Summary
Clinically prescribed insulin analogues are putatively linked with increased cancer risk. We developed a framework for the mandated regulatory in vitro evaluation of cancer-relevant bioassays for comparisons of insulin analogues, and showed that the cell-specific IGF-IR/IR ratio is crucial for interpretation.
Abstract
Epidemiological and laboratory studies raise the possibility of a link between clinically prescribed insulin analogues and increased cancer risk. Accordingly, there is a regulatory mandate for cancer-related pre-clinical safety evaluation during insulin analogue development, but currently, there is no standardized framework for such in vitro evaluation. We tested human insulin; the super-mitogenic insulin, X10 and insulin-like growth factor I, in four cancer cell lines with a range of insulin-like growth factor-I receptor (IGF-IR)/IR (insulin receptor) ratios (HCT 116, HT-29, COLO 205 and MCF7) and related these to IGF-IR and IR expression in 17 human adenocarcinomas. All cell types were IR-A isoform dominant. We determined IGF-IR/IR signalling pathway endpoints in dose- and time-varying experiments, and performed mitogenic dose–response equivalent assays to derive EC50 values, and correlated these with IGF-IR/IR ratios. We superimposed relative EC50 values onto data from the literature in a meta-analysis. The IGF-IR/IR ratios varied from <1 to 12 in the selected cell lines; similar pattern ranges were observed in human adenocarcinomas. The three ligands demonstrated differential IR/IGF-IR and Akt phosphorylation, which correlated with cell-specific IGF-IR/IR ratios. Mitogenic profiles of X10 mimicked those for insulin-like growth factor I (IGF-I) and correlated with IGF-IR/IR ratios. The meta-analysis, adding data from five additional studies, supported the hypothesis that ligand mitogenic potency, relative to human insulin, increases with increasing cell-specific IGF-IR/IR ratio. This study established a framework for the in vitro evaluation of cancer-relevant bioassays for comparisons of insulin analogues, and specifically consolidated earlier studies that determination of the cell-specific IGF-IR/IR ratio is crucial for the interpretation of ranking relative biological activities.
Introduction
Approximately 15–20% of patients with type 2 diabetes are prescribed some form of long-term insulin therapy (1). Interest in molecular safety of insulin analogues was stimulated by four epidemiological studies, published simultaneously in Diabetologia in June 2009 (2–5), three of which speculated a link between the use of insulin analogue, glargine (Lantus®, Sanofi, France) and increased incident cancer risk (2,4,5). These studies had several methodological limitations, such that definitive interpretation was not possible (6). Several additional analyses of observational data followed, but with inconsistent results (7). In 2012, the ORIGIN trial—a randomized trial of insulin glargine versus standard care, in 12 537 patients with impaired glucose tolerance or type 2 diabetes, reported no differences between treatment arms for all cancer incidence or deaths (8). However, here too, and in the extended ORIGIN analysis (9), there were limitations for definitive interpretation as: (i) there is a rapid drop-off after the median 6.2 year follow-up (arguably too short to evaluate the full effect of exposure on cancer risk); (ii) there was interrupted glargine exposure in the intervention arm and (iii) there was considerable contamination across the arms (10,11). Thus, there is a continued need for cancer risk assessment in insulin analogues and this includes pre-clinical safety evaluation.
In the laboratory, the potential for increased mitogenic potential among modified insulin molecules (relative to human insulin) has been recognized since a prototype rapid-acting analogue, insulin X10 (B10Asp), was found to increase the incidence of mammary tumours in female Sprague–Dawley rats (12,13). Subsequent investigations showed that X10 and other analogues have increased affinity for the insulin-like growth factor-I receptor (IGF-IR) relative to the insulin receptor (IR), in contrast with human insulin (14–17); and increased residence time at the IR, eliciting prolonged IR activation (15,18). Additionally, it is suggested that X10’s mitogenic potency is stronger in cells characterized by a high IGF-IR/IR ratio (19), though this is not seen in all studies (20). These properties represent plausible mechanisms by which X10 and other analogues could evoke an increased mitogenic response compared with human insulin (13,21). For safety evaluation, traditional animal toxicological studies with long-acting insulin analogues are hampered by limited dose ranges due to premature animal death from hypoglycaemia at higher doses, such that the emphasis has shifted to molecular characterization of insulin analogues in pre-clinical studies (19,22,23). This is borne out in regulatory guidance—e.g. the European Medicine Agency state that ’before initiating clinical development, non-clinical studies should be performed. These studies should be comparative in nature …………….. and should not just assess the response per se’ (24).
Although there have been several in vitro laboratory studies evaluating the mitogenic properties (directly or indirectly) of insulin analogues, there have been three broad categories of limitations in these bioassays relevant to comparative assessment of cancer risk: (i) cell lines have been either in a non-neoplastic state, frequently engineered to an extreme molecular phenotype (16,17,25,26) or limited to one neoplastic cell system only (15,17,19); (ii) cancer-relevant endpoints have mainly been limited to mitogenic properties determined indirectly using markers of DNA synthesis (17,19,25–28), rather than directly quantifying cell growth or (iii) comparisons of cellular activity have been suboptimally performed based on equimolar doses of different insulins (20,29,30), rather than using dose–response equivalent doses, as advocated by the EMA [‘It is important that assays are …………. based on a sufficient number of dilutions per curve to characterize the whole concentration–response relationship’] and explained elsewhere (21).
We recently reported detailed in vitro comparative assessments of the binding affinity of insulin, X10 and IGF-I for IR isoforms and IR/IGF-IR hybrids, while simultaneously performing cancer-relevant signalling pathway analyses (e.g. pAkt) and direct mitogenic assays in a mammary epithelial cell line (19). We additionally showed general correlations between ligand activation of key signalling pathways and IGF-IR/IR ratio in a murine colon cancer line (22). Here, we extend these works reporting a series of in vitro experiments to establish a pre-clinical framework for evaluating cancer-relevant bioassays comparing insulin, IGF-I and X10 in three colon and one breast cancer cell line. Our previous work showed that these cells have a stepwise range from low to high IGF-IR/IR ratios (31).
