Abstract
Interleukin-33 (IL-33) has been a focus of study because of its variety of functions shaping CD4+ T-cell biology. In the present work, we evaluated the modulatory effect of IL-33 on suppressor cells in an in vivo transplantation model. C57BL/6 wild-type mice were grafted with syngeneic or allogeneic skin transplants and treated with exogenous IL-33 daily. After 10 days of treatment, we analysed draining lymph node cellularity and found in allogeneic animals an increment in myeloid-derived suppressor cells, which co-express MHC-II, and become enriched upon IL-33 treatment. In line with this observation, inducible nitric oxide synthase and arginase 1 expression were also increased in allogeneic animals upon IL-33 administration. In addition, IL-33 treatment up-regulated the number of Foxp3+ regulatory T (Treg) cells in the allogeneic group, complementing the healthier integrity of the allografts and the increased allograft survival. Moreover, we demonstrate that IL-33 promotes CD4+ T-cell expansion and conversion of CD4+ Foxp3− T cells into CD4+ Foxp3+ Treg cells in the periphery. Lastly, the cytokine pattern of ex vivo-stimulated draining lymph nodes indicates that IL-33 dampens interferon-γ and IL-17 production, stimulating IL-10 secretion. Altogether, our work complements previous studies on the immune-modulatory activity of IL-33, showing that this cytokine affects myeloid-derived suppressor cells at the cell number and gene expression levels. More importantly, our research demonstrates for the first time that IL-33 allows for in vivo Foxp3+ Treg cell conversion and favours an anti-inflammatory or tolerogenic state by skewing cytokine production. Therefore, our data suggest a potential use of IL-33 to prevent allograft rejection, bringing new therapeutics to the transplantation field.
Keywords: Foxp3+ regulatory T cells, interleukin-33, tolerance, transplantation
Introduction
After tissue damage or the disabling of an organ, transplantation becomes the only option to restore the homeostasis of the organism and recover the initial function of the injured tissue or organ. However, graft rejection remains the main problem in transplantation, despite multiple efforts to circumvent the inflammatory response initiated against the graft. Furthermore, the immune reactions generated affect not only the graft itself but also the whole organism, causing serious side effects such as graft-versus-host-disease, infections (due to immunosuppression) and cardiovascular pathologies, among others,1 making the chance of a successful transplant even more difficult. Understanding the basis of the inflammatory response during transplant rejection is crucial, so that the design of cellular therapies to achieve graft acceptance can be developed.
Many reports have been published about interleukin-33 (IL-33), a member of the IL-1 superfamily, which plays different roles during immune responses. Interleukin-33 was initially described as a promoter of T helper type 2 (Th2) cell responses2,3 and its membrane receptor (ST2) is highly expressed on activated Th2 cells, mast cells4 and innate lymphoid cells.5 In contrast with the mentioned observations, it has been shown that IL-33 has a role in the induction of Th1-type immune responses,6 and it can also promote antiviral responses,7 classifying IL-33 as a pleiotropic cytokine with a wide range of functions.
However, recent reports demonstrated that IL-33 can promote tolerance through the expansion of CD11b+ Gr1+ cells [or myeloid-derived suppressor cells (MDSCs)] and Foxp3+ regulatory T (Treg) cells, and several groups have shown these effects in vitro in myeloid cells8 and in animal settings after exogenous administration of IL-33, including models of intestinal inflammation9,10 and transplantation.11,12 In transplantation, the groups of Brunner11 and Turnquist12 showed that treatment with IL-33 improves heart graft survival after allogeneic transplantation, which was linked to the expansion of the two populations previously mentioned. In a different model, IL-33 indirectly reduces intestinal inflammation by the expansion of CD103+ retinoic acid-producing dendritic cells, ameliorating experimental colitis in mice.13 Complementing the beneficial role of IL-33, additional studies have indicated that a faster healing process is seen when mice bearing skin wounds are treated with this cytokine, due to an increase in collagen deposition, which allows a better re-epithelialization of the tissue.14 This last observation is of great importance for the transplantation field, where a correct healing of the injured tissue is necessary for the appropriate function of the new organ.
These data show that IL-33 is a cytokine with an interesting variety of functions during immune responses,15 and could be a novel target for ameliorating graft rejection.16
Taking into consideration all the information described here, in this work we aimed to study the cellular dynamics in IL-33-treated skin-transplanted mice, focusing on cell populations such as MDSCs or Foxp3+ Treg cells, and the production of key cytokines. Using the mentioned in vivo model, we found that: (i) in draining lymph nodes (dLNs) from allogeneic-grafted mice the number of MDSCs and expression of their key genes are increased upon IL-33 treatment, (ii) IL-33 administration enriches for Foxp3+ Treg cells, which correspond to induced Treg (iTreg) cells, and (iii) IL-33 inhibits Th1/Th17 differentiation, favouring the production of IL-10 from ex vivo-stimulated dLN cells. These results suggest that IL-33 may be an interesting novel target to achieve tolerance, by modulating MDSCs and CD4+ T-cell biology to control immunity against allogeneic transplants.
