Abstract
From January 1 to April 3, 2015, 159 people from 18 states and the District of Columbia were reported as having measles. Most cases are part of an outbreak linked to a California amusement park. Because measles was eliminated in the United States in 2000, most U.S. clinicians are unfamiliar with the condition. We reviewed information on the current outbreak, measles manifestations, diagnostic methods, treatment, and infection-control recommendations. To identify information on measles and pregnancy, we reviewed reports with 20 or more measles cases during pregnancy that included data on effects on pregnant women or pregnancy outcomes. These reports were identified through MEDLINE from inception through February 2015 using the following strategy: (((pregnan*) AND measles) AND English[Language]) NOT review[Publication Type]. Reference lists also were reviewed to identify additional articles. Pregnant women infected with measles are more likely to be hospitalized, develop pneumonia, and die than nonpregnant women. Adverse pregnancy outcomes, including pregnancy loss, preterm birth, and low birth weight, are associated with maternal measles; however, the risk of congenital defects does not appear to be increased. No antiviral therapy is available; treatment is supportive. Early identification of possible cases is needed so that appropriate infection control can be instituted promptly. The recent measles outbreak highlights the role that obstetric health care providers play in vaccine-preventable illnesses; obstetrician–gynecologists should ensure that patients are up to date on all vaccines, including measles-containing vaccines, and should recommend and ideally offer a measles-containing vaccine to women without evidence of measles immunity before or after pregnancy.
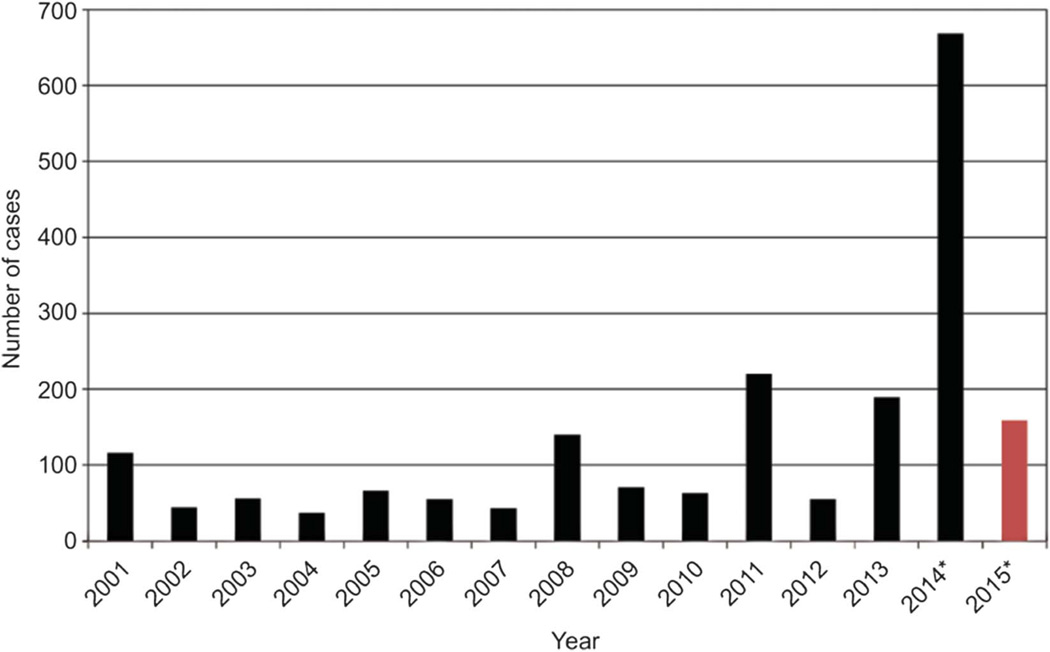
From January 1 to April 3, 2015, 159 people from 18 states and the District of Columbia have been reported as having measles.1 Most cases are part of a large, ongoing outbreak linked to a California amusement park. Because of a highly successful vaccination program, measles elimination (defined as absence of endemic disease transmission, ie, a chain of transmission that continues for 12 or more months)2 was declared in the United States in 2000. However, elimination does not suggest that no cases will occur: measles is endemic in many countries throughout the world, and outbreaks continue to occur in the United States when unvaccinated persons are exposed to imported measles virus either during international travel or by foreign visitors infected with measles.3 Since 2000, the annual number of measles cases in the United States has ranged from a low of 37 in 2004 to a high of 668 in 2014 (Fig. 1).1 Before measles vaccination was available, measles was primarily a childhood disease; however, since 2000, about 40% of cases occurred in adults, with about a quarter among persons 20–39 years of age.2
Fig. 1.
