Abstract
Alcohol induces widespread changes in cytokine expression, with recent data from our laboratory having demonstrated that, during acute ethanol intoxication, adult rats exhibit consistent increases in interleukin (IL)-6 mRNA expression in several brain regions, while showing reductions in IL-1 and TNFα expression. Given evidence indicating that adolescence may be an ontogenetic period in which some neuroimmune processes and cells may not yet have fully matured, the purpose of the current experiments was to examine potential age differences in the central cytokine response of adolescent (P31–33 days of age) and adult (69–71 days of age) rats to either an acute immune (lipopolysaccharide; LPS) or non-immune challenge (ethanol). In Experiment 1, male Sprague-Dawley rats were given an intraperitoneal (i.p.) injection of either sterile saline, LPS (250 µg/kg), or ethanol (4-g/kg), and then trunk blood and brain tissue were collected 3 hr later for measurement of blood EtOH concentrations (BECs), plasma endotoxin, and central mRNA expression of several immune-related gene targets. In Experiment 2, the response to intragastrically (i.g.) administered ethanol was examined and compared to animals given tap water (i.g.). Results showed that LPS stimulated robust increases in expression of IL-1, IL-6, TNFα, and IκBα in the hippocampus, PVN, and amygdala, and that these increases were generally less pronounced in adolescents relative to adults. Following an i.p. EtOH challenge, IL-6 and IκBα expression were significantly increased in both ages in the PVN and amygdala, and adults exhibited even greater increases in IκBα than adolescents. I.g. administration of ethanol also increased IL-6 and IκBα expression in all three brain regions, with hippocampal IL-6 expression elevated even more so in adults compared to adolescents. Furthermore, assessment of plasma endotoxin concentrations revealed (i) whereas robust increases in plasma endotoxin were observed in adults injected with LPS, no corresponding elevations were seen in adolescents after LPS; and (ii) neither adolescents nor adults demonstrated increases in plasma endotoxin concentrations following i.p. or i.g. ethanol administration. Analysis of BECs indicated that, for both routes of exposure, adolescents exhibited lower BECs than adults. Taken together, these data suggest that categorically different mechanisms are involved in the central cytokine response to antigen exposure versus ethanol administration. Furthermore, these findings confirm once again that acute ethanol intoxication is a potent activator of brain cytokines, and calls for future studies to identify the mechanisms underlying age-related differences in the cytokine response observed during ethanol intoxication.
Keywords: Rat, Adolescent, Ethanol, Lipopolysaccharide, Cytokines, Endotoxin
1. Introduction
Ethanol exposure has been recognized as significantly influencing a variety of neurotransmitter systems, including but not limited to GABA, glutamate, dopamine, serotonin, and endocannabinoid systems (for review see [1,2]). Yet, research has now demonstrated that ethanol can also profoundly impact inflammatory-related processes, both peripherally as well as in the brain. Historically, the role of inflammatory pathways in alcohol-related liver disease and organ damage has been the focus of research, with studies in this area commonly examining the effects of acute or chronic ethanol exposure on expression of immune factors after an antigen challenge. Results from experiments such as these have shown a complex relationship between ethanol exposure and the immune response, with ethanol sometimes dampening cytokine expression in response to an antigen [e.g., 3,4–6], yet exacerbating the cytokine response to bacterial challenge in other instances [e.g., 7,8,9].
Studies of this nature have been crucial in elucidating the mechanisms responsible for organ damage under conditions of chronic ethanol consumption, as well as an enhanced susceptibility to infection that is often observed among alcoholics (for review see [10]). More recently, however, evidence indicates that ethanol can also dramatically alter inflammatory-related factors in the absence of an immune challenge. In humans, for example, elevations in several plasma cytokines were observed during withdrawal following an acute alcohol challenge [11,12]. Additionally, post-mortem examination of the brains of alcoholics demonstrated that monocyte chemotactic protein (MCP)-1, a chemokine, was increased in the ventral tegmental area, substantia nigra, hippocampus, and amygdala, with microglial markers also increased in certain brain regions [13]. In parallel, chronic ethanol administration or long term ethanol consumption has been shown to significantly elevate expression of a variety of cytokines in the brain, including interleukin (IL)-1β, IL-6, tumor necrosis factor alpha (TNFα), and MCP-1 in both rats [14,15] and mice [8,16,17]. Furthermore, increased expression of these inflammatory factors has been observed across several different brain regions such as hippocampus [e.g., 14], cortex [e.g., 16], and cerebellum [e.g., 18].
While studies such as those described above have consistently demonstrated that chronic ethanol exposure can influence inflammatory factors, other research has shown that manipulation of neuroimmune pathways/processes can alter ethanol intake, ethanol responsivity, and ethanol reward/reinforcement. Studies utilizing knock-out mice have indicated that deletion of several immune-related genes resulted in significant reductions in ethanol intake and preference when compared to wild-type mice, while also increasing sensitivity to ethanol-induced conditioned taste aversion [19,20]. Furthermore, when mice were injected with minocycline (a putative microglia inhibitor), ethanol intake was similarly reduced [21]. In contrast, however, stimulation of immune processes by lipopolysaccharide (LPS) administration led to increased ethanol intake in mice [22]. Moreover, acute ethanol-induced sedation and motor impairment were also affected by alterations in cytokine signaling, as administration of minocycline or interleukin-1 receptor antagonist (IL-1ra) to adult mice differentially impacted these measures of ethanol responsivity [23].
Given evidence that alterations in neuroimmune pathways have been shown to influence ethanol responsivity and intake, it is not surprising that the effects of ethanol exposure during adolescence on immune-related factors have now begun to receive attention. Indeed, adolescence is now known to be an ontogenetic period characterized by elevated ethanol consumption [24,25], as well as a unique sensitivity to ethanol (for review see [26]). For example, using an animal model of adolescence (for review see [27]), studies have shown that adolescent rodents are less sensitive to many consequences of ethanol exposure, including the sedative/hypnotic [28], motor impairing [29], hangover [30], aversive [31], and social inhibitory [32] effects. Yet, on the other hand, adolescents are seemingly more sensitive to other consequences of ethanol exposure, such as ethanol-induced memory impairment [33], ethanol-related deficits in hippocampal LTP [34], and the social stimulatory effects of acute ethanol exposure [32]. As these studies identified the sensitivity of the hippocampus to adolescent ethanol exposure, several research groups have begun to explore the effects of adolescent binge ethanol administration on the hippocampus, as well as other brain regions known to undergo profound neurodevelopmental changes during adolescence. For instance, McClain et al. (2011) reported that adolescent binge ethanol exposure led to an upregulation of partially activated microglia (the resident macrophage of the brain) in the hippocampus [35]. Additionally, BrdU/Iba-1 co-labeling demonstrated that adolescent binge ethanol led to increased proliferation of new microglia in this brain region, with these microglia remaining up to 30 days after ethanol exposure [35]. Crews and colleagues have also reported that a similar adolescent binge ethanol exposure upregulated RAGE/TLR-4 and HMGB1 in the PFC of adolescent rats, as well as gene expression of numerous other neuroimmune factors, and these effects persisted well into adulthood [36,37]. Notably, a recent study [38] would suggest that some of these effects of adolescent binge ethanol administration are specific to exposure during adolescence, as an ethanol-related up-regulation of expression of TLR-2, TLR-4, IL-1, and TNFα in the PFC was only observed in adolescent but not adult mice under these circumstances.
While studies such as these have demonstrated that adolescent rodents exhibit significant alterations in neuroimmune processes following chronic binge-like ethanol exposure, there have been few studies in which adolescents and adults have been directly compared, and even fewer studies investigating effects of acute ethanol exposure. In adults, we have recently shown that marked changes in cytokine gene expression are apparent in the adult brain during the first ethanol exposure, with these changes dose-, time-, and regionally-dependent in nature [39]. More specifically, an acute 4-g/kg ethanol challenge consistently and significantly increased expression of IL-6 during intoxication in the paraventricular nucleus of the hypothalamus (PVN), hippocampus, cerebellum, and amygdala, whereas decreased expression of IL-1 and TNFα was generally observed in these same structures during intoxication [39]. In contrast, withdrawal from an acute ethanol challenge was not shown to result in marked alterations in cytokine expression, with IL-1 expression sometimes elevated relative to non-ethanol-exposed controls.
As (i) adolescence is a developmental period during which the first exposure to alcohol typically occurs, (ii) acute ethanol exposure has been shown to significantly alter cytokine expression in adults, and (iii) binge ethanol exposure during adolescence results in significant alterations in immune processes, the primary purpose of the current series of experiments was to directly compare central cytokine responses of adolescent and adult rats to an acute ethanol challenge. In doing so, it was deemed necessary to include a more fundamental comparison of antigen exposure in both adolescents and adults to help inform how neuroimmune responses to naturally occurring immunogens might vary in these two age groups. Thus, rats of both ages were exposed to either LPS, ethanol, or vehicle in a controlled series of experiments, and potential age differences in plasma endotoxin responses evoked by LPS or ethanol were examined. Data from these animals also served as a positive verification of cytokine detection procedures employed in subsequent analyses of ethanol-exposed rats. Considering recent findings that showed ethanol administration via gastric gavage increased apparent endotoxin concentration in blood obtained from the hepatic portal vein [40,41], separate groups of rats received an acute ethanol challenge via either an i.p. or i.g. route of administration. These groups provided a key comparison between intra-lumenal (i.g.) versus abdominal (i.p.) ethanol as potential drivers of endotoxin transit into blood, and whether such responses might differentially impact the action of ethanol on brain cytokines following these two commonly used modes of ethanol administration. Our a priori hypothesis was that ethanol exposure (regardless of route or age) would evoke central cytokine changes consistent with our previous findings (i.e., increased IL-6, decreased IL-1 and TNF; see [39]), and that these changes would occur independent of plasma endotoxin alterations. Together, the side-by-side comparison of LPS and ethanol challenge with multiple, within-subject, physiological measures (e.g., plasma measures of corticosterone, endotoxin, and blood ethanol content; plus cytokine measures in several key CNS structures) was expected to fully elucidate the nature of age-related differences in neuroimmune consequences of ethanol, and to inform future directions for identifying the mechanisms underlying any developmental differences observed.
2. General Methods
2.1.1 Subjects
Adolescent [postnatal day (P) 22–24 at arrival] and adult (P60–62 at arrival) male Sprague-Dawley rats (total N = 84) were purchased from Harlan Laboratories (Frederick, MD) and acclimated to the colony for 1 week. The experiment began, therefore, at P29–31 for adolescents and P67–69 for adults. At this time, rats were briefly handled (3–5 min) for 2 days prior to the start of experimental procedures. Colony conditions were maintained at 22 ± 1 °C with 12:12 light:dark cycle (lights on 06:00 h). Animals were pair-housed in standard Plexiglas bins and provided ad libitum access to both food and water. In all experiments, animals were maintained and treated in accordance with the guidelines set forth by the “Guide for the Care and Use of Laboratory Animals” [42], and with protocols approved by the IACUC committee at Binghamton University.
2.1.2 Drugs
When administered i.p., ethanol (95%) was diluted fresh daily with pyrogen-free physiological saline (0.9%, Teknova, Hollister, CA) to a final concentration of 20% (v/v) and sterile saline alone was used for vehicle. When delivered i.g., ethanol was mixed with tap water (20% v/v), with tap water alone delivered isovolumetrically to the ethanol intubations as the vehicle. LPS (from E. coli serotype 0111:B4, Sigma-Aldrich, St. Louis, MO) was initially diluted in sterile, pyrogen-free saline and aliquots were stored at −20 °C until needed. On the day of experimentation, a frozen aliquot was thawed and diluted to the required concentration [250 µg/kg (i.p. at 1.0 ml/kg)], also in pyrogen-free physiological saline.
