Abstract
After immune interactions, membrane fragments can be transferred between cells. This fast transfer of molecules is transient and shows selectivity for certain proteins; however, the constraints underlying acquisition of a protein are unknown. To characterize the mechanism and functional consequences of this process in natural killer (NK) cells, we have compared the transfer of different NKG2D ligands. We show that human NKG2D ligands can be acquired by NK cells with different efficiencies. The main findings are that NKG2D ligand transfer is related to immune activation and receptor–ligand interaction and that NK cells acquire these proteins during interactions with target cells that lead to degranulation. Our results further demonstrate that NK cells that have acquired NKG2D ligands can stimulate activation of autologous NK cells. Surprisingly, NK cells can also re-transfer the acquired molecule to autologous effector cells during this immune recognition that leads to their death. These data demonstrate that transfer of molecules occurs as a consequence of immune recognition and imply that this process might play a role in homeostatic tuning-down of the immune response or be used as marker of interaction.
Keywords: glycosyl-phosphatidyl-inositol-anchored proteins, human natural killer cells, immune synapse, NKG2D, trogocytosis
Introduction
Transfer of molecules after cell–cell contact has been described in the last few years and its consequences are an active matter of debate. The term trogocytosis has been adopted to describe the fast transfer of membrane fragments from a donor cell resulting in the acquisition of proteins by an acceptor cell.1 Many of the previously described instances of protein transfer involve cells participating in the immune response, such as from antigen-presenting cells to T or B cells.2,3 In particular in T cells, trogocytosis has been reported to require active signalling via T-cell receptors and to cause internalization of the receptor.4,5 In natural killer (NK) cells, trogocytosis was first reported in a murine system, in which NK cells that had acquired MHC-I showed reduced cytotoxicity.6 The ability of human NK cells to acquire membrane fragments was demonstrated later7 and since then, several activating and inhibitory receptor–ligand systems have been shown to be transferred from and to NK cells, for example, during NK inhibitory interactions, both HLA-C and the receptor KIR2DL1 can be transferred from targets to NK cells and vice versa, respectively.8–10 Natural-killer-activating interactions result also in transfer of proteins, such as CD96-mediated acquisition of poliovirus receptor (CD155)11 and 2B4-GFP transfer from NK cell membrane connective structures to susceptible target cells.12 MHC-II acquisition by murine NK cells has also been reported to regulate CD4+ T cells.13
NKG2D is a potent activating receptor expressed by all human NK cells and CD8+ T cells that recognizes two families of ligands: major histocompatibility complex class I-related chain (MICA/B) and UL16 binding proteins (ULBP1–6) (for review see refs 14,15). These two MHC-related families are encoded within two different regions of chromosome 6 and share two biochemical features: some NKG2D ligands (NKG2D-L) are transmembrane proteins while others are anchored via glycosyl-phosphatidyl-inositol (GPI).16,17 NKG2D-mediated recognition is affected by many viral escape mechanisms, confirming the importance of this system for modulation of immune responses.18 In fact, NKG2D has been reported to mediate elimination of both cancer cells and virus-infected cells.19
Previous work has demonstrated the transfer of MICB and MICA from target cells to human NK cells20,21 and the transfer of murine NKG2D-L;22 however, the transfer of human GPI-anchored NKG2D-L has not been previously explored. Recent reports have described several functional aspects of trogocytosis, but the mechanism of capture, the fate of the transferred proteins and the physiological role of this phenomenon are still unclear. With the aim of investigating in more detail the characteristics and significance of the transfer of proteins from target cells to effector cells, we have analysed the transfer of NKG2D-L to effector cells and explored the functional consequences using a model with human primary NK cells. As the direction of transfer depends on the nature of both the donor and acceptor cells,23,24 here we have extensively characterized the kinetics of ULBP transfer, comparing different target and effector cells: we have used a panel of donor cells transfected with several NKG2D-L and confirmed the data using cells that express these same proteins endogenously. We also compared resting with interleukin-2 (IL-2) -activated NK cells as well as NKG2D+ T cells. We show that the transfer of NKG2D-L does not depend on the membrane anchor type (GPI versus transmembrane), instead, it depends on the activation status of the NK cell and on receptor–ligand interaction and can be modulated by other cell processes such as metalloprotease cleavage of the ligand. Further, the amount of protein transferred is related to the formation of a productive immune synapse and effector cell degranulation. Strikingly, transferred NKG2D-L can stimulate cytotoxicity by autologous NK cells and the acquired protein can then be re-transferred during the interaction with this second set of effector NK cells, suggesting that this process could play a role in the regulation of the immune response.
Materials and methods
Reagents
Reagents were purchased from Sigma (St Louis, MO), unless otherwise stated. BB94 (Batimastat) was from Tocris Bioscience (Bristol, UK).
Cell lines
Chinese hamster ovary (CHO) cells transfected with NKG2D-L were previously reported25,26 and grown in Ham’s F12 medium, supplemented with 10% fetal calf serum, 1 mm glutamine and 1 mm sodium pyruvate, 0·1 mm non-essential amino acids, 100 U/ml penicillin, 100 U/ml streptomycin (Biowest, Kansas City, MO). U373 cells have been described27 and were grown in complete DMEM medium (Lonza, Basel, Switzerland). Bladder cancer cell lines (J82, RT-112) were obtained from F.X. Real (CNIO, Madrid, Spain), genotyped by the StemElite ID System (Promega, Madison, WI) at the Genomics Service (IIB-CSIC), and grown in complete Eagle’s minimal essential medium (Lonza, Basel, Switzerland) with 2·5 μg/ml amphotericin B (Lonza). K562, Daudi and RPMI-8866 cells were grown in complete RPMI-1640 medium (Lonza).
Human NK cells
Polyclonal NK cell lines were prepared as previously described.20 Briefly, peripheral blood mononuclear cells were isolated by centrifugation on Ficoll–HyPaque from healthy donor buffy coats [(Transplant Centre, Madrid, Spain) approval from local ethics committees (Transplant Centre and CSIC-UAM Committees) and informed consent from all participants were obtained; experiments were conducted according to the principles expressed in the Declaration of Helsinki]. NK cells were isolated by negative selection using the MACS NK Cell Isolation Kit (Miltenyi Biotec, Bergisch Gladbach, Germany). NK cells were cultured in the presence of irradiated feeder cells (autologous peripheral blood mononuclear cells, Daudi and RPMI-8866 B-cell lines) in RPMI-1640 medium (Lonza) supplemented with 10% fetal bovine serum, 10% male AB-negative human serum (the first week, and later with 5% fetal bovine serum, 5% human serum), 4 mm l-glutamine, 0·1 mm non-essential amino acids, 1 mm sodium pyruvate, 100 U/ml penicillin, 100 U/ml streptomycin, 10 mm HEPES, 50 μm β-mercaptoethanol (Biowest) and 50 U/ml recombinant human lL-2 (Peprotech, Rocky Hill, NJ). NK cell cultures that were > 95% pure were re-stimulated weekly and the NK phenotype was monitored by flow cytometry. Functional experiments with NK cells were performed 4–5 days after IL-2 stimulation. For experiments using freshly isolated NK cells, cells were purified by negative selection and cultured for 16 hr in the presence or absence of IL-2 (50 U/ml).