Number of measles cases in the United States by year, 2001–present (April 3, 2015). Red bar indicates partial data. *Provisional data reported to Centers for Disease Control and Prevention’s National Center for Immunization and Respiratory Diseases through April 3, 2015. Modified from Centers for Disease Control and Prevention. Measles Cases and Outbreaks. Atlanta (GA): Centers for Disease Control and Prevention; 2015. Available at: http://www.cdc.gov/measles/cases-outbreaks.html.
Rasmussen. Measles and Pregnancy. Obstet Gynecol 2015.
In previous studies, pregnant women have been shown to be at increased risk for complications associated with measles, including adverse pregnancy outcomes.4–6 Therefore, it is essential that obstetric health care providers are aware of measles and its effects on pregnant women and their newborns. Here we review the clinical features, diagnostic methods, infection-control measures, and treatment of measles, as well as information on the effects of measles during pregnancy and recommendations for pregnant women and newborns. To identify information on measles and pregnancy, we reviewed reports with 20 or more measles cases during pregnancy that included data on effects of measles on pregnant women or pregnancy outcomes. These reports were identified through MEDLINE from inception through February 2015 using the following search strategy: (((pregnan*) AND measles) AND English[Language]) NOT review[Publication Type]. Reference lists from selected articles were also reviewed to identify additional articles.
MEASLES
Measles (rubeola) is a highly contagious respiratory illness caused by a single-stranded, enveloped RNA virus that is a member of the genus Morbillivirus in the Paramyxoviridae family.3 Measles is a condition clinically and virologically distinct from rubella (sometimes referred to as German or 3-day measles). Persons infected with measles typically present with a prodrome of high fever and malaise and cough, coryza (runny nose), and conjunctivitis (the “three Cs”). The measles prodrome typically occurs 3–4 days before appearance of the rash. Near the end of the prodrome, Koplik’s spots (small white lesions on an erythematous base) may appear on the buccal mucosa, followed by a maculopapular rash that typically spreads from the head to the trunk to the lower extremities. The measles rash gradually fades, first from the face and last from the lower extremities. The average incubation period of measles (from exposure until prodrome onset) is 10–12 days; onset of the rash typically occurs about 14 (range 7–21) days after exposure.3 Treatment of measles is supportive; no specific antiviral therapy is available.
Complications and death from measles are more common in infants, young children, and adults compared with older children and adolescents.3 Measles complications include pneumonia and encephalitis. In addition, subacute sclerosing panencephalitis, a degenerative disease of the central nervous system characterized by intellectual decline and behavior changes, followed by seizures, dementia, and death, can occur on average 4–10 years after acute measles illness, although latency periods have ranged from less than a year to several decades.7 Incidence has been estimated to be 4–11 cases per 100,000 cases of measles.7
Before licensure of the live attenuated measles vaccine in 1963, approximately 3–4 million persons were estimated to have been infected with measles in the United States each year; of those, 48,000 were hospitalized and 500 died.3 The Centers for Disease Control and Prevention recommends that the first dose of measles-containing vaccine (measles–mumps–rubella (MMR) vaccine or measles–mumps–rubella–varicella vaccine—both referred to hereafter as MMR vaccine) be given at 12 through 15 months of age, with a second dose recommended at 4 through 6 years of age or at least 28 days after the first dose.3 The first dose of measles-containing vaccine is about 93% effective at preventing measles if exposed to the virus; two doses are about 97% effective. Immunity appears to be long-term and probably life-long in most people.3
Measles is a highly contagious illness transmitted by direct contact with infectious droplets or by airborne spread. Up to 90% of susceptible persons develop measles after exposure.3 The virus continues to be infectious on surfaces and in the air for up to 2 hours. Persons with measles are infectious from 4 days before through 4 days after rash onset and should be isolated during that period.
