberg HR, Kremer JA, Oyen WJ, van den Heuvel BL, Wetzels JF, Levtchenko EN: Long-term outcome of biopsy-proven, frequently relapsing minimal-change nephrotic syndrome in children. Clin J Am Soc Nephrol 4: 1593–1600, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ponticelli C, Zucchelli P, Imbasciati E, Cagnoli L, Pozzi C, Passerini P, Grassi C, Limido D, Pasquali S, Volpini T, Sasdelli M, Locatelli F: Controlled trial of methylprednisolone and chlorambucil in idiopathic membranous nephropathy. N Engl J Med 310: 946–950, 1984 [DOI] [PubMed] [Google Scholar]
- 8.Trompeter RS, Lloyd BW, Hicks J, White RH, Cameron JS: Long-term outcome for children with minimal-change nephrotic syndrome. Lancet 1: 368–370, 1985 [DOI] [PubMed] [Google Scholar]
- 9.Hodson EM, Knight JF, Willis NS, Craig JC: Corticosteroid therapy for nephrotic syndrome in children. Cochrane Database Syst Rev (1): CD001533, 2005 [DOI] [PubMed] [Google Scholar]
- 10.ISKDC : Nephrotic syndrome in children: Prediction of histopathology from clinical and laboratory characteristics at time of diagnosis. A report of the International Study of Kidney Disease in Children. Kidney Int 13: 159–165, 1978 [DOI] [PubMed] [Google Scholar]
- 11.Filler G: Treatment of nephrotic syndrome in children and controlled trials. Nephrol Dial Transplant 18 Suppl 6: vi75–78, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Ghiggeri GM, Catarsi P, Scolari F, Caridi G, Bertelli R, Carrea A, Sanna-Cherchi S, Emma F, Allegri L, Cancarini G, Rizzoni GF, Perfumo F: Cyclosporine in patients with steroid-resistant nephrotic syndrome: An open-label, nonrandomized, retrospective study. Clin Ther 26: 1411–1418, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Ravani P, Magnasco A, Edefonti A, Murer L, Rossi R, Ghio L, Benetti E, Scozzola F, Pasini A, Dallera N, Sica F, Belingheri M, Scolari F, Ghiggeri GM: Short-term effects of rituximab in children with steroid- and calcineurin-dependent nephrotic syndrome: A randomized controlled trial. Clin J Am Soc Nephrol 6: 1308–1315, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iijima K, Sako M, Nozu K, Mori R, Tuchida N, Kamei K, Miura K, Aya K, Nakanishi K, Ohtomo Y, Takahashi S, Tanaka R, Kaito H, Nakamura H, Ishikura K, Ito S, Ohashi Y, Rituximab for Childhood-onset Refractory Nephrotic Syndrome (RCRNS) Study Group : Rituximab for childhood-onset, complicated, frequently relapsing nephrotic syndrome or steroid-dependent nephrotic syndrome: A multicentre, double-blind, randomised, placebo-controlled trial. Lancet 384: 1273–1281, 2014 [DOI] [PubMed] [Google Scholar]
- 15.Ravani P, Ponticelli A, Siciliano C, Fornoni A, Magnasco A, Sica F, Bodria M, Caridi G, Wei C, Belingheri M, Ghio L, Merscher-Gomez S, Edefonti A, Pasini A, Montini G, Murtas C, Wang X, Muruve D, Vaglio A, Martorana D, Pani A, Scolari F, Reiser J, Ghiggeri GM: Rituximab is a safe and effective long-term treatment for children with steroid and calcineurin inhibitor-dependent idiopathic nephrotic syndrome. Kidney Int 84: 1025–1033, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruggenenti P, Ruggiero B, Cravedi P, Vivarelli M, Massella L, Marasà M, Chianca A, Rubis N, Ene-Iordache B, Rudnicki M, Pollastro RM, Capasso G, Pisani A, Pennesi M, Emma F, Remuzzi G, Rituximab in Nephrotic Syndrome of Steroid-Dependent or Frequently Relapsing Minimal Change Disease Or Focal Segmental Glomerulosclerosis (NEMO) Study Group : Rituximab in steroid-dependent or frequently relapsing idiopathic nephrotic syndrome. J Am Soc Nephrol 25: 850–863, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Magnasco A, Ravani P, Edefonti A, Murer L, Ghio L, Belingheri M, Benetti E, Murtas C, Messina G, Massella L, Porcellini MG, Montagna M, Regazzi M, Scolari F, Ghiggeri GM: Rituximab in children with resistant idiopathic nephrotic syndrome. J Am Soc Nephrol 23: 1117–1124, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamei K, Takahashi M, Fuyama M, Saida K, Machida H, Sato M, Ogura M, Ito S: Rituximab-associated agranulocytosis in children with refractory idiopathic nephrotic syndrome: case series and review of literature [published online ahead of print August 1, 2014]. Nephrol Dial Transplant 10.1093/ndt/gfu258 [DOI] [PubMed] [Google Scholar]
