Abstract
Empirically based lifting criteria established by the National Institute for Occupational Safety and Health (NIOSH) to reduce the risk of overexertion injuries in the general US working population were evaluated for application to pregnant workers. This report proposes criteria to guide decisions by medical providers about permissible weights for lifting tasks performed at work over the course of an uncomplicated pregnancy. Our evaluation included an extensive review of the literature linking occupational lifting to maternal and fetal health. Although it has been 29 years since the American Medical Association’s Council on Scientific Affairs published its report on the Effects of Pregnancy on Work Performance, these guidelines continue to influence clinical decisions and workplace policies. Provisional clinical guidelines derived from the NIOSH lifting criteria that account for recent evidence for maternal and fetal health are presented and aim to improve the standard of care for pregnant workers.
Keywords: lifting, occupational hazard, pregnancy, recommendations
Clinicians play an important role in decisions concerning work activity restrictions during pregnancy. This role is underscored in the United States where federal regulations for occupational lifting are lacking and limited opportunities for paid antenatal leave are available.1,2 Findings from a recent survey show that recommendations to pregnant workers in physically demanding jobs vary widely, possibly because of limited clinician training in occupational health3 and the absence of current authoritative guidelines.4
To address the need for updated empirically based recommendations that can be uniformly applied, this paper presents provisional clinical guidelines for occupational lifting in pregnancy based on an extensive review of the literature linking occupational lifting to maternal and fetal health and application of the National Institute for Occupational Safety and Health (NIOSH) lifting equation.
Literature review criteria
An extensive review of the literature linking occupational lifting to maternal and fetal health was conducted to inform the application of the NIOSH lifting equation to pregnant workers. Using the PubMed MESH Thesaurus terms, pregnancy, lifting, occupational diseases, preeclampsia, hypertension, pregnancy complications, pelvis, sacroiliac joint, pubic symphysis, range of motion articular, joint instability, relaxin, estradiol, biomechanics, stress mechanical, occupational exposure, low back pain, human engineering, and gait, we conducted an electronic search of PubMed, Web of Science, Cochrane Library, the OSH References Collection, CINAHL, Directory of Published Proceedings, EMBASE, Google Scholar, PsycNET, ScienceDirect, CISDOC, OCLC First Search, EBSCOHost, and OSH References Collection. This electronic search was initially completed in July 2009 (inclusive of all preceding dates) and then updated in August 2012 (since July 2009). The search terms were used to search the Defense Technical Information Center and Google for possible unpublished research. The initial electronic database search was supplemented by manual searches of published reference lists, review articles, and conference abstracts.
Occupational lifting and fetal-maternal health outcomes
Several etiological mechanisms are thought to influence maternal and/or fetal health for pregnant women-working in jobs with high exertion demands, such as heavy manual lifting. These mechanisms include venous insufficiency, excessive intraabdominal pressure, ligament laxity, and increased demands on the musculoskeletal system because of fetal load. Venous insufficiency is thought to play a role in the relationship between occupational physical activity and fetal health (eg, growth retardation) 5,6 and preeclampsia.7 Mechanical compression, altered venous tone, and poor venous return from the lower extremities may be exacerbated by constrained postural demands (eg, prolonged standing, stooping), inducing conditions of fetal hypoxia. Increased intraabdominal pressure has been hypothesized to explain significant associations between forward flexion of the upper body (or stooping) and preterm delivery8 and spontaneous abortion.9
The bulk of the epidemiological evidence shows a small increased risk of lower birthweight for gestational age in relation to heavy physical work.10–12 The evidence is strongest in research involving women in developing countries, which may increase the likelihood of maternal nutrition influences but also may signal more strenuous working conditions coupled with more limited opportunities to avoid or reduce exposures.6
Evidence on the association between lifting and miscarriage also shows a generally consistent pattern of a slightly elevated significant risk, with odds ratios (ORs) most often in the range of 1.5–2.013–15; however, a Finnish case control study of physiotherapists found a notably higher OR of 3.5, 95% confidence interval (CI), 1.1–9.0 between heavy lifting (often related to patient transfers) and spontaneous abortion.16 Investigations on the relationship between occupational lifting and preterm birth is more limited than for other fetal health outcomes, and the findings more consistently suggest no association.17–20 For a more detailed summary of the epidemiological evidence related to fetal health outcomes, we recommend the systematic reviews conducted by Bonzini et al (2007)20 and the 2009 Guideline Development Group of the Royal College of Physicians.12
Fewer studies have investigated the association between maternal health outcomes and heavy physical work load, despite evidence showing a higher use of antenatal sick leave21,22 and hospital visits23 among those employed in heavy work. One previous study showed a positive association between heavy lifting (10–20 kg or 22–44 lb) in early pregnancy (occurring more than 20 times per week) and preeclampsia.24 Additionally, a 2-fold increased risk of preeclampsia was found for pregnant women with high physical activity at work (composite score).7
An explanatory model by Paul et al25 (1994) suggests that pregnancy-related musculoskeletal problems arise, at least in part, from reduced load-bearing capacity associated with joint laxity. Although the mechanisms underlying laxity are unknown, the condition presents early in pregnancy and persists beyond 6 weeks postpartum.26 The associated reduction in ligament rigidity is believed to weaken joint stability, increasing demand on stabilizing muscles.
