Abstract
Multiple sclerosis (MS) is a chronic inflammatory disease that causes demyelination of neurons in the central nervous system. Traditional therapies for MS have involved anti-inflammatory and immunosuppressive drugs with significant side effects that often only provide short-term relief. A more desirable outcome of immunotherapy would be to protect against disease before its clinical manifestation or to halt disease after its initiation. One attractive approach to accomplish this goal would be to restore tolerance by targeting immunoregulatory cell networks. Although much of the work in this area has focused on CD4+ Foxp3+ regulatory T cells, other studies have investigated natural killer T (NKT) cells, a subset of T cells that recognizes glycolipid antigens in the context of the CD1d glycoprotein. Studies with human MS patients have revealed alterations in the numbers and functions of NKT cells, which have been partially supported by studies with the experimental autoimmune encephalomyelitis model of MS. Additional studies have shown that activation of NKT cells with synthetic lipid antigens can, at least under certain experimental conditions, protect mice against the development of MS-like disease. Although mechanisms of this protection remain to be fully investigated, current evidence suggests that it involves interactions with other immunoregulatory cell types such as regulatory T cells and immunosuppressive myeloid cells. These studies have provided a strong foundation for the rational design of NKT-cell-based immunotherapies for MS that induce tolerance while sparing overall immune function. Nevertheless, additional pre-clinical and clinical studies will be required to bring this goal to fruition.
Keywords: experimental autoimmune encephalomyelitis, glycolipid antigens, immunotherapy, multiple sclerosis, natural killer T cells
Introduction
Multiple sclerosis (MS) is an autoimmune disease of the central nervous system (CNS) mediated by T cells, resulting in paralysis and loss of cognitive functions.1 In the USA, approximately 250 000–350 000 patients suffer from MS with 200 new patients being diagnosed with MS each week. Most treatments for MS and other autoimmune diseases require long-term treatment and work by broadly suppressing immune function, which leaves the patients vulnerable to infections and possibly cancer.2 More recent strategies for treatment of MS involve disease-modifying therapies such as interferons, glatiramer acetate, sphingosine-1-phosphate receptor modulators and monoclonal antibody medications, which have decreased the numbers of MS attacks and have slowed disease progression in many patients. However, the ‘holy grail’ of MS treatment is to induce tolerance against the target antigens and to restore immune homeostasis, effectively curing the disease while leaving the rest of the body’s immune defences intact. One attractive approach to accomplish this goal is to target the immunoregulatory components of the immune system, ideally in an autoantigen-specific manner. While much of the work in this area has focused on CD4+ Foxp3+ regulatory T (Treg) cells, other investigators have studied natural killer T (NKT) cells, a subset of glycolipid-reactive T cells with potent immunomodulatory properties.3–9 Two subsets of NKT cells have been identified, and both of these subsets have been implicated in the progression of disease in MS and its mouse model, experimental autoimmune encephalomyelitis (EAE). Glycolipid antigens that selectively activate NKT cells have been tested for their capacity to modulate EAE. The mechanisms involved remain incompletely understood, but results from these studies have raised enthusiasm for the development of NKT cell-based therapies for MS and other autoimmune and inflammatory diseases. The studies on NKT cells will be discussed in this review article.
Natural killer T cells
Natural killer T cells are a subset of T lymphocytes that recognize lipid or glycolipid antigens presented by the MHC class I-related protein CD1d.3–9 Two subsets of NKT cells, Type I or invariant NKT (iNKT) cells, and Type II or variant NKT (vNKT) cells, with overlapping but distinct properties, have been identified (Fig.1). We will briefly discuss these NKT cell subsets as well as tools and experimental models that have been employed to study them.
Figure 1.
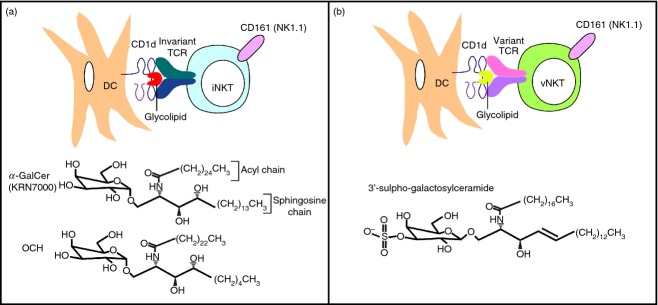
Natural killer T (NKT) cells and their prototypical antigens. (a) Type I or invariant NKT (iNKT) cells express a semi-invariant T-cell receptor (TCR) specific for glycolipid antigens such as the α-linked glycosphingolipids α-galactosylceramide (α-GalCer; the structure is shown of a synthetic optimized version, KRN7000, most commonly used in pre-clinical and clinical studies) and OCH (the α-GalCer analogue that induces a T helper type 2-biased cytokine production profile in iNKT cells) bound with CD1d on the surface of dendritic cells (DCs). (b) Type II or variant NKT (vNKT) cells express variant or oligoclonal TCRs that recognize β-linked glycolipid antigens bound with CD1d on the surface of DCs. The structure is shown of 3′-sulpho-galactosylceramide, a sulphatide that has been employed in pre-clinical studies and is recognized by the sulphatide-reactive vNKT cell subset.
Invariant NKT cells
Invariant NKT cells express a semi-invariant T-cell receptor (TCR), composed of an invariant Vα14-Jα18 chain paired with Vβ8, Vβ7 or Vβ2 chains in mice, or homologous Vα24-Jα18 and Vβ11 chains in humans.3–7 The iNKT cells also constitutively express a number of surface receptors such as CD25, CD44 and CD69 that are characteristic of activated or memory T cells. Most iNKT cells express the TCR co-receptor CD4, and the remaining cells express neither CD4 nor CD8, although in humans a small subset of CD8+ iNKT cells has been identified. In addition to T-cell markers, mouse iNKT cells express a variety of activating receptors such as NK1.1 (CD161) and inhibitory receptors such as members of the Ly49 family of C-type lectins that are characteristically expressed by natural killer (NK) cells. Human iNKT cells similarly express a variety of NK-cell markers. The iNKT cells also express receptors for innate cytokines such as interleukin-12 (IL-12) and IL-18. Invariant NKT cells are most abundant in liver and adipose tissue, represent a substantial T-cell subset in spleen, peripheral blood and bone marrow, and are also present in mucosal tissues such as lung and intestine, but are quite rare in lymph nodes. Interestingly, iNKT cells are more abundant in mice than humans, and their prevalence varies among different mouse strains and even more so among different human subjects.
Invariant NKT cells react most strongly with α-anomeric glycosphingolipids such as α-galactosylceramide (α-GalCer; Fig.1a), which was originally isolated as an anti-metastatic agent from a marine sponge,10 but is probably derived from environmental bacteria that colonize the sponge. The iNKT cells bind with CD1d–glycolipid complexes in a parallel configuration and engage the glycolipid antigen mainly via the TCR-α chain.11 Glycosphingolipids and diacylglycerols that can activate iNKT cells have been isolated from various microbial pathogens (e.g. Streptococcus pneumoniae, Aspergillus fumigatus and Borrelia burgdorferi) as well as from non-pathogenic microbes that are widely distributed in the environment (e.g. Sphingomonas species). A lot of debate in the field has focused on the endogenous antigens that drive the development and function of iNKT cells.12 Although it has been long assumed that mammalian cells only produce β-anomeric glycosphingolipids, recent studies have provided evidence for the presence of minute amounts of α-anomeric glycosphingolipids that can activate iNKT cells.13,14 The enzymatic activities responsible for the synthesis of these antigens in mammalian tissues remain to be defined.