- 19.Le Berre L, Bruneau S, Naulet J, Renaudin K, Buzelin F, Usal C, Smit H, Condamine T, Soulillou JP, Dantal J: Induction of T regulatory cells attenuates idiopathic nephrotic syndrome. J Am Soc Nephrol 20: 57–67, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shalhoub RJ: Pathogenesis of lipoid nephrosis: A disorder of T-cell function. Lancet 2: 556–560, 1974 [DOI] [PubMed] [Google Scholar]
- 21.McCarthy ET, Sharma M, Savin VJ: Circulating permeability factors in idiopathic nephrotic syndrome and focal segmental glomerulosclerosis. Clin J Am Soc Nephrol 5: 2115–2121, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Wei C, Trachtman H, Li J, Dong C, Friedman AL, Gassman JJ, McMahan JL, Radeva M, Heil KM, Trautmann A, Anarat A, Emre S, Ghiggeri GM, Ozaltin F, Haffner D, Gipson DS, Kaskel F, Fischer DC, Schaefer F, Reiser J, PodoNet and FSGS CT Study Consortia : Circulating suPAR in two cohorts of primary FSGS. J Am Soc Nephrol 23: 2051–2059, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fervenza FC, Abraham RS, Erickson SB, Irazabal MV, Eirin A, Specks U, Nachman PH, Bergstralh EJ, Leung N, Cosio FG, Hogan MC, Dillon JJ, Hickson LJ, Li X, Cattran DC, Mayo Nephrology Collaborative Group : Rituximab therapy in idiopathic membranous nephropathy: A 2-year study. Clin J Am Soc Nephrol 5: 2188–2198, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stasi R, Cooper N, Del Poeta G, Stipa E, Laura Evangelista M, Abruzzese E, Amadori S: Analysis of regulatory T-cell changes in patients with idiopathic thrombocytopenic purpura receiving B cell-depleting therapy with rituximab. Blood 112: 1147–1150, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Vallerskog T, Gunnarsson I, Widhe M, Risselada A, Klareskog L, van Vollenhoven R, Malmström V, Trollmo C: Treatment with rituximab affects both the cellular and the humoral arm of the immune system in patients with SLE. Clin Immunol 122: 62–74, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Reiser J, von Gersdorff G, Loos M, Oh J, Asanuma K, Giardino L, Rastaldi MP, Calvaresi N, Watanabe H, Schwarz K, Faul C, Kretzler M, Davidson A, Sugimoto H, Kalluri R, Sharpe AH, Kreidberg JA, Mundel P: Induction of B7-1 in podocytes is associated with nephrotic syndrome. J Clin Invest 113: 1390–1397, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu CC, Fornoni A, Weins A, Hakroush S, Maiguel D, Sageshima J, Chen L, Ciancio G, Faridi MH, Behr D, Campbell KN, Chang JM, Chen HC, Oh J, Faul C, Arnaout MA, Fiorina P, Gupta V, Greka A, Burke GW, 3rd, Mundel P: Abatacept in B7-1-positive proteinuric kidney disease. N Engl J Med 369: 2416–2423, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishio M, Fujimoto K, Yamamoto S, Endo T, Sakai T, Obara M, Kumano K, Yamaguchi K, Takeda Y, Goto H, Sato N, Koizumi K, Mukai M, Koike T: Delayed redistribution of CD27, CD40 and CD80 positive B cells and the impaired in vitro immunoglobulin production in patients with non-Hodgkin lymphoma after rituximab treatment as an adjuvant to autologous stem cell transplantation. Br J Haematol 137: 349–354, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Saadoun D, Rosenzwajg M, Landau D, Piette JC, Klatzmann D, Cacoub P: Restoration of peripheral immune homeostasis after rituximab in mixed cryoglobulinemia vasculitis. Blood 111: 5334–5341, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Sfikakis