Co-culture experiments for analysis of transfer
Target cells were allowed to reach confluency and mixed with NK cells at an effector : target (E : T) ratio of 1 : 3. NK cells and target cells were co-incubated for 5 min to 16 hr as indicated in each experiment. NK cells were recovered from co-culture supernatant for subsequent experiments. For transwell experiments, Transwell inserts (Corning, Schiphol-Rijkk, the Netherlands) 0·4-μm pore size were used. NK cells were cultured in the upper chamber separated from CHO-ULBP1/2/3 cells by the semi-permeable membrane. For phosphoinositide-specific phospholipase C (PI-PLC; Invitrogen, Carlsbad, CA) digestion, cells were washed with PBS, and resuspended in buffer (Tris–HCl 10 mm, NaCl 150 mm pH 7·4) containing PI-PLC (0·1 enzyme units/105 cells). After 30 min at 37°C, treatment was stopped by washing cells with PBS containing 1% BSA, 0·1% sodium azide (PBA). For acid wash treatment, NK cells were recovered from the co-culture, washed twice in PBS and resuspended for 4 min at room temperature in citrate buffer (0·133 m citric acid and 0·066 m Na2HPO4 pH 3·3). Treatment was stopped by addition of PBS/5% FBS and washing. Loss of binding of the conformation-dependent human MHC class I specific antibody (HP-1F728) was used to check efficiency of the acid treatment. The viability of the cells after acid wash was checked by propidium iodide staining. For NKG2D blocking, Clone 149810 from R&D Systems (Abingdon, UK) was used. In experiments with CD3+ cells, polyclonal populations of T cells cultured with IL-2 were used as effectors.
Flow cytometry
Natural killer cells recovered from transfer experiments were pre-incubated in PBA with 10% human serum. Cells were then incubated with the appropriate antibodies: monoclonal antibodies specific for ULBPs were purchased from R&D Systems; monoclonal anti-Flag was from Sigma; conjugated antibodies were from Biolegend (Cambridge, UK) and Immunotools (Friesoythe, Germany); 1H10 antibody specific for MICA was described before29 and, when necessary, bound antibody was visualized using either phycoerythrin- or FITC-labelled F(ab’)2 fragments of goat anti-mouse immunoglobulin (Dako, Glostrup, Denmark). Samples were analysed using either FACScalibur, Gallios (Becton Dickinson, Franklin Lakes, NJ) or FC500 (Beckman Coulter, Brea, CA) flow cytometers. Where necessary, dead cells were excluded by staining with propidium iodide. Analysis of the experiments was performed using the Kaluza, Summit (Beckman coulter) or FlowJo (Tree Star, Inc, Ashland, OR) programs.
Immunoprecipitation and Western blot
NKG2D immunoprecipitation was performed as described previously.30 Briefly, 2 × 107cells were lysed in 0·5% Triton X-100 for 30 min at 4°. Nuclei were removed by centrifugation at 13 000 g and, after preclearing with Pansorbin (Calbiochem, Darmstadt, Germany), the lysates were divided into aliquots for immunoprecipitation with either NKG2D antibody (clone 5C6; eBioscience, San Diego, CA) or immunoglobulin control. Incubation with Protein G-coupled beads was carried out for 16 hr at 4°C. After washing, aliquots were run on SDS–PAGE (10–12%). Proteins were transferred on to Immobilon-P (Millipore, Billerica, MA) membrane. The membrane was blocked using PBS containing 0·1% Tween-20 and 5% non-fat dried skimmed milk powder. Detection of ULBP was performed by incubation with biotinylated-goat polyclonal antibody (R&D Systems), followed by horseradish peroxidase-conjugated streptavidin. NKG2D was detected as control with clone 1D11 (Santa Cruz Biotechnology, Santa Cruz, CA), followed by horseradish peroxidase-conjugated secondary antibody. Proteins were visualized using the ECL system (GE Healthcare, Chalfont St Giles, UK).
Degranulation and cytotoxicity experiments
Natural killer cells were co-cultured with target cells for 2 hr at an E : T ratio of 1 : 3. Surface expression of LAMP1 (CD107a) was analysed by flow cytometry. For cytotoxicity experiments, NK cells that had been in co-culture, either with targets or with medium (to evaluate spontaneous death), were labelled with 0·2 μm PKH2 and used as target cells. Autologous NK cells were used as effector cells at an E : T ratio of 3 : 1. K562 cells were used as positive control targets. Cell death was calculated by the analysis of 7-aminoactinomycin D staining.
NK cell sorting
Natural killer cells recovered after co-culture with CHO-ULBP3 cells, were resuspended in PBS 5 mm EDTA and separated from the contaminating CHO-ULBP3 cells by cell sorting (Moflow XDP, Beckman Coulter). The sorted NK cells, used as target cells, were cultured with autologous NK cells, labelled with 2 μm CellTrace™ Violet Cell Proliferation Kit (Molecular Probes, Eugene, OR) at an E : T ratio of 1 : 5 for 1 hr. ULBP3 expression was analysed by flow cytometry in effector and target NK cells before and after their co-culture.
Results
NKG2D-L are transferred from target cells to NK cells by trogocytosis
To compare the transfer of the different types of NKG2D-L after cell–cell contact, primary NK cells were co-cultured with CHO cells expressing ULBP1, ULBP2 or ULBP3. The three ULBPs were transferred to the effector cell as soon as 30 min after co-incubation (Fig.1a,b) (see Supplementary material, Fig. S1a, for full gating and analysis strategy; and Fig. S1b for ULBP expression on transfectants). Incubation with control parental CHO cells was included in all the experiments reported in this paper, but as it was always negative for ULBP transfer, as in Fig.1(a), this control is not shown in the remaining figures. Different transfer percentages were obtained because of donor to donor variation; however, as shown in Fig.1(b), where 11 experiments with different donors are summarized, the pattern of percentages for ULBP1 compared with ULBP2 and ULBP3 transfer was maintained in all cases. The transfer of NKG2D-L required cell–cell contact, as demonstrated in transwell experiments (Fig.1c), and resulted in a stable insertion of proteins in the plasma membrane in a native orientation, as GPI proteins could be removed by PI-PLC digestion, but not by acid treatment (Fig.1d,e), which dissociates charge-based interactions.31 Although the percentage of ULBP-positive cells remained unchanged after acid treatment, NKG2D expression decreased (data not shown). These data suggest that NKG2D-L were not non-specifically bound to the plasma membrane of the acceptor cell, but rather were correctly anchored to the membrane. In addition, these data imply that receptor and ligand are not interacting through the recognition interface. Moreover, only the ULBP transfected in the donor cell was ever found at the NK cell surface in each experiment, i.e. no ULBP2 was detected on NK cells when the target was ULBP1 and the same was true for other ULBPs, arguing against the possibility of de novo ULBP expression by NK cells (see Supplementary material, Fig. S1c). This option was further excluded in experiments detecting the FLAG-tag present in our constructs on the NK cell surface (see Supplementary material, Fig. S1d,e). Altogether, these data demonstrate that NKG2D-L are quickly transferred from donor to acceptor cells, in a cell–cell contact-dependent manner and in the correct orientation, features that correspond to the phenomenon known as trogocytosis.1
Figure 1.
