Abstract
Several recent studies, using molecular, electrophysiological, or structural approaches, have investigated how synapses are affected by sleep, spontaneous wake, chronic sleep restriction, and acute sleep deprivation. Overall, the results have found that even a few hours of sleep or wake can modify the molecular composition of excitatory synapses, change their efficacy, and make synapses grow or shrink. Moreover, partial and total loss of sleep affect the ability of synapses to undergo long-term potentiation, an effect that may underlie some of the negative consequences of sleep deprivation on memory and other cognitive functions.
INTRODUCTION
Acute total sleep deprivation and chronic sleep restriction consistently lead to impairment of many cognitive functions, from attention and working memory to verbal fluency, innovative thinking, and even humor appreciation [1,2]. The human brain uses up to 25% of the whole body glucose consumption, despite accounting for only 2% of body mass [3]. Most of this energy is used to sustain the various components of synaptic activity, from the release of neurotransmitter vesicles and their recycling to the restoration of Na+ and K+ gradients following postsynaptic potentials [4,5]. It is not surprising, therefore, that many hypotheses about the functions of sleep have focused on its effects on synapses, suggesting that sleep may allow the recovery of the synapses that underwent plastic changes during wake [6], the further strengthening of the synapses activated by learning during wake [7], the stimulation of the synapses that were not sufficiently activated during wake [8–10], or the generalized downregulation of synaptic strength after wake-induced synaptic potentiation [11]. Irrespective of the specific hypothesis, the underlying assumption is that sleep, or lack of it, will affect how synapses work, with significant consequences for brain functions. Below is a brief summary of some of the recent studies that assessed the effects of sleep, wake, and sleep deprivation on synaptic activity.
MOLECULAR CHANGES AT THE SYNAPSE DURING SLEEP AND WAKE
Over the last decade, microarray studies have shown that there are hundreds of genes whose brain expression is affected by sleep and wake, and several of them are involved in synaptic plasticity. In general, putative markers of synaptic potentiation, including several immediate early genes and brain derived neurotrophic factor (BDNF), are expressed at higher levels during spontaneous wake and short sleep deprivation than during sleep [12–16**]. Most of these studies pooled transcripts from large brain regions, for example the entire cerebral cortex or the whole forebrain, and thus the results suggest that sleep/wake effects on the expression of genes involved in synaptic plasticity are widespread. Other studies [17–19] used rats previously subjected to lesions of the locus coeruleus, to deplete the cerebral cortex of noradrenergic fibers. In general, loss of sleep is followed by a sleep rebound, i.e. sleep is longer and deeper, an homeostatic response that strongly suggests that sleep has important functions. The rats with noradrenergic lesions showed a blunted homeostatic response after sleep deprivation. In these animals most cortical transcripts upregulated by wake and short sleep deprivation were unaffected by the noradrenergic depletion, with the exception of plasticity-related genes, whose upregulation during wake was reduced or completely eliminated. These findings suggested that the diffuse upregulation of plasticity-related genes during wake is not just an epiphenomenon of wake-related learning, but may be causally involved in determining sleep need. This conclusion has been strengthened by the results of a recent study [15**] whose results are complementary to those reported in rats with noradrenergic lesions. The authors assessed sleep homeostatic mechanisms and sleep/wake-dependent brain gene expression in normal mice and in mice subjected to adrenalectomy, which abolishes the increase in corticosterone levels often seen during acute sleep deprivation. They found that most of the brain transcripts upregulated after a few hours of sleep deprivation in controls were no longer upregulated in adrenalectomized, sleep deprived mice. Crucially, however, genes involved in synaptic plasticity were among the few still upregulated after adrenalectomy [15**]. Moreover, adrenalectomy did not blunt the sleep homeostatic response; in other words, adrenalectomized mice still slept deeper and longer after acute sleep deprivation, and did so in a manner indistinguishable from that of controls. Together, the recent study [15**] and the older experiments suggest that sleep need is more strongly linked to the wake-dependent expression of plasticity-related genes than to the expression of other genes controlled by the classical stress pathway.