Materials and methods
Mice
Six- to eight-week-old C57BL/6 wild-type mice (syngeneic skin donors) and C57BL/6 Foxp3/GFP reporter mice (kindly provided by J. Rodrigo Mora, Harvard Medical School, Cambridge, MA) were used in this study. BALB/c × C57BL/6 (or F1dxb) mice (skin allograft donors) were obtained by crossing BALB/c mice (H2d) with wild-type (WT) C57BL/6 mice (H2b). Mice were maintained in accordance with the Bioethical Committee guidelines from the Facultad de Medicina, Universidad de Chile.
Skin transplantation
Skin grafting was performed as described previously.17 Briefly, tail skin (∼ 1 cm2) from C57BL/6 (syngeneic) or F1 (allogeneic) donors was transplanted onto the dorsal area of C57BL/6 WT or C57BL/6 Foxp3/GFP reporter recipient animals. Survival of skin allografts was evaluated twice per week and grafts were considered rejected when 80% of the original graft had disappeared or become necrotic.
Flow cytometry
Flow cytometry analyses were performed using anti-mouse CD4 (clone RM4-5), CD25 (clone PC61.5), Gr1 (clone RB6-8C5), CD11c (clone N418), CD11b (clone M1/70), CD45.1 (clone A20, all from BioLegend, San Diego, CA) and ST2 (clone 245707, R&D Systems, Minneapolis, MN), all conjugated with FITC, phycoerythrin, peridinin chlorophyll protein or allophycocyanin. FACS data acquisition was performed with FACSCalibur (Beckton Dickinson, Franklin Lakes, NJ), using CellQuest software (BD Biosciences, San Jose, CA). Data were analysed using flowjo software (Tree Star, Canton, OH).
Cell sorting and adoptive transfer experiment
Draining LN cells were obtained from C57BL/6-Foxp3/GFP (Ly5.1) mice and stained with anti-CD4 and anti-CD25 in PBS 1× + 5% FBS. Effector CD4+ T cells were sorted based on CD4+ CD25− Foxp3/GFP– T-cell phenotype using a BD FACSAria III (Franklin Lakes, NJ). After sorting the population of interest was ≥ 98% pure, as seen in the Supplementary material (Fig. S2). For cell transfer, C57BL/6 mice (Ly5.2+) received 1 × 106 sorted cells (Ly5.1) intravenously.
ELISA test
Peripheral lymph nodes were harvested on Day 10, and cell suspensions were concentrated at 1 × 106 cells/ml/well and seeded in 24-well plates (BD Biosciences) in the presence of polyclonal activation (5 μg/ml anti-CD3 clone 2c11, BioLegend) for 3 days. Supernatants were collected and stored at −80° and later analysed for cytokine quantification by ELISA (sandwich) test using, anti-interferon-γ (IFN-γ), anti-IL-17, anti-IL-33 and anti-IL-10 antibodies (eBioscience, San Diego, CA) and recombinant murine cytokines for standard curves (BioLegend).
Quantitative RT-PCR
RNA from dLN cells was extracted using an E.Z.N.A. Total RNA Kit (Omega Bio-tek, Norcross, GA). The cDNA samples were prepared using an iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). The expression of inducible nitric oxide synthase (iNOS), arginase 1 (ARG1) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH; housekeeping gene) was performed using the Mx3000P quantitative PCR system (Agilent Technologies, Santa Clara, CA), using 5× HOT FIREPol® EvaGreen® qPCR Supermix (Solis BioDyne, Tartu, Estonia) as fluorescence detector. Primer sequences were: iNOS F: CCT TGG TGA AGG GAC TGA GC and R: CAA CGT TCT CCG TTC TCT TGC; ARG1 F: TTT TAG GGT TAC GGC CGG TG and R: CCT CGA GGC TGT CCT TTT GA; GAPDH F: CCA GGT TGT CTC CTG CGA CTT and R: CCT GTT GCT GTA GCC GTA TTC A.
Statistical analysis
Data were analysed using an unpaired Student’s t-test or a Mann–Whitney test (two-tailed). Survival rate was analysed by the Kaplan–Meier method, and comparisons were made by long-rank analysis. In all cases, P < 0·05 was considered with statistical significance. For data analysis, graphpad prism v5.0 (GraphPad Software, San Diego, CA) was used.
