Abstract
Better understanding how glucagon-like peptide 1 (GLP-1) promotes pancreatic β-cell function and/or mass may uncover new treatment for type 2 diabetes. In this study, we investigated the potential involvement of microRNAs (miRNAs) in the effect of GLP-1 on glucose-stimulated insulin secretion. miRNA levels in INS-1 cells and isolated rodent and human islets treated with GLP-1 in vitro and in vivo (with osmotic pumps) were measured by real-time quantitative PCR. The role of miRNAs on insulin secretion was studied by transfecting INS-1 cells with either precursors or antisense inhibitors of miRNAs. Among the 250 miRNAs surveyed, miR-132 and miR-212 were significantly up-regulated by GLP-1 by greater than 2-fold in INS-1 832/3 cells, which were subsequently reproduced in freshly isolated rat, mouse, and human islets, as well as the islets from GLP-1 infusion in vivo in mice. The inductions of miR-132 and miR-212 by GLP-1 were correlated with cAMP production and were blocked by the protein kinase A inhibitor H-89 but not affected by the exchange protein activated by cAMP activator 8-pCPT-2′-O-Me-cAMP-AM. GLP-1 failed to increase miR-132 or miR-212 expression levels in the 832/13 line of INS-1 cells, which lacks robust cAMP and insulin responses to GLP-1 treatment. Overexpression of miR-132 or miR-212 significantly enhanced glucose-stimulated insulin secretion in both 832/3 and 832/13 cells, and restored insulin responses to GLP-1 in INS-1 832/13 cells. GLP-1 increases the expression of miRNAs 132 and 212 via a cAMP/protein kinase A-dependent pathway in pancreatic β-cells. Overexpression of miR-132 or miR-212 enhances glucose and GLP-1-stimulated insulin secretion.
Glucagon-like peptide 1 (GLP-1), the incretin hormone secreted by intestinal L-cells after food intake, potentiates glucose-stimulated insulin secretion (GSIS) from pancreatic β-cells and inhibits glucagon secretion from α-cells. Chronic administration of GLP-1 also promotes insulin synthesis as well as β-cell proliferation and neogenesis in animal models of diabetes (1, 2). GLP-1 analogues and small molecule compounds that inhibit the GLP-1 degrading enzyme DPP-IV have become mainstream therapeutic agents for type 2 diabetes.
GLP-1 exerts its tropic effects on β-cell function and β-cell mass through the GLP-1 receptor (GLP-1R), which is mainly expressed in pancreatic β-cells. Upon binding to its ligands, GLP-1R, coupling through the G-protein Gαs, activates adenylyl cyclase, leading to cAMP production. The elevation of cAMP in turn leads to the activation of protein kinase A (PKA) and exchange protein activated by cAMP (Epac), also known as cAMP-regulated guanine nucleotide exchange factor II, which potentiates insulin secretion (3–5). GLP-1R activation also induces IRS-2 and other gene expression pathways via ERK1/2, protein kinase C (PKC), and phosphatidylinositol 3-kinase, and promotes cell growth, differentiation, and maintenance (6). Moreover, β-arrestin-1 was shown to play a role in GLP-1 signaling, leading to enhanced insulin secretion and β-cell survival (7, 8). The downstream molecular mechanisms of these signaling pathways in β-cells, however, remain to be fully understood.
microRNAs (miRNAs) are short, noncoding RNAs that regulate gene expression by pairing to 3′ untranslated region sequences of target mRNAs and directing their posttranscriptional repression (9, 10). Previous studies have demonstrated that miRNAs, such as miR-375, may directly regulate both embryonic islet development and islet function in adult animals (11–13). In this study, we investigated the involvement of miRNAs in the regulation of insulin secretion stimulated by glucose and GLP-1 in pancreatic β-cells. Our study indicated that GLP-1 selectively induces the expression levels of 2 miRNAs, miR-132 and miR-212, and increased expression of these miRNAs significantly augment glucose and GLP-1 induced insulin secretion in pancreatic β-cells.
Materials and Methods
Cell lines and treatment
Two INS-1-derived rat insulinoma cell sublines, 832/3 and 832/13, were used in this study (14, 15). Both lines exhibit robust GSIS, but only 832/3 cells exhibit significant enhancement of insulin secretion in response to GLP-1 (15). Cells were cultured in RPMI 1640 with 10% fetal bovine serum and 11mM glucose, as described (14). For GLP-1 treatment, GLP-1 (7–36) amide (BACHEM Biosciences) was added directly to culture medium for up to 48 hours without replenishment. In some cases, INS-1 832/3 cells were treated with the PKA inhibitor H-89 (EMD Chemicals) or the Epac activator Epac-selective cAMP analogue, 8-pCPT-2′-O-Me-cAMP-AM (ESCA) (Axxora, LLC), alone or in combination with GLP-1 (50nM), for 24 hours before being harvested for miRNA extraction and quantification.
Quantitative PCR based miRNA profiling
Total RNA was extracted from INS-1 832/3 cells with TRIzol reagent (Invitrogen). A total of 250 mature miRNA species were determined by the locked nucleic acid-based SYBR Green quantitative PCR (qPCR) methodology as previously described (16, 17). The threshold cycle values were converted into copy number per 10-pg total RNA (the approximate amount of RNA per cell) using standard curves established for each miRNA. Data of 3 replicates using cells at passages between 7 and 15 were analyzed using the Rosetta Resolver system, version 7.1 (Rosetta Biosoftware). There was no measurable difference in the responsiveness to GLP-1 for cells between passages 7 and 15.
TaqMan qPCR analysis of selective miRNAs
Fluorogenic TaqMan probes for miR-132, miR-212, and miR-375 were purchased from Applied Biosystems. Relative expression levels for miRNAs of interest were determined by real-time qPCR using the ABI PRISM 7900 Sequence Detection System from Applied Biosystems. 4.5S RNA or U6 snRNA (Applied Biosystems) was used as normalization control to determine the relative abundance of each miRNA in different samples.
Rat and mouse islet isolation and treatment
Pancreatic islets of Langerhans were isolated from normal Sprague-Dawley rats and C57Bl/6N mice (Taconic, Inc) by collagenase digestion and discontinuous Ficoll gradient separation (18). Islets were cultured overnight in RPMI 1640 medium with 11mM glucose. The next day, 100–200 islets were treated with 50nM GLP-1 (7–36) amide in the presence of 16mM glucose in the culture media for 24 (for rat) or 48 hours (for mouse). RNA was extracted with TRIzol reagent for quantification of miRNA species with TaqMan analysis.
Human pancreas procurement and islet isolation and treatment
The human islets from 2 nondiabetic cadaver organ donors were isolated at the University of Illinois in Chicago. Human pancreata were provided by organ procurement organizations, with formal research consent. The islet isolation procedures, including digestion, purification, and culture, were preformed according to the previously described protocol (19, 20). In brief, the pancreata were perfused, via the pancreatic duct, with Roche Liberase MTF C/T GMP Grade-Collagenase. Tissue digestion and islet dissociation were achieved using a modified Ricordi semiautomated method. The refined University of Illinois in Chicago-University of Wisconsin solution/Biocoll continuous density gradient (21) was used for islet purification using COBE 2991 Cell Separator (Terumo BCT). Isolated human islets were cultured in complete CMRL-1066 medium containing 5.5mM glucose (Invitrogen) supplemented with 10mM HEPES, 1% penicillin-streptomycin, and 5% human albumin. After 24 hours of recovery, human islets were tested in the [Ca2+]influx and insulin secretion assays to confirm their glucose responsiveness. For GLP-1 treatment, human islets were treated with 50–100nM GLP-1 (7–36) amid for 24 hours before being harvested for TaqMan analysis of miRNA expression.
