Abstract
Background
The annual cost of providing care for patients in their last year of life is estimated to account for approximately 9% of the Ontario health care budget. Access to integrated, comprehensive support and pain/symptom management appears to be inadequate and inequitable.
Objective
To evaluate the cost-effectiveness of end-of-life (EoL) care interventions included in the EoL care mega-analysis.
Data Sources
Multiple sources were used, including systematic reviews, linked health administration databases, survey data, planning documents, expert input, and additional literature searches.
Review Methods
We conducted a literature review of cost-effectiveness studies to inform the primary economic analysis. We conducted the primary economic analysis and budget impact analysis for an Ontario cohort of decedents and their families and included interventions pertaining to team-based models of care, patient care planning discussions, educational interventions for patients and caregivers, and supportive interventions for informal caregivers. The time horizon was the last year of life. Costs were in 2013 Canadian dollars. Effectiveness measures included days at home, percentage dying at home, and quality-adjusted life-days. We developed a Markov model; model inputs were obtained from a cohort of Ontario decedents assembled from Institute for Clinical Evaluative Sciences databases and published literature.
Results
In-home palliative team care was cost-effective; it increased the chance of dying at home by 10%, increased the average number of days at home (6 days) and quality-adjusted life-days (0.5 days), and it reduced costs by approximately $4,400 per patient. Expanding in-home palliative team care to those currently not receiving such services (approximately 45,000 per year, at an annual cost of $76–108 million) is likely to improve quality of life, reduce the use of acute care resources, and save $191–$385 million in health care costs. Results for the other interventions were uncertain.
Limitations
The cost-effectiveness analysis was based in part on the notion that resources allocated to EoL care interventions were designed to maximize quality-adjusted life-years (QALY) for patients and their family, but improving QALYs may not be the intended aim of EoL interventions.
Conclusions
In-home palliative team care was cost-effective, but firm conclusions about the cost-effectiveness of other interventions were not possible.
Plain Language Summary
Most people with a terminal illness say they would prefer to die at home, but we know that they are more likely to die in hospital. As part of an effort to improve end-of-life care in the Ontario health care system, we evaluated the cost-effectiveness of nine quality improvement strategies. We found that in-home team care increased people's chances of dying at home, increased the time spent at home in the last year of life, and reduced health care costs by about $4,400 per patient. Because of the limited data available, we could not make firm conclusions about the cost-effectiveness of the remaining strategies related to team care, patient care planning discussions, education for patients and caregivers, and support services for caregivers.
Background
The Toronto Health Economic and Technology Assessment (THETA) Collaborative was commissioned by Health Quality Ontario (HQO) to evaluate cost-effectiveness of palliative interventions to improve health-related quality of life of Ontarians who are nearing end-of-life and their family. This report summarizes the methods and results of the economic literature review and original economic evaluation developed for this analysis.
Health Quality Ontario conducts full evidence-based analyses, including economic analyses, of health technologies being considered for use in Ontario. These analyses are then presented to the Ontario Health Technology Advisory Committee, whose mandate it is to examine proposed health technologies in the context of available evidence and existing clinical practice, and to provide advice and recommendations to Ontario health care practitioners, the broader health care system, and the Ontario Ministry of Health and Long-Term Care.
DISCLAIMER: Health Quality Ontario uses a standardized costing method for its economic analyses. The main cost categories and associated methods of retrieval from the province's perspective are described below.
Hospital costs: Ontario Case Costing Initiative cost data are used for in-hospital stay, emergency department visit, and day procedure costs for the designated International Classification of Diseases diagnosis codes and Canadian Classification of Health Interventions procedure codes. Adjustments may be required to reflect accuracy in the estimated costs of the diagnoses and procedures under consideration. Due to difficulties in estimating indirect costs in hospitals associated with a particular diagnosis or procedure, Health Quality Ontario normally defaults to a consideration of direct treatment costs only.
Non-hospital costs: These include physician services costs obtained from the Ontario Schedule of Physician Benefits, laboratory fees from the Ontario Schedule of Laboratory Fees, drug costs from the Ontario Drug Benefit Formulary, and device costs from the perspective of local health care institutions whenever possible, or from the device manufacturer.
Discounting: As appropriate, a discount rate of 5% is applied (to both costs and effects/QALYs), as recommended by economic guidelines.
Downstream costs: All reported downstream costs are based on assumptions of population trends (i.e., incidence, prevalence, and mortality rates), time horizon, resource utilization, patient compliance, health care patterns, market trends (i.e., rates of intervention uptake or trends in current programs in place in the province), and estimates of funding and prices. These may or may not be realized by the Ontario health care system or individual institutions and are often based on evidence from the medical literature, standard listing references, and educated hypotheses from expert panels. In cases where a deviation from this standard is used, an explanation is offered as to the reasons, the assumptions, and the revised approach.
The economic analysis represents an estimate only, based on the assumptions and costing methods explicitly stated above. These estimates will change if different assumptions and costing methods are applied to the analysis.
NOTE: Numbers may be rounded to the nearest decimal point, as they may be reported from an Excel spreadsheet
In July 2013, the Evidence Development and Standards (EDS) branch of Health Quality Ontario (HQO) began work on developing an evidentiary framework for end of life care. The focus was on adults with advanced disease who are not expected to recover from their condition. This project emerged from a request by the Ministry of Health and Long-Term Care that HQO provide them with an evidentiary platform on strategies to optimize the care for patients with advanced disease, their caregivers (including family members), and providers.
After an initial review of research on end-of-life care, consultation with experts, and presentation to the Ontario Health Technology Advisory Committee (OHTAC), the evidentiary framework was produced to focus on quality of care in both the inpatient and the outpatient (community) settings to reflect the reality that the best end-of-life care setting will differ with the circumstances and preferences of each client. HQO identified the following topics for analysis: determinants of place of death, patient care planning discussions, cardiopulmonary resuscitation, patient, informal caregiver and healthcare provider education, and team-based models of care. Evidence-based analyses were prepared for each of these topics.
HQO partnered with the Toronto Health Economics and Technology Assessment (THETA) Collaborative to evaluate the cost-effectiveness of the selected interventions in Ontario populations. The economic models used administrative data to identify an end-of-life population and estimate costs and savings for interventions with significant estimates of effect. For more information on the economic analysis, please contact Murray Krahn at murray.krahn@theta.utoronto.ca.
The End-of-Life mega-analysis series is made up of the following reports, which can be publicly accessed at http://www.hqontario.ca/evidence/publications-and-ohtac-recommendations/ohtas-reports-and-ohtac-recommendations.
-
▸
End-of-Life Health Care in Ontario: OHTAC Recommendation
-
▸
Health Care for People Approaching the End of Life: An Evidentiary Framework
-
▸
Effect of Supportive Interventions on Informal Caregivers of People at the End of Life: A Rapid Review
-
▸
Cardiopulmonary Resuscitation in Patients with Terminal Illness: An Evidence-Based Analysis
-
▸
The Determinants of Place of Death: An Evidence-Based Analysis
-
▸
Educational Intervention in End-of-Life Care: An Evidence-Based Analysis
-
▸
End-of-Life Care Interventions: An Economic Analysis
-
▸
Patient Care Planning Discussions for Patients at the End of Life: An Evidence-Based Analysis
-
▸
Team-Based Models for End-of-Life Care: An Evidence-Based Analysis
Objective of Analysis
The objective of this analysis was to evaluate the cost-effectiveness of end-of-life (EoL) care interventions included in part of the EoL care mega-analysis. (1)
Clinical Need and Target Population
The end of life is “a phase of life when a person is living with an illness that will worsen and eventually cause death.” (2) The target population for EoL includes people whose health is in decline and are deemed to be terminal or dying in the foreseeable future. (3) In this report, end-of-life care and palliative care will be used interchangeably and are intended to mean care for the target population.
Between 2007 and 2011, 87,000 to 89,000 people died in Ontario each year. (1) Of those who died between 2007 and 2009, 99% were adults aged 18 and older, and causes of death included cancer (30%), heart disease (21%), cerebrovascular disease (6%), accidents (4%), chronic lower respiratory illness (4%), diabetes mellitus (3%), Alzheimer's disease (3%), influenza and pneumonia (2%), and kidney-related disease (1%). (1) From 2000 to 2009, death due to Alzheimer's disease had the largest relative increase, at 25%. (1) By 2026, the proportion of elderly Ontarians is expected to increase to 21% (from the current 13%). (4) As the population ages, the demand for EoL care services is likely to increase.
The annual cost of providing care for patients in their last year of life is estimated to account for approximately 9% of the Ontario health care budget. (5) EoL care in Canada tends to incorporate a consultation team in institutions and home care settings, with an emphasis on shared care approaches. (6) Still, access to integrated, comprehensive support and pain/symptom management appears to be inadequate and inequitable. (7) There is significant disparity across health regions in terms of access to 24/7 EoL care and interprofessional expertise, (8) because referrals to EoL care are either not made or made too late in the disease trajectory. Key supports for families and caregivers are lacking or inconsistently available. (8)
Communities, philanthropists, the private sector, and provincial governments support the provision of EoL care outside the statutes of the Canada Health Act, but programs are unevenly distributed across the country, small with regard to service capability, (7) rely heavily on volunteers, and vary in terms of service offerings. Currently, dying patients rely on care in emergency departments (EDs) and hospitals, where they may encounter treatment that is not beneficial or is inconsistent with their wishes and preferences and those of their family. (9)
Efforts are being made to improve EoL care at different levels of the Canadian health care system, (10) but cost-effectiveness data are needed to support decision-making, particularly data relevant to current EoL care practices in Canada. (8)
Interventions Under Evaluation
The purpose of the EoL care mega-analysis was to review the evidence in key areas, including team-based models for EoL care, patient care planning discussions, educational interventions for patients and caregivers, and supportive interventions for informal caregivers. (1) We evaluated the cost-effectiveness of evidence-based interventions in these areas. The EoL care mega-analysis also included reviews of determinants of place of death and cardiopulmonary resuscitation (CPR) in patients with terminal illness, but these areas were not considered in the cost-effectiveness analysis because no interventions were associated with the former and cost-effectiveness issues are generally not considered for the latter.
The key areas considered in this analysis are briefly outlined below. Specific interventions are described in details in the Methods section.
Team-Based Models of Care
People approaching the end of life need many health care services to support their physical, emotional, and spiritual needs; using a team-based model to deliver EoL care is generally accepted as optimal. (1) However, team-based models may differ in terms of core elements, including services offered, mode of patient contact, and setting.
Patient Care Planning Discussions
Patient care planning discussions occur between patients, surrogate decision-makers, and health care providers about the goals and desired direction of the patient's care. (11) Their objective is to create a care plan that reflects the patient's and family's wishes after considering factors such as disease status and progress, treatment options, preferences, goals, and values.
Educational Interventions for Patients and Caregivers
Education is “that multidisciplinary practice, which is concerned with designing, implementing, and evaluating educational programs that enable individuals, families, groups, organizations, and communities to play active roles in achieving, protecting, and sustaining health.” (12) Education of health care providers, patients nearing the end of life, and their informal caregivers plays a vital role in increasing their knowledge about the different care options available.
Supportive Interventions for Caregivers
An informal caregiver is an unpaid individual who cares for people who cannot care for themselves due to physically and/or psychologically limiting birth, trauma, or chronic health conditions. Often, relatives or friends become informal caregivers to people approaching the end of life. Caregiving can be burdensome, and studies have shown that it leads to negative health impacts for informal caregivers, including sleep problems, fatigue, depression, anxiety, burnout, and an increased risk of mortality. (13)
Economic Analysis
Research Question
What is the cost-effectiveness of EoL care interventions included in the EoL care mega-analysis?
Economic Literature Review
Research Methods
Literature Search
Search Strategy
An economic literature search was performed on October 22, 2013, using Ovid MEDLINE, Ovid MEDLINE In-Process and Other Non-Indexed Citations, Ovid Embase, and the Centre for Reviews and Dissemination/International Agency for Health Technology Assessment, for studies published from January 1, 2000, to October 22, 2013. (Appendix 1 provides details of the search strategies.) Abstracts were reviewed by a single reviewer and, for those studies meeting the eligibility criteria, full-text articles were obtained. Reference lists were also examined for any additional relevant studies not identified through the search.
Inclusion Criteria
English-language full-text publications
published between January 1, 2000, and October 22, 2013
full economic evaluations: cost-utility analyses or cost-effectiveness analyses
studies reporting on interventions pertaining to palliative care, EoL care, or care of patients with advanced disease
Exclusion Criteria
abstracts, posters, reviews, letters/editorials, foreign language publications, and unpublished studies
Data Abstraction
We used a predefined form to summarize the results of each included study (Appendix 2). The form was developed by members of the health economic team at Health Quality Ontario and has been used previously. (14)
Results of Economic Literature Review
The database search yielded 5,605 citations published between January 1, 2000, and September 28, 2011 (with duplicates removed). Articles were excluded based on information in the title and abstract. The full texts of potentially relevant articles were obtained for further assessment.
Six relevant studies met the inclusion criteria (1 systematic review and 5 cost-effectiveness studies). The reference lists of the included studies were hand-searched to identify other relevant studies, but no additional citations were included.
The results of each included study are described in Table 1. The evidence was inconclusive as to whether in-home palliative care was cost-effective (compared to usual care) for adults with advanced illness and their caregivers. Treatment strategies favouring hospitalization for long-term care (LTC) residents with advanced dementia were not cost-effective. Timely referral to palliative care was potentially cost-effective compared to usual care.
Table 1:
Results of Economic Literature Review—Summary
| Name, Year | Study Design, Perspective | Population | Interventions | Results | Authors’ Conclusions | Limitations | Applicability | ||
|---|---|---|---|---|---|---|---|---|---|
| Health Outcomes | Costs | Cost-Effectiveness | |||||||
| Gomes et al, 2013 (15) | Systematic review of the effectiveness/cost-effectiveness of home palliative care services for adults with advanced illness and their caregivers; cost-effectiveness data were reported in 5 RCTs and 1 controlled before-after study Perspective: societal (16;17) or health care perspective (18–21) | Patients with advanced illness and their family and caregivers (n = 2,047 patients and 1,678 caregivers) | Home palliative care services Usual care with various levels of primary care services, home health services, acute care services, and hospice care | Incremental health outcomes between interventions and controls varied across the 6 included studies | Incremental costs between interventions and controls varied across the 6 included studies | Intervention was cost-effective according to 2 included RCTs. (16;21) It was unclear whether the intervention was cost-effective in the other 4 studies (17–19;22) | More work is needed to study the cost-effectiveness of home palliative care services | Only 2 of the 6 included studies fulfilled the time criteria for this literature review (studies published between 2000 and 2009) | Given the systematic approach of the study, the conclusions are likely to be robust and applicable to similar patients in Ontario |
| Higginson et al, 2009 (16) | CEA; RCT of 12 weeks Perspective: societal, 2005 UK pounds | Patients with severe multiple sclerosis (n = 52) | Fast-track—immediate referral to a palliative care team (n = 26) Usual care (n = 26) | Patient outcomes: no significant differences in POS. A trend in pain reduction was reported for the intervention group, but pain increased for the usual care group Caregivers’ outcomes: intervention group had a significantly lower caregiver burden | Mean costs were £1,789 (95% CI £5,224–£1,902) lower for the intervention group | In-home palliative care significantly increased patient satisfaction while reducing use of medical services and costs of medical care at the end of life | Short-term palliative care for people with severe multiple sclerosis and their caregivers was cost-effective and warranted further study | Small pilot RCT | Intervention effect was studied in patients with severe multiple sclerosis only, limiting the applicability of the trial results to patients with EoL conditions |
| Goldfeld et al, 2013 (23) | Two CUAs in 1 study; prospective cohort study of residents from 22 nursing homes, 18 months’ follow-up Perspective: US Medicare; 2007 US $ | Nursing home residents with advanced dementia (n = 323) | CUA 1 No DNH order (n = 144) DNH order (n = 124) CUA 2 Hospitalization for suspected pneumonia (n = 18) No hospitalization (n = 113) | CUA 1 DNH associated with incremental survival of 3.7 QALDs CUA 2 Hospitalization associated with incremental reduction in survival of 9.7 QALDs | CUA 1 DNH associated with an incremental increase in Medicare expenditures of $5,972 CUA 2 Hospitalization associated with an incremental increase in Medicare expenditures of $3,697 | CUA 1 DNH associated with an estimated cost of approximately $589,000 per QALY gained CUA 2 Hospitalization dominated by no hospitalization | Treatment strategies favouring hospitalization for nursing home residents with advanced dementia were not cost-effective | Analyses based on data from an observational study, with a possibility of unmeasured confounding factors | Likely to be applicable to LTC residents in Ontario |
| Lowery et al, 2013 (24) | CEA and CUA (sensitivity analysis); CEA and CUA based upon a decision tree, 6-month time horizon Perspective: US Medicare; 2012 US$ | Patients with recurrent platinum-resistant ovarian cancer | Early referral to a palliative medicine specialist (EPC) plus usual care Usual care only | EPC associated with significant reductions in ED visits, hospitalizations, and chemotherapy admissions | EPC associated with a cost-saving of $1,285 per patient | EPC was dominant or cost-effective at $50,000 per QALY, unless the cost of outpatient EPC exceeded $2,400 | EPC had the potential to reduce costs associated with EoL care in patients with ovarian cancer | Unclear whether the health outcome estimates derived from an RCT of patients with metastatic NSCLC are applicable to patients with recurrent ovarian cancer in the current study | Overall, the methods were appropriate; likely to be applicable to similar patients in Ontario |
| Pace et al, 2012 (25) | CEA; observational study Perspective: not stated, but included only hospital costs for the last 2 months of life; Euros | Patients with primary brain tumours (n = 143) | Group 1 assisted at home (n = 72) Group 2 not assisted at home (n = 71) | Hospitalization rate of Group 1 was lower than that of Group 2 (16.7% vs. 38%, P = 0.001) | Costs of hospitalization differed substantially: €517 (95% CI €512–522) in Group 1 vs. €24,076 €24,040–24,112) in Group 2 | Group 1 was dominant compared to Group 2 | Home-care models may represent an alternative to in-hospital care for the management of brain tumour patients and may improve EoL quality of care | Unclear whether the 2 groups were similar with respect to factors that influence inputs into the CEA (e.g., re-hospitalization rates and hospital days) | Unclear whether the study results and the authors’ conclusions were valid |
| Ljungman et al, 2013 (26) | CUA; retrospective analysis of a population-based cohort Perspective: health care payer, 1,2,5 years for different patient groups; 2011 Euros | A population-based cohort of patients with exocrine pancreatic adenocarcinoma during 1998–2005 from 1 hospital (n = 444) | Patients with personalized palliative care (n = 21) Patients on standard palliative care for pain management (n = 284) Patients with pancreatic carcinoma resected for cure (n = 139) | QALYs for 1 year from diagnosis were 0.2 (95% CI 0.17–0.23) in patients on palliative care and 0.48 (95% CI 0.44–0.54) in resection patients | Total direct health care costs were 50% in patients on palliative care vs. costs for surgical resections (€23,701 and €50,950, respectively) | Costs per QALY were €118,418 for patients on palliative care and €106,146 for resection patients (95% CI €103,048–€139,418 and €94,352–€115,795, respectively) | Optimized palliative care of patients with exocrine pancreatic carcinoma had costs per achieved utility similar to those for surgical resections aimed at cure | Analysis involved patient groups with very different prognoses; it's unclear whether it was valid to compare the costs and health consequences of palliative patients to those of patients undergoing tumour resection for cure | Results may not be interpretable due to choices of comparators |
Abbreviations: CEA, cost-effectiveness analysis; CI, confidence interval; CUA, cost-utility analysis; DNH, do-not-hospitalize; ED, emergency department; EoL, end-of-life; EPC, early palliative care; LTC, long-term care; NSCLC, non-small cell lung cancer; POS, Palliative Outcome Scale; QALD, quality-adjusted life-day; QALY, quality-adjusted life-year; RCT, randomized controlled trial.
