Abstract
OBJECTIVES
Type I autoimmune pancreatitis (AIP) and IgG4-related sclerosing cholangitis (IgG4-related SC) are now recognized as components of a multisystem IgG4-related disease (IgG4-RD). We aimed to define the clinical course and long-term outcomes in patients with AIP/IgG4-SC recruited from two large UK tertiary referral centers.
METHODS
Data were collected from 115 patients identified between 2004 and 2013, and all were followed up prospectively from diagnosis for a median of 33 months (range 1–107), and evaluated for response to therapy, the development of multiorgan involvement, and malignancy. Comparisons were made with national UK statistics.
RESULTS
Although there was an initial response to steroids in 97%, relapse occurred in 50% of patients. IgG4-SC was an important predictor of relapse (P < 0.01). Malignancy occurred in 11% shortly before or after the diagnosis of IgG4-RD, including three hepatopancreaticobiliary cancers. The risk of any cancer at diagnosis or during follow-up when compared with matched national statistics was increased (odds ratio = 2.25, CI = 1.12–3.94, P = 0.02). Organ dysfunction occurred within the pancreas, liver, kidney, lung, and brain. Mortality occurred in 10% of patients during follow-up. The risk of death was increased compared with matched national statistics (odds ratio = 2.07, CI = 1.07–3.55, P = 0.02).
CONCLUSIONS
Our findings suggest that AIP and IgG4-SC are associated with significant morbidity and mortality owing to extrapancreatic organ failure and malignancy. Detailed clinical evaluation for evidence of organ dysfunction and associated malignancy is required both at first presentation and during long-term follow-up.
INTRODUCTION
IgG4-related disease (IgG4-RD) is a systemic fibro-inflammatory condition that is characterized by the development of mass lesions, with similar histopathological findings in involved organs (1). Autoimmune pancreatitis (latterly termed type I AIP) was the first described pancreatic manifestation of the disease, upon which the early diagnostic criteria were based (2,3). Biliary disease (IgG4-related sclerosing cholangitis; IgG4-SC) is a common extrapancreatic manifestation of IgG4-RD. The disease has been described in Asia, the United States, and throughout Europe, with geographical variations in its clinical presentation, the utility of serum IgG4 in diagnosis, the presence of extrapancreatic manifestations, treatment regimens, and outcome (4-6). A separate histological variant, named idiopathic duct centric pancreatitis or type II AIP, has been described (7), but it is not considered part of the IgG4-RD spectrum and is not discussed in this paper.
There have been no prospective randomized controlled studies of optimal treatment regimens and limited short-term follow-up of patients in most documented series. A large multinational retrospective analysis of AIP therapy and relapse was recently published, but there have been no such evaluations of relapse rates in patients with extrapancreatic disease (5). The necessity and urgency of treatment oft en depends on the involvement of vital organs and risk of organ dysfunction or failure, although this risk is not currently defined. The majority of patients have a rapid response to corticosteroids, which is most significant in those with early inflammatory rather than fibrotic disease. Relapse after immunosuppressive therapy occurs commonly (8). Second-line immunosuppressive agents and B-cell depletion therapies have been used to treat relapsing disease (9). The risk of malignancy in IgG4-RD has received recent attention, but it remains largely undefined (10,11).
This study describes a large UK cohort of prospectively recruited patients with IgG4-RD (AIP and IgG4-SC), with particular emphasis on treatment response and relapse, second-line immunosuppressive medication, and longer-term outcomes including organ failure, malignancy, and mortality.
METHODS
Data were collected from two UK tertiary referral centers: University College Hospital, London, and the John Radcliffe Hospital, Oxford. These are currently the largest units treating patients with IgG4-RD in the United Kingdom. Patients referred from February 2004 (University College Hospital) or February 2005 (John Radcliffe Hospital, Oxford) to February 2013 were included. All patients were followed up prospectively and data were stored in a database. Patients were identified from three sources: (i) general medicine and hepatopancreaticobiliary outpatient clinics, (ii) a histopathology database where biopsy and resection specimens were recorded, and (iii) a clinical immunology database where all serum IgG subclasses were recorded. The study was reviewed by the Oxfordshire Research Ethics Committee (ref: 10/H0604/51).
Diagnoses were initially made using the Japan Pancreas Society criteria (12), and since 2007 using the HISORt criteria (3,13). The recent International Consensus Diagnostic Criteria were applied since 2011 and retrospectively to the rest of the cohort. A total of 115 patients met HISORt criteria for AIP or IgG4-SC, and all patients with AIP met International Consensus Diagnostic Criteria definite type I AIP criteria (14). Patients with type II AIP (1 patient histologically confirmed) were excluded from the analysis. The Boston Consensus Histopathological Criteria for IgG4-RD (2012) were applied to those with surgical resection specimens (15).
Clinicopathological information was collated from the two sites including demographics, clinical presentation, organ involvement, autoimmune and immune-related conditions, imaging features (including pancreatic mass, diffuse pancreatic enlargement, pancreatic duct abnormalities, distal common bile duct stricture, extrapancreatic cholangitis), IgG4 serology, histopathological sampling and diagnosis, treatment and response, relapse, biliary stenting, other complications thought to be associated with the disease, cancer, and death. For comparison, national age and sex incidence rates for cancer and cancer mortality were obtained from Public Health England Cancer Research UK. Age and sex incidence for mortality were obtained from National UK Statistics (http://www.cancerresearchuk.org/cancer-info/cancerstats/mortality/).
Serum IgG and subclasses were measured in clinical immunology by nephelometry (Siemens BN2 Nephelometer at Oxford and Sheffield, UK). Imaging modalities of the pancreas and ducts included dedicated computed tomography pancreas, magnetic resonance cholangiopancreatography, endoscopic retrograde cholangiopancreatogram, and endoscopic ultrasound. Histopathological evaluation was performed at each site by a dedicated hepatopancreaticobiliary pathologist. Morphological assessment was performed for characteristic features including a lymphoplasmacytic infiltrate, storiform fibrosis, and obliterative phlebitis. Tissues were immunostained with IgG4 monoclonal antibody. The IgG4 count was reported as the average number of IgG4-positive plasma cells in three high-powered fields (HPF).
Patients presenting with AIP and IgG4-SC underwent a multi-detector-row computed tomography scan of the abdomen and magnetic resonance cholangiopancreatography, and then an endoscopic retrograde cholangiopancreatogram and/or endoscopic ultrasound as required. Affected organs on imaging were noted on the clinical database, and further investigations were then performed depending on the patients’ signs and symptoms. Repeat imaging was performed after 4 weeks of corticosteroids and/or upon completion of medical treatment and as dictated by clinical developments. Blood tests including liver function tests and serum IgG4 were performed at each clinic visit.
