Abstract
Hydrophilic polymers are commonly applied as surface coatings on vascular devices and have been shown to dissociate during endovascular use, causing hydrophilic polymer embolism (HPE). Adverse effects related to this phenomenon have been recognized and reported. The prevalence of this complication is unknown. We conducted a retrospective study to determine the prevalence of HPE among hospital autopsies over a 29-month period. Postmortem tissue was histologically evaluated for the presence, location(s) and extent of HPE. HPE findings were correlated with documented clinical and laboratory data and patient outcome. Of 136 hospital autopsies examined, 18 (13%) showed evidence of HPE involving the lungs (n = 18), heart (n = 1) or central nervous system (n = 1). Localized pulmonary HPE was seen in 12 patients (9%). Multifocal pulmonary HPE was found in 6 patients (4%) and was associated with clinical vasculitis (33%; P < .0001), suspected pulmonary ischemia (50%; P = .008), coagulopathy (67%; P = .002), and constitutional disease (83%; P = .01). Within affected lung, associated histopathologic changes included occlusive intravascular or perivascular inflammation (89%), intravascular fibrous response (56%), microthrombus formation (44%), vasculitis (28%), and/or pulmonary microinfarction (28%). Statistically significant differences in hospital days (P = .008) and number of vascular interventions (P = .01) were noted between affected and unaffected patients. We conclude that HPE is an underdiagnosed phenomenon with primary involvement of the lungs, where secondary vascular changes are common. Additional studies may be needed to clarify risks and to identify preventative strategies for this iatrogenic complication of catheterizations and “minimally invasive” endovascular techniques.
Keywords: catheterization, drug delivery vehicle, endovascular procedure, HPE, hydrophilic polymer embolism, iatrogenic complication, vasculopathy
Introduction
Hydrophilic polymers are increasingly used for biomedical applications. Enhanced lubrication and biocompatibility, made possible by hydrophilic coats on cardiovascular and neurointerventional devices, allow for less invasive approaches for common endovascular procedures [1-3]. The advent of drug-eluting polymers additionally allows for sustained, targeted release of intravascular drugs which improve therapeutic efficacy and compliance, while reducing systemic drug toxicities. With advanced nano-technologies and evolution of bioengineered insertable and implantable “smart devices”, manufacturers will continue to incorporate this material on new and emerging vascular devices [4-6].
Despite their technological advances, polymer coating materials have been shown to dissociate from device surfaces during endovascular manipulation [7-16], or following implantation in patients [9]. These foreign materials may then deposit in unexpected locations within the body. Recent studies conducted by our group document morbidity and mortality attributable to embolization of polymer particles within the bloodstream [12,13]. Thus, Hydrophilic Polymer Embolism (HPE), a term we introduced in 2010, has recently been established as a potentially fatal iatrogenic disease [13], although its frequency in populations at risk has not been clear.
While vascular devices undergo friction, durability, and particulate trials required by the United States Food and Drug Administration (USP XXII sec 788) [17], risks associated with intravascular polymer applications are not fully recognized by the medical community [12,13,18,19]. To date, the clinicopathological effects associated with HPE in patients have not been systematically evaluated. The recent observation of widespread polymer microemboli in a new fatal case prompted us to perform a retrospective analysis at a tertiary care hospital. Herein, we report the frequency of this condition and analyze associated pulmonary vascular changes in affected patients who died in a hospital setting and underwent autopsy.
Materials and Methods
Autopsy Material and Tissue Processing
During a 29-month interval, from January 1, 2010 to May 30, 2012, 2766 patients died at the University of Maryland, Baltimore, Medical System. Of the total deaths, 198 inhospital autopsy requests were made during this period. Of these, 54 fetal, stillborn, or infant pediatric autopsies were excluded from this study; slides were unavailable in our files on 6 cases; 2 cases consisted of gross examination only. Corresponding tissue slides for the remaining 136 cases were evaluated.
During routine autopsies, standard blocking protocols were used, with expanded organ sectioning utilized in cases with prominent gross pathology (e.g., infarction or hemorrhage) or restricted autopsies. Autopsy blocks ranged from 10-71 (mean, 36 total blocks), per case. Overall mean per organ (and range in sampled organs) were: 10 from the central nervous system (range: 11-38); 6 from the lungs (range: 4-20); 6 from the heart (range: 3-29); 2 from the gastrointestinal tract (range: 1-5); 2 from the genitourinary organs (range: 2-4); 1 from the liver (range: 1-5); 1 from vertebral bone (range: 1-2); 1 from skin (range: 1-3); 1 from muscle and peripheral nerve (range: 1-2); and 1 from aorta (range: 1-4). Tissue blocks were processed per standard protocol, including formalin fixation (5-10 days duration), paraffin embedment, 5-μm thick sectioning, and hematoxylin and eosin (H&E) staining.
Quantification of Polymer Emboli and Autopsy Diagnoses
Autopsy cases were routinely diagnosed by attending staff pathologists at the University of Maryland Medical Center, and were retrospectively re-reviewed by an autopsy pathologist who was blinded to demographic and clinical information. Slides were scanned by light microscopy (200X and 600X) and in some cases were additionally evaluated on serial step cuts and special staining [13,20,21]. Localized HPE was defined by polymer deposition within a single organ, whereas multifocal HPE was defined by widespread involvement of both lungs and/or spread to multiple organ systems. HPE-positive cases were re-reviewed by a senior autopsy pathologist to confirm presence of foreign polymer materials. Alternate diagnoses were ruled out on histologic evaluation conducted by an autopsy pathologist with expertise on vascular diseases.
Clinical Data and Hospital Course
Demographic and clinical data were collected from electronic medical records by a co-investigator who was blinded to autopsy results. Age, sex, previous medical history, and drug habits were noted from admission notes. Inpatient vascular procedures, clinical signs, symptoms and clinically suspected cause of death were determined by review of inpatient and death notes originating during the final month of life. Number and duration of admissions during the final year of life were also tabulated. Post-admission laboratory values were recorded from inpatient flowsheets.