Primary Economic Evaluation
The published economic evaluations included in the literature review addressed some EoL care interventions of interest, but none of these studies comprehensively evaluated those from the mega-analysis. (1) Because of these limitations, we conducted a primary cost-effectiveness analysis.
Research Methods
Type of Analysis
We conducted a cost-effectiveness analysis from the health care payer's perspective. For the base case analysis, health outcomes were days at home in the last year of life and percentage dying at home. We selected these outcomes in part because a high proportion of EoL patients express a preference for dying at home rather than in hospital. (18)
We also conducted sensitivity analyses to inform decisions about allocating resources to EoL care rather than to other health care interventions (see Limitations). For the sensitivity analyses, we used quality-adjusted life-years (QALYs) as an outcome measure. QALYs are widely used in cost-effectiveness analyses for pharmaceuticals, public health programs, surgical procedures, and diagnostic tests; (27) however, they have limited use as an outcome measure for evaluating the cost-effectiveness of EoL interventions (see Limitations).
Interventions Evaluated
We considered 8 interventions identified from the EoL care mega-analysis. (1) These interventions were selected because they are supported by sufficient clinical evidence to be put forward for policy considerations. Table 2 describes the characteristics of the interventions. We compared each intervention to usual care (current EoL care practice in Ontario), because these interventions are not mutually exclusive and can be used in combination to improve the quality of EoL care. The interventions included in the analysis were as follows:
Table 2:
Subgroups and Timing of Intervention Strategies
| Intervention | Description | Subgroup | Timing of Intervention |
|---|---|---|---|
| Usual care | Current patterns of EoL care; decedents were identified with a palliative prognosis if they received at least 1 palliative care service (e.g., physician billing for palliative consultation) | All decedents (with and without a palliative prognosis in their last year of life); the former received additional interventions listed below | Current patterns of EoL care observed from linked health administrative databases at ICES |
| Palliative Team Care | |||
| PTC: In-home | An interprofessional core team that coordinates and delivers palliative services in the home, including the patient and family, a physician, nurse, social worker, and other team members (e.g., a bioethicist, a chaplain) (21) | Decedents with a palliative prognosis who received home care | When a palliative prognosis is detected in a decedent receiving home care |
| PTC: Inpatient | A team that includes a palliative care physician, a nurse, a hospital social worker, and a chaplain. The team assesses the needs of patients with respect to symptom management, psychosocial and spiritual support, and EoL care planning, and provides care and support for patients and informal caregivers (28;29) | Decedents with a palliative prognosis who received inpatient care | When a palliative prognosis is detected in a decedent receiving hospital care |
| PTC: Comprehensive | A team with an outpatient clinic and an inpatient consultant team. The core intervention includes consultation and follow-up in the clinic by a physician and a nurse. The team communicates with family physicians. Home care physicians from the team provide back-up support to family physicians doing house calls or direct care (30) | Decedents with a palliative prognosis who received home care or inpatient care | When a palliative prognosis is detected in a decedent receiving home care or hospital care |
| Patient Care Planning Discussions | |||
| PCPDs: Identifying LTC residents with EoL goals and preferences for EPC | A structured interview is used to identify LTC residents with a palliative prognosis. Residents’ physicians are notified and asked to authorize a visit by a member of an in-home palliative care team (31) | Decedents with a palliative prognosis in LTC | When a palliative prognosis is detected in a LTC resident |
| PCPDs: Ethics consultation for ICU patients with treatment conflicts | ICU nurses identify ICU patients with treatment conflicts that could lead to incompatible courses of action. An ethics consultant discusses the conflicts in easily understood ethical terms with the involved parties (e.g., patients, family, attending physicians), facilitates communication, and explores ways to address and resolve the conflicts (32) | Decedents admitted to ICU in the last month of life | When treatment conflicts are identified by ICU nurses |
| PCPDs: Improving family conferences for relatives of patients dying in the ICU | A proactive EoL conference involving the ICU team members caring for the patient and family and a brochure to facilitate communication during the conference. The aim of the family conference is to lessen the effects of bereavement for caregivers (33) | Decedents in the ICU and their families | Last ICU stay |
| Educational Interventions for Patients and Caregivers | |||
| Multicomponent psycho-educational interventions for patients and families | Education is delivered by APNs with palliative care specialty training. The APNs conduct 4 initial structured educational and problem-solving sessions by phone with the patient and caregiver. The educational approach is designed to encourage patient activation, self-management, and empowerment. The APNs also conduct monthly telephone follow-up until the patient dies (34–36) | Decedents with a palliative prognosis and their families | When a palliative prognosis is detected |
| Supportive Interventions for Informal Caregivers | |||
| Supportive interventions for informal caregivers | Direct support for caregivers (e.g., breaks from caregiving), increasing coping skills (e.g., by providing programs that develop problem-solving) and enhancing well-being (e.g., by providing counselling, relaxation or psychotherapy) (37) | Caregivers of decedents with a palliative prognosis | When a palliative prognosis is detected |
Abbreviations: APN, advance practice nurse; EoL, end-of-life; EPC, early palliative care; ICES, Institute for Clinical Evaluative Sciences; ICU, intensive care unit; LTC, long-term care; PCPD, patient care planning discussion; PTC, palliative team care.
-
palliative team care
-
–
in-home palliative team care
-
–
inpatient palliative team care
-
–
comprehensive palliative team care (in which a single team is in charge of care coordination across all settings)
-
–
-
patient care planning discussions
-
–
identifying LTC residents with EoL goals and preferences for early palliative care
-
–
ethics consultation for intensive care unit (ICU) patients with treatment conflicts among providers, patients and family that could lead to incompatible courses of action
-
–
improving family conferences for relatives of patients dying in the ICU
-
–
-
educational interventions for patients and caregivers
-
–
multicomponent psycho-educational interventions for patients and families
-
–
supportive interventions for informal caregivers
Perspective
The analysis was conducted from the perspective of the Ontario Ministry of Health and Long-Term Care. Costs were expressed in 2013 Canadian dollars.
Discounting and Time Horizon
No discounting was used for health outcomes and costs. We used the cohort's last year of life to define a 1-year time horizon.
Target Population
Using observed, population-based, setting-specific patterns of EoL care services, we conducted a cost-effectiveness analysis for a cohort of Ontarian decedents (average age 72 years, approximately 50% female) and their primary informal caregivers (average age 56 years, approximately 68% female).
Variability and Uncertainty
Parameter uncertainty was characterized by probability distributions representing point estimates and variances. We conducted several probabilistic, 1-way sensitivity analyses to explore key sources of variability and uncertainty in the simulation model. (38) One-way sensitivity analysis refers to the process of varying 1 parameter in a range between an upper and lower limit while all other parameters are kept constant. A series of 1-way sensitivity analyses is the easiest way to identify which parameters have the greatest effect on the optimal decision. The point at which the decision shifts from 1 alternative to another is often referred to as the cross-over point or the threshold.
Results of the probabilistic sensitivity analyses were summarized by the probability that an intervention would be more cost-effective than usual care at fixed values of a hypothetical cost-effectiveness threshold. Interventions with an incremental cost per QALY gained of < $50,000 were cost-effective, and interventions with an incremental cost per QALY gained of $50,000 to $100,000 were possibly cost-effective. These thresholds are arbitrary but widely used in cost-effectiveness analyses of pharmaceuticals, public health programs and surgical procedures. (39)
Generalizability
Findings of this cost-effectiveness analysis are likely to be generalizable to EoL care practice in Ontario, since inputs were derived from population-based data reflecting Ontario demographics and patterns of EoL care services.
Model Structure
We adopted a decision analytic modelling approach to evaluate a range of evidence-based interventions. We developed an Ontario End-of-Life Care Decision Model using population-based inputs from linked health administration databases at the Institute for Clinical Evaluative Sciences and used the decision model to simulate usual care and the included interventions.
We structured the Ontario End-of-Life Care Decision Model using feedback from the Health Quality Ontario Expert Advisory Panel on End-of-Life Care. We used a Markov model to simulate patterns of EoL care and related health care utilization for a cohort of decedents in their last year of life, as well as to simulate recurrent events experienced by the target population (e.g., ED visits, hospital admissions). Patterns of care and health care utilization were assumed to change overtime, with a higher likelihood of receiving EoL care and higher health care utilization closer to the time of death.
We selected a cycle length of 1 day, because events in the model were typically reported in daily increments (e.g., hospital days, ICU days). The simulation started at the first day of the last year of life and tracked daily events for each of the next 365 days. All simulated decedents were assumed to die on the 365th day.
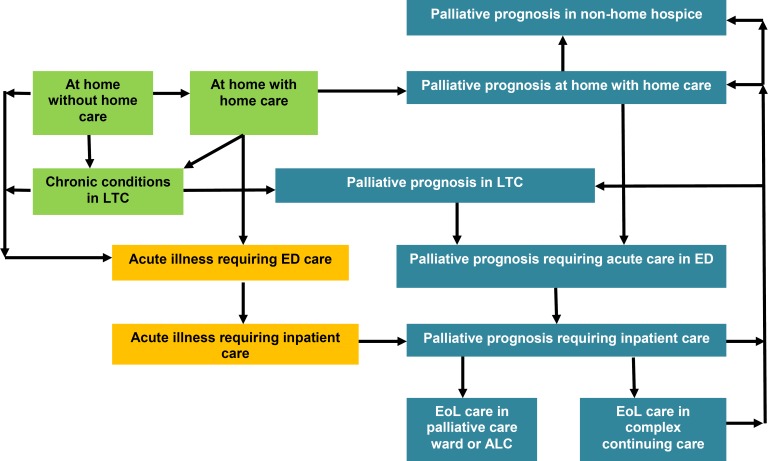
Figure 1 outlines the model structure, including health states and transitions between health states. Health states were defined by location (e.g., home, ED, acute care ward, or LTC), health care services used, and palliative prognosis. We assumed that information about location and health care services used would be meaningful for inferring the health status of simulated patients. For example, a patient would likely be in stable health if he/she was at home without home care, in less stable health if at home with home care, and so on. Corresponding health states were also defined for simulated patients who received EoL services and were designated with a palliative prognosis. Simulated individuals with a palliative prognosis would also receive care and eventually die in various location-specific health states.
Figure 1: Structure and Health States, Ontario End-of-Life Care Decision Model.
Abbreviations: ALC, alternate level of care; ED, emergency department; LTC, long-term care.
Blue boxes represent health states for patients identified with a palliative prognosis. Green boxes represent health states in which patients are at home or in LTC. Yellow boxes represent patients receiving ED or hospital care for acute conditions.
At the beginning of the last year of life, simulated patients began in different health states according to the initial distribution from the linked health administration databases. The model accounted for a proportion of simulated patients who were designated with a palliative prognosis before the last year of life. On any day, simulated patients could begin receiving home care services, be admitted to LTC, visit the ED, or be admitted to hospital. Home care and LTC could be requested from home or upon hospital discharge. Simulated patients with a palliative prognosis could receive a combination of acute or palliative services at home, in LTC, in the ED, or in hospital.
To track the timing of multiple events (e.g., hospital days and ICU days) and previous pathways (e.g., hospital admission from private or LTC home), we generated pathways, associated health outcomes, and costs for each patient in the cohort (microsimulation). We derived average health outcomes and costs by summing the simulated data.
The simulation was run from the first day to the final day of the last year of life, when all simulated patients were assumed to die. In terms of health outcomes, days at home were accumulated and places of death were recorded. Simulated days in the last year of life were weighted using QALY weights to derive quality-adjusted life-days (QALDs).
Key Assumptions
We assumed that only patients receiving EoL care services in current practice were designated with a palliative prognosis. The simulation model did not account for patients with a terminal illness who did not receive EoL care services in their last year of life. The target population that may benefit from effective EoL interventions is larger than the population with a designated palliative prognosis used in our simulation, but the effect of this difference on the results is unclear.
We also assumed that the beginning of the last year of life was known for all simulated decedents. The target population consisted of decedents with different death trajectories, including terminal illness (approximately 31%), organ failure (approximately 31%), and illness related to old age (approximately 30%). Other trajectories included sudden death (4%) and others, such as multiple causes (4%). In practice, clinical predictions of patients who will die within a year (or 6 months) using simulated trajectories have low accuracy. (40)
Finally, we assumed that the EoL care interventions included in this analysis did not affect the survival time of simulated patients. Although this assumption is conceptually reasonable, mean survival times have been reported to be slightly different in participants randomized to alternative EoL interventions. (21;30) It is unclear how this assumption affected the results of the cost-effectiveness analysis.
Data Sources
The model structure and inputs were informed by the data sources outlined below.
Systematic reviews of EoL care interventions conducted by Health Quality Ontario as part of the EoL mega-analysis (1)
Summary data from 2 EoL cohorts from linked health administration databases at ICES (5)
Inputs from the Expert Advisory Panel on End-of-Life Care (1)
Survey data of EoL services in Ontario hospices and hospitals (41)
Summary characteristics of 11 in-home palliative expert consult teams from different health regions of Ontario (42)
Planning documents from the Ontario Long-Term Care Association (personal communication, Ms. Paula Neves, Director of Health Planning and Research, Ontario Long-Term Care Association, December 12, 2013)
Inputs from the Ontario Association of Community Care Access Centres (personal communication, Misses Janet McMullan and Eva Haratsidis, Client Services Specialists, Ontario Association of Community Care Access Centres, December 18, 2013)
Inputs from the Bridgepoint Active Healthcare facility (a complex continuing care facility) in Toronto (personal communication, Mr. Michael Gekas, Director of Ambulatory Care and Business Operations, Bridgepoint Active Healthcare, December 16, 2013).
Inputs from Rouge Valley Health System on ethics consultation services (personal communication, Dr. Christopher De Bono, bioethicist, Rouge Valley Health System, February 11, 2014)
Additional literature searches of published and unpublished studies for specific model inputs
We obtained summary data from 2 EoL cohorts; both tracked patterns of care and health care resource utilization in the 12 months before death from linked health administration databases at ICES. The first cohort consisted of 256,284 Ontario decedents from January 1, 2007, to December 31, 2009. Data from this cohort were generated specifically for this analysis (a data creation plan submitted to ICES is available from the authors of this report upon request). In the pages that follow, these data will be referred to as the Health Quality Ontario (HQO) ICES cohort. (43)
The second cohort consisted of 175,478 Ontarian decedents from April 1, 2010, to March 31, 2012; this cohort was developed by 2 members of the Health Quality Ontario Expert Panel on End-of-Life Care as part of a research project on EoL care in Ontario. (5) Summary data from this cohort were also used to inform model inputs. In the pages that follow, these data will be referred to as the Ottawa Hospital Research Institute (OHRI) ICES cohort.
Data from the HQO ICES cohort (e.g., transition rate and cost estimates) were reported on a monthly basis; data from the OHRI cohort were reported on an annual basis, with breakdowns by months from death (including 6 to 12 months, 3 to 6 months, and within 3 months). These data were used to estimate daily transition rates and daily costs in the model.
Model Input Parameters: Natural History
Target Population for EoL Interventions
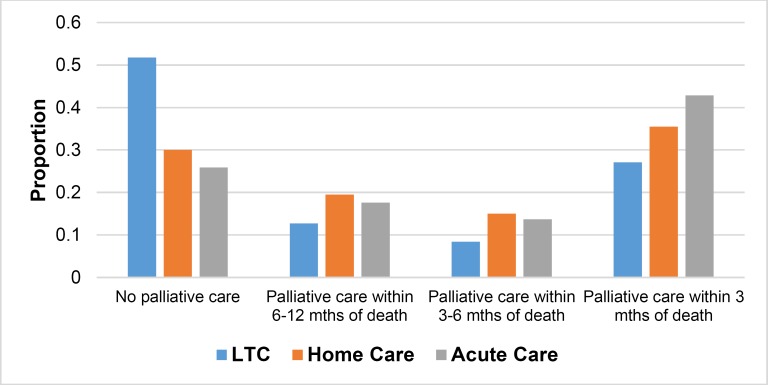
The proportion of patients with a palliative prognosis was derived using the OHRI ICES summary data. (5) A substantial proportion of decedents received no EoL care (Figure 2). Of those who did receive EoL care, it was most likely to be delivered in the last 3 months of life.
Figure 2: Identifying Decedents With a Palliative Prognosis.