Human islet calcium influx and insulin secretion measurements
Intracellular calcium influxes and insulin secretion of human islets were determined using microfluidic-based perifusion and imaging system (22).
In vivo treatment with Exendin-4 in C57Bl/6N mice
Alzet osmotic minipumps (D-1007; DURECT Corp) were implanted sc in 13-week-old lean C57Bl/6N mice (Taconic, Inc). Exendin-4 (Ex-4) (Sigma) was delivered at 10 or 30 μg/kg·d, and saline (0.9%) was used as vehicle. After 48-hour treatment, pancreatic islets, liver and lung tissues were isolated for quantification of miRNA species. In separate cohorts of mice, oral glucose tolerance tests (OGTTs) were performed to verify the effectiveness of GLP-1 infusion. Mice were fasted for 4 hours before glucose challenge (oral gavage, 5 g/kg). Blood glucose levels were measured by hand-held glucometer (Onetouch Ultra, LifeSpan) at 0, 15, and 30 minutes after glucose challenge. Blood samples were also collected at 0, 15, and 30 minutes during OGTT for the measurement of plasma insulin levels by ELISA kit (ALPCO).
Transfection of INS-1 cells with pre-miRNA and anti-miRNA oligos
Chemically modified pre-miR miRNA precursors and anti-miR miRNA antisense inhibitors were purchased from Ambion. The oligonucleotide molecules were delivered to the cells by the Nucleofector System (Amaxa). In brief, INS-1 cells were trypsinized, centrifuged, and resuspended in 100-μL Nucleofector solution V. RNA oligonucleotides (100–500 pmol) were transfected into 3 million cells with the Amaxa Nucleofector Device. Pre-miR and Anti-miR negative control 1 (Ambion) were used with the same transfection method. After electroporation, cells were transferred to regular culture medium and seeded into 96-well microtiter plates. Insulin secretion, miRNA quantification, and cAMP assays were performed 48 hours after electroporation.
GSIS assay in INS-1 cells
The GSIS assay was performed with cells grown to near confluency in 96-well plates as previously described (23). Briefly, cells were preincubated at 37°C in freshly prepared Krebs-Ringer buffer without glucose for 2 hours, followed by another 2 hours of final incubation in Krebs-Ringer buffer medium with 2mM or 16mM glucose, or 16mM glucose with 10nM GLP-1 (7–36) amide. Insulin concentration in the media was assayed by Ultrasensitive Rat Insulin ELISA kit (ALPCO) or insulin immunoassay by Gyrolab workstation (GYROS AB). Insulin was extracted into acid ethanol for measurement of total insulin content. Total protein was measured by Pierce 660nm Protein Assay from Pierce Biotechnology.
Measurement of intracellular cAMP levels in INS-1 cells
INS-1 832/3 and 832/13 cells were grown to near confluency in 384-well plates. Cells (∼9000 cells/well) were incubated with increasing concentrations of GLP-1 or Ex-4 in Hank's Balanced Salt Solution buffer containing 5mM IBMX (3-isobutly-1-methylxanthine stimulation buffer in the user's manual of the Lance cAMP kit) for 1 hour at 37°C. cAMP levels were measured using LANCE cAMP assay kit according to manufacturer's protocol (PerkinElmer). The ratio of 665-nm to 615-nm photon counts was detected by the EnVison Plate-reader (PerkinElmer) and was converted to cAMP concentrations using a cAMP standard curve. Each condition was measured in quadruplicates.
Statistics
Data are expressed as mean ± SE. For miRNA profiling data, the expression data were processed with Rosetta Resolver (Rosetta Biosoftware). Data from replicates of the same treatment group were combined by calculating geometric mean (24). The “replicates combined mean” values were computed as the arithmetic mean of the logarithm-transformed values of copy numbers per 10-pg RNA of replicates, followed by using the exponentiation to return the computation to the original scale. Statistical analysis for other data was conducted using either ANOVA followed by Dunnett's post hoc test using Prism when there were 3 or more groups for comparison (version 4.0.3; GraphPad) or Student's t test for 2 group comparison, as described in the figure legend. Statistical significance was defined as 2-tailed P < .05.
Results
GLP-1 induces miR-132 and miR-212 expression in pancreatic β-cells in vitro
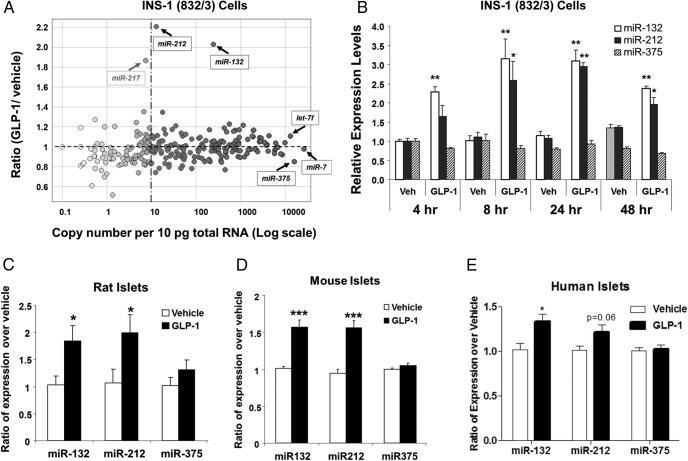
To survey miRNAs expressed in pancreatic β-cells and determine their response to GLP-1, we profiled the expression levels of 250 distinct, well-characterized miRNAs in INS-1 832/3 cells cultured in the presence or absence of 50nM GLP-1 for 24 hours using quantitative RT-PCR. The INS-1 832/3 cell is a rat insulinoma cell line with robust insulin secretion upon stimulation with glucose and GLP-1. Of the 250 miRNAs measured, 162 miRNAs were present with greater than 10 copies per 10-pg total RNA in the INS-1 832/3 cells cultured in the absence of GLP-1 treatment. The most abundantly expressed miRNAs were miR-7, miR-375, and let-7f. Figure 1A depicts the average copy number and the fold induction by GLP-1 of all 250 mRNAs in INS-1 832/3 cells. The actual copy numbers of miRNAs in cells cultured in the absence and presence of GLP-1 are enlisted in Supplemental Table 1.
Figure 1.
GLP-1 induces miR-132 and miR-212 expressions in pancreatic β-cells in vitro. A, miRNA expression profiling of GLP-1-treated INS-1 832/3 cells. miRNA profiling was performed by real-time qRT-PCR. Data represent the replicate-combined average of 3 independent measurements with cells at different passages. The x-axis of the scatter plot represents the copy number of miRNAs per 10-pg total RNA, and the y-axis is the ratio of miRNA expression changes in response to 50nM GLP-1 for 24 hours. Data from replicates of the same treatment group were combined by using the Rosetta Resolver. B, Time course of miRNA induction by GLP-1 in INS-1 832/3 cells. The 832/3 cells were treated with GLP-1 (50nM) for the time periods as labeled. Levels of miRNAs were measured by TaqMan with specific primer/probes (ABI system) and normalized to 4.5S RNA. The relative expression levels in GLP-1-treated cells were expressed relative to that observed for vehicle alone. Data represent mean ± SE of 3 independent treatment groups. C, miRNAs 132, 212, and 375 levels in GLP-1-treated rat islets. Pancreatic islets were isolated from male Sprague-Dawley rats and cultured in RPMI 1640 medium with or without 50nM GLP-1 for 24 hours. Levels of the miRNAs were determined by TaqMan PCR and normalized to 4.5S RNA. Data are the mean ± SE of 3 independent islet preparations. D, miRNAs 132, 212, and 375 levels in GLP-1-treated mouse islets. Islets were isolated from male C57Bl/6N mice and cultured in RPMI 1640 medium with or without 50nM GLP-1 for 48 hours. Data are the mean ± SE of 6 independent islet preparations. E, miRNAs 132, 212, and 375 levels in GLP-1-treated human islets. Islets were isolated from human donors and cultured in complete CMRL-1066 medium in the present or absence of GLP-1 for 24 hours. Data are the mean ± SE of 3–6 replicates of each islet preparation. For B–E, 2-tailed Student's t test was performed for the GLP-1-treated group vs the vehicle group comparison; *, P < .05; **, P < .01; ***, P < .001.