EFFECTS OF MEASLES IN THE PREGNANT WOMAN
Pregnant women are at increased risk of morbidity and mortality associated with measles based on many studies (Table 1). Many of these studies are case series and lack comparison groups, but two studies that include comparison groups of nonpregnant women with measles deserve particular attention. In a study by Eberhart-Phillips et al,5 58 pregnant women with measles were identified in an outbreak in Los Angeles during the period from 1988–1991. Compared with 748 nonpregnant women with measles, pregnant women were significantly more likely to be hospitalized, to develop pneumonia, and to die. In a study in Saudi Arabia in 1993,6 pregnant women with measles were significantly more likely to be hospitalized than those who were not pregnant. Fever and elevated liver enzymes were also more common among pregnant women with measles than among nonpregnant women. Although these studies suggest that pregnant women with measles are more likely to be hospitalized and many hospitalized women had pneumonia, the reasons for hospital admission usually were not specified. Some of these studies also included women infected during the postpartum period (Table 1), but numbers of cases are too small to assess whether postpartum women are also at high risk.
Table 1.
Summary of Case Series and Cohort Studies of Measles in Pregnancy
| Year Cases Occurred |
First Author | Location | Pregnant Women With Measles |
Control Group |
|---|---|---|---|---|
| 1938–1939 | Dyer17 | Oklahoma | 24 | None |
| 1951 | Christensen18 | Greenland | 76 pregnant; 7 postpartum | Nonpregnant women with measles in same age group |
| 1951–1962 (includes 10 epidemics) | Jespersen10 | Greenland | Total of 368 pregnant women, 327 with pregnancy information; 252 neonates with clinical examination | No formal control group, but compared outcomes with baseline rates during same time period |
| 1957–1964 | Siegel8,9,11 | New York (NY) | 65 pregnant women with measles with information on outcomes, 60 neonates born to women with measles | Pregnant women without measles; 62 neonates born to women without measles matched on obstetric service, maternal age, race, gravidity, date of last menstrual period |
| 1988–1991 | Eberhart-Phillips5 | Los Angeles (CA) | 58 | 748 nonpregnant women with measles of similar age reported to county health department during same period as cases |
| 1993 | Ali6 | Saudi Arabia | 40 | 37 nonpregnant women with measles; 120 pregnant women without measles |
| 2007–2008 | Yasunaga19 | Japan | 31 | None |
| 2009–2011 | Ogbuanu4 | Namibia | 55; 19% HIV-positive | 3 pregnant women without measles for each case; 15% HIV-positive |
| 2010–2011 | Stahl20 | France | 20 pregnant women | 440 nonpregnant persons hospitalized during the same time period |
| 2011–2012 | Ali21 | Eastern Sudan | 61 | None |
|
Maternal Outcome |
Fetal or Neonatal Outcome |
Congenital Defects |
|---|---|---|
| 1 epistaxis; 1 postpartum sepsis; no maternal deaths | 1 spontaneous abortion at 18 wk of gestation; 2 preterm deliveries at 33 and 35 wk; among 24 liveborn infants, (including 1 set of twins), one infant death at 1 mo of age (infant died from pneumonia) | No mention of congenital defects among 24 liveborn neonates |
| No increased lung complications; heart failure more common among pregnant and postpartum women | 7 spontaneous abortion; 6 preterm delivery with 3 neonatal deaths. Among term neonates, 2 deaths within 24 h of life | No congenital defects among 69 liveborn neonates |
| Among 20 women infected in first 8 wk of pregnancy, 10 (50%) had spontaneous abortion; 6/64 (9%) of women infected in 1st trimester had fetal deaths; rates of low birth weight (11%) and infant mortality (11%) higher than typically seen during time period | Among 300 liveborn neonates, 8 (2.7%) had congenital malformations, a rate not higher than expected. 5/58 (9%) of neonates exposed in the 1st trimester had severe congenital malformations, but no specific pattern of malformations noted | |
