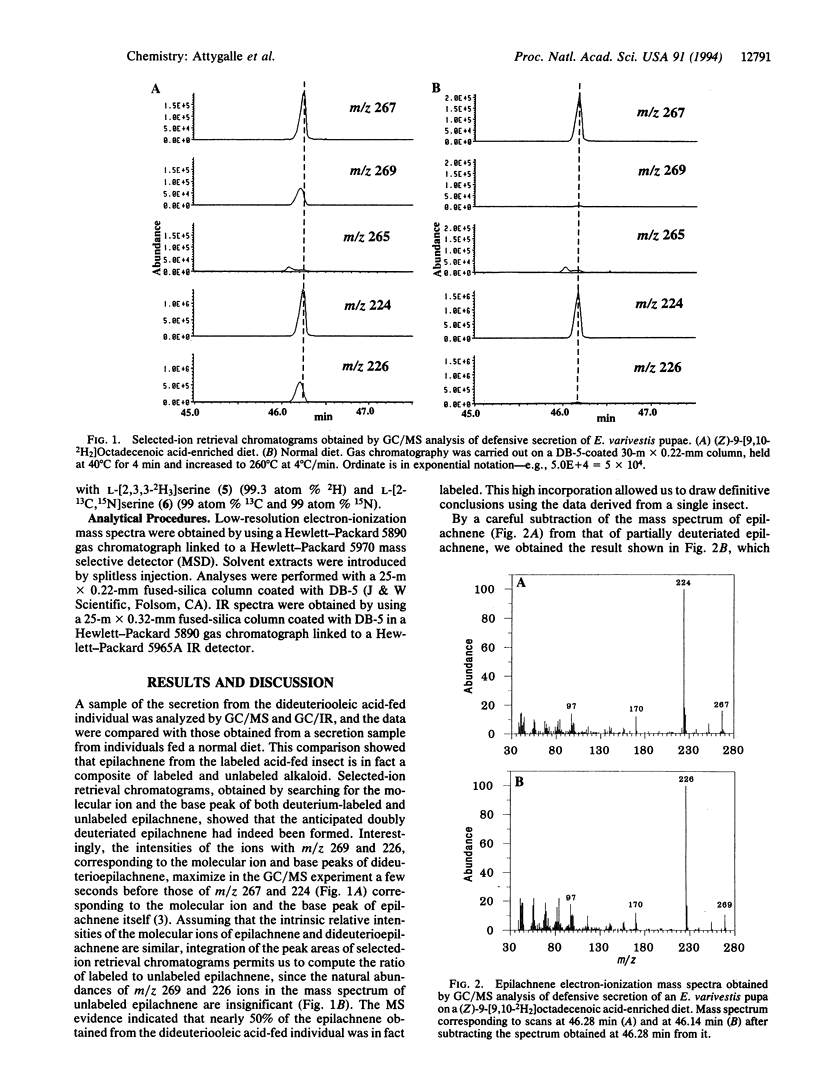
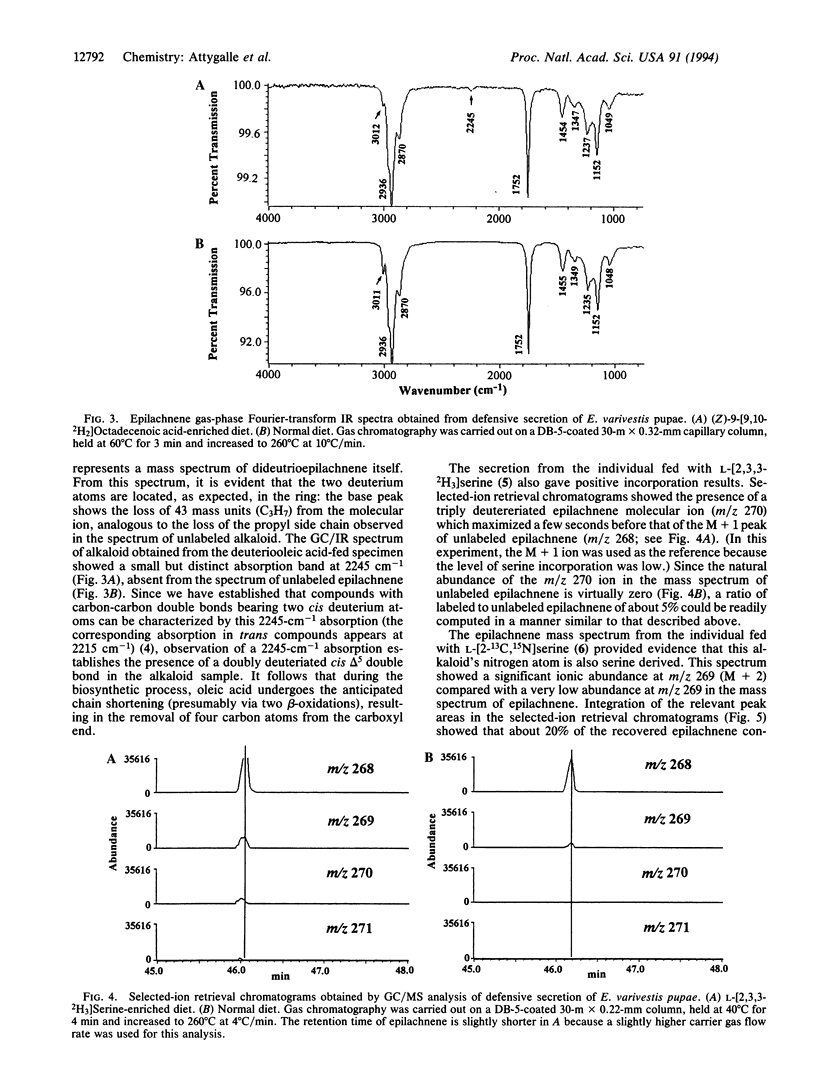
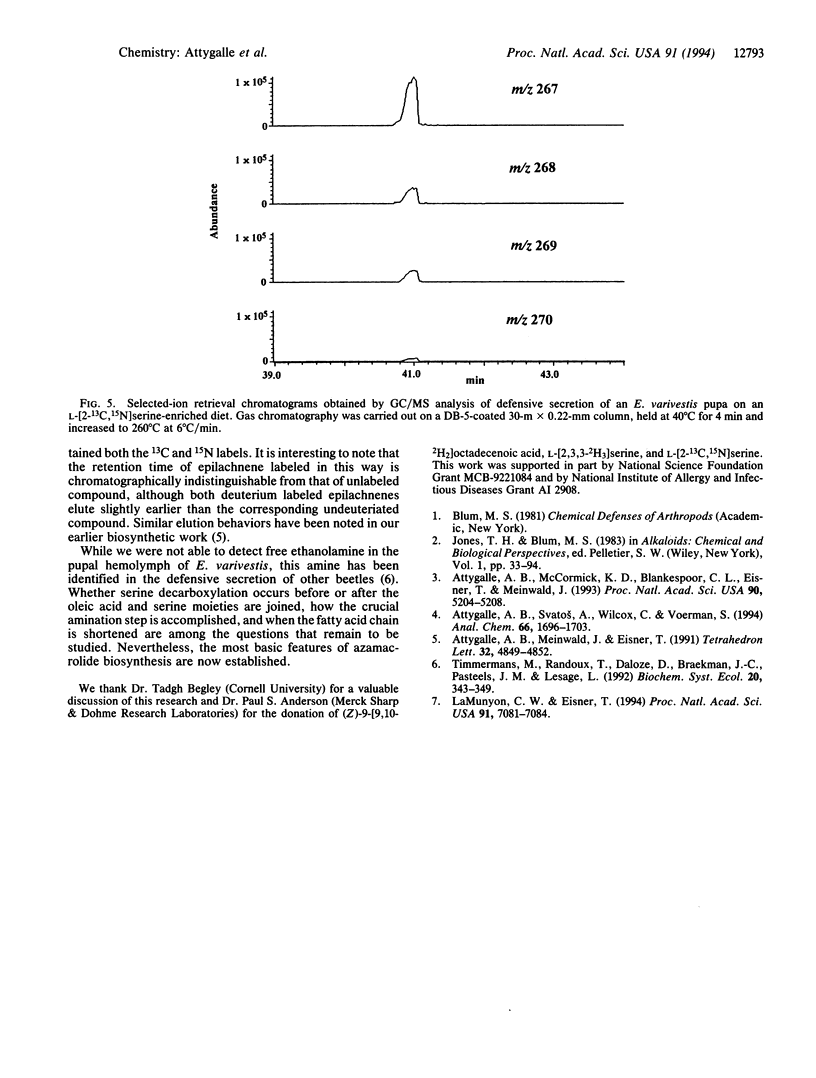
Abstract
The biosynthesis of the azamacrolide epilachnene by the coccinellid beetle Epilachna varivestis has been studied with 2H-labeled oleic acid, 2H-labeled L-serine, and 13C,15N-labeled L-serine. The incorporation of these precursors into epilachnene defines the origin of the alkaloid's entire carbon/nitrogen skeleton. GC/MS and GC/IR studies of alkaloid produced by Epilachna fed with deuteriated oleic acid show that oleic acid loses four carbon atoms from its carboxyl end during the biosynthesis. Other details, including the mechanism of carbon-nitrogen bond formation between the fatty acid and amino acid moieties, remain to be established.