Many researchers have identified laxity as a contributing factor in pregnancy-related pelvic girdle pain,27,28 low back pain,29 and knee pain,26,30–34 although direct evidence is lacking. Although the hormonal basis for laxity has been questioned, laxity itself is a well-established phenomenon that deserves further attention, especially in relation to short- and long-term maternal health consequences of occupational lifting and other physical job demands. The pregnancy-related musculoskeletal risk model by Paul et al (1994)25 also calls attention to increased load on the musculoskeletal system because of increased abdominal mass and the change in the center of mass.35,36
Low back and pelvic girdle pain
Low back pain (LBP) and pelvic girdle pain (PGP) are common during pregnancy, with LBP occurring in up to two thirds of pregnancies and PGP occurring in nearly 20%.37–39 Data from numerous studies show that LBP prevalence is most elevated in months 6 and 7.38–40 Because women often underreport LBP/PGP to their prenatal provider,41 the topic may not garner sufficient clinical attention. Two studies indicate that the prevalence of severe LBP and/or PGP symptoms ranges from 15% to 20%40,42; however, studies investigating antecedents for pregnancy-related low back and/or pelvic girdle pain have rarely considered occupational exposures. Importantly, severe cases of pregnancy-related LBP/PGP have been reported to trigger or exacerbate comorbid conditions, affecting patient well-being and functional status. Two studies report increased sleep disturbance and impaired daily living.41,43 Other research shows elevated depression among those with pregnancy-related LBP/PGP.44,45
Activity limitations resulting from LBP/PGP during pregnancy and the postpartum period have been shown to interfere with weight loss and resumption of leisure-time physical activity levels needed for health maintenance.46 Additionally, patients with both LBP and PGP have been found to be at greatest risk of persistent pain postpartum 47 and to experience greater disability.48 Although back pain spontaneously resolves postpartum for most,49 those with persistent pain were more likely to have had back pain prior to pregnancy, present with early onset of symptoms, and exhibit higher pain severity during pregnancy.50,51
Existing guidance on occupational lifting
For the past 29 years, clinical management for physical job activities, including lifting, has relied on the American Medical Association’s (AMA) Council on Scientific Affairs published guidance on the effects of pregnancy on work performance.52 These guidelines define permissible weight limits “that healthy employees with normal uncomplicated pregnancies should be able to perform…without undue difficulty or risk to the pregnancy” (Table 1). Evidence suggests that these guidelines continue to inform physician practice and workplace policy.4,53
TABLE 1.
1984 AMA recommended weight limits for occupational lifting during pregnancy
| Week of gestation | Intermittent liftinga
|
Repetitivea
|
||
|---|---|---|---|---|
| Metric | US customary | Metric | US customary | |
| 20 | – | – | >23 kg | >51 lb |
|
| ||||
| 24 | – | – | 11–23 kg | 24–51 lb |
|
| ||||
| 30 | >23 | kg | >51 lb | – |
|
| ||||
| 40 | <14 kg | <31 lb | <11 kg | <24 lb |
AMA, American Medical Association.
Intermittent and repetitive were not defined in the AMA guidance.
MacDonald. Clinical guidelines for occupational lifting. Am J Obstet Gynecol 2013.
The AMA’s guidelines apply to repetitive lifting beginning in the 24th week or intermittent lifting beginning in the 30th week of pregnancy, permitting up to 51 pounds. The AMA’s recommended weight allowance drops in the final week of pregnancy to less than 24 pounds for repetitive and less than 31 pounds for intermittent lifting. An unpublished statement from the AMA’s 1999 Annual Meeting encourages physicians to “consider the potential benefits and risks of occupational activities and exposures on an individual basis, and work with patients and employers to define a healthy work environment for pregnant women and encourages employers to “minimize heavy lifting.”54
Certain aspects of the AMA guidelines are nonspecific (eg, repetitive and intermittent lifting were not defined), and they do not inform the clinician how to take into consideration lifting task conditions, such as object location at the time of the lift (eg, near or far from the front of the body), which may leave pregnant workers at risk of overexertion injury.
The following statement on pregnancy and work by the American College of Obstetricians and Gynecologists (ACOG) from 1979 highlights other nonspecific guidance provided to clinicians about patient employment conditions: “The normal woman with an uncomplicated pregnancy and a normal fetus in a job that presents no greater potential hazards than those encountered in normal daily life in the community may continue to work without interruption until the onset of labor and may resume work several weeks after an uncomplicated pregnancy.”55
Although acknowledging evidence associating physical job demands (standing, lifting) with preterm or small-for- gestational-age outcomes, specific recommendations on employment conditions are also absent from the recent American Academy of Pediatrics/ACOG Guidelines for Perinatal Care (6th edition), which state, “Women with medical or obstetric complications of pregnancy need to make adjustments based on the nature of their activities, occupations, and specific complications.”56 More recent guidance by the American College of Occupational and Environmental Medicine for reproductive and developmental hazard management does not address lifting or other physically strenuous work activities.57
All military services have policies exempting pregnant women from some work activities, yet few specifically address lifting in pregnancy. The policy of the US Army exempts soldiers from wearing “load-bearing equipment” after pregnancy has been confirmed,58 and the US Air Force policy precludes wearing “heavy gear” after 20 weeks’ gestation. 59 All military services designate the obstetrical health care provider as the authority for recommending restricted duty for pregnant personnel,58–61 although a newer Army policy also mandates an “occupational health interview” for pregnant service women.58 Occupational health consultation is optional in the US Navy and the US Marine Corps, but, when sought, these military services offer the only lifting-specific guidance.53
Citing both the 1984 AMA guidelines 52 and the 1991 Revised NIOSH lifting equation,62 the Navy and Marine Corps technical manual states that lifting may generally continue up to the level a woman was accustomed prior to pregnancy. The guidance adds that “additional restrictions in the third trimester of pregnancy include limiting or prohibiting … lifting weights that are bulky or awkward or that approach the woman’s maximal (prepregnancy) lifting capacity. As pregnancy progresses, it is wise to reduce the physical workload and ensure rest periods of adequate frequency and duration. In late pregnancy, a pregnant woman should not do any task that may require a Valsalva (bearing-down) maneuver.”
NIOSH lifting equation
Following a detailed scientific review by a panel of experts, the empirically derived NIOSH Work Practices Guide for Manual Lifting was published in 1981 to reduce overexertion injury in the general working population in association with 2-handed lifting of compact loads.63 After consideration of new evidence, the original NIOSH lifting guidelines were expanded and replaced in 1991 by the revised NIOSH lifting equation.62
The lifting equation is an ergonomic job assessment tool used to evaluate the specific conditions of a lifting task to compute a recommended weight limit (RWL). The RWL represents the weight of the load that nearly all healthy workers could lift, up to 8 hours per day, without an increased risk of developing lifting-related LBP. By healthy workers, NIOSH means workers who are free of adverse health conditions (or other conditions such as pregnancy) that may increase their risk of musculoskeletal injury. According to the authors of the lifting equation, the RWL provides weight limits that would be acceptable to 90% of healthy women.63
The lifting equation defines a maximum RWL of 51 pounds, which is considered safe for an ideal lift (ie, infrequent 2-handed lifting of compact loads close to the body without twisting, stooping, or reaching up or forward). Because lifting conditions deviate from this ideal, the RWL value is reduced in accordance with specific task conditions such as lifting frequency and location of the object at the start of the lift (eg, lifting from the floor, overhead, or far in front of the body).