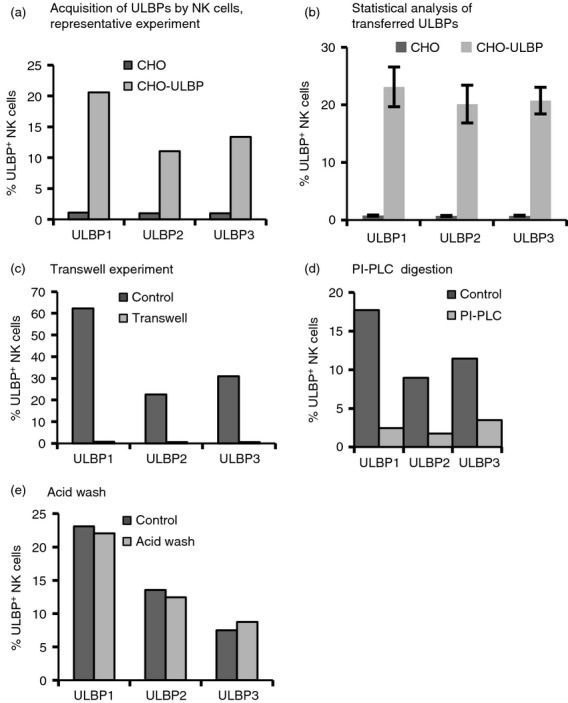
NKG2D-ligands are transferred to natural killer (NK) cells by trogocytosis. Primary NK cells were co-incubated with either Chinese hamster ovary (CHO) parental cells or UL16 binding protein (ULBP) transfectants for 30 min. Staining for NKG2D-L was analysed in the CD56+ gate, following the gating strategy explained in the Supplementary material (Fig. S1a). The percentage of ULBP+ NK cells is represented in bar graphs. (a) ULBP1, 2 and 3 are acquired by NK cells. A representative experiment is presented. (b) Statistical analysis of ULBP transfer to NK cells after 30 min co-culture with CHO-ULBP transfectants. The figure shows the mean and standard deviation data from 11 experiments. The percentage of ULBP-positive NK cells is depicted. (c) NKG2D-L transfer requires cell–cell contact. Cells were co-incubated in the presence and absence of cell contact in a transwell. (d) NKG2D-L are transferred to NK cells as full proteins in the correct orientation. After co-incubation, transferred glycosyl-phosphatidyl-inositol (GPI)-anchored proteins were digested with phosphoinositide-specific phospholipase C (PI-PLC) for 30 min or PI-PLC buffer for negative control. (e) Transferred NK ligands are inserted in the membrane. After co-incubation, cells were transiently washed with a pH 3·3 buffer, which can unfold proteins but does not extract proteins inserted in the membrane. In (b), (c) and (d), one representative experiment, out of three, are depicted.
NKG2D-L can be transferred with different kinetics and efficiency
To further characterize the transfer process, the kinetics of transfer of different NKG2D-L and the influence of the type of membrane anchor were compared in time–course experiments (Fig.2a): the transfer was very fast, 5 min being sufficient to observe that 10% of NK cells had received ULBPs. In general, more protein accumulated when the effector and target cells were in contact for longer times, reaching a maximum at 6 hr. After 18 hr, the amount of transferred protein started to decrease. The loss of transferred ULBP from NK cells could be the result of internalization of the protein or of shedding; however, it is not possible to distinguish between these two possibilities in the experiment shown. Another striking fact is that the transfer of ULBP1, -2 and -3 followed reproducibly different patterns: ULBP2 generally was transferred at lower amounts and reached a plateau sooner than ULBP1 and ULPB3. That ULBP2 was transferred with lower efficiency was also observed using anti-FLAG antibody (see Supplementary material, Fig. S1e), confirming that the differences were not due to differences in the affinities of the ULBP-specific antibodies. However, although this different pattern could be observed in various experiments, the differences did not achieve statistical significance. Hence, several experiments were performed to study these observations in more detail. First, ULBP transfer was compared with that of other NKG2D-L such as MICA. As shown in Fig.2(b), the type of membrane anchor is not a factor that enhances or limits NKG2D-L transfer. These observations were confirmed in experiments performed with other MICA alleles and MICB, which also transferred to NK cells (not shown). Second, experiments with different cell lines endogenously expressing different amounts of NKG2D-L were carried out. Two bladder cancer cell lines and U373 cells expressing different amounts of different NKG2D-L were used as donor cells (Fig.2c, d). Transfer of endogenous MICA, ULBP2 and ULBP3 from bladder cancer cells to NK cells could be detected, although lower levels of endogenous protein expression are associated with less transfer than that observed with CHO cells over-expressing the ULBPs. Even though U373 cells expressed lower amounts of endogenous ULBP3 than ULBP2, ULBP3 was transferred more efficiently than ULBP2, and this also happened with CHO transfectants. This indicates that, as previously reported for other proteins in T or B cells,24 the ability of different proteins to transfer to NK cells depends on the identity of the particular protein. It is interesting to note that this is yet another example in which different ULBPs behave differently, as has already been reported for many other aspects of their biology.15 As ULBP2 is more sensitive to metalloprotease shedding, whereas ULBP3 is preferentially released in exosomes,25,32 we hypothesized that susceptibility to proteolytic cleavage could influence the differences in transfer. To test this hypothesis, a metalloprotease-inhibition experiment was performed. As shown in Fig.2(e, f), more ULBP2 was detected on the NK cell membrane in the presence of the metalloprotease inhibitor BB94. This result suggests that the lower detection of transferred ULBP2 on the NK cells could be due to loss of ULBP2 expression as a consequence of the proteolytic process. However, it is not possible to know from this experiment whether cleavage occurs at the surfaces of the donor or acceptor cells. Altogether, these data show that the different NKG2D-L can be transferred to the effector cell depending on a combination of their intrinsic biochemical features, susceptibility to metalloprotease cleavage and their abundance at the plasma membrane of the donor cell. All of these different factors – efficiency of transfer, shedding and half-life in the acceptor cell – result in different amounts of ULBPs at the surface of NK cells after their encounter with a target cell.
Figure 2.