Most plasticity-related genes identified by microarray studies are only indirect markers of synaptic function. Recent studies, however, have measured how sleep and wake affect the number and phosphorylation levels of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs; [20–23]). The occurrence of synaptic potentiation and depression is believed to depend on the movement of AMPARs in and out of the synaptic membrane, respectively [24,25]. AMPARs containing the subunit GluA1 are permeable to calcium and their expression shows a supralinear relationship with the area of the post-synaptic density [26]. Thus, they may be in a unique position to affect synaptic strength. In an early study [20] CA1 neurons in the intact brain were infected with a GluA1-green fluorescent protein (GFP) virus, and hippocampal slices were prepared ~ 10 hours later, after the rats had spent most of the time either asleep or awake. Only after wake, but not after sleep, CA1 neurons showed increased rectification compared with nonexpressing neurons, indicating synaptic insertion of GluA1-containing AMPARs. GluA2-containing AMPARs, by contrast, could be inserted in both wake and sleep. A more recent study assessed the expression of GluA1-containing AMPARs in synaptoneurosomes, a preparation that enriches for synaptic proteins, and found a ~40% increase in wake relative to sleep in rat cortex and hippocampus [21]. In the same animals absolute levels of GluA1 phosphorylation at Ser831 and Ser845 were also higher in wake than in sleep. GluA1 phosphorylation at Ser831 enhances single channel conductance and is associated with long-term potentiation, while dephosphorylation of GluA1 at Ser845 leads to a decrease in the channel open probability and to internalization of AMPARs [24,25]. Another recent study performed whole-cell patch-clamp recordings from layer V pyramidal neurons of rat somatosensory cortex, and found that after the dark period, when the animals were mostly awake, calcium-permeable AMPA currents accounted for 25% of the total size of excitatory postsynaptic potentials, while after the light period, which rats spend mostly asleep, their contribution was much reduced, suggesting that GluA1-containing AMPARs are removed during sleep [22]. Finally, it was recently shown, in synaptoneurosomes obtained from mouse cortex (half cortical hemisphere), that GluA1 phosphorylation at Ser845 increases with time spent awake and decreases during sleep [23**]. Thus, in both mice and rats, sleep and wake lead to widespread changes in the expression of AMPARs in both cortex and hippocampus. These changes are consistent with the occurrence of an overall increase in synaptic strength in large brain regions after wake, and an overall decrease after sleep.
ELECTROPHYSIOLOGICAL CHANGES AT THE SYNAPSE DURING SLEEP AND WAKE
The effects of sleep and wake on electrophysiological markers of synaptic efficacy have also been measured in recent studies. The slope of the early (monosynaptic) response evoked by electrical stimulation delivered in vivo is a classical electrophysiological measure of synaptic strength, with a steeper slope indicating higher synaptic efficiency. Studies in rats found that in both cortex [21] and hippocampus [27], the first negative component of the evoked response increases with time spent awake and decreases with time spent asleep. A recent study found similar results in humans using transcranial magnetic stimulation to evoke a response in frontal cortex: the slope of the early response increased progressively in the course of 18 hours of continuous wake, and returned to baseline levels after one night of recovery sleep [28*]. These electrophysiological changes are consistent with the molecular changes described above, pointing to an overall increase in synaptic efficacy after wake. In contrast to these results, a recent study in cats reported that the amplitude of the cortical response increased, rather than decreased, after sleep [29]. Also in contrast to previous studies, whose effects were observed after several hours of sleep or wake, the effect in cats occurred after as little as 10 min of sleep, and rapidly saturated after two short sleep episodes. Whether the saturated responses eventually recovered was not investigated. While species-specific differences may exist, the EEG and intracellular recordings shown in this paper suggest that cats may have been sleepy while the responses were measured, since the membrane potential in the “awake” condition immediately post-sleep was actually hyperpolarized. In the previous studies in rats and humans, evoked responses were recorded some time after awakening, to avoid confounding effects due to sleep inertia.