Results
IL-33 treatment induces the accumulation of MDSCs and the expression of iNOS and ARG1 during allograft rejection
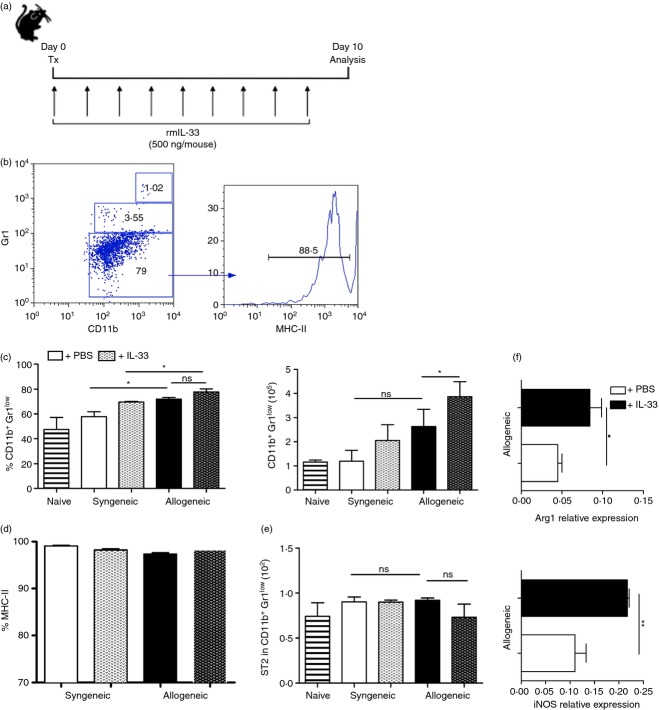
Several reports have shown that IL-33, an IL-1 family member, may modulate adaptive immunity by acting on distinct leucocyte populations.18–20 Although in allograft rejection IL-33 has been described as a protector molecule and as an inductor of Foxp3+ Treg cells,11,12 limited information is available on the cellular and molecular mechanisms involving IL-33 and its effect on the adaptive immune response against the transplant. To elucidate the role of IL-33 during transplant rejection, we decided to use a murine skin transplantation model already established in our laboratory. First, C57BL/6 recipient mice were grafted with syngeneic (C57BL/6) or allogeneic C57BL/6 × BALB/c F1 skin transplants, treated or not with exogenous IL-33 (daily). Ten days post-surgery, when signs of rejection appear, animals were killed and dLNs were collected for analysis (Fig.1a). Flow cytometry showed that during the rejection-driven inflammatory response there was no change, in either cell frequency or number of ST2+ cells. The treatment with IL-33 did not change this observation (see Supplementary material, Fig. S1). Next, we focused on CD11c, CD11b and Gr1 expressing cells because it has been reported that IL-33 treatment seems to impact their expansion and/or survival in cardiac allograft transplantation.11,21 As observed in Fig.1(b, c), we found that dLN-resident cells from IL-33-treated mice bearing an allograft accumulate more MDSCs (CD11b+ Gr1low cells, see gating strategy in Supplementary material, Fig. S3), in comparison with untreated allo-grafted mice (∼ 4 × 105 versus ∼ 2·5 × 105 cells, respectively). The expression of ST2 did not vary among all groups, but the expression of MHC-II was highly represented in this population suggesting that these MDSCs may behave as antigen-presenting cells, Fig.1(d, e). As MDSC immune-regulatory function is dependent on iNOS and Arg1 activity,22,23 we also measured the relative expression of these two genes, using GAPDH as the housekeeping control. Quantitative RT-PCR results for the indicated genes were obtained from allogeneic groups only (treated or not with IL-33) considering that IL-33 benefits this state and it would be relevant in a human setting. We did not include the syngeneic (treated or not) groups because there is no difference in the acceptance rate (Fig.2d). Our results show a twofold increment in the expression of these two key MDSC genes in rejecting animals treated with IL-33 (Fig.1f), suggesting that one of the IL-33 target cell population during transplant rejection corresponds to MDSCs, up-regulating their cell number and functional genes.
Figure 1.
Interleukin-33 (IL-33) favours the accumulation of myeloid-derived suppressor cells (MDSCs) and up-regulates the expression of inducible nitric oxide synthase (iNOS) and arginase 1 (Arg1) in draining lymph nodes (dLNs) of skin allo-transplanted mice. (a) C57BL/6-Foxp3/GFP reporter mice receive either C57BL/6 syngeneic or F1 (C57BL/6 × BALB/c) allogeneic skin grafts at Day 0. From Day 0 to Day 10 (post-surgery) animals received vehicle control or IL-33 (500 ng/mouse) injections intraperitoneally. At Day 10, dLNs were removed to obtain cell suspensions, which were stained with the indicated fluorochrome-conjugated antibodies to further analyse cell subsets by flow cytometry. (b) Dot plot showing the gating strategy for the study of different Gr-1+ cells according to the level of expression, and a representative histogram of MHC-II expression in the CD11b+ Gr1low population. (c) Percentage (left) and number (right) of CD11b+ Gr1low cells in dLNs. (d) Frequencies of CD11b+ Gr1low cells expressing MHC-II. (e) Frequencies of CD11b+ Gr1low cells expressing ST2. (f) The iNOS and Arg1 expression in dLN cells from allogeneic transplanted mice treated or not with IL-33. GAPDH was used as housekeeping gene. Bars correspond to the standard deviation (SD), and the statistical significance was assessed by analysis of Mann–Whitney test: ns, non-significant, *P = 0·05 and **P = 0·01; two independent experiments, with two to four mice per group in each experiment.