PP, Souliotis VL, Fragiadaki KG, Moutsopoulos HM, Boletis JN, Theofilopoulos AN: Increased expression of the FoxP3 functional marker of regulatory T cells following B cell depletion with rituximab in patients with lupus nephritis. Clin Immunol 123: 66–73, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Bertelli R, Trivelli A, Magnasco A, Cioni M, Bodria M, Carrea A, Montobbio G, Barbano G, Ghiggeri GM: Failure of regulation results in an amplified oxidation burst by neutrophils in children with primary nephrotic syndrome. Clin Exp Immunol 161: 151–158, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Couser WG: Basic and translational concepts of immune-mediated glomerular diseases. J Am Soc Nephrol 23: 381–399, 2012 [DOI] [PubMed] [Google Scholar]
- 33.Liu LL, Qin Y, Cai JF, Wang HY, Tao JL, Li H, Chen LM, Li MX, Li XM, Li XW: Th17/Treg imbalance in adult patients with minimal change nephrotic syndrome. Clin Immunol 139: 314–320, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Pierson ER, Stromnes IM, Goverman JM: B cells promote induction of experimental autoimmune encephalomyelitis by facilitating reactivation of T cells in the central nervous system. J Immunol 192: 929–939, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vaglio A, Buzio C, Zwerina J: Eosinophilic granulomatosis with polyangiitis (Churg-Strauss): State of the art. Allergy 68: 261–273, 2013 [DOI] [PubMed] [Google Scholar]
- 36.van de Veerdonk FL, Lauwerys B, Marijnissen RJ, Timmermans K, Di Padova F, Koenders MI, Gutierrez-Roelens I, Durez P, Netea MG, van der Meer JW, van den Berg WB, Joosten LA: The anti-CD20 antibody rituximab reduces the Th17 cell response. Arthritis Rheum 63: 1507–1516, 2011 [DOI] [PubMed] [Google Scholar]
- 37.Yamamoto A, Sato K, Miyoshi F, Shindo Y, Yoshida Y, Yokota K, Nakajima K, Akiba H, Asanuma Y, Akiyama Y, Mimura T: Analysis of cytokine production patterns of peripheral blood mononuclear cells from a rheumatoid arthritis patient successfully treated with rituximab. Modern Rheumatol 20: 183–187, 2010 [DOI] [PubMed] [Google Scholar]
- 38.Kallenberg CG: Pathophysiology of ANCA-associated small vessel vasculitis. Curr Rheumatol Rep 12: 399–405, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fornoni A, Sageshima J, Wei C, Merscher-Gomez S, Aguillon-Prada R, Jauregui AN, Li J, Mattiazzi A, Ciancio G, Chen L, Zilleruelo G, Abitbol C, Chandar J, Seeherunvong W, Ricordi C, Ikehata M, Rastaldi MP, Reiser J, Burke GW, 3rd: Rituximab targets podocytes in recurrent focal segmental glomerulosclerosis. Sci Transl Med 3: 85ra46, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lopez-Olivo MA, Tayar JH, Martinez-Lopez JA, Pollono EN, Cueto JP, Gonzales-Crespo MR, Fulton S, Suarez-Almazor ME: Risk of malignancies in patients with rheumatoid arthritis treated with biologic therapy: A meta-analysis. JAMA 308: 898–908, 2012 [DOI] [PubMed] [Google Scholar]
- 41.Schwartz GJ, Gauthier B: A simple estimate of glomerular filtration rate in adolescent boys. J Pediatr 106: 522–526, 1985 [DOI] [PubMed] [Google Scholar]
- 42.Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL: New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20: 629–637, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hogg RJ, Portman RJ, Milliner D, Lemley KV, Eddy A, Ingelfinger J: Evaluation and management of proteinuria and nephrotic syndrome in children: Recommendations from a pediatric nephrology panel established at the National Kidney Foundation conference on proteinuria, albuminuria, risk, assessment, detection, and elimination (PARADE). Pediatrics 105: 1242–1249, 2000 [DOI] [PubMed] [Google Scholar]
- 44.Wolfe R, Carlin JB: Sample-size calculation for a log-transformed outcome measure. Control Clin Trials 20: 547–554, 1999 [DOI] [PubMed] [Google Scholar]