The analysis of the frequency and amplitude of miniature excitatory postsynaptic currents (mEPSCs) in slices is another classical electrophysiological method to assess synaptic strength. It was found that both frequency and amplitude of mEPSCs increase in layers II-III of frontal cortex after wake and short total sleep deprivation, suggesting an increase in both presynaptic and postsynaptic efficacy [41]. In infragranular layers of medial prefrontal cortex, instead, only limited mEPSCs changes were seen after acute sleep restriction that targeted REM sleep but spared 50% of NREM sleep, with a small decrease in amplitude but no change in frequency [42]. This latter study suggests that stronger synaptic effects are seen only when both NREM sleep and REM sleep are prevented.
Recent studies have also focused on the effects of different durations of sleep deprivation on the ability of synapses to undergo long-term synaptic potentiation or depression. It has been known for a long time that electrical stimuli delivered in vivo can easily induce long-term potentiation in the hippocampus during wake and REM sleep but not during NREM sleep [30,31]. Intriguingly, after learning, long-term potentiation can still be induced during REM sleep but not during wake [32], suggesting that learning impairs the further strengthening of synapses, at least until the animal is allowed to sleep. Several more recent studies, mostly in vitro, have assessed how sleep deprivation affects the ability to induce synaptic potentiation or synaptic depression in the hippocampus, mainly in the CA1 region. Chronic sleep restriction for up to 72 hours using the flower pot technique, or sleep restriction due to forced locomotion on a treadmill, strongly decrease REM sleep but spare a large fraction of NREM sleep. These treatments impair the induction of long-term potentiation but not that of synaptic depression [33–35]. One of the studies concluded that saturation was unlikely to account for the impaired potentiation, because synaptic depression was normal but not enhanced, and its induction could not rescue the induction of potentiation [33]. The same study also suggested that reduced membrane excitability was unlikely to explain the results, because it occurred in CA1 but not in the dentate gyrus, while potentiation was impaired in both regions [33]. Instead, the authors suggested a postsynaptic mechanism, which involved a decrease in N-methyl-D-aspartate (NMDA) currents associated with decreased surface expression of NMDA receptors [36]. Other recent studies have used short periods (4–5 hours) of total sleep deprivation, in which both NREM sleep and REM sleep are abolished. It was found that acute sleep loss also impairs the induction of synaptic potentiation, although the block was not absolute [37,38]. One study found that the induction of potentiation was negatively affected only when using patterns of stimulation, such as massed stimulation, which depend on cAMP signaling, since acute sleep loss increased phosphodiesterase activity [38]. In the second study potentiation could still be induced by repeated high frequency stimulation but not by single stimulations at 10 or 100 Hz [37], again suggesting that saturation was unlikely to account for the failed potentiation. Instead, a decrease in NMDA currents was observed, associated with changes in receptor subunit composition resulting in an increased NR2A/NR2B ratio, pointing again to a postsynaptic mechanism centered on altered NMDA function. Of note, this study also found that the range of stimulation frequencies able to induce synaptic depression had expanded to the right. In other words, synaptic depression could be induced by stimulation not only at 1 Hz, as expected, but also at 5 Hz, which in controls was ineffective [37]. Thus, in the hippocampus, REM sleep deprivation seems to specifically impair synaptic potentiation but leaves depression unmodified, while the loss of both NREM and REM sleep impairs potentiation and facilitates depression.
