Abstract
Objective
Neutrophil recruitment is a key process in the pathogenesis of stroke, and may provide a valuable therapeutic target. Targeting the melanocortin receptors (MC) has previously shown to inhibit leukocyte recruitment in peripheral inflammation, however it is not known whether treatments are effective in the unique cerebral microvascular environment. Here, we provide novel research highlighting the effects of the melanocortin peptides on cerebral neutrophil recruitment, demonstrating important yet discrete roles for both MC1 and MC3.
Approach and Results
Using intravital microscopy, in two distinct murine models of cerebral ischemia-reperfusion (I/R) injury we have investigated melanocortin control over neutrophil recruitment. Following global I/R, pharmacological treatments suppressed pathological neutrophil recruitment. MC1 selective treatment rapidly inhibited neutrophil recruitment while a non-selective MC agonist provided protection even when co-administered with an MC3/4 antagonist, suggesting the importance of early MC1 signaling. However by 2h reperfusion, MC1 mediated effects were reduced, and MC3 anti-inflammatory circuits predominated. Mice bearing a non-functional MC1 displayed a transient exacerbation of neutrophil recruitment following global I/R, which diminished by 2h. However importantly, enhanced inflammatory responses in both MC1 mutant and MC3-/- mice resulted in increased infarct size and poor functional outcome following focal I/R. Furthermore we utilized an in vitro model of leukocyte recruitment to demonstrate these anti-inflammatory actions are also effective in human cells.
Conclusions
These studies reveal for the first time melanocortin control over neutrophil recruitment in the unique pathophysiological context of cerebral I/R, whilst also demonstrating the potential therapeutic value of targeting multiple MCs in developing effective therapeutics.
Keywords: Stroke, BCCAo, MCAo, Melanocortins, Neutrophil
Introduction
Inflammation plays a central role in cerebral I/R injury. Infiltrating neutrophils contribute to a highly neurotoxic milieu as illustrated by the reduced infarct size and improved functional outcome in models of cerebral I/R following depletion of circulating neutrophils1. Anti-inflammatory strategies focused on inhibiting neutrophil recruitment by blocking adhesion molecules have however thus far proven ineffective in clinical trials1. The most probable limiting factor to such therapies is that the inflammatory response is a robust system, propagated by diverse pathways, and as such, cannot be effectively subdued by neutralizing a single component. Thus, harnessing endogenous mechanisms for the resolution of inflammation, which impact multiple elements of the inflammatory response, may provide a fruitful strategy.
Five G-protein coupled melanocortin receptors (MC1-5) and the endogenous agonists, adrenocorticotrophic hormone, α, β and γ melanocyte-stimulating hormones (MSH) make up the melanocortin receptor system 2. Over the last fifteen years, research by our team has been pivotal in helping to unravel the biological effects of peptides within this system demonstrating robust anti-inflammatory actions in a number of inflammatory situations including gouty, rheumatoid, osteoarthritis as well as in cardiovascular and I/R models 3-5. These actions are proposed to be mediated primarily through inhibition of NF-κB. Furthermore, leukocytes are both a target for, and source of, melanocortins suggesting that the melanocortin receptor system may provide a self-limiting anti-inflammatory loop, serving to promote inflammatory resolution2. Such pleiotropic anti-inflammatory actions make these receptors a promising therapeutic candidate to address aberrant inflammation in stroke6.
Of the five MCs identified, anti-inflammatory actions have been attributed primarily to MC1 and MC32. These receptors have both been shown to be expressed at varying levels in the brain and also on endothelial cells and immune cells (MC1 expression on neutrophils; monocytes and macrophages; dendritic cells; natural killer cells and B lymphocytes. MC3 expression on monocytes; macrophages and B lymphocytes)2. However, the exact anti-inflammatory role of MC subtypes remains unclear, and may vary with the pathophysiological environment.
In this study, we utilize two distinct murine models of cerebral I/R to evaluate the dynamic recruitment of neutrophils in the cerebral microcirculation. Using both pharmacological and genetic approaches, we have demonstrated potent inhibitory actions of the melanocortins on cerebral leukocyte trafficking, and gained an insight into the relative importance of MC subtypes in mediating these effects.
Materials and Methods
Materials and Methods are available in the online-only data supplement.
Results
α-MSH abrogates neutrophil recruitment following cerebral I/R
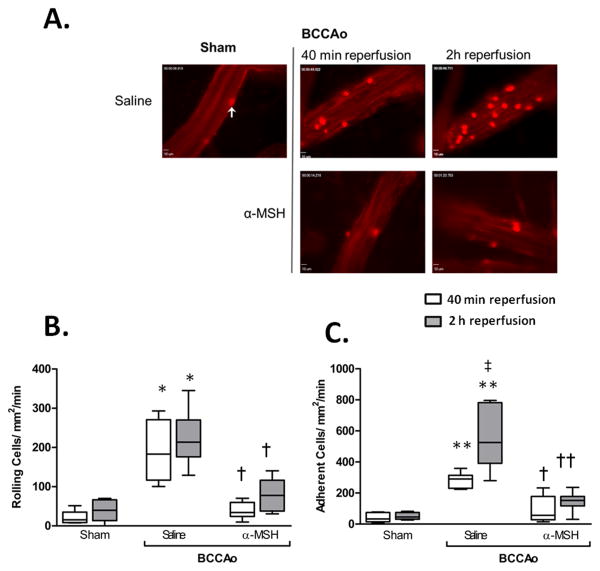
To investigate leukocyte recruitment in the cerebral microvasculature, global ischemia was induced, followed by 40min or 2h reperfusion and leukocyte to endothelial cell (L-E) interactions in pial vessels were assessed by IVM (Figure 1). Sham surgery, produced little to no leukocyte recruitment, however I/R caused significant leukocyte rolling (191.0±31.49cells/mm2/min) and adherence (282.3±49.4cells/mm2/min), with 2h reperfusion resulting in a further significant increase in adhesion to 555.2±85.3cells/mm2/min (Figure 1C, supplemental video I and II). Correlating with the observed effects on leukocyte recruitment was an enhanced serum soluble E-selectin by 2h reperfusion, as detected by ELISA, and a trend toward an increase in the number of ICAM-1 and VCAM-1 positive vessels detected in the brain. No effect was observed with respect to soluble P-selectin (supplemental figure II). α-MSH (10μg i.p.), a non-selective MC agonist, given at the start of reperfusion strongly inhibited leukocyte recruitment at 40min, reducing rolling by 80% and adhesion by 68%, to levels comparable to sham animals. Furthermore these protective effects remained highly significant even after 2h of reperfusion (supplemental video III). In line with the reduced leukocyte recruitment at 2h, α-MSH was also found to reduce levels of soluble E-selectin and a modest reduction in vessel ICAM-1and VCAM-1 expression.