The task conditions of a lift are associated with corresponding metabolic and biomechanical loads or demands so, for example, as the distance between a worker and a load lifted in front of the body increases, the RWL for that lifting task would be reduced from the ideal lift starting value of 51 pounds (the condition when an object is held close to the body) to a maximum value of 20 pounds (the condition when an object is held very far from the body). Those interested in knowing more about the lifting equation and the task parameters used to compute the RWL are encouraged to access the Applications Manual from the NIOSH web site (http://www.cdc.gov/niosh/docs/94-110/) and to read the article by Waters et al.62
Provisional clinical guidelines for occupational lifting
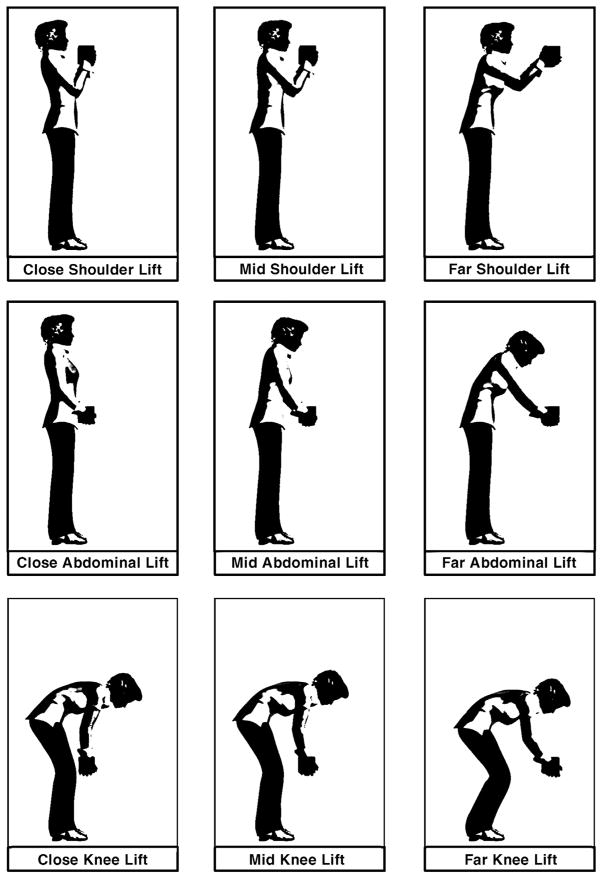
Motivated by the need for practical, evidence-based weight limits to aid clinical decision making, we applied the NIOSH lifting equation to define RWLs for a broad range of lifting patterns for pregnant workers. Criteria related to the distance objects are held in front of the body while lifting and the height of the object lifted relative to the floor, task conditions that influence the metabolic and biomechanical load, were used to define 9 “lifting zones.” Visual representations of these lifting zones are shown in Figure 1.
FIGURE 1. Visual representation of lifting a compact load in each lifting zone.
Graphical illustration of 9 work postures typically associated with lifting a compact load at each of 3 vertical and 3 horizontal distances in front of the body. The postural requirements of a lift, in addition to the weight of the object lifted, influence the risk of an overexertion musculoskeletal injury. Accordingly, recommended weight limits are reduced as work posture deviates from the ideal (ie, the close abdominal lift) to compensate for risk because of postural loading. The term “mid” refers to the “middle” horizontal lifting distance (between the close and far).
MacDonald. Clinical guidelines for occupational lifting. Am J Obstet Gynecol 2013.
Determination of the horizontal boundary points involved consideration of the minimum distance needed for clearance in front of the body, abdominal depth in the first and second half of pregnancy, and maximum reach distances. Prospectively recorded data on abdominal depth among pregnant women reported by Perkins and Blackwell 64 (1998) were used to inform the dimensional reference points for clearance in front of the body in the first half of pregnancy, which we defined as close (15 inches), and for the second half of pregnancy, which we defined as mid (20 inches). Importantly, the RWLs derived for the close lift and mid lift zones were computed using horizontal distance values of 15 and 20 inches, respectively; the RWLs therefore encompass the full range of expected clearance distances required in the first and second half of pregnancy. Vertical height values for shoulder, knuckle, and tibia locations were derived from reports of average US female anthropometry and defined to be 52 inches (shoulder), 28 inches (knuckle), and 17 inches (tibia), respectively.65
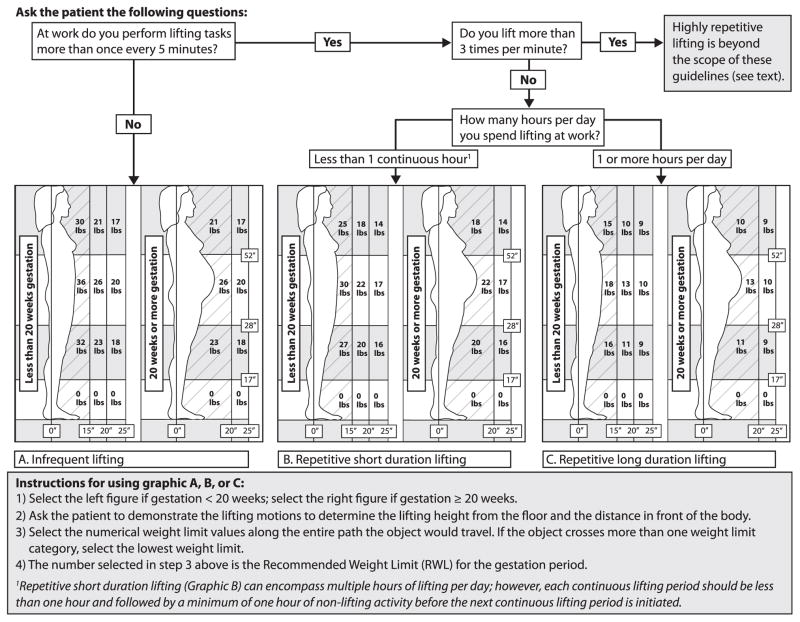
Using the horizontal and vertical reference points noted above, RWLs for each lifting zone were derived, as specified in Figure 2, for 3 lifting patterns and 2 gestational periods (less than 20 weeks and 20 weeks or more). A flow diagram at the top of Figure 2 indicates the sequence of questions to ask a patient to determine which of 3 lifting patterns to select (ie, infrequent lifting [graphic A], repetitive short-duration lifting [graphic B], or repetitive long-duration lifting [graphic C]). After selecting graphic A, B, or C, the clinician then follows the instructions at the bottom of Figure 2 to determine the RWL (ie, the maximum recommended weight that can be safely handled in accordance with the selected lifting pattern and gestational period).