| Increased frequency of low birth weight in neonates born to women with measles (17%) vs neonates born to women without measles (3%); no differences in pregnancy loss rates | Rate of congenital defects similar in neonates born to mothers with measles vs controls (1.7% vs 1.6%) | |
| Among pregnant women: 35 (60%) hospitalized; 15 (26%) had pneumonia; 2 (3%) died; vs controls, pregnant women more likely to be hospitalized (RR 1.8; 95% CI 1.5–2.3), to develop pneumonia (RR 2.6; 95% CI 1.6–4.3), and to die (RR 6.4; 95% CI 1.2–34.5) | Among 58 pregnancies, there were 37 term deliveries, 3 therapeutic abortions, 5 spontaneous abortions, and 13 premature deliveries. 29 (50%) of pregnancies ended within 14 d of the onset of measles rash | 2 congenital defects; slight clubfoot in neonate born to mother infected at 39 wk of gestation; polydactyly in neonate born to mother infected at 37 wk of gestation. No defects in 5 liveborn neonates born to mothers infected in the 1st trimester |
| Vs nonpregnant women, pregnant women more likely to be hospitalized (80% vs 8%; P<.001), have high fever (33% vs 11%, P=.04), have elevated liver enzymes (65% vs 32%, P<.008). Among pregnant women, 4 (10%) had pneumonia, 2 requiring oxygen therapy and 1 required mechanical ventilation vs 5% (2) with pneumonia among nonpregnant controls (P=.4), neither required oxygen or mechanical ventilation | Vs pregnant women without measles, neonates born to women with measles were more likely to be born preterm (25% vs 6.7%, P=.003), to be admitted to the neonatal intensive care unit (17.5% vs 1.7%, P<.001), and to have a longer stay in the neonatal intensive care unit (P<.001). Rates of spontaneous abortion, intrauterine fetal demise and neonatal mortality higher in pregnant women with measles vs pregnant women without measles, but findings not statistically significant | No difference in rate of congenital defects among neonates born to women with and without measles during pregnancy |
| All hospitalized (hospital-based case ascertainment); 1 pneumonia, 1 intestinal complication, 1 conjunctivitis | 10 (32.3%) spontaneous abortions or fetal deaths | No mention of congenital defects among 21 liveborn neonates |
| 53 (96%) hospitalized; diarrhea (60%), pneumonia (40%), encephalitis (5%); 5 deaths including 3 among HIV-positive and 2 with unknown HIV status. After adjustment for maternal age, vs pregnant women without measles, pregnant women with measles were more likely to die (RR 9.6; 95% CI 1.3–70.0) | Vs pregnant women without measles, pregnant women with measles were at increased risk for spontaneous abortion (adjusted RR 5.9; 95% CI 1.8–19.7), intrauterine fetal death (adjusted RR 9.0; 95% CI 1.2–65.5) and low birth weight (adjusted RR 3.5; 95% CI 1.5–8.2). | No congenital defects among 31 liveborn neonates |
| All hospitalized (hospital-based case ascertainment); no deaths; no pregnant women with severe illness (admission to intensive care unit), vs 6% of nonpregnant cases; 1 pneumonia and elevated serum transaminases, 2 pneumonia only, 10 with elevated serum transaminases only, 7 with a “nonstandard rash” | 9 outcomes of pregnancy “favorable,” 1 spontaneous abortion, 10 outcomes of pregnancy not documented | No mention of congenital defects in 9 patients with “favorable” outcomes |
| All hospitalized (hospital-based case ascertainment); 11 maternal deaths (18.0%); reported causes of death were pneumonia (9), encephalitis (1), intracranial hemorrhage (1) | Among 53 women followed until delivery, 6 (11.3%) spontaneous abortions, 4 (7.5%) preterm births, 3 (5.7%) fetal deaths | No mention of congenital defects among 44 liveborn neonates |
RR, relative risk; CI, confidence interval; HIV, human immunodeficiency virus.