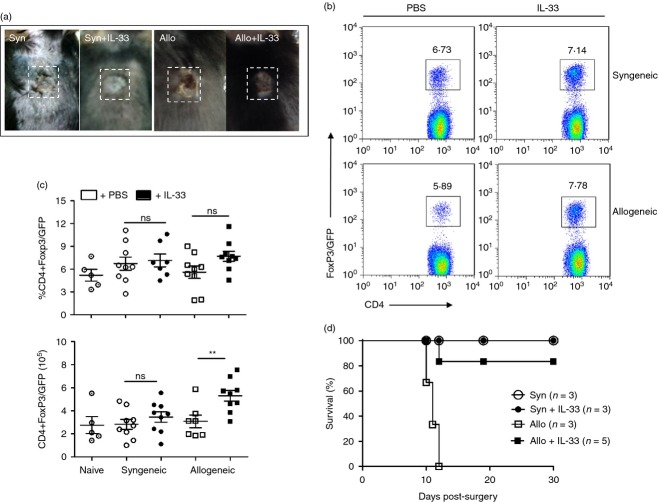
Figure 2.
Interleukin-33 (IL-33) expands Foxp3+ regulatory T (Treg) cells in skin-transplanted mice and induces allograft acceptance. Skin transplants were performed as described earlier. From Day 0 to Day 10 (post-surgery) animals received vehicle control or IL-33 (500 ng/mouse) injections intraperitoneally. At Day 10, draining lymph nodes (dLNs) were removed to obtain cell suspensions, which were stained with the indicated fluorochrome-conjugated antibodies to further analyse cell subsets by flow cytometry. (a) Picture showing the integrity of the skin grafts in all experimental groups. (b) Characteristic dot plots depicting the frequency of CD4+ Foxp3/GFP+ Treg cells present in dLNs of experimental animals. (c) Graphs compiling the frequencies (top) and number (bottom) of CD4+ Foxp3/GFP+ Treg cells. (d) Plot depicting skin graft survival in animals receiving syngeneic or allogeneic transplants, treated or not with IL-33 for 30 days. Bars correspond to the standard deviation (SD), and the statistical significance was assessed by analysis of Mann–Whitney test, **P = 0·01; three independent experiments, with two or three mice per group in each experiment.
Administration of exogenous IL-33 favours Foxp3+ Treg cell accumulation and permits the conversion of Foxp3− T cells to Foxp3+ Treg cells in skin transplant recipient animals
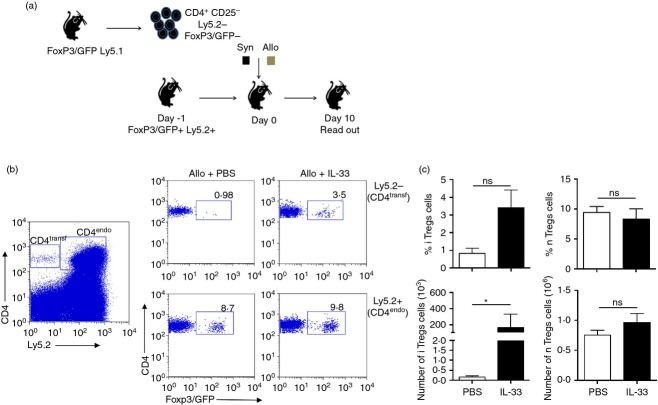
In addition to the effect on myeloid cells, administration of exogenous IL-33 into heart-transplanted animals results in an increased frequency of Foxp3+ Treg cells, positively impacting graft survival.11,12 Based on this, we decided to analyse the effect of IL-33 on CD4+ T cells during skin graft rejection. Similar to the experiments described above, Foxp3/GFP reporter animals (C57BL/6 background) were transplanted with syngeneic or allogeneic skin grafts and received recombinant murine IL-33 via intraperitoneal (500 ng/mouse in PBS 1×), or PBS 1× alone (vehicle control). As shown in Fig.2(a), both syngeneic and allogeneic skin grafts from IL-33-treated animals looked much healthier than vehicle-treated controls. Supporting this observation, we found a slight increment in CD4+ Foxp3/GFP+ Treg cell frequency in the dLN cells from IL-33-treated allogeneic grafted animals (Fig.2b, c) with an elevated number of Foxp3+ Treg cells compared with the syngeneic counterpart (∼ 5 × 105 cells versus ∼ 3 × 105 cells in syngeneic condition, Fig.2c, bottom graph). Moreover, a transplant survival experiment indicates that IL-33 induces graft tolerance (Fig.2d). As the impact of IL-33 in Foxp3+ Treg cells could be at the (de novo) differentiation and/or proliferation stages, we sought to answer this question by performing adoptive transfer experiments. For this approach, we sorted and transferred CD4+ CD25– Ly5.2– Foxp3/GFP– T cells into Foxp3/GFP+ Ly5.2+ recipients via intravenous injection (Day –1) (Fig.3a). The next day (Day 0), recipient mice were skin grafted as described above. Interleukin-33 treatment started at Day –1 and continued daily until the end of the experiment. At Day 10, flow cytometry analysis showed that the frequencies and cell numbers of both transferred (Ly5.2−) and exogenous (Ly5.2+) CD4+ T cells are increased upon IL-33 treatment (see