miR-132 and miR-212 were 2 top-ranked miRNA species by fold of change regulated by GLP-1 in INS-1 832/3 cells (Figure 1A). In contrast, GLP-1 did not alter the expression of most miRNAs by greater than 2-fold, including those previously known to be involved in the regulation of β-cell function, such as miR-9 and miR-124a (11, 25, 26). miR-375, whose expression was previously reported to be repressed by Ex-4 (27), had a nonsignificant, very modest down-regulation (∼20%) with GLP-1 treatment (Figure 1A). The inductions of miR-132 and miR-212 expression by GLP-1 were subsequently confirmed by RT-qPCR analysis in INS-1 832/3 cell samples prepared independently of those used for the profiling study. As shown in Figure 1B, the inductions of miR-132 and miR-212 in INS-1 832/3 cells were observed as early as 4 hours after GLP-1 treatment, peaked between 8 and 24 hours and sustained up to 48 hours. miR-217 was found to have an approximately 1.8-fold increase by GLP-1 in the initial miRNA profiling study (Figure 1A). However its abundance was less than 10 copies per cell and its up-regulation was not confirmed by subsequent RT-qPCR confirmation.
To determine whether GLP-1 regulates miR-212 and miR-132 expressions in primary β-cells, we analyzed their expression levels in freshly isolated rat, mouse, and human islets treated with GLP-1 in tissue culture. After a 24- to 48-hour treatment, the expression levels of both miR-132 and miR-212 were significantly increased in rat (Figure 1C) and mouse (Figure 1D) islets by approximately 2- and 1.5-fold, respectively. The expression of miR-375 was not significantly affected by GLP-1 treatment in either rat or mouse islets (Figure 1, C and D). A 24-hour treatment of human islets with GLP-1 resulted in a similar increase in the expression levels of miR-132 and miR-212, and there was no change in the expression of miR-375 (Figure 1E). The quality of human islets used in the GLP-1 treatment experiment was evaluated by intracellular calcium influx and insulin secretion in response to 25mM glucose and 30mM KCl (Supplemental Figure 1).
Ex-4 increases miR-132 and miR-212 expression in pancreatic islets in vivo
To determine whether in vivo treatment with GLP-1 induces miR-212 and miR-132 expression in islets similarly to what we observed in vitro, Ex-4, a GLP-1 analog, was infused in C57BL/6N mice at 10 and 30 μg/kg·d with sc implanted osmotic minipumps. The administration of Ex-4 dose dependently reduced blood glucose (Figure 2A) and increased plasma insulin (Figure 2B) during an OGTT performed after Ex-4 infusion, confirming that the selected doses of Ex-4 engaged GLP-1R activation in vivo. The expression levels of miR-132 and miR-212 in islets were dose dependently increased at 48 hours after Ex-4 infusion, whereas the expression of miR-375 was not significantly affected by the treatment (Figure 2C). Dose-dependent up-regulation of miR-132 and miR-212 expression was also observed in the lung from Ex-4-treated mice, consistent with the presence of GLP-1R in that tissue (Supplemental Figure 2A). Conversely, we did not see significant change in miR-132 or miR-212 levels in liver where there is no detectable GLP-1R expression (Supplemental Figure 2B).
Figure 2.
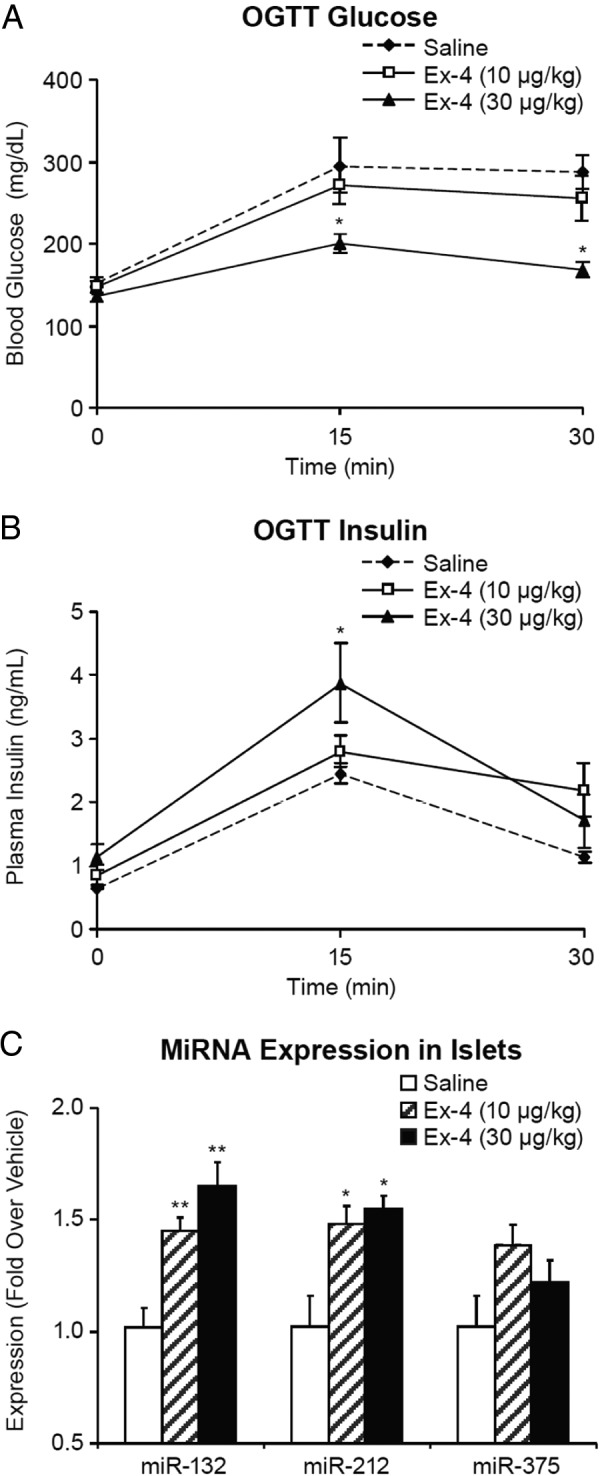
Infusion of Ex-4 in mice improves glucose homeostasis and increases the expression levels of miRNAs 132 and 212 in pancreatic islets. C57Bl/6N lean mice were infused with Ex-4 (10 or 30 μg/kg·d) or saline through sc implanted osmotic minipumps. Glucose (A) and insulin (B) levels were measured during an OGTT after Ex-4 infusion. C, Pancreatic islets were isolated after Ex-4 infusion for RNA extraction. miRNAs 132, 212, and 375 were determined by TaqMan PCR. Data are the mean ± SE of 5 mice per group. One-way ANOVA followed by Dunnett's post hoc test was performed for the 2 Ex-4-treated and the saline groups; *, P < .05; **, P < .01 as compared with the saline group.