Associated Factors and Statistical Analysis
To investigate factors associated with a diagnosis of HPE, several demographic, clinical, laboratory and pathology variables were examined. The number of percutaneous vascular interventions incorporating polymer coated catheters and devices during the final month of life were tabulated. Numbers of tissue blocks evaluated at autopsy were additionally analyzed. Two-group comparisons were made among patients with HPE (multifocal, localized, or all) and without HPE. Statistical analysis was performed using JMP software (Cory, NC, USA). Categorical variables were compared using the Chi-squared (Pearson) test. Continuous variables were compared using ANOVA one-way test. A P value of less than 0.05 was considered to indicate statistical significance.
Results
Patient Autopsies
A total of 4794 tissue slides originating from 136 adult and adolescent hospital autopsies were evaluated (patient age range, 10-96 years; 53% male). Per autopsy consent, 85 cases were unrestricted autopsies; 26 cases excluded examination of the head; 19 cases consisted only of chest examination; 4 autopsies were limited to examination of the heart, abdomen, pancreas, or liver only; while 2 cases were limited to examination of the brain.
Of 136 autopsy cases examined, 18 cases (13%) showed histologic evidence of HPE. One decedent had multifocal, systemic evidence of HPE with widespread involvement of the lungs, heart and central nervous system; 5 decedents had multifocal intrapulmonary HPE with involvement of all lobes; 12 decedents had only rare HPE with localized involvement of 1 or more lobes of lung (Table 1). Affected patients included 13 men, 4 women, and 1 adolescent male (age range, 16-73 years) who had no documented heritable coagulopathies or history of active drug use. Additional demographical information is detailed in Table 2. No statistically significant differences in mean age or number of autopsy tissue blocks evaluated were found between affected and unaffected patients (Table 3).
Table 1.
Disease burden and anatomical distribution of HPE in 18 patients with Multifocal or Localized involvement.
| Multifocal HPE (n=6) | Localized HPE (n=12) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | 1 | 2 | 3 | 4 | 5 | 6 | 7a | 8a | 9 | 10 | 11 | 12a | 13 | 14 | 15 | 16 | 17 | 18a |
| Anatomical Region | ||||||||||||||||||
| LUNGS | ||||||||||||||||||
| Right upper lobe | ++ | ++ | ++ | +++ | +++ | ++ | 0 | 0 | + | + | + | 0 | + | 0 | 0 | + | + | 0 |
| Right middle lobe | ++ | ++ | + | ++ | +++ | ++ | + | + | ++ | 0 | 0 | 0 | + | 0 | 0 | 0 | + | + |
| Right lower lobe | ++ | ++ | ++++ | ++ | +++ | ++ | + | + | 0 | 0 | 0 | 0 | + | 0 | 0 | 0 | 0 | + |
| Left upper lobe | +++ | +++ | ++ | +++ | ++ | ++ | + | + | 0 | + | 0 | + | 0 | 0 | 0 | 0 | 0 | 0 |
| Left lower lobe | ++ | +++ | ++ | +++ | ++ | ++ | + | + | 0 | 0 | 0 | + | 0 | + | + | 0 | 0 | 0 |
| HEART | + | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| BRAIN | ||||||||||||||||||
| Cerebral cortex | ++ | 0 | 0 | 0 | 0 | 0 | N/A | N/A | 0 | 0 | 0 | N/A | 0 | 0 | 0 | 0 | 0 | N/A |
| White matter | ++ | 0 | 0 | 0 | 0 | 0 | N/A | N/A | 0 | 0 | 0 | N/A | 0 | 0 | 0 | 0 | 0 | N/A |
| Subcortical nuclei | ++ | 0 | 0 | 0 | 0 | 0 | N/A | N/A | 0 | 0 | 0 | N/A | 0 | 0 | 0 | 0 | 0 | N/A |
| Leptomeninges | ++ | 0 | 0 | 0 | 0 | 0 | N/A | N/A | 0 | 0 | 0 | N/A | 0 | 0 | 0 | 0 | 0 | N/A |
| Cerebellum | + | 0 | 0 | 0 | 0 | 0 | N/A | N/A | 0 | 0 | 0 | N/A | 0 | 0 | 0 | 0 | 0 | N/A |
| Brainstem | + | 0 | 0 | 0 | 0 | 0 | N/A | N/A | 0 | 0 | 0 | N/A | 0 | 0 | 0 | 0 | 0 | N/A |
| SPINAL CORD | + | 0 | 0 | 0 | 0 | 0 | N/A | N/A | 0 | 0 | 0 | N/A | 0 | 0 | 0 | 0 | 0 | N/A |
| Number of Vessels with HPE | Mean = ~70 (Range: 25 – over 200) | Mean = ~7 (Range: 1 – 10) | ||||||||||||||||
| Size of Vessels with HPE | Mean = ~100 μm (Range: 7 μm – 1.3 mm) | Mean = ~100 μm (Range: 13 μm – 370 μm) | ||||||||||||||||
Semiquantitative scores: 0, none; +, sparse (1-5); ++, moderate (6-10); +++, common (11-15); ++++, widespread (>15)
Abbreviations: HPE, hydrophilic polymer emboli; N/A, not available
Central nervous system examination was excluded on restricted autopsy
Table 2.
