Abstract
Background
“Social smoking” - smoking mostly or even only with others – may be an important pattern that implies smoking motivated extrinsically by social influences. Non-daily smokers (intermittent smokers; ITS) are often assumed to be social smokers, with some authors even assuming that all ITS are social smokers (SS+). We sought to identify and characterize social smokers in a sample of ITS.
Methods
204 adult ITS (smoking 4–27 days/month) recorded the circumstances of smoking in their natural settings using Ecological Momentary Assessment, while also recording their circumstances in nonsmoking moments. SS+ were defined as ITS who were with others when they smoked most of their cigarettes, and who were ≥ 50% more likely to be with others when smoking than when not.
Results
Only 13% of ITS were SS+. Although defined solely on the basis of presence of others, SS+ showed a distinct pattern of smoking across multiple dimensions: Compared to other ITS (who were significantly less likely to smoke when with others), SS+ smoking was more associated with socializing, being with friends and acquaintances, drinking alcohol, weekends, evening or nighttime, being in other people’s homes, but not their own home. SS+ smoking was low in the morning and increased in the evening. SS+ smoked fewer days/week and were less dependent, but did not differ demographically.
Conclusions
Social smoking does constitute a highly distinct smoking pattern, but is not common among adult ITS.
Keywords: smoking, non-daily smoking, social smoking, ecological momentary assessment
1. INTRODUCTION
The dominant models of smoking behavior explain smoking as an attempt by smokers to maintain a certain level of nicotine in order to avoid withdrawal symptoms (Benowitz, 2010; Benowitz et al., 1990). These fit the smoking patterns seen in most smokers – smoking every day, at rates that can maintain steady-state nicotine levels (Benowitz, 2010). However, an increasing number of smokers do not smoke every day, making it impossible to maintain nicotine levels. Such intermittent smokers (ITS) now constitute up to 38% of adult smokers in the US (Centers for Disease Control and Prevention, 2008a, 2008b; Substance Abuse and Mental Health Services Administration, 2009), and are also prevalent elsewhere (Lindstrom and Ostergren, 2001). While the literature has described and characterized ITS (Shiffman et al., 2012b, 2012c), their smoking behavior has not been explained. We have suggested that their smoking is largely under stimulus control by particular environmental cues (Shiffman et al., in press).
We recently described a sample of adult ITS who smoked an average of 4.4 days per week, consuming 4.4 cigarettes on those days (Shiffman et al., 2012c). These individuals had been smoking for 19 years, having consumed over 40,000 cigarettes. ITS’ nicotine intake was in line with their smoking rate, and their nicotine metabolism was normal (Shiffman et al., 2014a). A study using Ecological Momentary Assessment (EMA; Shiffman, 2009) to assess smoking patterns in real time in real-world contexts showed that ITS’ smoking patterns differed from those of daily smokers (Shiffman et al., 2014b). In particular, ITS’ smoking was more closely related to being in a bar, socializing, with friends and acquaintances, drinking, and being around others who are also smoking.
The EMA findings suggest that ITS might be “social smokers” who smoke primarily - or even solely - for social reasons, perhaps representing a particularly strong form of stimulus control. Indeed, some authors have equated non-daily smoking with social smoking, calling all non-daily smokers social smokers, even without any evidence that non-daily smoking is motivated by social factors (Philpot et al., 1999). The social-smoking hypothesis has important theoretical and clinical implications, emphasizing as it does social and extrinsic motives for smoking, and minimizing intrinsic motives, including those related to pharmacological effects of nicotine. If ITS are social smokers, their smoking patterns and motives might be regarded as analogous to those of young teens who are just beginning to experiment with smoking as a means of fitting in with peers (Aloise-Young et al., 1994), and whose smoking motives are deemed to be more social than pharmacological. This would help explain why they smoke, even if not why they do not progress to daily and dependent smoking.
The most commonly-used definition characterizes as social smokers those who smoke predominantly when others are present (Biener and Albers, 2004; Gilpin, 2005; Levinson et al., 2007; Lisha et al., 2014; Moran et al., 2004). So, for example, young adult smokers, such as college students, are often characterized as social smokers, because many (27–70%) (Lisha et al., 2014; Moran et al., 2004; Waters, 2006) of them say they most often smoke with others (Waters, 2006); about 19% smoke only with others (Sutfin, 2009). To identify social smokers, surveys have simply asked smokers to estimate whether they smoke mainly with others (Biener and Albers, 2004; Gilpin, 2005; Levinson et al., 2007; Moran et al., 2004). However, evidence suggest that smokers are not able to accurately characterize their smoking patterns, due to limitations of autobiographical memory (Shiffman, 1993).
EMA methods (Shiffman, 2009), which have smokers record the circumstances of smoking in real time, in real-world settings, aim to assess smoking patterns without relying on recall or global self-perceptions. In this study, we sought to empirically assess the prevalence of social smoking among ITS. Using EMA methods, ITS recorded the context of each smoking occasion as it occurred (Shiffman et al., 2014b; “event-contingent” assessment; Shiffman et al., 2008). Consistent with the definition above, we identified social smokers as those who smoked the majority of cigarettes when they were with others. However, a definition that relies solely on the situations in which respondents smoke is logically incomplete, because it does not establish an association between smoking and social setting. This requires data on the base rate of being with others (i.e., even when not smoking; Paty et al., 1992). As in a case-control study, one needs to contrast the presence of others in smoking occasions (“cases”) against their presence in non-smoking occasions (“controls”). An EMA design that collects data on non-smoking occasions allows for such controls, and can thus evaluate the association between social context and smoking. In the present study, the context of non-smoking occasions was assessed by “beeping” participants at random when they were not smoking, and administering an assessment (Shiffman et al., 2014b; “signal-contingent” assessment; Shiffman et al., 2008). Taking this into account, the definition of a social smoker required that the likelihood of being with others be at least 50% higher when smoking than when not smoking. Using this definition, we estimated the proportion of ITS who qualified as social smokers.
While the defining feature of social smokers (SS+) is that they smoke with others, they are also thought to have very distinct smoking patterns in much broader respects. SS+ were the subjects of intense interest and research by the tobacco industry for many years in the ‘70s and ‘80s, as summarized by Schane et al. (2009b), which suggested that SS+ smoke primarily on evenings and weekends, especially after eating food or drinking alcohol in venues such as bars and restaurants. SS+ were also posited to be more sensitive to social constraints on smoking, and more sensitive to the needs of nonsmokers, but also more likely to impose their own restrictions on their smoking (Morris, 1989). They were hypothesized to identify less as smokers, to see themselves as less vulnerable to adverse effects of smoking, less nicotine-dependent and more confident they could quit. We addressed these hypotheses by contrasting the smoking patterns and other characteristics of SS+ to those of other ITS who did not qualify as social smokers (i.e., SS−).
2. METHODS
2.1. Subjects
Subjects were 204 ITS. The sample largely overlaps with that reported in several papers (Shiffman et al., 2012a, b, c, 2013a, b). To be eligible, volunteers had to be at least 21 years old, report smoking for at least three years, smoking at their current rate for at least three months, and not be planning to quit within the next month. ITS had to report smoking 4 to 27 days per month, with no restrictions on number of cigarettes. We oversampled African-American (AA) smokers, because national surveys indicate they are more likely to be ITS (Trinidad et al., 2009), and weighted the data to re-balance the ethnic mix.