2.1.3 Tissue and Blood Collection
Animals were rapidly decapitated (unanesthetized) 3 h after drug administration and trunk blood was collected into EDTA-coated glass blood collection tubes (BD Vacutainers, VWR cat. no. VT6450, Radnor, PA). Plasma was separated through refrigerated centrifugation, aliquoted, and stored at −20 °C until time of assay. Brains were removed and immediately submerged in ice-cold saline for approximately 1 min before dissection in a cold brain matrix. Coronal slices (2 mm) were dissected and incubated overnight in RNALater (Qiagen, cat. no. 76106, Valencia, CA), followed by storage at −20 °C. Individual brain structures—PVN, amygdala, and hippocampus (Figure 1a, b, and c, respectively)—were then microdissected using a dissecting microscope and reference coordinates obtained from the rat brain atlas (Paxinos and Watson, 1998) [68]. After microdissection, structures were again stored in RNAlater reagent at −20 °C until the time of tissue processing.
Figure 1.
Dissection guide for sites of interest, based on the Paxinos and Watson (1998) rat brain atlas, with the (a) The paraventricular nucleus of the hypothalamus (PVN), (b) amygdala, and (c) hippocampus collected for analysis.
2.1.4 Reverse-transcription Polymerase Chain Reaction
A Qiagen Tissue Lyser (Qiagen, Valencia, CA) provided rapid, thorough and consist homogenization of brain samples. Each structure was placed into a 2.0 ml Eppendorf tube containing 500 µl of Trizol® RNA reagent (Invitrogen, Grand Island, NY) and a 5 mm stainless steel bead, and was then rapidly shaken for 2 min for complete disruption/homogenization of the tissue. Chloroform (100 µl) was then added to the Trizol solution, the samples briefly shaken, and then samples were centrifuged for 15 min at 4 °C. Equal volume of 70% ethanol was added to the supernatant and purified through RNeasy mini columns (Qiagen, Valencia, CA) according to the manufacturer’s instructions. Columns were washed with buffer and eluted with 30 µL of RNase-free water (65 °C). RNA yield and quality was determined using a Nanodrop micro-volume spectrophotometer (NanoDrop 2000, Thermo Scientific, Wilmington, DE), with total RNA stored at −80 °C until the time of cDNA synthesis. Synthesis of cDNA was performed on 0.1–1.0 µg of normalized total RNA from each sample using the QuantiTect® Reverse Transcription Kit (Cat. No. 205313, Qiagen, Valencia, CA) which included a DNase treatment step. All cDNA was stored at −20 °C until time of assay.
Probed cDNA amplification was performed in a 10 µL reaction consisting of 5 µL IQ SYBR Green Supermix (Bio-Rad, cat. no. 170-8882, Hercules, CA), 0.5 µL primer (final concentration 250 nM), 0.5 µL cDNA template, and 4 µL Rnase-free water run in triplicate in a 384 well plate (BioRad, cat. no. HSP-3805) and captured in real-time using a PCR detection system (BioRad, model no. CFX384). Following a 3-min hot start (95 °C), samples underwent denaturation for 30 s at 95 °C, annealing for 30 s at 60 °C and extension for 30 s at 72 °C for 50 cycles. An additional denaturation (95 °C, 1 min) and annealing cycle (55 °C, 1 min) were conducted to ensure proper product alignment prior to melt curve analysis. For melt curve analysis, samples underwent 0.5 °C changes every 15 s ranging from 55 °C to 95 °C. A single peak expressed as the negative first derivative of the change in fluorescence as a function of temperature indicated primer specificity to the target gene. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a reference gene in these experiments, as studies from our laboratory have revealed more stable gene expression across ethanol treatment conditions with this gene [e.g., 39]. Primer sequences and a brief description of gene function can be found in Table 1.
Table 1.
RT-PCR Primers and Sequences Utilized in Experiments 1 and 2.
| Gene Target |
Accession # | RT-PCR Primer sequences | Functional Role |
|---|---|---|---|
| IL-6a | NM_012589 | Forward: 5’-TAGTCCTTCCTACCCCAACTTCC-3’ | Cytokine produced in response to infection or injury and a potent inducer of the acute phase response, particularly as a pyrogen; has both pro- and anti-inflammatory properties depending on nature of inflammatory insult |
| Reverse: 5’-TTGGTCCTTAGCCACTCCTTC-3’ | |||
| IL-1βb | NM_031512 | Forward: 5’-AGGACCCAAGCACCTTCTTT-3’ | Pro-inflammatory cytokine that is a robust immune system activator; involved in the production of sickness behaviors and fever following infection; often used as an immediate early gene marker of microglia activation |
| Reverse: 5’-AGACAGCACGAGGCATTTTT-3’ | |||
| TNFαc | NM_012675 | Forward: 5’-AACCACCAAGCAGAGGAGCA-3’ | Cytokine produced by a variety of different cell types, with pleiotropic actions including activation of macrophages and endothelial cells during an immune response; typically one of the first cytokines evoked by LPSd exposure |
| Reverse: 5’-ATGGCAAATCGGCTGACGGT-3’ | |||
| IκBαe | NM_080899 | Forward: 5’-CTGTTGAAGTGTGGGGCTGA-3’ | When NF-κBf is activated and translocates to the nucleus, IκBα is rapidly induced and sequesters further NF-κB translocation, thus IκBα gene expression is often used as a reporter of NF-κB activation |
| Reverse: 5’-AGGGCAACTCATCTTCCGTG-3’ | |||
| GAPDHg | NM_017008 | Forward: 5’-ATGACTCTACCCACGGCAAG-3’ | Enzyme important for cellular metabolic function, but also has been associated with apoptosis, ER-Golgi functioning, and axonal transport; was used as a stable reference gene against which target gene expression was examined |
| Reverse: 5’-AGCATCACCCCATTTGATGT-3’ |
Interleukin-6
interleukin-1 beta
tumor necrosis factor alpha
lipopolysaccharide
nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha
nuclear factor kappa-light-chain-enhancer of activated B cells
Glyceraldehyde 3-phosphate dehydrogenase
2.1.5 Plasma Measurement of Blood Ethanol Concentrations and Corticosterone
All blood ethanol concentrations (BECs) were determined in 5-µl aliquots using an Analox AM-1 alcohol analyzer (Analox Instruments, Lunenburg, MA). The machine was first calibrated using a 100 mg% industry standard, with BECs recorded in milligrams per deciliter (mg%). Accuracy was confirmed with a quality control solution provided by Analox Instruments, which contained a known concentration of ethanol. After confirmation with the quality control, experimental samples were measured, and counterbalanced across groups with respect to the order in which they were processed. Accuracy of the machine was systematically rechecked by reading the quality control following measurement of every 12–15 samples, as well as after the final sample.
Quantitative determination of plasma CORT was assessed by a commercially available ELISA kit (Cat No: ADI-901-097; Enzo Life Sciences, Farmingdale, NY). The CORT assay had a sensitivity of 27.0 pg/mL and inter-assay coefficient of 10.29%. Samples were diluted 1:30 and heat inactivated to denature endogenous corticosteroid binding globulin (CBG) by immersion in 75 °C water for 60 min, which produces a much more reliable and uniform denaturation of CBG than the enzyme cleavage step provided by the kit (unpublished observations). After heat inactivation of CBG, samples were processed according to the directions provided by the kit.
2.1.6 Measurement and Analysis of Plasma Endotoxin
Plasma endotoxin concentrations were measured using the limulus amebocyte lysate (LAL) assay (LAL kit QCL-1000, Cat No. 50-647U, Lonza, Walkersville, MD), with all steps conducted using pyrogen-free microplates (Lonza, Cat. No. 25-340) and glass test tubes (Lonza, Cat. No. N207). Plasma samples (obtained from trunk blood collected at the time of tissue harvest) were first diluted 1:10 in pyrogen-free LAL reagent water provided by the kit manufacturer (Cat. No. W50-640, Lonza), and then heated to 75 °C for 10 min in a hot water bath such that plasma endotoxin binding proteins could be denatured prior to assay. Concentration of endotoxin was determined using a standard curve generated from an Endotoxin standard provided by the kit. Using the microplate method, absorbance of standards and samples were measured in duplicate at 405 nm. Data were calculated in endotoxin units (EU) per ml, as the potency/reactivity within the LAL assay is highly variable across endotoxin molecules and, therefore, across different lots/assays.
In the analysis of ethanol-exposed animals, samples were measured at a 1:10 dilution, as this resulted in absorbance values that were within the limits of the standard curve provided by the kit. For LPS-exposed adolescent rats, a dilution series (1:10, 1:25, 1:50, 1:100) demonstrated that a 1:10 dilution was optimal for detecting endotoxin in this group as well. However, in LPS-exposed adults, endotoxin concentrations were well above the upper-limit of the standard curve at a 1:10 dilution, and hence had to be further diluted. A dilution series (1:100, 1:200, 1:400, 1:800) conducted on this group revealed that a 1:400 dilution was best for 7 out of 8 LPS-exposed adult samples, with these data then used for final analysis. It was necessary to use data obtained from the 1:800 dilution for one animal in this group, as the 1:400 dilution resulted in an absorbance value that exceeded the highest standard. Thus, plasma endotoxin data presented in Figure 3d-3f are the product of an intensive dilution series trial, designed to ensure that all samples were extrapolated from the linear portion of the standard curve.
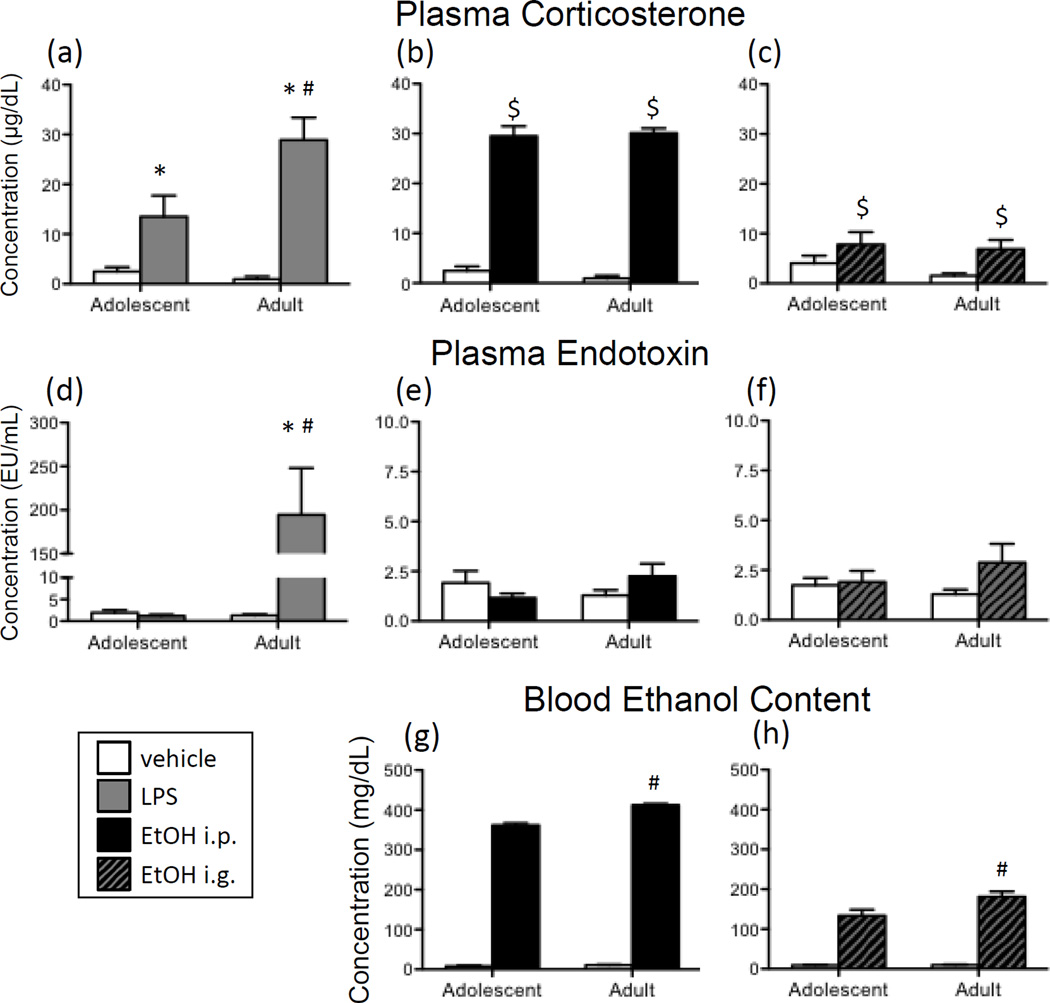
Figure 3.