Summary of comorbidities, recent procedures, and clinicopathological course in 18 patients with Multifocal or Localized HPE.
| Case Age Sex | Comorbid Conditions and → Suspected Contributing Procedure(s) | Documented Clinical Cause of Death | Documented Cause(s) of Death on Original Autopsy | Documented Signs and Diagnoses (Final Month of Life) | Findings on Retrospective CP Review |
|---|---|---|---|---|---|
|
Multifocal HPE (n=6)
| |||||
| 1 73F |
Hypertrophic cardiomyopathy; HTN; DM; CKD requiring HD → Hemodialysis; CVVH; Cardiac cath; CVC |
Cardiac insufficiency | Per clinical history, probable cardiac insufficiency | ■ Possible pneumonia ■ Pulmonary embolism ■ Vasculitis (p-ANCA +) ■ Lymphadenopathy ■ Sepsis with DIC, MODS |
aMultiorgan failure; Widespread HPE; Hypertrophic cardiomyopathy |
| 2 65M |
Aortic stenosis requiring prior valve replacement; A. fib; HTN; DM; Recent cardioversion complicated by Clostridium difficile colitis → CVC |
Respiratory insufficiency | Respiratory failure/DIC | ■ Possible pneumonia ■ Hemoptysis ■ Decubitus ulcer ■ Sepsis with DIC, MODS |
aMultiorgan failure; DIC; HPE; Scattered pulmonary and cerebral infarcts |
| 3 64M |
HTN; DM; CKD; A. fib; Ischemic cardiomyopathy; recent OHT (on immunosuppressive therapy) → CVC; ECMO; Hemodialysis; CVVH |
Septic shock | Pneumonia; Septic shock; Peripheral pulmonary emboli noted | ■ Mediastinitis ■ Groin infection ■ Pneumonia ■ Pulmonary embolism ■ Upper GI bleed ■ Acute PEA + SVT ■ Septic shock with MODS |
a, bSeptic shock; Pneumonia; Pulmonary HPE; Pulmonary microinfarct |
| 4 69M |
CAD; prior CABG; HL; recent MVA w/ Brown Sequard syndrome complicated by empyema and sepsis → CVC |
Sepsis; leptomeningitis; brainstem infarcts | Sepsis; leptomeningitis; brainstem infarcts | ■ Leptomeningitis ■ Embolic stroke ■ A fib with RVR ■ DVT ■ Septic shock with DIC, MODS |
Sepsis; Leptomeningitis; Brainstem infarcts; Pulmonary HPE |
| 5 41M |
Remote h/o aortic dissection s/p repair; HTN; hepatitis C; osteogenesis imperfecta; Presented to ED with acute diarrhea → CVC; Arterial Line; Hemodialysis |
Septic shock; embolic stroke | Multiple embolic infarcts involving lungs, brain, kidneys, liver, spleen | ■ Vasculitis (p-ANCA −) ■ Embolic stroke ■ Septic shock with DIC, MODS ■ Anoxic brain injury |
cMultiple embolic infarcts involving lungs, brain, kidneys, liver, spleen secondary to septic emboli; Pulmonary HPE |
| 6 59M |
Abdominal liposarcoma requiring recent colectomy → PICC; CVC; Arterial line |
Acute Pulmonary embolism | Acute pulmonary embolism due to foreign material | ■ Pulmonary embolism ■ Sudden death |
a,b,dAcute pulmonary embolism due to foreign material |
|
Localized HPE (n=12) | |||||
| 7 57F |
h/o breast CA; dilated cardiomyopathy; PFO; HTN; CKD; A. fib requiring warfarin; Admitted for cellulitis requiring IV Ab → CVC; Cardiac Cath |
Nonischemic cardiomyopathy | Multiorgan failure associated with cardiomyopathy | ■ Pulmonary embolism ■ Pneumonia ■ A fib with RVR ■ DVT ■ SIRS/shock with DIC, MODS |
Multiorgan failure; Cardiomyopathy; Pulmonary HPE |
| 8 21F |
Morbid obesity; Admitted for spontaneous pneumothorax; complicated by pneumonia, empyema requiring IV Ab and R. lung wedge resection → CVC; ECMO |
Pneumonia; sepsis; Cor pulmonale | Pneumonia; sepsis; DAD; Cor pulmonale | ■ Pneumonia ■ Pulmonary embolism ■ Septic shock ■ Coagulopathy with DIC, MODS |
bPneumonia; sepsis; DAD; Cor pulmonale; Pulmonary HPE |
| 9 55M |
Aortic regurgitation; aortic aneurysm; HTN; HL; CHF; Admitted for aortic valve replacement → Cardiac Bypass |
Acute PEA | Acute PEA; Anoxic brain injury | ■ Acute PEA ■ Anoxic brain injury |
Acute PEA; Pulmonary HPE; Anoxic brain injury |
| 10 65M |
Rectal CA s/p recent resection; CAD; HTN; HL; Recent pneumonia requiring IV Ab → CVC; Arterial line |
Acute pneumonia | Acute pneumonia | ■ Resolving perineal wound ■ Acute pneumonia ■ Acute hypoxemia ■ Acute PEA |
Acute pneumonia; Pulmonary HPE |
| 11 61M |
h/o CVA; MI; HTN; HL; CKD; Admitted for upper GI bleed/newly diagnosed signet ring CA (gastric) → CVC |
Hemorrhagic shock; signet ring CA | Hemorrhagic shock; signet ring CA | ■ GI hemorrhage/signet ring CA ■ Hemorrhagic shock ■ ARDS ■ MODS |
Hemorrhagic shock; Signet ring CA; Pulmonary HPE |
| 12 16M |
Anomalous left coronary artery requiring surgical repair → ECMO; Swan-Ganz |
