Abstract
Results of recent clinical trials and experimental studies indicate that whereas atherosclerotic renovascular disease can accelerate both systemic hypertension and tissue injury in the poststenotic kidney, restoring vessel patency alone is insufficient to recover kidney function for most subjects. Kidney injury in atherosclerotic renovascular disease reflects complex interactions among vascular rarefication, oxidative stress injury, and recruitment of inflammatory cellular elements that ultimately produce fibrosis. Classic paradigms for simply restoring blood flow are shifting to implementation of therapy targeting mitochondria and cell-based functions to allow regeneration of vascular, glomerular, and tubular structures sufficient to recover, or at least stabilize, renal function. These developments offer exciting possibilities of repair and regeneration of kidney tissue that may limit progressive CKD in atherosclerotic renovascular disease and may apply to other conditions in which inflammatory injury is a major common pathway.
Keywords: renal artery stenosis, oxidative stress, macrophages, mesangial cells
Few clinical renal diseases have undergone more dramatic conceptual pendulum swings than renovascular disease and ischemic nephropathy. For decades, the search for renovascular hypertension with the goal of curing secondary hypertension with renal revascularization had been an established principle of clinical practice. In 2008 we reviewed the expanding application of endovascular stenting for renal artery stenosis, noting that stent procedures for atherosclerotic renovascular disease (ARVD) in Medicare recipients had risen from 7660 to 18,520 between 1996 and 2000 and was estimated to be >35,000 by 2005.1,2 A skeptical Center for Medicare and Medicaid Services review panel conducted in 2007 acknowledged equipoise uncertainty about the added value of stenting to medical therapy alone and the need for prospective randomized trials.1,3 Since then, publication of the Cardiovascular Outcomes for Renal Artery Lesions (CORAL) trial in 2014 and results of the previous Angioplasty and Stenting for Renal Atherosclerotic Lesions (ASTRAL) and Stent Placement in Patients with Atherosclerotic Renal Artery Stenosis and Impaired Renal function trials in 2009 have substantially lowered enthusiasm for renal revascularization.4 Detection and intervention for even high-grade ARVD are now limited mainly to refractory clinical syndromes of progressive renal functional loss, episodes of flash pulmonary edema, and intractable hypertension.5
Despite their individual weaknesses and recruitment difficulties, results of these prospective trials have been consistent: for most patients with ARVD, restoring vessel patency either with surgery or endovascular stenting fails to materially recover kidney function or to add clinical benefit beyond that achievable with current medical therapy, which most often includes blockade of the renin-angiotensin system and statin therapy. It must be emphasized that ARVD develops in older patients with heterogeneous backgrounds of preexisting kidney injury, hypertension, and susceptibility of varying degrees of reduced perfusion. What often is overlooked in this discussion is the equally consistent identification of a subset of patients with persistent and progressive renal dysfunction, as we have discussed.4,6 The CORAL, ASTRAL, and Stent Placement in Patients with Atherosclerotic Renal Artery Stenosis and Impaired Renal studies each identified subsets of between 16% and 22% of subjects developing a renal end point, variously characterized as a 20%–30% fall in GFR, doubling of serum creatinine, and/or ESRD. Remarkably, average rates of developing such end points do not differ between those with or without renal revascularization.
These human treatment trials have been conducted in parallel with experimental and clinical studies that are increasing our understanding and changing many fundamental paradigms of vascular disease and chronic kidney injury. The goal of this review is to briefly summarize changes in these paradigms for nephrologists and to outline where future therapeutic strategies in this field may be directed. Many of these mechanistic advances for repairing kidney injury in ARVD may be applicable more broadly to other forms of both microvascular injury and inflammatory pathways in CKD.