(a) Adolescent and adult male rats were given an acute intraperitoneal (i.p.) challenge of sterile saline (white bars) or lipopolysaccharide (LPS; 250 µg/kg; gray bars), and plasma corticosterone concentrations were assessed 3 hours later (Experiment 1). (b) Additionally, in Experiment 1, plasma corticosterone was examined in adolescent and adult rats 3 hours after (i.p.) injection of sterile saline (white bars) or 4-g/kg ethanol (20%v/v; black bars). (c) Following intragastric (i.g.) intubation with 4-g/kg ethanol (20% v/v; hatched bars) or tap water in Experiment 2, plasma corticosterone concentrations were also assessed. Endotoxin in plasma was measured in animals from Experiments 1 and 2 three hours after administration of (d) LPS, (e) i.p. ethanol, or (f) i.g. ethanol. Note the marked difference in scale of the y-axis for LPS-exposed animals in panel d versus ethanol-exposed rats in panels e and f. In both Experiments 1 and 2, blood ethanol concentrations were measured in adolescent and adult rats following either (g) i.p. or (h) i.g. administration of vehicle versus ethanol. Main effects of Drug are indicated by a dollar sign ($) above the bar for each Age group, whereas main effects of Age are denoted by a horizontal line with a plus symbol (+). In the case of a significant Age x Drug interaction, asterisks (*) denote a significant effect of Drug within a given Age group. A pound sign (#) indicates a significant difference between adolescents and adults within a Drug condition.
2.2 Experiment 1
Currently, there is a paucity of studies that have directly compared the immune response to an antigen, such as LPS, in adolescent and adult rodents. Whereas a recent study [43] reported that, relative to adults, adolescent males exhibited an abbreviated LPS-related increase in plasma IL-6, as well as fewer FOS-positive cells in the PVN 8 h after LPS administration, alterations in central cytokines in response to LPS were not examined. Thus, in Experiment 1, potential age-related differences in LPS-induced expression of several brain cytokines were assessed in both adolescent and adult male rats following an acute injection of LPS.
Additionally, Experiment 1 sought to examine potential age differences in the cytokine response to an acute ethanol challenge across ontogeny. Previous studies from our laboratory have identified highly reproducible changes in central cytokine expression following an acute ethanol challenge in adult rats [Gano et al., in prep; 39]. While these ethanol-related alterations in brain cytokine expression are dependent upon brain area of interest, cytokine of interest, and the time at which cytokine expression is examined after ethanol administration, a consistent increase in IL-6 gene expression has been observed in the hippocampus, PVN, amygdala, and cerebellum in adult male rats during the intoxication phase after a binge-like dose (4 g/kg) of ethanol. Since adolescent animals are known to exhibit differential sensitivity relative to adults to many of ethanol’s effects during this developmental period, another purpose of this experiment was to directly compare the brain cytokine response to acute ethanol during adolescence versus adulthood.
Thus, a 2 (Age) x 3 (Drug Condition) factorial design was employed where adolescent (n = 8 per group) and adult (n = 8 per group) male rats (N = 48) were given an i.p. injection of sterile saline, LPS (250 µg/kg) or ethanol (4 g/kg) sometime between 0900 and 1100 h. Cage mates were assigned to the same drug exposure condition. Three hours later, trunk blood and brains were collected according to the procedures outlined above, and expression of several cytokine targets was assessed in the hippocampus, amygdala, and PVN. The hippocampus and amygdala were selected for analysis as these brain regions have consistently demonstrated acute ethanol-related increases in IL-6 expression in adults [e.g., 39]. The hippocampus has also been reported to exhibit increased IL-1 levels following systemic administration of LPS [44]. Given that the hypothalamus, and in particular the PVN, has been demonstrated to exhibit robust increases in cytokines after acute immune challenge with LPS [e.g., 44,45], this brain structure was also chosen for examination.
2.3 Experiment 2
In prior studies using adults, the central cytokine response to an acute ethanol challenge has been examined following both i.p. and i.g. routes of administration. While both modes of ethanol delivery generally resulted in similar alterations in brain cytokine expression, subtle differences do seem to exist [see 39; Gano et al., under revision]. Given that i.g. administration is a method of ethanol delivery that more closely mimics the route via which human alcohol exposure occurs, however, assessment of brain cytokine changes following i.g. exposure provides an aspect of face validity that is important for generalizing results to humans.
For Experiment 2, therefore, a 2 (Age) x 2 (Drug Condition) factorial was used in which adolescent (n = 8–10 per group) and adult (n = 8–10 per group) male rats (N = 36) were given an acute intubation of either tap water or ethanol (4 g/kg), sometime between 0900 and 1100 h. Three hours after gavage, brains and trunk blood were collected as described in the General Methods.
2.4 Statistical Analyses
In the analyses of cytokine targets, plasma corticosterone concentrations, and plasma endotoxin concentrations, data were analyzed using a 2 (Age: Adolescent vs. Adult) x 2 (Drug: Vehicle vs. Ethanol or LPS) factorial ANOVA (p < 0.05), with Fisher’s Least Significant Difference (LSD) test used for post hoc examination of any significant 2-way interactions (p < 0.05) that were observed. Comparisons of BECs achieved between adolescent and adult animals in both Experiments 1 and 2 were made using an independent groups t test (p < 0.05). Data from Experiment 1 were first analyzed using a full 2 (Age) x 3 (Drug Exposure) ANOVA. Indeed, in these analyses significant Age x Drug Exposure interactions were observed for nearly all cytokine targets in the three brain regions of interest. However, when post hoc tests were employed to identify the loci of significant differences between these Age and Exposure groups, only LPS-exposed animals were determined to be significantly different from saline controls, as the magnitude of the ethanol-related alterations in brain cytokines was overall much lower than that induced by LPS-exposure. Subsequently, separate 2 (Age) x 2 (Drug Exposure) ANOVAs were conducted to analyze LPS- versus ethanol-exposed rats. Thus the meaningful, although overall lower, alterations in brain cytokines in response to ethanol were not overpowered by the effects of LPS on cytokine expression, thereby minimizing the probability of committing a Type 2 Error.
Outliers were defined as data points that were more extreme than ± 2 standard deviations from a given experimental group’s mean. One adult animal administered i.p. ethanol was an outlier for nearly all targets in the PVN and thus was eliminated from all analyses within that region. In the analyses of hippocampal data, two adult i.g. vehicle-exposed animals were extreme outliers for almost all cytokine targets and were therefore removed from all analyses within the this structure. Given the logarithmic amplification of RT-PCR data, it is not usual that there were a few instances in which a sample was an extreme data point in the analysis of only one target within a structure. In these situations (one i.p. vehicle-exposed adult animal in the hippocampal IL-6 analysis; one adult LPS-exposed rat in the PVN IL-6 analysis; one adult i.g. vehicle-intubated rat in the PVN IL-1 analysis), the data point was only removed in the analysis of that particular dependent variable. Unfortunately, due to experimental error 11 amygdala samples were lost during cDNA synthesis, and thus could not be used for RT-PCR: 2 adolescent and 2 adult LPS-exposed samples, 2 adolescent and 2 adult i.g. ethanol samples, 2 adult i.p. ethanol samples, and 1 i.g. vehicle-exposed adult sample.
Before conducting analyses of cytokine data, GAPDH expression was first examined as a separate target in order to assess any possible differences across groups in expression of this reference gene. Overall, few significant differences were present for GAPDH expression—a slight, but statistically significant, age difference in GAPDH expression was detected in the hippocampus and amygdala, as well as a minor overall increase in GAPDH expression in i.p. ethanol-exposed animals relative to vehicle controls in the hippocampus. While statistically significant, the magnitude of these group differences was quite small, especially in comparison to the robust increases in cytokine expression induced by LPS. Hence, in all analyses, gene expression of cytokine targets was quantified relative to expression of GAPDH using the 2-ΔΔC(T) method [46].
3. Results
3.1 Experiment 1
3.1.1 Age Differences in Responsiveness to LPS
3.1.1.1 Cytokine Expression
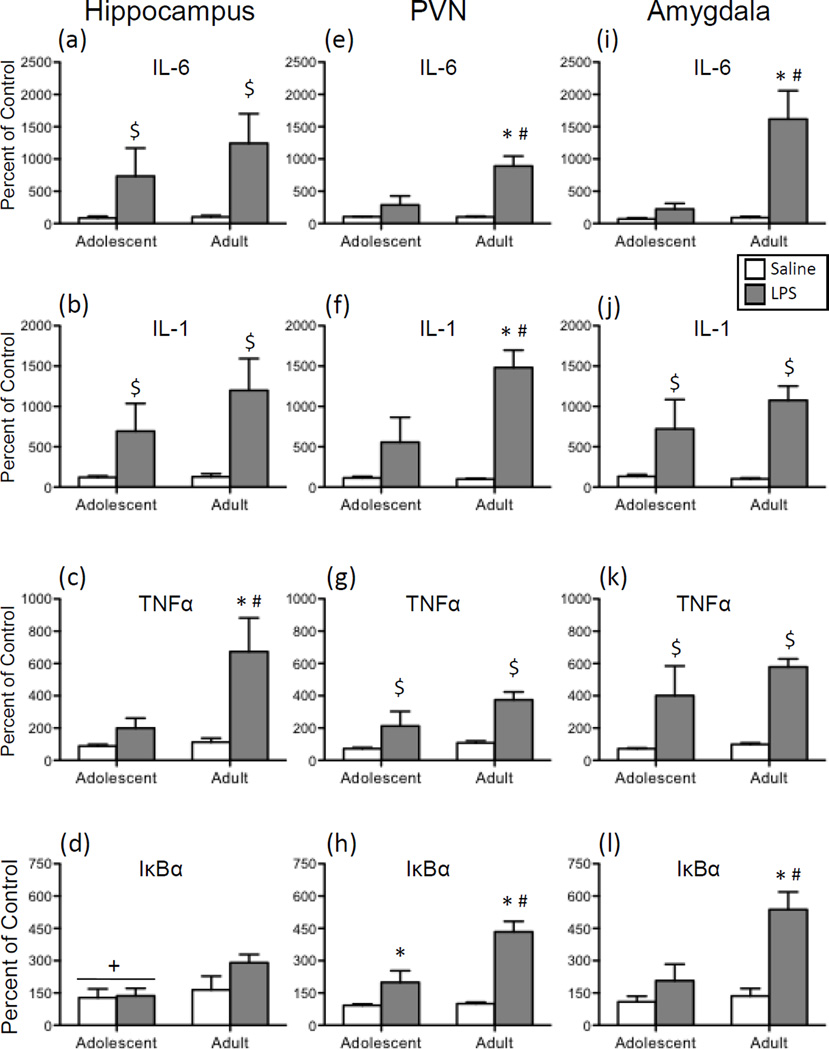
In the hippocampus, a main effect of Drug was observed in the analysis of both IL-6 (Figure 2a) and IL-1 (Figure 2b) expression [F(1,27) = 7.40, p < 0.05; F(1,28) = 9.78, p < 0.01, respectively]. Overall, LPS exposure induced significantly greater expression of IL-6 and IL-1 in this brain structure in comparison to vehicle-injected animals. For both of these cytokines, no significant main effects or interactions involving Age were observed. In contrast, a significant interaction of Age and Drug was found for TNFα (Figure 2c) expression in this brain region [F(1,28) = 4.24, p < 0.05]: LPS administration resulted in a significant increase in TNFα expression that was only observed among adults. The analysis of IκBα expression (Figure 2d) in the hippocampus demonstrated that adolescents had significantly lower levels of gene expression overall, with LPS not leading to significant elevations in IκBα in either age group [main effect of Age: F(1,28) = 4.30, p < 0.05].