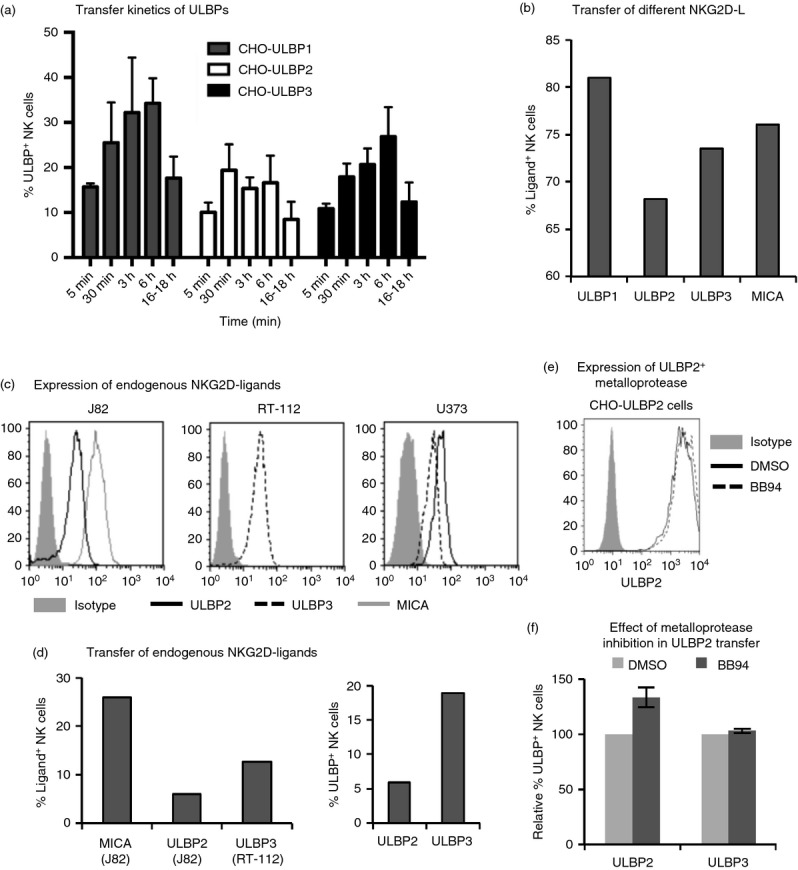
Transfer of different NKG2D-ligands to natural killer (NK) cells. (a) Kinetics of transfer of UL16 binding proteins (ULBP1, 2 and 3). NK cells were co-incubated as in Fig.1 with ULBP transfected Chinese hamster ovary (CHO) cells for the indicated times. The graph depicts the average and SEM of the percentage of ULBP-positive NK cells from three experiments performed with three different donors. (b) Transfer of glycosyl-phosphatidyl-inositol (GPI) -anchored and transmembrane NKG2D-L. NK cells were co-incubated with the indicated CHO transfectants (ULBP1–3 and MICA*019) for different NKG2D-L for 1 hr. Data are representative of five experiments. (c,d) Transfer of endogenous NKG2D-L expressed at different intensities at the cell surface. NK cells were co-incubated for 1 hr with bladder cancer cells (J82, RT-112) or with U373 cells endogenously expressing different NKG2D-L. (c) fluorescence intensity of the different NKG2D-L in each cell line. (d) percentage of NKG2D-L+ NK cells. Data are representative of three experiments. (e, f) Effect of metalloprotease inhibition in the transfer of ULBP to NK cells. ULBP2 and ULBP3 CHO transfectants were pre-treated with either vehicle (DMSO) or 5 μm BB94 for 2 hr. NK cells were co-incubated for 1 hr with these targets maintaining DMSO or BB94 treatment. (e) ULBP2 expression on CHO-transfected cells treated with DMSO or BB94. (f) Percentage of ULBP+ NK cells in BB94-treated cells is referred to the percentage in the DMSO treated. The average and standard deviation of four experiments are depicted. Statistical significance was determined using the one-way analysis of variance-Tukey’s multiple comparison test between DMSO and BB94. **P < 0·01.
NKG2D-L transfer requires activation and degranulation of the effector cell
NKG2D is expressed by all human NK cells and CD8+ T cells; however, not all the NK cells within an IL-2-cultured NK cell line acquired NKG2D-L, suggesting that there was some selectivity in the process. As primary cell lines comprise a diversity of NK cell sub-populations with different phenotypes, in a first approach to understand the mechanism of NKG2D-L trogocytosis, we evaluated whether transfer of NKG2D-L was associated with subsets of NK cells defined, for example, by expression of activating and inhibitory NK cell receptors, such as killer cell immunoglobulin-like receptors and CD94; however, none of these markers coincided with the sub-population acquiring NKG2D-L (not shown). Hence, the influence of NK cell activation on transfer was analysed next. For this purpose, the acquisition of ULBPs was monitored in freshly isolated, resting NK cells or NK cells that had been cultured with IL-2 for 16 hr (Fig.3a), as well as in CD3+ T cells expressing NKG2D (see Supplementary material, Fig. S2a,b). The activation status of freshly isolated NK cells was analysed by staining for CD69 expression (see Supplementary material, Fig. S2c). After 16 hr of incubation with IL-2, CD69 increased slightly although the fluorescence intensity was still much lower than that of the purified NK lines grown in IL-2 for longer periods. The experiment shows that NK cells activated with IL-2 for 16 hr acquired NKG2D-L more efficiently than NK cells incubated without IL-2 (Fig.3a), suggesting that NK activation plays a role in the transfer process. Next, the ability to initiate a cytotoxic response of NK cells was evaluated in the context of ULBP transfer, in experiments where NK degranulation and ULBP acquisition were analysed simultaneously (Fig.3b,c; see Supplementary material, Fig. S3). First, ULBP-positive NK cells showed most of the surface expression of LAMP1, indicating that those NK cells that had acquired ULBPs, in general, were the ones that had also degranulated (Fig.3b; see Supplementary material, Fig. S3a); second, LAMP1-negative NK cells were low for ULBPs (Fig.3c; see Supplementary material, Figure S3b), suggesting that the acquisition of ULBPs by those NK cells that failed to degranulate was inefficient. These data imply that transfer correlates with the activation of the effector cell lytic machinery, after receptor–ligand interaction and granule mobilization. It could be inferred that a productive cytotoxic immune synapse had been formed, although this was not evaluated here. Antibody blocking experiments demonstrate that NKG2D recognition was necessary for the transfer of ligands to the effector cell to occur (Fig.3d), supporting the theory that transfer correlates with activation. The mean fluorescence intensity (MFI) of the transferred ULBPs and the percentage of ULBP-positive NK cells decreased with NKG2D-blockade in all the cases (open symbols in the figure) (four experiments with different donors, three ULBPs); however, the blocking efficiency was different depending on the particular ULBP ranging from 40% to 80% blockade. In these experiments, blocking of ULBP2 transfer was somewhat less than that seen for ULBP1 and ULBP3. As ULBP2 also shows different kinetics of transfer, this result might reflect that additional mechanisms to receptor–ligand interaction are involved in the transfer of this molecule. Altogether, these data demonstrate that transfer of NKG2D-L depends mainly on immune activation following receptor–ligand interaction.
Figure 3.