Figure 1. BCCAo induces leukocyte recruitment, which is abrogated by α-MSH treatment.
A) representative IVM video stills show interactions of leukocytes (white arrow) on the cerebral vessel wall, with clearly observable increases following BCCAo. Images taken on an Olympus BW61WI microscope, magnification x 40. B-C) Leukocyte recruitment in the cerebral microcirculation was quantified in terms of: (B) number of cells rolling along the vessel wall per mm2 (termed rolling cell flux); and C) those cells stationary for 30sec or longer (Adhesion: cells/mm2/min). Values represent mean ± SEM. n=6 mice/group. * denotes significance to sham *P<0.01,**P<0.001. † = significance to saline treated BCCAo group P<0.01, †† P<0.001. ‡ = significant increase to 40min reperfusion group. P<0.05 is considered significant
Finally, in order to ascertain the role of neutrophils, some mice were depleted of neutrophils prior to I/R. Following I/R, neutropenic mice displayed significant reductions of leukocyte rolling (81%) and adhesion (76%), consistent with the majority or all cells observed being neutrophils (Supplemental Figure I).
Effect of α-MSH on NF-κB related cytokine and mRNA expression
The effect of cerebral I/R on serum cytokines was investigated using multi-cytokine analysis (Supplemental Figure IIIA-C). Expression of IL-12p70, IFN-γ and MCP-1 remained below the reliable detection range across all treatments (Data not shown). I/R induced a significant increase in serum TNF-α by 2h, which was abolished by α-MSH treatment. The anti-inflammatory cytokine IL-10 showed a trend toward an increase at 2h reperfusion (to 34.7pg/ml), but significantly increased (88.7pg/ml) following α-MSH treatment. Levels of the pleotropic cytokine IL-6 remained unchanged following I/R, however treatment with α-MSH was found to result in a significant up-regulation of IL-6 by 2h. Considering IL-6 signaling via STAT3 has been shown to reduce neutrophil recruitment7, we also investigated levels of tyrosine 705 phosphorylated STAT-3 in leukocyte nuclear fractions by western blot, finding a slight enhancement of STAT3 in α-MSH treated animals at 2h (supplemental figure IV).
To investigate whether this influence over serum cytokines could be due to NF-κB inhibition, mRNA levels of the NF-κB regulatory protein IκB (which closely corresponds to NF-κB activation8) were assessed in both blood and brain using qRT-PCR (Supplemental Figure IVA and B). IκB levels were not significantly elevated 40min following I/R, however by 2h, IκB was significantly increased in the blood, and this was suppressed by α-MSH treatment. Suppression of NF-κB activation in leukocytes at 2h reperfusion was further confirmed by western blot analysis of serine 536 phophorylated NF-kB p65 in leukocyte nuclear fractions.
Effect of MC1 and MC3 agonists on neutrophil recruitment and circulating cytokines
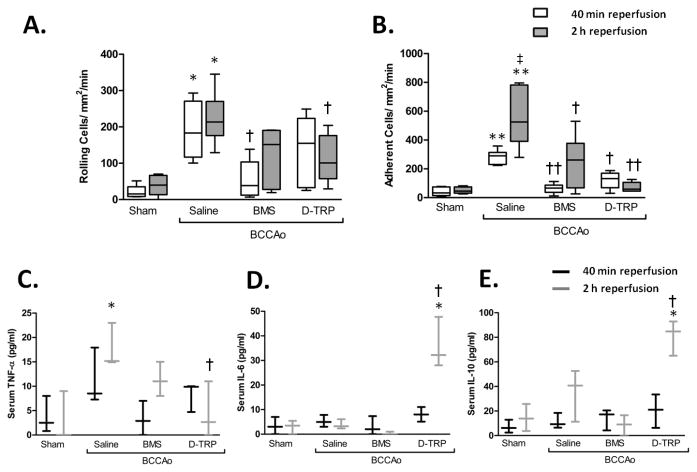
The relative contribution of MC subtypes on IR induced neutrophil recruitment was investigated using more selective MC agonists. Activation of MC1 by BMS-470539 provided a potent inhibition of BCCAo induced neutrophil rolling and adhesion at 40min reperfusion (Figure 2A and B) however, became less effective by 2h, with only the level of adhesion being significant vs. vehicle. On the other hand, treatment with [DTRP8]-γ-MSH, which has a high affinity for MC3, only inhibited cell adhesion at 40min reperfusion, yet by 2h this effect was stronger, with neutrophil rolling also being significantly reduced. Furthermore [DTRP8]-γ-MSH significantly reduced BCCAo induced TNF-α release by 2h whilst also elevating serum levels of the pleiotropic cytokine IL-6 and the anti-inflammatory IL-10 (Figure 2C-E).
Figure 2. The effect of pharmacologically selective treatments on L-E interactions and circulating cytokines.
Leukocyte recruitment in the cerebral microcirculation was quantified in terms of: A) rolling cell flux and B) adhesion (cells/mm2/min), at either 40min or 2h following 5min BCCAo. Treatments were given i.p. at the start of reperfusion using either the MC1 selective BMS-470539 (BMS) or the MC3 agonist [D-TRP8]-γ-MSH (DTRP). Values represent mean ± SEM. Statistical analysis was performed across different treatments within the same time of reperfusion. n=6 mice/group. * denotes significance to sham P<0.01,**P<0.001, † denotes significance to saline treated BCCAo group P<0.01, †† P<0.001. Serum levels of circulating cytokines C)TNF-α, D) IL-6 and E) IL-10 were assessed using a cytometric bead array, n=3 mice/group and performed in duplicate.