Figure 2.
Adolescent and adult male rats were given an acute intraperitoneal (i.p.) challenge of sterile saline (white bars) or lipopolysaccharide (LPS; 250 µg/kg; gray bars), with brains collected for analysis 3 hours later. Interleukin (IL)-6, IL-1, tumor necrosis factor alpha (TNFα), and nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha (IκBα) gene expression was examined in the hippocampus (a, b, c, and d, respectively), paraventricular nucleus of the hypothalamus (PVN) (e, f, g, and h, respectively), and the amygdala (i, j, k, and l). Data were calculated as a relative change in gene expression using the 2ΔΔC(t) method, with GAPDH used as a reference gene and the saline-exposed adult animals serving as the ultimate control group. Bars denote group means ± standard error of the mean (represented by vertical error bars). Main effects of Age are denoted by a horizontal line with a plus symbol (+), whereas main effects of Drug are indicated by a dollar sign ($) above the bar for each Age group. In the case of a significant Age x Drug Condition interaction, asterisks (*) signify a significant effect of Drug within a given Age group, with the pound sign (#) indicating a significant difference between adolescents and adults within a Drug condition.
When gene expression data for IL-6 (Figure 2e), IL-1 (Figure 2f), and IκBα (Figure 2h) in the PVN were analyzed, significant main effects of both Age [IL-6: F(1,27) = 9.17, p < 0.01; IL-1: F(1,28) = 5.85, p < 0.05; IκBα: F(1,28) = 10.93, p < 0.01] and Drug [IL-6: F(1,27) = 24.07, p < 0.0001; IL-1: F(1,28) = 23.53, p < 0.05; IκBα: F(1,28) = 36.18, p < 0.00001] were observed, as well as significant interactions of these two variables [IL-6: F(1,27) = 9.38, p < 0.01; IL-1: F(1,28) = 6.20, p < 0.05; IκBα: F(1,28) = 9.59, p < 0.01]. Post hoc analyses of the 2-way interactions revealed a similar pattern for all three cytokines, with LPS resulting in significant increases in IL-6, IL-1, and IκBα expression in adults relative to their saline-exposed counterparts, and LPS-injected adolescents demonstrating much smaller increases in expression of these cytokine targets compared to their saline-treated counterparts as well as LPS-exposed adults. Only in the case of IκBα expression did LPS elicit a significant increase in adolescents when compared to adolescent saline controls. A significant main effect of Drug was observed in the analysis of TNFα expression (Figure 2g) in the PVN, as LPS administration resulted in significant increases in this cytokine relative to saline controls [F(1,28) = 15.56, p < 0.001]. Although there was a trend for LPS-related activation of TNFα expression to be greater among adults compared to adolescents, this age difference approached but did not achieve statistical significance (p = 0.06).
Expression of IL-6 (Figure 2i) and IκBα (Figure 2l) in the amygdala was differentially impacted by LPS exposure in adolescents relative to adults [Age x Drug interaction for IL-6: F(1,24) = 12.26, p < 0.001; for IκBα: F(1,24) = 7.89, p < 0.01]. In both cases, adults but not adolescents demonstrated significant increases in expression of these genes following a systemic LPS injection. While LPS also significantly increased IL-1 (Figure 2j) and TNFα (Figure 2k) expression in the amygdala, these increases were comparable across age [main effect of Drug for IL-1: F(1,24) = 20.36, p < 0.001; for TNFα: F(1,24) = 24.95, p < 0.0001].
3.1.1.2 Plasma Measures
Plasma endotoxin concentrations were analyzed in animals that were given either sterile saline or LPS via i.p. injection. As can be seen in Figure 3d, LPS administration resulted in a significant increase in plasma endotoxin in adult rats, whereas neither adolescent LPS-exposed rats, nor saline-exposed rats at either age, demonstrated elevations in endotoxin concentrations [Age x Drug interaction: F(1,28) = 13.14, p < 0.01]
Analysis of plasma corticosterone (Figure 3a) demonstrated that LPS administration elicited a significant increase in corticosterone in both ages compared to their vehicle-treated counterparts, with the LPS-induced increase even greater for adults relative to adolescents [Age x Drug interaction: F(1,28) = 7.20, p < 0.05].
3.1.2 Examination of responsiveness to an acute intraperitoneal ethanol challenge across age
3.1.2.1 Cytokine Expression
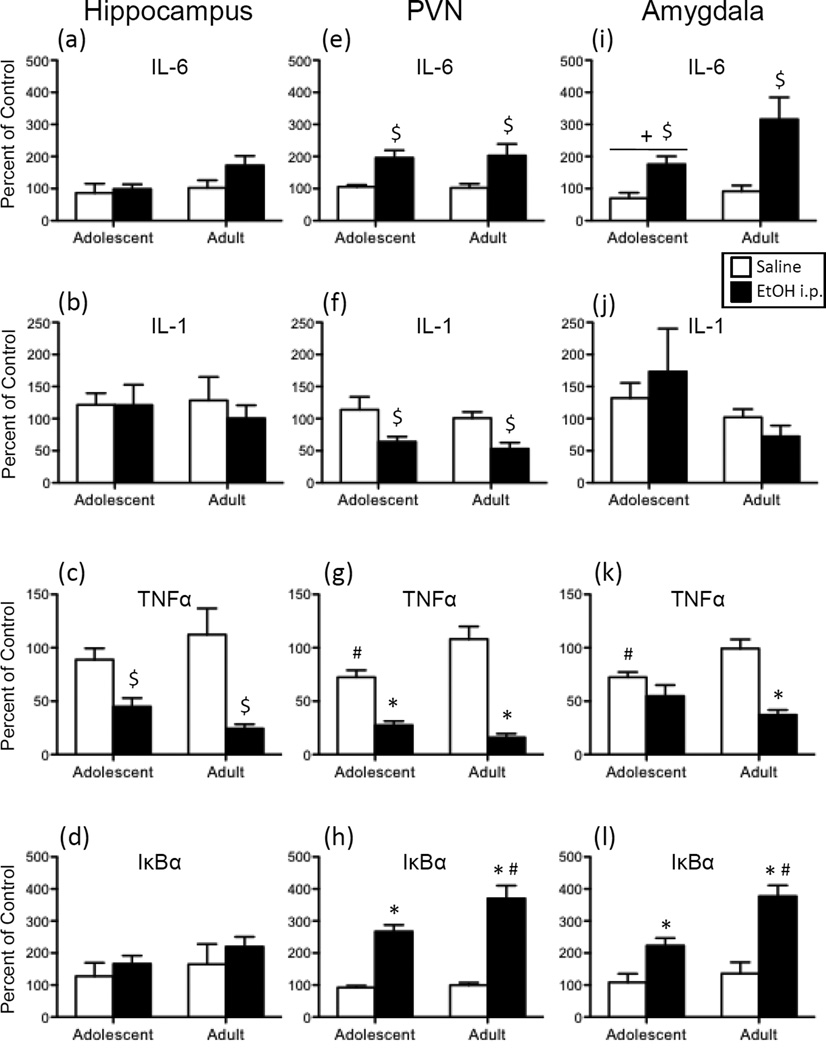
In the hippocampus, TNFα expression (Figure 4c) was significantly decreased by i.p. ethanol exposure [main effect of Drug: F(1,28) = 21.90, p < 0.0001], with no main effects or interactions involving Age observed. While there was a trend for adults to exhibit an ethanol-induced increase in IL-6 expression (Figure 4a) in this brain region (p = 0.11), no other significant effects of Age or Drug were observed for any of the other cytokine targets in the hippocampus.
Figure 4.
Adolescent and adult male rats were given an acute intraperitoneal (i.p.) challenge of sterile saline (white bars) or 4-g/kg ethanol (EtOH; 20% v/v; black bars), with brains collected for analysis three hours later. Interleukin (IL)-6, IL-1, tumor necrosis factor alpha (TNFα), and nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha (IκBα) gene expression was examined in the hippocampus (a, b, c, and d, respectively), paraventricular nucleus of the hypothalamus (PVN) (e, f, g, and h, respectively), and the amygdala (i, j, k, and l). Data were calculated as a relative change in gene expression using the 2ΔΔC(t) method, with GAPDH used as a reference gene and the saline-exposed adult animals serving as the ultimate control group. Bars denote group means ± standard error of the mean (represented by vertical error bars). Main effects of Drug are indicated by a dollar sign ($) above the bar for each Age group, whereas main effects of Age are denoted by a horizontal line with a plus symbol (+). In the case of a significant Age x Drug interaction, asterisks (*) denote a significant effect of Drug within a given Age group, with the pound sign (#) indicating a significant difference between adolescents and adults within a Drug condition.
Following acute ethanol exposure, both adolescents and adults exhibited increased expression of IL-6 (Figure 4e) in the PVN [main effect of Drug: F(1,27) = 19.75, p < 0.001] when compared to vehicle controls. In contrast, ethanol was found to decrease IL-1 expression in both age groups (Figure 4f) relative to controls [main effect of Drug: F(1,27) = 14.42, p < 0.001]. Although TNFα (Figure 4g) expression was significantly higher in vehicle-exposed adults relative to adolescent controls, both ages evinced a significant ethanol-induced decrease in TNFα expression in this structure when compared to their saline-injected counterparts [Age x Drug Interaction: F(1,27) = 10.03, p < 0.01]. In contrast, administration of ethanol resulted in an increase in IκBα expression at both ages (Figure 4h), with the ethanol-related increase in expression of this target significantly greater for adults when compared to adolescents [Age x Drug Interaction: F(1,27) = 5.06, p < 0.05].
When IL-6 in the amygdala (Figure 4i) was examined, i.p. administered ethanol was found to significantly increase expression of this gene in both adolescents and adults [main effect of Drug: F(1,26) = 9.85, p < 0.01], with adolescents also exhibiting overall lower levels of IL-6 expression than adults in this brain structure [main effect of Age: F(1,26) = 6.09, p < 0.05]. Ethanol delivered i.p. also increased expression of IκBα in the amygdala (Figure 4l) in both age groups, although these ethanol-related increases were greater in adults relative to adolescents [Drug x Age interaction: F(1,26) = 4.51, p < 0.05]. Ethanol injection significantly decreased expression of TNFα (Figure 4k) in this brain region in adults. Although adolescents did not demonstrate ethanol-related changes in TNFα expression in this region, vehicle-exposed adolescents exhibited significantly lower TNFα expression than their adult counterparts [Drug x Age interaction: F(1,26) = 7.97, p < 0.01].
3.1.2.2 Plasma Measures
As shown in Figure 3e, acute i.p. administration of ethanol did not significantly impact plasma endotoxin concentrations in either age group. In contrast, significant elevations in plasma corticosterone (Figure 3b) were observed following i.p. ethanol, and were comparable in both age groups [main effect of Drug: F(1,28) = 324.50, p < 0.00001]. While this ethanol challenge led to extremely high blood ethanol concentrations (Figure 3g), analysis of only the ethanol-exposed rats revealed that adults reached BECs that were significantly higher than adolescent animals [t(14) = 7.86, p < 0.00001.].