Methods
Cell lines and ligands
Three colon cancer cell lines—HCT 116, HT-29 and COLO 205 were selected as representatives of different mutations in KRAS, BRAF, PI3KCA and TP53 (Supplemenatry Table S1, available at Carcinogenesis Online) (31). The breast cancer cell line, MCF7, was used as a positive control, as it is known to be responsive to insulin and IGF-I (32). All cell lines were from the American Type Culture Collection and were grown in RPMI-1640 medium supplemented with 10% fetal calf serum (FCS) in a humidified atmosphere at 37°C and 5% CO2. The cell lines were validated by DNA sequencing using the Applied Biosystems AmpF/STR Identifier kit.
Cells were treated with the following ligands: human insulin (hereafter referred to as insulin), insulin X10 (X10) and IGF-I. All the agents were provided by NovoNordisk. The stock concentrations were: insulin (593.9 μM), X10 (606 μM) and IGF-I (116 μM).
Chemicals and antibodies
All chemicals were of the highest laboratory quality available: sources are detailed in the Supplementary Material
Determination of IR and IGF-IR expression
The relative expressions of IR and IGF-IR in all cell lines were determined using two methods: (i) western blotting and densitometry and (ii) a fluorescence-activated cell sorting -based receptor quantification system using QIFIKIT (indirect immunofluorescence staining in flow cytometry) (Dako, Denmark) according to manufacturer’s protocols using either the murine monoclonal antibody 83–7 against the human IR (10 ug/ml), 24–31 against the human IGF-IR (10 ug/ml), or an isotype control antibody (Dako, X0931, 10 ug/ml). Cells were then analysed using an LSRFortessa (BD Biosciences, Franklin Lakes, NJ).
Determination of IR isoform expression in cell lines
To address the potential differential effects of insulin and IGF-I in cells with different proportions of IR isoforms (IGF-I has higher affinity for IR-A compared with affinity for IR-B), we quantified hIR-A/B levels in our cells using the primers and probes described by Huang et al. (33) Briefly, RNA was purified using the Qiagen RNeasy mini Kit according to manufacturers’ protocol. The reverse transcription reaction was carried out with 0.6 μg total RNA using the iScript syntheses kit from Bio-Rad according to manufacturers’ protocol. A 2-fold total RNA dilution series from hIR-A or hIR-B overexpressing Baby hamster kidney cells served as standard to ensure a linear range (Ct versus relative copy number) of the amplification. The reverse transcription reaction was diluted 10 times in water and 10 µl aliquots were subsequently used for Real-Time PCR amplification using the Applied Biosystems 7900 Real-Time PCR instrument, as described elsewhere (33).
Western blotting and immunoprecipitation
Cells were seeded in six-well plates in complete medium and starved in serum-free medium for 24h and treated with insulin, X10 and IGF-I at the indicated concentrations and times, using standard western blotting and immunoprecipitation methods (detailed in Supplementary material, available at Carcinogenesis Online). Phosphorylation levels of downstream pathway activation were compared within each cell line following densitometric analysis. To compare time point and time course-dependent phosphorylations between cell lines, the potency of target phosphorylation by X10 and IGF-I was derived as the fold increase in phosphorylation compared with that for insulin as referent.
Immunohistochemical quantification
The relationship between IR and IGF-IR expression was determined in serial tumour sections from 17 patients with colonic adenocarcinomas immune-stained as described previously in our laboratory (34). Stained slides were scanned using a Mirax SCAN automated slide scanning system (Zeiss). Analysis was performed on whole slides using Tissue Studio 2 software (Definiens) trained to identify epithelial tumour material and then scored based on the total cellular Diaminobenzidene intensity.
Proliferation assays
We assessed cell growth using two assays: (i) cell counting (shown as main results) and (ii) MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide]. For cell counting, cells were seeded in 24-well plates in complete medium and allowed to grow for 24h, followed by two washes with phosphate-buffered saline and starvation in serum-reduced medium containing 0.1% FCS for 24h. Stimulation with the ligands (concentrations: 0, 0.001, 0.01, 0.1, 1, 10 and 100nM) was in media containing 0.1% FCS for 5 days. At the end of the experiment, the cells were trypsinized and counted using a CASY counter (Roche Applied Science, UK).
For the MTT assay, cells were seeded in 96-well plates in medium containing 0.1% FCS and 0.5% premium grade bovine serum albumin (free of insulin-like growth factor contaminants (35)), and ligands added after 24h (concentrations: 0, 0.002, 0.01, 0.1, 0.2, 0.625, 1.5, 2.5, 5, 10, 20, 50 and 200nM). The experiment lasted for 5 days and ligands replaced every 48h. After 96h of incubation, MTT reagent (10µl) was added to each well and incubated for a further 24h. Insoluble formazan that was formed at the bottom of the wells was solubilized and absorbance was read at 560nm.
Apoptosis assay
To quantify apoptosis, we used the Caspase Glo 3/7 assay purchased from Promega Corporation (Madison) and performed according to the manufacturer’s instructions. Briefly, the cells were plated in 96-well plates in complete medium; then, after 24h, the plates were washed twice with phosphate-buffered saline, and the medium changed to starvation medium containing 0.1% FCS (for 24h). All the cells were control treated or treated with three concentrations (1, 10 and 100nM) of the ligands for either 24 or 48h. Caspase Glo 3/7 agent was added at the end of the each incubation time for 1h and luminescence was measured.
Data and statistical analysis
In accordance with the European Pharmacopoeia (36), IR and IGF-1R receptor ligand activation data were fitted using a four parameter sigmoidal algorithm developed for bioassays (37). Curves were fitted using GraphPad Prism 5 (GraphPad Software) and potencies were calculated (if appropriate) relative to that of the human insulin standard [EC50 (insulin)/EC50 (analogue) × 100%]. Comparisons of continuous variables were performed using Student’s t-test. All computations were performed using Stata™ 11.1 (College Station, TX).
Meta-analysis
We performed a systematic search using the MeSH (Medical Subject Heading) terms ‘insulin’, ‘in vitro’; ‘mitogenic assay’ to add to a previous review from one of the authors (38). Inclusion criteria were studies reporting: (i) mitogenic potency (or EC50 values), (ii) IGF-IR/IR ratio (or data from which to estimate this) and (iii) limited to neoplastic human cell lines (to be clinically relevant). All three criteria had to be met. Unweighed regressions were performed to generate ‘fit’ lines stratified for IGF-I, X10 and glargine and mitogenic potencies plotted relative to human insulin at 100% (log scale).