Effects of cAMP, PKA and Epac-modulating agents on miR-132 and miR-212 expression in β-cells
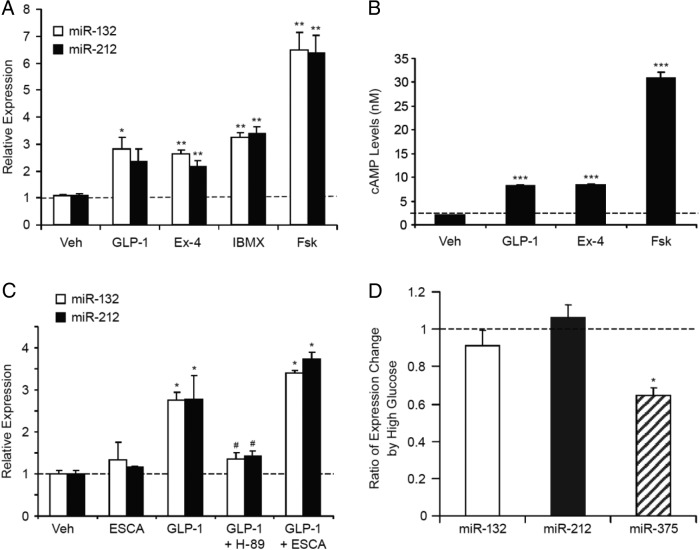
miRNAs 132 and 212 are mapped to the first intron of a noncoding mRNA gene on chromosome 11 in the mouse genome. A 500-bp region upstream of the exon 1, which is conserved in mouse and human, and the intron 1 of this mRNA contain multiple cAMP-responsive element consensus sites (28). cAMP response element-binding protein has been previously suggested to regulate miR-132 transcription (29, 30). Forskolin, a nonselective activator of adenylyl cyclases, yields a greater increase in intracellular cAMP than GLP-1 or Ex-4. We assessed whether forskolin and IBMX (a pan phosphodiesterase inhibitor) were effective in inducing miR-132 and miR-212 expression in INS-1 832/3 cells. The expression levels of both miR-132 and miR-212 were more robustly increased by forskolin than by GLP-1 or Ex-4 (Figure 3A). Furthermore, the fold inductions of miRNAs 132 and 212 were proportional to the degree of cAMP elevation by these cAMP raising agents (Figure 3B). IBMX treatment also resulted in a similar level of induction of miR-132 and miR-212 expression as observed by GLP-1 or Ex-4 treatment. However, we could not correlate its effect with cAMP elevation because 5mM IBMX is present in the cAMP assay buffer. miR-375 expression was not changed by any of these cAMP raising agents (data not shown). These results suggest that cAMP mediates the effect of GLP-1 on miR-132 and miR-212 expression in pancreatic β-cells.
Figure 3.
GLP-1 induces miRNAs 132 and 212 in a PKA-dependent manner. A, Effects of cAMP enhancing agents on miR-212 and miR-132 expression in INS-1 832/3 cells. INS-1 832/3 cells were treated in RPMI medium (containing 16mM glucose) with 50nM GLP-1, 50nM Ex-4, 5μM IBMX, or 1μM forskolin (Fsk) for 24 hours before being harvested for RNA extraction and TaqMan analysis of miR-132 and miR-212 expression. B, Effects of cAMP-enhancing agents on intracellular cAMP levels. The INS-1 832/3 cells, grown to near confluence in 96-well plates, were treated with 50nM GLP-1, 50nM Ex-4, or 1μM Fsk for 1 hour in Hank's Balanced Salt Solution buffer containing 0.5mM IBMX. Intracellular cAMP accumulation during the 1-hour stimulation was measured with immunoassay using the Lance cAMP detection kit from PerkinElmer. C, Effects of PKA/Epac modulators on miRNAs 132 and 212 expression. INS-1 832/3 cells were treated during tissue culture in RPMI 1640 medium (with 11mM glucose and 10% fetal bovine serum) with GLP-1 (50nM) with or without H-89 (PKA inhibitor, 10μM), ESCA (Epac-specific activator, 10μM) for 24 hours before being harvested for RNA extraction and miRNA measurement. Data represent the mean ± SE of 3 independent experiments. D, Effects of 16mM glucose on miR132 and miR-212 expression in INS-1 832/3 cells. The 832/3 cells were cultured in RPMI 1640 medium with either 2mM or 16mM glucose for 24 hours before RNA extraction and real-time qRT-PCR analysis. Data represent the mean ± SE of 3 independent experiments. For A–C, one-way ANOVA followed by Dunnett's post hoc test compared with the vehicle group was performed; *, P < .05; **, P < .01; ***, P < .001 as compared with the vehicle control; #, P < .05, as compared with the GLP-1-treated group. For D, 2-tailed Student's t test was performed for the comparison of the high-glucose group vs the low-glucose group; *, P < .05.
The biological effects of cAMP in β-cells are mediated by 2 parallel signaling pathways, PKA and Epac2 (4–6). To determine which pathway mediates the effect of GLP-1 on the regulation of miRNAs 132 and 212, we tested the effects of a PKA inhibitor (H-89) and an Epac activator (ESCA) (31, 32) on GLP-1-induced miR-132 and miR-212 expression in INS-1 832/3 cells. As depicted in Figure 3C, the ability of GLP-1 to induce miRNAs 132 and 212 expression was largely blocked by H-89. In contrast, ESCA did not alter miRNAs 132 and 212 expression significantly either in the presence or absence of GLP-1. These results demonstrate that PKA activity is required for GLP-1 to induce the expression of miR-132 and miR-212.
We asked whether glucose regulates the expression of miR-132 and miR-212. Comparing INS-1 832/3 cells cultured in 16mM glucose vs in 2mM glucose, the expression levels of miR-132 and miR-212 were unchanged, whereas miR-375 expression was significantly down-regulated (Figure 3D), similar to the findings reported by others (33).
Overexpression of miR-132 augmented GSIS and its response to GLP-1 in INS-1 832/3 cells
We recently reported that miRNAs 132 and 212 enhance insulin secretion in pancreatic β-cells via targeting the carnitine acyl-carnitine translocase (CACT) gene (34). Having confirmed the specific induction of miR-132 and miR-212 by GLP-1 in β-cells, we explored the significance of the 2 miRNAs on the ability of GLP-1 to enhance GSIS in INS-1 832/3 cells. Overexpression of miR-132 by transfecting the 832/3 cells with oligonucleotide precursors of miR-132 significantly augmented GSIS with or without the presence of GLP-1 (Figure 4A). The fold increase of GSIS afforded by GLP-1 was nevertheless not affected by miR-132 overexpression, potentially reaching the limit by which insulin secretion could be enhanced in this model. Similar results were obtained in the experiments with miR-212 overexpression (data not shown). Overexpression of miR-375 had no significant effect on GLP-1-stimulated insulin secretion (Figure 4A).
Figure 4.