Supplementary material, Fig. S4). Surprisingly, when we analysed the expression of Foxp3/GFP in these populations, we observed that the frequency and number of endogenous CD4+ Foxp3/GFP+ Ly5.2+ remained unchanged under all conditions (Fig.3b, c right column); but the transferred CD4+ Foxp3/GFP– Ly5.2– T cells became Foxp3/GFP+, indicating that the administration of IL-33 induces the conversion of CD4+ Foxp3/GFP− T cells into Foxp3/GFP+ Treg cells (or iTreg cells, Fig.3b, c, left column). Therefore, our results validate previous studies where IL-33 affects positively the outcome of a transplant by expanding Foxp3+ Treg cells, and demonstrate for the first time that IL-33 triggers iTreg cell differentiation in vivo.
Figure 3.
Interleukin-33 (IL-33) promotes de novo induced-Foxp3+ regulatory T (Treg) cells. (a) Scheme explaining the experimental design. CD4+ CD25– Foxp3/GFP– T cells were sorted from a C57BL/6-Foxp3/GFP (Ly5.2−) reporter mouse, with > 98% of purity. Cells (2 × 106) were adoptively transferred into C57BL/6-Foxp3/GFP (Ly5.2+) recipient animals, which received either C57BL/6 syngeneic or F1 (C57BL/6 × BALB/c) allogeneic skin grafts at Day 0. IL-33 treatment (500 ng/mouse) was administered every day until Day 10, when the animals are killed for tissue and organ collection. At this time-point, draining lymph nodes (dLNs) were removed to obtain cell suspensions, which were stained with the indicated fluorochrome-conjugated antibodies to further analyse cell subsets by flow cytometry. (b) Characteristic dot plots depicting the frequency of transferred and endogenous CD4+ T-cell populations (left), and the frequencies of natural Treg cells (bottom) or induced (top) CD4+ Foxp3/GFP+ Treg cells present in dLNs of experimental animals. (c) Graphs compiling the frequencies and number of induced-Foxp3/GFP+ Treg cells (left column) and natural Treg cells (right column). Bars correspond to the standard deviation (SD), and the statistical significance was assessed by analysis of Mann–Whitney test, *P = 0·05; one independent experiment, with four mice per group.
IL-33 inhibits Th1/Th17 differentiation and stimulates IL-10 production in dLNs from transplanted animals
How IL-33 confers protective properties on transplant recipients is not well understood. To complement the modulatory mechanism of IL-33, we re-stimulated dLN cells from transplanted animals ex vivo and collected supernatants after 72 hr in culture for cytokine analysis. As shown in Fig.4, IFN-γ and IL-17 production, two characteristic cytokines for Th1 and Th17 responses,24,25 is much higher in the allogeneic group (∼ 2000 pg/ml for IFN-γ, and ∼ 150 pg/ml for IL-17) versus the syngeneic counterpart (< 500 pg/ml for IFN-γ, and ∼ 100 pg/ml for IL-17). Interestingly, the administration of IL-33 into transplanted mice dampened the production of both cytokines in the allogeneic condition, to ∼ 250 pg/ml and ∼ 50 pg/ml for IFN-γ and IL-17, respectively. Conversely, allogeneic transplanted animals treated with IL-33 showed enhanced IL-10 production in dLNs compared with untreated animals (∼ 110 pg/ml versus ∼ 40 pg/ml). These data suggest that IL-33 may modulate T-cell adaptive immunity against the allograft, skewing the balance from an inflammatory milieu towards a regulatory microenvironment.
Figure 4.