Results
Ratios of IR to IGF-IR vary considerably from cell line to cell line
The relative expressions of IR and IGF-IR, and IGF-IR/IR ratios, in the four cell lines are shown in Figure 1. The results from both techniques—western blotting and QIFIKIT–despite the difference in their specificity and sensitivity, showed the same patterns. IR expression was greater than IGF-IR expression in HCT 116 cells; for the remainder, IGF-IR was the dominant receptor expression with ratios increasing in a stepwise manner from HCT 116, HT-29, COLO 205 through to MCF7 cells. For the four cell lines, the changes in the IR/IGF-IR ratio mainly reflected increasing expression of IGF-IR, which contrasted with the pattern observed in human colonic adenocarcinoma (see below). Nonetheless, the range and magnitude of the IR/IGF-IR ratios in the selected cell lines mirrored those seen in the human tissue.
Figure 1.
Western blots (a) and densitometry (b) for expression of IR and IGF-IR in four cell lines: HCT 116, HT-29, COLO 205 and MCF7. IR and IGF-IR expression determined using the QIFIKIT method (c). The values for IGF-IR/IR ratio in (c) were used subsequently in Figures 5 and 6. SABC, specific antigen binding capacity.
We determined the relative expression of the IR isoforms, IR-A and IR-B in all four cell lines and found that IR-A was by far the predominant isoform accounting for 86, 91, 95 and 95%, respectively, for MCF7, HCT 116, HT-29, COLO 205 cell lines (Supplementary Table 2, available at Carcinogenesis Online).
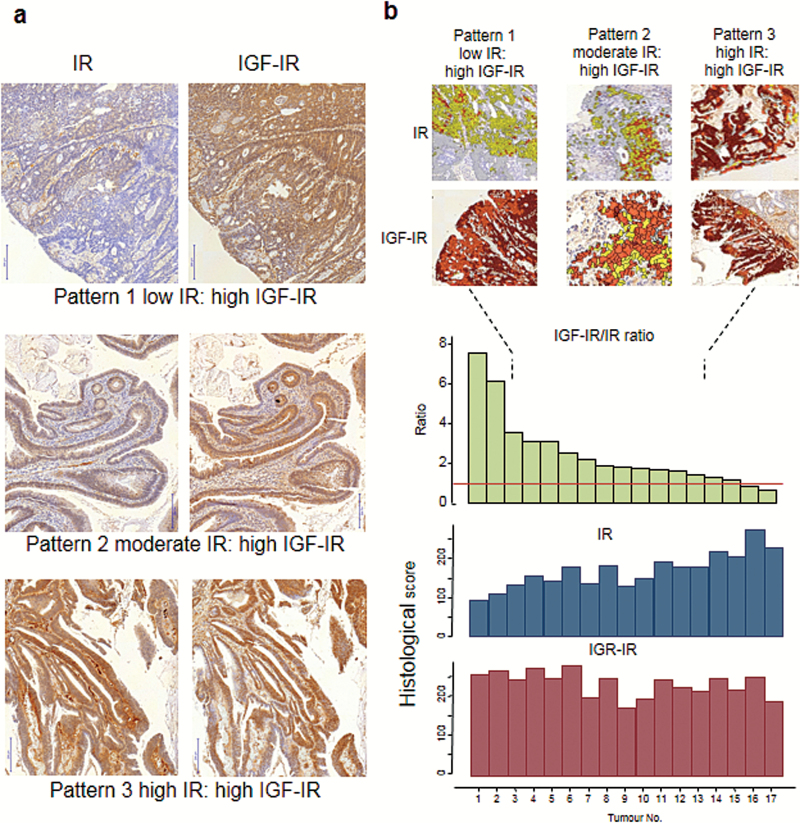
IGF-IR/IR ratio varies in human adenocarcinoma
The immunohistochemical expressions of IR and IGF-IR in 17 colonic adenocarcinomas are shown in Figure 2. Three patterns emerged—low IR: high IGF-IR, medium IR: high IGF-IR and high IR: high IGF-IR. Quantification of the immune-expressions, using Definiens software, showed that the expression of IGF-IR is relatively constant across colonic adeocarcinomas, but the variation in the IGF-IR/IR ratio is attributed to varying expressions of IR.
Figure 2.
Patterns of IR and IGF-IR expression in 17 human colonic adenocarcinomas (serial sections) (a). Quantification of immune expression of IR and IGF-IR using Definiens software (b). The expression of IGF-IR is relatively constant in the tumours, but the variation in the IGF-IR/IR ratio is mainly attributed to the variation in IR expression.
The three ligands differentially phosphorylate IR, IGF-IR and IRS-1
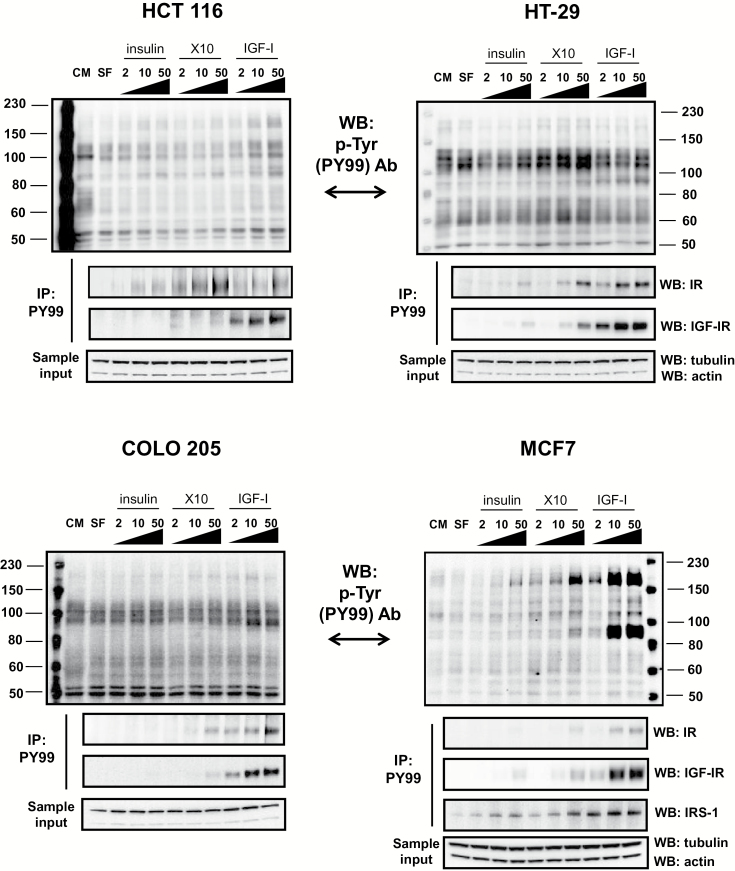
To better understand activation of downstream signalling pathways by insulin, X10 and IGF-I, we determined whether the ligands phosphorylate IR and/or IGF-IR, and/or activate insulin receptor substrate (IRS)-1. Following 15-min ligand stimulation, direct western blotting (using antibody PY99) showed phosphotyrosine rich residues at ~100 and 180 kD in all cell lines (Figure 3).