The goals of treatment with corticosteroid therapy are not completely defined in IgG4-RD. In our clinical practice, the aims were to (i) improve symptoms, (ii) reverse active disease, and (iii) halt or delay progression of disease including organ failure and additional organ involvement. Furthermore, we aimed to reduce the need for endoscopic intervention, namely biliary stenting in AIP and IgG4-related SC and to also achieve long-term maintenance of treatment benefits. The steroid treatment protocol at both sites was to give 0.5 mg/kg of prednisolone for 2–4 weeks and then reduce the dose by 5 mg per 1–2 weeks, with regular monitoring of biochemistry and repeated imaging after week 4, with an expected cessation of treatment by 4–6 months. Relapse was defined as progression of disease on imaging or deterioration in liver function tests (but not owing to stent dysfunction). Relapse was treated with repeat further courses of steroid therapy, and/or additional second-line immunosuppressive therapy, at the discretion of the investigators. As there are currently no international guidelines for the treatment of relapse in IgG4-RD, our protocol broadly reflected that used in other major US and European centers (5). Patients who required repeated or long-term courses of steroids were evaluated for steroid-related complications; clinical assessment, bone densitometry, and regular blood glucose measurements were performed.
Statistical analysis
Statistical analysis for disease relapse was univariate using a two-tailed Fisher’s exact test. The Poisson distribution was used to determine whether the number of cancer cases, cancer-associated mortality, and all-cause mortality in the IgG4-RD patients observed significantly differed from that expected by using rates from PHE cancer research UK registry and national statistics UK (taking account of the number of patients that fell into each of the sex/age categorizations and the duration of follow-up of each patient).
The incidence ratio and 95% confidence intervals were calculated as previously described (16). All of the statistics were calculated using the R programming language (http://www.R-project.org). A P-value of < 0.05 was considered statistically significant.
RESULTS
Demographics
A total of 115 patients with AIP/IgG4-SC were included: 85 male (74%), median age 61 years (range 16–85 years), with a median follow-up from diagnosis of 32.5 months (range 0.8–107). At least 12-month follow-up data were available for 88 patients (77% of the cohort).
Clinical presentation
Eighty-five patients (74%) had jaundice at presentation. In all, 42 patients (37%) described a history of abdominal pain, but there were only 4 (3%) confirmed cases of IgG4-RD presenting as acute pancreatitis. Twenty-four patients (21%) had undergone surgery for suspected pancreaticobiliary cancer. Of these, 17 had a resection (14 pancreatic and 3 hilar) with the aim of achieving a surgical cure; in these patients, IgG4-RD was subsequently diagnosed on assessment of resected tissue. The other seven patients underwent biliary bypass for presumed unresectable adenocarcinoma.
Pancreatic disease
Overall, 106 patients (92%) had pancreatic disease, of whom 60 (56%) also had IgG4-SC; 9 patients in the cohort (8%) had IgG4-SC only with no involvement of the intrapancreatic portion of the common bile duct or the pancreatic parenchyma. A total of 98 patients (85%) had pancreatic abnormalities on imaging; of these, 50 (43%) had a diffusely enlarged ‘sausage-shaped’ pancreas and 62 (54%) had imaging evidence of a discrete pancreatic mass, with or without diffuse enlargement. Eighty-three patients (72%) had evidence of pancreatic duct abnormalities (strictures, side branch dilatation, diffusely abnormal ducts) on imaging, but only 9% had pancreatic calcification or ductal stones. Sixty-nine percent had a low common bile duct stricture, usually in association with an inflammatory mass in the head of the pancreas. Of the patients who did not have a low common bile duct stricture, 50% had proximal biliary strictures.
IgG4-related SC
Extrapancreatic disease was common and occurred in 84 patients in the cohort (73%). Biliary involvement was the most frequent feature, occurring in 68 patients (59%). IgG4-SC was defined as patients who had evidence of biliary stricturing disease proximal to the intrapancreatic bile duct.
Extrapancreaticobiliary disease
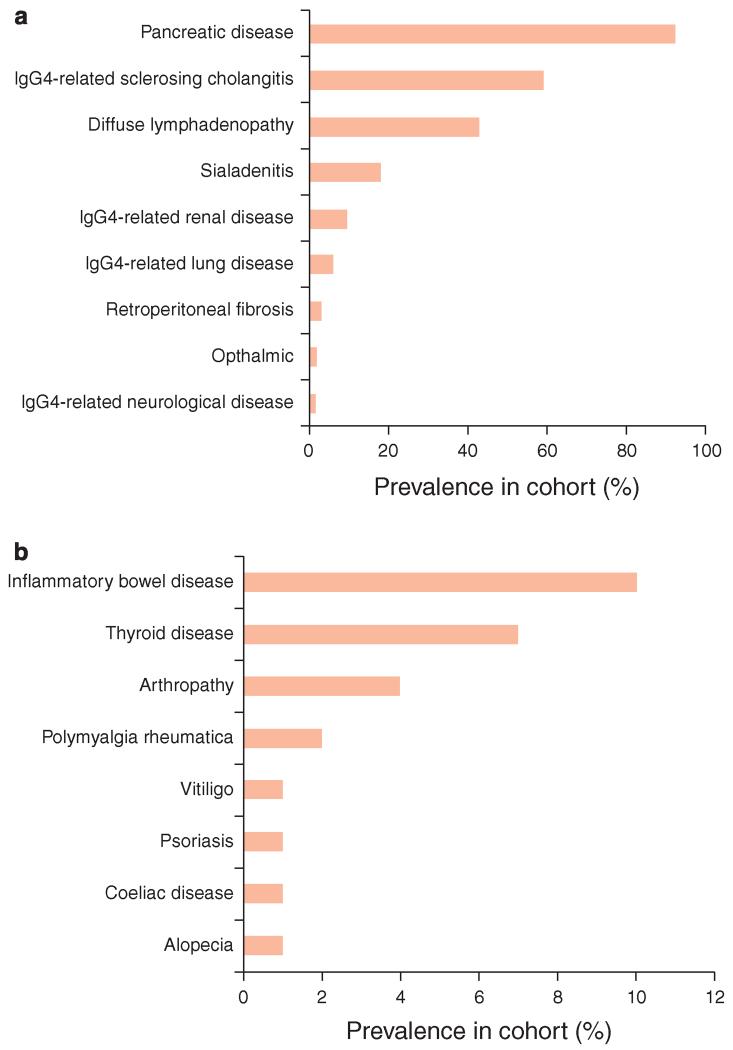
Forty-one patients (36%) had disease beyond the biliary tree or pancreas (Figure 1). Diffuse lymphadenopathy with enlarged intraabdominal, chest, or peripheral nodes (e.g., axillary or iliac) was the most common manifestation in 43% of patients. IgG4-related sialadenitis was seen in 18%, renal infiltrates or masses in 9%, pulmonary disease with nodules, interstitial lung disease, or pulmonary fibrosis in 6%, retroperitoneal fibrosis in 3%, ocular manifestations with proptosis and retro-orbital disease in 2%, and neurological sequelae including hypopituitarism caused by a pituitary mass and a rapidly progressive autoimmune encephalitis in 2%.