Akin to conventional T cells, iNKT cells develop in the thymus, but they branch away from conventional T-cell development at the CD4+ CD8+ (double-positive) thymocyte stage.15 Whereas conventional T cells require MHC expression by thymic epithelial cells for their development, iNKT cell development requires CD1d expression by cortical thymocytes. A variety of intracellular signalling pathways and transcription factors have been implicated in regulating iNKT cell development. Key pathways include the SLAM (signalling lymphocytic activation molecule)–SAP (SLAM family receptor adaptor protein)–Fyn pathway, the TCR signalling cascade with induction of the classical nuclear factor-κB pathway, and the IL-15 pathway. The innate characteristics of iNKT cells are largely imparted by the transcription factor promyelocytic leukaemia zinc finger.15
Following TCR engagement, iNKT cells rapidly produce a variety of cytokines and exhibit cytotoxic activity.16,17 One unusual property of iNKT cells is their capacity to produce mixtures of cytokines typically associated with either pro-inflammatory [e.g. interferon-γ (IFN-γ), tumour necrosis factor-α) or anti-inflammatory (e.g., IL-4, IL-10 and IL-13) immune responses. It is clear that several of these cytokines may be produced simultaneously by individual cells, but emerging evidence indicates the presence of subsets of iNKT cells with divergent cytokine profiles and transcription factor expression. This includes Tbet+ NKT1 cells producing IFN-γ, GATA3+ NKT2 cells producing IL-4, RORγt+ NKT17 cells producing IL-17A, IL-21 and IL-22,18 Bcl6+ follicular helper NKT (NKTFH) cells producing IL-21,19 and several subsets of regulatory iNKT cells.20–22
Invariant NKT cells can interact with multiple cell types of both the innate and acquired immune systems, including antigen-presenting cells, NK cells, B cells and conventional T cells.23 As such, iNKT cells have been implicated in a wide variety of immune responses.3–9 The iNKT cells become activated during a number of infections and play a protective role in host immunity against some pathogens. They also provide natural immunity against certain metastatic tumours. Generally, iNKT cells play a suppressive role during autoimmunity, but promote the development of atherosclerosis. These cells are also important for the development of allergic inflammation, contact hypersensitivity, oxazolone-induced colitis and concanavalin A-induced hepatitis. Invariant NKT cells also contribute to the rejection of tissue grafts, graft-versus-host disease, ischaemia–reperfusion injury and obesity-associated insulin-resistance. Finally, these cells play a critical role in the induction and/or maintenance of tolerance in a variety of experimental systems.
Following activation with glycolipid antigens such as α-GalCer, iNKT cells interact with other cells of the immune system, resulting in their activation.23 For example, α-GalCer-activated iNKT cells promote NK cells to produce IFN-γ and become cytotoxic, B cells to produce antibodies, and dendritic cells (DCs) to become activated.7,23 Activation of iNKT cells can also influence the differentiation of T helper (Th) cells, typically skewing the response towards Th2 cytokine production, especially when multiple α-GalCer challenges are performed.24–26 There is also evidence that iNKT cells can promote the induction and function of Foxp3+ CD4+ Treg cells.25,27
The immunomodulatory activities of several structural variants of α-GalCer have been evaluated.25 For example, α-GalCer analogues with shortened hydrocarbon chains, such as the reagent called OCH (Fig.1a), have reduced capacity to activate iNKT cells, but elicit a Th2-biased cytokine production profile. α-GalCer analogues containing unsaturated acyl chains have similar properties, in that they bias the iNKT cell response towards Th2 cytokine production. In contrast, a C-glycoside analogue of α-GalCer, α-C-GalCer, was a more potent agonist of iNKT cells and promoted Th1 cytokine production by these cells. Various other iNKT cell antigens that can selectively induce the divergent effector functions of iNKT cells have been described.
The immunomodulatory activities of α-GalCer have been exploited for therapeutic purposes.23,25 Pre-clinical studies have demonstrated that α-GalCer and certain structural analogues have potent adjuvant activities and protect mice against a variety of diseases, including certain cancers, infections and autoimmune and inflammatory conditions. Clinical studies with α-GalCer have also been performed in cancer patients and individuals infected with hepatitis viruses. 23,25
Variant NKT cells
While vNKT cells express more diverse TCRs than iNKT cells, this population includes oligoclonal subsets, with preferential usage of Vα3/Vα1-Jα7/Jα9 and Vβ8.1/Vβ3.1–Jβ2.7 chains in mice.28,29 Like iNKT cells, most vNKT cells express T-cell activation markers and NK cell receptors such as NK1.1, and contain CD4+ and CD4− CD8− subsets in mice, as well as a small CD8+ subset in humans.8,29 The tissue distribution of vNKT cells appears similar to that of iNKT cells. Interestingly, however, whereas vNKT cells are less prevalent than iNKT cells in mice, this NKT cell subset is more prevalent in humans.30,31
In sharp contrast with the bias of iNKT cells for α-anomeric glycolipids, vNKT cells preferentially recognize β-linked glycolipids (Fig.1b).8,29 Interestingly, a major subset of vNKT cells was shown to react with sulphatide, a self-glycolipid that is abundant in the myelin sheaths that coat and insulate nerve fibres in the CNS.32 The sulphatide-reactive vNKT cells also reacted with other self-lipids, including β-GlcCer and β-GalCer,33 as well as with lysophospholipids such as lysophosphatidylcholine.34 The TCRs of vNKT cells interact with CD1d–glycolipid complexes in a perpendicular manner and predominantly engage the ligand using the β-chain rather than the α-chain.35
Like iNKT cells, vNKT cells develop in the thymus, with a requirement for CD1d expression by haematopoietic cells, as well as the adaptor protein SAP and the transcription factor promyelocytic leukaemia zinc finger.36 Variant NKT cells display innate effector functions and can produce both Th1 and Th2 cytokines.8,29 Variant NKT cells have been implicated in protective immune responses against autoimmunity, exacerbation of colitis and obesity-associated insulin-resistance, protection against graft-versus-host disease, and modulation of immune responses against some infections.8,29 Curiously, vNKT cells sometimes exhibit opposing functions to iNKT cells.37 For example, vNKT cells suppress tumour immunity whereas iNKT cells promote tumour immunity. Another interesting feature of vNKT cells is that they can regulate iNKT cells. For example, they can suppress the anti-tumour effects of iNKT cells.38
Sulphatide has been shown to protect mice against several autoimmune diseases, suppress anti-tumour immunity, and ameliorate sepsis.8,29 Sulphatide and lysophosphatidylcholine also suppress concanavalin A-induced hepatitis mediated by iNKT cells.34,39
Tools and experimental models to study NKT cells
Because markers expressed by NKT cells are not uniquely expressed by these cells, multimeric CD1d molecules loaded with glycolipid antigens have been extensively employed to identify these cells. CD1d/α-GalCer-tetramers specifically identify iNKT cells,40 whereas CD1d/sulphatide-tetramers interact with sulphatide-specific vNKT cells.32 Whereas CD1d/α-GalCer-tetramers robustly stain with iNKT cells, they also react with a small population of α-GalCer-reactive NKT cells expressing canonical TCR Vα10-Jα50 rearrangements with unknown functions.41 Such tetramers have also been employed for the positive enrichment of iNKT cells in adoptive transfer studies, with the potential caveat that tetramers may induce some TCR signalling. Consistent with the relatively weak affinity of sulphatide-reactive TCRs with sulphatide, CD1d/sulphatide-tetramers react only rather weakly with sulphatide-reactive vNKT, which has complicated studies with these cells.32
Several experimental animal models of NKT cell deficiency or over-representation have been employed to analyse the functions of these cells. Initial studies were often performed by comparing β2-microglobulin-deficient mice with mice deficient in the transporter associated with antigen processing (TAP) peptide transporter that supplies peptides to classical MHC class I molecules. Although both of these animals have defects in CD8+ T cells, the former mice, but not the latter, lack NKT cells. Because these animals also have deficiencies in other MHC class I and class I-related molecules, studies obtained by comparing the immune responses of these animals have been difficult to interpret. CD1d-deficient animals42–44 lacking both iNKT and vNKT cells have been extensively employed to study these cells, and mice with a floxed CD1d1 allele for tissue-specific or conditional CD1d ablation studies are also available.45 Mice that are selectively deficient in the Jα18 gene segment have been employed as a model of selective iNKT cell deficiency,46 although a more recent study has revealed that these animals lack certain conventional TCR specificities,47 raising some concerns about interpreting data obtained with these animals. Nevertheless, phenotypic differences between CD1d-deficient and Jα18-deficient mice have often been interpreted as being mediated by vNKT cells. Transgenic animals expressing the murine invariant Vα14-Jα18 TCR chain48 or the human Vα24-Jα18 TCR chain49,50 have been employed as models of iNKT cell enrichment, and transgenic mice over-expressing a murine vNKT cell TCR (this TCR does not react with sulphatide) have been developed as well.51
In additional to these tools, mice over-expressing murine CD1d molecules52,53 and mice expressing a human CD1d allele50,54 have also been employed. Antibodies directed against CD1d molecules in an attempt to block interactions with NKT cell TCRs have been employed, although some of these antibodies appear to activate CD1d-expressing cells,55 complicating data interpretation.