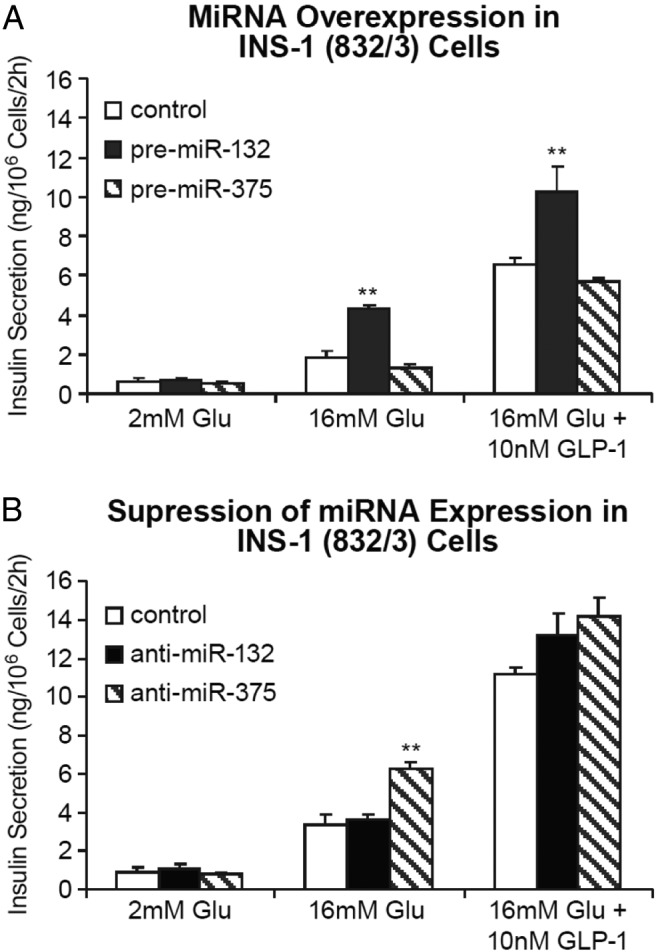
miRNA-132 augments GLP-1-stimulated insulin secretion in INS-1 832/3 cells. A, Effects of miRNA overexpression on GLP-1-stimulated insulin secretion. INS-1 832/3 cells were transfected by electroporation with precursors for miR-132, miR-375, or a Pre-miR negative control, and insulin secretion was determined 48 hours after transfection in response to 16mM glucose in the absence or presence of GLP-1 (10nM). Data are the mean ± SE of 3 independent experiments. B, Effects of miRNA antagonist on glucose and GLP-1-stimulated insulin secretion. The INS-1 832/3 cells were transfected with anti-miRNA oligonucleotides or negative control oligonucleotides by electroporation. Forty-eight hours after transfection, insulin secretion was measured in response to 2mM or 16mM glucose, or 16mM glucose plus 10nM GLP-1. Data are the mean ± SE of 3 replicate. One-way ANOVA followed by Dunnett's post hoc test compared with the control group was performed for both A and B; **, P < .01 as compared with the negative control.
Antisense oligonucleotides (ASOs) specific for miR-132 and miR-375 were used to determine whether suppression of endogenous miRNAs alters insulin secretion. Transfection of miR-132-ASO in INS-1 832/3 cells resulted in an approximately 42% decrease in the expression of endogenous miR-132 but was ineffective in altering GSIS or its potentiation by GLP-1 (Figure 4B). In contrast, miR-375-ASO significantly increased GSIS in response to an approximately 45% decrease in endogenous miR-375 expression (Figure 4B). Because the endogenous expression level of miR-212 is very low in INS-1 832/3 cells, knockdown experiment was not pursued with miR-212-ASO.
Overexpression of miR-132 and miR-212 restored GLP-1 potentiation in INS-1 832/13 cells
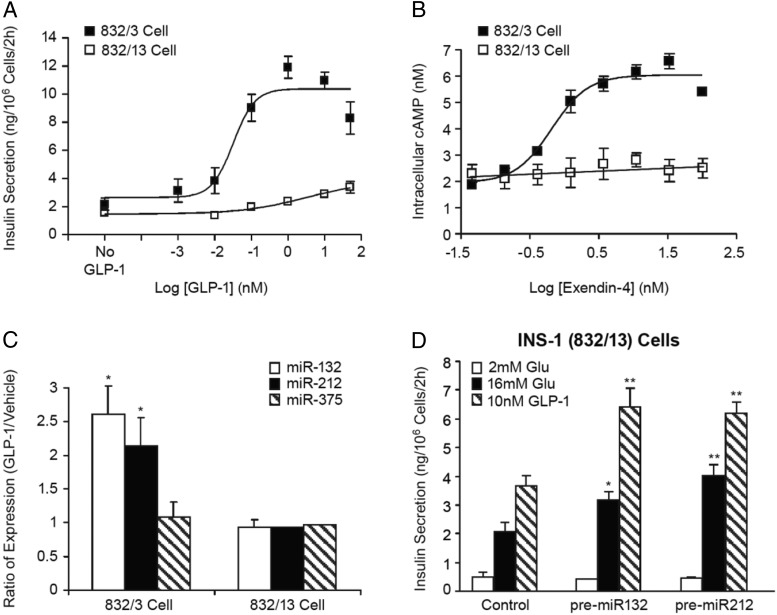
The INS-1 832/13 cell is a sister subclone of the 832/3 line that has similar GSIS function but much less responsiveness to GLP-1 than the 832/3 subclone of cells in terms of insulin secretion (15). In the presence of 16mM glucose, GLP-1 yields more than 300% enhancement of insulin secretion from the 832/3 cells but only approximately 50% enhancement from the 832/13 cells (Figure 5A). Ex-4 resulted in a dose-dependent increase in cellular cAMP (∼3-fold) in the 832/3 cells, whereas it was ineffective to elevate cAMP in the 832/13 cells (Figure 5B). Consistent with what we observed in the previous series of experiments, the lack of cAMP increase after GLP-1 treatment in the 832/13 cells correlated with an absence of miR-132 or miR-212 induction in the 832/13 cells (Figure 5C).
Figure 5.
GLP-1 induces the expression of miRNAs 132 and 212 and enhances insulin secretion in parallel with an increase in cellular cAMP. A, Potentiation of GSIS by GLP-1 in the INS-1 832/3 and 832/13 cells. Insulin secretion was measured from both INS-1 clonal cell lines in response to 16mM glucose in the absence and presence of varying concentrations of GLP-1. Data are the mean ± SE of 3 replicates. B, cAMP responses to Ex-4 in the 832/3 and 832/13 cells. The INS-1 clonal cells were incubated with varying concentrations of Ex-4 for 1 hour at 37°C. Cells were then lysed and cAMP concentration measured as described in Materials and Methods. Data are the mean ± SE of 4 independent experiments. C, miR-132 and miR-212 expression changes in response to GLP-1 in the 832/3 and 832/13 cells. The INS-1 clonal cells were treated with GLP-1 (50nM) for 24 hours, followed by RNA extraction and miRNA expression measurements by TaqMan PCR. Data are the mean ± SE of 3 independent experiments. Student's t test was performed for the GLP-1-treated group vs the vehicle group comparison in each cell line; *, P < .05. D, Effects of miR-132 and miR-212 overexpression on glucose and GLP-1-stimulated insulin secretion in the INS-1 832/13 cells. Cells were transfected with precursors of miR-132, miR-212, or Pre-miR control oligonucleotides. Insulin secretion was measured as described in Figure 4. Data are mean ± SE of 5 replicates. One-way ANOVA followed by Dunnett's post hoc test was performed for the comparisons of the miRNA precursor-transfected groups vs the control oligonucleotides-transfected group under each treatment condition; *, P < .05; **, P < .01 as compared with the negative control under the same treatment condition.