Abbreviation: LTC, long-term care; ICES, Institute for Clinical Evaluative Sciences; OHRI, Ottawa Hospital Research Institute.
Source: Summary data from the OHRI ICES cohort. (5)
Daily Transition Rates
We used monthly data from the HQO ICES cohort to estimate daily transition rates (e.g., from home to the ED, from home to LTC). (43) Typically, monthly data elements were calculated by dividing the number of events in a particular month by the total number of patient-months among cohort members who were at risk for the event during the month of interest. We derived the daily event rate from the monthly event rate, assuming a constant average daily event rate over the month. (38)
Table 3 displays daily transition rates between health states at selected months in the last year of life, stratified by home or LTC, as well as estimated length of stay in the hospital and ICU for patients admitted from home and LTC.
Table 3:
Transition Rate Estimates and Hospital Length of Stay in the Last Year of Life
| Time to Death | Distribution | |||||
|---|---|---|---|---|---|---|
| 12 Months | 6 Months | 3 Months | 2 Months | 1 Month | ||
| Transitions From Home, Daily Transition Rate Estimate | ||||||
| ED visit, daily rate per 1,000 person-days (SD) | 3.69 (0.70) | 4.98 (1.13) | 9.56 (3.33) | 14.04 (5.34) | 37.26 (12.87) | Gamma |
| Probability [hospitalization | ED visit] (SD) | 0.46 (0.08) | 0.49 (0.08) | 0.57 (0.09) | 0.65 (0.09) | 0.85 (0.06) | Beta |
| Probability [ICU | hospitalization] (SD) | 0.15 (0.02) | 0.16 (0.03) | 0.16 (0.05) | 0.18 (0.07) | 0.29 (0.15) | Beta |
| Home care, daily rate per 1,000 person-days (SD) | 7.96 (2.63) | 9.86 (3.18) | 13.73 (4.98) | 16.34 (6.54) | 22.33 (10.97) | Gamma |
| LTC admission, daily rate per 1,000 person-days (SD) | 0.10 (0.06) | 0.13 (0.08) | 0.19 (0.10) | 0.25 (0.14) | 0.29 (0.15) | Gamma |
| Transitions From LTC Home, Daily Transition Rate Estimate | ||||||
| ED visit, daily rate per 1,000 person-days (SD) | 1.99 (0.47) | 2.36 (0.42) | 3.84 (0.80) | 5.67 (1.15) | 15.39 (5.81) | Gamma |
| Probability [hospitalization | ED visit] (SD) | 0.43 (0.03) | 0.44 (0.05) | 0.50 (0.05) | 0.58 (0.05) | 0.66 (0.02) | Beta |
| Probability [ICU | hospitalization] (SD) | 0.07 (0.05) | 0.07 (0.05) | 0.08 (0.04) | 0.09 (0.01) | 0.11 (0.01) | Beta |
| Hospital Stay for Patients Admitted From Home | ||||||
| Number of hospital days (SD) | 14.16 (26.35) | 16.44 (27.38) | 20.71 (22.63) | 20.16 (15.96) | 9.07 (7.19) | Gamma |
| Number of ICU days (SD) | 7.31 (19.40) | 8.60 (18.91) | 11.83 (17.00) | 11.73 (12.53) | 5.54 (5.16) | Gamma |
| Hospital Stay for Patients Admitted From LTC Home | ||||||
| Number of hospital days (SD) | 9.35 (16.07) | 9.02 (11.39) | 10.31 (10.62) | 11.55 (9.83) | 7.76 (5.51) | Gamma |
| Number of ICU days (SD) | 5.36 (4.37) | 4.93 (7.05) | 6.68 (10.22) | 7.21 (8.32) | 4.98 (4.17) | Gamma |
Abbreviations: ED, emergency department; HQO, Health Quality Ontario; ICES, Institute for Clinical Evaluative Sciences; ICU, intensive care unit; LTC, long-term care; SD, standard deviation. Note: All estimates were derived using summary data from the HQO ICES cohort. (43) Daily transition rate estimates for each of the 12 months in the last year of life were used in the decision model. Source: Summary data from the HQO ICES cohort. (43)
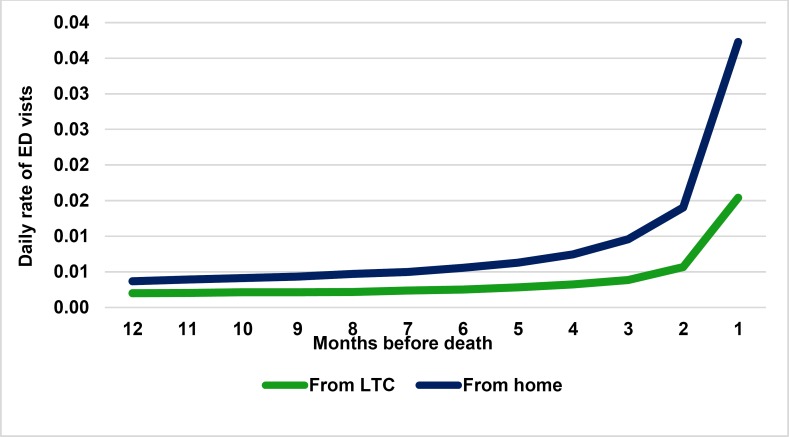
Monthly transitions were used to capture the trend of increasing transitions in the last few months before death (Figure 3).
Figure 3: Daily Rate of ED Visits.
Abbreviations: ED, emergency department; HQO, Health Quality Ontario; ICES, Institute for Clinical Evaluative Sciences; LTC, long-term care. Source: Data from the HQO ICES cohort. (43)
Model Input Parameters: Usual Care
We used summary data from the ICES cohorts to quantify the patterns of current EoL care practice in Ontario, so usual care includes some provision of services related to the intervention strategies. For example, in-home palliative team care is targeted at decedents identified with a palliative prognosis and receiving home care services. Approximately 21% of the target population already receives in-home palliative team care (see Budget Impact Analysis, below). The effectiveness evidence in support of in-home palliative team care was derived from a randomized controlled trial (RCT) comparing this intervention with a control group that received some palliative team care. Therefore, the results of the cost-effectiveness analysis for in-home palliative team care are subject to differences between usual care in Ontario and the care provided to patients in the control groups of the RCT.
In this cost-effectiveness analysis, we did not explicitly take into account the fact that some interventions are currently provided as part of usual care. This represents an important limitation of our cost-effectiveness analysis, but the results were interpreted taking this limitation into account.
Model Input Parameters: Intervention Summary Estimates
Table 4 summarizes effectiveness estimates for the interventions. These estimates were derived using data from RCTs included in the evidence-based analyses that were part of the EoL care mega-analysis. However, the mega-analysis included other outcome measures (e.g., satisfaction with care, quality of death) that were not part of the cost-effectiveness analysis.
Table 4:
Effectiveness of Included Interventions
| Intervention | Population | Outcome | Outcome Measure | Estimate (95% CI) | Patients, n | GRADE | Source |
|---|---|---|---|---|---|---|---|
| Usual care | |||||||
| Palliative Team Care | |||||||
| PTC: In-home | Cancer, CHF, COPD | ED visits | Rate ratio (I/C) | 0.61 (0.41–0.90) | 310 | Low | Brumley et al, 2007(21) |
| PTC: Inpatient | Cancer, CHF, COPD, advanced dementia | HRQOL | SMD (I-C) | 0.05 (−0.07 to 0.17) | 517; 261; 99 | Low | Gade et al, 2008 (28) Hanks et al, 2002 (45) Ahronheim et al, 2000 (29) |
| ICU admissions | Rate ratio (I/C) | 0.54 (0.27–1.07) | 517 | Low | Gade et al, 2008 (28) | ||
| Hospital days | Difference (I-C) | 0.27 (−0.83 to 1.38) | 517; 261; 99 | Moderate | Gade et al, 2008 (28) Hanks et al, 2002 (45) Ahronheim et al, 2000 (29) | ||
| PTC: Comprehensive | Cancer | HRQOL | SMD (I-C) | 0.14 (−0.25 to 0.53) | 434; 461; 151 | Moderate | Jordhoy et al, 2000 (46) Zimmermann et al, 2014 (30) Temel et al, 2010(47) |
| ED visits | Rate ratio (I/C) | 0.93 (0.66–1.32) | 151 | Low | Temel et al, 2010 (47) | ||
| Hospital admissions | Rate ratio (I/C) | 0.87 (0.62–1.12) | 434; 151 | Low, moderate | Jordhoy et al, 2000 (46); Temel et al, 2010 (47) | ||
| Hospital days | Difference (I-C) | −1.00 (−2.09 to 0.55) | 434 | Moderate | Jordhoy et al, 2000 (46) | ||
| Patient Care Planning Discussions | |||||||
| PCPD: Identifying LTC residents with EoL goals and preferences for EPC | LTC residents | Hospital admissions | Rate ratio (I/C) | 0.57 (0.33–0.98) | 205 | High | Casarett et al, 2005 (31) |
| Hospital days | Difference (I-C) | −1.8 (−0.53 to −3.07) | 205 | High | Casarett et al, 2005 (31) | ||
| PCPD: Ethics consultation for ICU patients with treatment | ICU patients | Hospital days | Difference (I-C) | −2.96 (−4.55 to −1.37) | 551 | High | Schneidermann et al, 2003 (32) Gilmer et al, 2005 (48) |
| ICU days | Difference (I-C) | −1.44 (−2.49 to −0.39) | 551 | High | Schneidermann et al, 2003 (32) Gilmer et al, 2005 (48) | ||
| PCPD: Improving family conferences for relatives of patients dying in the ICU | Patients dying in ICU | ICU days | Difference (I-C) | −2.00 (−8.43 to 4.43) | 126 | High | Lautrette et al, 2007 (33) |
| Reduced depression symptoms (relatives) | % Difference (I-C) | 27.2 (8.6–43.4) | 126 | High | |||
| Educational Interventions for Patients and Caregivers | |||||||
| Multicomponent psychoeducational interventions for patients and families | Advanced cancer | HRQOL (patients) | SMD (I-C) | 0.09 (−0.06 to 0.24) | 661 | Low | Bakitas et al, 2009 (34) |
| HRQOL (caregivers) | Pooled SMD (I-C) | 0.15 (0.06–0.25) | 720 | Moderate | Meyers et al, 2011 (35) | ||
| McMillan et al, 2006 (36) | |||||||
| Hospital days | Difference (I-C) | 0.1 (−0.03 to 0.23) | 322 | Moderate | Bakitas et al, 2009 (34) | ||
| ED visits | Rate ratio (I/C) | 1.37 (0.52–3.60) | 322 | Moderate | Bakitas et al, 2009 (34) | ||
| Supportive Interventions for Informal Caregivers | |||||||
| Supportive interventions for informal caregivers | Informal caregivers | HRQOL (caregivers) | SMD (I-C) | 0.08 (−0.11 to 0.26) | 631 | Low | Candy et al 2011 (37) |
Abbreviations: CHF, congestive heart failure; C, control; CI, confidence interval; COPD, chronic obstructive pulmonary disease; ED, emergency department; EoL, end-of-life; EPC, early palliative care; GRADE, Grading of Recommendations Assessment, Development and Evaluation; HRQOL, health-related quality of life; I, intervention; ICU, intensive care unit; LTC, long-term care; PCPD, patient care planning discussion; PTC, palliative team care; SMD, standardized mean difference.
Where appropriate, we calculated pooled intervention effect estimates using a random-effects approach. We chose this approach because it accounts for both sampling variation and heterogeneity in individual trial estimates; however, it also assigns larger weights for estimates from small trials relative to a fixed-effects approach. (44) We inspected instances of large differences between fixed- and random-effects estimates and conducted sensitivity analyses if indicated.
Model Input Parameters: Health-Related Quality of Life
We did not use intervention effect estimates of health-related quality of life (HRQOL) measures in the base case analysis, but we did use them in a sensitivity analysis to calculate QALDs and as an aggregate outcome measure. The sensitivity analysis provided additional information for interpretation, especially when an intervention was associated with increased health care costs but improved health outcomes. Using incremental cost per QALY gained in the sensitivity analysis helped us determine the relative cost-effectiveness of EoL care interventions (e.g., in-home palliative team care) compared to other interventions (e.g., primary and secondary prevention of cardiovascular events).
Model Input Parameters: Intervention Costs
Table 5 summarizes the daily costs of services (e.g., ED visit, hospital, home care, LTC costs), stratified by the time before death. (49)
Table 5:
Time-Specific Daily Health Care Costs in the Last Year of Life
| Type of Care | Mean Daily Cost, $ (SD) | Distribution | Sourcea | ||||
|---|---|---|---|---|---|---|---|
| 12 Months | 6 Months | 3 Months | 2 Months | 1 Month | |||
| ED visit | 520 (388) | 554 (404) | 630 (425) | 684 (432) | 807 (432) | Gamma | HQO ICES cohort |
| Hospital care | 846 (1,201) | 803 (927) | 829 (1,046) | 824 (1,023) | 820 (996) | Gamma | HQO ICES cohort |
| Home care | 34 (36) | 37 (39) | 45 (49) | 49 (56) | 63 (76) | Gamma | HQO ICES cohort |
| LTC | 91 (11) | 92 (14) | 94 (18) | 94 (19) | 107 (20) | Gamma | HQO ICES cohort |
| Rehabilitation | 3.09 (0.95) | 0.49 (0.05) | 0.49 (0.05) | 0.49 (0.06) | 0.38 (0.06) | Gamma | OHRI ICES cohort |
| Outpatient visit | 8.67 (0.99) | 10.21 (0.39) | 9.44 (0.39) | 8.72 (0.32) | 8.40 (0.32) | Gamma | OHRI ICES cohort |
| Physician | 48.08 (14.37) | 9.37 (0.52) | 8.35 (0.52) | 7.66 (0.31) | 7.34 (0.31) | Gamma | OHRI ICES cohort |
| Drugs/devices | 9.58 (0.36) | 8.62 (0.08) | 8.46 (0.08) | 8.19 (0.10) | 8.17 (0.10) | Gamma | OHRI ICES cohort |
| Otherb | 1.38 (0.08) | 1.42 (0.03) | 1.37 (0.03) | 1.32 (0.02) | 1.30 (0.02) | Gamma | OHRI ICES cohort |
Abbreviations: ED, emergency department; HQO, Health Quality Ontario; ICES, Institute for Clinical Evaluative Sciences; LTC, long-term care; OHRI, Ottawa Hospital Research Institute.
Cohort used in the calculation.
Including costs for Ontario Health Insurance Plan laboratory billings and nonphysician billings.
Table 6 summarizes other daily costs.
Table 6:
Other Daily Health Care Costs in the Last Year of Life
| Type of Care | Mean Daily Cost, $ (SD) | Distribution | Source |
|---|---|---|---|
| ICU stay | 644 (223) | Gamma | HQO ICES cohort (43) |
| CCC stay | 560 (722) | Gamma | Input from a local CCC facilitya |
| Non–home hospice stay | 376 (484) | Gamma | Central East Residential Hospice Working Group (50) |
| ALC, PCW stay | 592 (841) | Gamma | HQO ICES cohort (43) |
Abbreviations: ALC, alternate level of care; CCC, complex continuing care; HQO, Health Quality Ontario; ICES, Institute for Clinical Evaluative Sciences; ICU, intensive care unit; PCW, palliative care ward; SD, standard deviation.
Personal communication, clinical expert, December 16, 2013.
Table 7 summarizes the resources required to deliver each of the 8 interventions included in the analysis.
Table 7:
Resources Required for Included Interventions
| Intervention | Physician | Nurse | Other Personnel | Mean Program Duration | Patients, n | Sources |
|---|---|---|---|---|---|---|
| Palliative Team Care | ||||||
| PTC: In-home | 0.5–11.5 FTEs | 1–8 RN FTEs | CCAC resources | 73 days | 45–415/y | Data from 11 teams in Ontario (51) Lukas et al 2013 (52) |
| PTC: Inpatient | 2 FTEs | 1 NP FTE | Hospital resources | 7 days | 900–1,200/y | HQO EoL Expert Panel and published inputs (53) |
| PTC: Comprehensive | Inputs for PTC: | In-home and PTC: | Inpatient, above | — | — | — |
| Patient Care Planning Discussions | ||||||
| PCPD: Identifying LTC residents with EoL goals and preferences for EPC | Inputs for PTC: In-home | 30 min of RN time for screening and referral | Inputs for PTC: In-home | 89 days for +19% enrolled residents | Per patient | Published inputs (31) |
| PCPD: Ethics consultation for ICU patients with treatment conflicts | NA | NA | Hospital bioethicist | ICU stays in the last month of life | 100/y | HQO EoL Expert Panel and published inputs (48) |
| PCPD: Improving family conferences for relatives of patients dying in ICU | 2 FTEs | 1 RN | 1 SW | Increase conference from 30 min to 1 h | Per patient | HQO EoL Expert Panel and published inputs (33) |
| Educational Interventions for Patients and Caregivers | ||||||
| Multicomponent psycho-educational interventions for patients and families | NA | 4 phone training sessions | 3 phone follow-up sessions | 9 h | Per patient | Published inputs (54) |
| Supportive Interventions for Informal Caregivers | ||||||
| Supportive interventions for informal caregivers | NA | 2–6 visits | 0–2 phone sessions | 4–11 h | Per patient | Inputs from 6 RCTs included in a SR (37) |
Abbreviations: CCAC, Community Care Access Centre; EoL, end-of-life; EPC, early palliative care; FTE, full-time equivalent; HQO, Health Quality Ontario; ICU, intensive care unit; LTC, long-term care; NA, not applicable; NP, nurse practitioner; PCPD, patient care planning discussions; PTC, palliative team care; RCT, randomized controlled trial; RN, registered nurse; SR, systematic review; SW, social worker.
Table 8 summarizes the estimated mean total cost of delivering each intervention included in the analysis. The estimated costs for comprehensive palliative team care included both in-home and inpatient costs.