NKT cells in MS
Multiple sclerosis is a chronic inflammatory disease that causes demyelination of the neurons in the CNS, resulting in muscular weakness, loss of coordination, and speech and visual disturbances, ultimately leading to paralysis. T lymphocytes, especially MHC class II-restricted CD4+ T cells that infiltrate the CNS, are the primary mediators of MS.1 Activated T cells, together with activated macrophages and microglia, induce active lesions in the CNS, which causes demyelination of neurons.
Invariant NKT cells in MS
A few studies have shown that iNKT cells are decreased in the peripheral blood of MS patients.56,57 Importantly, the prevalence and function of iNKT cells was restored in patients in remission due to treatment with IFN-β.58 Another study analysed untreated patients in remission, and found instead a further reduction in iNKT cells but evidence for a Th2-bias in the cytokine profile of iNKT cell lines established from these patients.59 A Th2 bias in the cytokine profile of iNKT cells was also observed in patients treated with oral corticosteroids, suggesting that iNKT cells might exert immunoregulatory effects in MS by producing Th2 cytokines.60
Variant NKT cells in MS
The finding that a main subset of murine vNKT cells is specific to sulphatide,32 a major component of the myelin sheath, raised the possibility that vNKT cells may contribute to MS. Sulphatide-reactive human CD1-restricted T cells have been identified, but whether this includes CD1d-restricted NKT cells remains unclear.61 Studies regarding the status of vNKT cells in patients with MS have not yet been reported.
NKT cells in EAE
The most common mouse model of MS is EAE, which can be induced in multiple mouse strains by immunization with myelin antigens in the context of a potent adjuvant such as complete Freund’s adjuvant.62,63 Disease induction is usually further facilitated by breaking the blood–brain barrier with pertussis toxin. Several mouse strains, particularly those with the H2s (e.g., SJL/J) or H2u (e.g., PL/J and B10.PL) haplotypes, are most susceptible to EAE. The disease is characterized by multiple perivascular foci of mononuclear inflammation, leading to ascending weakness and paralysis.64 In addition to EAE, demyelinating disease with characteristics of MS can by induced in mice by infection with Theiler’s murine encephalitis virus.63 The disease observed in both of these experimental models can be adoptively transferred to naive animals by encephalitogenic T cells. Transgenic animals expressing TCRs derived from encephalitogenic T cells have also been developed to study disease pathogenesis and treatment modalities.
Invariant NKT cells in EAE
Studies with EAE-susceptible SJL/J mice showed that these animals have defects in iNKT cell numbers and functions,65 with the remaining cells exhibiting a Th1 cytokine production profile.66 Defects in these animals are owing to the absence of the Vβ8 gene segment, which is the preferred TCR-β gene segment of murine iNKT cells. These findings suggested that susceptibility of SJL/J mice to EAE might somehow be linked to alterations in the iNKT cell compartment, a possibility that remains to be validated.
One study investigated the fate of iNKT cells in the CNS of mice with EAE and found that numbers remain unchanged as compared with naive animals.67
The effects of CD1d- and Jα18-deficiency on the development of EAE have been controversial, with some studies reporting disease amelioration,8 no effect,68 or disease exacerbation69 in Jα18-deficient mice, and no affect67,68,70 or disease exacerbation71 in CD1d-deficient mice. The potential role of iNKT cells was more directly addressed by investigating Vα14-Jα18 transgenic mice, which were partially protected against the development of EAE in the non-obese diabetic (NOD) background.72 Protection from EAE in the latter model was associated with infiltration of iNKT cells into the CNS and suppression of encephalitogenic Th1 and Th17 cell responses in the spleen.73 In the Theiler’s murine encephalitis virus model, the absence of iNKT cells in Jα18-deficient mice had no effect on disease parameters in the C57BL/6 background.74 These divergent findings might be the result of differences in the EAE models, background strains, or genetically engineered animals employed, as well as differences in the microbiota of the animal facilities where the studies were performed. With regard to a possible role of the microbiota, one study implicated iNKT cells in the effects of gut flora on the development of EAE.75
Variant NKT cells in EAE
Sulphatide-reactive vNKT cells are expanded several-fold in the CNS of mice with EAE,32 suggesting that sulphatide may function as a self-ligand to activate vNKT cells during disease progression. Nevertheless, as discussed in the previous section, different laboratories have reported divergent effects of NKT-cell deficiency on the incidence and progression of EAE, leaving questions regarding the role of sulphatide-reactive vNKT cells in the progression of EAE unanswered.
Modulation of EAE by lipid-activated NKT cells
Based on the immunomodulatory activities of NKT cells and their potential role in the development of MS and EAE, many studies have investigated the capacity of glycolipid antigens for either iNKT or vNKT cells to influence EAE.