3.2 Experiment 2
3.2.1 Age Differences in responsiveness to intragastrically administered ethanol
3.2.1.1 Cytokine Expression
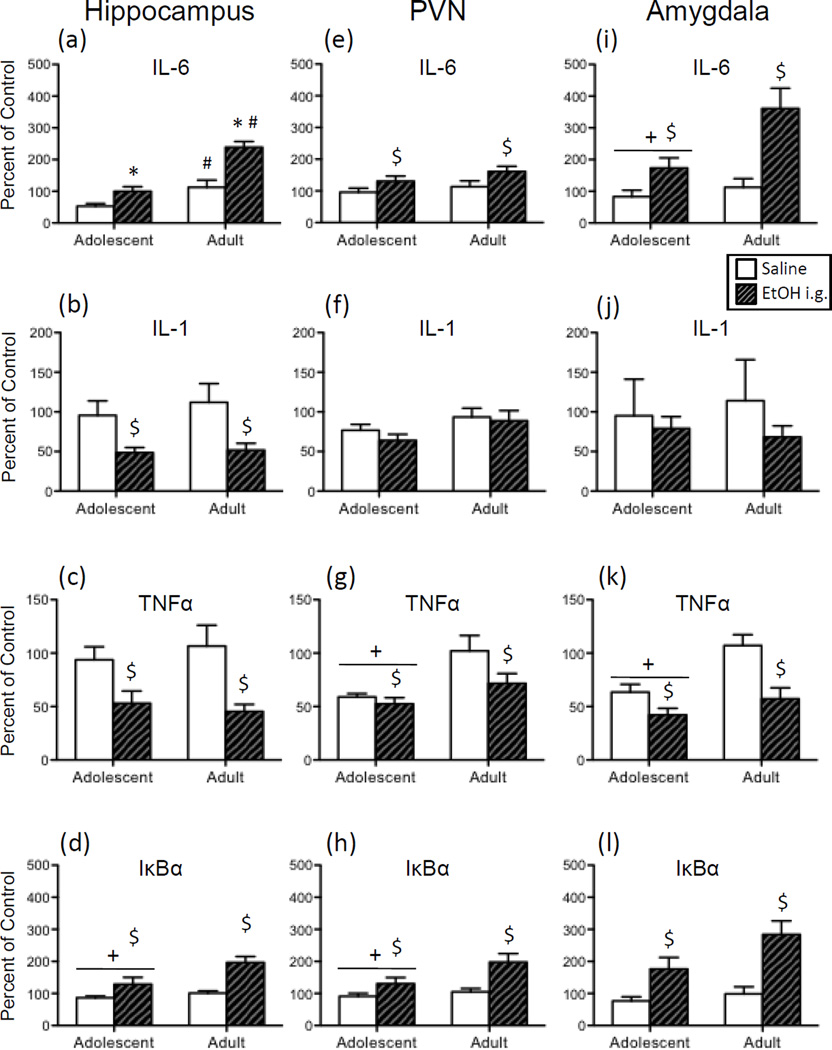
In both age groups, expression of IL-6 in the hippocampus (Figure 5a) was significantly increased by acute i.g. administration of ethanol when compared to the age-matched vehicle exposed controls. According to the significant Age x Drug interaction, however, the ethanol-evoked increase in hippocampal IL-6 was greater in adults compared to adolescents [F(1,30) = 5.78, p < 0.05]. Furthermore, IL-6 expression was slightly but significantly higher in water-exposed adolescents relative to adults. Overall, acute i.g. ethanol resulted in significant decreases in IL-1 (Figure 5b) and TNFα (Figure 5c), whereas ethanol increased IκBα expression (Figure 5d) in this brain region [main effect of Drug for IL-1: F(1,30) = 15.20, p < 0.001; for TNFα: F(1,30) = 17.82, p < 0.001; for IκBα: F(1,30) = 15.02, p < 0.001]. An additional main effect of Age for IκBα expression revealed that adolescents had overall lower IκBα expression than adults [F(1,30) = 5.40, p < 0.05].
Figure 5.
Adolescent and adult male rats were given an acute intragastric (i.g.) challenge of tap water (white bars) or 4-g/kg ethanol (EtOH; 20% v/v; hatched bars), with brains collected for analysis three hours later. Interleukin (IL)-6, IL-1, tumor necrosis factor alpha (TNFα), and nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha (IκBα) gene expression was examined in the hippocampus (a, b, c, and d, respectively), paraventricular nucleus of the hypothalamus (PVN) (e, f, g, and h, respectively), and the amygdala (i, j, k, and l). Data were calculated as a relative change in gene expression using the 2ΔΔC(t) method, with GAPDH used as a reference gene and the water-exposed adult animals serving as the ultimate control group. Bars denote group means ± standard error of the mean (represented by vertical error bars). Main effects of Age are signified by a horizontal line with a plus symbol (+), whereas main effects of Drug are indicated by a dollar sign ($) above the bar for each Age group. In the case of a significant Age x Drug interaction, asterisks (*) denote a significant effect of Drug within a given Age group, with the pound sign (#) indicating a significant difference between adolescents and adults within a Drug condition.
In the PVN, acute i.g. ethanol significantly increased expression of IL-6 (Figure 5e) and IκBα (Figure 5h) regardless of Age [main effect of Drug for IL-6: F(1,32) = 6.61, p < 0.05; for IκBα: F(1,32) = 11.67, p < 0.01], whereas the acute ethanol intubation decreased expression of TNFα (Figure 5g) in this structure [F(1,32) = 4.40, p < 0.05]. A main effect of Age was observed in the analysis of TNFα and IκBα expression, with adolescents overall exhibiting significantly lower levels of gene expression compared to adults for these cytokine targets [for TNFα: F(1,32) = 12.38, p < 0.01; for IκBα: F(1,32) = 4.38, p < 0.05].
Analysis of the effects of ethanol in the amygdala revealed that, similar to the hippocampus and PVN, expression of IL-6 (Figure 5i) and IκBα (Figure 5l) was generally increased by acute ethanol exposure in both age groups [main effect of Drug for IL-6: F(1,27) = 17.84, p < 0.001; for IκBα: F(1,27) = 21.73, p < 0.0001], whereas ethanol significantly reduced TNFα (Figure 5k) expression [F(1,27) = 20.39, p < 0.001] and marginally reduced IL-1 (Figure 5j) expression (p = 0.054). While the effects of ethanol administration did not significantly interact with Age for any gene target, there were general reductions in gene expression for adolescents relative to adults in the analysis of IL-6 and TNFα [main effect of Age for IL-6: F(1,27) = 7.33, p < 0.05; for TNFα: F(1,27) = 14.18, p < 0.001].
3.2.1.2 Plasma Measures
When acute ethanol was delivered i.g., plasma endotoxin concentrations were not found to differ significantly from vehicle controls in either age group (Figure 3f). There were, however, significant ethanol-induced elevations in plasma corticosterone concentrations (Figure 3c) as compared to animals intubated with tap water [main effect of Drug: F(1,32) = 5.85, p < 0.05]. No effects of Age occurred in the analysis of corticosterone. In the examination of ethanol-exposed rats, adults were found to reach BECs (Figure 3h) following an i.g. challenge that were significantly higher than those of adolescents [t(18) = 2.33, p < 0.05].
4.0 Discussion
Adolescence is a developmental period that is characterized by a unique sensitivity to alcohol, with individuals often times less sensitive to effects of ethanol exposure at this age, yet in some circumstances more susceptible to consequences of ethanol administration. Although it has been demonstrated that adolescent rodents exhibit significant alterations in neuroimmune processes following chronic binge-like ethanol exposure [35,36], there have been few studies in which adolescents and adults have been directly compared in terms of their sensitivity to ethanol-induced changes in brain immune factors. Recently, Pascual et al. (2013) reported that binge ethanol exposure resulted in increased gene expression of several neuroimmune genes in rats when the ethanol administration occurred during adolescence, but not adulthood [38]. Therefore, the primary goal of the current experiments was to further examine potential age differences in the central cytokine response to ethanol challenge, using an acute exposure paradigm, by comparing cytokine gene expression in the hippocampus, PVN, and amygdala of adolescent and adult rats.
In adult animals, chronic ethanol exposure has been shown to significantly impact expression of many cytokines, both peripherally and in the brain [for review see 47]. Yet, some studies have also shown that cytokine expression can be altered after acute ethanol. For example, 24 hours after an acute ethanol intubation, adult mice had elevated expression of TNFα and MCP-1 mRNA in brain [8]. When adult rats were given an acute injection of ethanol, TNF-α, IL-1β, and IL-6 protein were increased in the hypothalamus 48 hours after exposure [48]. Indeed, within our own laboratory, we have demonstrated that a 4-g/kg ethanol challenge, given either intraperitoneally or intragastrically, resulted in increased expression of IL-6 mRNA in several brain regions 3 hours after administration, with IL-1 mRNA expression tending to be increased during acute withdrawal [39]. In the present report, we again found that IL-6 mRNA expression was significantly increased in the hippocampus, PVN, and amygdala of adult rats 3 hours following a 4-g/kg ethanol challenge, with IL-1β and TNFα expression either decreased or unchanged at this time. For the first time, it was also found that acute intoxication elevated expression of IκBα—a reporter of NFκB activation. Thus, these results suggest that intoxication-related increases in IL-6 mRNA expression are a consistent result of ethanol exposure in adults, with IκBα increases implicating IL-6 signaling-related interaction with the NF-κB signaling cascade via phosphatidylinositide 3-kinase (PI3K) or other pathways [49].
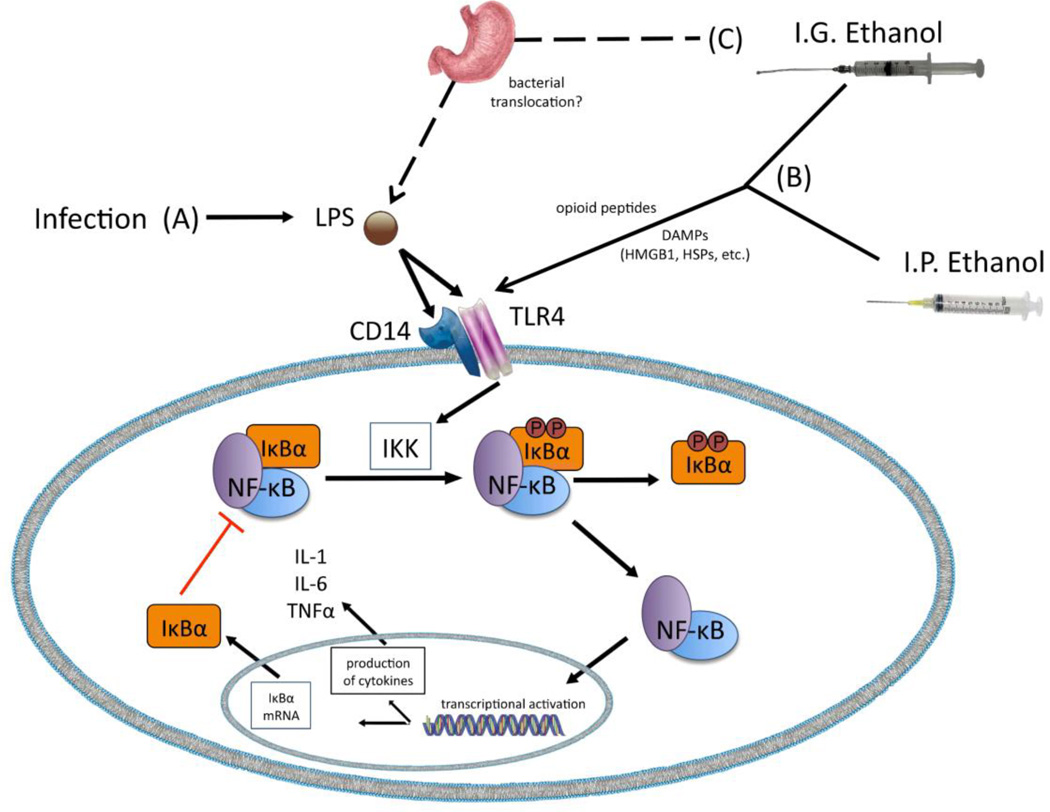
The mechanisms responsible for these ethanol-related alterations in cytokines in adults remain unclear, but it is likely that acute ethanol exposure represents a nonpathogenic challenge that induces a sterile inflammatory response via activation of TLR4s through danger-associated molecular patterns (DAMPs), such as heat-shock proteins (e.g., hsp72) or high-mobility group box 1 (HMBG-1) [50,51], as illustrated in Figure 6. Ethanol’s action on opioid peptides is yet another mechanism through which ethanol might stimulate TLR4s, since TLR4s have been shown to be sensitive to opioid action [52]. Alternatively, it may also be that ethanol delivered intragastrically is altering immune defenses of the gut, as well as intestinal permeability to endotoxin [53]. Elevated proliferation of gastrointestinal bacterial and/or increased passage of bacteria through the intestinal lumen may lead to “leaking” of bacteria into the general circulation, thus initiating a cytokine response (as indicated in Figure 6, pathway C). However, our data suggest that ethanol’s effect on gut bacteria and/or transit of endotoxin to blood is not likely to account for the brain cytokine changes observed after acute ethanol, or the age differences in ethanol sensitivity observed here. Indeed, the lack of an ethanol-related plasma endotoxin response in both adolescent and adult rats in the present experiments indicates that there was no indirect blood borne endotoxin signal that could induce a central cytokine response. Instead, these data seem to suggest that ethanol’s passage into brain allows for direct action on the CNS to impact inflammatory-related factors. Regardless of the mechanisms responsible, these experiments replicate the elevated IL-6 expression observed during intoxication with concomitant reductions of IL-1 and TNFα expression.