Hemodynamics and the Focus on Blood Flow
Some of the ambiguity regarding ARVD derives from simply defining and measuring properties of vascular occlusive lesions. Epidemiologic and imaging studies frequently refer to occlusion >50%–60% lumen occlusion, as if that information were readily available. Most of these studies estimate the presence of vascular lesions on the basis of two-dimensional imaging from arterial catheter images, gadolinium-contrast magnetic resonance (MR) angiography, or iodinated contrast computed tomography angiography, which are known to have limited validity and reproducibility.7 Detailed studies using latex casts and hemodynamic measurements indicate that measurable reductions in translesional pressures or blood flow develop only when lumen occlusion reaches some more severe or critical level, usually >70%–80% occlusion. Studies identifying release of renal renin into the renal vein indicate that activation of these pressor systems occurs only after translesional pressure gradients exceed 10–20 mmHg.8,9 Hence, many lesions using basic imaging tools are hemodynamically insignificant. Many investigators also have observed highly variable hemodynamic effects of vascular lesions even in those with <60% occlusion, in part related to fluctuating flow volumes (e.g., cardiac output). In some of these instances, a given lesion can generate widely variable gradients and likely have widely variable poststenotic waveforms depending on flow characteristics. Although renovascular disease remains a prototype of angiotensin-dependent hypertension, these hormonal responses are sometimes transient.10 It is likely that developing vascular occlusion is associated with intermittent reductions in renal perfusion pressures and flows that resolve as systemic pressures rise, despite a gradient between peak aortic and renal pressures.11 It must be recognized that antihypertensive drug therapy to lower systemic pressures inherently threatens to reduce renal perfusion pressures in this setting. One cannot exclude the possibility that ARVD presents a long-term process of repeated acute ischemic injury episodes from which the kidney normally can recover. Such a paradigm is entirely consistent with the observation of chronically elevated injury biomarkers, such as neutrophil gelatinase–associated lipocalin (NGAL) obtained from renal vein sampling in humans.12 Repeated episodes of experimental AKI with apparent tubular recovery can later lead to interstitial fibrosis and inflammation.13 Even when moderate arterial occlusion reaches a steady state, progression to more severe stenosis can restart this process, eventually producing malignant-phase hypertension if untreated.14 Perhaps just as importantly, even some severe vascular occlusive lesions either fail to activate pressor responses or elicit counter regulatory changes that limit blood pressure changes.15 For some subjects, cardiac dysfunction limits systemic pressor responses as part of a true cardiorenal syndrome.16,17 In such patients, progressive, severe occlusion may fail to elicit hypertension altogether, despite leading to advanced parenchymal fibrosis in the affected kidney.18
How vascular lesions develop over time may have important additional effects. Fibromuscular disease that often appears in younger subjects infrequently leads to parenchymal renal injury, short of vessel occlusion. Studies in human atherosclerotic renovascular lesions indicate that subclinical lesions already have localization of inflammatory T-regulatory cells within vessel walls and modification of T cell–associated cytokine release even before reaching critical hemodynamic occlusion.19 In other patients, segmental vascular occlusion is related to localized dissection and/or shelving of calcified debris that may be unrelated to inflammatory processes altogether. It should not be surprising that effects of large-vessel occlusive lesions may have widely variable effects on arterial pressure regulation and poststenotic processes within the kidney.
As a result, simply identifying and treating patients on the basis of a visualized ARVD lesion by radiographic imaging produces a widely heterogeneous population of subjects with widely differing responses to restoring vessel patency. Effective therapy depends on establishing the linkage between ARVD and clinical manifestations for that individual patient, specifically accelerating hypertension, parenchymal kidney injury, and/or circulatory congestion/pulmonary edema. Although measurement of renal vein renin levels is now rarely performed, lateralization of renin release to a poststenotic kidney was a consistently reliable predictor of potential BP reduction after surgical revascularization in >90% of patients.20,21 Identification of valid biomarkers to predict recovery of kidney function after revascularization remains an important unmet need.
Tissue Damage within the Poststenotic Kidney
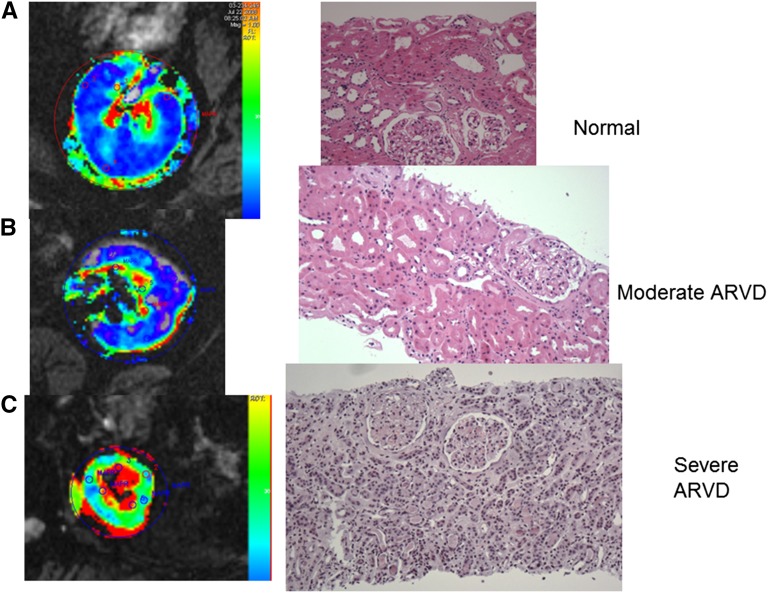
Perhaps the most important shift in thinking about ARVD derives from our understanding of tissue oxygenation and microvascular injury within the poststenotic kidney. Reduction in renal perfusion pressure activates multiple pressor systems, including the renin-angiotensin system22 and renal adrenergic nerves.23 When detected and treated soon after vascular occlusion, as in the case of vessel dissection or occlusion by an aortic stent graft, restoring blood flow can reverse this process and systemic hypertension, often without evident injury to the kidney.7 Much more commonly, large-vessel occlusive ARVD develops slowly, most often combined with other major cardiovascular risk factors, including smoking, preexisting hypertension, and dyslipidemia. Somewhat surprisingly, moderate single-kidney reductions of cortical and medullary blood flow of nearly 30%–40% (as measured by contrast transit times using multidetector computed tomography) sufficient to reduce GFR and activate the renin-angiotensin system most often do not produce measurable tissue deoxygenation in human studies (Figure 1, A and B, left panels).24 Our human studies focus on tissue oxygenation in both essential hypertension and renovascular disease, using formal measurements of cortical and medullary deoxyhemoglobin as measured by blood oxygen level–dependent (BOLD) MR under standardized conditions of sodium balance and antihypertensive drug therapy.25 BOLD MR consistently demonstrates large gradients in tissue oxygenation with remarkably low levels of deoxyhemoglobin in kidney cortex, evolving to areas of higher deoxygenation in deeper medullary areas,25,26 confirmed with the use of oxygen probes.27 The complexity of tissue oxygenation within the kidney reflects multiple factors, including reduced postglomerular blood supply, active oxygen consumption by energy-consuming solute transport in tubular segments, and arteriovenous shunting.28 Preservation of tissue oxygenation in moderate ARVD likely reflects the fact that blood flow to the kidney normally is far in excess of its metabolic requirements because of its role as a filtering organ.29 These observations are supported by finding minimal histologic changes in the poststenotic kidney on transjugular biopsy (Figure 1B, right panel). Furthermore, preservation of progressively hypoxic oxygen gradients extending into medullary regions within these kidneys likely reflects limited solute filtration and therefore reduced energy requirements for active solute reabsorption.30 Taken together, these data demonstrate remarkable ability of the kidney to adapt to moderate blood flow reductions with preservation of both oxygen gradients and function. We interpret these data to explain the relative stability of renal function in many patients with ARVD during antihypertensive drug therapy as reported in trials such as ASTRAL and CORAL.