FIGURE 2. Recommended weight limits in early and late pregnancy for 3 lift frequency patterns.
Graphics A, B, and C depict different recommended weight limits (RWLs) in 9 lifting zones as a function of lifting frequency and duration. Distance in inches is represented by the symbol (").
Lbs, pound.
MacDonald. Clinical guidelines for occupational lifting. Am J Obstet Gynecol 2013.
The highest RWL occurs when gestation is less than 20 weeks (left side of graphics A, B, and C), when abdominal protrusion is nominal, and objects lifted close to the body can be within 15 inches of the lower spine. At gestation of 20 weeks or more (right side of graphics A, B, and C), abdominal protrusion increases the distance (or load moment) between the lower spine and the object being lifted, requiring a lower RWL to prevent overexertion of the musculoskeletal system. Details on the decision rules applied in the development of these provisional guidelines and the technical aspects of the RWL computations are available in a companion paper.66
Guideline application restrictions
Although our goal was to include a wide spectrum of broadly applicable lifting patterns, lift condition restrictions of the original NIOSH lifting equation apply to these provisional guidelines. These restrictions, summarized in Table 2, represent lifting conditions that pose higher risk of musculoskeletal injury (eg, 1-handed lifting, lifting more than 8 hours per day, lifting unstable loads). To avoid overcomplicating our provisional guidelines, very high frequency lifting is not covered. When these higher-risk task conditions are present in a patient’s job, we suggest that obstetric providers use their best clinician judgment to decide the best course of action for their patient. These actions may range from work restrictions that prohibit lifting for the duration of pregnancy, choosing the lift condition in Figure 2 that most closely approximates the lifting conditions of the patient and reduce the RWL by an amount judged to mitigate the increased risk, or recommending in a letter to the patient’s employer that a formal job analysis be conducted by an occupational health professional to determine maximum weight limits based on actual lifting conditions for the gestational period.
TABLE 2.
Work conditions not covered by the NIOSH RNLE (and the clinical guidelines)
| • Lifting/lowering with 1 hand |
| • Lifting/lowering for more than 8 hours |
| • Lifting/lowering while seated or kneeling |
| • Lifting/lowering in a restricted work space |
| • Lifting/lowering unstable objects |
| • Lifting/lowering while carrying, pushing, or pulling |
| • Lifting/lowering with wheelbarrows or shovels |
| • Lifting/lowering with high-speed motion (faster than about 30 inches/ second) |
| • Lifting/lowering with unreasonable foot/floor coupling (less than 0.4 coefficient of friction between the sole and the floor) |
| • Lifting/lowering in an unfavorable environment (temperature significantly outside 66–79°F [19–26°C] range; relative humidity outside 35–50% range). |
| • (Highly repetitive lifting more than 3 times per minute) |
| • (Lifting/lowering from the floor with hands below midshin) |
| • (Lifting overhead) |
NIOSH RNLE, National Institute for Occupational Safety and Health Revised NIOSH lifting equation.
MacDonald. Clinical guidelines for occupational lifting. Am J Obstet Gynecol 2013.
Our provisional guidelines recommend no lifting/lowering from the floor with hands below midshin. Manually lifting/lowering objects at or near the floor is generally accomplished by forward flexion of the torso during the downward reach motion (Figure 1). Frequent or prolonged torso flexion is a significant risk factor for back injury.67,68
Accordingly, the American Conference of Governmental Industrial Hygienists (ACGIH) lifting threshold limit value restricts most lifting from the floor in its guidance for the general working population.69 Practical application of the ACGIH lifting threshold limit value after 20 weeks’ gestation would preclude lifting from the floor because abdominal protrusion would extend the distance that objects are handled in front of the body.
Additionally, research among those who are pregnant indicates that a significant majority of women entering the third trimester have difficulty in picking up objects from the floor,70,71 and evidence by Bonzini et al8 (2009) and Florack et al9 (1993) each showed a nearly 3-fold increased risk of preterm labor and spontaneous abortion, respectively, for women whose job required bending at the waist more than 1 hour per day. We further recommend no overhead lifting because of reports of increased task performance difficulties71 coupled with an increased risk of postural instability42,72 and an increased anteroposterior postural sway73 because of center-of-mass changes with the increased gestation.
Comment
Guidelines on occupational lifting during pregnancy need to be updated to aid decision making and clinical management, ensuring that pregnant workers are properly advised and afforded ample protections in accordance with accumulated scientific evidence. This need is underscored in the United States where federal regulations for occupational lifting are lacking and limited opportunities for paid antenatal leave are available.1,2
As detailed in this paper, existing lifting guidelines published by the AMA in 1984 and by NIOSH in 1981 (revised in 1991) are incongruent. The AMA guidelines did not define the terms repetitive and intermittent lifting, limiting their application. Evidence used in creating the NIOSH lifting guideline did not encompass pregnancy-related physiological and physical changes that may increase overexertion risk, and the guidelines did not consider evidence linking lifting demands with reproductive or developmental effects.