Figure 6.
Proposed pathways through which ethanol administration elicits alterations in cytokine gene expression. (A) Bacterial infection induced by administration of lipopolysaccharide (LPS) stimulates an immune response via activation of the TLR4/CD14 receptor complex. In the resting state IκBα binds to NF-κB, thus keeping NF-κB inactive and sequestered in the cytoplasm. Once LPS binds to TLR4s, a signaling cascade is induced that results in activation of the IKK complex. The IKK complex then phosphorylates IκBα, which liberates NF-κB and allows NF-κB to translocate to the nucleus where it activates gene transcription. Ultimately, cytokines including IL-1, IL-6, and TNFα are produced by this NF-κB activation. Additionally, NF-κB activation leads to increased expression of IκBα, with newly synthesized IκBα then binding to NF-κB and suppressing further activation of this pathway. (B) Ethanol delivered via the intragastric (i.g.) or intraperitoneal (i.p.) routes is believed to activate the TLR4 signaling pathway. Ethanol’s actions on opioid peptides and damage-associated molecular pattern molecules (DAMPs) are considered likely mechanisms by which ethanol would stimulate TLR4s. (C) In the case of i.g. administered ethanol, another possible mechanism leading to TLR4 activation is via bacterial translocation in the gut. Under normal healthy conditions, mucosal membranes of the gastrointestinal tract create a physical barrier that contains bacteria within the gut and intestines. Other physiological processes also keep bacteria within the GI tract at bay. Ethanol has been shown to impair these defense mechanisms, with ethanol promoting bacterial growth/accumulation within the gut and increasing intestinal permeability. The latter of these ethanol effects may then allow bacteria to “leak” from the gut to the liver and general circulation, thereby activating the TLR4 pathway.
Another issue worth addressing here is the potential role that ethanol-induced IL-6 expression might play within the context of these neuroimmune alterations. Although IL-6 is often regarded as a pro-inflammatory cytokine, emerging evidence suggests that IL-6 might have anti-inflammatory properties under some conditions [54,55], thus raising the possibility that early elevations in IL-6 may be responsible for suppression of other cytokines like IL-1 and TNFα, while also potentially initiating processes that lead to other downstream neuroimmune consequences of ethanol. Current studies in our laboratory are aimed at more fully delineating ethanol-related changes in IL-6 signaling pathways.
Given that adolescent rats were more sensitive to ethanol-induced impairments in spatial memory [33], showed greater ethanol-related suppression of hippocampal LTP [34], and exhibited more pronounced activation of neuroimmune factors in the cortex with intermittent binge ethanol exposure [38] compared to adults, it was expected that adolescents in the current series of experiments would be more sensitive to acute ethanol-induced alterations in brain cytokines. Instead, the present results indicated few age differences in ethanol’s effects on cytokine expression. And, in the instances in which significant age differences were apparent, adolescents evinced a less marked change in cytokine gene expression following ethanol administration. More specifically, increased IκBα expression in the PVN and amygdala with acute i.p.-delivered ethanol, as well as increased IL-6 expression in the hippocampus with i.g.-intubated ethanol, was significantly attenuated in adolescents relative to adults.
The mechanisms responsible for this adolescent-related insensitivity are not presently known. One possibility is that the dose of ethanol selected resulted in BECs that were slightly, but significantly, lower in adolescents versus adults, regardless of route of exposure. While this certainly could have contributed to a lessened response in adolescents, it seems unlikely as the magnitude of both increased and decreased cytokine expression for several cytokines in all three brain structures were comparable in both ages. If reduced BECs in adolescents were responsible for these age effects, a consistent attenuation of ethanol effects in adolescents would have been seen for all cytokine genes and across all brain regions examined. Instead, it is likely that age differences in other ethanol-related effects are responsible. More specifically, as proposed in Figure 6, part (B), adolescents could have differential sensitivity to ethanol’s activation of DAMPs, which could alter stimulation of the TLR4s. Yet another possibility is ontogenetic differences in ethanol’s actions on opioid peptides during adolescence [Figure 6, part (B)], which would also alter ethanol-TLR4 interactions, an issue which we have yet to explore. Finally, attenuated ethanol-induced increases in bacterial translocation from the gut to the general circulation is another potential explanation for reduced ethanol effects on cytokines in adolescents relative to adults [Figure 6, part (C)]. In the current experiments, however, plasma endotoxin levels were unaffected by ethanol administration, suggesting that indirect effects of ethanol mediated through endotoxin translocation into blood are not likely to be the key culprit for alterations in central cytokines. Ongoing studies in our laboratory are addressing these potential mechanisms already.
Interestingly, a recent report comparing neuroimmune consequences of chronic binge ethanol exposure in both adolescent and adult mice found that adolescents demonstrated fewer alterations in brain immune factors in hippocampus, cortex, and cerebellum when compared to their mature counterparts [17]. These results are more similar to those of the current study, though in contrast to those of Pascual et al. (2013) [38]. Thus, it seems that the available literature, which has directly compared ethanol-related changes in neuroimmune factors in adolescence and adulthood, has produced disparate results with respect to adolescent sensitivity. Certainly methodological factors (e.g., species used, age of animals, dose of ethanol, duration of ethanol exposure, pattern of ethanol exposure, timing of cytokine assessments relative to ethanol exposure, brain region of interest) are likely contributors to these contrasting results, and will have to be carefully considered in future ontogenetic studies. Nevertheless, taken together these developmental studies strongly suggest that age differences in the neuroimmune consequences of ethanol are apparent and should be the focus of future investigations.
The effects of administration of an immunogen, such as LPS, on central cytokines have been extensively studied in adult animals ([44,45]; for review see [56]). Both peripheral and central injection of LPS has been reported to lead to profound activation of immune pathways, with cytokines such as IL-1β and TNFα robustly increased in the brain [e.g., 45]. Not surprisingly, adults in the present study also exhibited significant elevations in gene expression of IL-1β, TNFα, IL-6, and IκBα in the PVN in adults after systemic injection of LPS, with similar LPS-induced increases also observed in the hippocampus and amygdala during adulthood. When adolescents were compared to adults, however, these juvenile animals demonstrated significant attenuations in LPS-related cytokine activation, particularly with respect to cytokine expression in the PVN and amygdala. Similar to the ethanol literature, there is a dearth of studies directly comparing age differences in the immune response to an antigen, such as LPS. A recent study [43] measured plasma cytokine expression in prepubertal and adult male rats after LPS administration, as well as c-fos immunoreactivity in the PVN and measures of HPA axis activation. While few age differences were observed in the first few hours after LPS injection, it was reported that adolescents showed faster resolution of the HPA axis response to LPS, exhibited an abbreviated IL-6 response in plasma, and demonstrated fewer FOS-positive cells in the PVN 8 hours after LPS administration [43]. These results are similar to those reported here, as adolescents in Experiment 1 also exhibited lower concentrations of plasma corticosterone 3 hr after LPS injection, in conjunction with the attenuated brain cytokine response. In contrast, when exposed to a stressor such as restraint, the HPA axis response has been shown to be resolved less quickly in adolescents compared to adults [e.g., 57,58,59]. Thus, it does not seem that adolescents generally evince an abbreviated HPA axis response to a challenge, as this was specific to exposure to an antigen. Developmental differences in the innate immune response during adolescence, as well as general ontogenetic characteristics of the immune system and immune cells at this age, have not been extensively studied, however.
Although the mechanisms involved in this altered sensitivity to an immune challenge during adolescence are unknown at this time, there are several potential explanations for these ontogenetic differences in response to LPS. First, it is a possibility that adolescent animals may have immature immune systems, which would lead to a less efficient immune response to antigen exposure and perhaps attenuated cytokine expression. While there are few studies that have directly examined immune system function specifically during adolescence, there is some evidence to suggest that primary cells of the immune system (e.g., microglia) are fully mature by P30 [60], thus it seems that an immature immune system is not likely the case for adolescents. Another possibility is that adolescents may be exhibiting some form of endotoxin tolerance—a lessened immune response, including brain cytokine production [61,62], in response to endotoxin exposure, due to previous repeated exposures to bacterial challenge [63,64]. Theoretically, however, endotoxin tolerance should grow ontogenetically, as more mature adult rats would be assumed to have had more lifetime exposure to gram-negative bacteria expressing the LPS motif relative to their younger adolescent counterparts. Thus, this possibility seems unlikely for adolescents.
Instead, the present data assessing plasma endotoxin concentrations following LPS exposure would indicate that adolescence may be an ontogenetic period in which transit of LPS from the i.p. cavity to the bloodstream is attenuated. Movement of endotoxin from the peritoneal cavity to the blood has been shown to be necessary for induction of plasma cytokines by endothelial cells or monocytes, which is thought to initiate a brain cytokine response and underlie individual differences in responsiveness to LPS among adults [65,66]. In Experiment 1, LPS administration indeed resulted in profound increases in plasma endotoxin in adults, which were likely contributing to a combination of neural and blood borne signals that orchestrated a neuroinflammatory response to the antigen. In contrast, adolescents given LPS exhibited no alterations in plasma cytokines when compared to either their saline controls or LPS-exposed adults. Among adolescents, the lack of endotoxin in blood suggests that endotoxin was being sequestered in the peritoneal cavity, which eliminated the blood borne component of the signal that would induce expression of central cytokine in response to LPS. Examination of the raw brain cytokine data from the current experiment provides further support for this possibility, as a greater number of adolescent rats appeared “non-responsive” to LPS (i.e., did not elicit a clear and robust increase in cytokine gene expression) when compared to adults. Ultimately, these quantitative differences in LPS-induced central cytokines between adolescents and adults indicate that categorically different mechanisms are responsible for a neuroinflammatory response to peripheral endotoxin exposure across age. Future studies will be necessary to further examine potential age-related differences in reactivity to LPS.