Impaired synaptic potentiation associated with facilitated induction of synaptic depression has been described in several cases, including in mice expressing a mutant form of CamKII, in which Threonine 286 is constitutively phosphorylated. The mutation results in increased levels of Ca++-independent CaMKII activity, similar to that seen following the induction of synaptic potentiation [39]. Other cases include developmental maturation and exposure to sensory stimulation, both of which trigger an increase in the NR2A/NR2B ratio. During brain development, an increase in the NR2A/NR2B ratio with age is believed to explain why synaptic depression becomes progressively easier to induce, while a decrease in the NR2A/NR2B ratio during monocular deprivation is believed to favor the late potentiation of the open eye [40]. Of note, wake and sleep deprivation increase the phosphorylation of CamKII at Threonine 286 [21], as well as the NR2A/NR2B ratio [37]. Thus, there may be different mechanisms by which sleep loss impairs potentiation but facilitates depression. Impaired potentiation may depend heavily on reduced NMDA currents and decreased cAMP signaling, while facilitated depression may be the result of sustained activation of CamKII and increased NR2A/NR2B ratio. Independent of the specific mechanism, it seems clear that by the end of prolonged wake, hippocampal synapses can be more easily depressed than potentiated. In line with the results in hippocampus, high frequency electrical stimulation during quiet wake fails to elicit synaptic potentiation in rat frontal motor cortex after several hours of wakefulness, while potentiation can be easily induced after a period of sleep [21]. The same study did not test synaptic depression but found increased cortical expression of AMPARs and no change in NR2A expression, suggesting that unlike in the hippocampus, occlusion may at least partially account for impaired potentiation in cortex.
STRUCTURAL CHANGES AT THE SYNAPSE DURING SLEEP AND WAKE
In general, structural changes in synapses are slower to occur than molecular and electrophysiological changes, but they can still happen in a matter of a few hours [43,44], a time line compatible with that of the physiological sleep/wake cycle. Recent studies have documented the growth of synapse number and/or size in the course of a few hours of wake in several neuronal circuits of the fly. For instance, axonal tips of the γ neurons of the mushroom bodies, which are involved in olfactory learning and sleep regulation, are larger after 7 hours of either spontaneous or forced wake than after 7 hours of sleep [45**]. Also, the number of dendritic spines in the first giant tangential neuron of the lobula plate vertical system, part of the visual system, increases after 7 hours of sleep deprivation [45**]. Of note, the number of spines in this neuron is similar in sleeping flies and in awake flies housed in isolation in a small, empty tube. By contrast, if 12 hours of spontaneous wake are spent in an enriched environment, both dendritic branch length and spine number increase significantly relative to the level in sleeping flies. Moreover, synaptic morphology goes back to pre-enrichment levels only if flies are allowed to sleep, but not if after enrichment they are forced to stay awake [45**]. Thus, in young adult flies, rapid synaptic growth can occur as a result of wake experience, and these morphological changes are reverted by sleep.
In mammals, structural synaptic changes due to sleep and wake have been investigated in yellow fluorescent protein (YFP)-H-expressing mice by using repeated two-photon microscopy. Branches of apical dendrites of layer V pyramidal neurons were identified and dendritic spines were counted twice within ~12–16 hours, after a period spent mostly asleep or awake. In adolescent, 1-month old mice, spine formation and spine elimination occurred at all times, but the former was larger than the latter during wake, while the opposite occurred during sleep, resulting in a net increase in spine density during wake, and in a net decrease during sleep [46*]. In adult mice spine turnover was very limited relative to that observed in adolescent mice, and was not affected by sleep and wake [46*]. Another study used the same mouse strain but younger mice, only 3-week old, an age that corresponds to the beginning of adolescence. The results were consistent with those seen in 1-month old animals: spine formation was greater than spine elimination during the dark period, when mice are mostly awake, while the opposite occurred during the day, when mice are mostly asleep [47*]. Similar sleep/wake effects were seen in filopodia, thin dendritic protrusions that are abundant in very young animals and may act as precursors of mature spines. Moreover, spine elimination was higher if the 2 hours between imaging sessions were spent asleep rather than awake [47*]. Although still limited, these studies suggest that sleep and wake can quickly affect the structure of synapses in the mammalian brain, at least in young animals.