Effects of iNKT cell activation on EAE
Treatment with α-GalCer has different effects on EAE, depending on the timing and dose of α-GalCer administration, and the genetic background of the animals employed.25 In C57BL/6 mice immunized with a peptide derived from the myelin oligodendrocyte glycoprotein (MOG), treatment with α-GalCer at the time of immunization protected the animals against disease in a CD1d-dependent manner.67,70,76 One study compared subcutaneous versus intraperitoneal α-GalCer administration and found that only subcutaneous administration effectively protected against EAE in this model,68 although this was not confirmed in a separate study.67,77 One study further reported that very high doses of α-GalCer (5 μg per animal) exacerbate rather than ameliorate EAE,77 which is also discordant with some other studies.67,70 In PL/J mice immunized with myelin basic protein (MBP), a co-treatment protocol similarly ameliorated disease.70 However, in B10.PL mice immunized with an MBP-derived peptide a co-treatment protocol exacerbated disease whereas a pre-treatment protocol protected against disease.67 Consistent with the Th1 bias in the cytokine production profile of their iNKT cells, α-GalCer treatment exacerbated disease in MBP-immunized SJL/J mice in a co-treatment protocol.70
With regard to the mechanisms by which α-GalCer-activated iNKT cells modulate EAE, the published studies consistently showed that disease protection was associated with a shift towards enhanced Th2 and/or reduced Th1/Th17 responses, whereas disease exacerbation had the opposite effect on Th cell profiles.67,70,78 These findings suggested a role for a shift in the cytokine profile of antigen-specific Th cells in the capacity of α-GalCer to modulate EAE. This possibility was consistent with the finding that a combination treatment of α-GalCer with anti-CD86 antibodies, which promotes Th2 cytokine production in iNKT cells, was more effective than α-GalCer treatment alone in protecting C57BL/6 mice against MOG-induced EAE.79 Similarly, the α-GalCer variant OCH, which induces a more Th2-biased cytokine production profile in iNKT cells, was more effective than α-GalCer in protecting mice against EAE.80 Similar findings have been obtained for additional Th2-biasing α-GalCer analogues.81 Additional studies with cytokine-deficient mice and cytokine neutralizing antibodies in MOG-immunized C57BL/6 mice revealed a role not only for the Th2 cytokines IL-4 and IL-10,67,70,82 but also for the Th1 cytokine IFN-γ.77,78 While this finding was consistent with a pathogenic role of Th17 cells in this EAE model, it suggested that a shift in the cytokine profile of iNKT cells might not be the domininant mechanism for the protective effects of these cells against EAE.
Additional mechanisms that might contribute to the capacity of iNKT cell antigens to protect against EAE have been explored. These studies have largely focused on myeloid lineage cells with immunosuppressive activities.69,83,84 One study showed that repeated treatment of C57BL/6 mice with α-GalCer induced a regulatory phenotype in splenic DCs that depended on IL-10 production by iNKT cells,83,85 and these DCs were able to suppress EAE upon adoptive transfer.83 A potential role of tolerogenic DCs in the immunomodulatory activities of α-GalCer in EAE is consistent with previous findings that α-GalCer similarly induces tolerogenic DCs in diabetes-prone NOD mice.86 Another study showed that protection against EAE was associated with a reduction in inflammatory monocytes and an increase in the proportion of non-inflammatory M2 macrophages in the CNS, in a manner that involved IL-4 production by iNKT cells.69 Yet another study provided evidence for a crucial role of myeloid-derived suppressor cells (MDSCs) in EAE protection.84 The MDSCs are a heterogeneous population of myeloid progenitor cells and immature myeloid cells that regulate immune responses during inflammation.87 These cells accumulate in various organs in response to inflammatory conditions (e.g. strong adjuvants) and have a strong capacity to suppress T-cell responses in vitro and in vivo, a property that has been exploited to prevent the generation of autoimmunity. Under homeostatic conditions, these cells lack immunosuppressive properties and quickly differentiate into various mature myeloid cells such as macrophages, DCs and granulocytes. Only in pathological situations do these cells expand vigorously, retain their immature phenotype, and acquire immunosuppressive functions. α-GalCer promoted a profound expansion of immunosuppressive MDSCs in the spleen of mice induced for EAE.87 Protection against disease in these animals also correlated with an influx of immunosuppressive MDSCs in the CNS. Induction of MDSCs involved granulocyte–macrophage colony-stimulating factor, IL-4 and IFN-γ production by iNKT cells, and disease protection involved IL-10 production by MDSCs. These findings are therefore consistent with the previously identified role of IFN-γ in the protective effects of α-GalCer against EAE.77,78 Because MDSCs can give rise to mature myeloid cells, an appealing possibility is that the immunosuppressive DCs and M2 macrophages that accumulate in response to α-GalCer treatment during EAE induction are derived from splenic MDSCs.
Studies on α-GalCer treatment of autoimmune diseases other than EAE might provide further insight into the protective effects of iNKT cell activation in EAE.23,25 In particular, a study on diabetes in NOD mice88 and another on experimental myasthenia gravis in C57BL/6 mice89 has provided evidence for a role of Foxp3+ Treg cells in disease protection afforded by α-GalCer.27 A possible role for Treg cells in the protective effects of iNKT cell antigens on EAE is therefore appealing. In this context, iNKT cells produce cytokines such as IL-2 and transforming growth factor-β, which might directly contribute to the induction of Treg cells. Additionaly, it has been shown that MDSCs and other tolerogenic myeloid lineage cells can promote the induction of Treg cells.90,91 Hence, activated iNKT cells might induce Treg cells either directly or indirectly via tolerogenic myeloid cells. Collectively, these findings suggest cooperative interactions between iNKT cells, tolerogenic myeloid cells and Treg cells in protecting mice against EAE and potentially other autoimmune and inflammatory diseases. A proposed model for the protective effects of α-GalCer and related glycolipids against EAE is depicted in Fig.2.
Figure 2.

Proposed model for the capacity of α-galactosylceramide (α-GalCer) and related invariant natural killer T (iNKT) cell antigens to protect mice against experimental autoimmune encephalomyelitis. α-GalCer-activated iNKT cells produce a variety of cytokines that can promote T helper type 2 (Th2) deviation of autoreactive T-cell responses, Foxp3+ regulatory T (Treg) cells, and immunosuppressive immature [e.g. myeloid-derived suppressor cells (MDSCs)] and mature [e.g. dendritic cells (DCs), M2 macrophages] myeloid cells. Tolerogenic myeloid lineage cells may also promote the induction of Treg cells. In turn, Th2 cells, Treg cells and suppressive myeloid cells suppress the generation and/or function of pathogenic autoantigen-specific Th1, Th17 and cytotoxic T cells (CTL) in the central nervous system (CNS). GM-CSF, granulocyte–macrophage colony-stimulating factor; IFN-γ, interferon-γ; IL-4, interleukin-4; TGF-β, transforming growth factor-β.
Effects of vNKT cell activation on EAE
A single dose of sulphatide injection before, at the time of, or after EAE induction protected against MOG-induced disease in C57BL/6 mice in a CD1d-dependent manner.32 Hence, compared with α-GalCer, the protective effects of sulphatide treatment on EAE appeared to be less dependent on the timing of treatment relative to the time of disease induction. A similar level of protection was observed in a co-treatment protocol in B10.PL mice immunized with an MBP-derived peptide.32 Sulphatide (but not α-GalCer) also was able to reverse ongoing relapsing–remitting EAE in SJL/J mice immunized with a proteolipid protein peptide.92 This was associated with a sharp reduction in the frequency of pathogenic Th1/Th17 responses.
Based on their previous findings that activation of sulphatide-reactive vNKT cells induces an anergic response in iNKT cells of C57BL/6 mice,39 these investigators performed experiments with iNKT cell-deficient Jα18 knockout mice, which revealed a surprising requirement for iNKT cells in the sulphatide-mediated inhibition of EAE.92 Additional studies revealed that sulphatide induced tolerance in peripheral DCs and CNS-resident microglia. Further, these tolerized DCs isolated from sulphatide-treated animals were able to protect naive animals against EAE upon adoptive transfer. Hence, these findings revealed an intricate immunoregulatory pathway involving interactions between sulphatide-reactive vNKT cells, anergic iNKT cells and tolerized DCs and microglia in suppressing EAE. A proposed, albeit speculative, model for the suppressive effects of sulphatide in EAE is shown in Fig.3.