Figure 1.
Multi-system manifestations associated with IgG4-related disease. Prevalence of other organ involvement in IgG4-related disease (a) and associated autoimmune conditions (b).
Other autoimmune diseases were seen in this patient group. Inflammatory bowel disease occurred in 11 patients (10%): ulcerative colitis in 8 patients, Crohn’s disease in 1 patient, and inflammatory bowel disease-unclassified in 2 patients. Thyroid disease affected 7% of patients. Four patients had evidence of an inflammatory arthropathy; two patients suffered from polymyalgia rheumatica; and vitiligo, celiac disease, psoriasis, and alopecia affected one patient each.
Serum IgG4
Serum IgG4 data were available for 110 patients. Serum IgG4 was raised (> 1.4 g/l) in 70% at diagnosis (in those with available serum values; n = 105), and in 77% of patients during follow-up. In those with a raised serum IgG4 at diagnosis and available levels following treatment, 55 patients (96%) had a fall in IgG4 with treatment, but levels returned to the normal range (< 1.4 g/l) in only 21 patients (37%).
Histology
Histology samples were obtained in 101 patients (88%) from a variety of sites including pancreas (n = 36), ampulla (n = 46), biliary tree (n = 23), liver (n = 22), kidney (n = 3), and peripheral lymph nodes (n = 10). Tissue IgG4 was raised (> 10 IgG4 + ve plasma cells per high-power field) in 75% of tissue biopsies that were stained for IgG4. Tissue IgG4 was raised (> 50 IgG4 + ve plasma cells per high-power field) in 11 of 16 patients who had surgical resections and IgG4 staining performed. Forty-one patients had biopsies taken from more than one site and 38 patients had nondiagnostic biopsies from at least one site.
Treatment and relapse
A total of 98 patients (87%) received oral corticosteroids for a median duration of 5.5 (range 1–73) months. Fourteen patients (14% of those treated) received less than 3 months, 38 patients (39%) received 3–6 months, 30 patients (31%) received 6–12 months, and 16 patients (16%) received over 12 months of corticosteroids. Starting doses were 30–40 mg of prednisolone in the majority, at the discretion of the treating clinician. A lower dose of 20 mg of prednisolone (eight patients) was used where patients had known diabetes or were deemed more likely to get steroid-induced complications. The remaining patients were not treated medically owing to retrospective diagnosis following resection of an affected organ and no subsequent objective disease activity. There was a 97% response rate to steroids, as defined by improvement in radiological findings and liver function tests. Sixty-one patients (53%) required biliary stenting with a mean of 2 stents in patients with available data (range 1–10 stents). It was possible to remove biliary stents in 77% of patients treated de novo during the first course of oral steroids.
Fifty-eight patients (50%) relapsed post steroids, at a median time of 4.6 (0–64) months after stopping the first course of steroids, with two of these patients relapsing while taking steroids. Univariate analysis of potential predictors of relapse showed that extrapancreatic disease (including IgG4-SC) predicted relapse (P < 0.01). When IgG4-SC was assessed independently, the presence of IgG4-SC predicted relapse (P < 0.01), but other organ involvement in the absence of IgG4-SC did not (P = NS). Relapse was not predicted by a raised serum IgG4 at diagnosis (P = 0.53), a lack of decline in serum IgG4 with treatment (P = 0.50), or a decline in serum IgG4 to normal levels (< 1.4 g/l; P = NS).
Seventy-six percent of patients who relapsed received additional second-line immunosuppression, with the remainder being treated solely with a second course of oral corticosteroids. The usual approach from both centers was to treat with a further course of steroids and add in azathioprine (to a dose of 2 mg/kg/day), which was started in 41 patients (36% of the cohort and 71% of those who relapsed; Table 1). Of these, 8 relapsed and 13 were intolerant of Azathioprine. Alternative immunosuppression in the event of azathioprine intolerance or side effects included five patients who received mycophenolate, four patients who received methotrexate, and two patients who received mercaptopurine. These agents were tolerated well. Two patients who were intolerant of azathioprine were treated with long-term corticosteroids. In those eight patients who relapsed on azathioprine, corticosteroids were restarted in all with an increased dose in azathioprine (4), or change in immunosuppression (4). Twenty-four patients remained on immunomodulatory monotherapy at the end of the study, without requirement for additional corticosteroids. Rituximab was not given to any patient in this cohort (9).
Table 1. Treatment protocol for patients in the cohort.