Acute cardiac tamponade; Pneumonia | Acute cardiac tamponade; Pneumonia | ■ Pulmonary hemorrhage ■ Pneumonia ■ Pulmonary embolism ■ Septic shock with MODS |
Cardiac tamponade; Pneumonia; Pulmonary HPE |
| 13 60M |
h/o CAD; DM; HL; ESRD on HD; Aortic stenosis; Schizophrenia; Admitted for acute MI → Hemodialysis; CVC; IVC Filter |
Cardiogenic shock | Cardiogenic shock | ■ Cardiogenic shock ■ Pneumonia ■ DVT ■ MODS ■ Upper GI bleed |
Cardiogenic shock; Pulmonary HPE |
| 14 47F |
Rheumatoid arthritis; nonischemic cardiomyopathy; Admitted for CHF →CVC; CVVH |
Nonischemic cardiomyopathy | Nonischemic cardiomyopathy; Pneumonia | ■ Cardiogenic shock ■ MODS |
Nonischemic cardiomyopathy; Pneumonia; Pulmonary HPE |
| 15 61M |
COPD; esophageal ulcers; recent admission for cysturethroscopy; Readmitted for syncope → CVC; Pulmonary Angiography |
Pulmonary thromboembolism | Pulmonary thromboembolism | ■ Pulmonary thromboembolism | bPulmonary thromboembolism; Pulmonary HPE |
| 16 48M |
h/o Fournier's gangrene admitted for debridement → CVC |
Septic shock | Septic shock; acute pulmonary hemorrhage | ■ Pneumonia ■ Pulmonary Embolism ■ Urinary tract infection ■ Gastrointestinal bleed ■ Possible DVT ■ Septic shock with MODS |
bSeptic shock, Acute pulmonary hemorrhage; PNA; Pulmonary HPE |
| 17 35M |
HTN; CAD; HL; Admitted for acute type A aortic dissection → ECMO |
Acute aortic dissection | Acute aortic dissection | ■ Acute MI ■ Acute aortic dissection ■ Anoxic brain injury ■ Cardiac tamponade ■ MODS |
Acute aortic dissection; Scattered cerebral infarcts; Pulmonary HPE |
| 18 71M |
h/o CAD; A. Fib; HL; PVD; Recent cardiac cath/stenting Recent lower extremity vascular bypass surgery → CVC; Lower Extremity Bypass; CVVH |
Septic shock; CHF | Septic shock; CHF | ■ Septic shock ■ Rhabdomyolysis ■ Small bowel ischemia ■ MODS |
Septic shock; CHF Pulmonary HPE |
Abbreviations: A. Fib, atrial fibrillation; B/L, bilateral; CA, adenocarcinoma; CABG, coronary artery bypass graft; CAD, coronary artery disease; cardiac cath, cardiac catheterization; CKD, chronic kidney disease; CP, clinicopathological; CVA, cerebral vascular accident; CVC, central venous catheterization; CVVH, continuous veno-venous hemofiltration; DIC, disseminated intravascular coagulation; DM, diabetes mellitus type II; DVT, deep venous thrombosis; ECMO, extracorporeal membrane oxygenation; ESRD, end-stage renal disease; HD, hemodialysis; HL, hyperlipidemia; h/o, history of; HPE, hydrophilic polymer emboli; HTN, hypertension; HL, hyperlipidemia; IV Ab, intravenous antibiotics; IVH, intravenous hydration; LAD, lymphadenopathy; MI, myocardial infarction; MODS, multiple organ dysfunction syndrome; OHT, orthotopic heart transplantation; PE, pulmonary embolism; PEA, pulseless electrical activity; PFO, patent foramen ovale; PICC, peripherally inserted central catheter; PNA, bronchopneumonia; R., right; RUL, right upper lobe of lung; RUQ, right upper quadrant; RVR, rapid ventricular response; SIRS, systemic inflammatory response syndrome; s/p, status post; SVT, supraventricular tachycardia; VSD, ventricular septal defect.
HPE may have been a primary cause of morbidity
“Rule out pulmonary embolism” specified on autopsy request
Scattered septic emboli were present, along with multifocal HPE
Mechanism of death is inconclusive (in addition to HPE, rare intravascular plant material is identified)
Table 3.
Documented demographic, clinical, and laboratory or pathology findings in 136 patients without or with (all, multifocal, or localized) HPE.
| No HPE (n=118) | All HPE (n=18) | Multifocal HPE (n=6) | Local HPE (n=12) |
P valued |
|||
|---|---|---|---|---|---|---|---|
| All HPE | Multifocal HPE | Local HPE | |||||
| Demographic Data | |||||||
| Age (years, mean) | 56 ± 14 | 54 ± 17 | 62 | 50 | 0.66 | ||
| Sex (% male) | 53 | 78 | 83 | 75 | 0.07 | ||
| Hospital days (mean, current admission) | 26 | 46 | 56 | 42 | 0.008* | 0.05 | 0.09 |
| ICU days (mean, current admission) | 13 | 25 | 39 | 19 | 0.01* | 0.02* | 0.40 |
| Total admissions (mean, prior year) | 2 | 2 | 2 | 2 | 0.99 | ||
| Vascular procedures (mean) | |||||||
| Total vascular interventions | 4 ±3.5 | 7 ± 5.5 | 8 | 6 | 0.01* | 0.02* | 0.08 |
| Left heart/arterial interventions | 2 ± 1.3 | 2 ± 1.8 | 2 | 2 | 0.16 | 0.17 | 0.40 |
| Right heart/venous interventions | 3 ± 2.6 | 5 ± 3.6 | 6 | 4 | 0.008* | 0.02* | 0.07 |
| Central catheters | 2 ± 1.7 | 3 ±1.7 | 3 | 3 | 0.03* | 0.07 | 0.14 |