Figure 3.
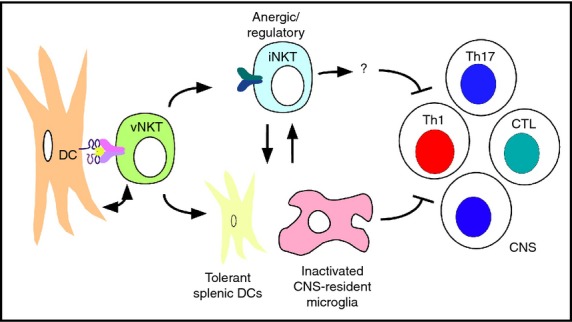
Proposed model for the capacity of sulphatide to protect mice against experimental autoimmune encephalomyelitis. Sulphatide-activated variant natural killer T (vNKT) cells induce an anergic or regulatory phenotype in invariant natural killer T (iNKT) cells and induce tolerance in splenic dendritic cells (DCs) as well as microglia in the central nervous system (CNS). In turn, these interactions suppress the generation and/or function of pathogenic autoantigen-specific T helper type 1 (Th1), Th17 and cytotoxic T lymphocytes (CTL) in the CNS. Note that many of the factors and interactions involved, and the locations where they take place, remain to be identified.
Conclusions and future perspectives
The ‘holy grail’ of MS treatment is to induce tolerance against the target antigens and to restore immune homeostasis, effectively curing the disease while leaving the rest of the body’s immune defences intact. One attractive approach to accomplish this goal is to target the immunoregulatory components of the immune system. NKT cells exhibit several appealing properties for therapeutic targeting during MS. The NKT cell system is highly conserved between mice and humans,93,94 so the findings obtained in mice should be relevant to human patients. Furthermore, because CD1 molecules are largely non-polymorphic, NKT cell-based therapies lack some of the limitations associated with therapies that target antigen-specific conventional T lymphocytes. Clinical trials with iNKT cell agonists such as α-GalCer have shown a favourable safety and tolerability profile,23,25 with promising clinical benefit in some cancer patients.95 Finally, pre-clinical studies in mice have shown that long-term treatment with α-GalCer does not impair host immunity to foreign antigens.26 These properties of NKT cells, together with the pre-clinical studies performed in mice with EAE, provide a strong foundation for the rational design of novel immunotherapies for MS that induce tolerance while sparing overall immune function. Nevertheless, additional studies will need to be performed to understand why α-GalCer (but not sulphatide) treatment can have such divergent outcomes depending on the treatment protocol employed, and to unravel the underlying mechanisms of protection involved.
Acknowledgments
We apologize to investigators whose work we were unable to cite due to space constraints or omission. We thank Dr Sebastian Joyce for helpful discussions and support. Work in our laboratory was supported by grants from the National Institutes of Health and the National Multiple Sclerosis Society.
Disclosures
The authors have no financial disclosures.
References
- Goverman J. Autoimmune T cell responses in the central nervous system. Nat Rev Immunol. 2009;9:393–407. doi: 10.1038/nri2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein A, Freeman J, Lo AC. Treatment of progressive multiple sclerosis: what works, what does not, and what is needed. Lancet Neurol. 2015;14:194–207. doi: 10.1016/S1474-4422(14)70231-5. [DOI] [PubMed] [Google Scholar]
- Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- Taniguchi M, Harada M, Kojo S, Nakayama T, Wakao H. The regulatory role of Vα14 NKT cells in innate and acquired immune response. Annu Rev Immunol. 2003;21:483–513. doi: 10.1146/annurev.immunol.21.120601.141057. [DOI] [PubMed] [Google Scholar]
- Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol. 2005;26:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- Brigl M, Brenner MB. CD1: antigen presentation and T cell function. Annu Rev Immunol. 2004;22:817–90. doi: 10.1146/annurev.immunol.22.012703.104608. [DOI] [PubMed] [Google Scholar]
- Van Kaer L, Parekh VV, Wu L. Invariant natural killer T cells: bridging innate and adaptive immunity. Cell Tissue Res. 2011;343:43–55. doi: 10.1007/s00441-010-1023-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viale R, Ware R, Maricic I, Chaturvedi V, Kumar V. NKT cell subsets can exert opposing effects in autoimmunity, tumor surveillance and inflammation. Curr Immunol Rev. 2012;8:287–96. doi: 10.2174/157339512804806224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. NKT cells: what’s in a name? Nat Rev Immunol. 2004;4:231–7. doi: 10.1038/nri1309. [DOI] [PubMed] [Google Scholar]
- Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, et al. CD1d-restricted and TCR-mediated activation of Vα14 NKT cells by glycosylceramides. Science. 1997;278:1626–9. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- Borg NA, Wun KS, Kjer-Nielsen L, Wilce MC, Pellicci DG, Koh R, et al. CD1d-lipid-antigen recognition by the semi-invariant NKT T-cell receptor. Nature. 2007;448:44–9. doi: 10.1038/nature05907. [DOI] [PubMed] [Google Scholar]
- Van Kaer L, Joyce S. The hunt for iNKT cell antigens: α-galactosidase-deficient mice to the rescue? Immunity. 2010;33:143–5. doi: 10.1016/j.immuni.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Brennan PJ, Tatituri RV, Heiss C, Watts GF, Hsu FF, Veerapen N, et al. Activation of iNKT cells by a distinct constituent of the endogenous glucosylceramide fraction. Proc Natl Acad Sci USA. 2014;111:13433–8. doi: 10.1073/pnas.1415357111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kain L, Webb B, Anderson BL, Deng S, Holt M, Costanzo A, et al. The identification of the endogenous ligands of natural killer T cells reveals the presence of mammalian α-linked glycosylceramides. Immunity. 2014;41:543–54. doi: 10.1016/j.immuni.2014.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gapin L. The making of NKT cells. Nat Immunol. 2008;9:1009–11. doi: 10.1038/ni0908-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh VV, Lalani S, Van Kaer L. The in vivo response of invariant natural killer T cells to glycolipid antigens. Int Rev Immunol. 2007;26:31–48. doi: 10.1080/08830180601070179. [DOI] [PubMed] [Google Scholar]
- Matsuda JL, Mallevaey T, Scott-Browne J, Gapin L. CD1d-restricted iNKT cells, the ‘Swiss-Army knife’ of the immune system. Curr Opin Immunol. 2008;20:358–68. doi: 10.1016/j.coi.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Holzapfel KL, Zhu J, Jameson SC, Hogquist KA. Steady-state production of IL-4 modulates immunity in mouse strains and is determined by lineage diversity of iNKT cells. Nat Immunol. 2013;14:1146–54. doi: 10.1038/ni.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinuesa CG, Cyster JG. How T cells earn the follicular rite of passage. Immunity. 2011;35:671–80. doi: 10.1016/j.immuni.2011.11.001. [DOI] [PubMed] [Google Scholar]