Figure 3.
Insulin, X10 and IGF-I induce phosphorylation of the IR, IGF-IR and IRS-1 in the four cell lines: HCT 116, HT-29, COLO 205 and MCF7. The doses of each ligand (2, 10 and 50) are in nM. Following 15-min ligand stimulation, direct western blotting (using the anti-phospho tyrosine antibody PY99) showed phosphotyrosine rich residues at ~100 and 180 kD in all cell lines. These were confirmed in the lower blots of each panel by immunoprecipitation. Sample input into the immunoprecipitations was determined using actin and tubulin detection in a small volume of total cell lysate. This experiment was performed three times and here is presented one example. CM, complete medium, SF, serum-free medium.
Next, proteins were immunoprecipitated using antibody PY99 and western blotting was performed using anti-IR, anti-IGF-IR and anti-IRS-1 antibodies (the latter only in the case of MCF7). X10 and IGF-I phosphorylated, in a dose–response manner, both the IR and the IGF-IR in HT-29, COLO 205 and MCF7 (IGF-IR dominant cell lines), whereas in the IR-rich HCT 116 cell line, only IGF-IR phosphorylation was seen after IGF-I stimulation. In addition, in MCF7 cells phosphorylated IRS-1 was present when the cells were stimulated with all the ligands and the strongest band was obtained when the cells were stimulated with IGF-I (all concentrations) and with the highest concentration of X10.
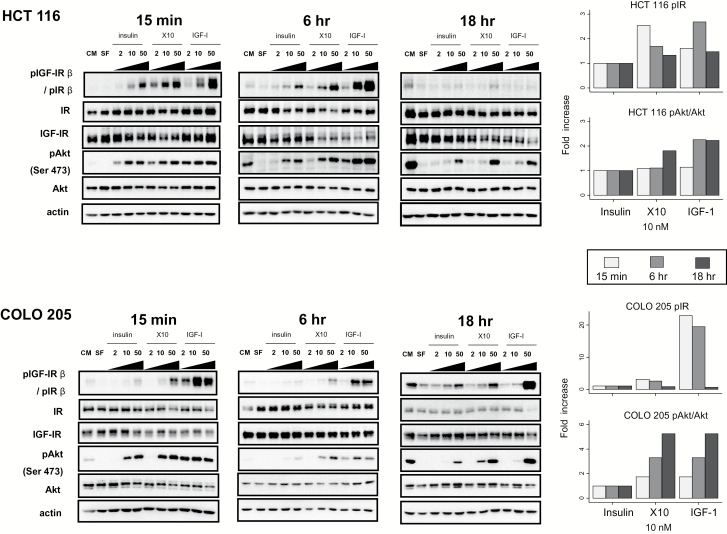
IGF-IR/IR and Akt phosphorylation correlates with cell-specific IGF-IR/IR ratios
We further explored downstream signalling pathways, for dose- and time-dependent patterns, using direct western blotting (Figure 4 and Supplementary Figures 1–4 is available at Carcinogenesis Online). After 15min stimulation with insulin, X10 and IGF-I, in broad terms, dose-dependent phosphorylations of IGF-IR/IR, IRS-1 and Akt were obtained. The densitometric analyses demonstrated patterns of activation determined by the IGF-IR/IR ratios. For example, after 15min IGF-I stimulation, weakest phosphorylation of IGF-IR/IR and Akt (Ser 473) was observed in IR-rich HCT 116 cells (note the y-axis scale on graphs indicating densitometric analysis of the bands in western blots); strongest phosphorylations of IGF-IR/IR and Akt were seen in the IGF-IR dominant cell lines. In the IR-dominant cell line (HCT 116), X10 induced greater receptor phosphorylation than that for insulin and IGF-I after 15min; by contrast, in IGF-IR dominant cells, the extent of receptor and Akt phosphorylation increased in a stepwise manner for insulin, X10 and IGF-I dose-equivalent stimulations. Low levels of extracellular signal-regulated kinase phosphorylation were detected in all cell lines, except COLO 205.
Figure 4.
Dose- and time-dependent activation of the Akt signalling pathway—exemplified by HCT 116 and COLO 205 cells. Western blots for HCT 116 (upper panel) and COLO 205 (lower panels) cells after 15min, 6 and 18h stimulation with three ligands (2, 10 and 50nM). Densitometry of the time-related changes (15min, 6 and 18h) in pIGF-IR/pIR and in pAkt (Ser 473) after 10nM stimulation with all three ligands. This concentration was selected as a mid-range dose (based on the western on the left-hand panels); and as a preferred concentration in the literature (21). Details of time-dependent target phosphorylation by X10 and IGF-I are described in the methods. This experiment was performed three times and here is presented one example. CM, complete medium; SF, serum-free medium.
We tested the effects of time (15min, 6 and 18h) on IGF-IR/IR and Akt (Ser 473) phosphorylation after stimulation with 10nM of each ligand (21), and compared by deriving phosphorylation ratios with insulin as referent (details in Methods) (Figure 4 and Supplementary Figure 1, available at Carcinogenesis Online). The three ligands demonstrated time-dependent IGF-IR/IR and Akt phosphorylation, which correlated with cell-specific IGF-IR/IR ratios. For example, IGF-IR/IR phosphorylation is an early event for all ligands in all cell lines (except HCT 116 after IGF-I stimulation). Furthermore, in HCT 116 cells, X10 induced the strongest phosphorylation of IGF-IR/IR after 15min stimulation suggesting that signalling might be through both receptors, whereas in other cell lines IGF-I was the strongest stimulus for receptor phosphorylation. In general, Akt (Ser 473) activation is later and a weaker event compared with IGF-IR/IR phosphorylation (in most cases it reaches maximum after 6 or 18h of stimulation). IGF-I is the ligand that induces the strongest phosphorylation of Akt in all cell lines. Across ligands, phosphorylation levels of both IGF-IR/IR and Akt were generally greatest for IGF-I and least for human insulin.