2.2 Measures
2.2.1 Questionnaire assessments
Participants provided demographic data and detailed smoking histories that included past quit attempts, and past history of daily smoking for six months or more (Shiffman et al., 2012c), and multiple measures of nicotine dependence: the Fagerstrom Test of Nicotine Dependence (FTND; Heatherton et al., 1991), Nicotine Dependence Syndrome Scale (NDSS; Shiffman et al., 2004), Wisconsin Inventory of Smoking Dependence Motives (WISDM; Piper et al., 2004), and the Hooked on Nicotine Checklist (HONC; DiFranza et al., 2002). Participants also completed the Smoker Identity Questionnaire (Shadel and Mermelstein, 1996), which assesses how central smoking is to a person’s identity, reported the strength of smoking restrictions at work and at home (US Department of Commerce Census Bureau, 2006), and completed Time Line Follow-Back (TLFB; Sobell et al., 1979) assessments of daily cigarette consumption over an average of 73 days.
2.2.2 Biochemical assessments
Exhaled carbon monoxide (CO) levels were averaged over a median of 9 study visits (Shiffman et al., 2013b, 2014b). Cotinine was assayed in a urine specimen collected during a representative period of smoking (Shiffman et al., 2014a).
2.2.3 EMA assessment
Participants monitored their smoking using EMA procedures described in Shiffman et al. (2014b). Briefly, participants were provided with a palmtop-computer-based Electronic Diary (ED; Palm Tungsten E2), running specialized software designed for the study (invivodata; Pittsburgh, PA). For an average of 21.60 (SD = 4.11) days, participants monitored their smoking. Participants were to record each cigarette upon lighting it, upon which the ED administered an assessment of the surrounding circumstances. In addition to recording cigarettes, participants were “beeped” 3–4 times per day (M = 3.93) at random times when they were not smoking (never within 15 minutes of recording a cigarette), and administered the same assessment. Participants responded to 88% of the prompts within two minutes. Subjects completed a total of 22,576 EMA assessments covering 7,767 smoking and 14,809 non-smoking occasions. Contrasting these assessments allowed for analysis of the association between situational stimuli and smoking (Paty et al., 1992; Shiffman et al., 2008).
ED administered assessments on a touch-screen. Participants could select one or multiple responses for categorical questions, and moved a Visual Analog Scale (VAS) pointer for continuous ratings of craving and mood. Assessments (see Shiffman et al., 2014b for details) covered whether others were present, current location, activity, recent food or beverage consumption (including alcohol and caffeinated drinks), whether others were smoking (and whether those were part of the group of people they were with or just someone in view), and smoking restrictions (self-imposed or imposed by others or by law). Participants also rated 14 mood adjectives (able to focus; active; angry/frustrated; bored; calm/relaxed; difficulty concentrating; enthusiastic; happy; irritable; miserable; nervous/tense; quiet/sleepy; restless; sad) on a 0–100 scale, as well as items characterizing overall mood and arousal level. Using factor analyses, the mood data were summarized into scales represented by standardized T -scores (M = 50, SD = 10): Negative Affect (NA), Positive Affect (PA), Arousal (AR), and Inattention (IA).
2.3 Analysis
Subjects were considered social smokers (SS+) if they met two criteria evaluated from EMA data: (1) they were with others on at least 50% of assessed smoking occasions, and (2) they were with others at least 50% more often when smoking than when not smoking.
Analyses contrasted ITS who were deemed SS+ to those deemed SS−. Demographics and smoking history contrasts used t-tests. To assess and compare the relationship between situational factors and smoking among SS+ and SS− in EMA data, Generalized Estimating Equations (GEE; Zeger et al., 1988) were used to ‘predict’ smoking (vs. non-smoking) from situational variables (Shiffman et al., 2014b), while accounting for the multi-level structure of the data. We used a logit link and a first-order auto-regressive correlation structure. For continuous variables, we examined curvilinear as well as linear effects. Since SS+ and SS− were defined and differentiated by the presence of others when they were smoking, for predictor variables related to social interaction or the presence of others, we also performed a sub-analysis limited to occasions when others were present, so that differences in this variable were held constant. Because many relationships were tested, we emphasize interpretation of effects where p < .005.
3. RESULTS
On average, ITS smoked 62.23% of their cigarettes when others were present, but others were almost as frequently present (58.26% of occasions) when subjects were not smoking. Overall, 68.64% of ITS smoked 50% or more of their cigarettes when others were present, but most of these were not 50% more likely to smoke when others were present; only 19.08% of them met this criterion (the overall rate among all ITS was 15.42%). Altogether, 13.06%, 95% CI: [8.42%, 17.70%] of ITS were classified as social smokers (SS+), on the basis that they met both criteria. The SS+ group smoked 82% of their cigarettes with others, but were with others only 40% of the time they were not smoking. Their GEE-estimated odds of smoking were 5 times greater when they were with others (p < .0001). In contrast, those who were not SS+ (designated SS−), smoked an average of 59% of their cigarettes when with others, but were actually more often with others (61% of the time) when they were not smoking, such that the odds of smoking were estimated by GEE as 24% lower when they were with others (p < .005). In other words, among SS− the presence of others significantly suppressed smoking, highlighting the differences between the SS+ and SS− groups.
3.1 Demographics and licit drug use
SS+ and SS− were similar in age, gender, race, education, income, and marital status (Table 1). The two groups reported consuming similar amounts of caffeinated beverages, but SS+ reported almost double the alcohol consumption reported by SS−.
Table 1.
Demographic and smoking history comparisons between social smokers (SS+) and others (SS−)
| SS− % / M (SD) |
SS+ % / M (SD) |
|
|---|---|---|
| Age (years) | 35.82 (12.03) | 32.06 (13.04) |
| Gender (% female) | 50.31% | 53.13% |
| Race (unweighted) | ||
| European-American | 64.77% | 86.96% |
| Education | ||
| HS graduate or less | 17.34% | 4.43% |
| Some college or college graduate | 62.26% | 60.10% |
| Graduate work or degree | 20.40% | 35.47% |
| Income ($1,000s) | 29.30 (24.42) | 37.30 (27.47) |
| Marital status | ||
| Never married | 61.54% | 71.04% |
| Currently married or cohabiting | 23.86% | 21.86% |
| Divorced or widowed | 14.60% | 7.10% |
| Caffeine (drinks per week) | 3.61 (2.74) | 3.13 (1.77) |
| Alcohol (drinks per week) *** | 8.73 (8.66) | 14.40 (11.32) |
| Previously a daily smoker * | 67.72% | 46.31% |
| % days smoke (TLFB) **** | 66.43% | 48.27% |
| Cigarettes per day, on smoking days (TLFB) | 4.47 (2.95) | 3.78 (2.91) |
| Max. duration of abstinence (days) * | 4.81 (4.15) | 6.71 (3.41) |
| Smoke ‘light’ cigarettes *** | 38.11% | 75.83% |
| Self-reported addiction (1–5) | 3.12 (1.25) | 2.27 (1.35) |
| Fagerstrom Test for Nicotine Dependence **** | 1.45 (1.66) | 0.16 (0.40) |
| Nicotine Dependence Syndrome Scale * | 29.79 (6.51) | 26.69 (4.23) |
| Hooked on Nicotine Checklist * | 4.80 (2.68) | 3.57 (2.84) |
| WISDM-Primary Dependence Motives ** | 2.33 (1.12) | 1.74 (0.71) |
| WISDM-Secondary Dependence Motives | 2.76 (0.92) | 2.67 (0.86) |
| Time to first cigarette (minutes)**** | 219.27 (254.83) | 502.52 (302.42) |
| Smoke within 30 minutes of waking *** | 27.42% | 0.00% |
| Confidence in quitting (0–10) * | 3.52 (0.86) | 3.88 (0.77) |
| Smoker Identity Questionnaire (1–10) | 2.12 (1.70) | 2.16 (1.55) |
| Present self as smoker (1–10) | 5.52 (2.97) | 4.45 (2.86) |
| Home smoking bans * | 73.42% | 96.42% |
| Workplace smoking restrictions | 83.33% | 78.57% |
| Perceived cancer risk (1–5) | 2.64 (0.86) | 2.55 (0.72) |
| Cotinine (ng/mL) * | 540.00 (665.91) | 170.36 (170.17) |
| CO (ppm) ** | 7.34 (7.12) | 3.54 (2.83) |
p<0.05,
p<0.01,
p<0.005,
p<0.001
3.2 Smoking behavior, history, and dependence
SS+ were marginally less likely to have ever smoked daily, but this difference was not significant. SS+ smoked on fewer days per week, but smoked similar numbers of cigarettes on the days they did smoke. On TLFB, SS+ reported longer runs of abstinence than did SS−. Unexpectedly, SS+ reported being more likely to smoke so-called “light” cigarettes.