Figure 1.
Cortical hypoxia and inflammation develop in severe ARVD. Parametric axial image maps of deoxyhemoglobin determined by BOLD MR (left panels) and transjugular or needle biopsy samples from (A) a normal subject (kidney donor implantation biopsy), (B) an individual with moderate ARVD (reduced blood flow and GFR, peak ultrasound velocity 350 cm/s), and (C) severe ARVD with loss of cortical volume and function and peak ultrasound velocity >450 cm/s. Panel (B) shows that despite reduced blood flow, tissue oxygenation in the cortex and medullary segments are remarkably preserved, consistent with near normal histologic appearance and stable renal function during antihypertensive drug therapy. Panel (C) demonstrates higher levels of cortical deoxyhemoglobin (green) associated with more severe ARVD, along with a larger fraction of the medullary segments being overtly hypoxic with elevated (yellow/red) deoxyhemoglobin. These findings were associated with histologic changes of interstitial inflammatory cell infiltrates (see additional information in the text) and tubular degeneration.
It is essential particularly for nephrologists to recognize the limits of adaptation to reduced blood flow to the kidney. Studies using BOLD MR in patients with higher-grade vascular occlusion demonstrate overt cortical hypoxia and increased fractional hypoxia (the proportion of kidney area exceeding a specified level of deoxyhemoglobin).31,32 Such individuals have more severe and sustained vascular occlusive disease than participants in many of the treatment trials. Biopsy samples from these kidneys demonstrate not only glomerular collapse, but also disruption of tubular structures, with appearance of interstitial inflammatory cells (Figure 1C, right panel). Histologic inflammation in patients with advanced ARVD is characterized by extensive T cell and macrophage infiltration in addition to fibrosis.33,34 Hence, it is equally clear that beyond the limits of tolerable reduction in blood flow, overt cortical hypoxia associated with more severe inflammatory injury eventually develops in the poststenotic kidney, as illustrated in Figure 2.
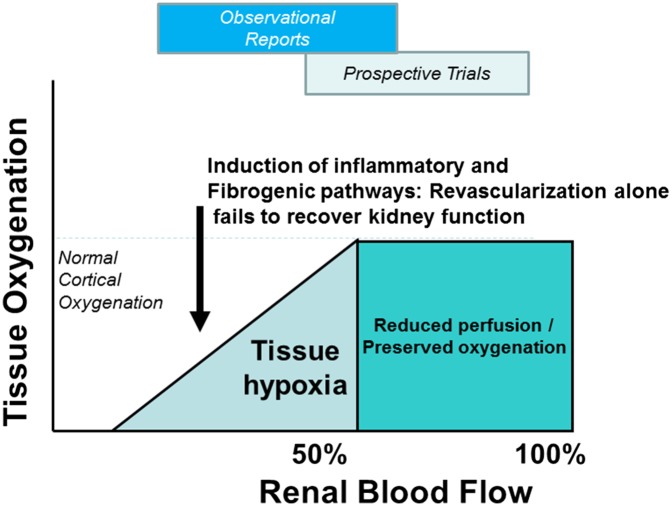
Figure 2.
Clinical results depend on the degree of blood flow reduction tissue hypoxia and the level of inflammatory and fibrotic injury. Schematic summary of the working paradigms related to ARVD. As a filtering organ, the kidney can adapt to moderate reductions of blood flow with no loss of tissue oxygenation. When one exceeds the lower limits of adaptation, eventual tissue hypoxia develops associated with activation of multiple injury pathways that may not be affected by simply restoring vessel patency and blood flow. Substantial differences between observational reports of ARVD and results from prospective trials likely reflect a degree of selection bias favoring moderate disease on the basis of vascular anatomy and hemodynamics alone (see additional information in the text).
