Abstract
Obsessive-compulsive disorder (OCD) has been associated with regional volumetric brain abnormalities, which provide promising intermediate phenotypes of the disorder. In this study, volumes of brain regions selected for a priori evidence of association with OCD (orbitofrontal cortex (OFC), anterior cingulate cortex (ACC), thalamus, caudate, putamen, globus pallidus and pituitary) were measured using structural magnetic resonance imaging (MRI) in 20 psychotropic-naïve pediatric OCD patients. We examined the association between these regional brain volumes and a total of 519 single nucleotide polymorphisms (SNPs) from nine glutamatergic candidate genes (DLGAP1, DLGAP2, DLGAP3, GRIN2B, SLC1A1, GRIK2, GRIK3, SLITRK1 and SLITRK5). These genes were selected based on either previous reported association with OCD in humans or evidence from animal models of OCD. After correcting for multiple comparisons by permutation testing, no SNP remained significantly associated with volumetric changes. The strongest trend toward association was identified between two SNPs in DLGAP2 (rs6558484 and rs7014992) and OFC white matter volume (P = 0.000565, Padjusted= 0.3071). Our other top ranked association findings were with ACC, OFC and thalamus. These preliminary results suggest that sequence variants in glutamate candidate genes may be associated with structural neuroimaging phenotypes of OCD.
Keywords: obsessive-compulsive disorder, glutamate, genetics association, structural magnetic resonance imaging, orbital frontal cortex
1. Introduction
Obsessive-compulsive disorder (OCD) is a common and often debilitating neuropsychiatric condition characterized by persistent intrusive thoughts (obsessions), repetitive ritualistic behaviours (compulsions) and excessive anxiety (American Psychiatric Association, 2000). It is estimated that the prevalence of pediatric OCD in the general population is around 1–3 % with the majority of cases of OCD having their onset during childhood or adolescence (Zohar et al., 1999; Kessler et al., 2005; Ruscio et al., 2010). Twin studies have revealed that heritability estimates for OCD symptoms in children range from 45%–65% (van Grootheest et al., 2005). Two controlled family studies have shown that the lifetime prevalence of OCD was significantly higher in first-degree relatives of pediatric probands compared with control relatives (Hanna et al, 2005; do Rosario-Campos et al., 2005).The odds ratios from these two studies are considerably higher than those reported in family studies with adult probands (Hettema et al., 2001).Taken together, family and twin studies indicate that genetic factors play a significant role in OCD, particularly in children and adolescents.
Dysfunction in cortical-striatal-thalamocortical (CSTC) circuitry has been postulated in the etiology of OCD and a growing body of evidence has suggested that the neurotransmission of glutamate, a major neurotransmitter in the CSTC circuit, is disrupted in OCD (MacMaster, 2010; Pittenger et al., 2011; Wu et al., 2012). Using 1H MRS, studies in OCD patients have revealed greater glutamatergic concentrations (Glx) in caudate (Rosenberg et al., 2000) and lower Glx in anterior cingulate cortex (ACC) regardless of medication status (Rosenberg et al., 2004; Yucel et al., 2008).Candidate gene studies have also implicated glutamate system genes in OCD. The strongest association findings for candidate glutamate genes in OCD have been for the neuronal glutamate transporter gene SLC1A1(Solute Carrier, Family 1, Member 1), which has been reported by several independent groups (Arnold et al., 2006; Dickel et al., 2006; Stewart et al., 2007; Shugart et al., 2009; Wendland et al., 2009). Our group has also reported the association between GRIN2B (glutamate receptor, ionotropic, N-methyl D-aspartate 2B), which encodes an NMDA receptor subunit and OCD (Arnold et al., 2004), as well as reduced Glx in the ACC of pediatric OCD patients (Arnold et al., 2009b). Finally, the use of transgenic or knockout mouse models has demonstrated that genetic alteration in regional glutamate signaling may induce behaviors similar to obsessive-compulsive symptoms in humans (Nordstrom et al., 2002; Welch et al., 2007; Shmelkov et al., 2010).
Given that OCD is a complex genetic trait with substantial genetic heterogeneity, one approach to minimize the complexity is to study intermediate phenotypes, such as quantitative traits that correlate with OCD but may be more closely linked with the actions of genes compared to clinical diagnosis (Meyer-Lindenberg et al., 2006). A substantial body of studies have demonstrated that individuals with OCD display regional volumetric brain abnormalities (Macmaster et al., 2008; Menzies et al., 2008; Radua et al., 2009). Therefore, neuroimaging measures of brain region volumes could serve as promising intermediate phenotypes for genetic analysis of OCD. In a pilot imaging genetic study conducted by our group (Arnold et al., 2009a), we identified positive associations between glutamate system genes and ventral prefrontal and thalamic volume. The rs1805476 variant of GRIN2B was associated with orbital frontal cortex (OFC) and anterior cingulate cortex (ACC) volume. The same variant was also associated with a non-significant trend for association with left caudate volume. The SLC1A1 rs3056 variant was associated with increased thalamic volume.
In this study, we expanded our previous work and conducted a more comprehensive analysis to examine the association between glutamatergic candidate genes and regional brain volumes in children with OCD. Here we focus on brain regions previously implicated in volumetric magnetic resonance imaging (MRI) studies of OCD: thalamus (Gilbert et al., 2000), ACC(Rosenberg et al., 1998; Rosenberg et al., 2004; Szeszko et al., 2004), globus pallidus (Szeszko et al., 2004), OFC(Szesko et al.,1999; Whiteside et al., 2006), caudate(Luxenberg et al., 1988; Rosenberg et al.,1997; Rosenberg et al., 2000), putamen(Rosenberg et al., 1997; Szeszko et al., 2008; Giedd et al., 2000) and pituitary volume (MacMaster et al., 2006). Nine candidate genes were selected for analysis based on previous candidate gene studies and animal models. Although we have expanded the number of genes and polymorphisms examined, we did not expand our sample size and therefore our intention was to perform a preliminary, hypothesis-generating study rather than to definitively test the association of glutamate system genes with imaging phenotypes in OCD.