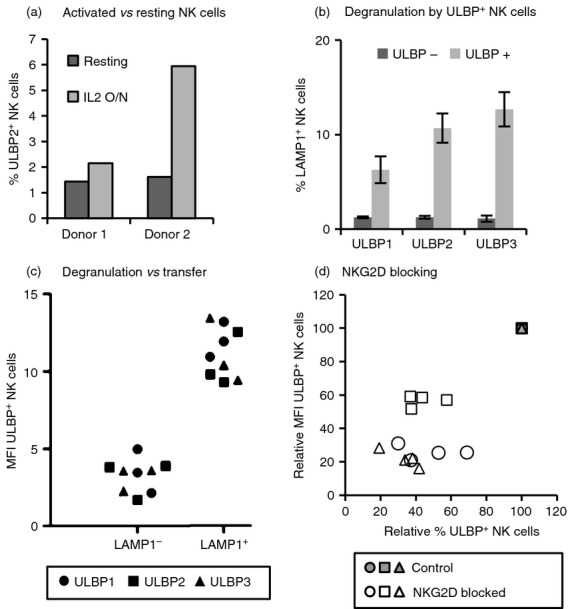
Transfer depends on ligand recognition and activation of the effector cell. (a) Transfer to resting versus activated natural killer (NK) cells. Resting or interleukin-2 (IL-2) activated NK cells were co-incubated as in Fig.1 with UL16 binding protein 2 (ULBP2) transfectants for 1 hr and analysed by flow cytometry. The percentages of ULBP2+ NK cells from two donors are shown. (b) Degranulation of ULBP+ NK cells. IL-2 activated NK cells were co-incubated for 2 hr with ULBP-transfectants and degranulation was measured as surface LAMP1 expression on NK cells. Data from ULBP-positive and -negative NK cells are shown separately (for gating strategy see Supplementary material, Fig. S3a). Degranulation against CHO-untransfected cells was around 1–2% on average. Six experiments were performed with each ULBP. Average and standard deviation are depicted. (c) NK cells that degranulate acquire more ULBP. The mean fluorescence intensity (MFI) in NK cells staining positive for ULBP that have degranulated (LAMP1+) and those that did not degranulate (LAMP1−) are shown (three co-culture experiments with each NKG2D-L) (for gating strategy see Supplementary material, Fig. S3b). The different symbols represent different ULBPs as shown in panel (d). Blocking NKG2D decreases ligand transfer. ULBP transfer experiments were performed in control, unblocked NK cells (grey symbols) and after blocking with anti-NKG2D monoclonal antibody (white symbols). Different symbols represent different ULBPs: ○ ULBP1, □ ULBP2, Δ ULBP3. The ULBP MFI versus the percentage of ULBP+ NK cells is shown relative to control conditions (unblocked, all grey symbols superimposed at the 100% value). Four experiments for each NKG2D-L are shown.
NKG2D-L acquired by NK cells are co-immunoprecipitated with NKG2D receptor
We explored the fate of the transferred molecules on the membrane of the acceptor cell. First, the association of the transferred ligand with the NKG2D receptor was analysed in co-immunoprecipitation experiments. NKG2D was immunoprecipitated from NK cells after co-culture with target cells and the presence of the transferred NKG2D-L was analysed by Western blot. As shown in Fig.4(a), co-immunoprecipitated ULBP3 was detected by Western blot in NK cells that had been co-incubated with CHO-ULBP3 cells, but not after co-incubation with untransfected CHO. In control experiments, ULBP3 was not detected by Western blotting in preparations of NKG2D immunoprecipitated from a mixture of NK lysates and CHO cell lysates (see Supplementary material, Fig. S4). The absence of co-precipitated ULBP3 in these experiments demonstrated that the ULBP3 shown in Fig.4a was associated with NKG2D on live cells and was not due to non-specific effects post-lysis. Acid wash experiments demonstrated that the NKG2D–ULBP interaction was not necessary to maintain the acquired ligand at the NK cell membrane, so this experiment most probably reflects the inclusion of the two proteins in the same membrane microdomain. This association likely does not occur via the normal interaction site since the NKG2D antibody used for immunoprecipitation blocks the interaction among receptor and ligand.33 In conclusion, these co-immunoprecipitation experiments demonstrate that the transferred NKG2D-L is still associated with the receptor in the NK cell membrane after transfer, at least after 2 hrs of co-culture. A second group of experiments examined the stability of the transferred NKG2D-L on the plasma membrane of the effector cells (Fig.4b). Most NK cells that had acquired ULBPs (at least 60%) remained ULBP positive after 4–6 hr of the co-culture whereas only a small percentage of NK cells still had ULBP at the plasma membrane after 24 hr, suggesting that: (1) ULBP+ NK cells could be eliminated from the culture and (2) there could be a redistribution of transferred ULBP molecules among NK cells within the culture. This hypothesis is further supported by the observation of an increased number of dead NK cells after co-culture with a ULBP+ target. Interestingly, within the ULBP+ NK cells, the intensity of ULBP fluorescence decreased quickly during the initial 30 min and then remained relatively stable on the NK cell surface for the following 24 hr, suggesting that those NK cells that acquired more ULBP molecules were lost more rapidly.
Figure 4.
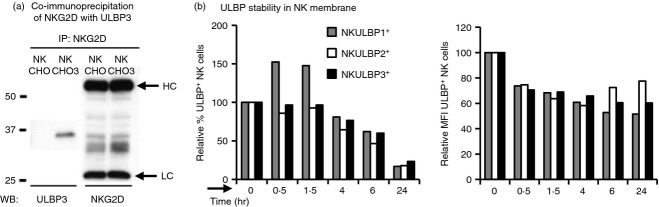
Fate of transferred UL16 binding protein (ULBP) molecules. (a) Acquired ULBP3 is closely associated with NKG2D. NKG2D was immunoprecipitated from natural killer (NK) cells previously co-incubated with either Chinese hamster ovary (CHO) parental cells (CHO) or ULBP3 transfectants (CHO3) for 2 hr. After separation in SDS–PAGE, co-immunoprecipitated ULBP3 and NKG2D were detected by Western blot. The bands for the heavy and light chains of the antibody are indicated (HC and LC). (b) Stability of transferred ULBP on NK cells. After co-incubation for 1 hr with ULBP transfectants, NK cells were separated from the adherent cells by pipetting and maintained in culture for the indicated times (hr) and then analysed by flow cytometry. The percentage and mean fluorescence intensity (MFI) of ULBP+ NK cells are shown as the proportion of NK cells that remained positive for ULBP, relative to time 0. Results representative from three to six experiments are shown.
NK cells with acquired NKG2D-L are targets for autologous NK cells and can sequentially transfer the acquired molecule
The observation that ligand transfer was intimately linked to NK cell activation for cytotoxicity (Fig.3b,c) accompanied by the rapid decrease in the percentage of NK cells expressing high amounts of ULBPs, and higher NK cell death after effector–target co-culture (Figs2a, 4b) suggested that ULBP+ NK cells could be targets for autologous NK cells. NK cells that had been incubated with ULBP3 expressing targets and that, in consequence, had acquired ULBP3 were therefore tested as targets for cytotoxicity experiments (Fig.5a). In these experiments, the death of the different sets of NK cells was analysed separately by labelling with a dye those NK cells that were used as targets and then measuring 7-aminoactinomycin D (7AAD) incorporation by flow cytometry, as a death marker. Similar results were obtained in experiments performed with ULBP1 transfectants (data not shown). These ULBP-positive NK cells were lysed by autologous NK cells whereas most control NK cells co-incubated with parental CHO cells survived. Interestingly, when ULBP3-expressing NK cells were isolated by cell sorting and used as targets for autologous acceptor NK cells, the NKG2D-L could be re-transferred to the new set of effectors (Fig.5b). A further observation from these experiments was that the number of NK cells initially positive for ULBP decreased as the number of ULBP-positive cells increased in the second set of NK cells. Moreover, the MFI of the re-transferred protein seemed to reach an equal level in both donor and acceptor NK cells (data not shown), suggesting that the process of recognition and re-transfer occurs quickly among NK cells. These data demonstrate that ULBPs can be transferred between NK cells. Although it seems likely that the mechanism would be the same, the experiments shown in Fig.5 did not explore the requirement for NKG2D interaction in this process. These data are consistent with the hypothesis that trogocytosis could constitute a mechanism for immune response homeostasis by eliminating highly activated NK cells.