The inter-relationships and reproducibility of these mechanisms are remarkably consistent between experimental swine models and human ARVD. Experimental studies in a gradually developing swine model demonstrate additional effects of the atherosclerotic environment itself (produced by cholesterol feeding) on microvascular density and endothelial function within the kidney cortex.35 These vessels demonstrate disturbances of oxidative pathways, generation of toxic oxygen species, and free radicals that accelerate intrarenal injury and fibrosis.36 To some degree these pathways can be modified by statin and/or antioxidant therapy in animal models.37,38 Importantly, prolonged reductions in renal perfusion pressures and flows in these models lead to microvascular rarefication with partial collapse and obliteration of the arteriolar microcirculation.39 These processes eventually activate an array of proinflammatory cytokine signals, including monocyte chemoattractant protein-1, TNF-α, ILs (IL-6 and IL-1), and diffuse tissue production of TGF-β.40,41 Blood levels of NGAL are elevated. Studies of TGF-β gene expression in a murine model confirm major upregulation in both the stenotic and contralateral kidneys.42 Studies of metabolomics profiles from both stenotic and contralateral kidneys underscore the cross-talk between kidneys that may participate in progressive injury to both.43 Measurement of cytokine signatures obtained from the renal vein of both human subjects and experimental models confirm a highly proinflammatory environment tilted toward homing signals that attract circulating peripheral blood monocytes, T cells, and differentiation toward proinflammatory macrophages.40,41 Biopsies from human subjects confirm that cellular infiltrates accumulate in poststenotic kidneys and that inflammatory signaling is correlated with the degree of tissue hypoxia within the poststenotic kidney cortex.32 Comparison of inflammatory cell infiltrates indicate T-lymphocyte (CD3+) and macrophage (CD68+) accumulation in ARVD intermediate between normal kidney donors and those with complete occlusion leading to nephrectomy.34 No such differences regarding B-lymphocyte (CD20+) cell counts were observed. Importantly, genetic knockout of downstream pathways (SMAD-3) for TGF-β in models of experimental renovascular disease protects the poststenotic kidney from occlusive vascular injury.44
Taken together, these data indicate that ARVD produces an initial hemodynamic reduction in blood flow that ultimately transitions into a proinflammatory state within the poststenotic kidney. Importantly, restoring blood flow by endovascular stenting can reverse the degree of cortical hypoxia32 and lead to partial recovery of blood flow, but it fails to reverse inflammatory signaling once established. Repeat sampling of renal vein cytokine signatures 3 months after renal artery stenting demonstrates persistent elevations of NGAL, TNF-α, and other inflammatory markers.32
Can Injury within the Poststenotic Kidney Be Prevented or Reversed?
Experimental studies in both swine and murine models identify complex pathways and interactions by which tissue injury develops in ARVD. As with many forms of CKD, a critical unresolved issue is whether these pathways can be targeted to repair or regenerate functional kidney tissue. Loss of microvessels, particularly in the renal cortex, results from perivascular fibrosis, metabolically driven injury to the vessel walls, and degradation of growth factors by reactive oxygen species.36 Local oxidative stress associated with mitochondrial dysfunction and apoptosis appears to participate in vessel loss and tubulointerstitial fibrosis. Consistent with these effects in the atherosclerotic environment produced by high-cholesterol feeding, aggressive administration of statins reduces oxidative stress injury in animal models and diminishes tissue TGF-β expression and fibrosis measurably in human nephrectomy samples.33,37 Administration of agents that block the renin-angiotensin-aldosterone system (RAAS) (specifically angiotensin-receptor blockers) improves cortical perfusion, increases microvascular density, and reduces markers of oxidative stress injury in the swine model.45 These observations extend and support clinical observations of reduced cardiovascular morbidity and mortality in patients with ARVD that have been treated with RAAS blockade.46,47 Indeed, the standard of medical care for ARVD in the CORAL study included RAAS blockade universally, which was achieved by providing candesartan to all participants.48
What additional measures might reduce the potential for oxidant injury, consequent to either disturbed mitochondrial function or to reperfusion injury after renal revascularization? Therapeutic administration of antioxidants did attenuate renal remodeling and partially protected microvascular architecture, but not GFR.49 Intravenous administration of a mitochondrial-targeted peptide (SS31, Bendavia; Stealth Biopharmaceuticals, Boston, MA) during renal artery angioplasty in a swine model restored measures of mitochondrial biogenesis and limited vascular rarefication, apoptosis, oxidative stress, and fibrosis.50 Remarkably, blood flow and GFR in the treated animals were restored to control levels, suggesting that sustained mitochondrial dysfunction may severely limit the potential benefits of restoring renal blood flow. Mitochondrial swelling and distortion are among the earliest histologic changes observed in temporary renal artery clamping in humans.51 Daily subcutaneous administration of this peptide also attenuates kidney damage and improves oxygenation, suggesting that mitochondrial dysfunction may participate both in acute and chronic injury pathways in experimental ARVD (Figure 3).52 These data suggest that targeting mitochondrial function and preventing oxidative stress injury at the time of revascularization and/or during chronic therapy may offer substantial benefit. This approach merits further study in human subjects.