EFFECTS OF MEASLES ON THE FETUS
Adverse pregnancy outcomes also have been commonly reported among pregnant women with measles in several studies (Table 1). Analyses that include a comparison group of pregnant women without measles are the most informative. In a cohort study from New York City during the period from 1957–1964,8 measles was associated with an increased frequency of low birth weight (less than 2,500 g), which appeared to be due to preterm labor. However, in this cohort, pregnancy loss was not more frequent among measles-infected pregnant women compared with pregnant women without measles.9 In the study from Saudi Arabia,6 neonates born to women with measles were significantly more likely to be born preterm, to be admitted to the neonatal intensive care unit, and to have longer intensive care unit stays than neonates born to women without measles. Rates of spontaneous abortion, intrauterine fetal demise, and neonatal mortality were higher among pregnant women with measles, but these findings were not statistically significant. In a study from Namibia,4 women with measles were significantly more likely to have spontaneous abortion, intrauterine fetal demise, or neonates with low birth weight than women without measles.
Because maternal rubella infection is a well-recognized cause of congenital defects, concerns have been raised regarding whether measles might also be teratogenic. In a case series from Greenland of 300 liveborn neonates prenatally exposed to measles in 10 epidemics during the period from 1951–1962,10 eight (2.7%) had congenital malformations, a rate not higher than expected. However, 5 of 58 (9%) neonates with first-trimester exposures had severe congenital malformations, leading the authors to speculate that maternal measles infection could cause congenital defects, although no specific pattern of malformations was seen and no comparison group of non–measlesinfected women was included. Subsequent studies have not substantiated this association (Table 1). In two studies that included comparison groups of neonates born to women without measles,6,11 no differences were seen in the rate of congenital defects among neonates born to women with and without measles during pregnancy.
TRANSMISSION OF MATERNAL MEASLES TO THE FETUS (CONGENITAL MEASLES)
Transmission of measles virus from a pregnant woman to her fetus (congenital measles) has been reported in neonates born to women who had measles within 10 days of delivery.12 Congenital measles is defined as presence of the rash at birth or within the first 10 days of life (this early timing excludes transmission from mother to newborn postdelivery) in a newborn whose mother was infected with measles late in pregnancy. Provision of pooled human immune globulin after delivery can decrease the risk of congenital measles; however, cases have been observed even after its receipt, although these cases may be milder.12 Infants who develop congenital measles are at increased risk for mortality and for subacute sclerosing panencephalitis, which is more common when measles is diagnosed in infancy.7 In addition, subacute sclerosing panencephalitis in newborns infected with measles either congenitally or shortly after birth appears to be more severe, with a shorter latency and rapidly progressive course.7
DOCUMENTATION OF MEASLES IMMUNITY
A two-dose MMR vaccine schedule has been recommended by the Advisory Committee on Immunization Practices and the American Academy of Pediatrics since 1989.3 Despite these national vaccine recommendations, obstetric health care providers cannot assume that pregnant women in the United States were vaccinated with MMR vaccine in childhood. Recent studies have documented high levels of nonimmunity to measles among pregnant women in the United States; in a study by Haas et al of women presenting for prenatal care in 2004, 16.5% of women were not immune to measles. In that study, three quarters of women did not recall whether they had received the recommended second MMR vaccination, and even among those who reported receiving a second dose of vaccine, only 85% were measles-immune,13 raising questions about maternal report of vaccination. One might assume that rubella immunity (a routine part of prenatal care) could serve as a surrogate for measles immunity; however, in a study in Iowa, only 88% of those who were rubella-immune were also immune to measles, leading these authors to recommend that pregnant women who are exposed to measles be tested to document measles immunity.14 These results emphasize the importance of written documentation of MMR vaccination.