The NIOSH lifting equation was adapted for use in a clinical setting after epidemiological and other evidence on the association between occupational lifting and maternal and fetal health was reviewed. The provisional guidelines presented here account for the influence of abdominal depth on the minimum distance an object can be handled in front of the body in the first and second half of pregnancy. Certain simplifying assumptions were made to distill the NIOSH lifting equation to a set of 1-page guidelines.
These assumptions state that certain known risk factors for overexertion injury, such as rotation of the spine while lifting, are not present. Additionally, our provisional guidelines do not apply to very repetitive tasks. It is suggested therefore that these guidelines be applied judiciously and reflect a maximum recommended weight for the applicable gestational period. When feasible, weight limits would be computed using the full NIOSH lifting equation to ensure maximum protection.62,63,74
Based on our review of the available evidence, the RWLs in these provisional guidelines represent lifting thresholds that most pregnant workers with uncomplicated pregnancies should be able to perform without increased risk of adverse maternal and fetal health
consequences. The RWLs are considerably lower and potentially more protective than the AMA guidelines, and, except for restrictions for lifting from the floor and overhead, they are compatible with NIOSH lifting recommendations for the general US workforce. Although provisional, if widely adopted, we believe these guidelines will narrow the variability shown to exist among health care providers in making clinical decisions about restricted employment activities.3,4
These provisional guidelines do not account for the effects of joint laxity. Although plausible, to our knowledge, no data exist demonstrating that pregnancy-related joint laxity reduces the load-bearing capacity of pregnant women. Research is warranted to examine joint stabilization of the spine and pelvic girdle regions during pregnancy and the postpartum period to determine whether laxity is associated with increased muscle recruitment and co-contraction, factors known to significantly increase spinal loading.75
Pelvic girdle pain was shown to be associated with occupational lifting of at least 11 kg more than 10 times per day in a very large Danish study.76 Although the effect estimates in this study were small to moderate (OR, 1.24; 95% CI, 1.05–1.45 for lifting 11–20 kg more than 10 times per day and OR, 1.64; 95% CI, 1.06–2.54 for any lifting more than 20 kg), the findings underscore the need for more research on musculoskeletal injury risk among employed pregnant women and those returning to work within 6 weeks postpartum.
Additionally, it is important to acknowledge limitations of the epidemiological evidence that can reduce the likelihood of finding a significant association between occupational lifting and any fetal/maternal health outcome. These limitations include inadequate sample size, significant potential for selection bias, inadequate attention to exposure contrast, and poor specification and measurement of exposure.12,20 Furthermore, significant selection biases in epidemiological studies of heavy lifting have been reported for investigations involving nonpregnant workers,77 and such biases are likely to be more pronounced among pregnant workers because antenatal leave is more common among those employed in heavy physical work.78
In summary, we propose the first evidence-based clinical recommendations in the United States to address occupational lifting during pregnancy in nearly 3 decades. During this time, there has been a large increase in the number of women employed outside the home and remaining in the workforce during pregnancy. 2 We hope that the adoption of these provisional guidelines by obstetric and occupational health medical providers will improve the standard of care for pregnant workers by increasing the uniformity of clinical decisions regarding employment restrictions for lifting.
Further research is needed to inform appropriate RWLs for highly repetitive lifting during pregnancy and for lifting during the postpartum period. Although testing these provisional guidelines is beyond the scope of this paper, we encourage clinical researchers and professional organizations such as the American College of Obstetricians and Gynecologists and the American College of Occupational and Environmental Medicine to evaluate their application, to participate in and encourage more research on physical job demands and maternal-fetal health, and to suggest revisions to these provisional guidelines as new research findings become available.
Acknowledgments
We thank Dr Christina Lawson for giving her early input on the composition of our working group and overall project support; Kathy Connick for conducting literature searches, generating EndNote libraries, addressing our document retrieval needs, and providing editorial contributions; Ellen Galloway for text editing; Dr John Meyer for scientific peer review; and Greg Hartle for developing illustrations for this paper. None of these acknowledged individuals have a conflict of interest.
Footnotes
The views expressed herein are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health or the official policy or position of the Department of the Army, the Department of the Navy, the Department of Defense, or the United States Government.
The authors report no conflict of interest.
Reprints not available from the authors.
References