The lack of robust cAMP production and insulin secretory responses to GLP-1 in the 832/13 line of INS-1 cell is not due to a reduction in the GLP-1R expression at the mRNA level (J.S., Y.F., N.A.T., A.D.H., Y.-P.Z., unpublished data), pointing to a possible defect in GLP-1R signaling or cAMP metabolism in the 832/13 cells. Hence we assessed whether overexpression of miRNA-132 and miRNA-212 in the 832/13 line via in vitro transfection would bypass cAMP production and augment GSIS response to GLP-1. miRNAs 132 or 212 overexpression restored the responsiveness of the 832/13 cells to GLP-1, as the magnitude of insulin release in the presence of high glucose and GLP-1 became similar to that observed in the 832/3 cells when measured during the same period of time (Figure 5D). The overexpression of the 2 miRNAs also promoted GSIS per se in the 832/13 cells (Figure 5D), as was observed in the 832/3 cells (Figure 4B).
Discussion
In this study, we set out to identify specific miRNAs that may mediate the action of GLP-1 on β-cell function. Of the 250 well-validated miRNAs at the time of this survey, miR-132 and miR-212 were regulated by GLP-1 by greater than 2-fold in the GLP-1 responsive INS-1 832/3 β-cell line (Figure 1A). The inductions of miR-132 and miR-212 were also observed in rodent and human islets treated with GLP-1 or Ex-4 in vivo or ex vivo (Figures 1, C–E, and 2C). Mechanistically, our data demonstrates that the induction of miR-132 and miR-212 by GLP-1 is PKA dependent. A potent PKA inhibitor (H-89), which has been shown to suppress insulin secretion stimulated by GLP-1 in β-cells (4, 32), can largely block GLP-1 dependent induction of miR-132 and miR-212 expression (Figure 3C). Our findings suggest a new model linking GLP-1 to improved β-cell function by inducing the expressions of miRNAs 132 and 212 via the cAMP-PKA pathway (Supplemental Figure 3).
GLP-1 appears to increase miR-132 and miR-212 expression levels via cAMP production and subsequent activation of PKA in β-cells. We found that not only was the expression of miR-132 and miR-212 up-regulated by multiple cAMP-raising agents in addition to GLP-1, such as Ex-4 and forskolin (Figure 3A), but also the magnitude of up-regulation correlated with their effects on increasing intracellular cAMP levels (Figure 3B). Moreover, using the PKA inhibitor H-89, we were able to show that up-regulation of miRNAs was abolished when PKA activity was inhibited (Figure 3C). Finally, in INS-1 832/13 cells in which there was no detectable increase of intracellular cAMP in response to GLP-1 analog Ex-4 (Figure 5B), miRNAs 132 and 212 were not up-regulated by GLP-1 treatment (Figure 5C), further supporting that the regulation of these miRNAs by activation of GLP-1R is mediated by cAMP elevation. Taken together, our data suggest that GLP-1 regulates miR-132 and miR-212 expression in β-cells via activation of the Gαs-cAMP-PKA pathway. Further work in model systems like islets from the GLP-1R knockout mice would provide additional evidence for a direct induction of these 2 miRNAs by GLP-1. It has been well recognized that the Epac2 pathway is also important downstream of the GLP1R activation in β-cells (3–5). Additionally, GLP-1 can activate PKCα and PKCϵ, and these GLP-1-activated PKCs may contribute considerably to insulin secretion (35). Our results do not exclude the importance of the EPAC or PKCs-mediated pathways for GLP-1. In fact, the observation that GLP-1 can still exert a significant effect on potentiating insulin secretion in INS-1 cells with miR-132 overexpression (Figures 4A and 5D) supported the notion that an miR-132-independent effect for GLP-1 activation exists in β-cells.
The up-regulation of miR-132 and miR-212 by GLP-1 initially observed in the insulinoma INS-1 832/3 cells (Figures 1, A and B, and 3A) was consistently reproduced in ex vivo isolated mouse and rat islets (Figure 1, C and D) and in vivo in mice (Figure 2C). It is important to point out that miRNAs may have species-specific targets, which may result in species-specific phenotypes. From translational perspective, it is important to examine whether the regulation of miR-132 and miR-212 by GLP-1 also occurs in human islets. We hence conducted similar experiments in isolated human islets from 2 healthy donors, and our result suggested that the regulation on miR-132 and miR-212 expression by GLP-1 is conserved in human islets (Figure 1E).
A number of miRNAs have been implicated in the regulation of nutrient-induced insulin secretion and insulin gene expression (36). The overexpression of miR-375 has been shown to suppress insulin secretion, whereas inhibition of endogenous miR-375 enhances insulin secretion (11, 27) by targeting PDK1 and insulin gene expression (33). In our study, we took advantage of these effects of miR-375 to serve as a control for our study on miR-132 and miR-212. miR-9 was reported to play a role in controlling insulin secretion via targeting Onecut-2 (25). miR-124a was found to directly target forkhead box A2, which regulates the expression of several key β-cell genes, including pancreatic and duodenal homeobox 1, inwardly rectifying potassium channel KIR6.2, and sulfonylurea receptor 1 (26). The overexpression of miR-124a was shown to decrease GSIS by directly targeting Ras-related protein Rab-27A and other components of the exocytosis pathway in MIN6B1 cells (37). Furthermore, miR-124a along with miRNAs 29a and 29b has been shown to contribute to the β-cell-specific silencing of the monocarboxylate transporter 1 transporter and may thus affect insulin release (38). miRNAs 21 and 34a have been implicated as novel players in β-cell failure via targeting Vesicle-associated membrane protein 2 and Rab3a (39). Recently, miR-204 was found to block insulin production by directly targeting and down-regulating transcription factor v-maf musculoaponeurotic fibrosarcoma oncogene homolog A (40). The increased expression of miR-187 in human islets from type 2 diabetic patients has been associated with reduced GSIS (41). Although these miRNAs have been reported to involve in regulating insulin secretion, miRNA species that can mediate the potentiation of GSIS by incretin hormone GLP-1 have not been reported. To our knowledge, miR-132 and miR-212 are the first miRNA species that are up-regulated in response to GLP-1 treatment. Overexpression of miR-132 or miR-212 enhances GSIS and its potentiation by GLP-1 in INS-1 832/3 cells (Figure 4A). Delivery of exogenous miR-132 or miR-212 to the INS-1 832/13 cells, a subclone that lacks robust cAMP and insulin secretory responses to GLP-1, restored GLP-1 responsiveness (Figure 5D). It will be important to determine whether these 2 miRNAs exert the same broad range of effects, including regulation of β-cell replication and survival, as reported for GLP-1. It is worth noting that in addition to these 2 miRNAs, there are a number of miRNAs (with >10 copy number per cell) whose expression was modestly up-regulated by GLP-1 (by ∼1.2-fold) (Figure 1A). They may potentially play important roles in mediating cellular processes in β-cells, as the effects of small changes in miRNA expression may be dramatically magnified if the downstream targets of the miRNAs are important transcription factors. Therefore, the potential importance of these miRNA species could be further investigated in future studies.