Pharmacological investigations using the MC3/4 antagonist SHU9119
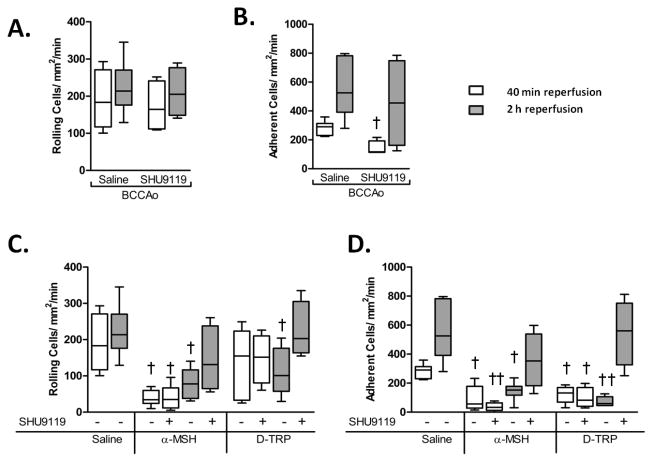
BMS-470539 is almost entirely selective for MC1, whilst [DTRP8]-γ-MSH may activate other MCs than MC39. To further examine the roles of specific MC subtypes, the MC3/4 antagonist SHU9119 was co-administered with either α-MSH or [DTRP8]-γ-MSH (Figure 3C and D), revealing that MC3/4 antagonism caused no increase in rolling or adhesion at 40min reperfusion vs. α-MSH or [DTRP8]-γ-MSH alone. In fact SHU9119 administered alone, or in conjunction with α-MSH, reduced neutrophil rolling. However by 2h, co-administration of SHU9119 blunted the α-MSH induced reductions in rolling and adhesion, and prevented the protective effects of [DTRP8]-γ-MSH.
Figure 3. Effects of MC3/4 antagonism on the actions of α-MSH and [D-TRP8]-γ-MSH.
Leukocyte recruitment in the cerebral microcirculation was quantified in terms of: rolling cell flux and adhesion (cells/mm2/min), following BCCAo and 40min or 2h reperfusion. Affect of the MC3/4 antagonist SHU9119 when administered 10μg i.p at the start of reperfusion on A) leukocyte rolling cell flux and B) leukocyte adhesion. Leukocyte rolling C) and adhesion D) was also assessed in mice treated with either α-MSH or [D-TRP8]-γ-MSH alone (10μg) or in combination with SHU9119 (10μg). α-MSH 40min co-treatment group n=6 mice/group all other co-treatment groups n=4. Comparisons were made to singular treatment groups from Figure 2. Statistical analysis was performed comparing different treatments within the same time of reperfusion. † represents statistical significance to saline treated BCCAo group P<0.01. †† = P<0.001
Melanocortin receptor expression
To determine whether the delayed importance of MC3 was due to change in receptor expression antibody based investigations into receptor expression were undertaken. However initial analysis of MC1 and MC3 protein expression using western blotting revealed antibody binding in MC3-/- mice, using both the Sigma Aldrich (M4937) and Acris (AP10124PU-N) MC3 antibodies, despite PCR confirmation of the MC3-/- (Supplemental figure V). This suggests that both antibodies tested display non-specific binding to protein at a similar molecular weight to MC3, as has been previously described10. Therefore, qRT-PCR was used to quantify MC expression at the mRNA level. MC1,3,4&5, was detected in both blood and brain, however BCCAo induced no changes at either 40min or 2h following BCCAo (Supplemental Figure VI).
Physiological effects of MC1 and 3 in cerebral I/R-induced inflammation
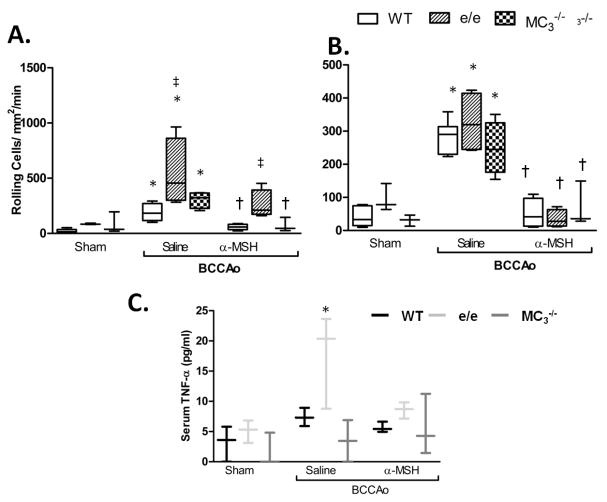
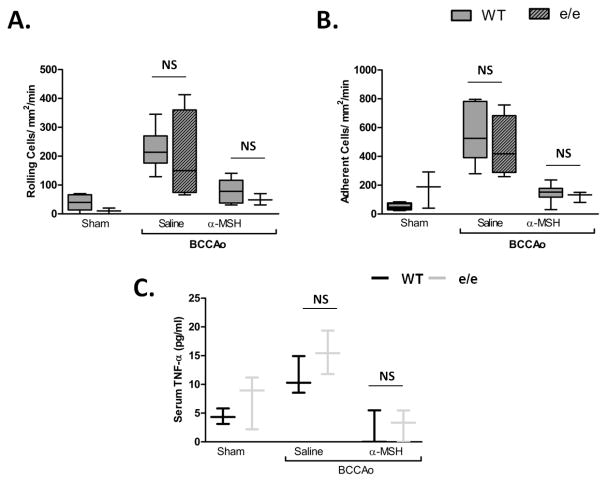
We tested whether the physiological effects of receptor deficiency would support our findings from pharmacological treatments. Recessive yellow (e/e) MC1 mutant mice displayed enhanced (nearly 3 times) neutrophil rolling at 40min following I/R vs. WT (Figure 4A) accompanied by elevated serum TNF-α (Figure 4C). In the absence of a functional MC1, α-MSH reduction of rolling was also hampered at this time point, however e/e mice displayed no derangements in cell adhesion and were able to respond to α-MSH treatment. MC3 null mice showed no significant differences in neutrophil recruitment, or in their ability to respond to α-MSH at 40min. This is consistent with the predominat anti-inflammatory role of MC1 at early reperfusion. In agreement with the diminished role of MC1 observed in pharmacological studies at 2h, at this time point the inflammatory phenotype was not maintained, with TNF-α levels and neutrophil rolling and adhesion being comparable to WT (Figure 5).