Table 8:
Summary of Intervention Costs
| Intervention | Mean Total Cost, $ (SD) |
|---|---|
| Palliative Team Care | |
| PTC: In-home (cost per patient) | 1,700 (998) |
| PTC: Inpatient (cost per hospital stay) | 409 (162) |
| PTC: Comprehensive | |
| In-home (cost per patient) | 1,700 (998) |
| Inpatient (cost per hospital stay) | 409 (162) |
| Patient Care Planning Discussions | |
| PCPD: Identifying LTC residents with EoL goals and preferences for EPC (cost per patient) | 915 (361) |
| PCPD: Ethics consultation for ICU patients with treatment conflicts (cost per patient) | 950 (280) |
| PCPD: Improving family conferences for relatives of patients dying in ICU (cost per patient) | 219 (153) |
| Educational Interventions for Patients and Caregivers | |
| Multicomponent psychoeducational interventions for patients and families (cost per dyad of patient and caregiver) | 316 (45) |
| Supportive Interventions for Informal Caregivers | |
| Supportive interventions for informal caregivers (cost per caregiver) | 305 (224) |
Abbreviations: EoL, end-of-life; EPC, early palliative care; ICU, intensive care unit; LTC, long-term care; PTC, palliative team care. PCPD: patient care planning discussion.
Model Input Parameters: Additional Information for In-home Palliative Team Care
Of the cost estimates for the interventions in Table 8, those for in-home palliative team care were most uncertain, so we sought additional details for this estimate. Table 9 describes the characteristics and composition of 11 palliative care teams from different health regions in Ontario, according to a survey conducted by Seow et al. (51) These teams gave patients access to interprofessional EoL expertise and 24/7 services. They were selected from approximately 30 palliative care teams with varying capacity (personal communication, clinical experts, March 28, 2014).
Table 9:
In-Home Palliative Team Care—Resources Required (Fiscal Years 2009–2011)
| Team | Deaths in Region, n | Admission to Palliative Care Team, n | Date Team Established | Mean Time in Program Before Death, Days (SD) | Palliative Care Physicians, FTE | Nurses, FTE | Other Team Members, FTE |
|---|---|---|---|---|---|---|---|
| 1 | 16,243 | 830 | 2009 | 68 (79) | 1 | 8 | 2 |
| 2 | 2,240 | 221 | 2009 | 97 (117) | 1 | 2 | 1.5 |
| 3 | 1,534 | 144 | 2009 | 83 (102) | 1 | 1 | 0.6 |
| 4 | 1,670 | 125 | 2009 | 66 (86) | 1 | 2 | 1 |
| 5 | 3,102 | 105 | 2009 | 72 (85) | 0.5 | 1 | 0.2 |
| 6 | 1,185 | 90 | 2009 | 93 (97) | 2 | 2 | 1.2 |
| 7 | 7,629 | 676 | 1986 | 71 (83) | 11.5 | 1 | 5.9 |
| 8 | 5,264 | 497 | 2007 | 82 (93) | 2 | 2 | 1 |
| 9 | 840 | 775 | 1998 | 73 (98) | 1.3 | 3 | 1.7 |
| 10 | 737 | 268 | 2004 | 60 (96) | 0.6 | 1 | 2.5 |
| 11 | 689 | 181 | 1979 | 63 (102) | 6 | 2 | 4.7 |
| Pooled | 41,133 | 3,912 | — | 73 (92) | — | — | — |
Abbreviations: FTE, full-time equivalent; SD, standard deviation.
Source: Seow et al, 2013. (52)
The mean cost (and distribution) of in-home palliative team care depended on whether the team was a primary care expert palliative care team that does direct care or an expert consult team that may see the patient and family once or twice while a patient is in a palliative care program; primary care providers included the family physician and the home care team (personal communication, Dr. Mary Lou Kelly, Northern Ontario School of Medicine, February 6, 2014).
We assumed that in-home palliative team care was delivered by an expert consult team working with the family physician and the home care team; current palliative care teams tend to do a mix of both primary care and consulting care. The teams would be expert consult or shared care teams, with primary care offered by the primary care providers, but when the primary care team was either unwilling or unavailable, the consult team would become the primary care team (personal communication, Dr. Mary Lou Kelly, Northern Ontario School of Medicine, February 6, 2014).
We also assumed that in-home visits were conducted primarily by nurse practitioners with support from palliative care specialists. We used a ratio of 2.8 nurse practitioner full-time equivalents and 0.2 palliative care specialist full-time equivalents. (52) We further assumed that the costs of home care services were covered as part of the health care costing items described in Table 5. For the base case analysis, we estimated the annual mean cost of services using the annual salary of nurse practitioners and the annual average specialist fee for service (Table 10). Per-patient costs were estimated for each of the 11 teams in Table 9 and then averaged to derive a mean per-patient cost.
Table 10:
Unit Costs
| Intervention-Related Cost | Median, $ | Low, $ | High, $ | Source |
|---|---|---|---|---|
| Annual fee-for-service payment per specialist FTE | 302,387 | 229,967 | 384,001 | CIHI 2012, Table A.6.1 (55) |
| Nurse practitioner annual salary | 74,217 | 66,690 | 85,040 | CFNU 2013 (56) |
| Registered nurse annual salary | 63,667 | 58,831 | 83,557 | CFNU 2013 (3+ years of experience) (56) |
| Bioethicist annual salary | 90,000 | 50,000 | 150,000 | Expert inputa |
| Social worker annual salary | 58,181 | 35,625 | 82,688 | Living in Canada (57) |
| Registered nurse hourly rate 3 (1–5) years of experience | 31.78 | 29.79 | 35.15 | CFNU 2013 (3+ years of experience) (56) |
| Social worker hourly rate | 31.03 | 19.00 | 44.10 | Living in Canada (57) |
Abbreviations: CFNU, Canadian Federation of Nurses Unions; CIHI, Canadian Institute for Health Information; FTE, full-time equivalent.
Personal communication, clinical expert, February 11, 2014.
Klinger et al described resource utilization and costs for 95 patients enrolled in an expanded home-based palliative care service from the Niagara West End-of-Life Shared-Care Project. Resources were reported separately for home care services and other services provided by the expanded home-based palliative team. (10) The authors estimated that the average cost for the expanded home-based palliative team was $2,431 per patient ($16.75 per patient day). This cost included additional nursing services (61%); medication, transportation, and equipment charges (22%); and palliative care physician consultations (6%), among others. We used this average cost to conduct the sensitivity analysis.
Model Input Parameters: Quality Weights and Quality-Adjusted Life Years
In Table 4, some of the intervention effects are reported as an effect size — the absolute mean difference in a continuous measure divided by the standard deviation of the measurement. An effect size of ≤ 0.20 is generally considered to be small, 0.50 is moderate, and 0.80 is large. (58)
Using estimates from 3 RCTs included in the EoL care mega-analysis, (30;46;47) we estimated that comprehensive palliative team care was associated with a pooled effect size of 0.14 (95% confidence interval −0.25 to 0.53, Table 4) using HRQOL scales specific to EoL care (Functional Assessment of Chronic Illness Therapy—Spiritual Weil-Being scale, (59) European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-C30, (60) and Functional Assessment of Cancer Therapy–Lung scale (61)).
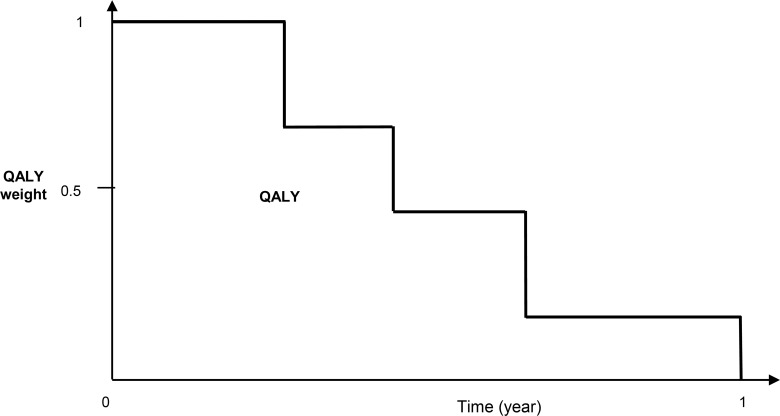
Figure 4 illustrates the use of QALY weights to adjust survival time and estimate QALYs using the area under the curve. QALY weights are generally derived from generic HRQOL instruments (e.g., European Quality of Life 5 Dimensions [EQ-5D] or the Health Utilities Index [HUI-2]). We assumed that the generic instruments would be slightly less responsive than EoL-specific instruments by a relative reduction of 0.8 (range, 0.4–1.2), and based on this assumption, we converted the pooled effect size to an estimated effect size of 0.11 (0.14*0.8), ranging from −0.20 to 0.42 on the QALY-weight scale. We estimated a standard deviation of 0.18 on the QALY-weight scale for patients with terminal illnesses. (39) The absolute QALY-weight change scores were estimated to be 0.02 (0.11*0.18), ranging from 0.04 to 0.07.
Figure 4: QALY Weights and QALY Calculation.
Abbreviations: QALY, quality-adjusted life-year.
QALY is the measure of the area under the curve.
We applied the absolute QALY-weight change scores associated with comprehensive palliative team care to the QALY weight of patients with a palliative prognosis during their hospital days and post-discharge days. According to the summary data for the HQO ICES cohort, decedents were identified with a palliative prognosis approximately 3 months prior to death. This was also the duration effect for the QALY-weight change scores associated with comprehensive palliative team care.
We conducted a specific literature search to obtain estimates of decrements in QALY weight when patients had acute conditions that required ED visits, hospital days, and ICU days (Table 11). We also estimated decrements in QALY weights for caregivers. Because we accounted for intervention effect on HRQOL and decrements in QALY weights with respect to ED visits, hospital days and ICU days, there was the potential for double-counting; we took this issue into account when we interpreted the results of the cost-effectiveness analysis.
Table 11:
Quality-Adjusted Life-Year Weights
| Estimate | SD | Distribution | Source | |
|---|---|---|---|---|
| Patient QALY | ||||
| Decrement in QALY weight due to ED visits | 0.014 | 0.0015 | TN | Church et al, 2011 (62) |
| Decrement in QALY weight due to hospitalization | 0.06 | 0.085 | TN | Ghatnekar et al, 2013 (63) |
| Decrement in QALY weight due to ICU stay | 0.108 | 0.022 | TN | Dinglas et al, 2013 (64) |
| Caregiver QALY (average of 56 years old) | ||||
| QALY weight without caregiving | 0.92 | 0.07 | TN | Mittmann et al, 1999 (65) |
| Decrement in QALY weight due to caregiving | 0.062 | 0.024 | TN | Davidson et al, 2008 (66) |
| Decrement in QALY weight of not having a break from caregiving | 0.006 | 0.009 | TN | Davidson et al, 2008 (66) |
| Decrement in QALY weight due to mild depression during bereavement | 0.103 | 0.037 | TN | Mann et al, 2009 (67) |
Abbreviations: ED, emergency department; ICU, intensive care unit; QALY, quality-adjusted life-year; SD, standard deviation; TN, truncated normal.
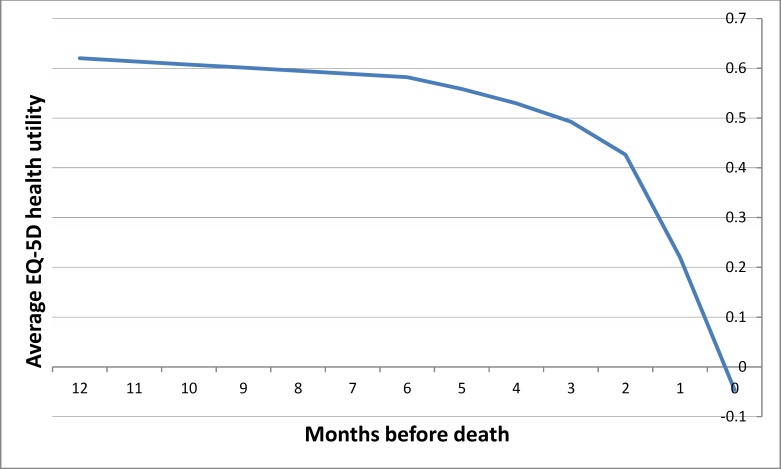
A plot of the time-specific QALY weights for decedents in their last year of life is shown in Figure 5. This QALY-weight curve was extracted from a cost-utility analysis of short- versus long-course palliative radiotherapy in patients with non-small-cell lung cancer. (68) In the study, patients filled out mailed questionnaires (at baseline, every week for 12 weeks, and every other week for 40 weeks) asking about patients’ symptoms and quality of life measured using the EQ-5D. (The EQ-5D assesses general health status using 5 questions on mobility, self-care, usual activities, pain/discomfort, and anxiety/depression.) This QALY weight reflected the general public's valuation of the health states defined by the EQ-5D questions, ranging from 1.00 (optimal health), to 0.00 (as bad as death), to −0.594 (worse than death). (69)
Figure 5: QALY Weights in the Last Year of Life.
Abbreviations: EQ-5D, EuroQOL 5 Dimensions questionnaire; QALY, quality-adjusted life-year.
Source: Reproduced from Van den Hout et al 2006 with permission. (68)
Model Calibration
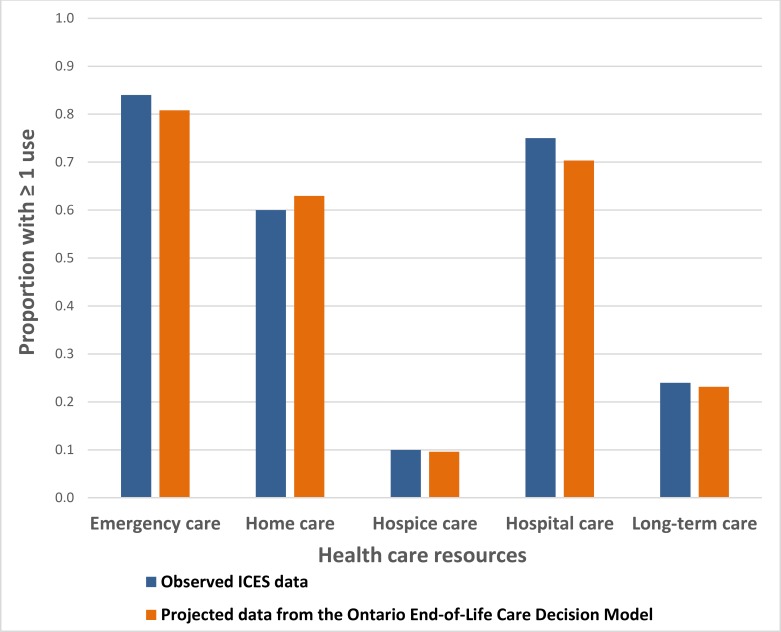
The Ontario End-of-Life Care Decision Model (Figure 1) is a simplified version of actual patterns of care and transitions in the last year of life. Because the model is an imperfect version of reality, we used model calibration to adjust inputs and ensure that projections were consistent with observed data from the HQO ICES cohort and the OHRI ICES cohort. We applied scaling factors to the daily rates of home care services, ED visits, hospitalizations, and LTC admissions; we then varied those scaling factors and projected resources used. We visually inspected projected utilization to ensure it was close to observed utilization (Figure 6).
Figure 6: Observed and Projected Use of Health Care Resources.
Abbreviations: EoL, end-of-life; ICES, Institute for Clinical Evaluative Sciences; OHRI, Ottawa Hospital Research Institute.
Note: Hospice care includes ≥ 1 use of complex continuing care, alternative level of care in hospitals, palliative care wards, and non–home hospice care among EoL patients.
Source: Summary data from OHRI ICES cohort. (5)
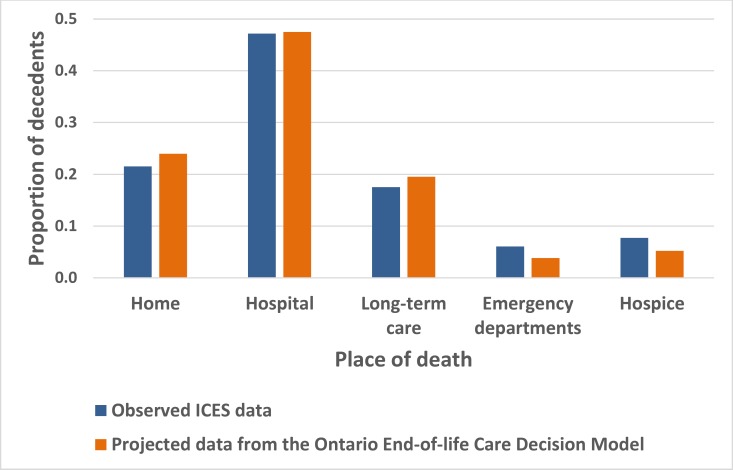
We used a similar trial-and-error procedure to calibrate place of death. We applied scaling factors to rates of ED visits, hospitalizations, and discharges from hospital within 2 weeks before death to ensure consistency between projected and observed place of death (Figure 7).
Figure 7: Observed and Projected Place of Death.
Abbreviations: HQO, Health Quality Ontario; ICES, Institute for Clinical Evaluative Sciences.
Source: HQO ICES cohort. (43)
Results of Primary Economic Evaluation
Table 12 summarizes results of the primary economic evaluation. Table 13 summarizes the results of the probabilistic sensitivity analysis, including estimates of the probability that an intervention is more cost-effective than usual care at a willingness-to-pay of $50,000 per QALY gained. Table 14 summarizes the results of the one-way sensitivity analysis regarding the cost-effectiveness of palliative team care.