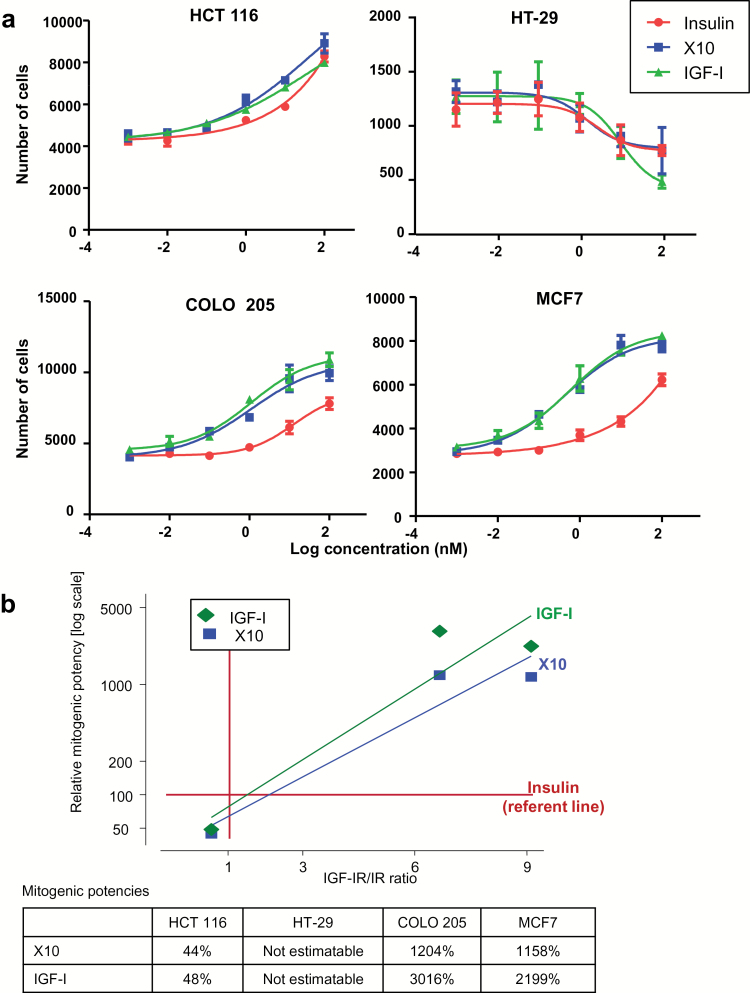
Mitogenic profiles correlate with cell-specific IGF-IR/IR ratios
In cell-counting experiments, insulin, X10 and IGF-I showed different proliferative profiles in each cell line, except HT-29 (Figure 5). In the HCT 116 cells (low IGF-IR/IR ratio), there are little differences between X10 and IGF-I, versus insulin (as referent). By contrast, in the COLO 205 and MCF7 cells (high IGF-IR/IR ratio), the dose–response curves demonstrated left-shifts for X10 and IGF-I, compared with human insulin, indicating increased mitogenic potencies. To summarize these relationships, we plotted the relative mitogenic potencies versus the IGF-IR/IR ratios, for X10 and IGF-I, with insulin as referent. Figure 5 demonstrates that mitogenic profiles correlate with cell-specific IGF-IR/IR ratios. We obtained near identical patterns for the growth curves with the MTT assays (Supplementary Figure 5, available at Carcinogenesis Online).
Figure 5.
Dose–response curves for increasing doses of the three ligands—insulin, X10 and IGF-I (a). For these experiments, we included zero ligand concentrations (data not shown)—there were no differences with effects for 1 pmol for any ligand, indicating no ‘background’ effect from the FCS. Each point in the figure represents the mean ± SD of a triplicate determination. The experiment was performed three times. From these curves, EC50 values were derived using GraphPad Prism and mitogenic potencies calculated relative to insulin as 100% (b). Regression line fitted to demonstrate the relationship between increasing IGF-IR/IR ratio and relative mitogenic potency. The ‘non-responder’ data for HT-29 are not included.
In both proliferation assays, the three ligands failed to increase cell growth in HT-29 cells—indeed, the number of cells decreased with increasing ligand concentrations. We explored this further quantifying caspase 3/7 activity, as an indicator of apoptosis. A moderate increase in caspase 3/7 activity was observed following insulin, whereas IGF-I and the highest concentration of X10 (100nM) caused significant increases in caspase 3/7 activity after 24h (Supplementary Figure 6, available at Carcinogenesis Online). At longer incubation (48h), all three ligands induced 2- to 5-fold increases in caspase 3/7 activity. These changes were not observed in HCT 116 cells.