Figure 5.
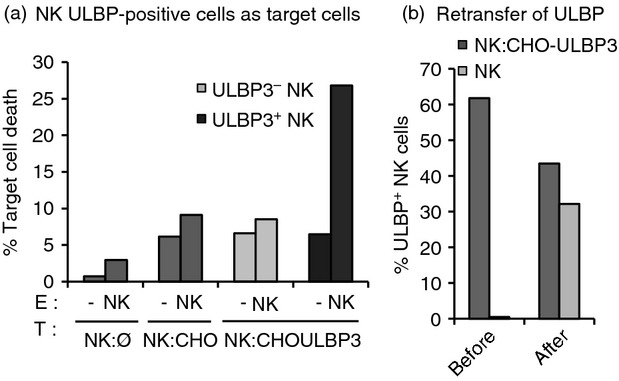
UL16 binding protein (ULBP) -positive natural killer (NK) cells become targets for autologous NK cells and re-transfer the acquired NKG2D-ligand. (a) ULBP3+ NK cells as target cells. NK cells were labelled with PKH2 and incubated either alone (NK:Ø) or co-incubated for 1 hr with Chinese hamster ovary (CHO) parental (NK:CHO) or ULBP3 transfectants (NK:CHOULBP3). After co-incubation, these green NK cells were used as targets (T) in degranulation assays in which the effectors (E) were autologous unlabeled NK cells (NK). Target cells were also maintained alone (−) in culture to determine the levels of spontaneous cell death. Death was analysed by 7-aminoactinomycin D-staining and flow cytometry. Cell death is shown separately for ULBP-negative and -positive NK cells. (b) ULBP re-transfer. NK cells were co-incubated for 1 hr with ULBP3 transfectants (NK:CHOULBP3), NK cells from co-incubation experiments were sorted and used as targets in co-culture experiments with autologous effector cells (NK) labelled using the Cell Trace Violet kit. The percentage of ULBP+ NK cells before and after co-culture within each population of NK cells is shown. (a) and (b) show one representative result of three experiments.
Discussion
The work presented here compares the transfer of several distinct NKG2D-L from target cells to NKG2D-receptor-expressing effector cells and evaluates the consequences for those NK cells that have acquired the activating ligands. We describe that the fast transfer of NKG2D-L has properties corresponding to the phenomenon known as trogocytosis1 and occurs after ligand–receptor interaction. In this sense, the transfer of NKG2D-L to effector cells seems to be the consequence of a productive immune synapse formation leading to degranulation. We further demonstrate that the different ULBP ligands for NKG2D transfer with different efficiencies depending on aspects of the cell biology of each protein, such as processing and metalloprotease cleavage. Lastly, the paper describes how NK cells that acquire NKG2D ligands by transfer become susceptible to NK cell attack and that successive transfer of activating ligands can occur, a process that could represent a novel way of regulating the intensity of the immune response.
Natural killer cells were able to acquire different amounts of NKG2D-L and, curiously, not all the cells in the culture acquired the proteins. The data suggest that the variability in the capacity of effector cells to acquire NKG2D-L could be related to the response made by an NK cell against a particular target. The link between the need for the formation of a cytotoxic immune synapse and transfer is demonstrated in several approaches: the requirement for cell–cell contact (Fig.1c), the dependence of ULBP transfer on effector cell activation (Fig.3a), and, more definitively, the degranulation levels of those effector cells that had acquired the ligands (Fig.3b,c). Further, the proximity of NKG2D and the transferred ULBPs at the NK cell membrane (Fig.4a), as well as the role of receptor–ligand interaction (Fig.3d) have been demonstrated. The apparent lack of ULBP staining in the CD56bright cell subset (which is generally considered to have reduced cytotoxicity) (see Supplementary material, Figs S3 and S5) would be consistent with a requirement for a cytotoxic encounter for transfer. As the NK cells used were IL-2-stimulated and the intensity of CD56 is quite homogeneous in these NK cell lines, the significance of this observation was not explored further.
This paper demonstrates that both types of human NKG2D-L, transmembrane and GPI-anchored molecules, can be transferred to NK cells. However, it is interesting to note that variations in transfer were observed even within the group of GPI-anchored ligands. ULBP1, -2 and -3 reproducibly had different kinetics of trogocytosis. It is difficult to know whether the different affinities for NKG2D binding of the three ULBPs could play any role in this phenomenon, but again the biology of the three receptors seems to have distinct functional consequences. We have shown that ULBP transfer results in different half-lives at the plasma membrane, probably as the result of the combination of several processes: first, the efficiency of the transfer and second, different internalization or shedding events, the latter influenced by their different susceptibilities to metalloprotease cleavage. Once again, the biology of the three GPI-anchored ULBPs is not equivalent and their function/activity is affected by cellular processes that regulate the expression of these proteins.15
The work presented here suggests that, after the transfer and once on the NK cell membrane, the transferred ULBPs maintain an interaction with NKG2D, but it is not clear if this association depends solely on the receptor binding site (see Supplementary material, Fig. S5). The antibody used to immunoprecipitate NKG2D blocks binding to the ligand;33 however, the monoclonal antibodies used to detect transferred ULBPs in flow cytometry only partially block the interaction with NKG2D, so it is possible that, after transfer, some ULBPs continue to associate with NKG2D via the receptor-binding site, but that other ULBP molecules remain tightly proximal to NKG2D in the same membrane microdomain. This might be related to the observation that ligand-binding by NKG2D induces the recruitment of this receptor to GM1-enriched detergent-resistant membrane microdomains.34,35 Furthermore, the experiments using PI-PLC showed that transferred ULBPs were inserted in the membrane via their GPI-anchors (Fig.1c) and the loss of NKG2D after acid wash (not shown), support that not all the ULBPs detected on the surface of NK cells were bound to the NK cells through the interaction with NKG2D, but rather were inserted in the membrane.
The functional effects of protein transfer on cell function have been approached previously and the consequences vary depending on the identity of the protein being transferred. For example, HLA-G acquisition by NK cells stops proliferation and abrogates cytolytic function,36 whereas acquisition of MHC-I from virus-infected cells by dendritic cells stimulates CD8+ memory T cells.37 Recently, Nakamura et al. published a study in which the authors describe that death of murine NK cells can occur in an NKG2D-dependent manner after the transfer of Rae-1δ and ε from transfected RMA-S cells.22 Here, ULBP acquisition by human NK cells led to increased susceptibility to lysis by autologous NK cells and, further, the retransfer of the ULBP ligand to these NK cells. In this sense, it is thought-provoking that those NK cells that had acquired more ULBP molecules (as reflected by a higher ULBP MFI) disappeared very quickly after co-incubation with autologous NK cells. This could reflect that they became targets and rapidly initiated the re-transfer of the acquired molecule. That MICA uptake could render NK cells susceptible to killing by other NK cells has previously been shown and it was suggested that fratricide was occurring.21,22 Also, a decrease of cytotoxicity was observed in NK cell lines that had acquired MICB.20 This could be related to a down-modulation of NKG2D after encounter with the target cell.
In conclusion, the data presented here demonstrate that transfer of NKG2D-L occurs only to those effectors cells that have undergone degranulation and suggest that acquisition of proteins in acceptor/effector cells could be used as a marker for receptor–ligand interaction. Further, these results strongly support a possible role of trogocytosis in modulation of immune response.