Table 12:
Cost-Effectiveness Analysis
| Intervention | Health Outcomes | |||||||
|---|---|---|---|---|---|---|---|---|
| Cost, $ | Days at Home | Dying at Home, % | QALD, Patients | QALD, Caregivers | Total QALD | Cost per QALY | Categorya | |
| Usual care | 50,129 | 336.05 | 45.41 | 198.71 | 319.82 | NA | NA | Absolute values |
| Palliative Team Care | ||||||||
| PTC: In-home | −4,424 | 5.75 | 10.32 | 0.44 | 0.03 | 0.47 | Dominant | Incremental effect (I-C) |
| PTC: Inpatient | −1,643 | 0.65 | −0.15 | 0.26 | 0.00 | 0.27 | Dominant | Incremental effect (I-C) |
| PTC: Comprehensive | 527 | 1.44 | 1.74 | 2.64 | 0.01 | 2.65 | 72,717 | Incremental effect (I-C) |
| Patient Care Planning Discussions | ||||||||
| PCPD: Identifying LTC residents with EoL goals and preferences for EPC | −26 | 0.07 | 0.00 | 0.0049 | 0.0004 | 0.0053 | Dominant | Incremental effect (I-C) |
| PCPD: Ethics consultation for ICU patients with treatment conflicts | −85 | 1.05 | 0.31 | 0.09 | 0.01 | 0.10 | Dominant | Incremental effect (I-C) |
| PCPD: Improving family conferences for relatives of patients dying in ICU | 56 | 0.01 | −0.12 | 0.00 | 0.49 | 0.49 | 41,690 | Incremental effect (I-C) |
| Educational Interventions for Patients and Caregivers | ||||||||
| Multicomponent psychoeducational interventions for patients and families | 4,766 | −4.35 | −5.72 | 1.98 | 1.65 | 3.63 | 479,509 | Incremental effect (I-C) |
| Supportive Interventions for Informal Caregivers | ||||||||
| Supportive interventions for informal caregivers | 196 | 0.00 | 0.00 | 0.00 | 0.82 | 0.82 | 87,205 | Incremental effect (I-C) |
Abbreviations: C, control; EoL, end-of-life; EPC, early palliative care; I, intervention; ICU, intensive care unit; LTC, long-term care; NA, not applicable; PCPD, patient care planning discussion; PTC, palliative team care; QALD, quality-adjusted life-day; QALY, quality-adjusted life-year.
Incremental effect is the different between estimates for the intervention and usual care.
Table 13:
Probabilistic Sensitivity Analysis
| Intervention | Probability Statement | Probability Estimate |
|---|---|---|
| Usual care | — | — |
| Palliative Team Care | ||
| PTC: In-home (vs. usual care) | ↓Health care cost and ↑ health | 0.72 |
| PTC: Inpatient (vs. usual care) | ↓Health care cost and ↑ health | 0.38 |
| PTC: Comprehensive (vs. usual care) | Cost-effective at $50,000 per QALY | 0.32 |
| Patient Care Planning Discussions | ||
| PCPD: Identifying LTC residents with EoL goals and preferences for EPC (vs. usual care) | Cost-effective at $50,000 per QALY | 0.28 |
| PCPD: Ethics consultation for ICU patients with treatment conflicts (vs. usual care) | Cost-effective at $50,000 per QALY | 0.21 |
| PCPD: Improving family conferences for relatives of patients dying in ICU (vs. usual care) | Cost-effective at $50,000 per QALY | 0.52 |
| Educational Interventions for Patients and Caregivers | ||
| Multicomponent psychoeducational interventions for patients and families (vs. usual care) | Cost-effective at $50,000 per QALY | 0.26 |
| Supportive Interventions for Informal Caregivers | ||
| Supportive interventions for informal caregivers (vs. usual care) | Cost-effective at $50,000 per QALY | 0.28 |
Abbreviations: EoL, end-of-life; EPC, early palliative care; ICU, intensive care unit; LTC, long-term care; PCPD, palliative care planning discussion; PTC, palliative team care; QALY, quality-adjusted life-year.
Table 14:
One-Way Sensitivity Analysis
| Value | Cost Difference (I-C) | Difference in Days at Home (I-C) | |
|---|---|---|---|
| Palliative Team Care: In-home | |||
| ED Visits, Rate Ratio (I/C) | |||
| Base | 0.61 | −4,424 | 5.75 |
| Low | 0.41 | −7,983 | 9.10 |
| High | 0.90 | 33 | 1.35 |
| Intervention Cost | |||
| Base | 1,700 | −4,424 | 5.75 |
| Low | 636 | −6,163 | 5.75 |
| High | 3,789 | −3,017 | 5.75 |
| Alternative estimate | 2,431 | −3,822 | 5.75 |
| Cross-over threshold | 7,200 | 0 | 5.75 |
| Palliative Team Care: Inpatient | |||
| ICU Admission, Rate Ratio (I/C) | |||
| Base | 0.54 | −1,643 | 0.65 |
| Low | 0.27 | −3,904 | 1.36 |
| High | 1.07 | 838 | −0.03 |
| Hospital Days (I-C) | |||
| Base | 0.27 | −1,643 | 0.65 |
| Low | −0.83 | −3,166 | 2.16 |
| High | 1.38 | 653 | −0.23 |
| Intervention Cost | |||
| Base | 409 | −1,643 | 0.65 |
| Low | 386 | −2,252 | 0.65 |
| High | 462 | −1,441 | 0.65 |
| Cross-over threshold | 1570 | 0 | 0.65 |
| Palliative Team Care: Comprehensive | |||
| Hospital Admission, Rate Ratio (I/C) | |||
| Base | 0.87 | 527 | 1.44 |
| Low | 0.62 | 63 | 1.88 |
| High | 1.12 | 536 | 1.32 |
| Hospital Days (I-C) | |||
| Base | −1.00 | 527 | 1.44 |
| Low | −2.09 | −1,141 | 2.70 |
| High | 0.55 | 2,008 | −0.13 |
| In-home PTC Cost | |||
| Base | 1,700 | 527 | 1.44 |
| Low | 636 | 268 | 1.44 |
| High | 3,789 | 540 | 1.44 |
| Inpatient PTC Cost | |||
| Base | 409 | 527 | 1.44 |
| Low | 386 | 437 | 1.44 |
| High | 462 | 641 | 1.44 |
Abbreviations: C, control; I, intervention; ICU, intensive care unit; PTC, palliative team care.
We did not conduct one-way sensitivity analyses for patient care planning discussion, educational interventions for patients and caregivers, supportive interventions for informal caregivers, because the cost-effectiveness of these interventions was uncertain according to the probabilistic sensitivity analysis.
Palliative Team Care
In-Home Palliative Team Care (Versus Usual Care)
This strategy reduced the mean health care cost, increased the mean time at home, and increased the proportion of decedents dying at home (Table 12). Compared to usual care, it was a dominant strategy. Mean QALDs for in-home palliative team care were slightly higher than usual care, because patients with in-home palliative team care spent less time in the ED or hospital. Mean QALDs for caregivers were also slightly higher than usual care, because the analysis included a decrement in QALY weight for caregivers when patients were cared for in the ED or hospital (i.e., decrement in QALY weight of not having a break from caregiving). The results of the probabilistic sensitivity analysis suggested that this finding was less likely to change with additional data (Table 13).
These results were sensitive against variation in the estimate of the rate ratio of ED visits between the intervention and usual care (Table 14), but in-home palliative team care remained dominant when the cost estimates varied, unless the per-patient cost estimate increased to above $7,200.
Inpatient Palliative Team Care (Versus Usual Care)
Compared to usual care, this strategy appeared to be a dominant strategy. It reduced the mean health care cost, slightly increased the mean time at home and slightly decreased the percentage dying at home (Table 12). Inpatient palliative team care was associated with a small increase in HRQOL (Table 4), and this was projected to be associated with a small increase in QALDs (Table 12). However, this result was uncertain and might change with additional data (Table 13).
Results were sensitive to the associated rate ratio estimate of ICU admission, and differences in hospital days (Table 14), but were robust against changes in the per-patient cost estimate. The strategy remained cost-effective unless the per-patient cost estimate increased to above $1,570.
Comprehensive Palliative Team Care (Versus Usual Care)
This strategy increased the mean health care cost, slightly increased the mean time at home and slightly increased the percentage dying at home (Table 12). According to the effectiveness evidence, comprehensive palliative team care was associated with a nonsignificant increase in HRQOL (Table 4). This was projected to be associated with a small increase in QALDs, including improvement for patients and caregivers (Table 12). The probabilistic sensitivity analysis indicated that the result was uncertain and might change with additional data (Table 13).
The results were sensitive to relative risk estimates for hospital admission, differences in hospital days, and estimated costs of in-home and inpatient palliative team care (Table 14). The cost per QALY estimate was sensitive to the effect size estimate of HRQOL associated with the intervention but was robust against variation in the estimates of QALY weight decrements for ED visits and hospitalization (most likely due to the short duration of these events; data not shown).
Patient Care Planning Discussion
Identifying LTC Residents With EoL Goals and Preferences for Early Palliative Care (Versus Usual Care)
This strategy reduced the mean health care cost, increased the mean time at home by a fraction of a day, and had no impact on the percentage of dying at home (Table 12). Relative to usual care, this was a dominant strategy. This strategy was associated with a slightly higher mean QALD than usual care, but the probabilistic sensitivity analysis results were uncertain and might change with additional data (Table 13).
Ethics Consultation for ICU Patients With Treatment Conflicts (Versus Usual Care)
This strategy reduced mean health care costs, increased the mean time at home, and slightly increased the percentage of dying at home (Table 12). Relative to usual care, this was a dominant strategy. It was associated with a slightly higher mean QALD than usual care, but the probabilistic sensitivity analysis indicated that results were uncertain and might change with additional data (Table 13).
Improving Family Conferences for Relatives of Patients Dying in ICU (Versus Usual Care)
This strategy increased the mean health care cost, increased the time at home by a fraction of a day, and slightly decreased the percentage dying at home (Table 12). According to the effectiveness evidence, family conferences of sufficient duration for relatives of patients dying in ICU was associated with a reasonably large reduction in depressive symptoms among caregivers in the subsequent 3 month of bereavement period (Table 4). Compared to usual care, this strategy was cost-effective, but the probabilistic sensitivity analysis indicated that the results might change with additional data (Table 13).
Educational Interventions for Patients and Caregivers
Multicomponent Psychoeducational Training Interventions for Patients and Families (Versus Usual Care)
This strategy increased the mean health care cost, decreased the mean time at home, and decreased the percentage of dying at home (Table 12). These projected results were largely driven by an associated increase in ED visits and slightly more hospital days (Table 3). This strategy was dominated by usual care. However, the intervention was shown to increase HRQOL for caregivers (Table 4) and was projected to be associated with an increase in QALDs for both patients and caregivers (Table 12). Compared to usual care, this strategy was not cost-effective, but the probabilistic sensitivity analysis indicated that the results were uncertain and might change with additional data (Table 13).
Supportive Interventions for Informal Caregivers
Supportive Interventions for Informal Caregivers (Versus Usual Care)
This strategy increased the mean health care cost, and (due to a lack of effectiveness evidence) had no impact on the time at home or the percentage dying at home for the patients. Supportive interventions for caregivers led to a very small (nonsignificant) improvement in HRQOL (Table 4) and were projected to be associated with an increase in QALDs for caregivers (Table 12). Compared to usual care, this strategy may be cost-effective, but the probabilistic sensitivity analysis indicated that the results were uncertain and might change with additional data (Table 13).
Budget Impact Analysis
Of the 8 interventions evaluated in the cost-effectiveness analysis, the economic evidence was sufficiently robust for only in-home palliative team care, so we evaluated the budget impact of this single intervention. Expanding in-home palliative team care services to individuals nearing EoL who are at home or in LTC and are currently not supported with such services is likely to reduce the use of acute care resources without reducing patients’ quality of life.
We conducted the budget impact analysis from the perspective of the Ontario Ministry of Health and Long Term Care. All costs are reported in 2013 Canadian dollars.
Methods
Incident Population
The target population for in-home palliative team care was patients with a palliative prognosis, identified according to whether the patients received palliative care services in usual palliative care practice. We estimated the population to be approximately 56,000 individuals per year (Table 15).
Table 15:
Results of the Budget Impact Analysis
| Input | In-Home PTC | Sources |
|---|---|---|
| 1. Annual number of decedents in Ontario | 87,000 | HQO ICES cohort (43) |
| 2. Probability of being identified with a palliative prognosis | 0.65 | HQO ICES cohort (43) |
| 3. Decedents identified with palliative prognosis | 56,550 | Line 1* Line 2 |
| 4. Estimated decedents already received in-home PTC | 11,736 | Expert opiniona |
| 5. Annual number of decedents that may benefit from PTC | 44,814 | |
| 6. Per-patient cost of providing PTC | $1,700–$2,400 | Estimates |
| 7. Per-patient expected cost-saving (relative to usual care) | $2,200–$4,424 | Modelled projections |
| 8. Total cost of providing in-home PTC (million) | $76.18–$107.55 | Line 5* Line 6 |
| 9. Expected total cost-saving (million) | $191.40–$384.89 | Line 1* Line 7 |
Abbreviations: HQO, Health Quality Ontario; ICES, Institute for Clinical Evaluative Sciences; PTC, palliative team care.
Personal communication, clinical experts, March 28, 2014.
Resources
We assumed that in-home palliative team care was delivered by an expert consult team who delivered integrated interprofessional palliative care directly to patients in their home, in consultation with other primary care health care providers.
We estimated that in 2013, between 11 and 30 palliative care expert consult teams were operating in different health regions of Ontario, with varying capacity to deliver in-home palliative team care. At least 11 teams were available to patients and families on a 24/7 basis (Table 9). (51) We estimated that approximately 12,000 individuals in the target population currently receive in-home palliative team care, and approximately 45,000 individuals may benefit from in-home palliative team care but currently do not receive it (Table 15).
Estimated Costs
Based on the cost-effectiveness analysis, the per-patient cost of providing in-home palliative team care was estimated to be between $1,700 and $2,400.
Results of Budget Impact Analysis
Table 15 summarizes the results of the budget impact analysis. Expanding in-home palliative team care to individuals nearing the end of life who are at home or in LTC and are currently not supported with these services is likely to reduce patients’ use of acute care resources, leading to decreased associated health care costs without reducing quality of life. The expected cost saving is estimated to be $191 to $385 million per year.
Limitations
This analysis was the first approximation of the true cost-effectiveness of the EoL care interventions we evaluated, but it included a number of methodological and input uncertainties.
In the primary cost-effectiveness analysis, we used QALYs to capture intervention effects on the combined outcome of HRQOL and expected survival time. (27) However, improving QALYs may not be the intended aim of EoL care interventions, which tend to focus on comfort care rather than prolonging life; in fact, prolonging life may be inconsistent with patients’ wishes and preferences. It could be argued that the best outcome (e.g., a “good death”) may be one with the fewest QALYs. Conceptually, the QALY incorporates some but not all palliative domains. In particular, spiritual and psychosocial well-being is not included in conventional valuations of health states at the end of life (e.g., EQ-5D, HUI-2). (70) As well, quality-of-life improvements at the end of life tend to last for a short time, so it is challenging for EoL interventions to lead to high QALYs gained.
We resorted to the exploratory use of QALYs to address 2 key issues. For interventions that resulted in reduced health care costs, we used QALYs gained or lost to ensure that patients were not worse off as a result of lower expenditures. For interventions that resulted in increased in health care costs, QALYs gained or lost provided some indication of whether the additional expenditures were worth making. Still, we recognize that this use of QALYs was not ideal as an outcome measure.
We estimated intervention effects on QALY weights using effect estimates reported in different disease-specific HRQOL instruments. This estimation method was based on strong assumptions. Specifically, we assumed that there is a constant relative responsiveness between HRQOL generic scales (e.g., EQ-5D or HUI-2) and disease-specific HRQOL measures. In the cost-effectiveness analysis, we assumed that the relative responsiveness estimate was 0.8, suggesting that generic HRQOL measures would not be as responsive to change as disease-specific measures. We also assumed a wide range of uncertainty around this relative responsiveness estimate (0.4 to 1.2), including the possibility that generic instruments could be equally responsive to change. The assumption of constant relative responsiveness may not be plausible, since estimates of relative responsiveness depend on whether the measurement constructs and attributes are similar between generic and disease-specific instruments. Because of these limitations, we considered the cost-per-QALY estimates as only part of the sensitivity analysis.
In the simulation, we calculated the expected costs and health outcomes for a cohort of decedents in their last year of life. We assumed that those with a palliative prognosis could be identified (and therefore targeted for EoL interventions) according to a pattern of receiving EoL care services (e.g., physician billings). According to ICES health administration data, this pattern changed according to the proximity to death (e.g., 6 to 12 months, 3 to 6 months, and less than 3 months before death). We did not account for individuals nearing EoL who had a predictable palliative prognosis but did not receive EoL services, or individuals who received EoL services only before the last year of life. Because we used the time horizon of the last year of life, we also assumed that survival time was not affected by the EoL interventions we evaluated. This assumption will need to be verified in future work.
Our simulation approach was retrospective; we selected this approach because it was a conventional way of using health administration data to identify EoL care. (49) An alternative approach would have been to prospectively simulate a cohort of individuals with a palliative prognosis (e.g., identified using the “surprise” question) (71) until all simulated individuals died, allowing for EoL interventions to be evaluated early in the diagnosis of terminal conditions and accounting for the effects of EoL interventions on survival time. The challenge with this approach would have been uncertainty in the determination of a palliative prognosis, especially when the model inputs were determined using ICES health administration data. (49) At present, it is unclear how the cost-effectiveness results would have differed using a prospective simulation approach; this will need to be verified in future work.
This analysis focused primarily on resources and costs from a health care payer perspective. We attempted to conduct the analysis from a societal perspective, but there were insufficient data reporting the effects of EoL interventions on resources and costs (e.g., out-of-pocket expenses, third-party insurance, and costs of time lost from paid market labour and time lost from leisure and household work). Because of this lack of data, the results from a societal perspective were very similar to those from a health care payer perspective (data not shown). In conducting the cost-effectiveness analysis from the societal perspective, we attempted to highlight data gaps for future research.
In Ontario, home care and LTC are funded by the Ministry of Health and Long-Term Care. EoL care is financially supported by communities, philanthropists, the private sector and the provincial government. (10) From a health care payer perspective, findings from our analysis pointed to the benefits of increasing in-home palliative team care. However, we could not evaluate the cost-effectiveness of in-home palliative team care from a societal perspective—especially the impact of in-home palliative team care on the family. According to a recent study that estimated the cost of EoL care from a societal perspective, unpaid caregiving costs over the last year of life accounted for 77% of total EoL care expenses, followed by public costs (21%) and out-of-pocket expenditures (2%). (72) With an emphasis on in-home palliative team care, the burden on the family could be substantial; additional data are needed to update the current analysis from this perspective.