Our results suggest that the expression levels of miR-132 and miR-212 are increased in response to elevation of cellular cAMP, a signaling molecule that is critical for insulin secretion and β-cell survival. Numerous endocrinal and neural modulators of islet function affect cAMP levels in pancreatic β-cells through their cognate receptors (42). Therefore, it is possible that miR-132 and miR-212 are also involved in the action of various neuroendocrine modulators of islet function. We have previously shown that obesity induces the expressions of miR-132 and miR-212 in the islets of diabetes-resistant (B6) and diabetes-susceptible (Black and Tan, BRachyury) mice (17). Interestingly, the magnitude of induction was greater in B6 (∼13-fold) than Black and Tan, BRachyury (∼3-fold) mice, raising the possibility that these miRNAs may be involved in the pathogenesis of diabetes. It was recently reported that the expressions of miR-132 and miR-212, along with 28 other miRNAs, were elevated approximately 6-fold in the islets of Goto-Kakizaki rats, a nonobese model of type 2 diabetes (43). In this study, the authors proposed that the increased miRNA expressions underlie the reduced insulin secretion observed from Goto-Kakizaki vs wild-type rat islets. Moreover, miR-132 was found to belong to a group of islet miRNAs that display expression changes occurring long before the onset of diabetes and these expression changes have positive effects on promoting islet function (44). We recently reported that overexpression of miRNAs 132 and 212 enhances GSIS via the suppression of CACT (34), suggesting their up-regulation in diabetic animals may be a compensatory signal as opposed to causing dysregulation of β-cell function. In the present study, the overexpression of these 2 miRNAs in the INS-1 cells again augmented GSIS. We examined whether GLP-1 treatment resulted in up-regulation of CACT in INS-1 832/3 cells, but we did not observe this regulation. In our previous work, overexpression of miR-132 and miR-212 by transfection resulted in hundreds of fold increase in these miRNA species, whereas our current study indicated the GLP-1 induced up-regulation of miR-132 and miR-212 was about 2-fold. This difference in the magnitude of miRNA overexpression may explain the different observations in targeting CACT, pointing to the possibility that miR-132 and miR-212 may have other presumably important target genes. The biological significance of miR-132/212 in β-cell function was further indicated by the rescue of GSIS responses to GLP-1 in the 832/13 cell, a subline of the INS-1 cell that lacks robust cAMP and insulin secretary responses to GLP-1 treatment. The insulinotropic effect of GLP-1 is impaired in patients with type 2 diabetes (45), and the ability of miR-132 and miR-212 to restore GSIS responses to GLP-1 observed in this study could potentially be translated into a new therapy for type 2 diabetes.
It was reported by Keller et al that miR-375 expression was repressed by Ex-4 in rat islets, and that repression of pre-miR-375 by cAMP is mediated through PKA in INS-1 832/13 cells (27). In our study, treatment of rat islets with GLP-1 for 24 hours did not result in significant repression of mature miR-375 expression (Figure 1C), and GLP-1 treatment did not lead to suppression of miR-375 expression in INS-1 832/3 or 832/13 cells (Figures 1B and 5C). The apparent difference between these studies could be due to differences in treatment conditions, miRNA measurements (precursor vs mature miRNA), or subclones of cells (INS-1 832/3 cells vs INS-1 832/13 cells). As pointed by the authors in the previous study, the repression of the miR-375 precursor occurs rapidly in INS-1 832/13 cells although the mature miRNA declines more slowly due to the kinetics of RNA processing. It is possible that the difference in measuring the precursor molecules vs mature miRNA molecules may explain the different observations between the studies. Of note, our result of measuring miR-375 expression from in vivo study (Figure 2C) is consistent with the in vitro results in isolated rat and mouse islets (Figure 1, C and D).
In summary, we show that GLP-1 induces the expression levels of miR-132 and miR-212 in pancreatic β-cells, and that these miRNAs can augment glucose and GLP-1-stimulated insulin secretion. Thus, a new mechanism by which GLP-1 exerts its insulinotropic effect could be to induce the expressions of miR-132 and miR-212 via the cAMP-PKA pathway, which in turn modulates the function or biogenesis of the insulin secretory machinery in β-cells. Further exploration of this model may provide new avenues for novel therapies of type 2 diabetes.
Acknowledgments
We thank Weizhen Wu, Chris Thompson, Yakov Varganov, Liyang Wang, Shihua Xu, and Gino Castriota of Merck Research Laboratories for their technical support and colleagues of the former Rosetta Inpharmaceutic for the qPCR miRNA profiling of INS-1 cells. We also thank Dr Shubing Wang for his assistance on statistical analysis.
This work was supported by National Institutes of Health Grants DK56593 (to A.D.A.), DK66369 (to A.D.A.), and DK091526 (to Y.W.).
Disclosure Summary: J.S., J.L., Y.F., X.S., H.H.Z., A.D.H., N.A.T., and Y.-P.Z. are current or former employees of Merck & Co, Inc and may own stock and/or have stock options in the company. All other authors have nothing to disclose.
Footnotes
- ASO
- antisense oligonucleotide
- CACT
- carnitine acyl-carnitine translocase
- Epac
- exchange protein activated by cAMP
- ESCA
- Epac-selective cAMP analogue, 8-pCPT-2′-O-Me-cAMP-AM
- Ex-4
- Exendin-4
- GLP-1
- glucagon-like peptide 1
- GLP-1R
- GLP-1 receptor
- GSIS
- glucose-stimulated insulin secretion
- IBMX
- 3-isobutly-1-methylxanthine
- miRNA
- microRNA
- OGTT
- oral glucose tolerance test
- PKA
- protein kinase A
- PKC
- protein kinase C
- qPCR
- quantitative PCR.
References
- 1. Kim W, Egan JM. The role of incretins in glucose homeostasis and diabetes treatment. Pharmacol Rev. 2008;60:470–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153–165. [DOI] [PubMed] [Google Scholar]
- 3. Holz GG. Epac: a new cAMP-binding protein in support of glucagon-like peptide-1 receptor-mediated signal transduction in the pancreatic β-cell. Diabetes. 2004;53:5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kashima Y, Miki T, Shibasaki T, et al. Critical role of cAMP-GEFII–Rim2 complex in incretin-potentiated insulin secretion. J Biol Chem. 2001;276:46046–46053. [DOI] [PubMed] [Google Scholar]
- 5. Ozaki N, Shibasaki T, Kashima Y, et al. cAMP-GEFII is a direct target of cAMP in regulated exocytosis. Nat Cell Biol. 2000;2:805–811. [DOI] [PubMed] [Google Scholar]
- 6. Park S, Dong X, Fisher TL, et al. Exendin-4 uses Irs2 signaling to mediate pancreatic β cell growth and function. J Biol Chem. 2006;281:1159–1168. [DOI] [PubMed] [Google Scholar]
- 7. Sonoda N, Imamura T, Yoshizaki T, Babendure JL, Lu JC, Olefsky JM. β-Arrestin-1 mediates glucagon-like peptide-1 signaling to insulin secretion in cultured pancreatic β cells. Proc Natl Acad Sci USA. 2008;105:6614–6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Quoyer J, Longuet C, Broca C, et al. GLP-1 mediates antiapoptotic effect by phosphorylating Bad through a β-arrestin 1-mediated ERK1/2 activation in pancreatic β-cells. J Biol Chem. 2010;285:1989–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. [DOI] [PubMed] [Google Scholar]