2. Method
2.1 Subjects
Our sample included 20 (12 males, 8 females) children and adolescents with OCD, age 7 to 18 years (mean age = 12.1, S.D. = 3.2) recruited as part of on-going neuroimaging studies conducted at Wayne State University. All patients were medication-naive and none were receiving cognitive-behavioral therapy at the time of participation. The inclusion of only medication-naive children close to the onset of illness allowed us to limit the confounding effects of pharmacotherapy and chronic illness. Exclusion criteria included history of significant debilitating medical or neurologic condition, major depressive disorder, bipolar disorder, psychosis, substance use or dependence, eating disorder, attention deficit hyperactivity disorder, IQ < 80, pervasive developmental disorder, learning disorder or tic disorders. We have previously reported imaging genetics results from the same cohort (Arnold et al., 2009a; Arnold et al., 2009b), and the sample reported herein includes the subset of children for whom we had genome-wide data available. . Written informed consent was obtained from parent(s) and written assent was obtained from all participants prior to participation in the study. Patients received the Schedule for Affective Disorders and Schizophrenia – School-Age Children (Kaufman et al., 1997) and the following clinician-administered instruments included (mean ± S.D. in parentheses): Children’s Yale-Brown Obsessive Compulsive Scale (CY-BOCS) (mean = 26.5, s.d. =5.27 ) (Scahill et al., 1997), Hamilton Anxiety Rating Scale (HAM-A) (Hamilton, 1959); (8.00 ± 4.89), and Hamilton Depression Rating Scale (HAM-D) (Schwab et al., 1967) (7.2 ± 4.87). All patients had a CY-BOCS score of at least 17 consistent with significant dysfunction.
2.2 Imaging
Structural magnetic resonance imaging (MRI) data were collected at the Children’s Hospital of Michigan Imaging Center using a Sigma 1.5-Tesla unit (Horizon LX software, General Electric Medical Systems, Milwaukee, WI). Scanning methods, image acquisition and analysis procedures have been described in detail elsewhere (Rosenberg et al., 1997; Rosenberg et al., 1998; Rosenberg et al., 2000; Benazon et al., 2003; Mirza et al., 2004; Rosenberg et al., 2004; Szeszko et al., 2004). Briefly, a 3-dimensional spoiled gradient echo pulse sequence was used to obtain 124 1.5-mm-thick contiguous coronalimages. Parameters included: echo time=5 milliseconds, repetition time=25 milliseconds, acquisition matrix=256 × 256 pixels, field of view=24 cm, and flip angle=40°. Well-trained and reliable operators, blinded to the subject’s group membership and other identifying information, used a manual tracing technique to measure regional brain volumes with the MEDx program. Seven independent volumetric neuroimaging measures were chosen for the analysis based on a priori evidence of association with OCD: orbital frontal cortex (OFC), anterior cingulate cortex (ACC), caudate, putamen, thalamus, globus pallidus (GP) and pituitary volume. Regions of interest definitions are outlined briefly in the following paragraphs, some of which has previously been described in previous reports from our group (Szeszko et al., 2004; Arnold et al., 2009a). In addition to grey matter abnormalities, white matter differences are increasingly being recognized in OCD. Therefore, based on a priori evidence for differences between OCD patients and controls specific to either white or grey matter in OFC (Szeszko et al., 2008; Macmaster et al., 2010) or ACC (Szeszko et al., 2004; Togao et al., 2010) we analyzed total, white matter volume and grey matter volumes in these two regions.
2.2.1 Orbital Frontal Cortex (OFC)
The anterior boundary was the last appearance of the anterior horizontal ramus, and the posterior boundary the last appearance of the olfactory sulcus. The lateral boundary was the anterior horizontal ramus or circular sulcus of insula, while the medial boundary was the olfactory sulcus (Szeszko et al., 1999; Macmaster et al., 2010). Intraclass correlation coefficients (ICC) and inter-rater reliability between two highly trained raters were r=0.98 and r=0.98 respectively for the right OFC and r=0.92 and r=0.97 for the left OFC.
2.2.2 Anterior Cingulate Cortex (ACC)
The anterior boundary was the tip of the cingulate sulcus and the posterior border the connection of the superior and precentral sulci. The superior boundary was the cingulate sulcus and the inferior border the callosal sulcus (Rosenberg et al., 1998; Szeszko et al., 1999). ICC valuesand inter-rater reliability were r = 0.95 and r = 0.97 for the right ACC and r = 0.98 and r = 0.98 for the left ACC.
2.2.3 Caudate
The anterior boundary was the point at which it was first visible rostrally, and the posterior boundary was the point at which the tail of the caudate was no longer clearly visible. Raters took special care to exclude cerebrospinal fluid medially and to exclude the nucleus accumbens ventrally. The putamen was separated from the caudate by the internal capsule, the nucleus accumbens, and the globus pallidus (Rosenberg et al., 1997; Szeszko et al., 1999; Szeszko et al., 2004). ICC and inter-rater reliability values were r = 0.93 and r = 0.96 for the right caudate, and r = 0.95 and r = 0.95 for the left caudate.
2.2.4 Thalamus
The anterior boundary was the coronal slice with the mamillary bodies and interventricular foramen present. The posterior boundary was the coronal slice where the thalamus merged under the crux fornix. The lateral boundary was the internal capsule and the third ventricle the medial boundary. The superior boundary was the main body of the lateral ventricle and the inferior boundary was the hypothalamus (Gilbert et al., 2000). The ICC and inter-rater reliability values were r = 0.94 and r = 0.96 for the right thalamus and r = 0.93 and r = 0.94 for the left thalamus.
2.2.5 Putamen
The anterior boundary was the point at which the putamen was first visible rostrally (Rosenberg et al., 1997; Szeszko et al., 2004). The putamen was separated caudally from the caudate by the internal capsule, the nucleus accumbens, and finally the globus pallidus, which then became the medial border. Ventrally, the putamen was delimited by the anterior commissure and then the anterior perforated substance. Dorsally, the putamen was separated from the caudate by part of the anterior and posterior limbs of the internal capsule. Measurement was discontinued when the putamen was no longer visible. The ICC and inter-rater reliability values were r = 0.91 and r = 0.93 for the right putamen, and r = 0.94 and r = 0.94 for the left putamen.
2.2.6 Globus Pallidus
The globus pallidus is a small, deep brain structure and it is technically very difficult to measure the internal and external globus pallidus separately. Therefore, although we acknowledge there are important functional differences between internal and external globus pallidus we analyzed them as a single structure Measurement began when the globus pallidus was first visible rostrally (Szezko et al., 2004). The dorsomedial boundary was the internal capsule, and the lateral border was the putamen. Rostrally, the ventral border was the anterior commissure and the medial border was the point at which the internal capsule and anterior commissure intersect. Caudally, the ventral border was the substantia innominata (measurement included the ansa lenticularis) and the medial border was the internal capsule and/or the crus cerebri. The ICC and inter-rater reliability values were r = 0.93 and r = 0.95 for the right globus pallidus, and r = 0.90 and r = 0.94 for the left globus pallidus.
2.2.7 Pituitary
Please refer to MacMaster et al (MacMaster et al., 2006). for methods used to measure the pituitary. Briefly, serial coronal slices (1.45-mm each) were summed and multiplied by the slice thickness (mean of 6.77 +/−1.21 slices). Because pituitary volume was acquired in a three-dimensional mode, sagittal views were also used to guide and enhance measurement precision.The superior border was defined as the optic chiasm and infundibular recess of the third ventricle, and the inferior border at the sphenoid sinus. ICC and inter-rater reliability values were r = 0.98 and r = 0.92, respectively (MacMaster and Kusumakar, 2004).