Figure 4. Mice bearing a non-functional MC1 display acutely enhanced L-E interactions and TNF-α levels following I/R.
Wild type C57BL/6 (white bars), MC1 mutant e/e (lined bars) and MC3-/- (checked bars) mice were subjected to sham or BCCAo surgery with 40min reperfusion and treatment with either saline or 10μg α-MSH at the start of reperfusion. Leukocyte recruitment in the cerebral microcirculation was quantified in terms of: A) rolling cell flux and B) adhesion (cells/mm2/min). WT groups n=6 mice/group, e/e and MC3-/- sham and MC3-/- α-MSH groups n=3 all other groups n=4 mice/group. C) ELISA measurements of serum TNF-α levels n=3 mice/group and performed in duplicate. Statistical analysis was performed between mouse strains, within sham or saline treated groups to determine the phenotypic differences between strains, ‡ denotes significance to WT P<0.01. Statistical analysis was also performed between sham, saline and α-MSH treatment groups within the same strain. * shows significance to sham group P<0.05 and † shows significance to saline treated BCCAo group P<0.05.
Figure 5. The e/e mouse inflammatory phenotype is diminished by 2h.
WT (C57BL/6) (white bars) and e/e (lined bars) mice were subjected to sham or BCCAo and treated with saline or 10μg α-MSH at the start of reperfusion. Leukocyte recruitment was quantified at 2h of reperfusion in terms of: in terms of: A) rolling cell flux and B) leukocyte adhesion (cells/mm2/min). n = 4 mice/group for e/e saline group, and n=3 mice/group for sham and α-MSH e/e groups, comparisons were made to WT groups as in figure 1 (n=6 mice/group). Statistical analysis was performed between mouse strains, within the same treatment group to evaluate phenotypic differences between strains. NS denotes no statistical significance to WT group. C) ELISA measurements of serum TNF-α in WT and e/e mice. * denotes significance to sham groups P<0.01, † denotes significance to BCCAo P<0.05.
Physiological role in focal stroke model
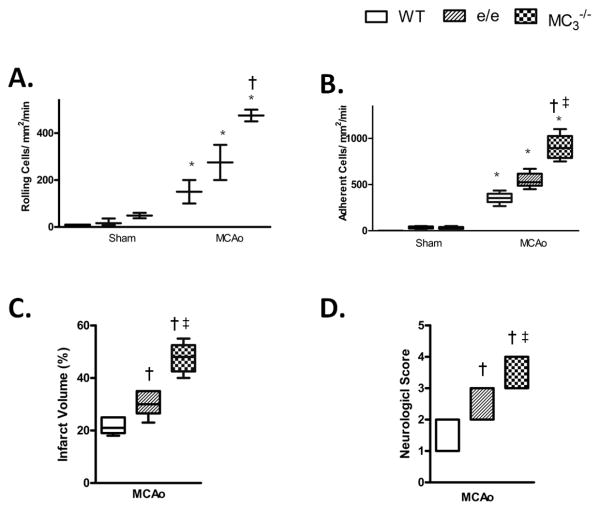
Stroke is highly variable in its severity and magnitude. The global model of cerebral I/R, represents human stroke conditions caused by atherosclerotic degeneration of the common carotid arteries and respiratory or cardiac arrest. To investigate whether the MC anti-inflammatory effects we were observing were specific to global I/R, we also undertook investigations using a focal stroke model (i.e. the middle cerebral artery occlusion (MCAo) model). We chose this model due to the fact that focal ischemia accounts for ∼80% of ischemic stroke. Figure 6 demonstrates that at 24h post ischemia while e/e mice showed elevated adhesion, MC3-/- displayed a more severe inflammatory phenotype with significantly enhanced rolling and adhesion. Importantly, the increased neutrophil recruitment observed, translated into elevated infarct volume and poor functional outcome (detected through neurological scoring) in animals with deficits in either receptor, suggesting both receptors to be potential pharmacological targets.
Figure 6. Physiological role of MC1 and MC3 in focal cerebral I/R.
(A and B) Leukocyte recruitment in the cerebral microcirculation was quantified following MCAo and 24h reperfusion in terms of: rolling cell flux and leukocyte adhesion (cells/mm2/min). C) Infarct volume and D) Neurological score were also assessed. Values represent mean ± SEM. n=4-6 mice/group. * denotes significance to corresponding sham group, +denotes significance to WT MCAo group. # denotes significance to WT e/e group. P<0.05 is considered significant.
In vitro investigations of melanocortin effects on neutrophil functioning
The melanocortin receptor system displays a number of disparities between humans and rodents and a number of MC agonists and antagonists have different selectivity in MCs from different species9,11. To assess the effectiveness of melanocortin treatments on human cells, we utilized the neutrophil flow chamber model and chemotaxis assay to investigate neutrophil inflammatory function (Supplemental Figure VII). In the flow chamber, treating neutrophils with just 10μg/ml of α-MSH (a comparable dose to in vivo studies) resulted in significant reductions in capture (54% reduction) adherence (68%) and transmigration (67%) vs. saline (Supplemental Figure VIIA). Treating HUVECs with α-MSH (≤100μg/ml), however failed to induce significant reductions in neutrophil recruitment (data not shown), suggesting neutrophils to be the effector cells of MC actions in this context. In the chemotaxis assay, pre-treatment with 10μg/ml of α-MSH or [DTRP8]-γ-MSH failed to inhibit neutrophil migration towards fMLP, however BMS-470539 significantly reduced this response, suppressing the number of migrated cells by ∼75% (Supplemental Figure VIIB), suggesting MC1 to play a specific role in inhibiting neutrophil chemotaxis.