| Other right heart and venous | 2 ± 1.0 | 2 ± 1.9 | 3 | 2 | 0.05 | 0.06 | 0.02* |
| Autopsy tissue blocks examined (mean) | |||||||
| Total blocks | 34 ±14 | 41 ± 10 | 40 | 42 | 0.06 | ||
| Lung blocks | 6 ± 3 | 7 ± 3 | 7 | 7 | 0.16 | ||
| Heart blocks | 6 ± 5 | 7 ± 3 | 8 | 6 | 0.88 | ||
| Central nervous system blocks | 10 ± 8 | 10 ± 7 | 9 | 11 | 0.74 | ||
| Clinical Sign or Symptom Prior to Deatha | |||||||
| Constitutional symptomsb | 46 (39%) | 14 (78%) | 6 (100%) | 8 (67%) | 0.002* | 0.003* | |
| Multiple organ dysfunction syndrome (MODS) | 20 (17%) | 14 (78%) | 5 (83%) | 9 (75%) | <0.001* | <0.0001* | <0.0001* |
| SIRS/sepsis | 38 (32%) | 10 (56%) | 5 (83%) | 5 (42%) | 0.054 | 0.01* | |
| Suspected pulmonary embolism | 14 (12%) | 8 (44%) | 3 (50%) | 5 (42%) | 0.006* | 0.008* | 0.005* |
| Thrombo/embolic stroke | 19 (16%) | 3 (17%) | 2 (33%) | 1 (8%) | 0.95 | ||
| Suspected vasculitis | 1 (1%) | 2 (11%) | 2 (33%) | 0 (0%) | 0.006* | <0.0001* | |
| Lymphadenopathy | 20 (17%) | 2 (11%) | 2 (33%) | 0 (0%) | 0.53 | ||
| Acute cardiac arrhythmia/PEA | 24 (20%) | 2 (11%) | 0 (0%) | 2 (17%) | 0.35 | ||
| Laboratory or Pathology Findinga§§ | |||||||
| Leukocytosis | 94 (80%) | 18 (100%) | 6 (100%) | 12 (100%) | 0.035* | ||
| ↑ D-dimer ↑ fibrin split products ↓ fibrinogen | 20 (17%) | 6 (33%) | 4 (67%) | 2 (17%) | 0.10 | 0.002* | |
| Acute pulmonary infarct(s) | 5 (4%) | 5 (28%) | 3 (50%) | 2 (17%) | <0.001* | <0.0001* | |
| Acute focal cerebral infarct(s) | 8 (7%) | 4 (22%) | 3 (50%) | 1 (8%) | 0.03* | 0.0002 | |
| ↑ Erythrocyte sedimentation rate (ESR) | 2 (2%) | 2 (11%) | 2 (33%) | 0 (0%) | 0.03* | 0.0001* | |
| ↑ C-reactive protein (CRP) | 2 (2%) | 3 (17%) | 2 (33%) | 1 (8%) | 0.002* | 0.0001* | |
| ↑ p-ANCA | 1 (1%) | 1 (6%) | 1 (17%) | 0 (0%) | 0.12 | 0.003* | |
| ↑ c-ANCA | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | N/A | ||
Plus-minus values are means ± SD; Abbreviations: DIC, disseminated intravascular coagulation; HPE, hydrophilic polymer emboli; ICU, intensive care unit; PEA, pulseless electrical activity; SIRS, Systemic inflammatory response syndrome
Indicates number of patients, and percent
Defined by fevers, chills, malaise, and sweats or weight loss
c Indicates patients with documented abnormality (not all patients were tested)
P values are based upon comparison of patients with no HPE
Indicates P<0.05
Quantification of Polymer Emboli and Associated Tissue Reactions
On histologic examination, occlusive polymer emboli were non-refractile, nonpolarizable, lamellated, and finely granular and basophilic on H&E stain (Figs. 1 and 2) [13]; and were red on Congo Red and Mucicarmine stains (Fig. 2G and 2H, respectively) [20], and blue on Masson trichrome stain (Fig. 2I) [13,20,21]. Affected vessels numbered from 1 to greater than 200 per case, and ranged from 7 μm – 1.3 mm in diameter (mean: ~100 μm). Concordance of findings among reviewers was high (>90%). Anatomical distribution of HPE for each case is delineated in Table 1; preferential involvement was noted within the lungs. Associated pulmonary intravascular and/or perivascular inflammation was present in 16 decedents (89%) (Figs. 1 and 2) [9,11-14], including macrophages (16 cases, 89%) (Fig. 1C and D); foreign body giant cell reaction (11 cases, 61%) (Figs. 1E); neutrophils (12 cases, 67%); and rare eosinophils (5 cases, 28%). Additional associated tissue changes included intravascular fibrous reaction in 10 cases (Masson Trichrome) (56%) (Figs. 1F); microthrombus formation in 8 cases (44%) (Fig.1G); fibrinoid vascular necrosis in 6 cases (33%); giant cell or granulomatous vasculitis in 5 cases (28%) (Fig. 2B); internal elastic damage in 5 cases (elastic stain) (28%); and intravascular and/or perivascular neutrophilic collection in 3 cases (17%) (Fig.2C). Associated hemorrhagic microinfarcts were also found (5 cases, 28%) [13]. Heterogeneous vascular reactions to polymer emboli were frequently observed (Fig. 2A-E). Punctate intravascular calcification was associated with luminal fibrous reaction in two cases (alizarin red). No definite tissue reactions were observed in two cases. On original autopsies, evidence of HPE was recorded only in 3 patients (17%), having been overlooked in the majority of affected decedents (83%).
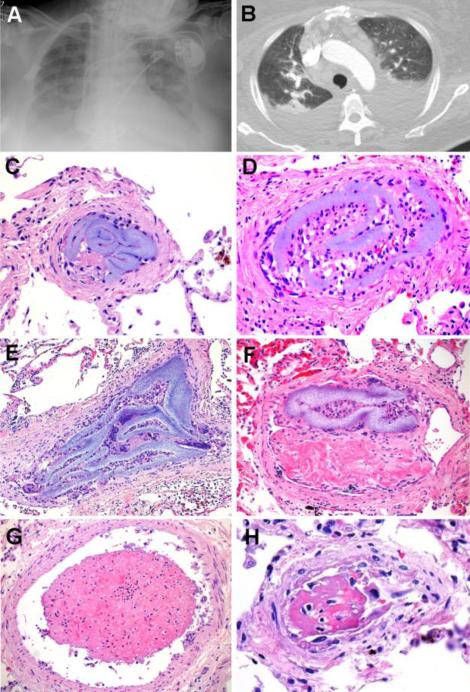
Figure 1. Summary, Patient 1.