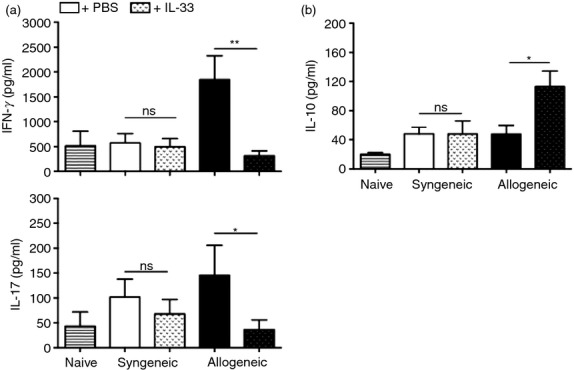
Interleukin-33 (IL-33) modulates T-cell immunity by inhibiting T helper type 1 (Th1)/Th17 differentiation and enhancing IL-10 production. Skin-transplanted animals were killed at Day 10 post-surgery, and draining lymph nodes (dLNs) were removed for analysis. Cell suspensions were prepared and cultured for 72 hr under polyclonal stimulation. After this time, supernatants were collected for cytokine quantification. (a) Graphs showing the amounts for interferon-γ (IFN-γ) (top) and IL-17 (bottom). (b) Graph depicting the amount of IL-10 obtained by ELISA. Bars correspond to the standard deviation (SD), and the statistical significance was assessed by analysis of Mann–Whitney test, *P = 0·05, **P = 0·01; two independent experiments, with two to four mice per group in each experiment.
Discussion
Recent evidence supports the idea of IL-33 as an inductor of tolerance by influencing different regulatory cell populations expressing its receptor ST2, such as MDSCs and Treg cells.12 Other studies have also reported tolerogenic effects associated with exogenous administration of IL-33,6,9,11–13,26 and it has been proven that this result depends on the presence of Treg cells.13 Additionally, it has been shown in animal models of transplantation, experimental autoimmune encephalomyelitis and atherosclerosis, that IL-33 is capable of diminishing the secretion of inflammatory cytokines such as IFN-γ (Th1) and IL-17 (Th17), so contributing to the creation of a favourable environment for recovering the homeostasis of the organism.6,11,27 Given the aforementioned reasons, we proposed that IL-33 could also modulate skin graft rejection through the induction of suppressor cells.
In the current study, we show that IL-33 treatment does not vary the expression of ST2 in dLN cells of skin transplanted mice, but it enriches the population of CD11b+ Gr1low cells, corresponding to MDSCs, as previously described.21 As IL-33 may signal via ST2 on MDSCs to control their numbers, this cytokine could be modulating their suppressor activity as well. To corroborate this, we measured the relative expression of two characteristic MDSC genes involved in their modulatory function, iNOS and Arg1.22,23 As expected, we found a marked (twofold) increase in iNOS and Arg1 expression in total dLN cells from IL-33-treated mice with allografts (Fig.1), which led us to consider MDSCs as a target for this cytokine, although a cleaner cell sample (sorted cells) should be used for the assay. On the other hand, we found that MDSCs express high levels of MHC-II, implying the ability to present antigen. Altogether, our data suggest that IL-33 expands or differentiates MDSCs, acting positively in their cell function during allograft rejection. As our results show a direct relationship between IL-33 administration and the integrity of the transplants [in the syngeneic group the grafts appeared to be better accepted and in the allogeneic group (even though the transplants were not fully accepted), the rejection kinetic seemed to be retarded (better graft status at Day 10)], one could propose that the enrichment of MDSCs (and other suppressor cells) may participate in these processes. The above correlates well with previous reports attributing to IL-33 a positive effect in wound healing14 and a beneficial role in graft survival.11,12 The fact that this population expresses high levels of MHC-II may also suggest that antigen presentation could take place in a regulatory environment, as supported by the cytokine data discussed below. Additionally, another key suppressive cell population with unquestionable positive effects in transplanted animals corresponds to Treg cells. In our experiments, we did not observe differences between the frequencies of Treg cells among syngeneic and allogeneic groups, but Treg cell numbers were increased upon IL-33 treatment in allogeneic transplanted mice, compared with their control (Fig.2). This result supports the increased graft survival obtained in those animals treated with IL-33 and grafted with allogeneic skin (long-term graft survival experiment). Altogether, our results suggest that: (i) peripheral Treg cells may be recruited into the dLNs, (ii) dLN-resident Treg cells may be expanding, and/or (iii) iTreg cells may be differentiating. Therefore, our next approach was to perform an adoptive transfer experiment to elucidate the origin of the IL-33-augmented Treg cells.
Our data show that IL-33 favours iTreg cell differentiation and expansion rather than impacting on natural Treg cells, because this population does not vary either in frequency or number (Fig.3). Even though the development of iTreg cells has been suggested before in transplantation models,28,29 this is the first time that IL-33 is associated with the generation of iTreg cells, contributing to the discovery of the functions of this pleiotropic cytokine.