- 10. Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Poy MN, Eliasson L, Krutzfeldt J, et al. A pancreatic islet-specific microRNA regulates insulin secretion. Nature. 2004;432:226–230. [DOI] [PubMed] [Google Scholar]
- 12. Kloosterman WP, Lagendijk AK, Ketting RF, Moulton JD, Plasterk RH. Targeted inhibition of miRNA maturation with morpholinos reveals a role for miR-375 in pancreatic islet development. PLoS Biol. 2007;5:e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Joglekar MV, Joglekar VM, Hardikar AA. Expression of islet-specific microRNAs during human pancreatic development. Gene Expr Patterns. 2009;9:109–113. [DOI] [PubMed] [Google Scholar]
- 14. Hohmeier HE, Mulder H, Chen G, Henkel-Rieger R, Prentki M, Newgard CB. Isolation of INS-1-derived cell lines with robust ATP-sensitive K+ channel-dependent and -independent glucose-stimulated insulin secretion. Diabetes. 2000;49:424–430. [DOI] [PubMed] [Google Scholar]
- 15. Ronnebaum SM, Jensen MV, Hohmeier HE, et al. Silencing of cytosolic or mitochondrial isoforms of malic enzyme has no effect on glucose-stimulated insulin secretion from rodent islets. J Biol Chem. 2008;283:28909–28917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Raymond CK, Roberts BS, Garrett-Engele P, Lim LP, Johnson JM. Simple, quantitative primer-extension PCR assay for direct monitoring of microRNAs and short-interfering RNAs. RNA. 2005;11:1737–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhao E, Keller MP, Rabaglia ME, et al. Obesity and genetics regulate microRNAs in islets, liver, and adipose of diabetic mice. Mamm Genome. 2009;20:476–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lacy PE, Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 1967;16:35–39. [DOI] [PubMed] [Google Scholar]
- 19. Gangemi A, Salehi P, Hatipoglu B, et al. Islet transplantation for brittle type 1 diabetes: the UIC protocol. Am J Transplant. 2008;8:1250–1261. [DOI] [PubMed] [Google Scholar]
- 20. Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343(4):230–238. [DOI] [PubMed] [Google Scholar]
- 21. Barbaro B, Salehi P, Wang Y, et al. Improved human pancreatic islet purification with the refined UIC-UB density gradient. Transplantation. 2007;84(9):1200–1203. [DOI] [PubMed] [Google Scholar]
- 22. Adewola AF, Lee D, Harvat T, et al. Microfluidic perifusion and imaging device for multi-parametric islet function assessment. Biomed Microdevices. 2010;12(3):409–417.2010. [DOI] [PubMed] [Google Scholar]
- 23. Waddleton D, Wu W, Feng Y, et al. Phosphodiesterase 3 and 4 comprise the major cAMP metabolizing enzymes responsible for insulin secretion in INS-1 (832/13) cells and rat islets. Biochem Pharmacol. 2008;76:884–893. [DOI] [PubMed] [Google Scholar]
- 24. Weng L, Dai H, Zhan Y, He Y, Stepaniants SB, Bassett DE. Rosetta error model for gene expression analysis. Bioinformatics. 2006;22:1111–1121. [DOI] [PubMed] [Google Scholar]
- 25. Plaisance V, Abderrahmani A, Perret-Menoud V, Jacquemin P, Lemaigre F, Regazzi R. MicroRNA-9 controls the expression of Granuphilin/Slp4 and the secretory response of insulin-producing cells. J Biol Chem. 2006;281:26932–26942. [DOI] [PubMed] [Google Scholar]
- 26. Baroukh N, Ravier MA, Loder MK, et al. MicroRNA-124a regulates Foxa2 expression and intracellular signaling in pancreatic β-cell lines. J Biol Chem. 2007;282:19575–19588. [DOI] [PubMed] [Google Scholar]
- 27. Keller DM, Clark EA, Goodman RH. Regulation of microRNA-375 by cAMP in pancreatic β-cells. Mol Endocrinol. 2012;26:989–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Remenyi J, Hunter CJ, Cole C, et al. Regulation of the miR-212/132 locus by MSK1 and CREB in response to neurotrophins. Biochem J. 2010;428:281–291. [DOI] [PubMed] [Google Scholar]
- 29. Klein ME, Lioy DT, Ma L, Impey S, Mandel G, Goodman RH. Homeostatic regulation of MeCP2 expression by a CREB-induced microRNA. Nat Neurosci. 2007;10:1513–1514. [DOI] [PubMed] [Google Scholar]
- 30. Vo N, Klein ME, Varlamova O, et al. A cAMP-response element binding protein-induced microRNA regulates neuronal morphogenesis. Proc Natl Acad Sci USA. 2005;102:16426–16431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chepurny OG, Kelley GG, Dzhura I, et al. PKA-dependent potentiation of glucose-stimulated insulin secretion by Epac activator 8-pCPT-2′-O-Me-cAMP-AM in human islets of Langerhans. Am J Physiol Endocrinol Metab. 2010;298:E622–E633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Seino S, Shibasaki T, Minami K. Dynamics of insulin secretion and the clinical implications for obesity and diabetes. J Clin Invest. 2011;121:2118–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. El Ouaamari A, Baroukh N, Martens GA, Lebrun P, Pipeleers D, van Obberghen E. miR-375 targets 3′-phosphoinositide-dependent protein kinase-1 and regulates glucose-induced biological responses in pancreatic β-cells. Diabetes. 2008;57:2708–2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Soni MS, Rabaglia ME, Bhatnagar S, et al. Downregulation of carnitine acyl-carnitine translocase by miRNAs 132 and 212 amplifies glucose-stimulated insulin secretion. Diabetes. 2014;63:3805–3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Suzuki Y, Zhang H, Saito N, Kojima I, Urano T, Mogami H. Glucagon-like peptides 1 activates protein kinase C through Ca2+ -dependent activation of phospholipase C in insulin-secreting cells. J Biol Chem. 2006;281:28499–28507. [DOI] [PubMed] [Google Scholar]
- 36. Plaisance V, Waeber G, Regazzi R, Abderrahmani A. Role of microRNAs in islet β-cell compensation and failure during diabetes. J Diabet Res. 2014;2014:618652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lovis P, Gattesco S, Regazzi R. Regulation of the expression of components of the exocytotic machinery of insulin-secreting cells by microRNAs. Biol Chem. 2008;389:305–312. [DOI] [PubMed] [Google Scholar]
- 38. Pullen TJ, da Silva Xavier G, Kelsey G, Rutter GA. miR-29a and miR-29b contribute to pancreatic β-cell-specific silencing of monocarboxylate transporter 1 (Mct1). Mol Cell Biol. 2011;31:3182–3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Roggli E, Britan A, Gattesco S, et al. Involvement of microRNAs in the cytotoxic effects exerted by proinflammatory cytokines on pancreatic β-cells. Diabetes. 2010;59:978–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Xu G, Chen J, Jing G, Shalev A. Thioredoxin-interacting protein regulates insulin transcription through microRNA-204. Nat Med. 2013;19:1141–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Locke JM, da Silva Xavier G, Dawe HR, Rutter GA, Harries LW. Increased expression of miR-187 in human islets from individuals with type 2 diabetes is associated with reduced glucose-stimulated insulin secretion. Diabetologia. 2014;57:122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ahrén B. Islet G protein-coupled receptors as potential targets for treatment of type 2 diabetes. Nat Rev Drug Discov. 2009;8:369–385. [DOI] [PubMed] [Google Scholar]
- 43. Esguerra JL, Bolmeson C, Cilio CM, Eliasson L. Differential glucose-regulation of microRNAs in pancreatic islets of non-obese type 2 diabetes model Goto-Kakizaki rat. PloS One. 2011;6:e18613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nesca V, Guay C, Jacovetti C, et al. Identification of particular groups of microRNAs that positively or negatively impact on β cell function in obese models of type 2 diabetes. Diabetologia. 2013;56:2203–2212. [DOI] [PubMed] [Google Scholar]
- 45. Ahrén B. Incretin dysfunction in type 2 diabetes: clinical impact and future perspectives. Diabetes Metab. 2013;39:195–201. [DOI] [PubMed] [Google Scholar]