Acknowledgments
The authors would like to thank members from the laboratoires of H.T. Reyburn and M. Valés-Gómez for helpful discussions; Drs F.X. Real and M. López-Botet for the kind gift of reagents; the CNB flow cytometry service. This work was supported by grants from Fondo de Investigación Sanitaria (PI11/00298)(PS09/00181); from the Spanish Ministry of Economy and Competitivity (MINECO) (SAF2012-32293), and from the Regional Government of Madrid (grant number S2010/BMD-2326 IMMUNOTHERCAN); SL-C was a recipient from a fellowship from the Spanish Ministry of Education, was partially supported by CSIC [grant 201020E086 (to MV-G)] and received the Immunotools IT-BOX-139 award 2012. EMG-C was a recipient of a fellowship from Fundación La Caixa; GR-C was a recipient of a JAE fellowship from CSIC.
Glossary
- GPI
glycosyl-phosphatidyl-inositol
- MICA/B
major histocompatibility complex class I-related chain
- NKG2D-L
NKG2D ligands
- PBA
PBS containing 1% BSA, 0·1% sodium azide
- ULBP
UL16 binding proteins
Contribution to authorship
SL-C performed experiments; SL-C, HTR and MV-G designed the research, analysed data and wrote the paper; GR-C, EMG-C and HTR contributed reagents/analytic tools.
Disclosures
There is no conflict of interest to disclose.
Supporting Information
Figure S1. (a) Gating strategy for transfer experiments. (b) ULBP expression in CHO transfectants. (c) Transfer specificity of ULBPs. (d) FLAG tag expression in CHO-ULBP transfectants. (e) Transfer of ULBPs as visualized using FLAG tag antibody.
Figure S2. (a) CD3+ T cells can acquire NKG2D-ligands. (b) NKG2D expression on CD3+ T cells. (c) CD69 expression in freshly isolated NK cells.
Figure S3. Gating strategy for experiments exploring NK cell degranulation vs ULBP transfer.(a) Degranulation of ULBP+ NK cells (b) NK cells that degranulate acquire more ULBP.
Figure S4. (a) ULBP3 is not detected after NKG2D immunoprecipitation from mixed lysates of NK + CHO-ULBP3 cells. (b) Detection of NKG2D receptor.
Figure S5. ULBP3 transfer to NK cells measured with either anti-ULBP3 antibody or NKG2D-Fc. (a) Flow cytometry of transferred ULBP3, representative experiment (b) Flow cytometry of transferred ULBP3, average of five experiments.
References
- Joly E, Hudrisier D. What is trogocytosis and what is its purpose? Nat Immunol. 2003;4:815. doi: 10.1038/ni0903-815. [DOI] [PubMed] [Google Scholar]
- Hudrisier D, Bongrand P. Intercellular transfer of antigen-presenting cell determinants onto T cells: molecular mechanisms and biological significance. FASEB J. 2002;16:477–86. doi: 10.1096/fj.01-0933rev. [DOI] [PubMed] [Google Scholar]
- Batista FD, Iber D, Neuberger MS. B cells acquire antigen from target cells after synapse formation. Nature. 2001;411:489–94. doi: 10.1038/35078099. [DOI] [PubMed] [Google Scholar]
- Hudrisier D, Riond J, Mazarguil H, Gairin JE, Joly E. Cutting edge: CTLs rapidly capture membrane fragments from target cells in a TCR signaling-dependent manner. J Immunol. 2001;166:3645–9. doi: 10.4049/jimmunol.166.6.3645. [DOI] [PubMed] [Google Scholar]
- Huang JF, Yang Y, Sepulveda H, et al. TCR-Mediated internalization of peptide-MHC complexes acquired by T cells. Science. 1999;286:952–4. doi: 10.1126/science.286.5441.952. [DOI] [PubMed] [Google Scholar]
- Sjostrom A, Eriksson M, Cerboni C, Johansson MH, Sentman CL, Karre K, Hoglund P. Acquisition of external major histocompatibility complex class I molecules by natural killer cells expressing inhibitory Ly49 receptors. J Exp Med. 2001;194:1519–30. doi: 10.1084/jem.194.10.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabiasco J, Espinosa E, Hudrisier D, Joly E, Fournie JJ, Vercellone A. Active trans-synaptic capture of membrane fragments by natural killer cells. Eur J Immunol. 2002;32:1502–8. doi: 10.1002/1521-4141(200205)32:5<1502::AID-IMMU1502>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Carlin LM, Eleme K, McCann FE, Davis DM. Intercellular transfer and supramolecular organization of human leukocyte antigen C at inhibitory natural killer cell immune synapses. J Exp Med. 2001;194:1507–17. doi: 10.1084/jem.194.10.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GS, Collinson LM, Brzostek J, Eissmann P, Almeida CR, McCann FE, Burshtyn D, Davis DM. Membranous structures transfer cell surface proteins across NK cell immune synapses. Traffic. 2007;8:1190–204. doi: 10.1111/j.1600-0854.2007.00603.x. [DOI] [PubMed] [Google Scholar]
- Vanherberghen B, Andersson K, Carlin LM, Nolte-’t Hoen EN, Williams GS, Hoglund P, Davis DM. Human and murine inhibitory natural killer cell receptors transfer from natural killer cells to target cells. Proc Natl Acad Sci U S A. 2004;101:16873–8. doi: 10.1073/pnas.0406240101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs A, Cella M, Giurisato E, Shaw AS, Colonna M. Cutting edge: CD96 (tactile) promotes NK cell-target cell adhesion by interacting with the poliovirus receptor (CD155) J Immunol. 2004;172:3994–8. doi: 10.4049/jimmunol.172.7.3994. [DOI] [PubMed] [Google Scholar]
- Roda-Navarro P, Mittelbrunn M, Ortega M, Howie D, Terhorst C, Sanchez-Madrid F, Fernandez-Ruiz E. Dynamic redistribution of the activating 2B4/SAP complex at the cytotoxic NK cell immune synapse. J Immunol. 2004;173:3640–6. doi: 10.4049/jimmunol.173.6.3640. [DOI] [PubMed] [Google Scholar]
- Nakayama M, Takeda K, Kawano M, Takai T, Ishii N, Ogasawara K. Natural killer (NK)-dendritic cell interactions generate MHC class II-dressed NK cells that regulate CD4+ T cells. Proc Natl Acad Sci U S A. 2011;108:18360–5. doi: 10.1073/pnas.1110584108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raulet DH, Gasser S, Gowen BG, Deng W, Jung H. Regulation of ligands for the NKG2D activating receptor. Annu Rev Immunol. 2013;31:413–41. doi: 10.1146/annurev-immunol-032712-095951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Messina L, Reyburn HT, Vales-Gomez M. Human NKG2D-ligands: cell biology strategies to ensure immune recognition. Front Immunol. 2012;3:299. doi: 10.3389/fimmu.2012.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosman D, Mullberg J, Sutherland CL, Chin W, Armitage R, Fanslow W, Kubin M, Chalupny NJ. ULBPs, novel MHC class I-related molecules, bind to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor. Immunity. 2001;14:123–33. doi: 10.1016/s1074-7613(01)00095-4. [DOI] [PubMed] [Google Scholar]