Lastly, during the immune response against an allograft, several cytokines are produced impacting T-cell differentiation, among other events.28 In this line, we found that the production of Th1/Th17 characteristic cytokines (IFN-γ and IL-17) is reduced in ex vivo-recalled dLN cells from IL-33-treated animals and, in contrast, the anti-inflammatory counterpart IL-10 is highly augmented (Fig.4). Hence, the IL-33 inhibitory effect on these cytokines is probably the result of the action of MDSCs and/or Treg cells against effector T cells that are mediating graft rejection.11
In summary, these data confirm the involvement of IL-33 on transplant immunity, favouring a balance towards an anti-inflammatory environment, so benefiting the transplanted organ. The current study opens new hopes in translating IL-33 into the clinic.
Acknowledgments
We are very grateful to Dr Arturo Ferreira for facilitating the performance of some experiments (facility/equipment), to Mrs Ruth Mora for technical assistance and Miss Petra Sergent (Dartmouth College) for manuscript editing. This work was supported by FONDECYT grant 11121309 and PMI grant UA1301.
Disclosures
The authors declare no conflict of interest.
Supporting Information
Figure S1. ST2 expression is not altered by exogenous interleukin-33 during the immune response to skin graft transplantation.
Figure S2. Foxp3 expression on freshly sorted Ly5.2− CD4+ T cells.
Figure S3. Gating strategy to identify myeloid-derived suppressor cells.
Figure S4. Interleukin-33 expands CD4+ T cells in vivo.
References
- Chinen J, Buckley RH. Transplantation immunology: solid organ and bone marrow. J Allergy Clin Immunol. 2010;125:S324–35. doi: 10.1016/j.jaci.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rank MA, Kobayashi T, Kozaki H, Bartemes KR, Squillace DL, Kita H. IL-33-activated dendritic cells induce an atypical TH2-type response. J Allergy Clin Immunol. 2009;123:1047–54. doi: 10.1016/j.jaci.2009.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–90. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Xu D, Jiang HR, Kewin P, Li Y, Mu R, Fraser AR, et al. IL-33 exacerbates antigen-induced arthritis by activating mast cells. Proc Natl Acad Sci USA. 2008;105:10913–8. doi: 10.1073/pnas.0801898105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spits H, Cupedo T. Innate lymphoid cells: emerging insights in development, lineage relationships, and function. Annu Rev Immunol. 2012;30:647–75. doi: 10.1146/annurev-immunol-020711-075053. [DOI] [PubMed] [Google Scholar]
- Jiang HR, Milovanovic M, Allan D, Niedbala W, Besnard AG, Fukada SY, et al. IL-33 attenuates EAE by suppressing IL-17 and IFN-γ production and inducing alternatively activated macrophages. Eur J Immunol. 2012;42:1804–14. doi: 10.1002/eji.201141947. [DOI] [PubMed] [Google Scholar]
- Bonilla WV, Frohlich A, Senn K, Kallert S, Fernandez M, Johnson S, et al. The alarmin interleukin-33 drives protective antiviral CD8+ T cell responses. Science. 2012;335:984–9. doi: 10.1126/science.1215418. [DOI] [PubMed] [Google Scholar]
- Mayuzumi N, Matsushima H, Takashima A. IL-33 promotes DC development in BM culture by triggering GM-CSF production. Eur J Immunol. 2009;39:3331–42. doi: 10.1002/eji.200939472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiering C, Krausgruber T, Chomka A, Frohlich A, Adelmann K, Wohlfert EA, et al. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature. 2014;513:564–8. doi: 10.1038/nature13577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matta BM, Lott JM, Mathews LR, Liu Q, Rosborough BR, Blazar BR, et al. IL-33 is an unconventional Alarmin that stimulates IL-2 secretion by dendritic cells to selectively expand IL-33R/ST2+ regulatory T cells. J Immunol. 2014;15(193):4010–20. doi: 10.4049/jimmunol.1400481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner SM, Schiechl G, Falk W, Schlitt HJ, Geissler EK, Fichtner-Feigl S. Interleukin-33 prolongs allograft survival during chronic cardiac rejection. Transpl Int. 2011;24:1027–39. doi: 10.1111/j.1432-2277.2011.01306.x. [DOI] [PubMed] [Google Scholar]