Discussion
We evaluated the cost-effectiveness of 8 interventions aimed at improving EoL care in Ontario; our analysis used population-based linked ICES health administration data to characterize the usual care (patterns of care and health care utilization) of Ontarian decedents in their last year of life. We showed that relative to usual care, in-home palliative team care for home care and LTC patients who are nearing EoL is likely to reduce health care costs and improve health outcomes. At the population level, extending in-home palliative team care to a high proportion of individuals nearing EoL who are currently not receiving such services is likely to substantially reduce health care costs.
We corroborated our findings related to in-home palliative team care with results from other studies. Seow et al conducted a retrospective cohort study of 11 expert consult teams (defined as a group of health care providers who delivered integrated, multidisciplinary, EoL care directly to patients in their homes and in consultation with other health care providers) from various regions of Ontario. (51) Using linked ICES health administration data, the authors showed that expert consult team care was associated with a significant reduction in hospital admission (relative risk, 0.71 [95% confidence interval, 0.64–0.79]) and ED visits (relative risk, 0.70 [0.63–0.77]) in the last 2 weeks of life. Expert consult team care was also associated with a significant reduction in the chance of dying in hospital (relative risk, 0.50 [0.44–0.56]). (51)
Although the economic evidence appeared to be in support of other interventions (patient care planning discussions and support services for caregivers), firm conclusions about their cost-effectiveness were not possible without additional data about their effects on patients and families. In particular, future studies should collect additional data on patterns of care, HRQOL (e.g., EQ-5D or HUI-2), resource utilization, and costs from a societal perspective, including comprehensive data relevant to the burden of dying for patients and their caregivers.
Conclusions
In-home palliative team care for individuals nearing EoL (at home and in LTC) reduced health care costs and improved health outcomes for patients nearing the end of life. The population impact of this intervention is potentially large—particularly the potential for reducing acute care utilization and improving in-home EoL care services.
With respect to the other interventions we evaluated, firm conclusions were not possible without additional data collected concurrently from patients and caregivers—especially QALY calculations.
Acknowledgements
Editorial Staff
Jeanne McKane, CPE, ELS(D)
Medical Information Services
Corinne Holubowich, BEd, MLIS
Kellee Kaulback, BA(H), MISt
Health Quality Ontario's Expert Advisory Panel on End-of-Life Care
| Panel Member | Affiliation(s) | Appointment(s) |
|---|---|---|
| Panel Co-Chairs | ||
| Dr Robert Fowler | Sunnybrook Research Institute University of Toronto | Senior Scientist Associate Professor |
| Shirlee Sharkey | St. Elizabeth Healthcare Centre | President and CEO |
| Professional Organizations Representation | ||
| Dr Scott Wooder | Ontario Medical Association | President |
| Health Care System Representation | ||
| Dr Douglas Manuel | Ottawa Hospital Research Institute University of Ottawa | Senior Scientist Associate Professor |
| Primary/Palliative Care | ||
| Dr Russell Goldman | Mount Sinai Hospital, Tammy Latner Centre for Palliative Care | Director |
| Dr Sandy Buchman | Mount Sinai Hospital, Tammy Latner Centre for Palliative Care | |
| Cancer Care Ontario | ||
| University of Toronto | Educational Lead | |
| Clinical Lead QI | ||
| Assistant Professor | ||
| Dr Mary Anne Huggins | Mississauga Halton Palliative Care Network; Dorothy Ley Hospice | Medical Director |
| Dr Cathy Faulds | London Family Health Team | Lead Physician |
| Dr José Pereira | The Ottawa Hospital | |
| University of Ottawa | Chief, Palliative Care program Professor | |
| Dean Walters | Central East Community Care Access Centre | Nurse Practitioner |
| Critical Care | ||
| Dr Daren Heyland | Clinical Evaluation Research Unit Kingston General Hospital | Scientific Director |
| Oncology | ||
| Dr Craig Earle | Ontario Institute for Cancer Research Cancer Care Ontario | Director of Health Services Research Program |
| Internal Medicine | ||
| Dr John You | McMaster University | Associate Professor |
| Geriatrics | ||
| Dr Daphna Grossman | Baycrest Health Sciences | Deputy Head Palliative Care |
| Social Work | ||
| Mary-Lou Kelley | School of Social Work and Northern Ontario School of Medicine, Lakehead University | Professor |
| Emergency Medicine | ||
| Dr Barry McLellan | Sunnybrook Health Sciences Centre | President and Chief Executive Officer |
| Bioethics | ||
| Robert Sibbald | London Health Sciences Centre University of Western Ontario | Professor |
| Nursing | ||
| Vicki Lejambe | Saint Elizabeth Healthcare | Advanced Practice Consultant |
| Tracey DasGupta | Sunnybrook Health Sciences Centre | Director, Inter-professional Practice |
| Mary Jane Esplen | De Souza Institute University of Toronto | Director Clinician Scientist |
Appendices
Appendix 1: Literature Search Strategies
Database(s): HTA & EED DB only,
Limits: 2000 to 2013 (with other limits)
Date Run: 25/10/13 17:37:23.87
| ID | Search | Hits | Description |
|---|---|---|---|
| #1 | MeSH descriptor: [Terminal Care] explode all trees | 296 | EoL Search |
| #2 | MeSH descriptor: [Palliative Care] explode all trees | 1288 | |
| #3 | ((End near/2 life near/2 care) or EoL care or (terminal* near/2 (care or caring or ill* or disease*)) or palliat* or dying or (Advanced near/3 (disease* or illness*)) or end stage*):ti,ab,kw (Word variations have been searched) | 14358 | |
| #4 | #1 or #2 or #3 | 14393 | |
| #5 | MeSH descriptor: [Cost-Benefit Analysis] explode all trees | 14344 | |
| #6 | cost* or cost effective:ti,ab,kw (Word variations have been searched) | 35233 | EconEvalFilter optimal balanced |
| #7 | #5 or #6 | 35233 | |
| #8 | letter or editorial or historical article:pt (Word variations have been searched) | 5891 | Publication Limit |
| #9 | #7 not #8 | 35158 | |
| #10 | MeSH descriptor: [Animals] explode all trees | 6200 | Humans only limit |
| #11 | MeSH descriptor: [Humans] explode all trees | 952 | |
| #12 | #10 not (#10 and #11) | 5248 | |
| #13 | #9 not #12 | 35023 | |
| #14 | #4 and #13 from 2000 to 2013, in Technology Assessments and Economic Evaluations | 99 | Time & Cochrane databases limit |
Database(s): Ovid MEDLINE(R), Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations
Limits: 2000 to October Week 1 2013 (with other limits)
Date Run: 25/10/13 17:37:23.87
Search Strategy
| # | Searches | Results |
|---|---|---|
| 1 | exp Terminal Care/ or exp Palliative Care/ or exp Terminally Ill/ or ((End adj2 life adj2 care) or EoL care or (terminal* adj2 (care or caring or ill* or disease*)) or palliat* or dying or (Advanced adj3 (disease* or illness*)) or end stage*).ti,ab. | 188995 |
| 2 | Cost-benefit analysis/ or costs.tw. or cost effective.tw. | 202521 |
| 3 | 1 and 2 | 3938 |
| 4 | limit 3 to yr=“2000 -Current” | 2734 |
| 5 | (letter or editorial or historical article or addresses or autobiography or bibliography or biography or comment or consensus development conference or consensus development conference, nih or directory or festschrift or guideline or interactive tutorial or interview or lectures or legal cases or legislation or news or newspaper article or patient education handout or periodical index or portraits or video-audio media or webcasts).pt. | 1869928 |
| 6 | interviews as topic/ or focus groups/ or narration/ or qualitative research/ or ((“semi-structured” or semistructured or unstructured or informal or “in-depth” or indepth or “face-to-face” or structured or guide) adj3 (interview* or discussion* or questionnaire*)).ti,ab. or (focus group* or qualitative or ethnograph* or fieldwork or “field work” or “key informant”).ti,ab. | 224770 |
| 7 | 5 or 6 | 2090844 |
| 8 | 4 not 7 | 2546 |
| 9 | limit 8 to english language | 2370 |
| 10 | remove duplicates from 9 | 2117 |
Database(s): Embase
Limits: 2000 to 2013 Week 40 (with other limits)
Date Run: 25/10/13 17:37:23.87
Search Strategy
| # | Searches | Results |
|---|---|---|
| 1 | exp Terminal Care/ or exp palliative therapy/ or exp terminally ill patient/ or ((End adj2 life adj2 care) or EoL care or (terminal* adj2 (care or caring or ill* or disease*)) or palliat* or dying or (Advanced adj3 (disease* or illness*)) or end stage*).ti,ab. | 232349 |
| 2 | (cost or costs).tw. | 387157 |
| 3 | 1 and 2 | 7347 |
| 4 | limit 3 to yr=“2000 -Current” | 5634 |
| 5 | (editorial or erratum or letter or note).pt. | 1984051 |
| 6 | (interview: or qualitative).tw. or exp health care organization/ | 1356255 |
| 7 | 5 or 6 | 3133860 |
| 8 | 4 not 7 | 3911 |
| 9 | limit 8 to english language | 3554 |
Appendix 2: Critical Appraisal of Included Studies
Table A1:
Gomes et al, 2013
| Gomes B, Calanzani N, Curiale V, et al. Effectiveness and cost-effectiveness of home palliative care services for adults with advanced illness and their caregivers. Cochrane Database Syst Rev 2013;(6):CD007760 | ||
| Methods | ||
| Study details | Population | Interventions |
|
Type of economic analysis: CEA alongside 5 RCTs and 1 controlled before/after study evaluating effectiveness. Stated as CEA (Higginson et al (16)) and CUA (Tramarin et al, 1992 (22)) Study design: Systematic review of effectiveness and cost-effectiveness studies Perspective: Mostly not stated, but can be inferred; societal (16;17) or health care perspective (18–21) Time horizon: Study enrollment to death, from 2 weeks to 6 months |
Patients with advanced illness and their family caregivers Mean age: varied by study Male: varied by study |
Home palliative care services vs. usual care, with various amounts and levels of primary care services, home health services, acute care services, and hospice care |
| Approach to analysis | ||
| CEA based mostly on data collected from the RCTs | ||
| Results | ||
| Costs | Health outcomes | Cost-effectiveness |
| All 6 studies reported lower costs in the intervention groups, with differences from 18% to 35%, except Greer et al, 1986 (17) in which the costs for the hospital-based intervention were 2% lower than conventional care, and 32% lower with the community-based intervention. Still, differences were statistically significant only in Brumley et al, 2007 (21). Differences in total costs were statistically nonsignificant in Higginson et al, 2009 (16) and Hughes et al, 1992 (19), although the existence of economically significant differences cannot be ruled out due to small sample sizes that were unlikely to have sufficient power to detect statistical significance. Statistical significance was not reported in Greer et al, 1986 (17), Tramarin et al, 1992 (22) or Zimmer et al, 1985 (18) | Four studies found significantly better outcomes with the intervention (increased probability of death at home and participant's ability to stay at home as long as desired; reduced probability of death in hospital; decreased symptom burden, pain, and caregiver burden; higher satisfaction with care; and better quality of death), but they all reported null results on other outcomes. Hughes et al, 1992 (19) reported a statistically significant negative intervention effect on caregiver morale at 6 months from enrollment and Greer et al, 1986 (17) found significantly higher caregiver burden over the last weeks of the patient's life in the group receiving the community-based intervention. The higher frequency of deaths at home in the intervention group in Zimmer et al, 1985 (18) failed to reach statistical significance (OR 2.86, 95% CI 0.78–10.53). It is also unclear whether the group differences in quality of life observed in Tramarin et al, 1992 (22) reached statistical significance | The 6 studies provided inconclusive evidence about the cost-effectiveness of home palliative care compared to usual care. In 2 studies, the data showed that the intervention was cost-effective. (16;21) It is unclear whether the intervention was cost-effective in the other 4 studies. (17–19;22) |
| Interpretation | ||
| Sensitivity analyses | Limitations and applicability | |
| Study-specific | Only 2 of the 6 included studies fulfilled the time criteria for this literature review—namely studies published between 2000 and 2009. Both studies were high-quality cost-effectiveness studies. (16;21) According to results from these 2 studies, the intervention with home palliative care services is likely to be cost-effective. Key assumptions: Study-specific. |
|
| Data sources | ||
| Clinical effectiveness: Data from RCTs (except the controlled before/after study of Greer et al, 1986 (17) | ||
| Costs: Study-specific | ||
| Quality of life: Study-specific | ||
| Funding | ||
| King's College London, Cicely Saunders Institute, Department of Palliative Care, Policy and Rehabilitation, UK. | ||
| Cicely Saunders International, UK and Calouste Gulbenkian Foundation, Portugal. | ||
Abbreviations: CEA, cost-effectiveness analysis; CI, confidence interval; CUA, cost-utility analysis; OR, odds radio; RCT, randomized controlled trial.
Table A2:
Higginson et al, 2009
| Higginson IJ, McCrone P, Hart SR, et al. Is short-term palliative care cost-effective in multiple sclerosis? A randomized phase II trial. J Pain Symptom Manage 2009;38(6):816–26 | ||
| Methods | ||
| Study details | Population | Interventions |
|
Type of economic analysis: CEA Study design: CEA using data from a randomized, controlled, fast-track phase II trial Perspective: Broad perspective, including costs to health, society, voluntary services and informal caregivers Time horizon: 12 weeks |
Patients severely affected by multiple sclerosis and deemed (by clinicians) to have 1 or more of unresolved symptoms, psychosocial concerns, EoL issues, progressive illness, or complex needs (i.e., palliative care needs) Mean age: 53 Male: 27%–35% |
Patients were randomly allocated immediately to a multiprofessional palliative care team (fast-track, n = 26) or the control care group, who continued best usual care for 3 months and then were offered the palliative care team (n = 26). |
| Approach to analysis | ||
| ITT analysis testing the differences between the 2 intervention groups with respect to changes from baseline using analysis of variance. The primary point of analysis was at 12 weeks. CEA was conducted by combining data on cost differences between groups with data on outcome differences with respect to POS-8 and ZBI. Uncertainty about the cost-effectiveness estimates was explored using the bootstrapping method | ||
| Results | ||
| Costs | Health outcomes | Cost-effectiveness |
|
Currency and cost year: 2005 pounds CEA: Total costs were £1789 lower for the fast-track group (95% CI £5224-£1,902) Discount rate: 5% |
Primary outcome: No significant difference over time in POS-8, but at week 12, caregiver burden ZBI scores had decreased in the fast-track group and increased slightly in the control group. The difference in change in burden was 4.47 (95% CI 1.05–7.89) | Primary ICER: Point estimates in the cost-effectiveness planes suggest that it was cost-saving, with equivalent outcomes on the POS-8 and improved outcomes for the ZBI |
| Interpretation | ||
| Sensitivity analyses | Limitations and applicability | |
| Sensitivity analyses were conducted to explore the impact of missing data and tested imputations (last value carried forward, next value carried backward, and mean value) Treatment effectiveness: As discussed in Health Outcomes, above. The results were similar in nonimputed and imputed data for all imputation methods |
Pilot RCT with small sample size. Intervention effect was studied in patients with severe multiple sclerosis only, limiting the applicability of the trial results to patients with EoL conditions Key assumptions: No major assumptions |
|
| Data Sources | ||
| Clinical effectiveness: Data collected from the pilot RCT | ||
| Costs: Resources and cost data collected from the pilot RCT using self-completed questionnaires | ||
| Quality of life: The POS-8 consists of 8 questions on anxiety, patient and carer concerns, and practical needs (each rated 0–4) | ||
| Funding | ||
| Multiple Sclerosis Society of the United Kingdom | ||
Abbreviation: CEA, cost-effectiveness analysis; CI, confidence interval; EoL, end-of-life; ICER, incremental cost-effectiveness ratio; ITT, intention-to-treat; POS, Palliative Care Outcome Scale; RCT, randomized controlled trial; ZBI, Zarit Carer Burden Inventory.