2.3 Candidate Gene and SNP selection
All subjects had previously been genotyped at the Broad Institute (Boston, MA) using the Illumina 610-Quad BeadChip as part of a genome-wide association study (GWAS) conducted by the International OCD Foundation Genetics Collaborative (IOCDF-GC) (Stewart et al., 2012). Nine glutamatergic candidate genes were selected for analysis based on either previous reported association with OCD in humans or evidence from animal models of OCD. A total of 519 SNPs in DLGAP1, DLGAP2, DLGAP3, GRIN2B, SLC1A1, GRIK2, GRIK3, SLITRK1 and SLITRK5 or within 10Kb of each gene’s flanking regions were obtained from the GWAS data and tested for association with the volumetric neuroimaging measures.
To evaluate the coverage of the candidate genes by the selected SNPs in this study, the genotype data of the same loci from HapMap release 28 were downloaded and analyzed using the Tagger function in Haploview (Barrett et al., 2005). SNPs in this study were used as tagging SNPs through the force-include method to determine what percentage of all the variants in each gene were captured by these SNPs using the pairwise tagging algorithm. Threshold for pairwise tagging were minor allele frequency (MAF) of 0.05 and r2 threshold of 0.75. Table 1 reports the result of the coverage analysis.
Table 1.
Glutamatergic Candidate Genes
| Chromosome | HapMap SNPs | Test SNPs | Captured SNPs | Coverage | r2 mean | |
|---|---|---|---|---|---|---|
|
| ||||||
| Gene Name | n | n | n | % | ||
| GRIN2B | 12 | 523 | 143(145) | 421 | 85% | 0.961 |
| DLGAP3 | 1 | 22 | 10 | 14 | 63% | 0.988 |
| SLC1A1 | 9 | 149 | 60 (62) | 113 | 79% | 0.973 |
| GRIK2 | 6 | 671 | 121(124) | 530 | 78% | 0.963 |
| GRIK3 | 1 | 183 | 21 | 101 | 55% | 0.973 |
| SLITRK5* | 13 | 10 | 2 | 5 | 50% | 0.981 |
| SLITRK1 | 13 | 2 | 1 | 1 | 50% | 1 |
| DLGAP1 | 18 | 285 | 96 | 226 | 79% | 0.960 |
| DLGAP2 | 8 | 223 | 58 | 195 | 87% | 0.958 |
HapMap SNPs: number of SNPs in the HapMap release 28 for each gene (up to 10kb flanking regions included).
Test SNPs: number of test SNPs used in the tagging algorithm (performed by using forced-include function in Haploview). Bracket indicates the number of actual SNPs used in this study due to some additional SNPs on the ChIP not genotyped in the HapMap sample.
Captured SNPs & Coverage: number and percentage of HapMap SNPs tagged by the test SNPs with an r2 > 0.75
r2 mean: average r2 between test SNPs and tagged HapMap SNPs
These 2 SNPs are located in the intergenic regions of SLITRK5, the SLTRK5 region was extended to include these 2 SNPs for the tagger algorithm
2.4 Data analysis
2.4.1 Quality Control
We repeated the same quality control procedures used in the OCFGC-GWAS in our smaller sample. Subjects with individual call rate less than 97% were removed from the analysis. Genotype data were available for a total of 590,924 SNPs. The SNPs were then filtered based on minor allele frequency (MAF), call rate and Hardy-Weinberg Equilibrium (HWE). SNPs were removed if their MAF was less than 0.1, call rate was less than 0.95 and if they deviated from HWE with a P value less than 10−5. After filtering, 450,951 SNPs remained.
To detect any underlying population stratification, multidimensional scaling analysis (MDS) was performed on a subset of autosomal SNPs. The subset of SNPs were generated by pruning for linkage disequilibrium (LD) using a sliding window algorithm (window size 1500 bp, r2>0.2, sliding length = 150 bp). The individuals from this study were compared against reference founder populations from HapMap release 23. There were no sub-clusters within the study sample. All subjects in the study sample clustered together with the European (CEU) HapMap population’s founders, which is in accordance with the self-reported ethnicity of these subjects.
2.4.2 Statistical Analysis
All quality control and genetic data analyses were performed using PLINK v1.07 (Purcell et al., 2007).Each SNP was tested for association with the volumetric measures using a linear regression model that assumed an additive contribution of each minor allele. All analyses were adjusted for age and intracranial volume. In order to control for multiple testing, permutation tests (n = 50,000) were used to generate corrected empirical p-values using the Max (T) function in PLINK. This permutation testing approach accounts for the correlational structure between SNPs, and thus is appropriate less stringent than a Bonferroni correction wich assumes all test (ie all SNP) are independent. Permutation testing was performed separately for each phenotype
3. Results
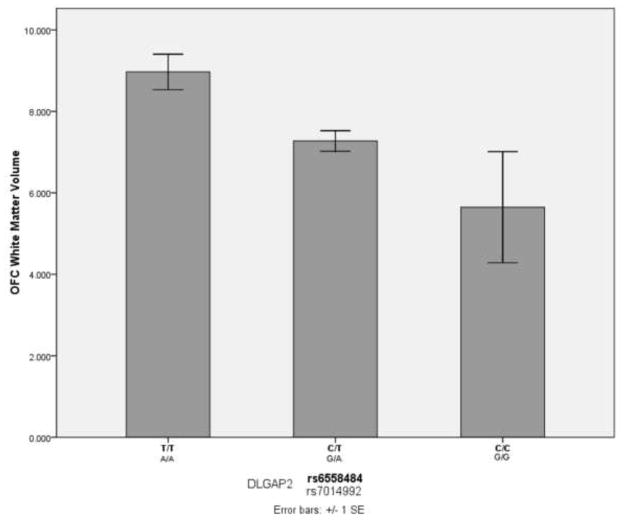
The association results were ranked by P value and those with raw P value less than 0.005 are displayed in Table 2. After correcting for multiple comparisons by permutation testing, no SNP remained significantly associated with volumetric changes (corrected empirical P < 0.05). However, two DLGAP2 SNPs, rs6558484 and rs7014992 showed the strongest trend toward association with OFC white matter volume (P = 0.000565, Padjusted= 0.3071, see Figure 1). These two SNPs were found to be in complete linkage disequilibrium with one another in our sample (D′ = 1, r2 = 1). Furthermore, SNPs in several candidate genes showed nominal association (P < 0.05) with specific brain regions (see Table S1, available online). Next to the DLGAP2 SNPs, two SNPs in DLGAP1 (rs1116345 and rs342484) also showed a strong trend towards association, this time with total ACC volume (P = 0.001282, Padjusted = 0.3884, see Table 2). Seven SNPs in DLGAP2 (rs6994849, rs1558024, rs6558484, rs7014992, rs2956913, rs10094463 and rs2956929) were nominally associated with total OFC volume. Total thalamus volume was nominally associated with SNPs in GRIN2B, SLC1A1 and SLITRK5.