CONCLUSIONS
It is obvious that sleep and wake can profoundly affect synaptic function, primarily by enhancing synaptic efficacy during wake and reducing it during sleep (Figure 1). Moreover, sleep deprivation limits the ability of synapses to increase their strength as a result of experience-dependent plasticity. Most experiments showing impairment of long-term potentiation after sleep deprivation have focused on the CA1 region of the hippocampus. In the future, therefore, it will be important to determine whether a similar impairment occurs in cortical and other subcortical regions. Similarly, electrophysiological and molecular changes in synaptic strength due to sleep and wake have been observed in cortex and hippocampus, but it is unknown whether they also occur in striatum, thalamus, hypothalamus, or brainstem. One study found that sleep deprivation increases the frequency and amplitude of mEPSCs in excitatory synapses on hypocretin/orexin neurons [48], but clearly more evidence is needed. Since these sleep/wake changes are driven by experience-dependent plasticity, their extent may vary in different regions of the brain, depending on how “plastic” these regions are.
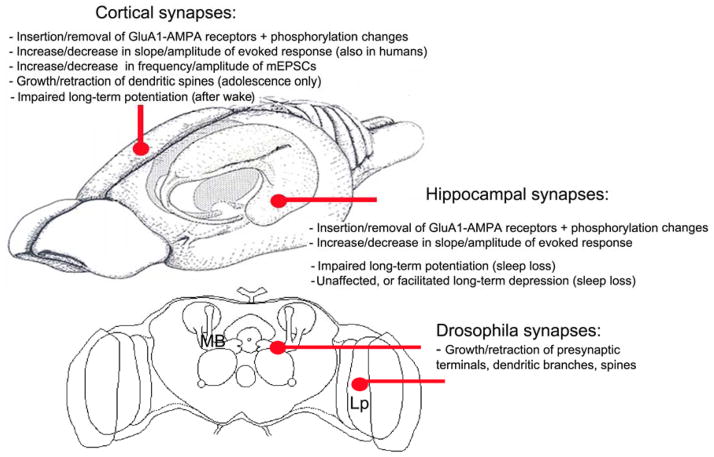
Figure 1.
Schematic diagram of some of the synaptic changes observed across the sleep/wake cycle and after sleep loss in the rodent brain (schematic view of the rat brain) and in the fly brain (frontal section). mEPSCs, miniature excitatory postsynaptic potentials; MB, mushroom bodies; Lp, lobula plate.
Highlights.
Synapses undergo molecular, electrophysiological, and structural changes due to the physiological sleep/wake cycle or as a consequence of partial or total sleep deprivation.
Sleep deprivation, especially when both NREM sleep and REM sleep are prevented, impairs the ability of synapses to undergo activity-dependent plastic changes.
The cognitive impairment caused by sleep deprivation is likely mediated by the effects of sleep loss on synaptic function.
Acknowledgments
This work was supported by NIMH (1R01MH091326).
References
* of special interest
** of outstanding interest
- 1.Banks S, Dinges DF. Behavioral and physiological consequences of sleep restriction. J Clin Sleep Med. 2007;3:519–528. [PMC free article] [PubMed] [Google Scholar]
- 2.Killgore WD. Effects of sleep deprivation on cognition. Prog Brain Res. 2010;185:105–129. doi: 10.1016/B978-0-444-53702-7.00007-5. [DOI] [PubMed] [Google Scholar]
- 3.Sokoloff L. Metabolism of the central nervous system in vivo. In: Field J, Magoun HW, editors. Handbook of Physiology. Neurophysiology. III American Physiological Society; 1960. pp. 1843–1864. [Google Scholar]
- 4.Mangia S, Giove F, Tkac I, Logothetis NK, Henry PG, Olman CA, Maraviglia B, Di Salle F, Ugurbil K. Metabolic and hemodynamic events after changes in neuronal activity: current hypotheses, theoretical predictions and in vivo NMR experimental findings. J Cereb Blood Flow Metab. 2009;29:441–463. doi: 10.1038/jcbfm.2008.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harris JJ, Jolivet R, Attwell D. Synaptic energy use and supply. Neuron. 2012;75:762–777. doi: 10.1016/j.neuron.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 6.Moruzzi G. The functional significance of sleep with particular regard to the brain mechanisms underlying consciousness. In: Eccles JC, editor. Brain and Conscious Experience. Springer-Verlag; 1966. pp. 345–388. [Google Scholar]
- 7.Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11:114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- 8.Kavanau JL. Memory, sleep and the evolution of mechanisms of synaptic efficacy maintenance. Neuroscience. 1997;79:7–44. doi: 10.1016/s0306-4522(96)00610-0. [DOI] [PubMed] [Google Scholar]