As hypothesized, SS+ rated themselves as less dependent, and were also scored as less dependent on FTND, NDSS, HONC, and on WISDM Primary dependence motives but not WISDM Secondary dependence motives. Moreover, there were very large differences in self-reported time to first cigarette and smoking within 30 minutes of waking, considered key measures of dependence (Baker et al., 2012, 2007); these differences persisted even when smoking rate was controlled. SS+ were modestly more confident they could quit smoking.
SS+ did not evidence weaker smoker identity. On questionnaires, SS+ were more likely to report having at least partial home bans, but reported similar strengths of workplace restrictions. SS+ did not differ in their perceived risk of getting cancer, even after controlling for the perceived risk of cancer to smokers in general.
3.3 Biochemical markers
SS+ had significantly lower cotinine and CO levels. However, this may have been due to underlying differences in cigarette consumption. When cigarette consumption was controlled, differences in cotinine and CO levels became only marginally significant.
3.4 EMA-Assessed Smoking patterns
3.4.1 Social setting and activity
The data (Table 2) show that although both groups’ odds of smoking were increased when they were with friends and acquaintances, this relationship was much stronger among SS+ than among SS−. In contrast, being with a spouse, family members, or co-workers tended to lower the odds of smoking similarly for SS+ and SS−. However, these differences were diminished when analysis was limited to situations where others were present, suggesting that who was present were less important.
Table 2.
Associations of smoking with situational characteristics, for social smokers (SS+) and other (SS−) intermittent smokers
| SS− (N=180) |
SS+ (N=24) | Difference/Interaction | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NS% | Cig% | OR (95%CI) |
p | NS% | Cig% | OR (95% CI) |
p | OR (95%CI) |
p | |
| Presence of others | ||||||||||
| With friends | 18.61 | 30.58 | 1.40 (1.15,1.70) |
0.0008 | 17.42 | 63.32 | 6.73 (4.34,10.44) |
<.0001 | 4.85 (2.98,7.90) |
<.0001 |
| When with others | 1.84 (1.50,2.25) |
<.0001 | 4.14 (2.27,7.56) |
<.0001 | 2.23 (1.17,4.25) |
0.0145 | ||||
| With acquaintances | 7.18 | 12.19 | 1.54 (1.19,1.98) |
0.0009 | 3.21 | 14.01 | 5.64 (3.18,10.01) |
<.0001 | 3.91 (2.11,7.27) |
<.0001 |
| When with others | 1.85 (1.45,2.37) |
<.0001 | 3.26 (1.75,6.06) |
0.0002 | 1.80 (0.93,3.49) |
0.0836 | ||||
| With family | 17.74 | 12.98 | 0.87 (0.68,1.11) |
0.2672 | 7.74 | 8.38 | 0.98 (0.50,1.92) |
0.9427 | 1.13 (0.56,2.30) |
0.7255 |
| When with others | 1.03 (0.81,1.30) |
0.8347 | 0.45 (0.24,0.83) |
0.0113 | 0.44 (0.22,0.86) |
0.0165 | ||||
| With coworkers | 13.18 | 7.93 | 0.45 (0.35,0.58) |
<.0001 | 9.67 | 6.46 | 0.51 (0.30,0.87) |
0.0128 | 1.13 (0.59,2.13) |
0.7172 |
| When with others | 0.49 (0.38,0.64) |
<.0001 | 0.23 (0.13,0.40) |
<.0001 | 0.46 (0.24,0.88) |
0.0180 | ||||
| With spouse | 18.49 | 17.82 | 0.83 (0.62,1.12) |
0.2276 | 7.50 | 9.94 | 0.85 (0.36,1.99) |
0.7106 | 1.02 (0.40,2.55) |
0.9736 |
| When with others | 0.96 (0.71,1.29) |
0.7714 | 0.38 (0.15,0.95) |
0.0386 | 0.39 (0.15,1.05) |
0.0614 | ||||
| Others smoking | ||||||||||
| Others’ smoking | ||||||||||
| No others smoking | 89.22 | 61.59 | 0.22 (0.18,0.28) |
<.0001 | 93.30 | 31.36 | 0.04 (0.03,0.07) |
<.0001 | 0.19 (0.11,0.32) |
<.0001 |
| When with others | 0.14 (0.11,0.18) |
<.0001 | 0.05 (0.03,0.08) |
<.0001 | 0.33 (0.18,0.61) |
0.0004 | ||||
| Others smoking in group | 7.28 | 27.84 | 4.50 (3.53,5.74) |
<.0001 | 4.33 | 56.35 | 25.12 (14.33,44.03) |
<.0001 | 5.54 (3.01,10.20) |
<.0001 |
| When with others | 6.59 (5.15,8.43) |
<.0001 | 18.35 (10.04,33.55) |
<.0001 | 2.76 (1.45,5.26) |
0.0020 | ||||
| Others smoking in view | 5.02 | 18.85 | 4.13 (3.32,5.15) |
<.0001 | 3.22 | 26.49 | 10.02 (5.97,16.80) |
<.0001 | 2.53 (1.43,4.49) |
0.0015 |
| When with others | 4.94 (3.88,6.29) |
<.0001 | 7.35 (4.25,12.71) |
<.0001 | 1.50 (0.82,2.74) |
0.1859 | ||||
| Combinations of others smoking (ref=no others smoking) | ||||||||||
| Both in group and in view | 1.52 | 8.28 | 8.37 (5.90,11.88) |
<.0001 | 0.85 | 14.20 | 53.16 (23.39,120.79) |
<.0001 | 6.19 (2.56,14.97) |
<.0001 |
| When with others | 13.46 (9.27,19.55) |
<.0001 | 43.83 (19.38,99.09) |
<.0001 | 3.17 (1.32,7.64) |
0.0100 | ||||
| In group only | 5.76 | 19.56 | 4.05 (3.04,5.40) |
<.0001 | 3.48 | 42.15 | 25.95 (13.88,48.52) |
<.0001 | 6.38 (3.22,12.64) |
<.0001 |
| When with others | 6.43 (4.81,8.59) |
<.0001 | 21.09 (10.52,42.25) |
<.0001 | 3.29 (1.57,6.91) |
0.0016 | ||||
| In view only | 3.50 | 10.58 | 3.53 (2.80,4.45) |
<.0001 | 2.37 | 12.29 | 9.84 (5.53,17.51) |
<.0001 | 2.74 (1.48,5.09) |
0.0014 |