- Sag D, Krause P, Hedrick CC, Kronenberg M, Wingender G. IL-10-producing NKT10 cells are a distinct regulatory invariant NKT cell subset. J Clin Invest. 2014;124:3725–40. doi: 10.1172/JCI72308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch L, Michelet X, Zhang S, Brennan PJ, Moseman A, Lester C, et al. Regulatory iNKT cells lack expression of the transcription factor PLZF and control the homeostasis of T(reg) cells and macrophages in adipose tissue. Nat Immunol. 2015;16:85–95. doi: 10.1038/ni.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro M, Almeida CF, Caridade M, Ribot JC, Duarte J, Agua-Doce A, et al. Identification of regulatory Foxp3+ invariant NKT cells induced by TGF-β. J Immunol. 2010;185:2157–63. doi: 10.4049/jimmunol.1000359. [DOI] [PubMed] [Google Scholar]
- Van Kaer L, Parekh VV, Wu L. Invariant NK T cells: potential for immunotherapeutic targeting with glycolipid antigens. Immunotherapy. 2011;3:59–75. doi: 10.2217/imt.10.85. [DOI] [PubMed] [Google Scholar]
- Singh N, Hong S, Scherer DC, Serizawa I, Burdin N, Kronenberg M, et al. Cutting edge: activation of NK T cells by CD1d and α-galactosylceramide directs conventional T cells to the acquisition of a Th2 phenotype. J Immunol. 1999;163:2373–7. [PubMed] [Google Scholar]
- Van Kaer L. α-Galactosylceramide therapy for autoimmune diseases: prospects and obstacles. Nat Rev Immunol. 2005;5:31–42. doi: 10.1038/nri1531. [DOI] [PubMed] [Google Scholar]
- Hong S, Wilson MT, Serizawa I, Wu L, Singh N, Naidenko OV, et al. The natural killer T-cell ligand α-galactosylceramide prevents autoimmune diabetes in non-obese diabetic mice. Nat Med. 2001;7:1052–6. doi: 10.1038/nm0901-1052. [DOI] [PubMed] [Google Scholar]
- La Cava A, Van Kaer L, Shi FD. CD4+CD25+ Tregs and NKT cells: regulators regulating regulators. Trends Immunol. 2006;27:322–7. doi: 10.1016/j.it.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Arrenberg P, Halder R, Dai Y, Maricic I, Kumar V. Oligoclonality and innate-like features in the TCR repertoire of type II NKT cells reactive to a β-linked self-glycolipid. Proc Natl Acad Sci USA. 2010;107:10984–9. doi: 10.1073/pnas.1000576107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhost S, Sedimbi S, Kadri N, Cardell SL. Immunomodulatory type II natural killer T lymphocytes in health and disease. Scand J Immunol. 2012;76:246–55. doi: 10.1111/j.1365-3083.2012.02750.x. [DOI] [PubMed] [Google Scholar]
- Arrenberg P, Halder R, Kumar V. Cross-regulation between distinct natural killer T cell subsets influences immune response to self and foreign antigens. J Cell Physiol. 2009;218:246–50. doi: 10.1002/jcp.21597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exley MA, Tahir SM, Cheng O, Shaulov A, Joyce R, Avigan D, et al. A major fraction of human bone marrow lymphocytes are Th2-like CD1d-reactive T cells that can suppress mixed lymphocyte responses. J Immunol. 2001;167:5531–4. doi: 10.4049/jimmunol.167.10.5531. [DOI] [PubMed] [Google Scholar]
- Jahng A, Maricic I, Aguilera C, Cardell S, Halder RC, Kumar V. Prevention of autoimmunity by targeting a distinct, noninvariant CD1d-reactive T cell population reactive to sulfatide. J Exp Med. 2004;199:947–57. doi: 10.1084/jem.20031389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhost S, Lofbom L, Rynmark BM, Pei B, Mansson JE, Teneberg S, et al. Identification of novel glycolipid ligands activating a sulfatide-reactive, CD1d-restricted, type II natural killer T lymphocyte. Eur J Immunol. 2012;42:2851–60. doi: 10.1002/eji.201142350. [DOI] [PubMed] [Google Scholar]
- Maricic I, Girardi E, Zajonc DM, Kumar V. Recognition of lysophosphatidylcholine by type II NKT cells and protection from an inflammatory liver disease. J Immunol. 2014;193:4580–9. doi: 10.4049/jimmunol.1400699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardi E, Maricic I, Wang J, Mac TT, Iyer P, Kumar V, et al. Type II natural killer T cells use features of both innate-like and conventional T cells to recognize sulfatide self antigens. Nat Immunol. 2012;13:851–6. doi: 10.1038/ni.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Weng X, Bagchi S, Wang CR. Polyclonal type II natural killer T cells require PLZF and SAP for their development and contribute to CpG-mediated antitumor response. Proc Natl Acad Sci USA. 2014;111:2674–9. doi: 10.1073/pnas.1323845111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V, Delovitch TL. Different subsets of natural killer T cells may vary in their roles in health and disease. Immunology. 2014;142:321–36. doi: 10.1111/imm.12247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosino E, Terabe M, Halder RC, Peng J, Takaku S, Miyake S, et al. Cross-regulation between type I and type II NKT cells in regulating tumor immunity: a new immunoregulatory axis. J Immunol. 2007;179:5126–36. doi: 10.4049/jimmunol.179.8.5126. [DOI] [PubMed] [Google Scholar]
- Halder RC, Aguilera C, Maricic I, Kumar V. Type II NKT cell-mediated anergy induction in type I NKT cells prevents inflammatory liver disease. J Clin Invest. 2007;117:2302–12. doi: 10.1172/JCI31602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald HR. CD1d-glycolipid tetramers: a new tool to monitor natural killer T cells in health and disease [comment] J Exp Med. 2000;192:F15–20. doi: 10.1084/jem.192.5.f15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uldrich AP, Patel O, Cameron G, Pellicci DG, Day EB, Sullivan LC, et al. A semi-invariant Vα10+ T cell antigen receptor defines a population of natural killer T cells with distinct glycolipid antigen-recognition properties. Nat Immunol. 2011;12:616–23. doi: 10.1038/ni.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendiratta SK, Martin WD, Hong S, Boesteanu A, Joyce S, Van Kaer L. CD1d1 mutant mice are deficient in natural T cells that promptly produce IL-4. Immunity. 1997;6:469–77. doi: 10.1016/s1074-7613(00)80290-3. [DOI] [PubMed] [Google Scholar]
- Smiley ST, Kaplan MH, Grusby MJ. Immunoglobulin E production in the absence of interleukin-4-secreting CD1-dependent cells. Science. 1997;275:977–9. doi: 10.1126/science.275.5302.977. [DOI] [PubMed] [Google Scholar]
- Chen YH, Chiu NM, Mandal M, Wang N, Wang CR. Impaired NK1+ T cell development and early IL-4 production in CD1-deficient mice. Immunity. 1997;6:459–67. doi: 10.1016/s1074-7613(00)80289-7. [DOI] [PubMed] [Google Scholar]