Table A3:
Goldfeld et al, 2013
| Goldfeld KS, Hamel MB, Mitchell S. Cost-effectiveness of the decision to hospitalize nursing home residents with advanced dementia. J Pain Symptom Manage 2013;46:640–51 | ||
| Methods | ||
| Study details | Population | Interventions |
|
Type of economic analysis: CUA Study design: CUA using individual patient data Perspective: Not stated, but inferred Medicare expenditures Time horizon: 18 months |
Nursing home residents with advanced dementia (323 residents of 22 nursing homes in the Boston area were followed in the prospective cohort study of Choices, Attitudes, and Strategies for Care of Advanced Dementia at the End-of-Life study, conducted between February 2003 and February 2009) Mean age: ∼ 85 years Male: 14% |
CUA 1: No DNH order CUA 2: Hospitalization for suspected pneumonia in nursing home residents with advanced dementia Advance directives to avoid future hospital transfers in the event of an acute illness and decisions not to hospitalize when an acute illness (i.e., pneumonia) occurred |
| Approach to analysis | ||
| Residents with and without DNH orders, or those who were and were not hospitalized for pneumonia, may have differed in ways that also could explain differences in expenditures, survival, and quality-adjusted survival. MSM was used to adjust for possible confounding. MSM provided estimates of mean Medicare expenditures, survival, and quality-adjusted survival. These were used to calculate the INBs of treatment vs. nontreatment. The INB was the primary measure. Bootstrap methods were used to estimate the standard error of the incremental expenditure and quality-adjusted survival estimates | ||
| Results | ||
| Costs | Health outcomes | Cost effectiveness |
|
Currency and cost year: 2007 US dollars CUA 1: Incremental (no DNH – DNH) $5,972 (SD $1,569) CUA 2: Incremental (hospitalization –no hospitalization): $3,697 (SD $5,981) Discount rate: 0% |
Primary outcome: QALY CUA 1: Incremental (no DNH–DNH): 0.01 (SD 0.01) CUA 2: Incremental (hospitalization – no hospitalization): −0.03 (SD 0.02) Discount rate: 0 |
Primary ICER CUA 1: ICER $589,000/year CUA 2: Hospitalization was dominated |
| Interpretation | ||
| Sensitivity analyses | Limitations and applicability | |
|
CUA 1: Not having a DNH order was not cost-effective at lower levels of WTP, assuming low to moderate levels of unmeasured confounding CUA 2: Hospitalization for pneumonia was not cost-effective for all WTP levels, and for all levels of unmeasured confounding related to expenditures and quality-adjusted survival (i.e., < 90% of INBs were positive) Treatment effectiveness CUA 1: 124 (46%) and 144 (54%) residents did and did not have DNH orders, respectively. Resident characteristics independently associated with not having a DNH order were: male, adjusted OR 2.3 95% CI 1.1–5.0; nonwhite, adjusted OR 5.6 95% CI 1.9–17.0; PEG tube adjusted OR 4.0 95% CI 1.1–14.5 CUA 2: Among residents with pneumonia, 113 (86%) were not hospitalized and 18 (14%) were hospitalized. Resident characteristics independently associated with a greater likelihood of hospitalization included: age 85 years or less, adjusted OR 3.8 (95% CI 1.1–13.0); male, adjusted OR 3.4 (95% CI 1.0–11.8); no DNH order, adjusted OR 13.2 (95% CI 1.6–111.4); COPD, adjusted OR 4.4)95% CI 1.0–19.0) |
Few limitations. This was a prospective cohort study. The methods were robust and transparent. QALY weights were estimated by mapping to health status using validated mapping methods. The conclusion is likely to be applicable to long-term care residents in Ontario | |
| Data Sources | ||
| Clinical effectiveness: Prospective cohort study Choices, Attitudes, and Strategies for Care of Advanced Dementia at the End-of-Life | ||
| Costs: Use of Medicare services was abstracted from the chart at each assessment: hospital admissions, ED visits, physician and other professional visits in the nursing home, hospice enrollment, and skilled nursing facility admission after hospitalization. Medicare expenditures attributable to these services were determined using publicly available sources and based on nationally representative rates from 2007 in US dollars | ||
| Quality of life: Data from 2 validated health status measures were collected from nurse interviews. The Symptom Management at the End-of-Life in Dementia Scale, ascertained quarterly, quantified the frequency with which residents experienced distressing symptoms (e.g., pain, depression, fear, anxiety, and agitation) over the preceding 90 days. The Comfort Assessment in Dying with Dementia Scale, ascertained within 14 days of death, quantified the frequency with which residents experienced distressing symptoms during the last week of life. We developed and validated a method that mapped the 2 scales to the HUI2. Possible HUI2 scores range from −0.025 to 1.00; perfect health is scored 1.00, death is scored 0.00, and a negative score implies a state worse than death. In the CASCADE study, the residents’ mean (SD) HUI2 score was 0.165 (0.060) (range −0.005 to 0.215) | ||
| Funding | ||
| This study was supported in part by grants R01AG024091 and K24AG033640 (Dr. Mitchell) from the National Institute on Aging | ||
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; CUA, cost-utility analysis; DNH, do-not-hospitalize; ED, emergency department; HUI2, Health Utility Index Mark 2; ICER, incremental cost-effectiveness ratio; INBs, incremental net benefits; MSM, marginal structured modelling; PEG, percutaneous endoscopic gastrostomy; QALY, quality-adjusted life-year; SD, standard deviation; WTP, willingness to pay.
Table A4:
Lowery et al, 2013
| Lowery WJ, Lowery AW, Barnett JC, et al. Cost-effectiveness of early palliative care intervention in recurrent platinum-resistant ovarian cancer. Gynecol Oncol 2013;130:426–30 | |||
| Methods | |||
| Study details | Population | Interventions | |
|
Type of economic analysis: CEA and CUA (sensitivity analysis) Study design: Decision tree model Perspective: Not stated, but can be inferred as a health care payers’ perspective Time horizon: 6 months |
Patients with recurrent, platinum-resistant ovarian cancer Mean age: NR Male: NR |
RC vs. RC plus early referral to a palliative medicine specialist (EPC) | |
| Approach to analysis | |||
| A decision model was constructed with a time horizon of 6 months to evaluate RC and EPC. The time horizon was chosen to reflect the early effects of palliative care intervention. In both strategies, a patient would receive RC at the discretion of the treating oncologist. EPC was defined as the initiation of outpatient palliative care at the time of diagnosis of platinum resistance. Patients receiving EPC would meet with a palliative care provider monthly to address symptoms, develop goals of care, and assist with decision-making about proposed treatments. Patients receiving RC would be referred to a palliative care provider only at the discretion of the treating physician or at the request of the patient or family. The primary model outcome was the average cost of care in each strategy. Model parameters included rates of inpatient admissions, ED visits, chemotherapy administration, and QOL. Costs of hospitalization, ED visits, chemotherapy, and EPC were included. | |||
| In the primary sensitivity analysis, potential QOL differences provided by EPC were modelled, with the assumption that the recurrent ovarian cancer population had the potential for a similar improvement in QOL to that observed by Temel et al (47) | |||
| Results | |||
| Costs | Health outcomes | Cost-effectiveness | |
|
Currency and cost year: 2012 US dollars Total costs (mean per person): RC $6303, EPC $5,017 incremental (RC–EPC) $1,285 Discount rate: 0% |
Primary outcome: QALY (in a sensitivity analysis) Total QALYs (mean per person): RC NR, EPC NR, incremental (RC–EPC): NR Discount rate: 0 |
Primary ICER: NR Other: Compared to RC, EPC was associated with a cost of $37,000 per QALY (in a sensitivity analysis) |
|
| Interpretation | |||
| Sensitivity analyses | Limitations and applicability | ||
| In the sensitivity analysis incorporating QOL, EPC was dominant (i.e., less costly and more effective) compared to routine care. In 1-way sensitivity analysis, the cost of EPC (estimate $468) had to exceed $1,753 before the average cost of the EPC strategy was higher than that of routine care. The cost of EPC had to exceed $2,400 before the ICER of EPC reached the common societal WTP threshold of $50,000/QALY, and had to exceed $3,000 before the ICER reached $100,000/QALY compared to routine care. When we assumed no clinical benefit of EPC other than QOL (i.e., no differences in chemotherapy administration, hospitalizations or ED visits between groups), EPC remained highly cost-effective, with an ICER of $37,440/QALY Treatment effectiveness: Outcomes that differed significantly between the EPC and RC strategies in the prior RCT (47) (rates of inpatient admissions, ED visits, and chemotherapy administration) were included in the model. The authors estimated inpatient hospitalizations, ED visits, and chemotherapy administrations in the RC group based on available ovarian cancer data. They calculated ORs for reductions in these events when EPC was introduced, based on what was observed in the prior RCT. The ORs for each clinical event (0.69 for hospitalizations, 0.74 for ED visits, and 0.77 for chemotherapy administration) were applied to the baseline event rates in ovarian cancer to determine their rates in the EPC group. This resulted in the base case assumption that EPC in the ovarian cancer population would potentially result in a reduction in hospitalizations from 70% to 48%, in ED visits from 30% to 22%, and in chemotherapy administration from 60% to 46% during the last 6 months of life |
Effectiveness evidence was from 1 source. (47) Extrapolation of the effectiveness evidence from patients with metastatic NSCLC to patients with recurrent platinum-resistant ovarian cancer. Assuming that the target population is treated reasonably similar between the US (North Carolina) and Ontario, the results could be considered applicable to Ontario Key Assumptions: (1) For purposes of cost calculation, all patients who were admitted to the hospital were assumed to be admitted once and all patients seen in the ED were assumed to be seen once during the 6-month time horizon; (2) the chemotherapy regimen was identical in both arms; for simplicity, the use of liposomal doxorubicin was assumed in this population; (3) patients receiving EPC were seen as outpatients for an initial visit, followed by 5 subsequent monthly visits; (4) QOL was not incorporated into the base case model; and (5) given that there are no data regarding the impact of EPC intervention on overall survival in patients with platinum-resistant ovarian cancer, we assumed equivalent survival between those receiving EPC and those receiving RC |
||
| Data Sources | |||
| Clinical effectiveness: Temel et al (47) | |||
| Costs: The cost of palliative care was estimated as the 2012 Medicare reimbursement for an initial high-complexity encounter, followed by moderately high-complexity visits every 4 weeks. The cost of hospitalization was estimated as the mean cost of inpatient hospitalization for a diagnosis of small bowel obstruction, 1 of the most common reasons for admission at the end of life in ovarian cancer, using the AHRQ's Healthcare Cost and Utilization Project. The cost of an ED visit was derived as the average total payment from all sources for an ED visit using the AHRQ's Medical Expenditure Panel Survey. The cost of chemotherapy was estimated using Medicare reimbursement data using Current Procedural Terminology codes and drug J codes, and included the costs of a physician visit, infusion room costs, routine laboratory panels, chemotherapy, and support drugs | |||
| Quality of life: The base case utility score representing HRQOL during treatment of recurrent ovarian cancer (0.67) was derived from a prospective elicitation of the preferences of a member of the public using the time trade-off method. From the RCT, (47) the odds ratio representing the potential change in QOL with the addition of EPC (1.07) was based on the global QOL score changes seen with incorporation of EPC. This OR was applied to the baseline utility score associated with treatment of recurrent ovarian cancer to produce the utility (0.72) representing QOL improvement in the EPC group. These favourable QOL changes were incorporated only over the final 3 months of the 6-month time horizon to account for the fact that there is likely a delay in the development of QOL differences between EPC and RC | |||
| Funding | |||
| The authors have no conflicts of interest to report | |||
Abbreviations: AHRQ, Agency for Healthcare Research and Quality; CEA, cost-effectiveness analysis; CUA, cost-utility analysis; ED, emergency department; EPC, early palliative care; HRQOL, health-related quality of life; ICER, incremental cost-effectiveness ratio; NR, not reported; NSCLC, non-small-cell lung cancer; OR, odds ratio; QALY, quality-adjusted life-year; QOL, quality of life; RC, routine care; RCT, randomized controlled trial; WTP, willingness to pay.
Table A5:
Pace et al, 2012
| Pace A, Di Lorenzo C, Capon A, et al. Quality of care and re-hospitalization rate in the last stage of disease in brain tumor patients assisted at home: a cost effectiveness study. J Palliative Med 2012;15:225–227 | ||
| Methods | ||
| Study details | Population | Interventions |
|
Type of economic analysis: CEA Study design: Individual-patient-data CEA Perspective: Not stated, but inferred health system payer perspective Time horizon: Last 2 months of life |
143 patients with primary brain tumours (a subgroup of glioblastoma) Mean age: ∼ 50 Male: 53% |
A CEA was carried out evaluating the rehospitalization rate in the last 2 months of life in a subgroup of patients (Group 1 assisted at home, 72 patients; Group 2 not assisted at home, 71 patients). |
| Approach to analysis | ||
| The main aim of the study was to evaluate the effectiveness of a home care model of assistance in reducing the rehospitalization rate; the model's cost-effectiveness was assessed based on administrative data on rehospitalization rate in the last 2 months of life in a subgroup of patients compared with a control group of brain tumour patients not receiving home care assistance at EoL | ||
| The CEA was carried out in a consecutive series of patients discharged after surgical procedures for glioblastoma from January to December 2006 in 1 hospital (Group 1). All patients of Group 1 received home care assistance. The control group was represented by glioblastoma patients discharged in the same period of time from the neurosurgical ward of the hospital (Group 2 was not assisted at home) | ||
| Results | ||
| Costs | Health outcomes | Cost-effectiveness |
|
Currency and cost year: Euro (year not reported) CEA: Assisted at home vs. not assisted at home; the costs of hospitalization were €517 (95% CI €512, €522) in Group 1 and €24,076 (€24,040, €24, 112) in Group 2 Discount rate: 0 |
Primary outcome: In the last month of life, 6 patients in Group 1 and 19 in Group 2 were rehospitalized. Crude hospitalization rate of Group 1 was lower than for Group 2 (8.3% vs. 26.8%), while Poisson regression age- and sex-adjusted IRR for Group 1 vs. group 2 was 0.29 (95% CI 0.12–0.74, P = 0.009) Discount rate: 0% |
Primary ICER: Not applicable |
| Interpretation | ||
| Sensitivity analyses | Limitations and applicability | |
| Not reported Treatment effectiveness: See primary outcomes |
It was unclear whether the 2 groups were similar with respect to factors that influence rehospitalization rates and hospital days. Because the costing study was conducted in Italy, the results may not be applicable to Ontario brain tumour patients | |
| Data Sources | ||
| Clinical effectiveness: Data from the pilot project, “Palliative home care for neuro-oncological patients” at the Regina Elena National Cancer Institute of Rome. (25) Data regarding the number of hospital readmissions in the last 2 months of life, and length and cost of hospitalizations in the 2 groups of patients were analyzed from hospital discharge records stored in the database of the regional public health agency | ||
| Costs: See above | ||
| Quality of life: Not applicable | ||
| Funding | ||
| The neuro-oncology home care program is supported by Latium Regional Health System (Regione Lazio, Italy) funds | ||
Abbreviations: CEA, cost-effectiveness analysis; CI, confidence interval; EoL, end-of-life; ICER, incremental cost-effectiveness ratio; IRR, incidence rate ratio.
Table A6:
Ljungman et al, 2012
| Ljungman D, Hyltander A, Lundholm K. Cost-utility estimations of palliative care in patients with pancreatic adenocarcinoma: a retrospective analysis. World J Surg 2013;37:1883–91 | ||
| Methods | ||
| Study details | Population | Interventions |
|
Type of economic analysis: CUA Study design: Individual-patient-data CUA Perspective: Health care payer perspective Time horizon: 1, 2, 5 years for different patient groups |
A population-based cohort of patients with exocrine pancreatic adenocarcinoma from 1998 to 2005 was evaluated retrospectively (n = 444) Mean age: 66–69 Male: Not reported |
A subgroup of 34 patients with pancreatic adenocarcinoma who were treated with personalized palliative care (e.g., indomethacin and erythropoietin treatment, nutritional support, and insulin treatment with the goal of providing the best individual palliative care for each patient by accounting for their clinical characteristics) Patients who had more conventional treatment, mainly based on sufficient pain treatment, are referred to as the standard palliative care group (n = 271) Results were compared to similar findings in a previously reported group of patients with pancreatic carcinoma resected for cure (n = 31) |
| Approach to analysis | ||
| The evaluation parameters included survival, direct health care costs, and QALY estimates (that were based upon the SF-6D health utility). The study report describes in details patients and data retrieval, HRQOL, cost measures, and statistical analysis. Nonparametric Mann–Whitney and Kruskal–Wallis tests were used for comparisons between groups. Survival from the date of diagnosis (date of surgery for resection patients) was analyzed according to the Kaplan-Meier test and tested by log rank. QALY calculations were performed across 1 year | ||
| Results | ||
| Costs | Health outcomes | Cost-effectiveness |
|
Currency and cost year: 2011 Euros CEA: The total health care costs were 50% on palliative care compared to costs for surgical R0 resections (€23,701 and €50,950, respectively) Discount rate: 5% |
Primary outcome: QALYs for 1 year from diagnosis were 0.2 (95% CI 0.17–0.23) in patients on palliative care and 0.48 (95% CI 0.44–0.54) in resection patients | Primary ICER: Costs per QALY were €118,418 and €106,146 (95% CI €103,048 €139,418 and €94,352–€115,795) for the palliative care group and the resection group, respectively |
| Interpretation | ||
| Sensitivity analyses | Limitations and applicability | |
| Not reported Treatment effectiveness: Patients on personalized palliative care showed significantly better survival than patients on standard palliative (without adjustment for baseline characteristics). Patients who underwent resection for cure showed better survival, as expected, compared to patients with unresectable tumours who experienced overall palliative care. Estimated QALYs over 1 year from diagnosis were 0.20 and 0.48 for palliation and resection patients, respectively (95% CI 0.17–0.23 and 0.44–0.54, respectively) |
Retrospective analysis of patients with quite different prognoses. The choice of comparators (comparing palliative care patients with patients receiving curative treatment with a high chance of prolonged life) made the results hard to interpret. Because the study was conducted in Sweden, the results (including the limitations described here) may not be applicable to the Ontario setting Key assumptions: The 3 groups were comparable |
|
| Data Sources | ||
| Clinical effectiveness: A consecutive retrospective database of 444 consecutive patients diagnosed with malignancy of the exocrine pancreas or ampulla (ICD-7: 155.3 or 157) at Sahlgrenska University Hospital from 1998–2005. This database contains survival and SF-36 data | ||
| Costs: The cost registry provided costs per patient, including health care interventions such as surgery, intensive care, radiologic examinations, drugs, and laboratory analyses. It also contained the basic charge for admission including bed and standardized provision of care in wards and staff salaries | ||
| Quality of life: Most calculations in the study were based on the entire consecutive cohort of 444 patients from 1998–2005, whereas the HRQOL data for palliative care group were based on information from the subgroup of 21 patients on personalized palliative care with complete data and from 31 resection patients | ||
| Funding | ||
| This study was supported in part by grants from the Assar Gabrielsson Foundation (AB Volvo), the Gothenburg Medical Society, the Swedish Government (LUA-ALF), the Swedish Cancer Society, and the Swedish Research Council (08712) | ||
Abbreviations: CEA, cost-effectiveness analysis; CI, confidence interval; CUA, cost-utility analysis; HRQOL, health-related quality of life; ICD-7, International Classification of Diseases, 7th edition; ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life-year.
References
- (1).OHTAC End-of-Life Collaborative. Health care for people approaching the end of life: an evidentiary framework. Ont Health Technol Assess Ser [Internet]. 2014 December; 14 (13): 1–45. Available from: http://www.hqontario.ca/evidence/publications-and-ohtac-recommendations/ontario-health-technology-assessment-series/eol-evidentiary-framework. [PMC free article] [PubMed] [Google Scholar]
- (2).Qaseem A, Snow V, Shekelle P, Casey Jr DE, Cross Jr JT, Owens DK. Evidence-based interventions to improve the palliative care of pain, dyspnea, and depression at the end of life: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2008; 148(2): 141–6. [DOI] [PubMed] [Google Scholar]
- (3).Fowler R, Hammer M. End-of-life care in Canada. Clin Invest Med. 2013; 36(3): 127–32. [DOI] [PubMed] [Google Scholar]
- (4).Foot D. A Canadian priorities agenda—policy choices to improve economic and social well-being. In: Leonard J, Ragan C, St-Hilaire Fe, editors. Population aging. Montreal: Institute for Research on Public Policy; 2007. p. 181–213.