| When with others | 4.70 (3.57,6.17) |
<.0001 | 9.73 (4.67,20.27) |
<.0001 | 2.06 (0.94,4.50) |
0.0703 | ||||
| Location | ||||||||||
| Home | 52.89 | 44.21 | 0.94 (0.79,1.13) |
0.5237 | 53.69 | 23.59 | 0.35 (0.21,0.58) |
<.0001 | 0.36 (0.21,0.62) |
0.0002 |
| Workplace | 16.35 | 10.34 | 0.46 (0.37,0.59) |
<.0001 | 19.43 | 5.53 | 0.29 (0.12,0.70) |
0.0054 | 0.68 (0.26,1.79) |
0.4390 |
| Other’s home | 9.54 | 9.95 | 0.97 (0.78,1.19) |
0.7531 | 5.36 | 18.68 | 2.92 (1.96,4.34) |
<.0001 | 3.04 (1.95,4.75) |
<.0001 |
| Bar | 1.39 | 10.17 | 6.00 (4.13,8.72) |
<.0001 | 3.54 | 30.83 | 12.02 (7.10,20.35) |
<.0001 | 2.04 (1.06,3.91) |
0.0323 |
| Restaurant | 1.88 | 1.80 | 0.77 (0.59,1.01) |
0.0625 | 1.83 | 1.36 | 1.28 (0.70,2.32) |
0.4197 | 1.64 (0.84,3.21) |
0.1502 |
| Outside | 8.98 | 16.98 | 1.98 (1.67,2.34) |
<.0001 | 6.83 | 12.65 | 1.68 (1.02,2.76) |
0.0402 | 0.84 (0.50,1.42) |
0.5238 |
| Vehicle | 4.67 | 3.73 | 0.72 (0.52,1.00) |
0.0473 | 4.70 | 1.95 | 0.44 (0.20,0.94) |
0.0335 | 0.58 (0.25,1.35) |
0.2054 |
| Other | 4.30 | 2.82 | 0.52 (0.41,0.67) |
<.0001 | 4.63 | 5.43 | 1.02 (0.48,2.16) |
0.9685 | 1.88 (0.80,4.41) |
0.1483 |
| Activity | ||||||||||
| Work | 27.63 | 16.29 | 0.51 (0.43,0.59) |
<.0001 | 32.95 | 10.44 | 0.29 (0.18,0.47) |
<.0001 | 0.56 (0.31,1.00) |
0.0501 |
| Controlling smoking restrictions | 0.58 (0.50,0.68) |
<.0001 | 0.34 (0.21,0.56) |
<.0001 | 0.56 (0.32,0.98) |
0.0437 | ||||
| Leisure | 36.33 | 35.53 | 1.01 (0.90,1.13) |
0.9095 | 36.22 | 26.07 | 0.79 (0.54,1.15) |
0.2145 | 0.77 (0.51,1.17) |
0.2257 |
| Interacting w/ others | 17.40 | 23.31 | 1.31 (1.09,1.59) |
0.0049 | 9.54 | 35.37 | 4.17 (3.11,5.59) |
<.0001 | 3.32 (2.33,4.71) |
<.0001 |
| Socializing | 12.49 | 19.47 | 1.47 (1.19,1.81) |
0.0003 | 7.62 | 33.31 | 4.95 (3.53,6.94) |
<.0001 | 3.51 (2.36,5.22) |
<.0001 |
| When with others | 1.84 (1.52,2.23) |
<.0001 | 2.78 (1.91,4.06) |
<.0001 | 1.53 (1.00,2.35) |
0.0519 | ||||
| Between activities | 16.13 | 21.11 | 1.33 (1.16,1.52) |
<.0001 | 14.09 | 9.86 | 0.76 (0.53,1.09) |
0.1304 | 0.54 (0.36,0.82) |
0.0041 |
| Other activities | 11.96 | 10.33 | 0.86 (0.71,1.03) |
0.1040 | 6.77 | 4.21 | 0.70 (0.38,1.30) |
0.2596 | 0.86 (0.44,1.69) |
0.6682 |
| Drinking alcohol | 3.61 | 16.17 | 3.50 (2.57,4.77) |
<.0001 | 6.10 | 43.00 | 13.14 (8.64,19.99) |
<.0001 | 3.61 (2.14,6.09) |
<.0001 |
| Controlling for bar, others smoking, restrictions, and leisure |
1.71 (1.28,2.27) |
0.0002 | 3.19 (1.47,6.92) |
0.0034 | 2.75 (1.47,5.15) |
0.0016 | ||||
| Smoking restrictions (ref=allowed) | ||||||||||
| Discouraged | 17.97 | 11.92 | 0.47 (0.34,0.64) |
<.0001 | 16.82 | 9.07 | 0.29 (0.14,0.59) |
0.0006 | 0.62 (0.29,1.34) |
0.2280 |
| Forbidden* | 21.73 | 10.51 | 0.30 (0.23,0.38) |
<.0001 | 20.51 | 6.85 | 0.20 (0.11,0.34) |
<.0001 | 0.62 (0.32,1.18) |
0.1420 |
| Reason forbidden (ref=not forbidden) | ||||||||||
| By law | 12.06 | 6.82 | 0.32 (0.25,0.42) |
<.0001 | 11.23 | 4.38 | 0.24 (0.13,0.48) |
<.0001 | 0.74 (0.36,1.54) |
0.4236 |
| By other’s rule | 15.90 | 7.87 | 0.34 (0.25,0.46) |
<.0001 | 11.75 | 6.53 | 0.33 (0.17,0.65) |
0.0012 | 0.95 (0.43,2.08) |
0.8923 |
| By own rule | 11.74 | 7.74 | 0.48 (0.32,0.71) |
0.0003 | 14.35 | 5.01 | 0.14 (0.04,0.54) |
0.0039 | 0.31 (0.08,1.11) |
0.0722 |
| When at home | 0.68 (0.42,1.10) |
0.1165 | 0.33 (0.09,1.21) |
0.0946 | 0.49 (0.12,1.93) |
0.3085 | ||||
SS+ and SS− did not differ in the percentage of time they were in smoking-restricted environments, either when smoking, or when not smoking.
The previous data characterize who was present, without regard to whether they were smoking. Examining others’ smoking behavior, we found that smoking by others in the participant’s immediate social group (e.g., people they are out to dinner with) increased the odds of SS+ smoking 25-fold, compared to 5-fold for SS−. The contrast was less marked when we considered the role of others smoking merely in view, which increased the odds of smoking 10-fold among SS+, but 4-fold among SS−. Conversely, being with others who were not smoking suppressed SS+’s smoking 5 times more than it did SS−’s smoking. Limiting the analysis to situations where others were present clarified these effects: others smoking in view affected SS+’s and SS−’s smoking similarly, whereas others in the subject’s group smoking still had a 2.8-times greater effect on SS+.
Both groups were more likely to smoke when interacting with others, especially when socializing, but this relationship was much stronger among SS+ (OR = 5.0 vs 1.5); however, this effect largely dissipated (p < .06) when the analysis was limited to situations where others were present. Other kinds of social interactions (e.g., a work meeting) were not associated with smoking in either group, and did not differentiate the groups.