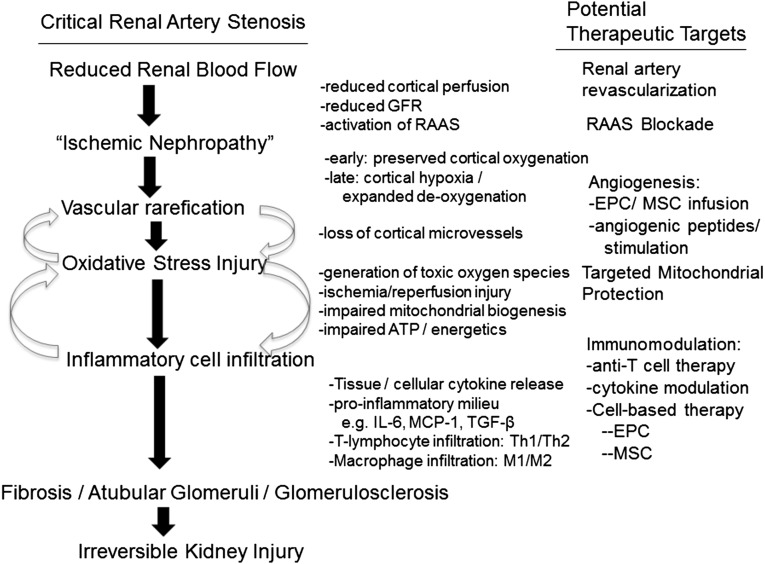
Figure 3.
Injury pathways and targets in ARVD. Working diagram highlighting recent experimental studies delineating specific pathways of oxidative stress injury and inflammatory injury pathways in the poststenotic kidney. The right panel identifies specific therapeutic targets that may alleviate these injury pathways, over and above simply restoring blood flow (see additional information in the text). Many of these pathways likely overlap in many forms of kidney injury. EPC, endothelial progenitor cells; MCP, monocyte chemoattractant protein.
Can inflammatory injury pathways within the poststenotic kidney be reversed or channeled to favor tissue repair and regeneration? Experimental studies using vascular growth factors (e.g., hepatocyte, vascular endothelial growth factors) indicate that angiogenesis is indeed possible and may modify the inflammatory environment. Intra-arterial injection of endothelial progenitor cells increases renal expression of angiogenic factors, proliferation, and maturation of new vessels, eventually leading to reduced fibrosis in the poststenotic kidney cortex, and improves blood flow and GFR.53 Their effectiveness appears to be magnified by enhanced expression of cognate receptors related to injury signals from the stenotic kidney that facilitate local adhesion and retention in the damaged kidney.54 Remarkably, adipose-derived mesenchymal stem/stromal cells (MSCs) share many of these properties and have practical advantages, including low immunogenicity and robust growth in culture. MSCs also are potent immunomodulators and are capable of modifying both macrophage and T cell function under specific conditions. Delivery of MSCs into the poststenotic renal artery in experimental swine ARVD attenuates interstitial fibrosis and inflammatory injury, improves microvascular density, and decreases oxidative stress.55 MSCs also reduce cytokine expression in poststenotic kidneys, specifically TNF-α and monocyte chemoattractant protein-1.56 It has become clear that macrophage polarity in ischemic injury has a role in determining the potential for regenerating injured tubules, at least in acute renal artery occlusion models. A model deficient in IL-1 receptor kinase-M, which is required for the transition from M1 (proinflammatory) to M2 (considered reparative) polarity, demonstrates failure to regenerate tubular epithelial cells at the glomerulotubular junction, leading to long-term atubular glomeruli, interstitial fibrosis, and loss of kidney function.57 Importantly, MSCs are capable of modulating the specific phenotypes and functions of multiple inflammatory cells, including directing macrophages toward a reparative M2 polarity, depending in part on the specific conditions within the local tissue microenvironment. Whether intrarenal administration of MSCs can modify the inflammatory conditions in human subjects with ARVD is currently under study (https://clinicaltrials.gov/).
Taken together, these studies offer the possibility that cell-based therapy may offer an opportunity to modulate local inflammatory injury in the kidney (Figure 3). ARVD presents an exceptional model to examine these possibilities within human subjects, insofar as individual kidneys can be treated without necessarily affecting the contralateral kidney. Moreover, this clinical condition provides a platform wherein the presumptive primary injury induced by vascular occlusion can be removed by successful revascularization. Hence, one has the opportunity to examine and modify tissue injury and repair without ongoing stimuli for disease on the basis of reduced blood flow or tissue hypoxia per se. These data underscore the enormous potential value of further clinical investigation of novel therapies in ARVD, potentially as a precursor to studies in other renal inflammatory disease as well.
Summary
Taken together, both clinical trials and experimental studies indicate that although ARVD is capable of triggering both accelerated systemic hypertension and tissue injury in the poststenotic kidney, restoring vessel patency alone is insufficient to recover kidney function for most subjects. Kidney injury in ARVD reflects complex interactions between vascular rarefication, oxidative stress injury, and recruitment of inflammatory cellular elements that ultimately produce fibrosis. Classic paradigms for simply restoring blood flow are shifting to implementation of therapy targeting mitochondria and cellular injury to allow regeneration of vascular, glomerular, and tubular structures sufficient to recover, or at least stabilize, renal function. These data offer exciting possibilities of repair and regeneration of kidney tissue that may limit progressive CKD and may apply to other conditions in which inflammatory injury is a major common pathway.
Disclosures
None.