Discussion
The present study provides, for the first time, evidence that melanocortin treatments can effectively inhibit neutrophil recruitment in the unique microenvironment of the cerebral microvasculature. Utilizing both selective ligands and MC mutant mice, we have gained an insight into MCs involved in modulating neutrophil recruitment in two separate models of cerebral I/R, finding both MC1 and MC3 to display important inhibitory roles. In addition, melanocortin treatment was effective in modulating human neutrophil inflammatory functioning, and may represent a novel treatment to stem post-stroke inflammation.
Both ischemic models used (BCCAo and MCAo) here resulted in a pronounced increase in neutrophil rolling and adhesion compared to sham, as has previously been observed12,13. Non-selective treatment with α-MSH caused an abrogation of BCCAo-induced neutrophil rolling and adhesion, congruent with a trend toward a reduction in ICAM-1 and VCAM-1 expression in the cerebral vascular. Considering these effects were not significant, other adhesion molecules and integrin activation state may also play a role in the melanocortin influence over leukocyte adhesion. While anti-ICAM-1 antibody Enlimomab failed clinical trials for stroke (possibly due to inflammatory side effects to the mouse monoclonal antibody) strategies inhibiting CAMs have shown great promise pre-clinically. Soluble P and E-selectin are early markers of endothelial activation. While levels of soluble P selectin were found only to produce a trend toward an increase following BCCAo which was unchanged by treatment, soluble E-selectin was increased by 2h post ischemia. Elevated levels of soluble E-selectin have been demonstrated in human stroke and during sepsis14. In vitro studies have also shown that endothelial cells stimulated with IL-1β, TNF-α, endotoxin or serum deprivation and TNF-α release E-selectin into the culture supernatant15,16. α-MSH treatment significantly reduced BCCAo induced soluble E-selectin, likely a reflection of reduced endothelial cell activation, consistent with the reduced levels of IL-1β and TNF-α observed.
Treatment also reduced serum TNF-α and IL-1β. IL-1β in particular plays a pivotal role in propagating inflammatory responses and is an established pathological factor in cerebrovascular disease, with significant pre-clinical and clinical evidence demonstrating blockade of IL-1β signaling to be beneficial in stroke17. MSH treatment also simultaneously enhanced anti-inflammatory IL-10 by 2h reperfusion. The ability of MCs to suppress pro-inflammatory responses whilst enhancing anti-inflammatory signals suggests that these receptors form an endogenous pro-resolving system. In humans α-MSH concentrations increases in myocardial infarction and infection2 and higher melanocortin levels correlate with better outcome in stroke patients18. Thus, given the results from the present study the melanocortin receptor system may prove a valuable therapeutic target for the treatment of stroke.
While others have demonstrated a contribution of either MC1 or MC3 in providing anti-inflammatory protection in different systemic inflammatory models, we have, for the first time, identified protective roles for both receptors in the cerebral-microvasculature. In particular, we have identified that MC1 mediated effects predominate in early protection, while MC3 actions are more delayed and induce pro-resolving factors. This study reveals an additional layer of complexity in MC inflammatory modulation, emphasizing the importance of drug treatment directed at both receptors.
Pharmacological investigations in the BCCAo model revealed the MC1 agonist BMS-470539, significantly inhibited early neutrophil rolling and adhesion, while activation of MC3 using [D-TRP8]-γ-MSH only reduced adhesion. However by 2h following BCCAo, despite a robust pharmacodynamic half-life of ∼8 h19, BMS-470539 lost its inhibitory actions on rolling and the reduction of adhesion was diminished slightly. However at 2h, [D-TRP8]-γ-MSH anti-inflammatory actions were enhanced. Thus suggesting MC3 mediated effects become more prominent at later time points. SHU9119 (MC3/4 antagonist) was used to further explore the role of MC subtypes. By 40min reperfusion, SHU9119 enhanced, rather than inhibited, α-MSH and [D-TRP8]-γ-MSH effects. Furthermore, administered alone, SHU9119 reduced neutrophil adhesion at 40min, possibly due to its seldom reported agonist actions at MC1/511, further supporting the predominance of MC1 mediated effects at early stages of reperfusion. By 2h reperfusion SHU9119 lost these anti-inflammatory effects, and co-treatments with SHU9119 inhibited the protective effects of α-MSH and [D-TRP8]-γ-MSH, illustrating role for MC3 in mediating delayed effects on neutrophil adhesion.
Despite the apparent change in MC engagement, no change in MC RNA expression was detected following BCCAo. Whether this is also reflected in protein expression is difficult to discern given the lack of specificity of the antibodies. Even if the surface expression of MC1 or MC3 is increased following stroke, α-MSH and [D-TRP8]-γ-MSH have short half-lives and therefore are most likely exerting their effects before such up-regulation occurs. The Shift in receptor importance may instead reflect activation of distinct mechanisms of action and/or effector cells. Neutrophils express MC1, while MC3 expression is limited to endothelial cells and macrophages/monocytes. Rapid inhibitory actions exerted by MC1 may be through direct interactions with neutrophils, while the more prolonged MC3 actions are likely via actions on other effector cells. Inhibition of NF-κB is a key element of the protective MC actions2. Given NF-κB DNA binding occurs only after 30min following TNF-α stimulation, followed by gene transcription 30min later8, NF-κB inhibition is unlikely to mediate the rapid effects on neutrophil recruitment at 40min. Indeed IκB transcript levels were not significantly increased at 40min, yet by 2h blood IκB elevated 25-fold and nuclear levels of phosphorlyated NF-κB protein were enhanced, which was inhibited by α-MSH. Given our finding of the delayed effect of MC3 targeted treatment on neutrophil this later effect by α-MSH on IκB may be mediated predominantly via MC3 signaling.