- Ashiru O, Lopez-Cobo S, Fernandez-Messina L, Pontes-Quero S, Pandolfi R, Reyburn HT, Vales-Gomez M. A GPI anchor explains the unique biological features of the common NKG2D-ligand allele MICA*008. Biochem J. 2013;454:295–302. doi: 10.1042/BJ20130194. [DOI] [PubMed] [Google Scholar]
- Jonjic S, Polic B, Krmpotic A. Viral inhibitors of NKG2D ligands: friends or foes of immune surveillance? Eur J Immunol. 2008;38:2952–6. doi: 10.1002/eji.200838823. [DOI] [PubMed] [Google Scholar]
- Ullrich E, Koch J, Cerwenka A, Steinle A. New prospects on the NKG2D/NKG2DL system for oncology. Oncoimmunology. 2013;2:e26097. doi: 10.4161/onci.26097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roda-Navarro P, Vales-Gomez M, Chisholm SE, Reyburn HT. Transfer of NKG2D and MICB at the cytotoxic NK cell immune synapse correlates with a reduction in NK cell cytotoxic function. Proc Natl Acad Sci U S A. 2006;103:11258–63. doi: 10.1073/pnas.0600721103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann FE, Eissmann P, Onfelt B, Leung R, Davis DM. The activating NKG2D ligand MHC class I-related chain A transfers from target cells to NK cells in a manner that allows functional consequences. J Immunol. 2007;178:3418–26. doi: 10.4049/jimmunol.178.6.3418. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Nakayama M, Kawano M, Amagai R, Ishii T, Harigae H, Ogasawara K. Fratricide of natural killer cells dressed with tumor-derived NKG2D ligand. Proc Natl Acad Sci U S A. 2013;110:9421–6. doi: 10.1073/pnas.1300140110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubeuf S, Lindorfer MA, Taylor RP, Joly E, Hudrisier D. The direction of plasma membrane exchange between lymphocytes and accessory cells by trogocytosis is influenced by the nature of the accessory cell. J Immunol. 2010;184:1897–908. doi: 10.4049/jimmunol.0901570. [DOI] [PubMed] [Google Scholar]
- Daubeuf S, Aucher A, Bordier C, et al. Preferential transfer of certain plasma membrane proteins onto T and B cells by trogocytosis. PLoS ONE. 2010;5:e8716. doi: 10.1371/journal.pone.0008716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Messina L, Ashiru O, Boutet P, Aguera-Gonzalez S, Skepper JN, Reyburn HT, Vales-Gomez M. Differential mechanisms of shedding of the glycosylphosphatidylinositol (GPI)-anchored NKG2D ligands. J Biol Chem. 2010;285:8543–51. doi: 10.1074/jbc.M109.045906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashiru O, Boutet P, Fernandez-Messina L, Aguera-Gonzalez S, Skepper JN, Vales-Gomez M, Reyburn HT. Natural killer cell cytotoxicity is suppressed by exposure to the human NKG2D ligand MICA*008 that is shed by tumor cells in exosomes. Cancer Res. 2010;70:481–9. doi: 10.1158/0008-5472.CAN-09-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vales-Gomez M, Browne H, Reyburn HT. Expression of the UL16 glycoprotein of Human Cytomegalovirus protects the virus-infected cell from attack by natural killer cells. BMC Immunol. 2003;4:4. doi: 10.1186/1471-2172-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Villar JJ, Melero I, Navarro F, et al. The CD94/NKG2-A inhibitory receptor complex is involved in natural killer cell-mediated recognition of cells expressing HLA-G1. J Immunol. 1997;158:5736–43. [PubMed] [Google Scholar]
- Vales-Gomez M, Winterhalter A, Roda-Navarro P, Zimmermann A, Boyle L, Hengel H, Brooks A, Reyburn HT. The human cytomegalovirus glycoprotein UL16 traffics through the plasma membrane and the nuclear envelope. Cell Microbiol. 2006;8:581–90. doi: 10.1111/j.1462-5822.2005.00645.x. [DOI] [PubMed] [Google Scholar]
- Park YP, Choi SC, Kiesler P, Gil-Krzewska A, Borrego F, Weck J, Krzewski K, Coligan JE. Complex regulation of human NKG2D-DAP10 cell surface expression: opposing roles of the gammac cytokines and TGF-beta1. Blood. 2011;118:3019–27. doi: 10.1182/blood-2011-04-346825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucey MA, Scarpellino L, Zimmer J, Guillaume P, Luescher IF, Bron C, Held W. Cis association of Ly49A with MHC class I restricts natural killer cell inhibition. Nat Immunol. 2004;5:328–36. doi: 10.1038/ni1043. [DOI] [PubMed] [Google Scholar]
- Ashiru O, Bennett NJ, Boyle LH, Thomas M, Trowsdale J, Wills MR. NKG2D ligand MICA is retained in the cis-Golgi apparatus by human cytomegalovirus protein UL142. J Virol. 2009;83:12345–54. doi: 10.1128/JVI.01175-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, Spies T. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–9. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- Endt J, McCann FE, Almeida CR, Urlaub D, Leung R, Pende D, Davis DM, Watzl C. Inhibitory receptor signals suppress ligation-induced recruitment of NKG2D to GM1-rich membrane domains at the human NK cell immune synapse. J Immunol. 2007;178:5606–11. doi: 10.4049/jimmunol.178.9.5606. [DOI] [PubMed] [Google Scholar]
- Mesecke S, Urlaub D, Busch H, Eils R, Watzl C. Integration of activating and inhibitory receptor signaling by regulated phosphorylation of Vav1 in immune cells. Sci Signal. 2011;4:ra36. doi: 10.1126/scisignal.2001325. [DOI] [PubMed] [Google Scholar]
- Caumartin J, Favier B, Daouya M, Guillard C, Moreau P, Carosella ED, LeMaoult J. Trogocytosis-based generation of suppressive NK cells. EMBO J. 2007;26:1423–33. doi: 10.1038/sj.emboj.7601570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakim LM, Bevan MJ. Cross-dressed dendritic cells drive memory CD8+ T-cell activation after viral infection. Nature. 2011;471:629–32. doi: 10.1038/nature09863. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. (a) Gating strategy for transfer experiments. (b) ULBP expression in CHO transfectants. (c) Transfer specificity of ULBPs. (d) FLAG tag expression in CHO-ULBP transfectants. (e) Transfer of ULBPs as visualized using FLAG tag antibody.
Figure S2. (a) CD3+ T cells can acquire NKG2D-ligands. (b) NKG2D expression on CD3+ T cells. (c) CD69 expression in freshly isolated NK cells.
Figure S3. Gating strategy for experiments exploring NK cell degranulation vs ULBP transfer.(a) Degranulation of ULBP+ NK cells (b) NK cells that degranulate acquire more ULBP.
Figure S4. (a) ULBP3 is not detected after NKG2D immunoprecipitation from mixed lysates of NK + CHO-ULBP3 cells. (b) Detection of NKG2D receptor.
Figure S5. ULBP3 transfer to NK cells measured with either anti-ULBP3 antibody or NKG2D-Fc. (a) Flow cytometry of transferred ULBP3, representative experiment (b) Flow cytometry of transferred ULBP3, average of five experiments.