3.4.2 Time and place
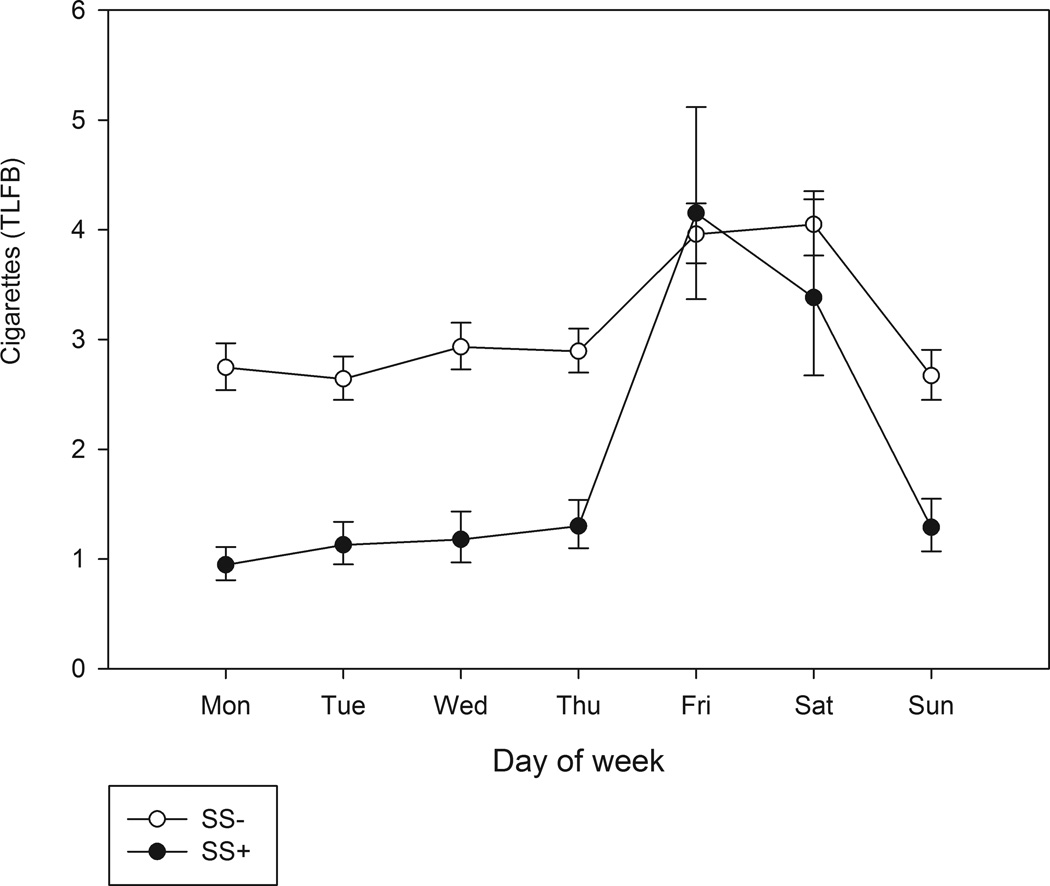
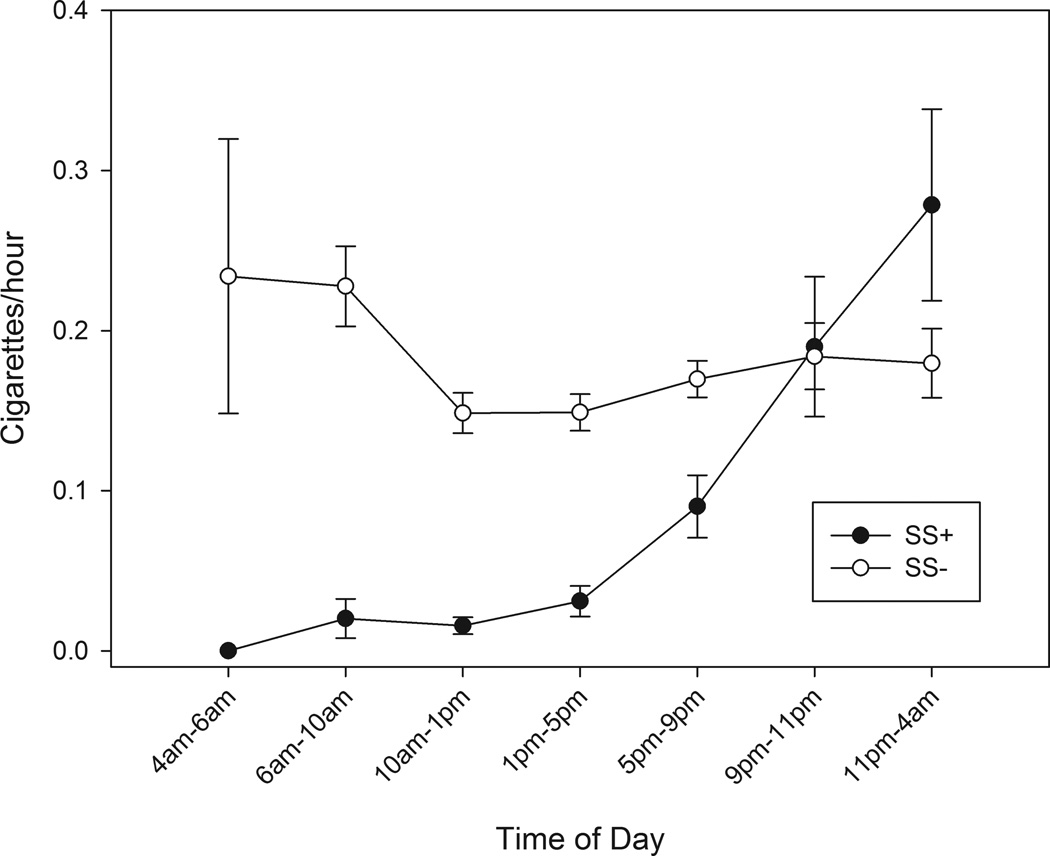
As seen in Figure 1, which is based on 10 weeks of TLFB data, smoking peaked on weekend days for both groups, but much more so for SS+ (p < .006). (EMA data, based only on three weeks, showed a similar but weaker pattern, with a distinct peak on Fridays.) SS+’s smoking was concentrated in the evening and nighttime. As Figure 2, based on EMA data, shows, SS+ rarely smoked in the morning or, indeed, before 5 p.m.; their cigarette consumption rose steeply after 5 p.m., peaking in the late hours after 11 p.m. In contrast, SS−’s smoking was highest in the morning, resulting in a significant difference in temporal patterns.
Fig 1.
Average number of cigarettes smoked by day of week, for social smokers (SS+) versus those who did not qualify as social smokers (SS−). Smoking data was obtained via Timeline Follow-back self-report.
Fig 2.
Average number of cigarettes smoked per hour over the course within time blocks over the course of a day, for social smokers (SS+) versus those who did not qualify as social smokers (SS−). Smoking data was obtained via Ecological Momentary Assessment.
For SS+, being at home was associated with a significantly lower probability of smoking, while being home did not change SS−’s risk of smoking. Conversely, being at another person’s home was associated with a 3-fold increase in the odds of SS+ smoking, but tended to be associated with a lower likelihood of smoking among SS−. Being in a bar was associated with increased smoking in both groups, with no significant group differences. Other environments, such as being in a restaurant or other locations also did not differentiate SS+ and SS−.
3.4.3 Activities and consumption
Generally, neither leisure nor work activities differentially influenced SS+’s and SS−’s smoking. However, interacting with others was more associated with smoking among SS+, even when limiting the analysis to when others were present. Being in between activities was more associated with SS−’s smoking than SS+’s smoking.
Although consuming alcohol was associated with increased risk of smoking in both groups, the relationship was much stronger among SS+, where drinking increased the odds of smoking 13-fold, in contrast to SS−, where the increase was 3.5-fold. Even after controlling for being in a bar, others smoking, smoking regulations, and engaging in leisure, alcohol still was a significantly more important factor in SS+’s smoking than in SS−’s smoking.
3.4.4 Smoking restrictions
Smoking restrictions were strongly associated with suppression of smoking, for both groups. SS+ were no more likely to report being restricted by their own rules (vs. legal restrictions) than were SS−, even when at home. Further, SS+ and SS− did not differ in exposure to smoking restrictions, either when smoking or when assessed on random nonsmoking occasions.