A 73-year-old woman with hypertrophic cardiomyopathy, sinus node dysfunction, hypertension, diabetes mellitus type 2 and chronic renal disease presented with pulmonary edema. Cardiac enzymes were negative; BNP and creatinine were elevated. Initially, the patient was improving with diuresis, intravenous fluids and dialysis. After admission, a CVC was placed and broad-spectrum antibiotics were administered for suspected pneumonia. The patient's respiratory symptoms subsequently worsened; however, transthoracic echocardiography and right and left cardiac catheterization revealed no definite cardiac etiology for her symptoms. During her hospitalization, she developed suspected PE, elevated ESR, p-ANCA+ vasculitis, coagulopathy of unknown cause, and prominent mediastinal lymphadenopathy. Chest X-Ray (A) and pulmonary CT angiography (B) showed pleural effusions and areas of pulmonary consolidation, with no evidence of PE. Despite multiple negative cultures, the patient developed multiple organ dysfunction syndrome and suspected sepsis, progressing to death. Autopsy, performed 1 month after admission, failed to reveal any natural etiology for her terminal symptoms. Retrospective histopathological analysis, however, showed multifocal HPE involving the bilateral peripheral pulmonary arteries (C-F), as well as the heart and central nervous system (not shown). Intravascular histiocytes (D), giant cells (E), and fibrous reaction (F) were associated with HPE. Adjacent vessels showed microthrombus formation (G) and areas of vascular injury (H). The cause of death was retrospectively attributed to widespread pulmonary HPE in the setting of hypertrophic cardiomyopathy [C-H: H&E, original matnification 600X].
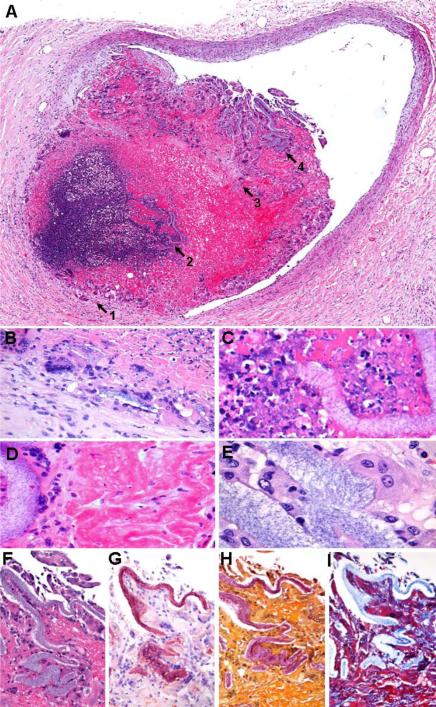
Figure 2. Summary, HPE Vasculopathy.
Heterogeneous reactions (A) elicited by intravascular polymer include foreign body giant cell vasculitis (arrow 1), intravascular microabscess formation (arrow 2); intravascular fibrous reaction (arrow 3); and histiocytic response (arrow 4) (magnified in panels B-E, respectively); high-power microscopy further demonstrates lamellated strips of foreign granular material, consistent with polymer [A-F: H&E; G: Congo Red; H: Mucicarmine; H: Masson Trichrome; A: 200X; B-I: 400X].
Clinical Presentation
Clinical signs, symptoms, and suspected patient diagnoses are shown in Tables 2 and 3. Suspected pulmonary embolism (PE) was documented in a greater proportion of patients with HPE, compared to those without HPE (P = 0.006); likewise, pulmonary infarcts were identified more commonly on routine autopsies (P < 0.001). Concurrent saddle thromboembolus was identified in one patient who had known deep venous thrombosis (case 15); another patient who died acutely from PE had widespread pulmonary HPE and rare intravascular plant material of unclear origin (case 6); and 1 patient had concurrent septic emboli (case 5) (Table 2). The remaining 15 patients with HPE showed no cholesterol, fat, or thrombo-emboli, or other significant polarizable or non-polarizable embolic sources on histopathologic evaluation, although bone marrow microemboli were incidentally found within the lungs of 6 patients who underwent attempted resuscitation prior to death. Although clinical suspicion of thromboembolic stroke was not more prevalent among affected patients, focal embolic cerebral infarcts were incidentally found in a greater proportion of patients, relative to those without HPE (P = 0.03) (Table 3). Patients with multifocal HPE were more likely to exhibit clinical evidence of vasculitis, ischemia and/or infarction, and constitutional disease, relative to those with localized or no HPE (Table 3).
Conditions of Hospitalization
Patients with HPE had undergone a significantly greater number of percutaneous vascular procedures incorporating polymer-coated devices, relative to patients with no HPE (P = 0.01), and had longer hospitalizations before death (P = 0.008) (Table 2). One affected patient had undergone insertion of a Cook Spectrum Glide central venous catheter (CVC) (Cook, Bloomington, IN), only [11]. In the remaining patients, definite causative device(s) could not be determined retrospectively [13]. However, continuous veno-venous hemofiltration was noted among a greater proportion of patients with HPE, relative to those without HPE (P = 0.001). Patients with multifocal HPE had additionally undergone placement of arterial lines, peripherally inserted central catheters, hemodialysis, cardiac catheterization and/or extracorporeal membrane oxygenation (ECMO) (Table 2).
Discussion
Hydrophilic coating materials are widely used on medical devices and have revolutionized catheterization and endovascular devices and techniques. Despite their numerous unique advantages, these substances occasionally dissociate from device surfaces and deposit within the bloodstream, thereby embolizing distally during clinical use [13]. Intracerebral polymer microemboli were first described by Barnwell et al following use of infusion microcatheters (Target Therapeutics, Fremont, CA) [7]. Subsequent reports illustrated localized polymer reactions at dermal access sites following transcutaneous cardiovascular procedures (Cook, Bloomington, IN) [8,9]. Recent studies further demonstrate polymer microembolism due to diverse procedures and illustrate potential for distal inflammatory and/or ischemic parenchymal damage within the vital organs, including the lungs [11,13], brain [7,12,13], heart [14, 16], kidney [15], liver (Mehta, unpublished observation) and lower limb [13]. Notably, foreign polymer materials have been documented also in peripheral arteriovenous grafts following dialysis as well as in explanted organs originating from patients who underwent invasive monitoring prior to transplantation [13,15]. The overall frequency of HPE in populations at risk, however, is not previously reported.