Meta-analysis
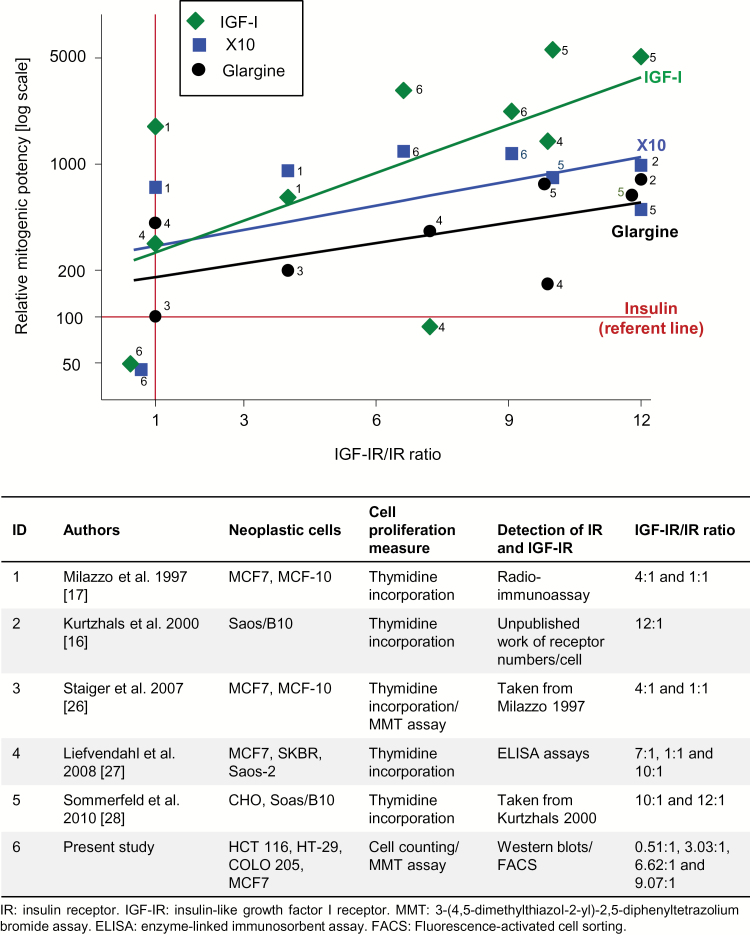
We identified five eligible studies (16,17,26–28), in addition to our data. Onto our model, we superimposed relative mitogenic potencies versus IGF-IR/IR ratios for cell types from these studies. We then derived regression lines (without intercept restrictions) across the studies were each ligand, IGF-I, X10 and glargine, and insulin as the referent. The plot in Figure 6 shows that with increasing IGF-IR/IR ratios, the mitogenic potency of each ligand increases (on a log scale). The descending effect order is IGF-I, X10 and glargine. In this model, in cells with a high IGF-IR/IR ratios, the mitogenic potencies for glargine are greater than insulin. In contrast, at low IGF-IR/IR ratios (<1), there are no material differences between the ligands and insulin. This model shows that cell-type IGF-IR/IR ratio explains inconsistent relative mitogenic potencies between insulin-related ligands reported in the literature.
Figure 6.
Meta-analysis of data from studies reporting mitogenic potencies versus IGF-IR/IR ratio. Mitogenic potencies relative to human insulin at 100% (log scale) with unweighed regression line stratified for IGF-I, X10 and glargine. The marker labels are the numbered studies listed in the lower panel table.
Discussion
Main findings
There is already a literature on the in vitro evaluation of cancer-relevant molecular characterizations and mitogenic potencies of insulin analogues, but within this, there are many inconsistencies. This study has built on that literature and established a cancer-relevant, dose–response equivalent and time-dependent framework for evaluating the effect of insulin analogues in vitro and identified the importance of determining the cell-specific IGF-IR/IR ratio for the interpretation of ranking relative biological activities. Specifically, we showed that: (i) the IGF-IR/IR ratio varies considerably between cell lines; (ii) similar ranges of IGF-IR/IR ratios occur in human adenocarcinomas; (iii) insulin, IGF-I and X10 differentially phosphorylate IR and IGF-IR, which in turn, correlate with the IGF-IR/IR ratio; (iv) activation of the Akt pathway varies by cell type, and is dose- and time-dependent, and thus, not a consistent ‘bioassay’ for ranking biological activities; but (v) that mitogenic potencies (relative to human insulin) derived from dose-equivalent assays correlate strongly with IGF-IR/IR ratios, and thus, is the preferred ‘bioassay’. This was substantiated in our meta-analysis.
Context with rest of literature
The study sets in place a framework to explain apparent inconsistencies amongst other reports. Thus, e.g. Sciacca et al. (39) studied insulin analogues in three engineered cell models (R−, IGF-IR-deprived mouse fibroblasts transfected with either only human IR-A or IR-B or IGF-IR) and concluded that these ‘cell models permit comparisons of the activity of insulin to that of insulin analogues …. and indicate that only minor differences exist.’ Our findings in human cancer lines and human adenocarcinoma show that such a model is too simplistic and that consideration of the relative expressions of IGF-IR and IR are clinically relevant.
Weinstein et al. (29) examined a number of cancer cells and concluded that detemir (Levemir®, Novo Nordisk, Denmark), along with several other insulin analogues, exhibited in vitro proliferative and anti-apoptotic activities comparable with human insulin. However, that study failed to show any significant effects of insulin and IGF-I in its dose–response assays, and was criticized by others for inconsistency in its experimental methodology (40). Sciacca et al. (39) additionally reported that the mitogenic potency of detemir was equivalent to that of glargine in their study, but the authors did not perform dose–response experiments for the measurement of mitogenic potencies and the observed responses were only very modest, even for X10, as a positive control. Similarly, Mayer et al. (20) determined cell proliferation rates in nine benign or malignant human mammary epithelial cell lines, with varying IGF-IR and IR expression patterns, and broadly found findings similar to our study, but ranking of the relative biological activities was not possible as dose-equivalent assays were reported. When comparing insulin analogues in cellular systems, it is necessary to perform full dose–response curves and to optimize the assay system to give an appropriate response (at least a 2-fold difference in maximal response) (21).
Relative to insulin, Sciacca et al. (39). also reported long-acting analogues more strongly activated signalling pathways, especially the extracellular signal-regulated kinase pathway, but this was not tested at different doses or times. Our findings show that signalling pathway activation is cell-specific with dose- and time-dependent profiles making it near impossible to rank biological activities based on such assays.
Strengths and limitations
Our study has several strengths. First, we undertook extensive series of dose–response and time-dependent experiments to demonstrate the limitations of using signalling pathway activation as surrogates of mitogenic effect. Second, we used several cell types with varying IGF-IR/IR ratios reflective of the clinical setting. Within this range, we showed that not all cells serve as suitable assays—e.g. we failed to elicit growth curves in HT-29 cells by either cell proliferation assay (a possible explanation to this is detailed in Supplementary material, available at Carcinogenesis Online). Third, we recognized that the expression of different IR (IR-A and IR-B) isoforms may be important as isoform IR-A is over-expressed in some cancers and associated with tumour progression (41). Additionally, as insulin analogues may have differential binding characteristics for IR-A versus IR-B, this raises the possibility that determination of the relative expressions of these isoforms may be relevant. However, despite this sound theoretical basis, we previously found balanced IR isoform binding, as well as IR isoform activation, among detemir, glargine and X10 (19), and in this study, we identified that our cell lines were almost exclusively IR-A expressors, suggesting that a differential effect through the IR isoforms is unlikely. Fourth, most previous reports determined cell proliferation indirectly using markers of DNA synthesis (17,19,25–28)—here, we determined cell growth using two assays (cell counts and MTT) and found similar results.