3.4.5 Mood
SS+ and SS− did not differ in the relationships of mood or arousal to smoking (Table 3). However, there were differences by attention disturbance: among SS+, the likelihood of smoking increased with increasing attention disturbance, then leveled off. In contrast, SS− were significantly less likely to smoke as attention disturbance increased, resulting in significant interactions in linear and quadratic trends.
Table 3.
Associations between craving and mood and smoking, among social smokers (SS+) and other (SS−) intermittent smokers
| SS− (N = 180) | SS+ (N = 24) | Interaction/Difference | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NS - Mean(SD) |
Cig - Mean(SD) |
OR (CI) | p | NS - Mean(SD) |
Cig - Mean(SD) |
OR (CI) | p | OR (CI) | p | |
| Negative affect | 52.10 (7.12) | 52.21 (7.45) | 53.30 (8.17) | 51.93 (7.35) | ||||||
| Linear | 0.90 (0.80,1.00) | 0.0515 | 1.06 (0.85,1.32) | 0.6213 | 1.21 (0.94,1.55) | 0.1467 | ||||
| Quadratic | 1.10 (1.04,1.16) | 0.0004 | 0.84 (0.71,1.00) | 0.0464 | 1.01 (0.99,1.04) | 0.2790 | ||||
| Positive affect | 50.78 (6.11) | 51.42 (6.09) | 49.99 (7.16) | 52.80 (6.56) | ||||||
| Linear | 1.14 (1.02,1.28) | 0.0171 | 1.35 (1.01,1.81) | 0.0413 | 1.18 (0.85,1.63) | 0.3298 | ||||
| Quadratic | 1.00 (0.94,1.07) | 0.8940 | 0.96 (0.81,1.14) | 0.6247 | 1.02 (0.98,1.05) | 0.3681 | ||||
| Arousal | 48.70 (4.55) | 50.11 (4.91) | 48.56 (4.79) | 51.70 (6.65) | ||||||
| Linear | 1.10 (1.02,1.18) | 0.0163 | 1.24 (1.03,1.49) | 0.0236 | 1.15 (0.93,1.41) | 0.2018 | ||||
| Adjusted for time of day | 1.12 (1.04,1.22) | 0.0051 | 1.58 (1.30,1.92) | <.0001 | 1.14 (0.93,1.40) | 0.2166 | ||||
| Quadratic | 0.90 (0.85,0.94) | <.0001 | 0.91 (0.81,1.02) | 0.0958 | 1.02 (0.99,1.04) | 0.1614 | ||||
| Adjusted for time of day | 0.89 (0.85,0.94) | <.0001 | 0.83 (0.75,0.93) | 0.0011 | 1.02 (0.99,1.04) | 0.1628 | ||||
| Attention disturbance |
53.05 (9.29) | 52.54 (9.08) | 55.21 (9.85) | 54.12 (9.20) | ||||||
| Linear | 0.86 (0.79,0.92) | <.0001 | 1.10 (0.97,1.25) | 0.1244 | 1.30 (1.11,1.51) | 0.0008 | ||||
| Quadratic | 1.02 (0.98,1.06) | 0.2616 | 0.88 (0.82,0.94) | 0.0002 | 1.02 (1.00,1.03) | 0.0057 | ||||
| Craving | 28.61 (20.05) | 61.48 (21.57) | 16.27 (16.06) | 57.45 (27.24) | ||||||
| Linear | 1.43 (1.35,1.52) | <.0001 | 1.81 (1.65,2.00) | <.0001 | 1.27 (1.13,1.42) | <.0001 | ||||
| Quadratic | 0.99 (0.97,1.01) | 0.3644 | 0.96 (0.93,1.00) | 0.0680 | 1.02 (1.01,1.04) | 0.0017 | ||||
Affect scales were standardized scores normed to mean = 50, SD = 10. Craving was assessed on a 0–100 scale; values were divided by 10 to estimate the OR, so it represents the change due to 10 points in the craving scale.
3.4.6 Craving
Craving was more closely tied to smoking among SS+, but not because their average craving was higher when smoking (it wasn’t; p > 0.36), but rather because it was lower when not smoking (p < .0006; table 3). Moreover, among SS+, the probability of smoking rose more steeply until craving reached an intensity of 80 (out of 100), then leveled off (a quadratic effect), which was not the case among SS−.
4. DISCUSSION
This study identified social smokers by their behavior, as recorded in detail in cigarette-by-cigarette EMA diary entries, and characterized their smoking patterns and characteristics. Whereas some literature implies or asserts that most or all ITS are social smokers, the data demonstrate that only a small minority of ITS – about one out of eight – are social smokers, as identified by a behavioral definition derived from the literature, and assessed through real-time records of smoking. This makes it less likely that most ITS smoking is motivated by extrinsic social motivations, which leaves open the door for intrinsic motivations, including nicotine-seeking.
We used a definition of social smokers drawn conceptually from the literature (Biener and Albers, 2004; Gilpin, 2005; Levinson et al., 2007; Lisha et al., 2014; Moran et al., 2004) but modified to draw on the strength of EMA data in evaluating smoking patterns. The definition seemed validated both by the striking differences in smoking patterns, but also by the fact that those defined as SS− were actually significantly less likely to smoke when others were present (which was not a requirement of the definition).
While the data suggested that social smoking was not prevalent among ITS, it strongly validated the concept of social smoking. Although the definition of social smoking concerned only the presence of others, the smokers identified in this way showed a different smoking pattern on many other dimensions. Compared to other ITS, social smokers were more likely to smoke on the weekends, in the evening and late at night, out at a bar or someone else’s home, and while socializing and drinking with friends or acquaintances who were themselves smoking. The fact that the influences on SS+ were truly social is highlighted by the fact that their smoking was particularly likely to increase when the smoking occurred among the people they were with socially, more so than just among strangers they could see smoking.
The strength of SS+’s patterns is also notable: for example, SS+ smoked nearly half their cigarettes when drinking, and a third while socializing. SS+ also differed from SS− in that they smoked less frequently, voluntarily engaged in longer runs of abstinence, and were less tobacco dependent, consistent with Lisha et al.’s (2014) observations among young adults. SS+ also had lower average craving when they weren’t smoking, but their average craving when smoking was similar to SS−. That is, SS+ ITS had highly coherent smoking patterns that fit existing descriptions of social smoking (Lisha et al., 2014; Schane et al., 2009a).
Some hypotheses about social smokers were not supported by the data. Whereas social smoking is often seen as a behavior of the young as they are learning to smoke, we found no difference between SS+ and SS− in age or years of smoking. The SS+ in this study averaged 32 years old and had been smoking for 15 years, demonstrating that social smoking occurs in mature smokers, though the prevalence of social smoking might have been higher in a younger cohort. Whereas social smoking is seemingly celebratory, and therefore might have been expected to be associated with positive affect, we found no distinct relationship between affect and smoking among social smokers – neither positive affect nor negative affect had any particular effects on social smokers’ smoking. Inferences from tobacco industry research (Morris, 1988, 1989) that SS+ would exercise more deliberate control over their own smoking were not confirmed, either in EMA data, which tracked self-imposed restrictions in real time, or in questionnaire data on home smoking bans. We also did not observe reliable differences in how much SS+ (vs SS−) identified as smokers.
One might expect that exposure to more smoking restrictions could be a strong influence on social smoking, by structuring smoking situations in one direction or the other, either forcing smoking to occur with others, e.g., in smoking-allowed areas, or by inhibiting smoking when others are present, e.g., in workplaces, restaurants, etc. However, SS+ and SS− did not differ in exposure to smoking restrictions, suggesting that such exposures are not a major driver (or confounder).