Melanocortin treatments inhibited neutrophil recruitment at 40min after BCCAo, a timeframe incompatible with transcriptional changes. Such a phenomenon has previously been observed in other models; in zymosan stimulated macrophages AP214 reduced IL-1β and TNF-α release with no effect on mRNA levels20, and α-MSH can act via MC1 to shed cell surface CD14 on macrophages21 and IL-8 receptors on neutrophils22 independently of mRNA changes. While the understanding of NF-κB independent MC effects is still in its infancy, these mechanisms may fit well with the early efficacy of MC1 treatments in suppressing neutrophil recruitment.
Differences in cytokine regulation between MC1 targeted activation and MC3 targeted/non-specific activation further support the hypothesis that MC1 and MC3 act via distinct mechanisms. Both α-MSH and [D-TRP8]-γ-MSH significantly reduced BCCAo induced TNF-α whilst enhancing anti-inflammatory IL-10. TNF-α has multiple roles in stroke pathology and early increases in blood TNF have been shown to correlate with stroke severity in humans23 while the anti-inflammatory cytokine IL-10 has been shown to be protective in a number of models of cerebral I/R24,25. BMS-470539 treatments however had no effect on these NF-κB controlled cytokines further supporting a distinct mechanism of action for the MC1 mediated early inhibition of neutrophil recruitment, while MC3 may be more prominent in initiating pro-resolving effects. Conflicting reports have been made as the effect of melanocortin treatments on IL-6 levels, however in the current investigations both α-MSH and [D-TRP8]-γ-MSH increased this pleiotropic cytokine at 2h, despite a decrease in NF-κB levels. As such the observed IL-6 release may be as a result of elevated release of pre-synthesized IL-6. α-MSH has previously been shown to influence IL-6 levels via MC326, thus, explaining why IL-6 induction was not observed in BMS-470539 treated animals. While IL-6 is an endogenous pyrogen with chemotactic activity, IL-6 KO mice show no protection following experimental stroke27. Indeed IL-6 signaling via STAT3, has been shown to limit the inflammatory recruitment of neutrophils7, given that IL-10 may also activate STAT3 and levels were increased following α-MSH treatment such findings appear to be consistent with the present results. Furthermore it has been demonstrated that NF-κB signalling may induce expression of IL-6 and IL-10 in cells with a high level of STAT3 phosphorylation28. In line with such observations we found nuclear extracts from α-MSH treated BCCAo animals to show a trend toward an elevation of phosphorylated STAT-3 protein.
Our investigations using MC mutant mice, in two independent models of stroke, further support a temporal difference in MC1 and MC3 actions. Recessive yellow e/e mice displayed a transiently enhanced neutrophil rolling and elevated TNF-α at the very onset of the BCCAo induced inflammatory response, while MC3-/- mice showed no inflammatory phenotype at this early time point. It is possible that compensatory up regulation of other MCs may mask the anti-inflammatory role of this receptor in MC3-/-. Montero-Melendez et al., have previously shown that while in macrophages isolated from wild type and e/e zymosan challenge induced no change in MC1 MC3 or MC5 expression, in MC3−/− inflammatory challenge led to marked gene activation for MC1 and MC5 20 perhaps indicating compensatory regulation of other MC in the absence of MC3. However in the focal stroke model, at 24h MC3-/- animals displayed enhanced rolling and adhesion further supporting a delayed role of this receptor, while e/e animals only showed elevated adhesion compared to WT, perhaps as a result enhanced rolling prior to the observation period. Previous reports and unpublished data from our laboratory have shown no significant differences in the basal circulating levels of leukocytes between WT, e/e and MC3-/- 3. As such the observed enhanced leukocyte recruitment is unlikely to be due to an initially high leukocyte count in these animals. As observed in BCCAo animals both receptors appear to be of importance with MC3 anti-inflammatory circuits predominating at later time points.
Previously, Leoni et al., demonstrated that the MC3-/- mouse displays higher levels of leukocyte adhesion and emigration in the mesenteric microcirculation following I/R, while the response of the e/e mouse was not significantly different from WT 3. Yet in the same model the MC1 agonist BMS-470539 was effective in reducing leukocyte recruitment (an effect which was absent in e/e mice)4. These results led the authors to postulate that the receptors show different physiological roles but that both may be harnessed pharmacologically. The present findings demonstrate a physiological role for both receptors in the cerebral microcirculation. Indeed the observed of temporally distinct roles of these two receptors may help to explain such apparently conflicting findings across studies investigating the anti-inflammatory actions of these receptors. Crucially, our study demonstrates that the absence of signaling from either receptor resulted in enhanced infarct size and worse functional outcome compared to WT, further illustrating that both receptors are important pharmacological targets.
Our studies have further demonstrated that melancortin peptides may effect neutrophil inflammatory functioning in human cells. As the melanocortin receptor system displays a number of disparities in organization and function between humans and rodents. Such as differences in potency and selectivity of melanocortin ligands, and receptor expression between species, we also investigated MC roles in human neutrophil functioning. The facts that α-MSH treatment was found to suppress neutrophil recruitment to HUVECs via actions on neutrophils, and that MC1, but not MC3, is expressed on neutrophils supports the concept of an MC1 specific effect. These effects were rapid, occurring within 30min following treatments, again a timeframe incompatible with transcriptional mechanisms. MC1 specific direct effects on neutrophils were further illustrated by the ability of MC1 targeted treatments to significantly reduce neutrophil chemotaxis (performed in the absence of other cell types). Further investigation however, will need to be made to establish the effector cell type of the delayed, but potent, effects of MC3 on neutrophil recruitment.