Table 2.
Summary of top SNPs associated with volumetric measures
| SNP | CHR | A1 | Genes | MAF | N | BETA | STAT | P | Adjusted Empirical P value | Phenotype |
|---|---|---|---|---|---|---|---|---|---|---|
| rs6558484 | 8 | C | DLGAP2 | 0.45 | 17 | −1.373 | −4.53 | 0.000565 | 0.3071 | OFC (White Matter) |
| rs7014992 | 8 | G | DLGAP2 | 0.45 | 17 | −1.373 | −4.53 | 0.000565 | 0.3071 | OFC (White Matter) |
| rs1116345 | 18 | T | DLGAP1 | 0.3 | 19 | 1.726 | 3.951 | 0.001282 | 0.3884 | ACC Volume |
| rs342483 | 18 | C | DLGAP1 | 0.3 | 19 | 1.726 | 3.951 | 0.001282 | 0.3884 | ACC Volume |
| rs6994849 | 8 | A | DLGAP2 | 0.475 | 17 | −3.605 | −4.061 | 0.001348 | 0.4504 | OFC Volume |
| rs10814988 | 9 | A | SLC1A1 | 0.2 | 19 | 2 | 3.86 | 0.001541 | 0.4032 | ACC (Grey Matter) |
| rs1558024 | 8 | G | DLGAP2 | 0.475 | 17 | 3.343 | 3.985 | 0.001555 | 0.4856 | OFC Volume |
| rs3738081 | 8 | C | GRIK3 | 0.475 | 19 | 0.9366 | 3.789 | 0.001781 | 0.45 | ACC (White Matter) |
| rs6558484 | 8 | C | DLGAP2 | 0.45 | 17 | −3.728 | −3.911 | 0.001789 | 0.5223 | OFC Volume |
| rs7014992 | 8 | G | DLGAP2 | 0.45 | 17 | −3.728 | −3.911 | 0.001789 | 0.5223 | OFC Volume |
| rs8097308 | 18 | C | DLGAP1 | 0.475 | 17 | 0.9312 | 3.899 | 0.001828 | 0.5664 | OFC(White Matter) |
| rs2956913 | 8 | G | DLGAP2 | 0.45 | 17 | 3.087 | 3.754 | 0.002411 | 0.6038 | OFC Volume |
| rs11612284 | 12 | A | GRIN2B | 0.2 | 20 | 2.138 | 3.565 | 0.002584 | 0.6546 | Thalamus Volume |
| rs10094463 | 8 | A | DLGAP2 | 0.475 | 17 | 4.265 | 3.685 | 0.002749 | 0.6425 | OFC Volume |
| rs301430 | 9 | C | SLC1A1 | 0.45 | 20 | −1.469 | −3.456 | 0.003251 | 0.6401 | Thalamus Volume |
| rs11842338 | 13 | A | SLITRK5 | 0.175 | 20 | −2.229 | −3.436 | 0.003394 | 0.6546 | Thalamus Volume |
| rs12215960 | 6 | G | GRIK2 | 0.15 | 19 | 0.7756 | 3.451 | 0.003567 | 0.6794 | ACC(White Matter) |
| rs10459061 | 12 | A | GRIN2B | 0.1 | 19 | −2.444 | −3.39 | 0.004038 | 0.7135 | ACC(Grey Matter) |
| rs17833967 | 12 | C | GRIN2B | 0.1 | 19 | −2.444 | −3.39 | 0.004038 | 0.7135 | ACC(Grey Matter) |
| rs7541937 | 1 | T | DLGAP3 | 0.45 | 17 | 0.8712 | 3.463 | 0.004203 | 0.7758 | OFC(White Matter) |
| rs2301963 | 8 | C | DLGAP2 | 0.475 | 17 | −1.355 | −3.443 | 0.004366 | 0.7844 | OFC(White Matter) |
| rs10485270 | 6 | A | GRIK2 | 0.175 | 19 | 0.7689 | 3.328 | 0.00459 | 0.76 | ACC(White Matter) |
| rs9390764 | 6 | A | GRIK2 | 0.175 | 19 | 0.7689 | 3.328 | 0.00459 | 0.76 | ACC(White Matter) |
| rs1116345 | 18 | T | DLGAP1 | 0.3 | 19 | 1.255 | 3.327 | 0.004596 | 0.7934 | ACC(Grey Matter) |
| rs342483 | 18 | C | DLGAP1 | 0.3 | 19 | 1.255 | 3.327 | 0.004596 | 0.7543 | ACC(Grey Matter) |
| rs1558024 | 8 | G | DLGAP2 | 0.475 | 17 | 1.071 | 3.406 | 0.004692 | 0.7844 | OFC(White Matter) |
| rs2956929 | 8 | A | DLGAP2 | 0.5 | 17 | 2.833 | 3.401 | 0.004733 | 0.7898 | OFC Volume |
| rs2300266 | 12 | G | GRIN2B | 0.175 | 20 | 1.797 | 3.275 | 0.004766 | 0.7638 | Thalamus Volume |
A1: Minor allele
MAF: minor allele frequency
N: number of subjects with non-missing data for the brain region volume.
Beta: Regression coefficient (non-standardized)
STAT: Coefficient t-statistic
Adjusted Empirical P value: based on max (T) permutation (n = 50,000)
ACC: Anterior Cingulate Cortex
OFC: Orbital Frontal Cortex
Fig 1.
OFC white matter volume and DLGAP2 SNPs (rs6558484 and rs7014992)
All of our top ranked findings (Table 2) were for associations with volume of the ACC, OFC or thalamus rather than the basal ganglia regions or pituitary. The top findings for each of the other four regions tested were (with unadjusted P values in parentheses: 1) GRIN2B-rs2300238 and globus pallidus (P=0.0054), 2) GRIK2-rs4839797 and putamen (P=0.006), 3) GRIN2B -rs12826869 and pituitary (P=0.006), and 4) GRIK3-rs3767088 and caudate (P=0.007) (Table S1).