Potential limitations are as follows: First, we did not test glargine or detemir, as our primary motivation was to establish X10 as a positive control in growth assays, as stipulated by the European agency for the evaluation of medicinal products (42). However, we were able to evaluate the effects of glargine in the context of X10, and IGF-I through our meta-analysis. There are currently too few studies with combined mitogenic potency data and IGF-IR/IR to include other insulin analogues, such as detemir. It is important to note that the clinical interpretation of in vitro assays for glargine and detemir need to take account that glargine is rapidly metabolized to at least two low mitogenic potency metabolites (32), and that the pharmacokinetics of detemir is heavily influenced by binding with albumin and its dihexamerized state in blood (43). Second, we recognize that receptor expression may be a poor indicator of receptor saturation—as receptors are seldom fully saturated. The motivation of our study manuscript was to demonstrate a principle in vitro that can be taken to clinical tissues specimens ex vivo (where receptor saturation cannot be determined). We draw analogy with the established clinical importance of the relative expression of tumour receptors in breast cancer (oestrogen and progesterone receptors) and drug effect (for example, tamoxifen). Third, we did not determine insulin/IGF-I hybrid receptor expression. At least one study has suggested a differential effect of insulin glargine (increased activation) on insulin/IGF-IR hybrids (32). However, in the IGF-IR rich cancer cell lines, the effects (for example, receptor phosphorylation) of IGF-I versus insulin activation are of many magnitude differences. Thus, any influences through IR/IGF-IR hybrid receptors in these cells are likely to have minimal functional impact. Fourth, one cannot currently extrapolate the relative mitogenic potencies (i.e. the slopes in Figure 6) to clinical effect (44).
Future research
Given the findings from this study, there is now a need to re-visit epidemiological cohorts and re-characterize IGF-IR and IR expression in tumours arising in these cohorts where human insulin and insulin analogues have been implicated. For example, a future nested case–control study within a cohort of patients with diabetes, with follow-up to incident cancer, might explore the relative tumour expressions of IGF-IR and IR ratio, and relate these back to cumulative exposure of insulin analogues. This will allow the research community get directly to the biological question and minimize the need for animal experimentation. Such models will allow a more detailed correlation between in vitro molecular characteristics and in vivo tumour-promoting effects of insulin and insulin analogues, and an enhanced understanding of the clinical epidemiology of insulin use and cancer risk (7,10)—ultimately better informing patient decisions.
Supplementary material
Supplementary Tables 1 and 2 and Supplementary Figures 1–6 can be found at http://carcin.oxfordjournals.org/
Funding
Novo Nordisk (A.G.R.)
Conflict of Interest Statement: None declared.
Supplementary Material
Glossary
Abbreviations
- FCS
fetal calf serum
- IGF-I
insulin-like growth factor I
- IGF-IR
insulin-like growth factor-I receptor
- IR
insulin receptor
- IRS
insulin receptor substrate
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide
References
- 1. Holden S.E., et al. (2014) How many people inject insulin? UK estimates from 1991 to 2010. Diabetes Obes. Metab., 16, 553–539. [DOI] [PubMed] [Google Scholar]
- 2. Colhoun H.M. (2009) Use of insulin glargine and cancer incidence in Scotland: a study from the Scottish diabetes research network epidemiology group. Diabetologia, 52, 1755–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Currie C.J., et al. (2009) The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia, 52, 1766–1777. [DOI] [PubMed] [Google Scholar]
- 4. Hemkens L.G., et al. (2009) Risk of malignancies in patients with diabetes treated with human insulin or insulin analogues: a cohort study. Diabetologia, 52, 1732–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jonasson J.M., et al. (2009) Insulin glargine use and short-term incidence of malignancies-a population-based follow-up study in Sweden. Diabetologia, 52, 1745–1754. [DOI] [PubMed] [Google Scholar]
- 6. Smith U., et al. (2009) Does diabetes therapy influence the risk of cancer? Diabetologia, 52, 1699–1708. [DOI] [PubMed] [Google Scholar]
- 7. Renehan A.G. (2012) Insulin analogues and cancer risk: the emergence of second-generation studies. Diabetologia, 55, 7–9. [DOI] [PubMed] [Google Scholar]
- 8. Gerstein H.C., et al. (2012) Basal insulin and cardiovascular and other outcomes in dysglycemia. N. Engl. J. Med., 367, 319–328. [DOI] [PubMed] [Google Scholar]
- 9. Bordeleau L., et al. (2014) The association of basal insulin glargine and/or n-3 fatty acids with incident cancers in patients with dysglycemia. Diabetes Care, 37, 1360–1366. [DOI] [PubMed] [Google Scholar]
- 10. Badrick E., et al. (2014) Diabetes and cancer: 5 years into the recent controversy. Eur. J. Cancer, 50, 2119–2125. [DOI] [PubMed] [Google Scholar]
- 11. Zanders M.M., et al. (2014) Comment on Bordeleau et al. The association of basal insulin glargine and/or n-3 fatty acids with incident cancers in patients with dysglycemia. Diabetes Care 2014;37:1360-1366. Diabetes Care, 37, e221–e222. [DOI] [PubMed] [Google Scholar]
- 12. Drejer K. (1992) The bioactivity of insulin analogues from in vitro receptor binding to in vivo glucose uptake. Diabetes Metab. Rev., 8, 259–285. [DOI] [PubMed] [Google Scholar]
- 13. Hansen B.F., et al. (2011) Insulin X10 revisited: a super-mitogenic insulin analogue. Diabetologia, 54, 2226–2231. [DOI] [PubMed] [Google Scholar]
- 14. Bornfeldt K.E., et al. (1991) Binding and biological effects of insulin, insulin analogues and insulin-like growth factors in rat aortic smooth muscle cells. Comparison of maximal growth promoting activities. Diabetologia, 34, 307–313. [DOI] [PubMed] [Google Scholar]
- 15. Drejer K., et al. (1991) Receptor binding and tyrosine kinase activation by insulin analogues with extreme affinities studied in human hepatoma HepG2 cells. Diabetes, 40, 1488–1495. [DOI] [PubMed] [Google Scholar]