- Scanlon ST, Thomas SY, Ferreira CM, Bai L, Krausz T, Savage PB, et al. Airborne lipid antigens mobilize resident intravascular NKT cells to induce allergic airway inflammation. J Exp Med. 2011;208:2113–24. doi: 10.1084/jem.20110522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Shin T, Kawano T, Sato H, Kondo E, Toura I, et al. Requirement for Vα14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278:1623–6. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- Bedel R, Matsuda JL, Brigl M, White J, Kappler J, Marrack P, et al. Lower TCR repertoire diversity in Traj18-deficient mice. Nat Immunol. 2012;13:705–6. doi: 10.1038/ni.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendelac A, Hunziker RD, Lantz O. Increased interleukin 4 and immunoglobulin E production in transgenic mice overexpressing NK1 T cells. J Exp Med. 1996;184:1285–93. doi: 10.1084/jem.184.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capone M, Cantarella D, Schumann J, Naidenko OV, Garavaglia C, Beermann F, et al. Human invariant Vα24-JαQ TCR supports the development of CD1d-dependent NK1.1+ and NK1.1− T cells in transgenic mice. J Immunol. 2003;170:2390–8. doi: 10.4049/jimmunol.170.5.2390. [DOI] [PubMed] [Google Scholar]
- Wen X, Rao P, Carreno LJ, Kim S, Lawrenczyk A, Porcelli SA, et al. Human CD1d knock-in mouse model demonstrates potent antitumor potential of human CD1d-restricted invariant natural killer T cells. Proc Natl Acad Sci USA. 2013;110:2963–8. doi: 10.1073/pnas.1300200110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skold M, Faizunnessa NN, Wang CR, Cardell S. CD1d-specific NK1.1+ T cells with a transgenic variant TCR. J Immunol. 2000;165:168–74. doi: 10.4049/jimmunol.165.1.168. [DOI] [PubMed] [Google Scholar]
- Chen YH, Wang B, Chun T, Zhao L, Cardell S, Behar SM, et al. Expression of CD1d2 on thymocytes is not sufficient for the development of NK T cells in CD1d1-deficient mice. J Immunol. 1999;162:4560–6. [PubMed] [Google Scholar]
- Wei DG, Lee H, Park SH, Beaudoin L, Teyton L, Lehuen A, et al. Expansion and long-range differentiation of the NKT cell lineage in mice expressing CD1d exclusively on cortical thymocytes. J Exp Med. 2005;202:239–48. doi: 10.1084/jem.20050413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann J, Pittoni P, Tonti E, Macdonald HR, Dellabona P, Casorati G. Targeted expression of human CD1d in transgenic mice reveals independent roles for thymocytes and thymic APCs in positive and negative selection of Vα14i NKT cells. J Immunol. 2005;175:7303–10. doi: 10.4049/jimmunol.175.11.7303. [DOI] [PubMed] [Google Scholar]
- Yue SC, Shaulov A, Wang R, Balk SP, Exley MA. CD1d ligation on human monocytes directly signals rapid NF-κB activation and production of bioactive IL-12. Proc Natl Acad Sci USA. 2005;102:11811–6. doi: 10.1073/pnas.0503366102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illes Z, Kondo T, Newcombe J, Oka N, Tabira T, Yamamura T. Differential expression of NK T cell Va24JaQ invariant TCR chain in the lesions of multiple sclerosis and chronic inflammatory demyelinating polyneuropathy. J Immunol. 2000;164:4375–81. doi: 10.4049/jimmunol.164.8.4375. [DOI] [PubMed] [Google Scholar]
- van der Vliet HJ, von Blomberg BM, Nishi N, Reijm M, Voskuyl AE, van Bodegraven AA, et al. Circulating Vα24+ Vβ11+ NKT cell numbers are decreased in a wide variety of diseases that are characterized by autoreactive tissue damage. Clin Immunol. 2001;100:144–8. doi: 10.1006/clim.2001.5060. [DOI] [PubMed] [Google Scholar]
- Gigli G, Caielli S, Cutuli D, Falcone M. Innate immunity modulates autoimmunity: type 1 interferon-β treatment in multiple sclerosis promotes growth and function of regulatory invariant natural killer T cells through dendritic cell maturation. Immunology. 2007;122:409–17. doi: 10.1111/j.1365-2567.2007.02655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki M, Kondo T, Gumperz JE, Brenner MB, Miyake S, Yamamura T. Th2 bias of CD4+ NKT cells derived from multiple sclerosis in remission. Int Immunol. 2003;15:279–88. doi: 10.1093/intimm/dxg029. [DOI] [PubMed] [Google Scholar]
- Sakuishi K, Miyake S, Yamamura T. Role of NK cells and invariant NKT cells in multiple sclerosis. Results Probl Cell Differ. 2010;51:127–47. doi: 10.1007/400_2009_11. [DOI] [PubMed] [Google Scholar]
- Shamshiev A, Donda A, Carena I, Mori L, Kappos L, De Libero G. Self glycolipids as T-cell autoantigens. Eur J Immunol. 1999;29:1667–75. doi: 10.1002/(SICI)1521-4141(199905)29:05<1667::AID-IMMU1667>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Baxter AG. The origin and application of experimental autoimmune encephalomyelitis. Nat Rev Immunol. 2007;7:904–12. doi: 10.1038/nri2190. [DOI] [PubMed] [Google Scholar]
- McCarthy DP, Richards MH, Miller SD. Mouse models of multiple sclerosis: experimental autoimmune encephalomyelitis and Theiler’s virus-induced demyelinating disease. Methods Mol Biol. 2012;900:381–401. doi: 10.1007/978-1-60761-720-4_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine S, Sowinski R. Experimental allergic encephalomyelitis in inbred and outbred mice. J Immunol. 1973;110:139–43. [PubMed] [Google Scholar]
- Yoshimoto T, Bendelac A, Hu-Li J, Paul WE. Defective IgE production by SJL mice is linked to the absence of CD4+, NK1.1+ T cells that promptly produce interleukin 4. Proc Natl Acad Sci USA. 1995;92:11931–4. doi: 10.1073/pnas.92.25.11931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh AK, Yang J-Q, Parekh VV, Wei J, Wang C-R, Joyce S, et al. The natural killer T cell ligand a-galactosylceramide prevents or promotes pristane-induced lupus in mice. Eur J Immunol. 2005;35:1143–54. doi: 10.1002/eji.200425861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahng AW, Maricic I, Pedersen B, Burdin N, Naidenko O, Kronenberg M, et al. Activation of natural killer T cells potentiates or prevents experimental autoimmune encephalomyelitis. J Exp Med. 2001;194:1789–99. doi: 10.1084/jem.194.12.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlan R, Bergami A, Cantarella D, Brambilla E, Taniguchi M, Dellabona P, et al. Activation of invariant NKT cells by a-GalCer administration protects mice from MOG35-55 induced EAE: critical roles for administration route and IFN-γ. Eur J Immunol. 2003;33:1830–8. doi: 10.1002/eji.200323885. [DOI] [PubMed] [Google Scholar]
- Denney L, Kok WL, Cole SL, Sanderson S, McMichael AJ, Ho LP. Activation of invariant NKT cells in early phase of experimental autoimmune encephalomyelitis results in differentiation of Ly6Chi inflammatory monocyte to M2 macrophages and improved outcome. J Immunol. 2012;189:551–7. doi: 10.4049/jimmunol.1103608. [DOI] [PubMed] [Google Scholar]
- Singh AK, Wilson MT, Hong S, Olivares-Villagomez D, Du C, Stanic AK, et al. Natural killer T cell activation protects mice against experimental autoimmune encephalomyelitis. J Exp Med. 2001;194:1801–11. doi: 10.1084/jem.194.12.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teige A, Teige I, Lavasani S, Bockermann R, Mondoc E, Homdahl R, et al. CD1-dependent regulation of chronic central nervous system inflammation in experimental autoimmune encephalomyelitis. J Immunol. 2004;172:186–94. doi: 10.4049/jimmunol.172.1.186. [DOI] [PubMed] [Google Scholar]