4. Discussion
The role of glutamate signalling in the CSTC model of OCD has been suggested through multiple lines of evidence ranging from neuroimaging to candidate gene studies. Briefly, reciprocal interaction between the direct (leading to thalamic stimulation of cortex) and indirect (leading to thalamic inhibition of cortex) pathways results in a dynamic balance without one pathway predominating. However, dysfunction in glutamate signalling can result in the hyperactivity of the direct pathway, or hypoactivity of the indirect pathway, which leads to the disinhibition of CSTC circuits and consequently overactivity of the cortex that is thought to result in cognitive and behavioural changes in OCD. Neuroimaging studies that support the CSTC model of OCD include studies performed by Rosenberg and colleagues(2000,2004) that suggested tonic-phasic dysregulation of glutamate in the CSTC results in reduced tonic glutamate level in ACC (as shown by reduced ACC Glx) and phasic overactivity in the striatum and orbitofrontal cortex (as shown by increased striatum and OFC Glx). (See Wu et al., 2012 for a detailed review of the role of glutamate signaling in the pathogenesis of OCD).
In this study, we conducted a preliminary analysis of the association between variants within glutamate system genes and volumes of brain regions previously implicated in OCD. Our results revealed no significant association between common variants in our nine candidate genes (GRIN2B, DLGAP3, SLC1A1, GRIK2, GRIK3, SLITRK5, SLITRK1, DLGAP1, and DLGAP2) and various volumetric phenotypes. There are strong trends toward association for two DLGAP2 SNPs in complete LD and orbital frontal cortex (OFC) white matter volume (Figure 1), as well as trends toward association between several glutamate system candidate genes and other volumetric neuroimaging measures (Table 1). Our group (MacMaster et al., 2010) recently reported that volume of right OFC white matter is significantly larger in patients with OCD compared to healthy controls. Previously, our group reported significant changes in grey matter in the OFC of OCD patients compared to healthy controls, which also correlated with increased OCD symptom severity among patients (Szeszko et al., 2008). In the current study, we did not identify any genetic association specific to OFC grey matter volume, although seven DLGAP2 SNPs were nominally associated with total OFC volume (Table 1). Decreased OFC volume has been reported in numerous MRI studies of OCD. Two studies reported bilateral reduction of OFC in OCD patients compared to healthy controls (Szeszko et al., 1999; Atmaca et al., 2007). We previously identified a preliminary association between a single SNP in GRIN2B (rs1805476) and OFC volume; however, this SNP was not included on the Illumina 610Quad array and the only nominal associations we identified between GRIN2B and OFC were relatively weak (minimum uncorrected p value = 0.01, Table S1) with SNPs that were at least 200 kB from rs1805476.
No studies have reported an association between DLGAP2 variants and OCD or related phenotypes. The variants for which we identified a trend towards an association with OFC white matter volume (rs6558484 and rs7014992) are located in introns, not coding or known regulatory regions of the gene. Should this association be confirmed in a larger sample, it would suggest a broader role for genes functionally related to DLGAP3, previously implicated in a knockout mouse model of OCD (Welch et al., 2007). Interestingly, copy number variants within DLGAP2 were implicated in a recent study of almost 1000 individuals with autism spectrum disorders (Pinto et al., 2010), a group of disorders in which prominent repetitive behaviours overlap with the symptoms of OCD (Jacob et al., 2009).
Although only nominally significant, it is worth noting the trend in our findings for association between rs301430 in SLC1A1 and thalamic volume (Beta =−1.469, P = 0.0033, Padjusted = 0.64), since SLC1A1 has the strongest a priori evidence for association with OCD and rs301430 is the SLC1A1 SNP which has been most often reported to be associated with OCD (Dickel et al., 2006; Stewart et al., 2007; Wendland et al., 2009). In addition, there is evidence from studies of post-mortem brain tissue and in vitro assays that rs301430, a common SNP in the 3′ region of the gene, may influence SLC1A1 expression (Wendland et al., 2009). Previously, we identified an association between decreased thalamic volume and rs3056, another SNP located in the 3′-untranslated region of SLC1A1.
This study has several strengths. By including only medication-naïve pediatric patients near the onset of illness, we minimize confounding factors on MRI measures due to chronic illnesses or pharmacotherapy. Furthermore, these subjects were genotyped using high density SNP arrays. This allowed for high coverage of the genes tested in this study (Table 1), which increased the detection power of our analysis. However, a crucial limitation to this study is our low sample size (n=20). The small sample size reduces the power to detect between-group differences, and therefore our analysis focuses only on genes and brain regions where there was good a priori evidence of association with OCD. Also due to the low sample size, we applied a minor allele frequency (MAF) threshold of 0.1, an approach which omits uncommon and rare variants that may exert casual effects on OCD. Finally, it is important to note the measures taken in this study to correct for multiple comparisons. We adjusted for multiple comparisons using the max(T) permutation function in PLINK to generate corrected empirical P values, This method effectively adjusts for the number of SNPs, while accounting for the correlation between SNPs in close linkage disequilibrium. However, there is still a risk of Type I error due to the number of different neuroimaging phenotypes tested, a risk which we attempted to minimize by focusing on brain regions where there was strong a priori evidence of association with OCD. Our overall aim in this hypothesis-generating study was to identify potential associations to follow up in independent samples with larger sample sizes, and therefore we believe our approach to multiple comparisons was appropriate in contrast to a more definitive, hypothesis-testing study.
In summary, in this preliminary study we have identified trends toward association between genetic variants in glutamate system genes and various volumetric neuroimaging phenotypes of OCD. Our results demonstrate the potential power of an imaging genomics approach in which variants genotyped using high density arrays are tested in subjects for association with quantitative brain imaging phenotypes. We are currently collecting a larger sample of 200 pediatric OCD cases and 200 age-matched healthy controls (funded by NIH) in order to test the same brain regions using a more comprehensive, genome-wide approach. Future studies by our group and others combining neuroimaging and genetic approaches should lead to improved understanding of how genetic variation can confer risk to OCD.