- 1.White LA. Institutions, constitutions, actor strategies, and ideas: explaining variation in paid parental leave policies in Canada and the United States. Int J Const Law. 2006;4:319–46. [Google Scholar]
- 2.Laughlin LL. Maternity leave and employment patterns of first-time mothers: 1961–2008. Washington, DC: US Department of Commerce, Economics and Statistics Administration, US Census Bureau; 2011. [Google Scholar]
- 3.Frazier LM, Ho HL, Molgaard CA. Variability in physician management of employment during pregnancy. Womens Health. 2001;34:51–63. doi: 10.1300/J013v34n04_04. [DOI] [PubMed] [Google Scholar]
- 4.Pompeii LA, Evenson KR, Delclos GL. Obstetricians’ practices and recommendations for occupational activity during pregnancy. J Reprod Med. 2011;56:17–24. [PubMed] [Google Scholar]
- 5.Spinillo A, Capuzzo E, Baltaro F, Piazza G, Nicola S, Iasci A. The effect of work activity in pregnancy on the risk of fetal growth retardation. Acta Obstet Gynecol Scand. 1996;75:531–6. doi: 10.3109/00016349609054666. [DOI] [PubMed] [Google Scholar]
- 6.Sternfeld B. Physical activity and pregnancy outcome. Review and recommendations Sports Med. 1997;23:33–47. doi: 10.2165/00007256-199723010-00004. [DOI] [PubMed] [Google Scholar]
- 7.Spinillo A, Capuzzo E, Colonna L, Piazzi G, Nicola S, Baltaro F. The effect of work activity in pregnancy on the risk of severe preeclampsia. Aust N Z J Obstet Gynaecol. 1995;35:380–5. doi: 10.1111/j.1479-828x.1995.tb02146.x. [DOI] [PubMed] [Google Scholar]
- 8.Bonzini M, Coggon D, Godfrey K, Inskip H, Crozier S, Palmer KT. Occupational physical activities, working hours and outcome of pregnancy: findings from the Southampton Women’s Survey. Occup Environ Med. 2009;66:685–90. doi: 10.1136/oem.2008.043935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Florack E, Zielhuis G, Pellegrino J, Rolland R. Occupational physical activity and the occurrence of spontaneous abortion. Int J Epidemiol. 1993;22:878–84. doi: 10.1093/ije/22.5.878. [DOI] [PubMed] [Google Scholar]
- 10.Woo GM. Daily demands during pregnancy, gestational age, and birthweight: reviewing physical and psychological demands in employment and non-employment contexts. Ann Behav Med. 1997;19:385–98. doi: 10.1007/BF02895158. [DOI] [PubMed] [Google Scholar]
- 11.Mogren IM. Previous physical activity decreases the risk of low back pain and pelvic pain during pregnancy. Scand J Public Health. 2005;33:300–6. doi: 10.1177/140349480503300410. [DOI] [PubMed] [Google Scholar]
- 12.Royal College of Physicians of London. Physical and shift work in pregnancy: occupational aspects of management—a national guideline. London: Royal College of Physicians; 2009. [Google Scholar]
- 13.Zhang H, Bracken M. Tree-based, two-stage risk factor analysis for spontaneous abortion. Am J Epidemiol. 1996;144:989–96. doi: 10.1093/oxfordjournals.aje.a008869. [DOI] [PubMed] [Google Scholar]
- 14.McDonald A, Armstrong B. Spontaneous abortion and occupation. J OccupMed. 1986;28:1232–8. [PubMed] [Google Scholar]
- 15.McDonald A, McDonald J, Armstrong B, Cherry NM, Côté R, Lavoie J, et al. Fetal death and work in pregnancy. Br J Ind Med. 1988;45:148–57. doi: 10.1136/oem.45.3.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taskinen H, Kyyronen P, Hemminki K. Effects of ultrasound, shortwaves, and physical exertion on pregnancy outcome in physiotherapists. J Epidemiol Community Health. 1990;44:196–201. doi: 10.1136/jech.44.3.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Magann EF, Evans SF, Chauhan SP, Nolan TE, Henderson J, Klausen JH, et al. The effects of standing, lifting and noise exposure on preterm birth, growth restriction, and perinatal death in healthy low-risk working military women. J Matern Fetal Neonatal Med. 2005;18:155–62. doi: 10.1080/14767050500224810. [DOI] [PubMed] [Google Scholar]
- 18.Ahlborg G, Bodin L, Hogstedt C. Heavy lifting during pregnancy—a hazard to the fetus? A prospective study. Int J Epimiol. 1990;19:90–7. doi: 10.1093/ije/19.1.90. [DOI] [PubMed] [Google Scholar]
- 19.Axelsson G, Lutz C, Rylander R. Exposure to solvents and outcome of pregnancy in university laboratory employees. Br J Ind Med. 1984;41:305–12. doi: 10.1136/oem.41.3.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonzini M, Coggon D, Palmer KT. Risk of prematurity, low birthweight and pre-eclampsia in relation to working hours and physical activities: a systematic review. Occup Environ Med. 2007;64:228–43. doi: 10.1136/oem.2006.026872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koemeester AP, Leegwater A, Broersen JPJ, Hoekstra EJ. Physical work load and the onset of maternity leave. J Occup Rehabil. 1997;7:75–82. [Google Scholar]
- 22.Strand K, Wergeland E, Bjerkedal T. Work load, job control and risk of leaving work by sickness certification before delivery, Norway 1989. Scand J Soc Med. 1997;25:193–201. doi: 10.1177/140349489702500308. [DOI] [PubMed] [Google Scholar]
- 23.Luke B, Avni M, Min L, Misiunas R. Work and pregnancy: the role of fatigue and the “second shift” on antenatal morbidity. Am J Obstet Gynecol. 1999;181:1172–9. doi: 10.1016/s0002-9378(99)70103-1. [DOI] [PubMed] [Google Scholar]
- 24.Wergeland E, Strand K. Working conditions and prevalence of pre-eclampsia, Norway 1989. Int J Gynaecol Obstet. 1997;58:189–96. doi: 10.1016/s0020-7292(97)00083-0. [DOI] [PubMed] [Google Scholar]
- 25.Paul J, van Dijk F, Frings-Dresen M. Work load and musculoskeletal complaints during pregnancy. Scand J Work Environ Health. 1994;20:153–9. doi: 10.5271/sjweh.1414. [DOI] [PubMed] [Google Scholar]
- 26.Schauberger CW, Rooney BL, Goldsmith L, Shenton D, Silva PD, Schaper A. Peripheral joint laxity increases in pregnancy but does not correlate with serum relaxin levels. Am J Obstet Gynecol. 1996;74:667–71. doi: 10.1016/s0002-9378(96)70447-7. [DOI] [PubMed] [Google Scholar]