Together, the results of these experiments demonstrated profound age differences in the cytokine response to endotoxin challenge, with adolescent insensitivity to LPS induction of central cytokine expression most pronounced in the PVN and occurring more often in the case of IL-6 and IκBα expression. In contrast, ethanol-induced alterations in central cytokines were generally comparable in adolescents and adults, as there were only three instances in which adolescents given ethanol differed significantly from adults: a blunted ethanol-related induction of IκBα expression in the PVN and amygdala after i.p. ethanol exposure; and attenuated IL-6 expression in the hippocampus after i.g. ethanol exposure. Given these very specific effects of LPS versus ethanol across age, it is tempting to hypothesize about the potential causes and consequences of these effects across brain regions and age.
There are, however, several minor limitations that require thoughtful consideration. While marked age differences were indeed observed in response to LPS, the response to endotoxin exposure was only investigated at one time point and one dose. In order to fully understand ontogenetic differences in sensitivity to both LPS and ethanol, a detailed time-course of cytokine responses across multiple doses will be necessary. Furthermore, it is important to note that age differences being reported here were examined only in male rodents. Although assessment of sex differences was beyond the scope of this series of experiments, it will be important that the neuroimmune consequences of both ethanol and immunogen exposure are examined in both males and females during adolescence and adulthood, as sensitivity to ethanol and/or LPS across ontogeny may vary according to sex. Additionally, it should be noted that central cytokine data were measured in brains without prior perfusion. Although it is possible, therefore, that cytokine signals present in the blood might contribute to the cytokine signal being measured in brain, our previous work reported that IL-1 protein content in the hippocampus was not altered by perfusion [see 67], indicating that the blood borne contribution to brain cytokine measures was minimal. Moreover, other recent work from our lab indicated a strong dissociation between cytokine measures obtained from multiple tissues from the same rats (blood, spleen and brain), further supporting the conclusion that the CNS cytokine measures here are not likely to be contaminated by blood-borne cytokines. Finally, examination of cytokines in fresh-frozen (rather than saline-perfused) brains has the added advantage that it prevents post-mortem degradation of cytokine signals and has therefore emerged as the method of choice for CNS cytokine measurements. We raise these minor concerns so as to be forthcoming with the reader of potential limitations and issues that will be addressed in subsequent studies from our lab.
In conclusion, adolescence is a developmental period in which numerous neural, hormonal, and behavioral transformations are apparent. Increases in risk-taking and sensation seeking increase the likelihood that adolescents will initiate alcohol consumption, while neural and hormonal alterations may change the responsivity of the individual to alcohol exposure at this age. Thus it is imperative that research examining the consequences of ethanol exposure continue to be investigated, with a greater focus given to less traditional neural systems, such as neuroimmune pathways. Emerging data from our laboratory and others suggests that adolescents uniquely respond to an ethanol challenge, and that the effects of ethanol on neuroimmune factors are different in adolescents, even upon the first exposure to the drug. Identification of developmental processes that contribute to ontogenetic differences in innate immunity and ethanol-induced neuroimmune activation are clearly needed in future studies, as this may provide information for novel targets in the prevention of development of alcohol use disorders.
Highlights.
LPS increased IL-6, IL-1, TNFα, and IκBα expression in hippocampus, PVN, and amygdala
Adolescents had lower LPS-related increases in cytokine expression versus adults
Plasma endotoxin was increased by LPS exposure in adults, but not adolescents
Ethanol did not alter plasma endotoxin, but elevated brain IL-6 and IκBα in both ages
Adolescents exhibited attenuated increases in IL-6 and IκBα expression by ethanol
Acknowledgements
Research reported in this publication was supported by the National Institute on Alcohol Abuse and Alcoholism of the National Institute of Health under Award Number P50AA017823 to T. Deak and the Center for Development and Behavioral Neuroscience at Binghamton University. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the above stated funding agencies.
We would like to gratefully acknowledge the impact of our long-term colleague and friend, Dr. Norman E. "Skip" Spear, for whom this Special Issue is dedicated. In addition to inspiring conversation and endless scientific support, Skip's dedication and attention to strong empirical scrutiny and rigorous hypothesis testing has provided a high-water mark for studies examining the ontogeny of learning, memory and responsiveness to ethanol. We eagerly will look forward to ongoing scientific conversation for years to come as he transitions toward Emeritus status.
Abbreviations
- CD14
cluster of differentiation 14
- HMGB1
high-mobility group box 1
- HSPs
heat shock proteins
- IκBα
nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha
- IKK
IκB kinase
- IL-1β
interleukin-1β
- IL-6
interleukin-6
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- TNFα
tumor necrosis factor alpha
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Cui C, Noronha A, Morikawa H, Alvarez VA, Stuber GD, Szumlinski KK, Kash TL, Roberto M, Wilcox MV. New insights on neurobiological mechanisms underlying alcohol addiction. Neuropharmacology. 2013;67:223–232. doi: 10.1016/j.neuropharm.2012.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vengeliene V, Bilbao A, Molander A, Spanagel R. Neuropharmacology of alcohol addiction. Br J Pharmacol. 2008;154:299–315. doi: 10.1038/bjp.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhatty M, Jan BL, Tan W, Pruett SB, Nanduri B. Role of acute ethanol exposure and TLR4 in early events of sepsis in a mouse model. Alcohol. 2011;45:795–803. doi: 10.1016/j.alcohol.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D’Souza NB, Bagby GJ, Nelson S, Lang CH, Spitzer JJ. Acute alcohol infusion suppresses endotoxin-induced serum tumor necrosis factor. Alcohol Clin Exp Res. 1989;13:295–298. doi: 10.1111/j.1530-0277.1989.tb00329.x. [DOI] [PubMed] [Google Scholar]
- 5.D’Souza NB, Nelson S, Summer WR, Deaciuc IV. Alcohol modulates alveolar macrophage tumor necrosis factor-alpha, superoxide anion, and nitric oxide secretion in the rat. Alcohol Clin Exp Res. 1996;20:156–163. doi: 10.1111/j.1530-0277.1996.tb01059.x. [DOI] [PubMed] [Google Scholar]
- 6.Kishore R, Hill JR, McMullen MR, Frenkel J, Nagy LE. ERK1/2 and Egr-1 contribute to increased TNF-alpha production in rat Kupffer cells after chronic ethanol feeding. Am J Physiol Gastrointest Liver Physiol. 2002;282:G6–G15. doi: 10.1152/ajpgi.00328.2001. [DOI] [PubMed] [Google Scholar]
- 7.Honchel R, Ray MB, Marsano L, Cohen D, Lee E, Shedlofsky S, McClain CJ. Tumor necrosis factor in alcohol enhanced endotoxin liver injury. Alcohol Clin Exp Res. 1992;16:665–669. doi: 10.1111/j.1530-0277.1992.tb00656.x. [DOI] [PubMed] [Google Scholar]
- 8.Qin L, He J, Hanes RN, Pluzarev O, Hong JS, Crews FT. Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. J Neuroinflammation. 2008;5:10. doi: 10.1186/1742-2094-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valles SL, Blanco AM, Azorin I, Guasch R, Pascual M, Gomez-Lechon MJ, Renau-Piqueras J, Guerri C. Chronic ethanol consumption enhances interleukin-1-mediated signal transduction in rat liver and in cultured hepatocytes. Alcohol Clin Exp Res. 2003;27:1979–1986. doi: 10.1097/01.ALC.0000099261.87880.21. [DOI] [PubMed] [Google Scholar]
- 10.Szabo G, Mandrekar P. A recent perspective on alcohol, immunity, and host defense. Alcohol Clin Exp Res. 2009;33:220–232. doi: 10.1111/j.1530-0277.2008.00842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez-Quintela A, Dominguez-Santalla MJ, Perez LF, Vidal C, Lojo S, Barrio E. Influence of acute alcohol intake and alcohol withdrawal on circulating levels of IL-6, IL-8, IL-10 and IL-12. Cytokine. 2000;12:1437–1440. doi: 10.1006/cyto.2000.0715. [DOI] [PubMed] [Google Scholar]
- 12.Kim DJ, Kim W, Yoon SJ, Choi BM, Kim JS, Go HJ, Kim YK, Jeong J. Effects of alcohol hangover on cytokine production in healthy subjects. Alcohol. 2003;31:167–170. doi: 10.1016/j.alcohol.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 13.He J, Crews FT. Increased MCP-1 and microglia in various regions of the human alcoholic brain. Exp Neurol. 2008;210:349–358. doi: 10.1016/j.expneurol.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tiwari V, Kuhad A, Chopra K. Suppression of neuro-inflammatory signaling cascade by tocotrienol can prevent chronic alcohol-induced cognitive dysfunction in rats. Behav Brain Res. 2009;203:296–303. doi: 10.1016/j.bbr.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 15.Valles SL, Blanco AM, Pascual M, Guerri C. Chronic ethanol treatment enhances inflammatory mediators and cell death in the brain and in astrocytes. Brain Pathol. 2004;14:365–371. doi: 10.1111/j.1750-3639.2004.tb00079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alfonso-Loeches S, Pascual-Lucas M, Blanco AM, Sanchez-Vera I, Guerri C. Pivotal role of TLR4 receptors in alcohol-induced neuroinflammation and brain damage. J Neurosci. 2010;30:8285–8295. doi: 10.1523/JNEUROSCI.0976-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kane CJ, Phelan KD, Douglas JC, Wagoner G, Johnson JW, Xu J, Phelan PS, Drew PD. Effects of ethanol on immune response in the brain: region-specific changes in adolescent versus adult mice. Alcohol Clin Exp Res. 2014;38:384–391. doi: 10.1111/acer.12244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lippai D, Bala S, Petrasek J, Csak T, Levin I, Kurt-Jones EA, Szabo G. Alcohol-induced IL-1beta in the brain is mediated by NLRP3/ASC inflammasome activation that amplifies neuroinflammation. J Leukoc Biol. 2013;94:171–182. doi: 10.1189/jlb.1212659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blednov YA, Bergeson SE, Walker D, Ferreira VM, Kuziel WA, Harris RA. Perturbation of chemokine networks by gene deletion alters the reinforcing actions of ethanol. Behav Brain Res. 2005;165:110–125. doi: 10.1016/j.bbr.2005.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blednov YA, Ponomarev I, Geil C, Bergeson S, Koob GF, Harris RA. Neuroimmune regulation of alcohol consumption: behavioral validation of genes obtained from genomic studies. Addict Biol. 2012;17:108–120. doi: 10.1111/j.1369-1600.2010.00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agrawal RG, Hewetson A, George CM, Syapin PJ, Bergeson SE. Minocycline reduces ethanol drinking. Brain Behav Immun. 2011;25(Suppl 1):S165–S169. doi: 10.1016/j.bbi.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blednov YA, Benavidez JM, Geil C, Perra S, Morikawa H, Harris RA. Activation of inflammatory signaling by lipopolysaccharide produces a prolonged increase of voluntary alcohol intake in mice. Brain Behav Immun. 2011;25(Suppl 1):S92–S105. doi: 10.1016/j.bbi.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu Y, Lousberg EL, Moldenhauer LM, Hayball JD, Robertson SA, Coller JK, Watkins LR, Somogyi AA, Hutchinson MR. Attenuation of microglial and IL-1 signaling protects mice from acute alcohol-induced sedation and/or motor impairment. Brain Behav Immun. 2011;25(Suppl 1):S155–S164. doi: 10.1016/j.bbi.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 24.Doremus TL, Brunell SC, Rajendran P, Spear LP. Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcohol Clin Exp Res. 2005;29:1796–1808. doi: 10.1097/01.alc.0000183007.65998.aa. [DOI] [PubMed] [Google Scholar]