Seeing that only a fraction of ITS were social smokers raises the question of what smoking patterns characterize the remainder. Other than identifying non-social smokers as smokers who are more likely to smoke alone than with others (perhaps a circular conclusion, given the definition of social smokers), the current analyses identified few unique patterns associated with non-social smokers. The most striking finding is that these ITS smoke more in the morning than later in the day – a pattern usually attributed to nicotine dependence, and associated with the need to replenish nicotine after clearing most of it overnight. This is unlikely to explain morning smoking among ITS, given that they abstain for days at a time (Shiffman et al., 2012b), and clear nicotine normally (Shiffman et al., 2014a). It seems likely that SS− are heterogeneous, with subsets showing different patterns that could potentially be identified by methods like cluster analysis.
Importantly, despite the strong contrasts observed between the smoking patterns of SS+ and SS−, some of the situational drivers of smoking that were prominent among SS+ were also observed among the SS−. For example, even though socializing, being with people who were smoking, and drinking alcohol were particularly strong correlates of smoking among SS+, correlations, albeit weaker ones, were also observed among the SS− and, indeed, among daily smokers (Shiffman et al., 2002, 2014b). Thus, it might be useful to think of social smoking as an influence on smoking that can vary continuously across a range of smokers, as well as a discrete group of smokers.
Like any study, ours was subject to certain limitations. We studied adult non-daily smokers in a convenience sample; ideally, the results would be replicated in broader samples, and among adolescent smokers, where the developmental trajectory of social smoking and dependence might be observed. We used a particular definition of social smoking; unlike surveys on social smoking, we required that smoking be substantially linked to the presence of others; other definitions might yield somewhat different results. Especially when analyzing subject characteristics, the study’s power was limited, particularly because of the uneven sample sizes: Only medium or larger effects (ES=0.59; Cohen, 1992) could be detected with 80% power. Power was much greater for the EMA analyses, as these were based on over 22,000 observations from the 204 subjects. Our study also had some unique strengths, notably our use of real-time real-world data collected via EMA to define social smoking, rather than relying on global self-report.
In summary, the data indicated that most ITS are not social smokers. It is possible that, like, daily smokers, they are motivated and reinforced by pharmacological effects of smoking. This would be consistent with analyses indicating that some ITS exhibit a modicum of dependence by traditional measures (Shiffman et al., 2012b) and with epidemiological data showing that many ITS have surprisingly low success rates in quitting (Tindle and Shiffman, 2011). Particularly because ITS do suffer morbidity and mortality due to smoking (Luoto et al., 2000; Schane et al., 2010), this in turn suggests that ITS should be encouraged to quit, and given help to quit when needed, as suggested by Schane et al. (2009a). In short, the smoking of most ITS cannot be dismissed as a mere “social habit.” We need to better understand what motivates and maintains ITS’ smoking, and how best to help them stop smoking.
Highlights for “Social Smoking among Intermittent Smokers”.
Only 13% of adult non-daily smokers were social smokers, based on Ecological Momentary Assessment (EMA) data
Social smokers were less dependent than other non-daily smokers
Social smokers differed dramatically in EMA-recorded smoking patterns
Smoking among non-daily smokers is not primarily motivated by social factors
Acknowledgements
The authors are grateful to Alex Cardy for assistance with manuscript preparation.
Role of Funding Source
This work was supported by grant R01-DA020742 (Shiffman) from the National Institutes of Health, National Institute on Drug Abuse. Additional support was provided by National Science Foundation Graduate Research Fellowship (Dunbar), National Cancer Institute grant (R25-CA057703-15 –Weissfeld (Dunbar), and Cancer Council Tasmania grant F0019238 (Ferguson). None of the aforementioned sources of funding had any further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Author Shiffman designed the study and authors Dunbar, Ferguson, Tindle, and Scholl participated in the development of the protocol. Author Li undertook the statistical analysis, and author Shiffman wrote the first draft of the manuscript and interpreted the results. All authors contributed to manuscript text and have approved the final manuscript.
Conflict of Interest
Dr. Shiffman consults to and has an interest in eRT, which provides electronic diary services for clinical research.
REFERENCES
- Aloise-Young PA, Graham JW, Hansen WB. Peer influence on smoking initiation during early adolescence: a comparison of group members and group outsiders. J. Appl. Psychol. 1994;79:281–287. doi: 10.1037/0021-9010.79.2.281. doi: http://dx.doi.org/10.1037/0021-9010.79.2.281. [DOI] [PubMed] [Google Scholar]
- Baker TB, Breslau N, Covey L, Shiffman S. DSM criteria for tobacco use disorder and tobacco withdrawal: a critique and proposed revisions for DSM-5. Addiction. 2012;107:263–275. doi: 10.1111/j.1360-0443.2011.03657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarhty DE, Bolt DM, Smith SS, Kim SY, Colby S, Conti D, Giovino GA, Hatsukami D, Hyland A, Krishnan-Sarin S, Niaura R, Perkins KA, Toll BA the Transdisciplinary Tobaccu Use Research Center (TTURC) Tobacco Dependence. Time to first cigarette in the morning as an index of ability to quit smoking: implications for nicotine dependence. Nicotine Tob. Research. 2007;9(Suppl. 4):S555–S570. doi: 10.1080/14622200701673480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz N. Nicotine addiction. N. Engl. J. Med. 2010;362:2295–2203. doi: 10.1056/NEJMra0809890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Porchet H, Jacob P., III . Pharmacokinetics, metabolism, and pharmacodynamics of nicotine. In: Wonnacott S, Russell MAH, Stolerman IP, editors. Nicotine Psychopharmacology: Molecular, Cellular, and Behavioral Aspects. New York: Oxford University Press; 1990. pp. 112–157. [Google Scholar]
- Biener L, Albers AB. Young adults: vulnerable new targets of tobacco marketing. Am. J. Public Health. 2004;94:326–330. doi: 10.2105/ajph.94.2.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Behavioral Risk Factor Surveillance System Survey Data. 2008a Retrieved from: http://www.cdc.gov/brfss/annual_data/annual_2008.htm.