Acknowledgments
The projects described were supported by the National Institute for Diabetes, Digestive and Kidney Diseases (NIDDK) (award number R01-DK100081) and the National Institutes of Health (NIH)/National Center for Research Resources Clinical and Translational Science Awards (grant number UL1-RR024150).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIDDK or NIH.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Textor SC: Atherosclerotic renal artery stenosis: Overtreated but underrated? J Am Soc Nephrol 19: 656–659, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Murphy TP, Soares G, Kim M: Increase in utilization of percutaneous renal artery interventions by medicare beneficiaries, 1996-2000. AJR Am J Roentgenol 183: 561–568, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Balk E, Raman G, Chung M, Ip S, Tatsioni A, Alonso A, Chew P, Gilbert SJ, Lau J: Effectiveness of management strategies for renal artery stenosis: A systematic review. Ann Intern Med 145: 901–912, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Herrmann SM, Saad A, Textor SC: Management of atherosclerotic renovascular disease after Cardiovascular Outcomes in Renal Atherosclerotic Lesions (CORAL). Nephrol Dial Transplant 30: 366–375, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ritchie J, Green D, Chrysochou C, Chalmers N, Foley RN, Kalra PA: High-risk clinical presentations in atherosclerotic renovascular disease: Prognosis and response to renal artery revascularization. Am J Kidney Dis 63: 186–197, 2014 [DOI] [PubMed] [Google Scholar]
- 6.Textor SC: Renovascular hypertension: is there still a role for stent revascularization? Curr Opin Nephrol Hypertens 22: 525–530, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Textor SC, Misra S, Oderich GS: Percutaneous revascularization for ischemic nephropathy: The past, present, and future. Kidney Int 83: 28–40, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Bruyne B, Manoharan G, Pijls NH, Verhamme K, Madaric J, Bartunek J, Vanderheyden M, Heyndrickx GR: Assessment of renal artery stenosis severity by pressure gradient measurements. J Am Coll Cardiol 48: 1851–1855, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Drieghe B, Madaric J, Sarno G, Manoharan G, Bartunek J, Heyndrickx GR, Pijls NH, De Bruyne B: Assessment of renal artery stenosis: Side-by-side comparison of angiography and duplex ultrasound with pressure gradient measurements. Eur Heart J 29: 517–524, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Zhu XY, Urbieta Caceres VH, Favreau FD, Krier JD, Lerman A, Lerman LO: Enhanced endothelial progenitor cell angiogenic potency, present in early experimental renovascular hypertension, deteriorates with disease duration. J Hypertens 29: 1972–1979, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Textor SC, Smith-Powell L: Post-stenotic arterial pressures, renal haemodynamics and sodium excretion during graded pressure reduction in conscious rats with one- and two-kidney coarctation hypertension. J Hypertens 6: 311–319, 1988 [PubMed] [Google Scholar]
- 12.Eirin A, Gloviczki ML, Tang H, Rule AD, Woollard JR, Lerman A, Textor SC, Lerman LO: Chronic renovascular hypertension is associated with elevated levels of neutrophil gelatinase-associated lipocalin. Nephrol Dial Transplant 27: 4153–4161, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nath KA, Croatt AJ, Haggard JJ, Grande JP: Renal response to repetitive exposure to heme proteins: Chronic injury induced by an acute insult. Kidney Int 57: 2423–2433, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Dzau VJ, Siwek LG, Rosen S, Farhi ER, Mizoguchi H, Barger AC: Sequential renal hemodynamics in experimental benign and malignant hypertension. Hypertension 3: I63–I68, 1981 [DOI] [PubMed] [Google Scholar]
- 15.Dustan HP, Humphries AW, Dewolfe VG, Page IH: Normal arterial pressure in patients with renal arterial stenoses. JAMA 187: 1028–1029, 1964 [DOI] [PubMed] [Google Scholar]
- 16.Messerli FH, Bangalore S, Makani H, Rimoldi SF, Allemann Y, White CJ, Textor S, Sleight P: Flash pulmonary oedema and bilateral renal artery stenosis: The Pickering syndrome. Eur Heart J 32: 2231–2235, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Urbieta-Caceres VH, Zhu XY, Jordan KL, Tang H, Textor K, Lerman A, Lerman LO: Selective improvement in renal function preserved remote myocardial microvascular integrity and architecture in experimental renovascular disease. Atherosclerosis 221: 350–358, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheung CM, Wright JR, Shurrab AE, Mamtora H, Foley RN, O’Donoghue DJ, Waldek S, Kalra PA: Epidemiology of renal dysfunction and patient outcome in atherosclerotic renal artery occlusion. J Am Soc Nephrol 13: 149–157, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Kotliar C, Juncos L, Inserra F, de Cavanagh EM, Chuluyan E, Aquino JB, Hita A, Navari C, Sánchez R: Local and systemic cellular immunity in early renal artery atherosclerosis. Clin J Am Soc Nephrol 7: 224–230, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Maxwell MH, Marks LS, Lupu AN, Cahill PJ, Franklin SS, Kaufman JJ: Predictive value of renin determinations in renal artery stenosis. JAMA 238: 2617–2620, 1977 [PubMed] [Google Scholar]