RECOMMENDATIONS (Updates Available at http://www.cdc.gov/measles/hcp/)
Recommendations for routine vaccination:
The American College of Obstetricians and Gynecologists recommends that health care providers review vaccination history as a routine part of a woman’s general preventive health care.15 Health care providers should recommend and, if possible, offer indicated vaccinations. Studies have shown that health care provider recommendation and offer of vaccines are strong factors in determining whether a patient gets vaccinated.15 Additional information on integrating immunizations into clinical practice is included at www.immunizationforwomen.org.
Health care providers should document that they provided counseling regarding indicated vaccines and the patient’s decision (accepted, refused, or intend to receive indicated vaccines at another facility) regarding these vaccines.15
As part of the vaccination history review, health care providers should ensure that all patients have documentation of measles immunity. Acceptable evidence of measles immunity includes written documentation of receipt of live measles virus–containing vaccine (two doses for adults at increased risk for exposure, such as students attending colleges or other post–high school educational institutions, health care personnel, and international travelers, and one dose for other adults aged 18 years or older), laboratory evidence of measles immunity (ie, presence of measles-specific immunoglobulin [Ig] G antibody in serum; equivocal results should be considered negative), laboratory confirmation of disease, or birth before 1957 (persons born before 1957 are presumed to be immune owing to natural measles infection).3 In an outbreak setting in which community-wide transmission is occurring among adults, a second dose should be considered for adults who have received only one dose.
If documentation of measles immunity is not available, women should be asked if they are pregnant or might become pregnant in the next 4 weeks. Because MMR vaccine is a live vaccine, women who are pregnant or planning to become pregnant should not be vaccinated. Others should be vaccinated after they are counseled to avoid becoming pregnant for the next 4 weeks because of the theoretical risk of infection with one of the live components of the vaccine.3 Routine pregnancy testing of women of reproductive age is not recommended before giving MMR vaccine.
If a woman inadvertently receives the MMR vaccine during pregnancy or becomes pregnant within 4 weeks after receiving the vaccine, she should be counseled about the theoretical risk of congenital rubella syndrome associated with receiving a rubella-containing vaccine; however, studies on neonates born to women who have inadvertently received MMR vaccination during pregnancy have not shown an increased risk of adverse outcomes.3 Because measles infection during pregnancy does not appear to be associated with congenital defects, exposure to the measles component is not believed to be of concern. MMR vaccination during pregnancy should not be considered a reason for pregnancy termination.3
Pregnant women without evidence of measles immunity or who are rubella-nonimmune should receive MMR vaccine after delivery—ideally before they are discharged from the hospital.3 Women who receive the MMR vaccine during the postnatal period can breastfeed without risk to their newborns.
Recommendations for persons who work in health care facilities:
All persons who work in health care facilities should have presumptive evidence of immunity to measles, which includes any of the following: 1) written documentation of vaccination with 2 doses of live measles or MMR vaccine (first dose at 1 year of age or older, second dose at least 28 days after the first dose), 2) laboratory evidence of immunity, 3) laboratory confirmation of disease, or 4) birth before 1957.16
Health care facilities should ensure that information on measles immunity of personnel is documented and readily available.
If measles exposure occurs in a health care facility, health care personnel without evidence of measles immunity should be excluded from the health care setting from 5 through 21 days after exposure.16
Recommendations for pregnant women without evidence of measles immunity exposed to measles:
Because of their increased risk for measles-related morbidity and mortality, pregnant women without evidence of measles immunity who are exposed to measles should receive intravenous immune globulin at a dose of 400 mg/kg within 6 days of exposure. Intravenous administration is recommended so that doses are high enough to reach levels of measles antibodies expected to be protective.3 A rapid IgG antibody test can be used to document measles immunity, provided that immune globulin administration is not delayed.3
Recommendations for pregnant women with suspected or known measles:
Measles should be considered in patients presenting with fever, rash, and other symptoms consistent with measles (eg, cough, coryza, and conjunctivitis). Ill patients should be asked about recent international travel, recent visits to venues frequented by international visitors, and history of measles illness in their communities.