- (5).Tanuseputro P, Wodchis W, Fowler R, Walker P, Bai YQ, Bronskill SE, et al. The health care cost of dying in Ontario: a retrospective population based cohort study of the last year of life. Poster session presented at: Canadian Association for Health Services and Policy Research Conference; 2013 May 28–30; Montreal (QC).
- (6).Howell D, Marshall D, Brazil K, Taniguchi A, Howard M, Foster G, et al. A shared care model pilot for palliative home care in a rural area: impact on symptoms, distress, and place of death. J Pain Symptom Manage. 2011; 42(1): 60–75. [DOI] [PubMed] [Google Scholar]
- (7).Collier R. Access to palliative care varies widely across Canada. CMAJ 2011; 183(2): E87–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Klinger CA, Howell D, Zakus D, Deber RB. Barriers and facilitators to care for the terminally ill: a cross-country case comparison study of Canada, England, Germany, and the United States. Palliative Med. 2014; 28(2): 111–20. [DOI] [PubMed] [Google Scholar]
- (9).Walker H, Anderson M, Farahati F, Howell D, Librach SL, Husain A, et al. Resource use and costs of end-of-life/palliative care: Ontario adult cancer patients dying during 2002 and 2003. J Palliative Care. 2011; 27(2): 79–88. [PubMed] [Google Scholar]
- (10).Klinger CA, Howell D, Marshall D, Zakus D, Brazil K, Deber RB. Resource utilization and cost analyses of home-based palliative care service provision: the Niagara West End-of-Life Shared-Care Project. Palliative Med. 2013; 27(2): 115–22. [DOI] [PubMed] [Google Scholar]
- (11).Fawole OA, Dy SM, Wilson RF, Lau BD, Martinez KA, Apostol CC, et al. A systematic review of communication quality improvement interventions for patients with advanced and serious illness. J Gen Intern Med. 2013; 28(4): 570–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Joint Committee on Health Education Terminology. Report of the 1990 Joint Committee on Health Education Terminology. J School Health. 1991;61 (6): 251–4. [DOI] [PubMed] [Google Scholar]
- (13).Hudson PL, Remedios C, Thomas K. A systematic review of psychosocial interventions for family carers of palliative care patients. BMC Palliative Care. 2010; 9: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Bermingham SL. The appropriate use of neuroimaging in the diagnostic work-up of dementia: an economic literature review and cost-effectiveness analysis. Ont Health Technol Assess Ser [Internet]. 2014 February; 14(2): 1–67. Available from: http://www.hqontario.ca/evidence/publications-and-ohtac-recommendations/ontario-health-technology-assessment-series/imaging-for-dementia. [PMC free article] [PubMed] [Google Scholar]
- (15).Gomes B, Calanzani N, Curiale V, McCrone P, Higginson IJ. Effectiveness and cost-effectiveness of home palliative care services for adults with advanced illness and their caregivers. Cochrane Database Syst Rev. 2013; (6): CD007760. [DOI] [PMC free article] [PubMed]
- (16).Higginson IJ, McCrone P, Hart SR, Burman R, Silber E, Edmonds PM. Is short-term palliative care cost-effective in multiple sclerosis? A randomized phase II trial. J Pain Symptom Manage. 2009; 38(6): 816–26. [DOI] [PubMed] [Google Scholar]
- (17).Greer DS, Mor V, Morris JN, Sherwood S, Kidder D, Birnbaum H. An alternative in terminal care: results of the national hospice study. J Chronic Dis. 1986; 39(1): 9–26. [DOI] [PubMed] [Google Scholar]
- (18).Zimmer JG, Groth-Juncker A, McCusker J. A randomized controlled study of a home health care team. Am J Public Health. 1985; 75(2): 134–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Hughes SL, Cummings J, Weaver F, Manheim L, Braun B, Conrad K. A randomized trial of the cost effectiveness of VA hospital-based home care for the terminally ill. Health Services Res. 1992; 26(6): 801–17. [PMC free article] [PubMed] [Google Scholar]
- (20).Grande GE, Todd CJ, Barclay SI, Farquhar MC. Does hospital at home for palliative care facilitate death at home? Randomised controlled trial. BMJ. 1999 Dec 4; 319(7223): 1472–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Brumley R, Enguidanos S, Jamison P, Seitz R, Morgenstern N, Saito S, et al. Increased satisfaction with care and lower costs: Results of a randomized trial of in-home palliative care. J Am Geriatr Soc. 2007; 55(7): 993–1000. [DOI] [PubMed] [Google Scholar]
- (22).Tramarin A, Milocchi F, Tolley K, Vaglia A, Marcolini F, Manfrin V, et al. An economic evaluation of home-care assistance for AIDS patients: a pilot study in a town in northern Italy. AIDS. 1992; 6(11): 1377–83. [DOI] [PubMed] [Google Scholar]
- (23).Goldfeld KS, Hamel MB, Mitchell SL. The cost-effectiveness of the decision to hospitalize nursing home residents with advanced dementia. J Pain and Symptom Manage. 2013; 46(5): 640–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Lowery WJ, Lowery AW, Barnett JC, Lopez-Acevedo M, Lee PS, Secord AA, et al. Cost-effectiveness of early palliative care intervention in recurrent platinum-resistant ovarian cancer. Gynecol Oncol. 2013; 130(3): 426–30. [DOI] [PubMed] [Google Scholar]
- (25).Pace A, Di Lorenzo C, Capon A, Villani V, Benincasa D, Guariglia L, et al. Quality of care and rehospitalization rate in the last stage of disease in brain tumor patients assisted at home: a cost effectiveness study. J Palliative Med. 2012; 15(2): 225–7. [DOI] [PubMed] [Google Scholar]
- (26).Ljungman D, Hyltander A, Lundholm K. Cost-utility estimations of palliative care in patients with pancreatic adenocarcinoma: a retrospective analysis. World J Surg. 2013; 37(8): 1883–91. [DOI] [PubMed] [Google Scholar]
- (27).Drummond M, Sculpher M, Torrance G, O'Brien B, Stoddard G. Methods for the economic evaluation of health care programmes. Oxford: Oxford University Press; 2005. 400 p.
- (28).Gade G, Venohr I, Conner D, McGrady K, Beane J, Richardson RH, et al. Impact of an inpatient palliative care team: a randomized control trial. J Palliative Med. 2008 Mar; 11(2): 180–90. [DOI] [PubMed] [Google Scholar]
- (29).Ahronheim JC, Morrison RS, Morris J, Baskin S, Meier DE. Palliative care in advanced dementia: A randomized controlled trial and descriptive analysis. J Palliative Med. 2000; 3(3): 265–73. [DOI] [PubMed] [Google Scholar]
- (30).Zimmermann C, Swami N, Krzyzanowska M, Hannon B, Leighl N, Oza A, et al. Early palliative care for patients with advanced cancer: a cluster-randomised controlled trial. Lancet. 2014; 383(9930): 1721–30. [DOI] [PubMed] [Google Scholar]
- (31).Casarett D, Karlawish J, Morales K, Crowley R, Mirsch T, Asch DA. Improving the use of hospice services in nursing homes: a randomized controlled trial. JAMA. 2005; 294(2): 211–7. [DOI] [PubMed] [Google Scholar]
- (32).Schneiderman LJ, Gilmer T, Teetzel HD, Dugan DO, Blustein J, Cranford R, et al. Effect of ethics consultations on nonbeneficial life-sustaining treatments in the intensive care setting: a randomized controlled trial. JAMA. 2003; 290(9): 1166–72. [DOI] [PubMed] [Google Scholar]
- (33).Lautrette A, Darmon M, Megarbane B, Joly LM, Chevret S, Adrie C, et al. A communication strategy and brochure for relatives of patients dying in the ICU. N Engl J Med. 2007; 356(5): 469–78. [DOI] [PubMed] [Google Scholar]
- (34).Bakitas M, Lyons KD, Hegel MT, Balan S, Brokaw FC, Seville J, et al. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the project ENABLE II randomized controlled trial. JAMA. 2009; 302(7): 741–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Meyers FJ, Carducci M, Loscalzo MJ, Linder J, Greasby T, Beckett LA. Effects of a problem-solving intervention (COPE) on quality of life for patients with advanced cancer on clinical trials and their caregivers: Simultaneous Care Educational Intervention (SCEI): linking palliation and clinical trials. J Palliative Med. 2011; 14(4): 465–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).McMillan SC, Small BJ, Weitzner M, Schonwetter R, Tittle M, Moody L, et al. Impact of coping skills intervention with family caregivers of hospice patients with cancer: a randomized clinical trial. Cancer. 2006; 106(1): 214–22. [DOI] [PubMed] [Google Scholar]
- (37).Candy B, Jones L, Drake R, Leurent B, King M. Interventions for supporting informal caregivers of patients in the terminal phase of a disease. Cochrane Database Syst Rev. 2011; (6): CD007617. [DOI] [PubMed]
- (38).Briggs A, Claxton K, Sculpher M. Further developments in decision analytic models for economic evaluation. New York: Oxford University Press; 2006. 237 p.
- (39).Koerkamp BG, Wang YC, Hunink MGM. Cost-effectiveness analysis for surgeons. Surgery. 2009; 145(6): 616–22. [DOI] [PubMed] [Google Scholar]
- (40).Selby D, Chakraborty A, Lilien T, Stacey E, Zhang L, Myers J. Clinician accuracy when estimating survival duration: the role of the patient's performance status and time-based prognostic categories. J Pain Symptom Manage. 2011; 42(4): 578–88. [DOI] [PubMed] [Google Scholar]
- (41).Towns K, Dougherty E, Kevork N, Wiljer D, Seccareccia D, Rodin G, et al. Availability of services in Ontario hospices and hospitals providing inpatient palliative care. J Palliative Med. 2012; 15(5): 527–34. [DOI] [PubMed] [Google Scholar]
- (42).Seow H, Barbera L, Howell D, Dy SM. How end-of-life home care services are used from admission to death: a population-based cohort study. J Palliative Care. 2010; 26(4): 270–8. [PubMed] [Google Scholar]
- (43).Pham B, Fowler R, Tanuseputro P, Manuel D, Sikich N, Baidoobonso S, et al. Cost-effectiveness of palliative team care for patients nearing end of life. Paper presented at: 36th Annual North American Meeting of the Society for Medical Decision Making; 2014. Oct 18–22; Miami (FL); 2014.
- (44).DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. 2007 Feb; 28(2): 105–14. [DOI] [PubMed] [Google Scholar]
- (45).Hanks GW, Robbins M, Sharp D, Forbes K, Done K, Peters TJ, et al. The imPaCT study: a randomised controlled trial to evaluate a hospital palliative care team. Br J Cancer. 2002; 87(7): 733–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Jordhoy MS, Fayers P, Saltnes T, Ahlner-Elmqvist M, Jannert M, Kaasa S. A palliative-care intervention and death at home: a cluster randomised trial. Lancet. 2000 Sep 9; 356(9233): 888–93. [DOI] [PubMed] [Google Scholar]
- (47).Temel JS, Greer JA, Muzikansky A, Gallagher ER, Admane S, Jackson VA, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010 Aug 19; 363(8): 733–42. [DOI] [PubMed] [Google Scholar]
- (48).Gilmer T, Schneiderman LJ, Teetzel H, Blustein J, Briggs K, Cohn F, et al. The costs of nonbeneficial treatment in the intensive care setting. Health Aff (Millwood). 2005 Jul-Aug; 24(4): 961–71. [DOI] [PubMed] [Google Scholar]
- (49).Tanuseputro P, Budhwani S, Bai Y, Wodchis W. Palliative care applied health research question: results for Central East Local Health Integration Network. Palliative Care Applied Health Research (AHRQ) 2013 [Internet]. Toronto (ON): Health System Performance Research Network; 2013. Jun [cited 2014 Oct 2]. 27 p. Available from: http://www.hsprn.ca/uploads/files/HSPRN_EoL%20AHRQ%201%20Evidence%20Brief%20June%202013%20%289-5-2014%29.pdf. [Google Scholar]
- (50).Stukel TA, Fisher ES, Alter DA, Guttmann A, Ko DT, Fung K, et al. Association of hospital spending intensity with mortality and readmission rates in Ontario hospitals. JAMA. 2012; 307(10): 1037–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Seow H, Brazil K, Sussman J, Pereira J, Marshall D, Austin PC, et al. Impact of community based, specialist palliative care teams on hospitalisations and emergency department visits late in life and hospital deaths: a pooled analysis. BMJ. 2014; 348: g3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Lukas L, Foltz C, Paxton H. Hospital outcomes for a home-based palliative medicine consulting service. J Palliative Med. 2013; 16(2): 179–84. [DOI] [PubMed] [Google Scholar]
- (53).Zaide GB, Pekmezaris R, Nouryan CN, Mir TP, Sison CP, Liberman T, et al. Ethnicity, race, and advance directives in an inpatient palliative care consultation service. Palliative Supportive Care. 2013 Feb; 11(1): 5–11. [DOI] [PubMed] [Google Scholar]
- (54).Bakitas M, Lyons KD, Hegel MT, Balan S, Barnett KN, Brokaw FC, et al. The project ENABLE II randomized controlled trial to improve palliative care for rural patients with advanced cancer: baseline findings, methodological challenges, and solutions. Palliative Supportive Care. 2009 Mar; 7(1): 75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Canadian Institute for Health Information. National Physician Database, 2010–2011—Data Release [Internet]. Ottawa (ON): Canadian Institute for Health Information; 2012. [cited 2014 Oct 7] 288 p. Available from: https://secure.cihi.ca/estore/productSeries.htm?pc=PCC476. [Google Scholar]
- (56).Canadian Federation of Nurses Unions. Overview of key nursing contract provisions [Internet]. Toronto: Canadian Federation of Nurses Unions; 2014. [cited 2014 Oct 7]. 16 p. Available from: https://nursesunions.ca/sites/default/files/contract_comparison_english.pdf [Google Scholar]
- (57).Living in Canada. Social Worker Salary Canada [Internet]. [location unknown]: Living in Canada; 2014. [cited 2014 Oct 26]. Available from: http://www.livingin-canada.com/salaries-for-social-workers.html.
- (58).Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. New York: Taylor & Francis Group; 1988. 590 p. [Google Scholar]
- (59).Peterman AH, Fitchett G, Brady MJ, Hernandez L, Cella D. Measuring spiritual well-being in people with cancer: the functional assessment of chronic illness therapy—Spiritual Well-being Scale (FACIT-Sp). Ann Behav Med. 2002 Winter; 24(1): 49–58. [DOI] [PubMed] [Google Scholar]
- (60).Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993 Mar 3; 85(5): 365–76. [DOI] [PubMed] [Google Scholar]
- (61).Cella DF, Bonomi AE, Lloyd SR, Tulsky DS, Kaplan E, Bonomi P. Reliability and validity of the Functional Assessment of Cancer Therapy-Lung (FACT-L) quality of life instrument. Lung Cancer. 1995 Jun; 12(3): 199–220. [DOI] [PubMed] [Google Scholar]
- (62).Church J, Goodall S, Norman R, Haas M. The cost-effectiveness of falls prevention interventions for older community-dwelling Australians. Aust N Z J Public Health. 2012 Jun; 36(3): 241–8. [DOI] [PubMed] [Google Scholar]
- (63).Ghatnekar O, Bondesson A, Persson U, Eriksson T. Health economic evaluation of the Lund Integrated Medicines Management Model (LIMM) in elderly patients admitted to hospital. BMJ Open. 2013; 3(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Dinglas VD, Gifford JM, Husain N, Colantuoni E, Needham DM. Quality of life before intensive care using EQ-5D: patient versus proxy responses. Crit Care Med. 2013 Jan; 41(1): 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Mittmann N, Trakas K, Risebrough N, Liu BA. Utility scores for chronic conditions in a community-dwelling population. Pharmacoeconomics. 1999 Apr; 15(4): 369–76. [DOI] [PubMed] [Google Scholar]
- (66).Davidson T, Krevers B, Levin LA. In pursuit of QALY weights for relatives: empirical estimates in relatives caring for older people. Eur J Health Econ. 2008 Aug; 9(3): 285–92. [DOI] [PubMed] [Google Scholar]
- (67).Mann R, Gilbody S, Richards D. Putting the “Q” in depression QALYs: a comparison of utility measurement using EQ-5D and SF-6D health related quality of life measures. Soc Psychiatry Psychiatr Epidemiol. 2009 Jul; 44(7): 569–78. [DOI] [PubMed] [Google Scholar]
- (68).van den Hout WB, Kramer GWPM, Noordijk EM, Leer JWH. Cost-utility analysis of short-versus long-course palliative radiotherapy in patients with non-small-cell lung cancer. J Natl Cancer Inst. 2006; 98(24): 1786–94. [DOI] [PubMed] [Google Scholar]
- (69).Dolan P. Modeling valuations for EuroQol health states. Med Care. 1997 Nov; 35(11): 1095–108. [DOI] [PubMed] [Google Scholar]
- (70).Murtagh FEM, Groeneveld EI, Kaloki YE, Calanzani N, Bausewein C, Higginson IJ. Capturing activity, costs, and outcomes: the challenges to be overcome for successful economic evaluation in palliative care. Progress Palliative Care. 2013; 21(4): 232–5. [Google Scholar]
- (71).You JJ, Fowler RA, Heyland DK. Just ask: Discussing goals of care with patients in hospital with serious illness. CMAJ. 2014; 186(6): 425–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Chai H, Guerriere DN, Zagorski B, Coyte PC. The magnitude, share and determinants of unpaid care costs for home-based palliative care service provision in Toronto, Canada. Health Soc Care Community. 2014; 22(1): 30–9. [DOI] [PubMed] [Google Scholar]