- 21.Simon G, Coleman CC: Captopril-stimulated renal vein renin measurements in the diagnosis of atherosclerotic renovascular hypertension. Am J Hypertens 7: 1–6, 1994 [DOI] [PubMed] [Google Scholar]
- 22.Basso N, Terragno NA: History about the discovery of the renin-angiotensin system. Hypertension 38: 1246–1249, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Wyss JM, Oparil S, Sripairojthikoon W: Neuronal control of the kidney: Contribution to hypertension. Can J Physiol Pharmacol 70: 759–770, 1992 [DOI] [PubMed] [Google Scholar]
- 24.Gloviczki ML, Glockner JF, Lerman LO, McKusick MA, Misra S, Grande JP, Textor SC: Preserved oxygenation despite reduced blood flow in poststenotic kidneys in human atherosclerotic renal artery stenosis. Hypertension 55: 961–966, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gloviczki ML, Glockner J, Gomez SI, Romero JC, Lerman LO, McKusick M, Textor SC: Comparison of 1.5 and 3 T BOLD MR to study oxygenation of kidney cortex and medulla in human renovascular disease. Invest Radiol 44: 566–571, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pruijm M, Hofmann L, Maillard M, Tremblay S, Glatz N, Wuerzner G, Burnier M, Vogt B: Effect of sodium loading/depletion on renal oxygenation in young normotensive and hypertensive men. Hypertension 55: 1116–1122, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Warner L, Gomez SI, Bolterman R, Haas JA, Bentley MD, Lerman LO, Romero JC: Regional decreases in renal oxygenation during graded acute renal arterial stenosis: A case for renal ischemia. Am J Physiol Regul Integr Comp Physiol 296: R67–R71, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Evans RG, Gardiner BS, Smith DW, O’Connor PM: Intrarenal oxygenation: Unique challenges and the biophysical basis of homeostasis. Am J Physiol Renal Physiol 295: F1259–F1270, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Heyman SN, Khamaisi M, Rosen S, Rosenberger C: Renal parenchymal hypoxia, hypoxia response and the progression of chronic kidney disease. Am J Nephrol 28: 998–1006, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Evans RG, Eppel GA, Michaels S, Burke SL, Nematbakhsh M, Head GA, Carroll JF, O’Connor PM: Multiple mechanisms act to maintain kidney oxygenation during renal ischemia in anesthetized rabbits. Am J Physiol Renal Physiol 298: F1235–F1243, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Gloviczki ML, Glockner JF, Crane JA, McKusick MA, Misra S, Grande JP, Lerman LO, Textor SC: Blood oxygen level-dependent magnetic resonance imaging identifies cortical hypoxia in severe renovascular disease. Hypertension 58: 1066–1072, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saad A, Herrmann SM, Crane J, Glockner JF, McKusick MA, Misra S, Eirin A, Ebrahimi B, Lerman LO, Textor SC: Stent revascularization restores cortical blood flow and reverses tissue hypoxia in atherosclerotic renal artery stenosis but fails to reverse inflammatory pathways or glomerular filtration rate. Circ Cardiovasc Interv 6: 428–435, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keddis MT, Garovic VD, Bailey KR, Wood CM, Raissian Y, Grande JP: Ischaemic nephropathy secondary to atherosclerotic renal artery stenosis: Clinical and histopathological correlates. Nephrol Dial Transplant 25: 3615–3622, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gloviczki ML, Keddis MT, Garovic VD, Friedman H, Herrmann S, McKusick MA, Misra S, Grande JP, Lerman LO, Textor SC: TGF expression and macrophage accumulation in atherosclerotic renal artery stenosis. Clin J Am Soc Nephrol 8: 546–553, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chade AR, Rodriguez-Porcel M, Grande JP, Krier JD, Lerman A, Romero JC, Napoli C, Lerman LO: Distinct renal injury in early atherosclerosis and renovascular disease. Circulation 106: 1165–1171, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Chade AR, Rodriguez-Porcel M, Grande JP, Zhu X, Sica V, Napoli C, Sawamura T, Textor SC, Lerman A, Lerman LO: Mechanisms of renal structural alterations in combined hypercholesterolemia and renal artery stenosis. Arterioscler Thromb Vasc Biol 23: 1295–1301, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Lavi R, Zhu XY, Chade AR, Lin J, Lerman A, Lerman LO: Simvastatin decreases endothelial progenitor cell apoptosis in the kidney of hypertensive hypercholesterolemic pigs. Arterioscler Thromb Vasc Biol 30: 976–983, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chade AR, Rodriguez-Porcel M, Herrmann J, Zhu X, Grande JP, Napoli C, Lerman A, Lerman LO: Antioxidant intervention blunts renal injury in experimental renovascular disease. J Am Soc Nephrol 15: 958–966, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Zhu XY, Chade AR, Rodriguez-Porcel M, Bentley MD, Ritman EL, Lerman A, Lerman LO: Cortical microvascular remodeling in the stenotic kidney: Role of increased oxidative stress. Arterioscler Thromb Vasc Biol 24: 1854–1859, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Eirin