- 16. Kurtzhals P., et al. (2000) Correlations of receptor binding and metabolic and mitogenic potencies of insulin analogs designed for clinical use. Diabetes, 49, 999–1005. [DOI] [PubMed] [Google Scholar]
- 17. Milazzo G., et al. (1997) ASPB10 insulin induction of increased mitogenic responses and phenotypic changes in human breast epithelial cells: evidence for enhanced interactions with the insulin-like growth factor-I receptor. Mol. Carcinog., 18, 19–25. [DOI] [PubMed] [Google Scholar]
- 18. Hansen B.F., et al. (1996) Sustained signalling from the insulin receptor after stimulation with insulin analogues exhibiting increased mitogenic potency. Biochem. J., 315(Pt 1), 271–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hansen B.F., et al. (2012) Molecular characterisation of long-acting insulin analogues in comparison with human insulin, IGF-1 and insulin X10. PLoS One, 7, e34274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mayer D., et al. (2008) Proliferative effects of insulin analogues on mammary epithelial cells. Arch. Physiol. Biochem., 114, 38–44. [DOI] [PubMed] [Google Scholar]
- 21. Pollak M., et al. (2010) Insulin analogues and cancer risk: cause for concern or cause célèbre? Int. J. Clin. Pract., 64, 628–636. [DOI] [PubMed] [Google Scholar]
- 22. Hvid H., et al. (2013) Treatment with insulin analog X10 and IGF-1 increases growth of colon cancer allografts. PLoS One, 8, e79710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hvid H., et al. (2011) In situ phosphorylation of Akt and ERK1/2 in rat mammary gland, colon, and liver following treatment with human insulin and IGF-1. Toxicol. Pathol., 39, 623–640. [DOI] [PubMed] [Google Scholar]
- 24. European Medicines Agency. (2012) Guideline on non-clinical and clinical development of similar biological medicinal products containing recombinant human insulin and insulin analogues. Committee for Medicinal products for Human (CHMP). [Google Scholar]
- 25. Eckardt K., et al. (2007) IGF-1 receptor signalling determines the mitogenic potency of insulin analogues in human smooth muscle cells and fibroblasts. Diabetologia, 50, 2534–2543. [DOI] [PubMed] [Google Scholar]
- 26. Staiger K., et al. (2007) Comparison of the mitogenic potency of regular human insulin and its analogue glargine in normal and transformed human breast epithelial cells. Horm. Metab. Res., 39, 65–67. [DOI] [PubMed] [Google Scholar]
- 27. Liefvendahl E., et al. (2008) Mitogenic effect of the insulin analogue glargine in malignant cells in comparison with insulin and IGF-I. Horm. Metab. Res., 40, 369–374. [DOI] [PubMed] [Google Scholar]
- 28. Sommerfeld M.R., et al. (2010) In vitro metabolic and mitogenic signaling of insulin glargine and its metabolites. PLoS One, 5, e9540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Weinstein D., et al. (2009) Insulin analogues display IGF-I-like mitogenic and anti-apoptotic activities in cultured cancer cells. Diabetes Metab. Res. Rev., 25, 41–49. [DOI] [PubMed] [Google Scholar]
- 30. Yehezkel E., et al. (2010) Long-acting insulin analogues elicit atypical signalling events mediated by the insulin receptor and insulin-like growth factor-I receptor. Diabetologia, 53, 2667–2675. [DOI] [PubMed] [Google Scholar]
- 31. Baricevic I., et al. (2014) Chronic insulin exposure does not cause insulin resistance but is associated with chemo-resistance in colon cancer cells. Horm. Metab. Res., 46, 85–93. [DOI] [PubMed] [Google Scholar]
- 32. Pierre-Eugene C., et al. (2012) Effect of insulin analogues on insulin/IGF1 hybrid receptors: increased activation by glargine but not by its metabolites M1 and M2. PLoS One, 7, e41992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Huang J., et al. (2011) Altered expression of insulin receptor isoforms in breast cancer. PLoS One, 6, e26177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Seenath M.M., et al. (2008) Reciprocal relationship between expression of hypoxia inducible factor 1alpha (HIF-1alpha) and the pro-apoptotic protein bid in ex vivo colorectal cancer. Br. J. Cancer., 99, 459–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Slaaby R., et al. (2008) IGF-I binding to the IGF-I receptor is affected by contaminants in commercial BSA: the contaminants are proteins with IGF-I binding properties. Growth Horm. IGF Res., 18, 267–274. [DOI] [PubMed] [Google Scholar]
- 36. European Pharmacopoeia. (2008) Statistical Analysis of Results of Biological Assays and Tests. 6th edn European Directorate for the Quality of Medicines, Strasbourg, France. [Google Scholar]
- 37. Vølund A. (1978) Application of the four-parameter logistic model to bioassay: comparison with slope ratio and parallel line models. Biometrics, 34, 357–365. [PubMed] [Google Scholar]
- 38. Hansen B.F. (2008) Insulin analogues with increased mitogenic potency–are they safe? Horm. Metab. Res., 40, 431–433. [DOI] [PubMed] [Google Scholar]
- 39. Sciacca L., et al. (2010) Insulin analogues differently activate insulin receptor isoforms and post-receptor signalling. Diabetologia, 53, 1743–1753. [DOI] [PubMed] [Google Scholar]
- 40. Kazda C., et al. (2010) Appraising the mitogenicity of insulin analogues relative to human insulin-response to: Weinstein D, Simon M, Yehezkel E, Laron Z, Werner H. Insulin analogues display IGF-I-like mitogenic and anti-apoptotic activity in cultured cancer cells. Diabetes Metab Res Rev 2009; 25(1): 41-9. Diabetes Metab. Res. Rev., 26, 145–149. [DOI] [PubMed] [Google Scholar]
- 41. Belfiore A., et al. (2009) Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocr. Rev., 30, 586–623. [DOI] [PubMed] [Google Scholar]
- 42. The European agency for the evaluation of medicinal products. (2001). Committee for proprietary medicinal products (CPMP). Points to consider document on the non-clinical assessment of the carcinogenic potential of insulin analogues. London. [Google Scholar]
- 43. Havelund S., et al. (2004) The mechanism of protraction of insulin detemir, a long-acting, acylated analog of human insulin. Pharm. Res., 21, 1498–1504. [DOI] [PubMed] [Google Scholar]
- 44. Varewijck A.J., et al. (2012) Insulin and its analogues and their affinities for the IGF1 receptor. Endocr. Relat. Cancer, 19, F63–F75. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.