We performed a histopathological analysis to assess how commonly polymer microparticle embolism is detected among adolescent and adult hospital decedents. In this retrospective 29-month autopsy study of 136 patients, we identify a prevalence rate of 13%. Most affected patients showed only localized arterial deposits within peripheral lung (9%); however, multifocal pulmonary parenchymal involvement was found in 6 patients (4%). Associated microvascular injuries were commonly found, including inflammatory and/or fibrous response, with vascular occlusion and occasional microinfarcts involving areas of peripheral lung. The diverse patterns of vascular damage were consistent with iatrogenic polymeric reactions elicited in experimental animal models [21,22].
HPE Vasculopathy: Potential Clinical Implications & Limitations of the Current Study
We speculated previously that ischemia and infarction due to HPE are clinically under-recognized [12-13]. Morever, growing evidence suggests that clinical sequelae attributable to HPE may be highly disparate [11-16], depending on organ and degree of involvement, among several other device and patient-related factors. The clinicopathological effects associated with HPE, however, have not yet been systematically evaluated.
In this study, we illustrate preferential HPE involvement within the distal pulmonary vasculature, wherein the body naturally filters systemic venous blood. To our knowledge, only 6 prior cases of pulmonary HPE have been reported in the literature [11,13]. One was that of a 22-year-old woman, reported by our group [13], who developed pulmonary infarction and death following central catheterization (device unknown). Another was the case of a 34-year-old woman, reported by Allan et al [11], who developed lung abscesses and vasculitis after central catheterization (Cook, Bloomington, IN) [11]. Sequelae in both patients were due to vascular damage and obstruction resulting from pulmonary HPE. The latter patient was found to have elevated p-antineutrophil cytoplasmic antibody (p-ANCA) and C-reactive protein in the setting of active Crohn colitis, and multiple lung wedge resections were required to determine the etiology of her transient pulmonary disease.
Herein, we report 18 additional cases of pulmonary HPE, including 6 (33%) with multifocal lung involvement. Among these, HPE appeared to be incidental in 14 patients (78%); however, widespread pulmonary HPE directly contributed to death of at least 1 patient (5%). Notably, this patient developed multiple symptoms following uneventful catheterization that were unexplainable at the time of her demise (case 1). She experienced an unusual constellation of findings including suspected PE (pulmonary computed tomography angiogram negative) and vasculitis (p-ANCA positive), with subsequent coagulopathy, lymphadenopathy and systemic inflammatory response disease (SIRS) [additional post-admission laboratory values: elevated CRP, ESR, leukocytes, PT, PTT, D-dimer, fibrin split products; low fibrinogen]. In the absence of other etiologies, the terminal findings were attributed to a syndromic effect resulting from widespread pulmonary HPE. In the remaining patients (17%), HPE may have contributed to morbidity, although definitively affects could not be evaluated due to comorbid disease.
This descriptive study sheds new light regarding the phenomenon of HPE in a select patient population; however, several limitations exist: Extensive tissue sampling of vital organs is currently necessary to confirm presence and distribution of HPE. In autopsy patients, controlling for confounding variables is a limitation to accurate assessment of iatrogenic injuries [23]. Because only limited representative tissues can be sampled retrospectively at autopsy, and because polymer materials gradually biodegrade, the frequency and burden recorded in these patients are likely underestimated. Although this study provides important new data, the true prevalence and significance of HPE cannot be accurately assessed by conventional diagnostic modalities.
Polymer surface coatings have been in wide clinical use for over two decades, suggesting that the complication of polymer embolization has gone undetected “under the radar” for several years. A heightened clinical awareness is necessary for premortem diagnosis of this iatrogenic phenomenon. Given the widespread use of polymer coatings and millions of catheterizations and endovascular procedures performed annually worldwide, morbidity associated with HPE is likely underrecognized. Although the current study highlights the utility of autopsies in this modern era of advanced procedures [24,25], additional investigations and novel diagnostic techniques (e.g., new serum tests) may be needed for additional clinical characterization of this vascular phenomenon. Ultimately, a better understanding of hydrophilic polymer embolism and associated vascular effects may lead to safer and more efficient methods of targeted drug and device delivery, and more importantly, to improved outcomes following catheterizations and “minimally invasive” endovascular procedures.
Acknowledgements
The authors thank Mrs. Stephanie Dampier and Mrs. Rekha Prasad for their excellent technical assistance. R.I.M. is supported by a grant from the National Institute of Neurological Disorders and Stroke (K08NS089830).
Footnotes
Disclosures:
The authors have no conflicts of interest to disclose.