Supplementary Material
Acknowledgments
Special thanks go to Dr. Lisa Strug, statistician in the Child Health Evaluative Sciences Program, Hospital for Sick Children, Toronto, for consultation regarding adjustment for multiple comparisons in our data analysis. Genotyping data for this report was obtained thanks to the International OCD Foundation Genetics Collaborative (IOCDF-GC, chair Dr. David Pauls). Grant support to the investigators was provided by the National Alliance on Research in Schizophrenia and Depression (Young Investigator Award, PDA), an Obsessive-Compulsive Foundation Research Award (PDA, JLK), the Canadian Psychiatric Research Foundation (PDA), the Miriam Hamburger Endowed Chair of Child Psychiatry at Children’s Hospital of Michigan and Wayne State University and the National Institute of Mental Health (RO1MH085300, K24MH02037).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnold PD, Rosenberg DR, Mundo E, Tharmalingam S, Kennedy JL, Richter MA. Association of a glutamate (NMDA) subunit receptor gene (GRIN2B) with obsessive-compulsive disorder: a preliminary study. Psychopharmacology (Berl) 2004;174:530–538. doi: 10.1007/s00213-004-1847-1. [DOI] [PubMed] [Google Scholar]
- Arnold PD, Sicard T, Burroughs E, Richter MA, Kennedy JL. Glutamate transporter gene SLC1A1 associated with obsessive-compulsive disorder. Archives of General Psychiatry. 2006;63:769–776. doi: 10.1001/archpsyc.63.7.769. [DOI] [PubMed] [Google Scholar]
- Arnold PD, Macmaster FP, Hanna GL, Richter MA, Sicard T, Burroughs E, Mirza Y, Easter PC, Rose M, Kennedy JL, Rosenberg DR. Glutamate system genes associated with ventral prefrontal and thalamic volume in pediatric obsessive-compulsive disorder. Brain Imaging and Behavior. 2009a;3:64–76. doi: 10.1007/s11682-008-9050-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold PD, Macmaster FP, Richter MA, Hanna GL, Sicard T, Burroughs E, Mirza Y, Easter PC, Rose M, Kennedy JL, Rosenberg DR. Glutamate receptor gene (GRIN2B) associated with reduced anterior cingulate glutamatergic concentration in pediatric obsessive-compulsive disorder. Psychiatry Research. 2009b;172:136–139. doi: 10.1016/j.pscychresns.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atmaca M, Yildirim H, Ozdemir H, Tezcan E, Poyraz AK. Volumetric MRI study of key brain regions implicated in obsessive-compulsive disorder. Prog Neuropsychopharmacol Biological Psychiatry. 2007;31:46–52. doi: 10.1016/j.pnpbp.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Benazon NR, Moore GJ, Rosenberg DR. Neurochemical analyses in pediatric obsessive-compulsive disorder in patients treated with cognitive-behavioral therapy. Journal of the American Academy of Child and Adolescent Psychiatry. 2003;42:1279–1285. doi: 10.1097/01.chi.0000087562.01900.de. [DOI] [PubMed] [Google Scholar]
- Dickel DE, Veenstra-VanderWeele J, Cox NJ, Wu X, Fischer DJ, Van Etten-Lee M, Himle JA, Leventhal BL, Cook EH, Jr, Hanna GL. Association testing of the positional and functional candidate gene SLC1A1/EAAC1 in early-onset obsessive-compulsive disorder. Archives of General Psychiatry. 2006;63:778–785. doi: 10.1001/archpsyc.63.7.778. [DOI] [PubMed] [Google Scholar]
- do Rosario-Campos MC, Leckman JF, Curi M, Quatrano S, Katsovitch L, Miguel EC, Pauls DL. A family study of early-onset obsessive-compulsive disorder. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2005;136B:92–97. doi: 10.1002/ajmg.b.30149. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Rapoport JL, Garvey MA, Perlmutter S, Swedo SE. MRI assessment of children with obsessive-compulsive disorder or tics associated with streptococcal infection. American Journal of Psychiatry. 2000;157:281–283. doi: 10.1176/appi.ajp.157.2.281. [DOI] [PubMed] [Google Scholar]
- Gilbert AR, Moore GJ, Keshavan MS, Paulson LA, Narula V, Mac Master FP, Stewart CM, Rosenberg DR. Decrease in thalamic volumes of pediatric patients with obsessive-compulsive disorder who are taking paroxetine. Archives of General Psychiatry. 2000;57:449–456. doi: 10.1001/archpsyc.57.5.449. [DOI] [PubMed] [Google Scholar]
- Hamilton M. The assessment of anxiety states by rating. British Journal of Medical Psychology. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Hanna GL, Himle JA, Curtis GC, Gillespie BW. A family study of obsessive-compulsive disorder with pediatric probands. Am J Med Genet B Neuropsychiatr Genet. 2005;134:13–19. doi: 10.1002/ajmg.b.30138. [DOI] [PubMed] [Google Scholar]
- Hettema JM, Neale MC, Kendler KS. A review and meta-analysis of the genetic epidemiology of anxiety disorders. American Journal of Psychiatry. 2001;158:1568–1578. doi: 10.1176/appi.ajp.158.10.1568. [DOI] [PubMed] [Google Scholar]
- Jacob S, Landeros-Weisenberger A, Leckman JF. Autism spectrum and obsessive-compulsive disorders: OC behaviors, phenotypes and genetics. Autism Research. 2009;2:293–311. doi: 10.1002/aur.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Luxenberg JS, Swedo SE, Flament MF, Friedland RP, Rapoport J, Rapoport SI. Neuroanatomical abnormalities in obsessive-compulsive disorder detected with quantitative X-ray computed tomography. American Journal of Psychiatry. 1988;145:1089–1093. doi: 10.1176/ajp.145.9.1089. [DOI] [PubMed] [Google Scholar]
- MacMaster FP, Kusumakar V. MRI study of the pituitary gland in adolescent depression. Journal of Psychiatric Research. 2004;38:231–236. doi: 10.1016/j.jpsychires.2003.11.001. [DOI] [PubMed] [Google Scholar]
- MacMaster FP, Russell A, Mirza Y, Keshavan MS, Banerjee SP, Bhandari R, Boyd C, Lynch M, Rose M, Ivey J, Moore GJ, Rosenberg DR. Pituitary volume in pediatric obsessive-compulsive disorder. Biological Psychiatry. 2006;59:252–257. doi: 10.1016/j.biopsych.2005.06.028. [DOI] [PubMed] [Google Scholar]