Patients with suspected measles should be isolated promptly in an airborne infection isolation room, and a facemask should be placed on the patient if feasible. All health care personnel caring for the patient should have evidence of measles immunity.3,16 Appropriate personal protective equipment (respiratory protection at least as protective as a fit-tested N95 respirator) should be used by all health care providers caring for the patient, even those with documented immunity.1 Cases of suspected measles should be reported immediately to the local health department.
The most common methods for confirming measles infection are detection of measles-specific IgM antibody and measles RNA by real-time polymerase chain reaction.1 Health care providers should collect both a serum sample and a throat (or nasopharyngeal) swab; collection of urine also might be helpful because testing both respiratory and urine samples can increase the likelihood of measles virus detection. Collection of virologic specimens allows viral genotyping. The local health department should be contacted for information about submitting specimens for testing. Additional information on laboratory testing is available at the Centers for Disease Control and Prevention website: http://www.cdc.gov/measles/lab-tools/index.html.
Pregnant women diagnosed with measles should receive symptomatic care. No specific treatment is available.
Recommendations for neonates born to women ill with measles:
Because of the increased risk of severe disease in neonates born to mothers who have measles within 10 days of delivery, obstetric health care providers need to ensure that the pediatrician caring for the neonate is aware of the potential for congenital measles. It is recommended that infants (younger than 12 months of age) with exposure to measles receive measles immune globulin intramuscularly at a dose of 0.5 mL/kg of body weight (max = 15 mL).3
Limited data are available regarding whether well neonates born to mothers who are ill with measles need to be separated from their mothers. Clinicians should discuss the specific situation with infection control and public health experts to weigh the benefits and risks of isolating the neonate from the mother until she is no longer infectious (4 days after onset of her rash).
Acknowledgments
The authors thank Dr. Greg Wallace, Lead, for CDC’s Measles, Mumps, Rubella, and Polio Team, for his helpful comments on the manuscript.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Financial Disclosure
The authors did not report any potential conflicts of interest.
REFERENCES
- 1.Centers for Disease Control and Prevention (CDC) Measles cases and outbreaks. [Retrieved March 20, 2015]; Available at: http://www.cdc.gov/measles/cases-outbreaks.html.
- 2.Parker Fiebelkorn A, Redd SB, Gallagher K, Rota PA, Rota J, Bellini W, et al. Measles in the United States during the post-elimination era. J Infect Dis. 2010;202:1520–1528. doi: 10.1086/656914. [DOI] [PubMed] [Google Scholar]
- 3.McLean HQ, Fiebelkorn AP, Temte JL, Wallace GS Centers for Disease Control and Prevention. Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: summary recommendations of the advisory committee on immunization practices (ACIP) MMWR Recomm Rep. 2013;62:1–34. [PubMed] [Google Scholar]
- 4.Ogbuanu IU, Zeko S, Chu SY, Muroua C, Gerber S, De Wee R, et al. Maternal, fetal, and neonatal outcomes associated with measles during pregnancy: Namibia, 2009–2010. Clin Infect Dis. 2014;58:1086–1092. doi: 10.1093/cid/ciu037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eberhart-Phillips JE, Frederick PD, Baron RC, Mascola L. Measles in pregnancy: a descriptive study of 58 cases. Obstet Gynecol. 1993;82:797–801. [PubMed] [Google Scholar]