| Relapse therapy | ||
|---|---|---|
|
| ||
| Treatment | Proportion of patients treated | |
| Steroids alone | Prednisolone 0.5 mg/kg for 2–4 weeks then taper by 5 mg/1–2 weeks | 34 % of relapses |
|
| ||
| Steroids + immunomodulator | Prednisolone 0.5 mg/kg for 2–4 weeks then taper by 5 mg/week | 76 % of relapses |
| 1st Line | Azathioprine 2 mg/kg | 71 % of relapses |
| 2nd Line | Mycophenolate 500 mg-1,000 mg b.d. | 5/13 |
| (If azathiopine intolerant, 13 patients) | Methotrexate 15 mg weekly | 4/13 |
| Mercaptopurine | 2/13 | |
| Low-dose prednisolone 5 mg o.d. | 2/13 | |
|
| ||
| If relapse on immunomodulator | Prednisolone | 8/8 |
| (8 patients) | + increase azathioprine dose or | 4/8 |
| + change immunomodulator | 4/8 | |
Organ dysfunction in patients with IgG4-RD
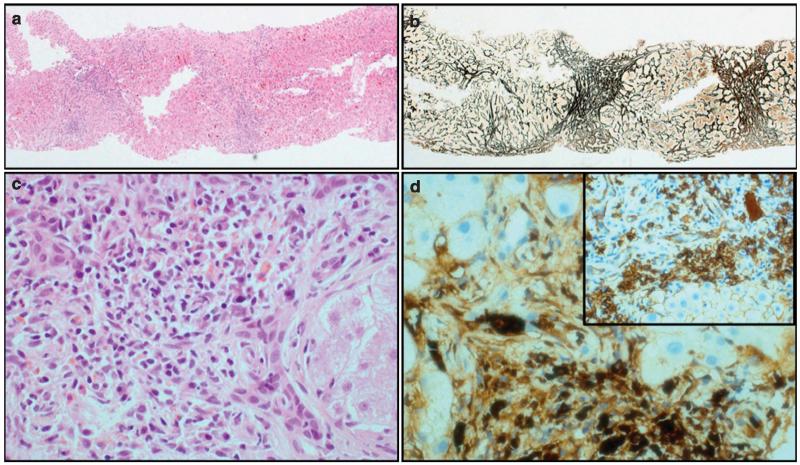
Six patients (5%) developed liver cirrhosis during follow-up. Cirrhosis was diagnosed on liver biopsy in two patients (Figure 2), and clinically in four patients (patients with signs of synthetic dysfunction and/or hepatic decompensation with consistent radiology). One patient underwent a successful liver transplantation. With regard to specific pancreatic complications, exocrine insufficiency occurred in 53% of patients, which was determined by measurement of fecal elastase (defined as < 200 μg in a solid stool sample; measured in 114 patients) and diabetes in 37%. Diabetes was pre-existent before steroid therapy in 89% of the diabetic patients. Twelve percent of patients developed biochemical renal impairment, ranging from stage 2 to 4 kidney disease. Portal and/or splenic vein thrombosis developed in 9%, but with no evidence of variceal bleeding in any patients. A list of non-malignant complications is shown in Table 2.
Figure 2.
Fibro-inflammatory changes in the liver. Liver biopsy (a: hematoxylin and eosin (H&E), ×40 and b: reticulin, ×40) showing thick fibrous bands with nodule formation. Inflammatory cell infiltrate rich in lymphocytes and plasma cells (c: H&E ×400). Large number of plasma cells expressing IgG4 (d: IgG4, ×400; inset: CD138, ×200).
Table 2. Non-malignant complications developing in the AIP and IgG4-SC cohort.
| Non-malignant complications | % Of 115 patients |
|---|---|
| Liver | |
| Cirrhosis and liver failure | 5 |
| Liver transplant | 0.9 |
| Pancreas | |
| Exocrine insufficiency | 53 |
| Endocrine insufficiency (diabetes) | 37 |
| Renal | |
| Impaired renal function—stage 2–4 CKD | 12 |
| Vascular | |
| Portal and splenic vein thrombosis | 9 |
AIP, autoimmune pancreatitis; CKD, chronic kidney disease; IgG4-SC, IgG4-related sclerosing cholangitis.
Malignancy in patients with IgG4-RD
In total, 13 patients (11%) were diagnosed with a malignancy within 3 years before the diagnosis of IgG4-RD (n = 4), concurrently (n = 2), or during follow-up (n = 7; Table 3). Three cancers were confirmed to originate from the pancreas or biliary tree (two cholangiocarcinomas and one pancreatic adenocarcinoma). The patients with concurrent pancreatic adenocarcinoma and cholangiocarcinoma had an elevated serum IgG4 (four times the upper limit of normal), diagnostic histological features for IgG4-RD, with diffuse disease in the resection specimens, and the patient with pancreatic adenocarcinoma had parotid IgG4-RD, which relapsed post surgery. The other patient with cholangiocarcinoma had diagnostic features of IgG4-RD, which predated the cancer diagnosis by 2 years. In total, two patients developed colorectal cancer, three developed prostate cancer, two developed transitional cell bladder tumors, one patient had a testicular cancer, one had lung cancer, and one had cancer of unknown primary. In addition to these cancers, one patient was found to have a low-grade pancreatic intraepithelial neoplasia lesion of the pancreas after Whipple’s resection, and another had a small gastrointestinal stromal tumor within the duodenum.
Table 3. Temporal relationship between the diagnosis of IgG4-RD and diagnosis of cancer in the cohort.
| Cancer diagnosis | Time in relation to IgG4-RD diagnosis (years) |
|---|---|
| Before diagnosis | |
| Prostate cancer | −3 |
| Testicular leiomyosarcoma | −3 |
| Transitional cell tumor of the bladder | −2 |
| Prostate cancer | −1 |
| Concurrent diagnoses (in resection specimen) | |
| Pancreatic cancer | |
| Cholangiocarcinoma | |
| After diagnosis | |
| Transitional cell tumor of the bladder | 1 |
| Colorectal cancer | 1.5 |
| Colorectal cancer | 2 |
| Cholangiocarcinoma | 2 |
| Lung cancer | 4 |
| Metastatic cancer (unknown primary) | 5 |
| Prostate | 10 |
IgG4-RD, IgG4-related disease.
Using public data from Cancer Research UK, the expected age- and sex-matched number of cancer cases to occur within the follow-up period was 4.5 cases. The actual number to have occurred was nine cases. Within this cohort, there was an increased incidence ratio of 2.25 (CI = 1.23–3.94, P = 0.02) for all cancers compared with the general population.
Mortality
Eleven patients (10%) in our cohort died during follow-up (Table 4). Four patients died from cancer--one from a cholangiocarcinoma, one from transitional cell tumor of the bladder, one from lung cancer, and a further one from cancer of unknown primary. One patient developed rapidly progressive biliary stricturing disease with progressive jaundice, and died from cholangitis before liver transplantation. One patient, with biopsy-proven multiorgan IgG4-RD, developed a rapidly progressive encephalitic illness, and a diagnosis of autoimmune encephalitis was made on the basis of characteristic features of encephalitis on imaging (gadolinium magnetic resonance imaging) and cerebrospinal fluid analysis. This patient died despite treatment with high-dose steroids and immunosuppression. Two patients died of pulmonary fibrosis, one died of pulmonary embolism, and one died of pneumonia. One patient died of postoperative complications of a Whipple’s resection performed for presumed cancer (the resection sample showing AIP).
Table 4. Causes of death in the 11 patients who died, with duration of IgG4-RD before death.
| Cause of death | Time after diagnosis of IgG4-RD |
|---|---|
| Pulmonary fibrosis | 6 Months |
| Post-operative death | 1.5 Years |
| Transitional cell carcinoma of bladder | 1.5 Years |
| Pneumonia | 2 Years |
| End-stage liver disease | 2.5 Years |
| Cholangiocarcinoma | 2.5 Years |
| Autoimmune encephalitis | 3 Years |
| Lung cancer | 4 Years |
| Pulmonary embolus | 5 Years |
| Metastatic cancer (unknown primary) | 5 Years |
| Pulmonary fibrosis | 9 Years |
IgG4-RD, IgG4-related disease.