Taken together, novel experimental data presented here highlights a role for utilization of melanocortin based treatments as potential therapeutics for stroke, and potentially other neurovascular diseases. This work also demonstrates important roles for both MC1 and MC3, showing MC1 effects to provide rapid inhibition of leukocyte recruitment via mechanisms independent of NF-κB regulation, while MC3 actions appear more robust at later time points. Given the complexity of MC regulation along with the variable nature of stroke, strategies targeting multiple MCs in a non-(or perhaps partially) selective manner may be more fruitful in providing robust protection, rather than targeting one MC alone. Indeed, it may be telling that currently the most promising melanocortin compounds (NDP-α-MSH and AP214) are both non-selective MC agonists. Further investigations into later time points of treatment and in animals with co-morbidities will help to reveal the full potential of this promising therapeutic target for stroke.
Supplementary Material
Significance.
Stroke is a leading cause of mortality and morbidity worldwide. Following the initial ischemic brain damage an ensuing inflammatory response may exacerbate injury. Neutrophils are key arbitrators of this damaging response and inhibiting neutrophil recruitment in cerebral ischemia reperfusion injury may provide therapeutic benefit. We have previously demonstrated that melanocortin treatments can reduce leukocyte recruitment in peripheral tissues, but these actions have yet to be demonstrated in the unique microcirculation of the brain. Here in, we show that melanocortins reduce post ischemic leukocyte recruitment in the brain, and that these effects are mediated by the distinct actions of both melanocortin receptors 1 and 3. Therefore, targeting these receptors provides a novel therapeutic strategy for treating stroke, and other cerebrovascular diseases.
Acknowledgments
PMH performed, designed and analyzed experiments and wrote the manuscript. PFD performed experiments, DC performed some flow chamber experiments and JG performed staining of cellular adhesion molecules. MP provided scientific input and provided the e/e and MC3-/- animals, SJG and FNEG designed and analyzed the experiments and wrote the manuscript. We also thank Monika Dowejko (University of Westminster, UK) for genotyping the MC3-/- mouse, Dr. Lucy Norling (William Harvey Research Institute, UK) for her help with the flow chamber model and Jonette Green (LSUHSC-S) for help with the immunofluorescent staining. Marjan Trutschl and Urska Cvek provided input on the statistical analysis and techniques.
Sources of funding: This study was funded by The British Heart Foundation (studentship FS/09/020/27184). Drs. Trutschl and Cvek's work reported in this publication was supported by the National Institute Of General Medical Sciences of the National Institutes of Health under Award Number P30GM110703.
Abbreviations
- BCCAo
Bilateral common carotid artery occlusion
- EC
Endothelial cell
- e/e
MC1 mutant recessive yellow e/e
- I/R
Ischemia-reperfusion
- IVM
Intravital microscopy
- L-E
Leukocyte, endothelial cell
- MC
Melanocortin Receptor
- MC3-/-
Melanocortin receptor 3 null
- MCAo
Middle cerebral artery occlusion
- MSH
Melanocyte stimulating hormone
Footnotes
Disclosures: none
References
- 1.Sughrue ME, Mehra A, Connolly ES, D'Ambrosio AL. Anti-adhesion molecule strategies as potential neuroprotective agents in cerebral ischemia: A critical review of the literature. Inflamm Res. 2004;53:497–508. doi: 10.1007/s00011-004-1282-0. [DOI] [PubMed] [Google Scholar]
- 2.Catania A, Gatti S, Colombo G, Lipton JM. Targeting Melanocortin Receptors as a Novel Strategy to Control Inflammation. Pharmacol Rev. 2004;56:1–29. doi: 10.1124/pr.56.1.1. [DOI] [PubMed] [Google Scholar]
- 3.Leoni G, Patel HB, Sampaio ALF, Gavins FN, Murray JF, Grieco P, Getting SJ, Perretti M. Inflamed phenotype of the mesenteric microcirculation of melanocortin type 3 receptor-null mice after ischemia-reperfusion. FASEB J. 2008;22:4228–4238. doi: 10.1096/fj.08-113886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leoni G, Voisin MB, Carlson K, Getting SJ, Nourshargh S, Perretti M. The melanocortin MC1 receptor agonist BMS-470539 inhibits leucocyte trafficking in the inflamed vasculature. Br J Pharmacol. 2010;160:171–180. doi: 10.1111/j.1476-5381.2010.00688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiao, Foster, Thomas, Lipton, Star Alpha-melanocyte-stimulating hormone reduces endotoxin-induced liver inflammation. J Clin Invest. 1996;97:2038–2044. doi: 10.1172/JCI118639. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holloway PM, Smith HK, Renshaw D, Flower RJ, Getting SJ, Gavins FNE. Targeting the melanocortin receptor system for anti-stroke therapy. Trends Pharmacol Sci. 2011;32:90–98. doi: 10.1016/j.tips.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 7.Fielding CA, McLoughlin RM, McLeod L, Colmont CS, Najdovska M, Grail D, Ernst M, Jones SA, Topley N, Jenkins BJ. IL-6 regulates neutrophil trafficking during acute inflammation via STAT3. J Immunol. 2008;181:2189–2195. doi: 10.4049/jimmunol.181.3.2189. [DOI] [PubMed] [Google Scholar]
- 8.Bottero V, Imbert V, Frelin C, Formento JL, Peyron JF. Monitoring NF-kappa B transactivation potential via real-time PCR quantification of I kappa B-alpha gene expression. Molecular diagnosis : a journal devoted to the understanding of human disease through the clinical application of molecular biology. 2003;7:187–194. doi: 10.1007/BF03260037. [DOI] [PubMed] [Google Scholar]