- 9.Krueger JM, Obal F. A neuronal group theory of sleep function. J Sleep Res. 1993;2:63–69. doi: 10.1111/j.1365-2869.1993.tb00064.x. [DOI] [PubMed] [Google Scholar]
- 10.Krueger JM, Obal F., Jr Sleep function. Front Biosci. 2003;8:d511–519. doi: 10.2741/1031. [DOI] [PubMed] [Google Scholar]
- 11.Tononi G, Cirelli C. Time to Be SHY? Some Comments on Sleep and Synaptic Homeostasis. Neural Plast. 2012;2012:415250. doi: 10.1155/2012/415250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cirelli C, Gutierrez CM, Tononi G. Extensive and divergent effects of sleep and wakefulness on brain gene expression. Neuron. 2004;41:35–43. doi: 10.1016/s0896-6273(03)00814-6. [DOI] [PubMed] [Google Scholar]
- 13.Mackiewicz M, Shockley KR, Romer MA, Galante RJ, Zimmerman JE, Naidoo N, Baldwin DA, Jensen ST, Churchill GA, Pack AI. Macromolecule biosynthesis - a key function of sleep. Physiol Genomics. 2007;31:441–457. doi: 10.1152/physiolgenomics.00275.2006. [DOI] [PubMed] [Google Scholar]
- 14.Maret S, Dorsaz S, Gurcel L, Pradervand S, Petit B, Pfister C, Hagenbuchle O, O’Hara BF, Franken P, Tafti M. Homer1a is a core brain molecular correlate of sleep loss. Proc Natl Acad Sci U S A. 2007;104:20090–20095. doi: 10.1073/pnas.0710131104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **15.Mongrain V, Hernandez SA, Pradervand S, Dorsaz S, Curie T, Hagiwara G, Gip P, Heller HC, Franken P. Separating the contribution of glucocorticoids and wakefulness to the molecular and electrophysiological correlates of sleep homeostasis. Sleep. 2010;33:1147–1157. doi: 10.1093/sleep/33.9.1147. A comprehensive analysis of the contribution of the corticosterone component of stress to the electroencephalographic and molecular markers of sleep homeostasis in mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **16.Thompson CL, Wisor JP, Lee CK, Pathak SD, Gerashchenko D, Smith KA, Fischer SR, Kuan CL, Sunkin SM, Ng LL, et al. Molecular and anatomical signatures of sleep deprivation in the mouse brain. Front Neurosci. 2010;4:165. doi: 10.3389/fnins.2010.00165. A comprehensive neuroanatomical, cellular, and molecular map of gene induction across sleep, wake, and sleep deprivation in the mouse brain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cirelli C, Pompeiano M, Tononi G. Neuronal gene expression in the waking state: A role for the locus coeruleus. Science. 1996;274:1211–1215. doi: 10.1126/science.274.5290.1211. [DOI] [PubMed] [Google Scholar]
- 18.Cirelli C, Tononi G. Locus ceruleus control of state-dependent gene expression. J Neurosci. 2004;24:5410–5419. doi: 10.1523/JNEUROSCI.0949-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cirelli C, Huber R, Gopalakrishnan A, Southard TL, Tononi G. Locus ceruleus control of slow-wave homeostasis. J Neurosci. 2005;25:4503–4511. doi: 10.1523/JNEUROSCI.4845-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qin Y, Zhu Y, Baumgart JP, Stornetta RL, Seidenman K, Mack V, van Aelst L, Zhu JJ. State-dependent Ras signaling and AMPA receptor trafficking. Genes Dev. 2005;19:2000–2015. doi: 10.1101/gad.342205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vyazovskiy VV, Cirelli C, Pfister-Genskow M, Faraguna U, Tononi G. Molecular and electrophysiological evidence for net synaptic potentiation in wake and depression in sleep. Nat Neurosci. 2008;11:200–208. doi: 10.1038/nn2035. [DOI] [PubMed] [Google Scholar]