Using National UK mortality statistics, the expected age- and sex-matched number of deaths to occur within the follow-up period was 5.4, and 11 were observed in this cohort. There was an increased incidence ratio of all-cause mortality of 2.07 (CI = 1.07–3.55, P = 0.02). The cancer-related mortality was not increased compared with the national statistics (expected 2.1 deaths and observed 4 deaths; incidence ratio = 1.95, CI = 0.6–4.51; P = 0.17).
DISCUSSION
Over recent years, a developing consensus on the pathology, clinical characteristics, and diagnostic features of this disease has led to important advances, including recognition that AIP is one component of a multisystem IgG4-RD (4,17,18), and that this is a global disease (6,19,20). Early studies of IgG4-RD focused on the predictable and gratifying clinical and radiological response to an initial course of steroids, and for this reason IgG4-RD is commonly perceived to run a benign disease course. However, disease relapse following an initial course of steroids is now recognized to occur in 30–50% of patients (21,22), and relapse frequently arises in organs other than the pancreas. Furthermore, long-term clinical outcome data in those both receiving and not receiving treatment remain limited (23-25), and the true morbidity and mortality associated with IgG4-RD are currently unclear.
In this study, we describe one of the largest prospective cohorts of IgG4-RD reported from Europe. We found that the pattern of clinical presentation, response to steroids, and subsequent disease relapse (50%) was similar to that reported in studies from Japan and the United States of America (5,19,26). Extrapancreatic disease was particularly common, occurring in 73% of patients, most frequently proximal biliary disease. Although serum IgG4 was previously believed to be highly sensitive and specific for AIP (27), our data show that raised serum IgG4 was present in only 70% of patients at diagnosis. This reinforces data from other studies that serum IgG4 cannot be used in isolation to make a firm diagnosis, or differentiate from other diseases (28-30). We found that pretreatment serum IgG4 level, or its change with treatment, did not predict relapse, but that patients with IgG4-SC were more likely to relapse and had a more complicated course. This supports the finding of other studies of IgG4-RD, showing that proximal/intrahepatic biliary disease strongly predicted relapse after an initial favorable steroid response (5,28).
The high relapse rate (50%) after corticosteroid therapy and the need for re-treatment or prolonged therapy, especially with IgG4-SC, raises two important issues. First, corticosteroid-related side effects should be anticipated and preventative measures taken, and, second, there is an urgent need for international prospective studies to evaluate rational treatment strategies, including the early use of second-line treatment regimens and B-cell depletion therapy to prevent relapse and long-term complications of disease.
In our patient cohort, long-term follow-up of up to 11 years (median 3 years) revealed a significant number of cases of organ dysfunction, with an associated morbidity and mortality. This occurred despite close clinical follow-up and treatment of clinically active IgG4-RD. Particular sites of organ dysfunction included the pancreas, liver, and kidneys. Pancreatic exocrine insufficiency occurred in > 50% of cases, and this included patients who had an excellent clinical and radiological response to steroid therapy, and without disease relapse. This finding is similar to other studies, and it reinforces the extent to which inflammation and subsequent fibrosclerosis can be a rapid process in IgG4-RD. Although steroid therapy undoubtedly has a marked and widely reported effect in resolving the pancreatic mass in IgG4-RD, it remains uncertain as to the extent to which steroids affect preserving pancreatic structure and function (31-33). Pancreatic exocrine failure can, of course, be managed relatively straightforwardly with enzyme supplementation; however, we found a number of cases of other organ dysfunction that included cerebral involvement (including one patient with pituitary failure due to a mass), fatal pulmonary fibrosis, and stage 4 chronic kidney disease, all of which have been previously reported in association with IgG4-RD (34-36).
In addition to a high frequency of IgG4-SC, six of our patients developed cirrhosis, two of whom developed end-stage liver disease resulting in death or liver transplantation. Although there can be diagnostic difficulty between IgG4-SC and primary sclerosing cholangitis (37), each of these patients fulfilled International Consensus Diagnostic Criteria for IgG4-RD and all of them had organ involvement outside the biliary tree and liver. There is ongoing interest concerning the observation that patients with primary sclerosing cholangitis who have an elevated IgG4 in serum or tissue have elevated parameters of liver disease severity and a more rapid progression to transplantation (38). Indeed, there are considerable radiographic similarities between primary sclerosing cholangitis and IgG4-SC, but the extent of pathogenic overlap between the two conditions remains uncertain (39-41). There is no doubt that close follow-up with clinical assessment, liver function tests, and regular imaging, along with aggressive management of progressive biliary stricturing, should be considered in all patients with IgG4-SC.
The fundamental question of whether IgG4-RD is associated with cancer (42), and an overall reduced life expectancy, remains unanswered. When comparing this cohort with expected age- and sex-matched number of cancer cases within the follow-up period from public health data from Cancer Research UK, there was an increased incidence ratio of 2.247 (CI = 1.23–3.939, P = 0.0176) for all cancers compared with the general population. In particular, the number of pancreaticobiliary cancers in this cohort (3 out of 115 patients) was higher than expected for the age-standardized incidence in the United Kingdom of 3 per 100,000 for pancreatic carcinoma and 0.5–1 per 100,000 for intrahepatic or extrahepatic cholangiocarcinoma, respectively. A recent retrospective analysis of pancreatic resection specimens showed that 82% (23 cases) of AIP showed pancreatic intraepithelial neoplasia and 25% (7 cases) showed grade 2 pancreatic intraepithelial neoplasia. In addition, 2 of 84 AIP patients developed pancreatic carcinoma 6 and 10 years after diagnosis, raising further concerns for an elevated risk of malignancy in AIP. Of 21 patients with AIP followed by Uchida et al., pancreatic cancer occurred in one patient after 50 months of follow-up (32). In a large international retrospective study of 978 patients with AIP type 1, pancreaticobiliary cancers were reported in 8 patients (of which 5 were pancreatic and 3 were cholangiocarcinoma) (5). In another recent paper by Shiokawa et al., cancers were identified in 13.9% of 108 AIP patients during a median follow-up of 3.3 years (10). The finding of an intense IgG4 + ve infiltrate in the cancer stroma of six of eight patients studied before steroid therapy, and an absence of AIP disease relapse in any of these six after successful cancer therapy, led the authors to suggest the possibility of AIP/IgG4-RD being a paraneoplastic phenomenon in some patients. This may be the case in two of our patients where there were histological features of IgG4-RD in resection specimens from pancreaticobiliary cancers. In our study, the development of cancer in 13 patients (11%) after, or within 3 years of, the diagnosis of AIP/IgG4-RD was higher than we expected. Although most of the cases are arguably unattributable to IgG4-RD, chronic pancreaticobiliary inflammation may be linked to the development of cancer in three patients with pancreaticobiliary malignancy.