References
- 1.Mann T, Cubeddu G, Bowen J, et al. Stenting in acute coronary syndromes: a comparison of radial versus femoral access sites. J Am Coll Cardiol. 1998;32:572–576. doi: 10.1016/s0735-1097(98)00288-5. [DOI] [PubMed] [Google Scholar]
- 2.Murayama Y, Tateshima S, Gonzalez NR, et al. Matrix and bioabsorbable polymeric coils accelerate healing of intracranial aneurysms: long-term experimental study. Stroke. 2003;34:2031–2037. doi: 10.1161/01.STR.0000083394.33633.C2. [DOI] [PubMed] [Google Scholar]
- 3.Gaba RC, Ansari SA, Roy SS, et al. Embolization of intracranial aneurysms with hydrogel-coated coils versus inert platinum coils: effects on packing density, coil length and quantity, procedure performance, cost, length of hospital stay, and durability of therapy. Stroke. 2006;37:1443–1450. doi: 10.1161/01.STR.0000221314.55144.0b. [DOI] [PubMed] [Google Scholar]
- 4.Peppas NA, Bures P, Leobandung W, Ichikawa H. Hydrogels in pharmaceutical formulations. Eur J Pharm Biopharm. 2000;50:27–46. doi: 10.1016/s0939-6411(00)00090-4. [DOI] [PubMed] [Google Scholar]
- 5.Qiu Y, Park K. Environment-sensitive hydrogels for drug delivery. Adv Drug Deliv Rev. 2001;53:321–39. doi: 10.1016/s0169-409x(01)00203-4. [DOI] [PubMed] [Google Scholar]
- 6.Chaterji S, Kwon IK, Park K. Smart Polymeric Gels: Redefining the Limits of Biomedical Devices. Prog Polym Sci. 2007;32:1083–122. doi: 10.1016/j.progpolymsci.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnwell SL, D'Agostino AN, Shapiro SL, et al. Foreign bodies in small arteries after use of an infusion microcatheter. Am J Neuroradiol. 1997;18:1886–1889. [PMC free article] [PubMed] [Google Scholar]
- 8.Kozak M, Adams DR, Ioffreda MD, et al. Sterile inflammation associated with transradial catheterization and hydrophilic sheaths. Cathet Cardiovasc Intervent. 2003;59:207–213. doi: 10.1002/ccd.10522. [DOI] [PubMed] [Google Scholar]
- 9.Fealey ME, Edwards WD, Giannini C, et al. Complications of endovascular polymers associated with vascular introducer sheaths and metallic coils in 3 patients, with literature review. Am J Surg Pathol. 2008;32:1310–1316. doi: 10.1097/PAS.0b013e318165582a. [DOI] [PubMed] [Google Scholar]
- 10.Meyers PM, Lavine SD, Fitzsimmons BR, et al. Chemical meningitis after cerebral aneurysm treatment using two second-generation aneurysm coils: report of two cases. Neurosurgery. 2004;55:1222. doi: 10.1227/01.neu.0000140987.71791.df. [DOI] [PubMed] [Google Scholar]
- 11.Allan RW, Alnuaimat H, Edwards WD, et al. Embolization of hydrophilic catheter coating to the lungs: report of a case mimicking granulomatous vasculitis. Am J Clin Pathol. 2009;132:794–797. doi: 10.1309/AJCPH2PGCCPA0ZJF. [DOI] [PubMed] [Google Scholar]
- 12.Mehta RI, Mehta RI, Fishbein MC, et al. Intravascular polymer material following coil embolization of a giant cerebral aneurysm. Hum Pathol. 2009;40:1803–1807. doi: 10.1016/j.humpath.2009.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehta RI, Mehta RI, Solis OE, et al. Hydrophilic polymer emboli: an under-recognized iatrogenic cause of ischemia and infarct. Mod Pathol. 2010;23:921–30. doi: 10.1038/modpathol.2010.74. [DOI] [PubMed] [Google Scholar]
- 14.El-Najjar V, Robinson M. Autopsy demonstration of intramyocardial polymer gel emboli associated with a giant-cell reaction following cardiac catheterization: a case report. Cardiovasc Pathol. 2012;21:59–61. doi: 10.1016/j.carpath.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Sequeira A, Parimoo N, Wilson J, Traylor J, Bonsib S, Abreo K. Polymer embolization from minimally invasive interventions. Am J Kidney Dis. 2013;61:984–7. doi: 10.1053/j.ajkd.2012.12.021. [DOI] [PubMed] [Google Scholar]
- 16.Sanon S, Maleszewski JJ, Rihal CS. Hydrophilic polymer embolism induced acute transcatheter aortic valve thrombosis: A novel complication. Catheter Cardiovasc Interv. 2014 Jan 8; doi: 10.1002/ccd.25353. [DOI] [PubMed] [Google Scholar]
- 17.U.S.-Pharmacopeia (788) Particle Matter in Injections. Available at http://www.pharmacopeia.cn/v29240/usp29nf24s0_c788.html.
- 18.Bates MC. Coating Delamination: Chink in the DES Armor? Catheterization and Cardiovascular Interventions. 2010;75:912–3. doi: 10.1002/ccd.22580. [DOI] [PubMed] [Google Scholar]
- 19.Denardo SJ, Carpinone PL, Vock DM, Batich CD, Pepine CJ. Changes to Polymer Surface of Drug-Eluting Stents During Balloon Expansion. JAMA. 2012;307:2148–50. doi: 10.1001/jama.2012.4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Babcock DE1, Hergenrother RW, Craig DA, Kolodgie FD, Virmani R. In vivo distribution of particulate matter from coated angioplasty balloon catheters. Biomaterials. 2013;34:3196–205. doi: 10.1016/j.biomaterials.2013.01.040. [DOI] [PubMed] [Google Scholar]
- 21.Yuki I, Uchiyama N, Murayama Y, Nien YL, Lee D, Ebara M, Ishii A, Chiang A, Vinters HV, Nishimura I, Wu BM, Vinuela F. Intravascular tissue reactions induced by various types of bioabsorbable polymeric materials: correlation between the degradation profiles and corresponding tissue reactions. Neuroradiology. 2010;52:1017–24. doi: 10.1007/s00234-010-0657-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simionescu A, Schulte JB, Fercana G, Simionescu DT. Inflammation in cardiovascular tissue engineering: the challenge to a promise: a minireview. Int J Inflam. 2011;2011:958247. doi: 10.4061/2011/958247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhan C, Miller MR. Excess length of stay, charges, and mortality attributable to medical injuries during hospitalization. JAMA. 2003;290:1868–74. doi: 10.1001/jama.290.14.1868. [DOI] [PubMed] [Google Scholar]
- 24.Shojania KG, Burton EC. The vanishing nonforensic autopsy. N Engl J Med. 2008;358:873–5. doi: 10.1056/NEJMp0707996. [DOI] [PubMed] [Google Scholar]
- 25.Sinard JH. Factors affecting autopsy rates, autopsy request rates, and autopsy findings at a large academic medical center. Exp Mol Pathol. 2001;70:333–43. doi: 10.1006/exmp.2001.2371. [DOI] [PubMed] [Google Scholar]