- 25.Vetter CS, Doremus-Fitzwater TL, Spear LP. Time course of elevated ethanol intake in adolescent relative to adult rats under continuous, voluntary-access conditions. Alcohol Clin Exp Res. 2007;31:1159–1168. doi: 10.1111/j.1530-0277.2007.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spear LP, Varlinskaya EI. Sensitivity to ethanol and other hedonic stimuli in an animal model of adolescence: implications for prevention science? Dev Psychobiol. 2010;52:236–243. doi: 10.1002/dev.20457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 28.Silveri MM, Spear LP. Decreased sensitivity to the hypnotic effects of ethanol early in ontogeny. Alcohol Clin Exp Res. 1998;22:670–676. doi: 10.1111/j.1530-0277.1998.tb04310.x. [DOI] [PubMed] [Google Scholar]
- 29.White AM, Truesdale MC, Bae JG, Ahmad S, Wilson WA, Best PJ, Swartzwelder HS. Differential effects of ethanol on motor coordination in adolescent and adult rats. Pharmacol Biochem Behav. 2002;73:673–677. doi: 10.1016/s0091-3057(02)00860-2. [DOI] [PubMed] [Google Scholar]
- 30.Doremus TL, Brunell SC, Varlinskaya EI, Spear LP. Anxiogenic effects during withdrawal from acute ethanol in adolescent and adult rats. Pharmacol Biochem Behav. 2003;75:411–418. doi: 10.1016/s0091-3057(03)00134-5. [DOI] [PubMed] [Google Scholar]
- 31.Anderson RI, Varlinskaya EI, Spear LP. Ethanol-induced conditioned taste aversion in male sprague-dawley rats: impact of age and stress. Alcohol Clin Exp Res. 2010;34:2106–2115. doi: 10.1111/j.1530-0277.2010.01307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Varlinskaya EI, Spear LP. Acute effects of ethanol on social behavior of adolescent and adult rats: role of familiarity of the test situation. Alcohol Clin Exp Res. 2002;26:1502–1511. doi: 10.1097/01.ALC.0000034033.95701.E3. [DOI] [PubMed] [Google Scholar]
- 33.Markwiese BJ, Acheson SK, Levin ED, Wilson WA, Swartzwelder HS. Differential effects of ethanol on memory in adolescent and adult rats. Alcohol Clin Exp Res. 1998;22:416–421. [PubMed] [Google Scholar]
- 34.Pyapali GK, Turner DA, Wilson WA, Swartzwelder HS. Age and dose-dependent effects of ethanol on the induction of hippocampal long-term potentiation. Alcohol. 1999;19:107–111. doi: 10.1016/s0741-8329(99)00021-x. [DOI] [PubMed] [Google Scholar]
- 35.McClain JA, Hayes DM, Morris SA, Nixon K. Adolescent binge alcohol exposure alters hippocampal progenitor cell proliferation in rats: effects on cell cycle kinetics. J Comp Neurol. 2011;519:2697–2710. doi: 10.1002/cne.22647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vetreno RP, Crews FT. Adolescent binge drinking increases expression of the danger signal receptor agonist HMGB1 and Toll-like receptors in the adult prefrontal cortex. Neuroscience. 2012;226:475–488. doi: 10.1016/j.neuroscience.2012.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vetreno RP, Qin L, Crews FT. Increased receptor for advanced glycation end product expression in the human alcoholic prefrontal cortex is linked to adolescent drinking. Neurobiol Dis. 2013;59:52–62. doi: 10.1016/j.nbd.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pascual M, Pla A, Minarro J, Guerri C. Neuroimmune activation and myelin changes in adolescent rats exposed to high-dose alcohol and associated cognitive dysfunction: a review with reference to human adolescent drinking. Alcohol Alcohol. 2013;49:187–192. doi: 10.1093/alcalc/agt164. [DOI] [PubMed] [Google Scholar]
- 39.Doremus-Fitzwater TL, Buck HM, Bordner K, Richey L, Jones ME, Deak T. Intoxication- and withdrawal-dependent expression of central and peripheral cytokines following initial ethanol exposure. Alcohol Clin Exp Res. 2014;38:2186–2198. doi: 10.1111/acer.12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mathurin P, Deng QG, Keshavarzian A, Choudhary S, Holmes EW, Tsukamoto H. Exacerbation of alcoholic liver injury by enteral endotoxin in rats. Hepatology. 2000;32:1008–1017. doi: 10.1053/jhep.2000.19621. [DOI] [PubMed] [Google Scholar]
- 41.Rivera CA, Bradford BU, Seabra V, Thurman RG. Role of endotoxin in the hypermetabolic state after acute ethanol exposure. Am J Physiol. 1998;275:G1252–G1258. doi: 10.1152/ajpgi.1998.275.6.G1252. [DOI] [PubMed] [Google Scholar]
- 42.Institute of Laboratory Animal Research CoLS, National Research Council. Guide for the Care and Use of Laboratory Animals. Washington, D.C: National Academy Press; 1996. [Google Scholar]
- 43.Goble KH, Bain ZA, Padow VA, Lui P, Klein ZA, Romeo RD. Pubertal-related changes in hypothalamic-pituitary-adrenal axis reactivity and cytokine secretion in response to an immunological stressor. J Neuroendocrinol. 2011;23:129–135. doi: 10.1111/j.1365-2826.2010.02085.x. [DOI] [PubMed] [Google Scholar]
- 44.Hansen MK, Nguyen KT, Goehler LE, Gaykema RP, Fleshner M, Maier SF, Watkins LR. Effects of vagotomy on lipopolysaccharide-induced brain interleukin-1beta protein in rats. Auton Neurosci. 2000;85:119–126. doi: 10.1016/s1566-0702(00)00230-7. [DOI] [PubMed] [Google Scholar]
- 45.Kakizaki Y, Watanobe H, Kohsaka A, Suda T. Temporal profiles of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in the plasma and hypothalamic paraventricular nucleus after intravenous or intraperitoneal administration of lipopolysaccharide in the rat: estimation by push-pull perfusion. Endocr J. 1999;46:487–496. doi: 10.1507/endocrj.46.487. [DOI] [PubMed] [Google Scholar]
- 46.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 47.Deak T, Okrainets A, Doremus-Fitzwater TL. Mechanisms of stress-dependent neuroinflammation and their implications for understanding consequences of alcohol exposure. In: Cui C, Grandison L, Noronha A, editors. Neural-immune interactions in brain function and alcohol related disorders. New York, Berlin: Springer; 2012. pp. 133–166. [Google Scholar]
- 48.Emanuele NV, LaPaglia N, Kovacs EJ, Emanuele MA. The impact of burn injury and ethanol on the cytokine network of the mouse hypothalamus: reproductive implications. Cytokine. 2005;30:109–115. doi: 10.1016/j.cyto.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 49.Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Crews FT, Qin L, Sheedy D, Vetreno RP, Zou J. High mobility group box 1/Toll-like receptor danger signaling increases brain neuroimmune activation in alcohol dependence. Biol Psychiatry. 2013;73:602–612. doi: 10.1016/j.biopsych.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Whitman BA, Knapp DJ, Werner DF, Crews FT, Breese GR. The cytokine mRNA increase induced by withdrawal from chronic ethanol in the sterile environment of brain is mediated by CRF and HMGB1 release. Alcohol Clin Exp Res. 2013;37:2086–2097. doi: 10.1111/acer.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu Y, Lousberg EL, Moldenhauer LM, Hayball JD, Coller JK, Rice KC, Watkins LR, Somogyi AA, Hutchinson MR. Inhibiting the TLR4-MyD88 signalling cascade by genetic or pharmacological strategies reduces acute alcohol-induced sedation and motor impairment in mice. Br J Pharmacol. 2012;165:1319–1329. doi: 10.1111/j.1476-5381.2011.01572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Purohit V, Bode JC, Bode C, Brenner DA, Choudhry MA, Hamilton F, Kang YJ, Keshavarzian A, Rao R, Sartor RB, and others Alcohol, intestinal bacterial growth, intestinal permeability to endotoxin, and medical consequences: summary of a symposium. Alcohol. 2008;42:349–361. doi: 10.1016/j.alcohol.2008.03.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813:878–888. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 55.Xing Z, Gauldie J, Cox G, Baumann H, Jordana M, Lei XF, Achong MK. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J. Clin. Invest. 1998;101:311–320. doi: 10.1172/JCI1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dantzer R. Cytokine, sickness behavior, and depression. Neurol Clin. 2006;24:441–460. doi: 10.1016/j.ncl.2006.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Doremus-Fitzwater TL, Varlinskaya EI, Spear LP. Social and non-social anxiety in adolescent and adult rats after repeated restraint. Physiol Behav. 2009;97:484–494. doi: 10.1016/j.physbeh.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Romeo RD, Bellani R, Karatsoreos IN, Chhua N, Vernov M, Conrad CD, McEwen BS. Stress history and pubertal development interact to shape hypothalamic-pituitary-adrenal axis plasticity. Endocrinology. 2006;147:1664–1674. doi: 10.1210/en.2005-1432. [DOI] [PubMed] [Google Scholar]
- 59.Romeo RD, McEwen BS. Stress and the adolescent brain. Annals Of The New York Academy Of Sciences. 2006;1094:202–214. doi: 10.1196/annals.1376.022. [DOI] [PubMed] [Google Scholar]
- 60.Bilbo SD, Smith SH, Schwarz JM. A lifespan approach to neuroinflammatory and cognitive disorders: a critical role for glia. J Neuroimmune Pharmacol. 2012;7:24–41. doi: 10.1007/s11481-011-9299-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen R, Zhou H, Beltran J, Malellari L, Chang SL. Differential expression of cytokines in the brain and serum during endotoxin tolerance. J Neuroimmunol. 2005;163:53–72. doi: 10.1016/j.jneuroim.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 62.Peck OM, Williams DL, Breuel KF, Kalbfleisch JH, Fan H, Tempel GE, Teti G, Cook JA. Differential regulation of cytokine and chemokine production in lipopolysaccharide-induced tolerance and priming. Cytokine. 2004;26:202–208. doi: 10.1016/j.cyto.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 63.Cook JA. Molecular basis of endotoxin tolerance. Ann N Y Acad Sci. 1998;851:426–428. doi: 10.1111/j.1749-6632.1998.tb09020.x. [DOI] [PubMed] [Google Scholar]
- 64.Ziegler-Heitbrock HW. Molecular mechanism in tolerance to lipopolysaccharide. J Inflamm. 1995;45:13–26. [PubMed] [Google Scholar]
- 65.Lenczowski MJ, Schmidt ED, Van Dam AM, Gaykema RP, Tilders FJ. Individual variation in hypothalamus-pituitary-adrenal responsiveness of rats to endotoxin and interleukin-1 beta. Ann N Y Acad Sci. 1998;856:139–147. doi: 10.1111/j.1749-6632.1998.tb08322.x. [DOI] [PubMed] [Google Scholar]
- 66.Lenczowski MJ, Van Dam AM, Poole S, Larrick JW, Tilders FJ. Role of circulating endotoxin and interleukin-6 in the ACTH and corticosterone response to intraperitoneal LPS. Am J Physiol. 1997;273:R1870–R1877. doi: 10.1152/ajpregu.1997.273.6.R1870. [DOI] [PubMed] [Google Scholar]
- 67.Nguyen KT, Deak T, Will MJ, Hansen MK, Hunsaker BN, Fleshner M, Watkins LR, Maier SF. Timecourse and corticosterone sensitivity of the brain, pituitary, and serum interleukin-1beta protein response to acute stress. Brain Res. 2000;859:193–201. doi: 10.1016/s0006-8993(99)02443-9. [DOI] [PubMed] [Google Scholar]
- 68.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4th ed. San Diego: Academic Press; 1998. [Google Scholar]