The epidemiology and etiopathogenesis of cancer in IgG4-RD merits ongoing attention. The concept of inflammation leading to malignancy is well recognized and has been described in a variety of disease states such as hepatocellular cancer and liver inflammation secondary to viral hepatitis, colonic cancer in inflammatory bowel disease, and cholangiocarcinoma on a background of primary sclerosing cholangitis. Therefore, in IgG4-RD, it is plausible that malignancy develops in a setting of chronic inflammation. However, a recent publication has identified a potential cellular mechanism whereby IgG4 antibodies are specifically generated in response to malignant disease (43); in human melanoma patients, tumor-associated B cells are polarized to produce IgG4, stimulated by a Th 2 (interleukin-10 secreting) tumor microenvironment. Strikingly, IgG4 antibodies were able to inhibit the antitumor effector functions of IgG1 antibodies, and IgG4 serum levels were associated with decreased patient survival. This study provides evidence for an alternative hypothesis linking malignancy to IgG4-RD whereby the generation of IgG4 antibodies, in the context of malignancy, represents a mechanism of tumor-induced immune escape.
Given the increasing evidence that IgG4-RD no longer runs an entirely benign course, we would recommend a policy of close observation early after initiating steroid therapy to confirm symptomatic improvement and steroid tolerance, and then assessment at weeks 6–8 with imaging (and biochemical assessment where appropriate) to confirm steroid responsiveness. Patients may then be assessed at 3 and 6 months to wean and discontinue steroids assuming a primary response to steroid therapy. We believe that repeat imaging (computed tomography or magnetic resonance imaging) should be performed between 6 months and 1 year after steroid discontinuation to assess the maintenance of the response. Further imaging is dictated by clinical or biochemical evidence of relapse. Importantly, we would recommend that a computed tomography of the chest, abdomen, and pelvis is performed at diagnosis and at 6–12 months post treatment both to assess any subclinical organ involvement and to detect malignancy.
The number of surgical operations carried out in this cohort for suspected pancreaticobiliary cancer was high (21%). The majority of resections were performed before 2008 when there was less awareness of AIP and IgG4-SC; however, it still highlights the fact that IgG4-RD is diffi cult to distinguish from localized malignancy. We would recommend that all patients with possible AIP or IgG4-SC are discussed in a hepatopancreaticobiliary multidisciplinary meeting involving medical, surgical, radiology, and histopathology input. Serum IgG4 levels should be routinely measured if there is clinical suspicion, although 30% of AIP/IgG4-SC patients will have a normal serum IgG4 and elevated levels are not specific for IgG4-RD. A level of > 4 times the upper limit of normal (5.6 g/l) can be helpful (45-46). We have recently reported the importance of careful review of imaging in helping to distinguish cholangiocarcinoma from IgG4-SC (47). Histological evidence of disease from the pancreas or other involved organs is important, where possible. All pancreatic biopsies should be reviewed for the key morphological features and immunostaining for IgG4 should be performed. Where there is diagnostic doubt, some physicians advocate a short (2 weeks) course of corticosteroid therapy to aid the diagnosis. However, this approach should be applied with caution, as a steroid-related improvement in symptoms, a fall in serum IgG4, reduction in liver function tests, and reduction of a mass on imaging may all occur in the presence of malignancy.
This study has a number of limitations. Although the cohort size and study follow-up period are relatively large for this rare disease, these values are still numerically small and there is therefore the possibility of underestimating the risk of organ dysfunction and malignancy (especially if pancreaticobiliary cancers are a consequence of chronic inflammation, as in alcohol-related chronic pancreatitis). Such studies are also at significant risk of ascertainment bias as the rate of malignancy may be artificially elevated by earlier discovery of cancer in patients having scans for evaluation of IgG4-RD. Furthermore, conclusions drawn from a comparison with national statistics should be treated with caution as they are not adjusted for other risk factors such as smoking, alcohol intake, and obesity. A diseased control cohort would have been preferable in this respect and would potentially eliminate some of these variables and may help explain the discrepancy between this study and a previous US study showing similar 5-year survival to the general population (44).
Our series adds to a growing body of evidence showing that IgG4-RD may run a clinical course that is associated with a significant morbidity and mortality. Importantly, we have shown that patients with proximal/intrahepatic biliary disease have a higher relapse rate and a more complicated course. Long-term follow-up is required in all of these patients. Ultimately, prospective studies of natural history and novel therapeutic agents, in very carefully defined patient cohorts, will be required to establish the true clinical course of the disease.
Study Highlights.
WHAT IS CURRENT KNOWLEDGE
-
✓
Autoimmune pancreatitis is one component of a multi-system IgG4-related disease (IgG4-RD).
-
✓
The majority of patients have a rapid response to corticosteroids, but many relapse.
-
✓
IgG4-RD has inflammatory and fibrotic components; the former is more corticosteroid responsive.
-
✓
There may be an increased risk of malignancy in IgG4-RD, but this risk is largely undefined.
WHAT IS NEW HERE
-
✓
Extrapancreatic disease affects over 70% of patients.
-
✓
Extrapancreatic disease at presentation, especially IgG4-related cholangitis, predicts patients who are more likely to relapse.
-
✓
There is a significant complication rate including endocrine and exocrine pancreatic insufficiency, renal failure, thrombosis, liver cirrhosis, and failure.
-
✓
There is substantial mortality from fibrotic disease, cancer, and inflammatory complications.
ACKNOWLEDGMENTS
We thank Faye Highfield (research NIHR portfolio facilitator in Oxford), who was involved in patient recruitment and data entry; Dr Ross Sadler (Department of Immunology, Churchill Hospital in Oxford), who was involved in creation and update of the immunology database; and George Nicholson (Department of Statistics Oxford University), who was involved in statistical analysis of cancer and mortality incidence rates.
Financial support: Funding for the Oxford work was obtained from a Wellcome Trust Research Fellowship into the natural history and pathogenesis of IgG4-RD (095160/Z10/Z) awarded to E.L. Culver and supervised by E. Barnes and R.W. Chapman. E. Barnes is supported by the MRC as an MRC Senior Clinical Fellow. The work that was undertaken at University College Hospital/UCL was supported in part by NIH program grant P01 CA084203 and the Department of Health’s National Institute for Health Research (NIHR) Biomedical Research Centres funding scheme.