A, Gloviczki ML, Tang H, Gössl M, Jordan KL, Woollard JR, Lerman A, Grande JP, Textor SC, Lerman LO: Inflammatory and injury signals released from the post-stenotic human kidney. Eur Heart J 34: 540–548a, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eirin A, Zhang X, Zhu XY, Tang H, Jordan KL, Grande JP, Dietz AB, Lerman A, Textor SC, Lerman LO: Renal vein cytokine release as an index of renal parenchymal inflammation in chronic experimental renal artery stenosis. Nephrol Dial Transplant 29: 274–282, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng J, Zhou W, Warner GM, Knudsen BE, Garovic VD, Gray CE, Lerman LO, Platt JL, Romero JC, Textor SC, Nath KA, Grande JP: Temporal analysis of signaling pathways activated in a murine model of two-kidney, one-clip hypertension. Am J Physiol Renal Physiol 297: F1055–F1068, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rhee EP, Clish CB, Pierce KA, Saad A, Lerman LO, Textor SC: Metabolomics of renal venous plasma from individuals with unilateral renal artery stenosis and essential hypertension. J Hypertens 33: 836–842, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Warner GM, Cheng J, Knudsen BE, Gray CE, Deibel A, Juskewitch JE, Lerman LO, Textor SC, Nath KA, Grande JP: Genetic deficiency of Smad3 protects the kidneys from atrophy and interstitial fibrosis in 2K1C hypertension. Am J Physiol Renal Physiol 302: F1455–F1464, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang X, Eirin A, Li ZL, Crane JA, Krier JD, Ebrahimi B, Pawar AS, Zhu XY, Tang H, Jordan KL, Lerman A, Textor SC, Lerman LO: Angiotensin blockade has protective effects on the post-stenotic porcine kidney. Kidney Int 84: 767–775, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hackam DG, Duong-Hua ML, Mamdani M, Li P, Tobe SW, Spence JD, Garg AX: Angiotensin inhibition in renovascular disease: A population-based cohort study. Am Heart J 156: 549–555, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Chrysochou C, Foley RN, Young JF, Khavandi K, Cheung CM, Kalra PA: Dispelling the myth: The use of renin-angiotensin blockade in atheromatous renovascular disease. Nephrol Dial Transplant 27: 1403–1409, 2012 [DOI] [PubMed] [Google Scholar]
- 48.Cooper CJ, Murphy TP, Cutlip DE, Jamerson K, Henrich W, Reid DM, Cohen DJ, Matsumoto AH, Steffes M, Jaff MR, Prince MR, Lewis EF, Tuttle KR, Shapiro JI, Rundback JH, Massaro JM, D’Agostino RB, Sr, Dworkin LD, CORAL Investigators : Stenting and medical therapy for atherosclerotic renal-artery stenosis. N Engl J Med 370: 13–22, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chade AR, Krier JD, Rodriguez-Porcel M, Breen JF, McKusick MA, Lerman A, Lerman LO: Comparison of acute and chronic antioxidant interventions in experimental renovascular disease. Am J Physiol Renal Physiol 286: F1079–F1086, 2004 [DOI] [PubMed] [Google Scholar]
- 50.Eirin A, Li Z, Zhang X, Krier JD, Woollard JR, Zhu XY, Tang H, Herrmann SM, Lerman A, Textor SC, Lerman LO: A mitochondrial permeability transition pore inhibitor improves renal outcomes after revascularization in experimental atherosclerotic renal artery stenosis. Hypertension 60: 1242–1249, 2012 [DOI] [PubMed] [Google Scholar]
- 51.Parekh DJ, Weinberg JM, Ercole B, Torkko KC, Hilton W, Bennett M, Devarajan P, Venkatachalam MA: Tolerance of the human kidney to isolated controlled ischemia. J Am Soc Nephrol 24: 506–517, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eirin A, Ebrahimi B, Zhang X, Zhu XY, Wollard JR, He Q, Textor SC, Lerman A, Lerman LO: Mitochondrial protection restores renal function in swine atherosclerotic renovascular disease. Cardiovasc Res 103: 461–472, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chade AR, Zhu X, Lavi R, Krier JD, Pislaru S, Simari RD, Napoli C, Lerman A, Lerman LO: Endothelial progenitor cells restore renal function in chronic experimental renovascular disease. Circulation 119: 547–557, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chade AR, Zhu XY, Krier JD, Jordan KL, Textor SC, Grande JP, Lerman A, Lerman LO: Endothelial progenitor cells homing and renal repair in experimental renovascular disease. Stem Cells 28: 1039–1047, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eirin A, Zhu XY, Krier JD, Tang H, Jordan KL, Grande JP, Lerman A, Textor SC, Lerman LO: Adipose tissue-derived mesenchymal stem cells improve revascularization outcomes to restore renal function in swine atherosclerotic renal artery stenosis. Stem Cells 30: 1030–1041, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ebrahimi B, Eirin A, Li Z, Zhu XY, Zhang X, Lerman A, Textor SC, Lerman LO: Mesenchymal stem cells improve medullary inflammation and fibrosis after revascularization of swine atherosclerotic renal artery stenosis. PLoS One 8: e67474, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lech M, Gröbmayr R, Ryu M, Lorenz G, Hartter I, Mulay SR, Susanti HE, Kobayashi KS, Flavell RA, Anders HJ: Macrophage phenotype controls long-term AKI outcomes--kidney regeneration versus atrophy. J Am Soc Nephrol 25: 292–304, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]