- 6.Ali ME, Albar HM. Measles in pregnancy: maternal morbidity and perinatal outcome. Int J Gynaecol Obstet. 1997;59:109–113. doi: 10.1016/s0020-7292(97)00196-3. [DOI] [PubMed] [Google Scholar]
- 7.Campbell H, Andrews N, Brown KE, Miller E. Review of the effect of measles vaccination on the epidemiology of SSPE. Int J Epidemiol. 2007;36:1334–1348. doi: 10.1093/ije/dym207. [DOI] [PubMed] [Google Scholar]
- 8.Siegel M, Fuerst HT. Low birth weight and maternal virus diseases. A prospective study of rubella, measles, mumps, chickenpox, and hepatitis. JAMA. 1966;197:680–684. [PubMed] [Google Scholar]
- 9.Siegel M, Fuerst HT, Peress NS. Comparative fetal mortality in maternal virus diseases. A prospective study on rubella, measles, mumps, chicken pox, and hepatitis. N Engl J Med. 1966;274:768–771. doi: 10.1056/NEJM196604072741404. [DOI] [PubMed] [Google Scholar]
- 10.Jespersen CS, Littauer J, Sagild U. Measles as a cause of fetal defects. A retrospective study of tem measles epidemics in Greenland. Acta Paediatr Scand. 1977;66:367–372. doi: 10.1111/j.1651-2227.1977.tb07909.x. [DOI] [PubMed] [Google Scholar]
- 11.Siegel M. Congenital malformations following chickenpox, measles, mumps, and hepatitis. Results of a cohort study. JAMA. 1973;226:1521–1524. [PubMed] [Google Scholar]
- 12.Charlier C, Hourrier S, Leruez-Ville M, Zahar JR, Floret D, Salomon LJ, et al. Polyvalent immunoglobulins in neonates after perinatal exposure to measles: benefits and long-term tolerance of immunoglobulins. J Infect. 2015 Jan 22; doi: 10.1016/j.jinf.2015.01.010. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 13.Haas DM, Flowers CA, Congdon CL. Rubella, rubeola, and mumps in pregnant women: susceptibilities and strategies for testing and vaccinating. Obstet Gynecol. 2005;106:295–300. doi: 10.1097/01.AOG.0000171110.49973.e3. [DOI] [PubMed] [Google Scholar]
- 14.Kennedy CM, Burns BA, Ault KA. Does rubella immunity predict measles immunity? A serosurvey of pregnant women. Infect Dis Obstet Gynecol. 2006;2006:13890. doi: 10.1155/IDOG/2006/13890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Integrating immunizations into practice. Committee Opinion No. 558. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2013;121:897–903. doi: 10.1097/01.AOG.0000428788.74725.90. [DOI] [PubMed] [Google Scholar]
- 16.Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention (CDC) Immunization of health-care personnel: recommendations of the advisory committee on immunization practices (ACIP) MMWR Recomm Rep. 2011;60:1–45. [PubMed] [Google Scholar]
- 17.Dyer I. Measles complicating pregnancy. South Med J. 1940;33:601–604. [Google Scholar]
- 18.Christensen PE, Schmidt H, Bang HO, Andersen V, Jordal B, Jensen O. An epidemic of measles in southern Greenland, 1951; measles in virgin soil. II. The epidemic proper. Acta Med Scand. 1953;144:430–449. doi: 10.1111/j.0954-6820.1953.tb15717.x. [DOI] [PubMed] [Google Scholar]
- 19.Yasunaga H, Shi Y, Takeuchi M, Horiguchi H, Hashimoto H, Matsuda S, et al. Measles-related hospitalizations and complications in Japan, 2007–2008. Intern Med. 2010;49:1965–1970. doi: 10.2169/internalmedicine.49.3843. [DOI] [PubMed] [Google Scholar]
- 20.Stahl JP, Salmon D, Bruneel F, Caumes E, Freymuth F, Bru JP, et al. Adult patients hospitalized for measles in France, in the 21st century. Med Mal Infect. 2013;43:410–416. doi: 10.1016/j.medmal.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Ali AA, Abdelhameed O, Abdallah TM. Case-fatality rate associated with measles during pregnancy in Kassala, eastern Sudan. Int J Gynaecol Obstet. 2014;124:261–262. doi: 10.1016/j.ijgo.2013.09.015. [DOI] [PubMed] [Google Scholar]