- Centers for Disease Control and Prevention (CDC) Cigarette smoking among adults --United States, 2007. MMWR. 2008b;57:1221–1226. [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis. Curr. Dir. Psychol. Sci. 1992;1:98–101. [Google Scholar]
- DiFranza JR, Savageau JA, Fletcher K, Ockene JK, Rigotti N, McNeill AD, Coleman M, Wood C. Measuring the loss of autonomy over nicotine use in adolescents: the Development and Assessment of Nicotine in Youths (DANDY) Study. Arch. Pediatr. Adolesc. Med. 2002;156:397–403. doi: 10.1001/archpedi.156.4.397. [DOI] [PubMed] [Google Scholar]
- Gilpin EA, White VM, Pierce JP. How effective are tobacco industry bar and club marketing efforts in reaching young adults. Tob. Control. 2005;14:186–192. doi: 10.1136/tc.2004.009712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br. J. Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Levinson AH, Campo S, Gascoigne J, Jolly O, Zakharyan A, Tran ZV. Smoking, but not smokers: identity among college students who smoke cigarettes. Nicotine Tob. Res. 2007;9:845–852. doi: 10.1080/14622200701484987. [DOI] [PubMed] [Google Scholar]
- Lindstrom M, Ostergren P. Intermittent and daily smokers: two different socioeconomic patterns, and diverging influence of social participation. Tob. Control. 2001;10:258–266. doi: 10.1136/tc.10.3.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisha NE, Delucchi KL, Ling PM, Ramo DE. Prevalence and correlates of social smoking in young adults: comparisons of behavioral and self-identified definitions. Nicotine Tob. Res. 2014 doi: 10.1093/ntr/ntu242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luoto R, Uutela A, Puska P. Occasional smoking increases total and cardiovascular mortality among men. Nicotine Tob. Res. 2000;2:133–139. doi: 10.1080/713688127. [DOI] [PubMed] [Google Scholar]
- Moran S, Wechsler H, Rigotti N. Social smoking among US college students. Pediatrics. 2004;114:1028–1034. doi: 10.1542/peds.2003-0558-L. [DOI] [PubMed] [Google Scholar]
- Morris P. 1988 00Bates No. 2057041812/1833. legacy.library.ucsf.edu/tid/acp96e00. [Google Scholar]
- Morris P. Casual (23 percent) social smoker. 1989 Bates No. 2057041843/1846. http://legacy.library.ucsf.edu/tid/ccp96e00. [Google Scholar]
- Paty JA, Kassel JD, Shiffman S. The importance of assessing base rates for clinical studies: an example of stimulus control of smoking. In: deVries MW, editor. The Experience of Psychopathology: Investigating Mental Disorders in their Natural Settings. New York: Cambridge University Press; 1992. pp. 347–352. [Google Scholar]
- Philpot SJ, Ryan SA, Torre LE, Wilcox HM, Jalleh G, Jamrozik K. Effect of smoke-free policies on the behavior of social smokers. Tob. Control. 1999;8:278–281. doi: 10.1136/tc.8.3.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Piasecki TM, Federman EB, Bolt DM, Smith SS, Fiore MC, Baker TB. A multiple motives approach to tobacco dependence: the Wisconsin Inventory of Smoking Dependence Motives (WISDM-68) J. Consult. Clin. Psychol. 2004;72:139–154. doi: 10.1037/0022-006X.72.2.139. [DOI] [PubMed] [Google Scholar]
- Schane RE, Glantz SA, Ling PM. Nondaily and social smoking: an increasingly prevalent pattern. Arch. Intern. Med. 2009a;169:1742–1744. doi: 10.1001/archinternmed.2009.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schane RE, Glantz SA, Ling PM. Social smoking: implications for public health, clinical practice, and intervention research. Am. J. Prev. Med. 2009b;37:124–131. doi: 10.1016/j.amepre.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schane RE, Ling PM, Glantz SA. Health effects of light and intermittent smoking: a review. Circulation. 2010;121:1518–1522. doi: 10.1161/CIRCULATIONAHA.109.904235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadel WG, Mermelstein R. Individual differences in self-concept among smokers attepting to quit: validation and predictive utility of measures of the smoker self-concept and abstainer self-concept. Ann. Behav. Med. 1996;18:151–156. doi: 10.1007/BF02883391. [DOI] [PubMed] [Google Scholar]
- Shiffman S. Assessing smoking patterns and motives. J. Consult. Clin. Psychol. 1993;61:732–742. doi: 10.1037//0022-006x.61.5.732. [DOI] [PubMed] [Google Scholar]
- Shiffman S. Ecological Momentary Assessment (EMA) in studies of substance abuse. Psychol. Assess. 2009;21:486–497. doi: 10.1037/a0017074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Dunbar M, Kirchner T, Li X, Tindle H, Anderson S, Scholl S. Smoker reactivity to cues: effects on craving and on smoking behavior. J. Abnorm. Psychol. 2013a;122:264–280. doi: 10.1037/a0028339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Dunbar MS, Benowitz NL. A comparison of nicotine biomarkers and smoking patterns in daily and non-daily smokers. Cancer Epidemiol. Biomarkers Prev. 2014a;23:1264–1272. doi: 10.1158/1055-9965.EPI-13-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Dunbar MS, Kirchner TR, Li X, Tindle HA, Anderson SJ, Scholl SM, Ferguson SG. Cue reactivity in non-daily smokers: effects on craving and on smoking behavior. Psychopharmacology. 2013b;226:321–333. doi: 10.1007/s00213-012-2909-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Dunbar MS, Li X, Scholl SM, Tindle HA, Anderson SJ, Ferguson SG. Smoking patterns and stimulus control in intermittent and daily smokers. PLoS One. 2014b;9:e89911. doi: 10.1371/journal.pone.0089911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Dunbar MS, Scholl SM, Tindle HA. Smoking motives of daily and non-daily smokers: a profile analysis. Drug Alcohol Depend. 2012a;126:362–368. doi: 10.1016/j.drugalcdep.2012.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Dunbar M, Ferguson S. Stimulus control in intermittent and daily smokers. Psychol. Addict. Behav. in press doi: 10.1037/adb0000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Ferguson SG, Dunbar MS, Scholl SM. Tobacco dependence among intermittent smokers. Nicotine Tob. Res. 2012b;14:1372–1381. doi: 10.1093/ntr/nts097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Gwaltney CJ, Balabanis M, Liu KS, Paty JA, Kassel JD, Hickcox M, Gnys M. Immediate antecedents of cigarette smoking: an analysis from ecological momentary assessment. J. Abnorm. Psychol. 2002;111:531–545. doi: 10.1037//0021-843x.111.4.531. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Stone AA, Hufford M. Ecological momentary assessment. Annu. Rev. Clin. Psychol. 2008;4:1–32. doi: 10.1146/annurev.clinpsy.3.022806.091415. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Tindle H, Li X, Scholl S, Dunbar M, Mitchell-Miland C. Characteristics and smoking patterns of intermittent smokers. Exp. Clin. Psychopharmacol. 2012c;20:264–277. doi: 10.1037/a0027546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Waters AJ, Hickcox M. The Nicotine Dependence Syndrome Scale: a multi-dimensional measure of nicotine dependence. Nicotine Tob. Res. 2004;6:327–348. doi: 10.1080/1462220042000202481. [DOI] [PubMed] [Google Scholar]
- Sobell MB, Sobell LC, Maisto SA. Time-Line Follow-Back assessment method [TLFB] In: Lettieri DJ, Nelson JE, Sayers MA, editors. Alcoholism Treatment Assessment Research Instruments (NIAAA Ttreatment Handbook Series, Vol 2) Rockville, MD: National Institute on Alcoholism and Alcohol Abuse; 1979. pp. 167–188. [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2008 National Survey on Drug Use and Health: National Findings. Rockville, MD: Office of Applied Studies, NSDUH Series H-36, HHS Publication No. SMA 09-4434; 2009. [Google Scholar]
- Sutfin EL, Reboussin BA, TP McCoy, Wolfson M. Are college student smokers really a homogeneous group? A latent class analysis of college student smokers. Nicotine Tob. Res. 2009;11:444–454. doi: 10.1093/ntr/ntp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tindle HA, Shiffman S. Smoking cessation behavior among intermittent smokers versus daily smokers. Am. J. Public Health. 2011;101:e1–e3. doi: 10.2105/AJPH.2011.300186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinidad DR, Perez-Stable EJ, Emery SL, White MM, Grana RA, Messer KS. Intermittent and light daily smoking across racial/ethnic groups in the United States. Nicotine Tob. Res. 2009;11:203–210. doi: 10.1093/ntr/ntn018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Department of Commerce Census Bureau. National Cancer Institute and Centers for Disease Control and Prevention Co-sponsored Tobacco Use Special Cessation Supplement to the Current Population Survey (2003) 2006 http://riskfactor.cancer.gov/studies/tus-cps/
- Waters H, Harris K, Hall S, Nazir N, Waigandt A. Characteristics of social smoking among college students. J. Am. Coll. Health. 2006;55:133–139. doi: 10.3200/JACH.55.3.133-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeger SL, Liang K, Albert P. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988;44:1049–1060. [PubMed] [Google Scholar]