Footnotes
CONFLICT OF INTEREST
Guarantor of the article: Matthew T. Huggett, BSc, BM, PhD, MRCP.
Potential competing interests: None.
Study registration: This study was registered on the UK NIHR portfolio as study number 10776.
REFERENCES
- 1.Stone JH, Zen Y, Deshpande V. IgG4-related disease. N Engl J Med. 2012;366:539–51. doi: 10.1056/NEJMra1104650. [DOI] [PubMed] [Google Scholar]
- 2.Okazaki K, Kawa S, Kamisawa T, et al. Clinical diagnostic criteria of autoimmune pancreatitis: revised proposal. J Gastroenterol. 2006;41:626–31. doi: 10.1007/s00535-006-1868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chari ST, Smyrk TC, Levy MJ, et al. Diagnosis of autoimmune pancreatitis: the Mayo Clinic experience. Clin Gastroenterol Hepatol. 2006;4:1010–6. doi: 10.1016/j.cgh.2006.05.017. quiz 934. [DOI] [PubMed] [Google Scholar]
- 4.Kamisawa T, Egawa N, Nakajima H. Autoimmune pancreatitis is a systemic autoimmune disease. Am J Gastroenterol. 2003;98:2811–2. doi: 10.1111/j.1572-0241.2003.08758.x. [DOI] [PubMed] [Google Scholar]
- 5.Hart PA, Kamisawa T, Brugge WR, et al. Long-term outcomes of autoimmune pancreatitis: a multicentre, international analysis. Gut. 2013;62:1771–6. doi: 10.1136/gutjnl-2012-303617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chari ST, Longnecker DS, Kloppel G. The diagnosis of autoimmune pancreatitis: a Western perspective. Pancreas. 2009;38:846–8. doi: 10.1097/MPA.0b013e3181bba281. [DOI] [PubMed] [Google Scholar]
- 7.Chari ST, Kloeppel G, Zhang L, et al. Histopathologic and clinical subtypes of autoimmune pancreatitis: the Honolulu consensus document. Pancreas. 2010;39:549–54. doi: 10.1097/MPA.0b013e3181e4d9e5. [DOI] [PubMed] [Google Scholar]
- 8.Sandanayake NS, Church NI, Chapman MH, et al. Presentation and management of post-treatment relapse in autoimmune pancreatitis/immunoglobulin G4-associated cholangitis. Clin Gastroenterol Hepatol. 2009;7:1089–96. doi: 10.1016/j.cgh.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 9.Hart PA, Topazian MD, Witzig TE, et al. Treatment of relapsing autoimmune pancreatitis with immunomodulators and rituximab: the Mayo Clinic experience. Gut. 2013;62:1607–15. doi: 10.1136/gutjnl-2012-302886. [DOI] [PubMed] [Google Scholar]
- 10.Shiokawa M, Kodama Y, Yoshimura K, et al. Risk of cancer in patients with autoimmune pancreatitis. Am J Gastroenterol. 2013;108:610–7. doi: 10.1038/ajg.2012.465. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto M, Takahashi H, Tabeya T, et al. Risk of malignancies in IgG4-related disease. Mod Rheumatol. 2012;22:414–8. doi: 10.1007/s10165-011-0520-x. [DOI] [PubMed] [Google Scholar]
- 12.Otsuki M, Chung JB, Okazaki K, et al. Asian diagnostic criteria for autoimmune pancreatitis: consensus of the Japan-Korea Symposium on Autoimmune Pancreatitis. J Gastroenterol. 2008;43:403–8. doi: 10.1007/s00535-008-2205-6. [DOI] [PubMed] [Google Scholar]
- 13.Bjornsson E, Chari ST, Smyrk TC, et al. Immunoglobulin G4 associated cholangitis: description of an emerging clinical entity based on review of the literature. Hepatology. 2007;45:1547–54. doi: 10.1002/hep.21685. [DOI] [PubMed] [Google Scholar]
- 14.Shimosegawa T, Chari ST, Frulloni L, et al. International consensus diagnostic criteria for autoimmune pancreatitis: guidelines of the International Association of Pancreatology. Pancreas. 2011;40:352–8. doi: 10.1097/MPA.0b013e3182142fd2. [DOI] [PubMed] [Google Scholar]
- 15.Deshpande V, Zen Y, Chan JK, et al. Consensus statement on the pathology of IgG4-related disease. Mod Pathol. 2012;25:1181–92. doi: 10.1038/modpathol.2012.72. [DOI] [PubMed] [Google Scholar]
- 16.Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. 3rd edn. Lippincott, Williams & Wilkins; Philadelphia, PA: 2008. [Google Scholar]
- 17.Kamisawa T. Is it time to reconsider autoimmune pancreatitis? J Gastroenterol. 2006;41:1240–1. doi: 10.1007/s00535-006-1968-x. [DOI] [PubMed] [Google Scholar]
- 18.Kamisawa T. Immunoglobulin G4-positive plasma cells in organs of patients with autoimmune pancreatitis. Clin Gastroenterol Hepatol. 2008;6:715. doi: 10.1016/j.cgh.2007.12.053. author reply 715. [DOI] [PubMed] [Google Scholar]
- 19.Kamisawa T, Chari ST, Giday SA, et al. Clinical profile of autoimmune pancreatitis and its histological subtypes: an international multicenter survey. Pancreas. 2011;40:809–14. doi: 10.1097/MPA.0b013e3182258a15. [DOI] [PubMed] [Google Scholar]
- 20.Varadarajulu S, Cotton PB. Autoimmune pancreatitis: is it relevant in the west? Gastroenterology. 2003;125:1557. doi: 10.1016/j.gastro.2003.02.006. [DOI] [PubMed] [Google Scholar]
- 21.Chari ST, Murray JA. Autoimmune pancreatitis, Part II: the relapse. Gastroenterology. 2008;134:625–8. doi: 10.1053/j.gastro.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 22.Kalaitzakis E, Webster GJ. Review article: autoimmune pancreatitis - management of an emerging disease. Aliment Pharmacol Ther. 2011;33:291–303. doi: 10.1111/j.1365-2036.2010.04526.x. [DOI] [PubMed] [Google Scholar]
- 23.Kamisawa T, Shimosegawa T, Okazaki K, et al. Standard steroid treatment for autoimmune pancreatitis. Gut. 2009;58:1504–7. doi: 10.1136/gut.2008.172908. [DOI] [PubMed] [Google Scholar]
- 24.Naitoh I, Nakazawa T, Ohara H, et al. Clinical significance of extrapancreatic lesions in autoimmune pancreatitis. Pancreas. 2010;39:e1–5. doi: 10.1097/MPA.0b013e3181bd64a1. [DOI] [PubMed] [Google Scholar]