- Turnquist HR, Zhao Z, Rosborough BR, Liu Q, Castellaneta A, Isse K, et al. IL-33 expands suppressive CD11b+ Gr-1int and regulatory T cells (Treg), including ST2L+ Foxp3+ cells, and mediates Treg-dependent promotion of cardiac allograft survival. J Immunol. 2011;187:4598–610. doi: 10.4049/jimmunol.1100519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan L, Chen J, Zhang H, Yang H, Zhu P, Xiong A, et al. Interleukin-33 ameliorates experimental colitis through promoting Th2/Foxp3+ regulatory T-cell responses in mice. Mol Med. 2012;18:753–61. doi: 10.2119/molmed.2011.00428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H, Li X, Hu S, Liu T, Yuan B, Gu H, et al. IL-33 accelerates cutaneous wound healing involved in upregulation of alternatively activated macrophages. Mol Immunol. 2013;56:347–53. doi: 10.1016/j.molimm.2013.05.225. [DOI] [PubMed] [Google Scholar]
- Villarreal DO, Weiner DB. Interleukin 33: a switch-hitting cytokine. Curr Opin Immunol. 2014;28:102–6. doi: 10.1016/j.coi.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Turnquist HR. Implications for interleukin-33 in solid organ transplantation. Cytokine. 2013;62:183–94. doi: 10.1016/j.cyto.2013.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos-Mora M, Morales RA, Perez F, Gajardo T, Campos J, Catalan D, et al. Neuropilin-1+ regulatory T cells promote skin allograft survival and modulate effector CD4+ T cells phenotypic signature. Immunol Cell Biol. 2015;93:113–9. doi: 10.1038/icb.2014.77. [DOI] [PubMed] [Google Scholar]
- Suzukawa M, Koketsu R, Iikura M, Nakae S, Matsumoto K, Nagase H, et al. Interleukin-33 enhances adhesion, CD11b expression and survival in human eosinophils. Lab Invest. 2008;88:1245–53. doi: 10.1038/labinvest.2008.82. [DOI] [PubMed] [Google Scholar]
- Pecaric-Petkovic T, Didichenko SA, Kaempfer S, Spiegl N, Dahinden CA. Human basophils and eosinophils are the direct target leukocytes of the novel IL-1 family member IL-33. Blood. 2009;113:1526–34. doi: 10.1182/blood-2008-05-157818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho KA, Suh JW, Sohn JH, Park JW, Lee H, Kang JL, et al. IL-33 induces Th17-mediated airway inflammation via mast cells in ovalbumin-challenged mice. Am J Physiol Lung Cell Mol Physiol. 2012;302:L429–40. doi: 10.1152/ajplung.00252.2011. [DOI] [PubMed] [Google Scholar]
- Gao X, Wang X, Yang Q, Zhao X, Wen W, Li G, et al. Tumoral expression of IL-33 inhibits tumor growth and modifies the tumor microenvironment through CD8+ T and NK Cells. J Immunol. 2015;194:438–45. doi: 10.4049/jimmunol.1401344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrilovich DI, Nagaraj S. Myeloid-derived-suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn JI, Gabrilovich DI. The biology of myeloid-derived suppressor cells: the blessing and the curse of morphological and functional heterogeneity. Eur J Immunol. 2010;40:2969–75. doi: 10.1002/eji.201040895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood KJ, Goto R. Mechanisms of rejection: current perspectives. Transplantation. 2012;93:1–10. doi: 10.1097/TP.0b013e31823cab44. [DOI] [PubMed] [Google Scholar]
- Schenk S, Kish DD, He C, El-Sawy T, Chiffoleau E, Chen C, et al. Alloreactive T cell responses and acute rejection of single class II MHC-disparate heart allografts are under strict regulation by CD4+ CD25+ T cells. J Immunol. 2005;174:3741–8. doi: 10.4049/jimmunol.174.6.3741. [DOI] [PubMed] [Google Scholar]
- Miller AM. Role of IL-33 in inflammation and disease. J Inflamm. 2011;8:22. doi: 10.1186/1476-9255-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman A, Ben-Shoshan J, Entin-Meer M, Maysel-Auslender S, Guzner-Gur H, Keren G. Interleukin-33 augments Treg cell levels: a flaw mechanism in atherosclerosis. Isr Med Assoc J. 2012;14:620–3. [PubMed] [Google Scholar]
- Wood KJ, Sakaguchi S. Regulatory T cells in transplantation tolerance. Nat Rev Immunol. 2003;3:199–210. doi: 10.1038/nri1027. [DOI] [PubMed] [Google Scholar]
- Burrell BE, Nakayama Y, Xu J, Brinkman CC, Bromberg JS. Regulatory T cell induction, migration, and function in transplantation. J Immunol. 2012;189:4705–11. doi: 10.4049/jimmunol.1202027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. ST2 expression is not altered by exogenous interleukin-33 during the immune response to skin graft transplantation.
Figure S2. Foxp3 expression on freshly sorted Ly5.2− CD4+ T cells.
Figure S3. Gating strategy to identify myeloid-derived suppressor cells.
Figure S4. Interleukin-33 expands CD4+ T cells in vivo.