- Mars LT, Laloux V, Goude K, Desbois S, Saoudi A, Van Kaer L, et al. Cutting edge: Vα14-Jα281 NKT cells naturally regulate experimental autoimmune encephalomyelitis in nonobese diabetic mice. J Immunol. 2002;168:6007–11. doi: 10.4049/jimmunol.168.12.6007. [DOI] [PubMed] [Google Scholar]
- Mars LT, Gautron AS, Novak J, Beaudoin L, Diana J, Liblau RS, et al. Invariant NKT cells regulate experimental autoimmune encephalomyelitis and infiltrate the central nervous system in a CD1d-independent manner. J Immunol. 2008;181:2321–9. doi: 10.4049/jimmunol.181.4.2321. [DOI] [PubMed] [Google Scholar]
- Kawai E, Sato F, Omura S, Martinez NE, Reddy PC, Taniguchi M, et al. Organ-specific protective role of NKT cells in virus-induced inflammatory demyelination and myocarditis depends on mouse strain. J Neuroimmunol. 2015;278:174–84. doi: 10.1016/j.jneuroim.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokote H, Miyake S, Croxford JL, Oki S, Mizusawa H, Yamamura T. NKT cell-dependent amelioration of a mouse model of multiple sclerosis by altering gut flora. Am J Pathol. 2008;173:1714–23. doi: 10.2353/ajpath.2008.080622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh VV, Singh AK, Wilson MT, Olivares-Villagomez D, Bezbradica JS, Inazawa H, et al. Quantitative and qualitative differences in the in vivo response of NKT cells to distinct α- and β-anomeric glycolipids. J Immunol. 2004;173:3693–706. doi: 10.4049/jimmunol.173.6.3693. [DOI] [PubMed] [Google Scholar]
- Qian G, Qin X, Zang YQ, Ge B, Guo TB, Wan B, et al. High doses of α-galactosylceramide potentiate experimental autoimmune encephalomyelitis by directly enhancing Th17 response. Cell Res. 2010;20:480–91. doi: 10.1038/cr.2010.6. [DOI] [PubMed] [Google Scholar]
- Mars LT, Araujo L, Kerschen P, Diem S, Bourgeois E, Van LP, et al. Invariant NKT cells inhibit development of the Th17 lineage. Proc Natl Acad Sci USA. 2009;106:6238–43. doi: 10.1073/pnas.0809317106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal E, Tabira T, Kawano T, Taniguchi M, Miyake S, Yamamura T. Costimulation-dependent modulation of experimental autoimmune encephalomyelitis by ligand stimulation of Vα14 NK T cells. J Immunol. 2001;166:662–8. doi: 10.4049/jimmunol.166.1.662. [DOI] [PubMed] [Google Scholar]
- Miyamoto K, Miyake S, Yamamura T. A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing TH2 bias of natural killer T cells. Nature. 2001;413:531–4. doi: 10.1038/35097097. [DOI] [PubMed] [Google Scholar]
- Shiozaki M, Tashiro T, Koshino H, Shigeura T, Watarai H, Taniguchi M, et al. Synthesis and biological activity of hydroxylated analogues of KRN7000 (α-galactosylceramide) Carbohydr Res. 2013;370:46–66. doi: 10.1016/j.carres.2013.01.010. [DOI] [PubMed] [Google Scholar]
- Oh SJ, Chung DH. Invariant NKT cells producing IL-4 or IL-10, but not IFN-γ, inhibit the Th1 response in experimental autoimmune encephalomyelitis, whereas none of these cells inhibits the Th17 response. J Immunol. 2011;186:6815–21. doi: 10.4049/jimmunol.1003916. [DOI] [PubMed] [Google Scholar]
- Kojo S, Seino K-I, Harada M, Watarai H, Wakao H, Uchida T, et al. Induction of regulatory properties in dendritic cells by Vα14 NKT cells. J Immunol. 2005;175:3648–55. doi: 10.4049/jimmunol.175.6.3648. [DOI] [PubMed] [Google Scholar]
- Parekh VV, Wu L, Olivares-Villagomez D, Wilson KT, Van Kaer L. Activated invariant NKT cells control central nervous system autoimmunity in a mechanism that involves myeloid-derived suppressor cells. J Immunol. 2013;190:1948–60. doi: 10.4049/jimmunol.1201718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Cho S, Ueno A, Cheng L, Xu BY, Desrosiers MD, et al. Ligand-dependent induction of noninflammatory dendritic cells by anergic invariant NKT cells minimizes autoimmune inflammation. J Immunol. 2008;181:2438–45. doi: 10.4049/jimmunol.181.4.2438. [DOI] [PubMed] [Google Scholar]
- Naumov YN, Bahjat KS, Gausling R, Abraham R, Exley MA, Koezuka Y, et al. Activation of CD1d-restricted T cells protects NOD mice from developing diabetes by regulating dendritic cell subsets. Proc Natl Acad Sci USA. 2001;98:13838–43. doi: 10.1073/pnas.251531798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condamine T, Gabrilovich DI. Molecular mechanisms regulating myeloid-derived suppressor cell differentiation and function. Trends Immunol. 2011;32:19–25. doi: 10.1016/j.it.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly D, Mi QS, Hussain S, Delovitch TL. Protection from type 1 diabetes by invariant NK T cells requires the activity of CD4+ CD25+ regulatory T cells. J Immunol. 2006;177:3695–704. doi: 10.4049/jimmunol.177.6.3695. [DOI] [PubMed] [Google Scholar]
- Liu R, La Cava A, Bai X-F, Jee Y-H, Price M, Campagnolo DI, et al. Cooperation of invariant NKT cells and CD4+ CD25+ regulatory T cells in the prevention of autoimmune myasthenia. J Immunol. 2005;175:7898–904. doi: 10.4049/jimmunol.175.12.7898. [DOI] [PubMed] [Google Scholar]
- Maldonado RA, von Andrian UH. How tolerogenic dendritic cells induce regulatory T cells. Adv Immunol. 2010;108:111–65. doi: 10.1016/B978-0-12-380995-7.00004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, et al. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66:1123–31. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- Maricic I, Halder R, Bischof F, Kumar V. Dendritic cells and anergic type I NKT cells play a crucial role in sulfatide-mediated immune regulation in experimental autoimmune encephalomyelitis. J Immunol. 2014;193:1035–46. doi: 10.4049/jimmunol.1302898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brossay L, Chioda M, Burdin N, Koezuka Y, Casorati G, Dellabona P, et al. CD1d-mediated recognition of an α-galactosylceramide by natural killer T cells is highly conserved through mammalian evolution. J Exp Med. 1998;188:1521–8. doi: 10.1084/jem.188.8.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spada FM, Koezuka Y, Porcelli SA. CD1d-restricted recognition of synthetic glycolipid antigens by human natural killer T cells. J Exp Med. 1998;188:1529–34. doi: 10.1084/jem.188.8.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii SI, Shimizu K, Okamoto Y, Kunii N, Nakayama T, Motohashi S, et al. NKT cells as an ideal anti-tumor immunotherapeutic. Front Immunol. 2013;4:409. doi: 10.3389/fimmu.2013.00409. [DOI] [PMC free article] [PubMed] [Google Scholar]