- 25.Takuma K, Kamisawa T, Tabata T, et al. Short-term and long-term outcomes of autoimmune pancreatitis. Eur J Gastroenterol Hepatol. 2011;23:146–52. doi: 10.1097/meg.0b013e3283431e23. [DOI] [PubMed] [Google Scholar]
- 26.Ghazale A, Chari ST, Zhang L, et al. Immunoglobulin G4-associated cholangitis: clinical profile and response to therapy. Gastroenterology. 2008;134:706–15. doi: 10.1053/j.gastro.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 27.Hamano H, Kawa S, Horiuchi A, et al. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med. 2001;344:732–8. doi: 10.1056/NEJM200103083441005. [DOI] [PubMed] [Google Scholar]
- 28.Ghazale A, Chari ST, Smyrk TC, et al. Value of serum IgG4 in the diagnosis of autoimmune pancreatitis and in distinguishing it from pancreatic cancer. Am J Gastroenterol. 2007;102:1646–53. doi: 10.1111/j.1572-0241.2007.01264.x. [DOI] [PubMed] [Google Scholar]
- 29.Kamisawa T, Takuma K, Tabata T, et al. Serum IgG4-negative autoimmune pancreatitis. J Gastroenterol. 2011;46:108–16. doi: 10.1007/s00535-010-0317-2. [DOI] [PubMed] [Google Scholar]
- 30.Sugumar A, Takahashi N, Chari ST. Distinguishing pancreatic cancer from autoimmune pancreatitis. Curr Gastroenterol Rep. 2010;12:91–7. doi: 10.1007/s11894-010-0098-z. [DOI] [PubMed] [Google Scholar]
- 31.Hirano K, Tada M, Isayama H, et al. Long-term prognosis of autoimmune pancreatitis with and without corticosteroid treatment. Gut. 2007;56:1719–24. doi: 10.1136/gut.2006.115246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uchida K, Yazumi S, Nishio A, et al. Long-term outcome of autoimmune pancreatitis. J Gastroenterol. 2009;44:726–32. doi: 10.1007/s00535-009-0049-3. [DOI] [PubMed] [Google Scholar]
- 33.Maruyama M, Arakura N, Ozaki Y, et al. Risk factors for pancreatic stone formation in autoimmune pancreatitis over a long-term course. J Gastroenterol. 2012;47:553–60. doi: 10.1007/s00535-011-0510-y. [DOI] [PubMed] [Google Scholar]
- 34.Wong S, Lam WY, Wong WK, et al. Hypophysitis presented as inflammatory pseudotumor in immunoglobulin G4-related systemic disease. Hum Pathol. 2007;38:1720–3. doi: 10.1016/j.humpath.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 35.Tsushima K, Tanabe T, Yamamoto H, et al. Pulmonary involvement of autoimmune pancreatitis. Eur J Clin Invest. 2009;39:714–22. doi: 10.1111/j.1365-2362.2009.02164.x. [DOI] [PubMed] [Google Scholar]
- 36.Yoneda K, Murata K, Katayama K, et al. Tubulointerstitial nephritis associated with IgG4-related autoimmune disease. Am J Kidney Dis. 2007;50:455–62. doi: 10.1053/j.ajkd.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 37.Kalaitzakis E, Levy M, Kamisawa T, et al. Endoscopic retrograde cholangiography does not reliably distinguish IgG4-associated cholangitis from primary sclerosing cholangitis or cholangiocarcinoma. Clin Gastroenterol Hepatol. 2011;9:800–3. e2. doi: 10.1016/j.cgh.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mendes FD, Jorgensen R, Keach J, et al. Elevated serum IgG4 concentration in patients with primary sclerosing cholangitis. Am J Gastroenterol. 2006;101:2070–5. doi: 10.1111/j.1572-0241.2006.00772.x. [DOI] [PubMed] [Google Scholar]
- 39.Webster GJ, Pereira SP, Chapman RW. Autoimmune pancreatitis/IgG4-associated cholangitis and primary sclerosing cholangitis--overlapping or separate diseases? J Hepatol. 2009;51:398–402. doi: 10.1016/j.jhep.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 40.Zen Y, Nakanuma Y, Portmann B. Immunoglobulin G4-related sclerosing cholangitis: pathologic features and histologic mimics. Semin Diagn Pathol. 2012;29:205–11. doi: 10.1053/j.semdp.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 41.Okazaki K. Is primary sclerosing cholangitis different from sclerosing cholangitis with autoimmune pancreatitis? J Gastroenterol. 2007;42:600–1. doi: 10.1007/s00535-007-2066-4. [DOI] [PubMed] [Google Scholar]
- 42.Ghazale A, Chari S. Is autoimmune pancreatitis a risk factor for pancreatic cancer? Pancreas. 2007;35:376. doi: 10.1097/MPA.0b013e318073ccb8. [DOI] [PubMed] [Google Scholar]
- 43.Karagiannis P, Gilbert AE, Josephs DH, et al. IgG4 subclass antibodies impair antitumor immunity in melanoma. J Clin Invest. 2013;123:1457–74. doi: 10.1172/JCI65579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sah RP, Chari ST, Pannala R, et al. Differences in clinical profile and relapse rate of type 1 versus type 2 autoimmune pancreatitis. Gastroenterology. 2010;139:140–8. doi: 10.1053/j.gastro.2010.03.054. quiz e12-3. [DOI] [PubMed] [Google Scholar]
- 45.Boostra K, Culver EL, Maillette de Buy Wenniger L, et al. Serum IgG4 and IgG1 for distinguishing IgG4-Associated Cholangitis from Primary Sclerosing Cholangitis. Heptology. 2014;59:1954–63. doi: 10.1002/hep.26977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oseini AM, Chaiteerakji R, Shire AM, et al. Utility of serum immunoglobulin G4 in distinguishing Immunoglobulin G4-Associated Cholangitis from Cholangiocrainoma. Hepatology. 2011;54:940–8. doi: 10.1002/hep.24487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lytras D, Kalatzakis E, Webster G, et al. Cholangiocarcinoma or IgG4-associated cholangitis: how feasible is it to avoid unnecessary surgical interventions? Ann Surg. 2012;256:1059–67. doi: 10.1097/SLA.0b013e3182533a0a. [DOI] [PubMed] [Google Scholar]