- 9.Joseph CG, Yao H, Scott JW, Sorensen NB, Marnane RN, Mountjoy KG, Haskell-Luevano C. γ2-Melanocyte stimulation hormone (γ2-MSH) truncation studies results in the cautionary note that γ2-MSH is not selective for the mouse MC3R over the mouse MC5R. Peptides. 2010;31:2304–2313. doi: 10.1016/j.peptides.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kathpalia PP, Charlton C, Rajagopal M, Pao AC. The natriuretic mechanism of Gamma-Melanocyte-Stimulating Hormone. Peptides. 2011;32:1068–1072. doi: 10.1016/j.peptides.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hrubu VJ, Lu D, Sharma SD, Castrucci AL, Kesterson RA, al-Obeidi FA, Hadley ME, Cone RD. Cyclic lactam .alpha.-melanotropin analogs of Ac-Nle4-cyclo[Asp5,D-Phe7,Lys10]-.alpha.-melanocyte-stimulating hormone-(4-10)-NH2 with bulky aromatic amino acids at position 7 show high antagonist potency and selectivity at specific melanocortin receptors. J Med Chem. 1995;38:3454–3461. doi: 10.1021/jm00018a005. [DOI] [PubMed] [Google Scholar]
- 12.Gavins FNE, Dalli J, Flower RJ, Granger DN, Perretti M. Activation of the annexin 1 counter-regulatory circuit affords protection in the mouse brain microcirculation. FASEB J. 2007;21:1751–1758. doi: 10.1096/fj.06-7842com. [DOI] [PubMed] [Google Scholar]
- 13.Ishikawa M, Cooper D, Russell J, Salter JW, Zhang JH, Nanda A, Granger DN. Molecular determinants of the prothrombogenic and inflammatory phenotype assumed by the postischemic cerebral microcirculation. Stroke. 2003;34:1777–1782. doi: 10.1161/01.STR.0000074921.17767.F2. [DOI] [PubMed] [Google Scholar]
- 14.Frijns CJ, Kappelle LJ, van Gijn J, Nieuwenhuis HK, Sixma JJ, Fijnheer R. Soluble adhesion molecules reflect endothelial cell activation in ischemic stroke and in carotid atherosclerosis. Stroke. 1997;28:2214–2218. doi: 10.1161/01.str.28.11.2214. [DOI] [PubMed] [Google Scholar]
- 15.Wayne Smith C. Potential Significance of Circulating E-Selectin. Circulation. 1997;95:1986–1988. doi: 10.1161/01.cir.95.8.1986. [DOI] [PubMed] [Google Scholar]
- 16.Harrington EO, Stefanec T, Newton J, Rounds S. Release of soluble E-selectin from activated endothelial cells upon apoptosis. Lung. 2006;184:259–266. doi: 10.1007/s00408-005-2589-5. [DOI] [PubMed] [Google Scholar]
- 17.Denes A, Pinteaux E, Rothwell NJ, Allan SM. Interleukin-1 and Stroke: Biomarker, Harbinger of Damage, and Therapeutic Target. Cerebrovasc Dis. 2011;32:517–527. doi: 10.1159/000332205. [DOI] [PubMed] [Google Scholar]
- 18.Zierath D, Tanzi P, Cain K, Shibata D, Becker K. Plasma alpha-Melanocyte Stimulating Hormone Predicts Outcome in Ischemic Stroke. Stroke. 2011;42:3415–3420. doi: 10.1161/STROKEAHA.111.627331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang L, McIntyre KW, Gillooly KM, et al. A selective small molecule agonist of the melanocortin-1 receptor inhibits lipopolysaccharide-induced cytokine accumulation and leukocyte infiltration in mice. J Leukoc Biol. 2006;80:897–904. doi: 10.1189/jlb.1204748. [DOI] [PubMed] [Google Scholar]
- 20.Montero-Melendez T, Patel HB, Seed M, Nielsen S, Jonassen TEN, Perretti M. The Melanocortin Agonist AP214 Exerts Anti-Inflammatory and Proresolving Properties. Am J Pathol. 2011;179:259–269. doi: 10.1016/j.ajpath.2011.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarkar A, Sreenivasan Y, Manna SK. alpha-Melanocyte-stimulating hormone inhibits lipopolysaccharide-induced biological responses by downregulating CD14 from macrophages. FEBS Lett. 2003;553:286–294. doi: 10.1016/s0014-5793(03)01029-9. [DOI] [PubMed] [Google Scholar]
- 22.Manna Sunil K, Sarkar A, Sreenivasan Y. Alpha-melanocyte-stimulating hormone down-regulates CXC receptors through activation of neutrophil elastase. Eur J Immunol. 2006;36:754–769. doi: 10.1002/eji.200535209. [DOI] [PubMed] [Google Scholar]
- 23.Zaremba J, Losy J. Early TNF-α levels correlate with ischaemic stroke severity. Acta Neurol Scand. 2001;104:288–295. doi: 10.1034/j.1600-0404.2001.00053.x. [DOI] [PubMed] [Google Scholar]
- 24.Liesz A, Bauer A, Hoheisel JD, Veltkamp R. Intracerebral interleukin-10 injection modulates post-ischemic neuroinflammation: An experimental microarray study. Neurosci Lett. 2014;579:18–23. doi: 10.1016/j.neulet.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 25.Spera PA, Ellison JA, Feuerstein GZ, Barone FC. IL-10 reduces rat brain injury following focal stroke. Neurosci Lett. 1998;251:189–192. doi: 10.1016/s0304-3940(98)00537-0. [DOI] [PubMed] [Google Scholar]
- 26.Lindberg, Hjorth, Post, Winblad, Schultzberg Cytokine production by a human microglial cell line: effects of beta-amyloid and alpha-melanocyte-stimulating hormone. Neurotox Res. 2005;8:267–276. doi: 10.1007/BF03033980. [DOI] [PubMed] [Google Scholar]
- 27.Clark WM, Rinker LG, Lessov NS, Al Masaoudi M, Sellge G, Nevzorova YA, Gassler N, Liedtke C, Cubero FJ, Trautwein C. Lack of Interleukin-6 Expression Is Not Protective Against Focal Central Nervous System Ischemia. Stroke. 2000;31:1715–1720. doi: 10.1161/01.str.31.7.1715. [DOI] [PubMed] [Google Scholar]
- 28.Lam LT, Wright G, Davis RE, Lenz G, Farinha P, Dang L, Chan JW, Rosenwald A, Gascoyne RD, Staudt LM. Cooperative signaling through the signal transducer and activator of transcription 3 and nuclear factor-{kappa}B pathways in subtypes of diffuse large B-cell lymphoma. Blood. 2008;111:3701–3713. doi: 10.1182/blood-2007-09-111948. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.