- 27.Mens JM, Pool-Goudzwaard A, Stam HJ. Mobility of the pelvic joints in pregnancy-related lumbopelvic pain: a systematic review. Obstet Gynecol Surv. 2009;64:200–8. doi: 10.1097/OGX.0b013e3181950f1b. [DOI] [PubMed] [Google Scholar]
- 28.Damen L, Buyruk HM, Guler-Uysal F, Lotgering FK, Snijders CJ, Stam HJ. Pelvic pain during pregnancy is associated with asymmetric laxity of the sacroiliac joints. Acta Obstet Gynecol Scand. 2001;80:1019–24. doi: 10.1034/j.1600-0412.2001.801109.x. [DOI] [PubMed] [Google Scholar]
- 29.Colliton J. Managing back pain during pregnancy. Medscape Womens Health. 1997;2:2. [PubMed] [Google Scholar]
- 30.Van Lunen BL, Roberts J, Branch JD, Dowling EA. Association of menstrual-cycle hormone changes with anterior cruciate ligament laxity measurements. J Athl Train. 2003;38:298–303. [PMC free article] [PubMed] [Google Scholar]
- 31.Charlton WP, Coslett-Charlton LM, Ciccotti MG. Correlation of estradiol in pregnancy and anterior cruciate ligament laxity. Clin Orthop Relat Res. 2001;387:165–70. doi: 10.1097/00003086-200106000-00022. [DOI] [PubMed] [Google Scholar]
- 32.Blecher AM, Richmond JC. Transient laxity of an anterior cruciate ligament-reconstructed knee related to pregnancy. Arthroscopy. 1998;14:77–9. doi: 10.1016/s0749-8063(98)70125-2. [DOI] [PubMed] [Google Scholar]
- 33.Dumas GA, Reid JG. Laxity of knee cruciate ligaments during pregnancy. J Orthop Sports Phys Ther. 1997;26:2–6. doi: 10.2519/jospt.1997.26.1.2. [DOI] [PubMed] [Google Scholar]
- 34.Marnach ML, Ramin KD, Ramsey PS, Song SW, Stensland JJ, An KN. Characterization of the relationship between joint laxity and maternal hormones in pregnancy. Obstet Gynecol. 2003;101:331–5. doi: 10.1016/s0029-7844(02)02447-x. [DOI] [PubMed] [Google Scholar]
- 35.Fries EC, Hellebrandt FA. The influence of pregnancy on the location of the center of gravity, postural stability, and body alignment. Am J Obstet Gynecol. 1943;46:374–80. [Google Scholar]
- 36.Jensen RK, Doucet S, Treitz T. Changes in segment mass and mass distribution during pregnancy. J Biomech. 1996;29:251–6. doi: 10.1016/0021-9290(95)00042-9. [DOI] [PubMed] [Google Scholar]
- 37.Cherry N. Physical demands of work and health complaints among women working late in pregnancy. Ergonomics. 1987;30:689–701. doi: 10.1080/00140138708969761. [DOI] [PubMed] [Google Scholar]
- 38.Fast A, Shapiro D, Ducommun EJ, Friedmann LW, Bouklas T, Floman Y. Low-back pain in pregnancy. Spine. 1987;12:368–71. doi: 10.1097/00007632-198705000-00011. [DOI] [PubMed] [Google Scholar]
- 39.Pennick VE, Young G. Interventions for preventing and treating pelvic and back pain in pregnancy. Cochrane Database Syst Rev. 2007:CD001139. doi: 10.1002/14651858.CD001139.pub2. [DOI] [PubMed] [Google Scholar]
- 40.Mantle MJ, Greenwood RM, Currey HL. Backache in pregnancy. Rheumatol Rehabil. 1977;16:95–101. doi: 10.1093/rheumatology/16.2.95. [DOI] [PubMed] [Google Scholar]
- 41.Wang SM, Dezinno P, Maranets I, Berman MR, Caldwell-Andrews AA, Kain ZN. Low back pain during pregnancy: prevalence, risk factors and outcomes. Obstet Gynecol. 2004;104:65–70. doi: 10.1097/01.AOG.0000129403.54061.0e. [DOI] [PubMed] [Google Scholar]
- 42.Mens JM, Huis in ‘t Veld YH, Pool-Goudzwaard A. Severity of signs and symptoms in lumbopelvic pain during pregnancy. Man Ther. 2012;17:175–9. doi: 10.1016/j.math.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 43.Skaggs CD, Prather H, Gross G, George JW, Thompson PA, Nelson DM. Back and pelvic pain in an underserved United States pregnant population: a preliminary descriptive survey. J Manipulative Physiol Ther. 2007;30:130–4. doi: 10.1016/j.jmpt.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 44.Gutke A, Josefsson A, Oberg B. Pelvic girdle pain and lumbar pain in relation to postpartum depressive symptoms. Spine. 2007;32:1430–6. doi: 10.1097/BRS.0b013e318060a673. [DOI] [PubMed] [Google Scholar]
- 45.Van De Pol G, Van Brummen HJ, Bruinse HW, Heintz AP, Van Der Vaart CH. Pregnancy-related pelvic girdle pain in the Netherlands. Acta Obstet Gynecol Scand. 2007;86:416–22. doi: 10.1080/00016340601151683. [DOI] [PubMed] [Google Scholar]
- 46.Pivarnik JM, Chambliss HO, Clapp JF, et al. Impact of physical activity during pregnancy and postpartum on chronic disease risk. Med Sci Sports Exerc. 2006;38:989–1006. doi: 10.1249/01.mss.0000218147.51025.8a. [DOI] [PubMed] [Google Scholar]
- 47.Gutke A, Ostgaard HC, Oberg B. Predicting persistent pregnancy-related low back pain. Spine. 2008;33:E386–93. doi: 10.1097/BRS.0b013e31817331a4. [DOI] [PubMed] [Google Scholar]
- 48.Noren L, Ostgaard S, Johansson G, Ostgaard HC. Lumbar back and posterior pelvic pain during pregnancy: a 3-year follow-up. Eur Spine J. 2002;11:267–71. doi: 10.1007/s00586-001-0357-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kristiansson P, Svardsudd K, von Schoultz B. Back pain during pregnancy: a prospective study. Spine. 1996;21:702–9. doi: 10.1097/00007632-199603150-00008. [DOI] [PubMed] [Google Scholar]
- 50.Rost CC, Jacqueline J, Kaiser A, Verhagen AP, Koes BW. Prognosis of women with pelvic pain during pregnancy: a long-term follow-up study. Acta Obstet Gynecol Scand. 2006;85:771–7. doi: 10.1080/00016340600626982. [DOI] [PubMed] [Google Scholar]
- 51.To WW, Wong MW. Factors associated with back pain symptoms in pregnancy and the persistence of pain 2 years after pregnancy. Acta Obstet Gynecol Scand. 2003;82:1086–91. doi: 10.1046/j.1600-0412.2003.00235.x. [DOI] [PubMed] [Google Scholar]
- 52.American Medical Association Council on Scientific Affairs. Effects of pregnancy on work performance. JAMA. 1984;251:1995–7. [PubMed] [Google Scholar]