- Macmaster FP, O’Neill J, Rosenberg DR. Brain Imaging in Pediatric Obsessive-Compulsive Disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2008 doi: 10.1097/CHI.0b013e318185d2be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMaster FP. Translational neuroimaging research in pediatric obsessive-compulsive disorder. Dialogues in Clinical NeuroScience. 2010;12:165–174. doi: 10.31887/DCNS.2010.12.2/fmacmaster. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMaster F, Vora A, Easter P, Rix C, Rosenberg D. Orbital frontal cortex in treatment-naive pediatric obsessive-compulsive disorder. Psychiatry Research. 2010;181:97–100. doi: 10.1016/j.pscychresns.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzies L, Chamberlain SR, Laird AR, Thelen SM, Sahakian BJ, Bullmore ET. Integrating evidence from neuroimaging and neuropsychological studies of obsessive-compulsive disorder: the orbitofronto-striatal model revisited. Neuroscience & Biobehavioral Reviews. 2008;32:525–549. doi: 10.1016/j.neubiorev.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Weinberger DR. Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Nature Reviews Neuroscience. 2006;7:818–827. doi: 10.1038/nrn1993. [DOI] [PubMed] [Google Scholar]
- Mirza Y, Tang J, Russell A, Banerjee SP, Bhandari R, Ivey J, Rose M, Moore GJ, Rosenberg DR. Reduced anterior cingulate cortex glutamatergic concentrations in childhood major depression. Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43:341–348. doi: 10.1097/00004583-200403000-00017. [DOI] [PubMed] [Google Scholar]
- Nordstrom EJ, Burton FH. A transgenic model of comorbid Tourette’s syndrome and obsessive-compulsive disorder circuitry. Molecular Psychiatry. 2002;7:617–25. 524. doi: 10.1038/sj.mp.4001144. [DOI] [PubMed] [Google Scholar]
- Pinto D, Pagnamenta AT, Klei L, Anney R, Merico D, Regan R, Conroy J, Magalhaes TR, Correia C, Abrahams BS, Almeida J, Bacchelli E, Bader GD, Bailey AJ, Baird G, Battaglia A, Berney T, Bolshakova N, Bolte S, Bolton PF, Bourgeron T, Brennan S, Brian J, Bryson SE, Carson AR, Casallo G, Casey J, Chung BH, Cochrane L, Corsello C, Crawford EL, Crossett A, Cytrynbaum C, Dawson G, de Jonge M, Delorme R, Drmic I, Duketis E, Duque F, Estes A, Farrar P, Fernandez BA, Folstein SE, Fombonne E, Freitag CM, Gilbert J, Gillberg C, Glessner JT, Goldberg J, Green A, Green J, Guter SJ, Hakonarson H, Heron EA, Hill M, Holt R, Howe JL, Hughes G, Hus V, Igliozzi R, Kim C, Klauck SM, Kolevzon A, Korvatska O, Kustanovich V, Lajonchere CM, Lamb JA, Laskawiec M, Leboyer M, Le Couteur A, Leventhal BL, Lionel AC, Liu XQ, Lord C, Lotspeich L, Lund SC, Maestrini E, Mahoney W, Mantoulan C, Marshall CR, McConachie H, McDougle CJ, McGrath J, McMahon WM, Merikangas A, Migita O, Minshew NJ, Mirza GK, Munson J, Nelson SF, Noakes C, Noor A, Nygren G, Oliveira G, Papanikolaou K, Parr JR, Parrini B, Paton T, Pickles A, Pilorge M, Piven J, Ponting CP, Posey DJ, Poustka A, Poustka F, Prasad A, Ragoussis J, Renshaw K, Rickaby J, Roberts W, Roeder K, Roge B, Rutter ML, Bierut LJ, Rice JP, Salt J, Sansom K, Sato D, Segurado R, Sequeira AF, Senman L, Shah N, Sheffield VC, Soorya L, Sousa I, Stein O, Sykes N, Stoppioni V, Strawbridge C, Tancredi R, Tansey K, Thiruvahindrapduram B, Thompson AP, Thomson S, Tryfon A, Tsiantis J, Van Engeland H, Vincent JB, Volkmar F, Wallace S, Wang K, Wang Z, Wassink TH, Webber C, Weksberg R, Wing K, Wittemeyer K, Wood S, Wu J, Yaspan BL, Zurawiecki D, Zwaigenbaum L, Buxbaum JD, Cantor RM, Cook EH, Coon H, Cuccaro ML, Devlin B, Ennis S, Gallagher L, Geschwind DH, Gill M, Haines JL, Hallmayer J, Miller J, Monaco AP, Nurnberger JI, Jr, Paterson AD, Pericak-Vance MA, Schellenberg GD, Szatmari P, Vicente AM, Vieland VJ, Wijsman EM, Scherer SW, Sutcliffe JS, Betancur C. Functional impact of global rare copy number variation in autism spectrum disorders. Nature. 2010;466:368–372. doi: 10.1038/nature09146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC. PLINK: a tool set for whole-genome association and population-based linkage analyses. American Journal of Human Genetics. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radua J, Mataix-Cols D. Voxel-wise meta-analysis of grey matter changes in obsessive-compulsive disorder. British Journal of Psychiatry. 2009;195:393–402. doi: 10.1192/bjp.bp.108.055046. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, Keshavan MS, O’Hearn KM, Dick EL, Bagwell WW, Seymour AB, Montrose DM, Pierri JN, Birmaher B. Frontostriatal measurement in treatment-naive children with obsessive-compulsive disorder. Archives of General Psychiatry. 1997;54:824–830. doi: 10.1001/archpsyc.1997.01830210068007. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, Keshavan MS. A.E. Bennett Research Award. Toward a neurodevelopmental model of of obsessive--compulsive disorder. Biological Psychiatry. 1998;43:623–640. doi: 10.1016/s0006-3223(97)00443-5. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, MacMaster F, Keshavan M, Fitzgerald K, Stewart C, Moore G. Decrease in caudate glutamatergic concentrations in pediatric obsessive-compulsive disorder patients taking paroxetine. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:1096–1103. doi: 10.1097/00004583-200009000-00008. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, Mirza Y, Russell A, Tang J, Smith JM, Banerjee SP, Bhandari R, Rose M, Ivey J, Boyd C, Moore GJ. Reduced anterior cingulate glutamatergic concentrations in childhood OCD and major depression versus healthy controls. Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43:1146–1153. doi: 10.1097/01.chi.0000132812.44664.2d. [DOI] [PubMed] [Google Scholar]
- Ruscio AM, Stein DJ, Chiu WT, Kessler RC. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Molecular Psychiatry. 2010;15:53–63. doi: 10.1038/mp.2008.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scahill L, Riddle MA, McSwiggin-Hardin M, Ort SI, King RA, Goodman WK, Cicchetti D, Leckman JF. Children’s Yale-Brown Obsessive Compulsive Scale: reliability and validity. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:844–852. doi: 10.1097/00004583-199706000-00023. [DOI] [PubMed] [Google Scholar]
- Schwab JJ, Bialow MR, Clemmons RS, Holzer CE. Hamilton rating scale for depression with medical in-patients. British Journal of Psychiatry. 1967;113:83–88. doi: 10.1192/bjp.113.494.83. [DOI] [PubMed] [Google Scholar]