- 22.Lante F, Toledo-Salas JC, Ondrejcak T, Rowan MJ, Ulrich D. Removal of synaptic Ca(2)+-permeable AMPA receptors during sleep. J Neurosci. 2011;31:3953–3961. doi: 10.1523/JNEUROSCI.3210-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **23.Hinard V, Mikhail C, Pradervand S, Curie T, Houtkooper RH, Auwerx J, Franken P, Tafti M. Key electrophysiological, molecular, and metabolic signatures of sleep and wakefulness revealed in primary cortical cultures. J Neurosci. 2012;32:12506–12517. doi: 10.1523/JNEUROSCI.2306-12.2012. A pioneer study that combined in vitro model and sleep-deprived animals to identify shared correlates of sleep and wake states. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 25.Collingridge GL, Isaac JT, Wang YT. Receptor trafficking and synaptic plasticity. Nat Rev Neurosci. 2004;5:952–962. doi: 10.1038/nrn1556. [DOI] [PubMed] [Google Scholar]
- 26.Shinohara Y, Hirase H. Size and Receptor Density of Glutamatergic Synapses: A Viewpoint from Left-Right Asymmetry of CA3-CA1 Connections. Front Neuroanat. 2009;3:10. doi: 10.3389/neuro.05.010.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lubenov EV, Siapas AG. Decoupling through Synchrony in Neuronal Circuits with Propagation Delays. Neuron. 2008;58:118–131. doi: 10.1016/j.neuron.2008.01.036. [DOI] [PubMed] [Google Scholar]
- *28.Huber R, Maki H, Rosanova M, Casarotto S, Canali P, Casali AG, Tononi G, Massimini M. Human Cortical Excitability Increases with Time Awake. Cereb Cortex. 2012 doi: 10.1093/cercor/bhs014. Long-term analysis of changes in cortical responsiveness due to sleep deprivation in humans using transcranial magnetic stimulation (TMS) combined with EEG, to measure directly how sleep loss affects the local and early electrical response of cortical neurons to TMS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chauvette S, Seigneur J, Timofeev I. Sleep oscillations in the thalamocortical system induce long-term neuronal plasticity. Neuron. 2012;75:1105–1113. doi: 10.1016/j.neuron.2012.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leonard BJ, McNaughton BL, Barnes CA. Suppression of hippocampal synaptic plasticity during slow-wave sleep. Brain Res. 1987;425:174–177. doi: 10.1016/0006-8993(87)90496-3. [DOI] [PubMed] [Google Scholar]
- 31.Bramham CR, Srebro B. Synaptic plasticity in the hippocampus is modulated by behavioral state. brain research. 1989;493:74–86. doi: 10.1016/0006-8993(89)91001-9. [DOI] [PubMed] [Google Scholar]
- 32.Bramham CR, Maho C, Laroche S. Suppression of long-term potentiation induction during alert wakefulness but not during ‘enhanced’ REM sleep after avoidance learning. Neuroscience. 1994;59:501–509. doi: 10.1016/0306-4522(94)90172-4. [DOI] [PubMed] [Google Scholar]
- 33.McDermott CM, LaHoste GJ, Chen C, Musto A, Bazan NG, Magee JC. Sleep deprivation causes behavioral, synaptic, and membrane excitability alterations in hippocampal neurons. J Neurosci. 2003;23:9687–9695. doi: 10.1523/JNEUROSCI.23-29-09687.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tartar JL, Ward CP, McKenna JT, Thakkar M, Arrigoni E, McCarley RW, Brown RE, Strecker RE. Hippocampal synaptic plasticity and spatial learning are impaired in a rat model of sleep fragmentation. Eur J Neurosci. 2006;23:2739–2748. doi: 10.1111/j.1460-9568.2006.04808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ravassard P, Pachoud B, Comte JC, Mejia-Perez C, Scote-Blachon C, Gay N, Claustrat B, Touret M, Luppi PH, Salin PA. Paradoxical (REM) sleep deprivation causes a large and rapidly reversible decrease in long-term potentiation, synaptic transmission, glutamate receptor protein levels, and ERK/MAPK activation in the dorsal hippocampus. Sleep. 2009;32:227–240. doi: 10.1093/sleep/32.