- 53.Navy and Marine Corps Public Health Center. Reproductive and developmental hazards: a guide for occupational health professionals. Portsmouth, VA: Occupational and Environmental Medicine Directorate; 2010. Technical Manual NMCPHC-TM-OEM 6260. 01C. [Google Scholar]
- 54.American Medical Association. Summaries and recommendations of the Council on Scientific Affairs Reports: effects of work on pregnancy. CSA Report 9, A-99. Presented at the 1999 Annual Meeting of the American Medical Association; 1999; Chicago, IL.. [Google Scholar]
- 55.Messite J. Guidelines on pregnancy and work developed by the American College of Obstetrics and Gynecology. J Environ Pathol Toxicol. 1979;2:319–23. [PubMed] [Google Scholar]
- 56.American Academy of Pediatrics, American College of Obstetricians and Gynecologists. March of Dimes Birth Defects Foundation. Guidelines for perinatal care. 6. Elk Grove Village, IL: American Academy of Pediatrics; American College of Obstetricians and Gynecologists; 2008. [Google Scholar]
- 57.Luderer U, Hieb M, Diaz J, Baker B. Reproductive and developmental hazard management guidance: ACOEM Task Force on Reproductive Toxicology. J Occup Environ Med. 2011;53:941–9. doi: 10.1097/JOM.0b013e318229a549. [DOI] [PubMed] [Google Scholar]
- 58.US Department of the Army. Profiling pregnant soldiers. Medical services-standards of medical fitness. Washington (DC): Superintendent of Documents, US Government Printing Office; 2007. [Google Scholar]
- 59.Secretary of the Air Force. Air Force Instruction 44-102—Medical care management. US Air Force; 2012. [Google Scholar]
- 60.US Department of the Navy. Guidelines concerning pregnant servicewomen. Office of the Chief of Naval Operations, OPNAV Instruction 6000.1B; 2003. [Google Scholar]
- 61.Commandant of the Marine Corps. Marine Corps policy concerning pregnancy and parenthood. US Department of the Navy, Marine Corps; 2004. Order 5000.12E W/CH 1-2. [Google Scholar]
- 62.Waters TR, Putz-Anderson V, Garg A, Fine LJ. Revised NIOSH equation for the design and evaluation of manual lifting tasks. Ergonomics. 1993;36:749–76. doi: 10.1080/00140139308967940. [DOI] [PubMed] [Google Scholar]
- 63.NIOSH. Work practices guide for manual lifting. Cincinnati, OH: National Institute for Occupational Safety and Health; 1981. [Google Scholar]
- 64.Perkins T, Blackwell S. Accommodation and occupational safety for pregnant military personnel: final report. (ADA364792) Dayton, OH: United States Air Force Research Laboratory; 1998. [Google Scholar]
- 65.Chengalur SN, Rodgers SH, Bernard TE. Kodak’s ergonomic design for people at work. 2. Hoboken, NJ: John Wiley & Sons; 2004. [Google Scholar]
- 66.Waters TR, MacDonald LA, Hudock SD, Goddard DE. Provisional recommended weight limits for manual lifting during pregnancy. Hum Factors. doi: 10.1177/0018720813502223. accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Punnett L, Fine LJ, Keyserling WM, Herrin GD, Chaffin DB. Back disorders and nonneutral trunk postures of automobile assembly workers. Scand J Work Environ Health. 1991;17:337–46. doi: 10.5271/sjweh.1700. [DOI] [PubMed] [Google Scholar]
- 68.Vandergrift JL, Gold JE, Hanlon A, Punnett L. Physical and psychosocial ergonomic risk factors for low back pain in automobile manufacturing workers. Occup Environ Med. 2012;69:29–34. doi: 10.1136/oem.2010.061770. [DOI] [PubMed] [Google Scholar]
- 69.American Conference of Governmental Industrial Hygienists. Lifting. 2012 TLVs and BEIs: based on the documentation of the threshold limit values for chemical substances and physical agents and biological exposure indices. Cincinnati, OH: ACGIH Signature Publications; 2012. pp. 177–80. [Google Scholar]
- 70.Nicholls JA, Grieve DW. Performance of physical tasks in pregnancy. Ergonomics. 1992;35:301–11. doi: 10.1080/00140139208967815. [DOI] [PubMed] [Google Scholar]
- 71.Cheng PL, Dumas GA, Smith JT, Leger AB, Plamondon A, McGrath MJ, et al. Analysis of self-reported problematic tasks for pregnant women. Ergonomics. 2006;49:282–92. doi: 10.1080/00140130500434929. [DOI] [PubMed] [Google Scholar]
- 72.Dunning K, Lemasters G, Bhattacharya A. A major public health issue: the high incidence of falls during pregnancy. Matern Child Health J. 2010;14:720–5. doi: 10.1007/s10995-009-0511-0. [DOI] [PubMed] [Google Scholar]
- 73.Oliveira L, Vieira T, Macedo A, Simpson D, Nadal J. Postural sway changes during pregnancy: a descriptive study using stabilometry. Eur J Obstet Gynecol Reprod Biol. 2009;147:25–8. doi: 10.1016/j.ejogrb.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 74.Waters T, Putz-Anderson V, Garg A. Applications manual for the revised NIOSH lifting equation. Cincinnati, OH: National Institute for Occupational Safety and Health; 1994. [Google Scholar]
- 75.Marras WS. The working back: a systems view. Hoboken, NJ: Wiley-Interscience; 2008. [Google Scholar]
- 76.Larsen PS, Strandberg-Larsen K, Juhl M, Svendsen SW, Bonde JP, Nybo Andersen AM. Occupational lifting and pelvic pain during pregnancy: a study within the Danish National Birth Cohort. Scand J Work Environ Health. 2013;39:88–95. doi: 10.5271/sjweh.3304. [DOI] [PubMed] [Google Scholar]
- 77.Waters TR, Baron SL, Piacitelli LA, Anderson VP, Skov T, Haring-Sweeney M, et al. Evaluation of the revised NIOSH lifting equation— a cross-sectional epidemiologic study. Spine. 1999;24:386–94. doi: 10.1097/00007632-199902150-00019. [DOI] [PubMed] [Google Scholar]
- 78.Saurel-Cubizolles MJ, Kaminski M. Pregnant women’s working conditions and their changes during pregnancy: a national study in France. Br J Ind Med. 1987;44:236–43.66. doi: 10.1136/oem.44.4.236. [DOI] [PMC free article] [PubMed] [Google Scholar]