Full text
PDF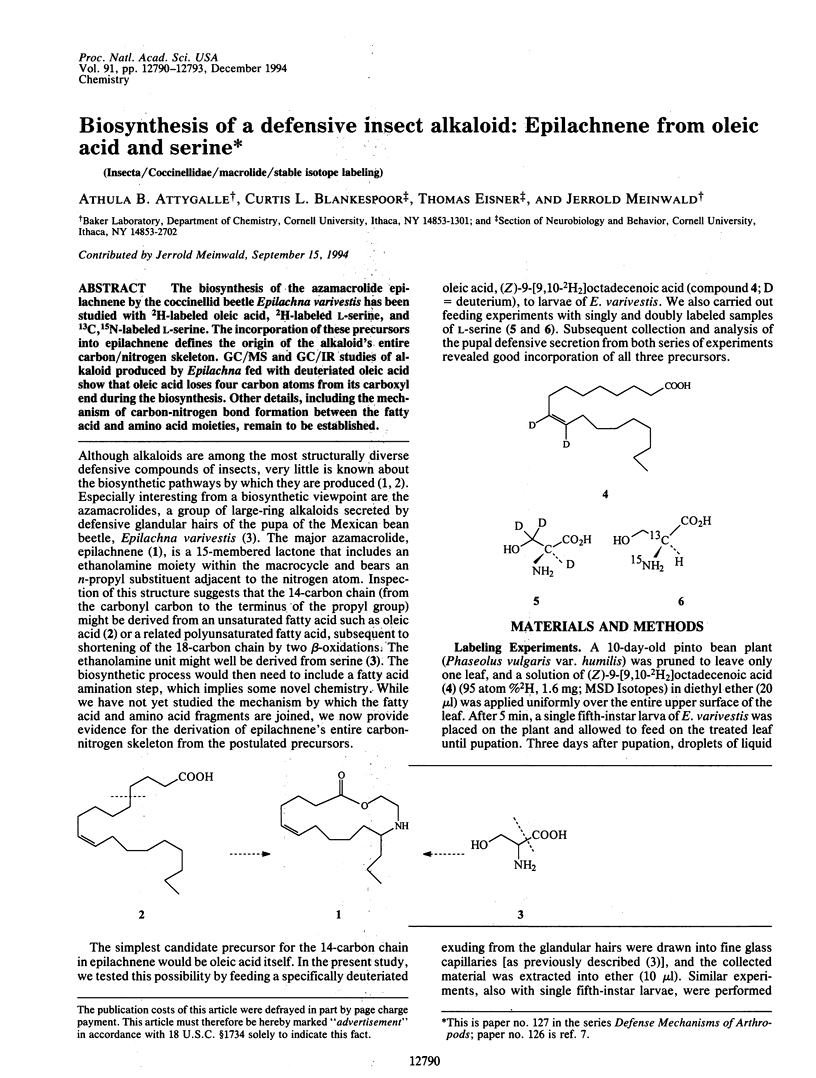
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attygalle A. B., McCormick K. D., Blankespoor C. L., Eisner T., Meinwald J. Azamacrolides: a family of alkaloids from the pupal defensive secretion of a ladybird beetle (Epilachna varivestis). Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5204–5208. doi: 10.1073/pnas.90.11.5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attygalle A. B., Svatos A., Wilcox C., Voerman S. Gas-phase infrared spectroscopy for determination of double bond configuration of monounsaturated compounds. Anal Chem. 1994 May 15;66(10):1696–1703. doi: 10.1021/ac00082a016. [DOI] [PubMed] [Google Scholar]
- LaMunyon C. W., Eisner T. Spermatophore size as determinant of paternity in an arctiid moth (Utetheisa ornatrix). Proc Natl Acad Sci U S A. 1994 Jul 19;91(15):7081–7084. doi: 10.1073/pnas.91.15.7081. [DOI] [PMC free article] [PubMed] [Google Scholar]