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McDermott CM, Hardy MN, Bazan NG, Magee JC. Sleep deprivation-induced alterations in excitatory synaptic transmission in the CA1 region of the rat hippocampus. J Physiol. 2006;570:553–565. doi: 10.1113/jphysiol.2005.093781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kopp C, Longordo F, Nicholson JR, Luthi A. Insufficient sleep reversibly alters bidirectional synaptic plasticity and NMDA receptor function. J Neurosci. 2006;26:12456–12465. doi: 10.1523/JNEUROSCI.2702-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vecsey CG, Baillie GS, Jaganath D, Havekes R, Daniels A, Wimmer M, Huang T, Brown KM, Li XY, Descalzi G, et al. Sleep deprivation impairs cAMP signalling in the hippocampus. Nature. 2009;461:1122–1125. doi: 10.1038/nature08488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mayford M, Wang J, Kandel ER, O’Dell TJ. CaMKII regulates the frequency-response function of hippocampal synapses for the production of both LTD and LTP. Cell. 1995;81:891–904. doi: 10.1016/0092-8674(95)90009-8. [DOI] [PubMed] [Google Scholar]
- 40.Smith GB, Heynen AJ, Bear MF. Bidirectional synaptic mechanisms of ocular dominance plasticity in visual cortex. Philos Trans R Soc Lond B Biol Sci. 2009;364:357–367. doi: 10.1098/rstb.2008.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu ZW, Faraguna U, Cirelli C, Tononi G, Gao XB. Direct evidence for wake-related increases and sleep-related decreases in synaptic strength in rodent cortex. J Neurosci. 2010;30:8671–8675. doi: 10.1523/JNEUROSCI.1409-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Winters BD, Huang YH, Dong Y, Krueger JM. Sleep loss alters synaptic and intrinsic neuronal properties in mouse prefrontal cortex. Brain Res. 2011;1420:1–7. doi: 10.1016/j.brainres.2011.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caroni P, Donato F, Muller D. Structural plasticity upon learning: regulation and functions. Nat Rev Neurosci. 2012;13:478–490. doi: 10.1038/nrn3258. [DOI] [PubMed] [Google Scholar]
- 44.Zatorre RJ, Fields RD, Johansen-Berg H. Plasticity in gray and white: neuroimaging changes in brain structure during learning. Nat Neurosci. 2012;15:528–536. doi: 10.1038/nn.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **45.Bushey D, Tononi G, Cirelli C. Sleep and synaptic homeostasis: structural evidence in Drosophila. Science. 2011;332:1576–1581. doi: 10.1126/science.1202839. Demonstration of the direct effects of sleep and wake on the morphology of Drosophila central synapses, independent of time of day effects. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *46.Maret S, Faraguna U, Nelson A, Cirelli C, Tononi G. Sleep and wake modulate spine turnover in the adolescent mouse cortex. Nature Neuroscience. 2011;14:1418–1420. doi: 10.1038/nn.2934. Demonstration that sleep and wake modulate the turnover of dendritic spines in the adolescent mouse cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *47.Yang G, Gan WB. Sleep contributes to dendritic spine formation and elimination in the developing mouse somatosensory cortex. Dev Neurobiol. 2011 doi: 10.1002/dneu.20996. Demonstration that sleep and wake modulate the turnover of dendritic spines and filopodia in the adolescent mouse cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rao Y, Liu ZW, Borok E, Rabenstein RL, Shanabrough M, Lu M, Picciotto MR, Horvath TL, Gao XB. Prolonged wakefulness induces experience-dependent synaptic plasticity in mouse hypocretin/orexin neurons. J Clin Invest. 2007;117:4022–4033. doi: 10.1172/JCI32829. [DOI] [PMC free article] [PubMed] [Google Scholar]