- Shmelkov SV, Hormigo A, Jing D, Proenca CC, Bath KG, Milde T, Shmelkov E, Kushner JS, Baljevic M, Dincheva I, Murphy AJ, Valenzuela DM, Gale NW, Yancopoulos GD, Ninan I, Lee FS, Rafii S. Slitrk5 deficiency impairs corticostriatal circuitry and leads to obsessive-compulsive-like behaviors in mice. Nature Medicine. 2010;16:598–602. doi: 10.1038/nm.2125. 1p following 602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shugart YY, Wang Y, Samuels JF, Grados MA, Greenberg BD, Knowles JA, McCracken JT, Rauch SL, Murphy DL, Rasmussen SA, Cullen B, Hoehn-Saric R, Pinto A, Fyer AJ, Piacentini J, Pauls DL, Bienvenu OJ, Riddle MA, Liang KY, Nestadt G. A family-based association study of the glutamate transporter gene SLC1A1 in obsessive-compulsive disorder in 378 families. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2009 doi: 10.1002/ajmg.b.30914. [DOI] [PubMed] [Google Scholar]
- Stewart SE, Fagerness JA, Platko J, Smoller JW, Scharf JM, Illmann C, Jenike E, Chabane N, Leboyer M, Delorme R, Jenike MA, Pauls DL. Association of the SLC1A1 glutamate transporter gene and obsessive-compulsive disorder. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2007;144B:1027–1033. doi: 10.1002/ajmg.b.30533. [DOI] [PubMed] [Google Scholar]
- Stewart SE, Yu D, Scharf JM, Neale BM, Fagerness JA, Mathews CA, Arnold PD, Evans PD, Gamazon ER, Osiecki L, McGrath L, Haddad S, Crane J, Hezel D, Illman C, Mayerfeld C, Konkashbaev A, Liu C, Pluzhnikov A, Tikhomirov A, Edlund CK, Rauch SL, Moessner R, Falkai P, Maier W, Ruhrmann S, Grabe HJ, Lennertz L, Wagner M, Bellodi L, Cavallini MC, Richter MA, Cook EH, Jr, Kennedy JL, Rosenberg D, Stein DJ, Hemmings SM, Lochner C, Azzam A, Chavira DA, Fournier E, Garrido H, Sheppard B, Umaña P, Murphy DL, Wendland JR, Veenstra-Vanderweele J, Denys D, Blom R, Deforce D, Van Nieuwerburgh F, Westenberg HG, Walitza S, Egberts K, Renner T, Miguel EC, Cappi C, Hounie AG, Conceição do Rosário M, Sampaio AS, Vallada H, Nicolini H, Lanzagorta N, Camarena B, Delorme R, Leboyer M, Pato CN, Pato MT, Voyiaziakis E, Heutink P, Cath DC, Posthuma D, Smit JH, Samuels J, Bienvenu OJ, Cullen B, Fyer AJ, Grados MA, Greenberg BD, McCracken JT, Riddle MA, Wang Y, Coric V, Leckman JF, Bloch M, Pittenger C, Eapen V, Black DW, Ophoff RA, Strengman E, Cusi D, Turiel M, Frau F, Macciardi F, Gibbs JR, Cookson MR, Singleton A, for the North American Brain Expression Consortium, Hardy J, for the UK Brain Expression Database, Crenshaw AT, Parkin MA, Mirel DB, Conti DV, Purcell S, Nestadt G, Hanna GL, Jenike MA, Knowles JA, Cox N, Pauls DL. Genome-wide association study of obsessive-compulsive disorder. Molecular Psychiatry. 2012 Aug 14; doi: 10.1038/mp.2012.85. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeszko PR, Christian C, Macmaster F, Lencz T, Mirza Y, Taormina SP, Easter P, Rose M, Michalopoulou GA, Rosenberg DR. Gray Matter Structural Alterations in Psychotropic Drug-Naive Pediatric Obsessive-Compulsive Disorder: An Optimized Voxel-Based Morphometry Study. American Journal of Psychiatry. 2008 doi: 10.1176/appi.ajp.2008.08010033. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, MacMillan S, McMeniman M, Chen S, Baribault K, Lim KO, Ivey J, Rose M, Banerjee SP, Bhandari R, Moore GJ, Rosenberg DR. Brain structural abnormalities in psychotropic drug-naive pediatric patients with obsessive-compulsive disorder. American Journal of Psychiatry. 2004;161:1049–1056. doi: 10.1176/appi.ajp.161.6.1049. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Robinson D, Alvir JM, Bilder RM, Lencz T, Ashtari M, Wu H, Bogerts B. Orbital frontal and amygdala volume reductions in obsessive-compulsive disorder. Archives of General Psychiatry. 1999;56:913–919. doi: 10.1001/archpsyc.56.10.913. [DOI] [PubMed] [Google Scholar]
- Togao O, Yoshiura T, Nakao T, Nabeyama M, Sanematsu H, Nakagawa A, Noguchi T, Hiwatashi A, Yamashita K, Nagao E, Kanba S, Honda H. Regional gray and white matter volume abnormalities in obsessive-compulsive disorder: a voxel-based morphometry study. Psychiatry Research. 2010;184:29–37. doi: 10.1016/j.pscychresns.2010.06.011. [DOI] [PubMed] [Google Scholar]
- van Grootheest DS, Cath DC, Beekman AT, Boomsma DI. Twin studies on obsessive-compulsive disorder: a review. Twin Research and Human Genetics. 2005;8:450–458. doi: 10.1375/183242705774310060. [DOI] [PubMed] [Google Scholar]
- Welch JM, Lu J, Rodriguiz RM, Trotta NC, Peca J, Ding JD, Feliciano C, Chen M, Adams JP, Luo J, Dudek SM, Weinberg RJ, Calakos N, Wetsel WC, Feng G. Cortico-striatal synaptic defects and OCD-like behaviours in Sapap3-mutant mice. Nature. 2007;448:894–900. doi: 10.1038/nature06104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendland JR, Moya PR, Timpano KR, Anavitarte AP, Kruse MR, Wheaton MG, Ren-Patterson RF, Murphy DL. A haplotype containing quantitative trait loci for SLC1A1 gene expression and its association with obsessive-compulsive disorder. Archives of General Psychiatry. 2009;66:408–416. doi: 10.1001/archgenpsychiatry.2009.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside SP, Port JD, Deacon BJ, Abramowitz JS. A magnetic resonance spectroscopy investigation of obsessive-compulsive disorder and anxiety. Psychiatry Research. 2006;146:137–147. doi: 10.1016/j.pscychresns.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Wu K, Hanna GL, Rosenberg DR, Arnold PD. The role of glutamate signaling in the pathogenesis and treatment of obsessive-compulsive disorder. Pharmacology, Biochemistry and Behaviour. 2012;100:726–735. doi: 10.1016/j.pbb.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yucel M, Wood SJ, Wellard RM, Harrison BJ, Fornito A, Pujol J, Velakoulis D, Pantelis C. Anterior cingulate glutamate-glutamine levels predict symptom severity in women with obsessive-compulsive disorder. Australian and New Zealand Journal of Psychiatry. 2008;42:467–477. doi: 10.1080/00048670802050546. [DOI] [PubMed] [Google Scholar]
- Zohar AH. The epidemiology of obsessive-compulsive disorder in children and adolescents. Child & Adolescent Psychiatric Clinics of North America. 1999;8:445–460. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.