Abstract
Cardiometabolic disease, emerging as a worldwide epidemic, is a combination of metabolic derangements leading to type 2 diabetes and cardiovascular disease. Genetic and environmental factors are linked through epigenetic mechanisms to the pathogenesis of cardiometabolic disease. Post-translational modifications of histone tails, including acetylation and deacetylation, epigenetically alter chromatin structure and dictate cell-specific gene expression patterns. The histone deacetylase (HDAC) family is comprised of 18 members that regulate gene expression by altering the acetylation status of nucleosomal histones and by functioning as nuclear transcriptional co-repressors. HDACs regulate key aspects of metabolism, inflammation, and vascular function pertinent to cardiometabolic disease in a cell- and tissue-specific manner. HDACs also likely play a role in the “metabolic memory” of diabetes, an important clinical aspect of the disease. Understanding the molecular, cellular, and physiological functions of HDACs in cardiometabolic disease is expected to provide insight into disease pathogenesis, risk factor control, and therapeutic development.
Keywords: cardiovascular, histone deacetylases, inflammation, metabolic disease, sirtuins
Introduction
Epigenetic processes influence gene expression and regulation without altering the information encoded by the primary DNA sequence. In the 1960s, Allfrey and colleagues discovered that chemical modification of histones influenced chromatin structure and RNA synthesis in eukaryotic cells, thus laying the foundation for modern epigenetics research1. Subsequently, proteins that possess intrinsic histone acetylase and deacetylase activities were identified2, 3, followed by enzymes involved in histone methylation, ubiquitination, and sumoylation4. Reversible modification of histone proteins is a fundamental mechanism of gene regulation in cell differentiation, organogenesis, growth, aging, etc.
HDACs are a class of enzymes that remove acetyl groups from an ε- N-acetyl lysine amino acid on histone or non-histone proteins. Deacetylation of histones promotes a closed chromatin structure, thereby impairing access of transcription factors to their regulatory sites and silencing gene expression. Some HDACs are multifunctional proteins that can also suppress gene expression by functioning as transcriptional co-repressors, independent of their deacetylase activity5, 6. To date, 18 evolutionary-conserved mammalian HDACs have been identified and are grouped into four classes based on their phylogenetic conservation7. HDACs exhibit a wide variety of functional activities and cellular and tissue distribution (Supplemental Table 1). Numerous studies describing HDAC protein regulation, structure, and functions pertinent to cardiovascular and metabolic diseases have been summarized in recent publications8–12. This review will highlight cellular mechanisms of HDAC action, in vivo data in animal models of cardiometabolic disease, and studies linking HDACs to cardiometabolic disease in humans.
There is a strong interplay between metabolic disease, inflammation, and cardiovascular risk factors. Metabolic syndrome is a pro-inflammatory state that is tightly linked to C-reactive protein levels in humans13, and vascular inflammation plays a role in all stages of atherosclerosis14. Dyslipidemia, hypertension, and insulin resistance, major components of metabolic syndrome, are powerful cardiovascular disease risk factors15, 16. Data from the longitudinal Framingham heart study suggest that hypertension and glucose intolerance are interrelated phenomena that predispose to the development of premature atherosclerotic disease17. Additionally, growing evidence suggests insulin resistance predisposes to heart failure18. A better understanding of the molecular mechanisms whereby HDACs regulate the interplay between metabolic disease, inflammation, and cardiovascular risk factors could lead to novel therapeutic approaches for cardiometabolic disease.
Potential Role of HDACs in Metabolic Disease
Class I and II HDACs have been reported to regulate a variety of metabolic processes, including differentiation of pancreatic islet cells and adipocytes11,12. HDAC5 or HDAC9 gene deletion increased pancreatic β-cell mass and insulin secretion but not β-cell proliferation, consistent with a primary effect on pancreatic β-cell differentiation19. This may have important clinical implications for optimization of stem cell transplantation therapy for diabetes20. In addition to the pancreas, adipose tissues factor prominently in metabolic disease. Failure of white adipocytes to differentiate and properly store excess calories in obesity is associated with adipose tissue inflammation, insulin resistance, and type 2 diabetes21. Conversely, thermogenic beige and brown adipocytes promote insulin sensitivity and weight loss22. HDAC3 inhibition was reported to regulate PPARγ acetylation and activity, thereby enhancing insulin signaling and glucose uptake in white adipocytes and improving insulin sensitivity in diet-induced obese (DIO) mice23. Adipose HDAC3 was also demonstrated to repress phosphoenolpyruvate carboxykinase, the key enzyme controlling glyceroneogenesis, resulting in reduced triglyceride biosynthesis and lipodystrophy. The mechanism was linked to activation of NFkβ by HDAC324.
HDAC9 overexpression repressed, while HDAC9 gene deletion accelerated, white adipocyte differentiation in vitro25. Interestingly, DIO was associated with upregulated HDAC9 expression in white adipose tissues and impaired adipocyte differentiation, which was abrogated by HDAC9 gene deletion. HDAC9 gene deletion led to diminished weight gain and hepatic steatosis along with improved glucose tolerance and insulin sensitivity25, 26. Additionally, HDAC9 deficiency promoted accumulation of beige adipocytes in subcutaneous adipose, which likely contributed to the lean body mass in these mice26.
Class III HDACs (sirtuins, or SIRTs) have been reported to favorably regulate key metabolic pathways, including adipogenesis, fatty acid metabolism, amino acid metabolism, and gluconeogenesis27, 28. For example, SIRT1 was reported to repress visceral white adipogenic genes associated with insulin resistance and stimulate brown adipogenic gene expression29. Mice lacking SIRT1 that were fed a high fat diet developed excessive hepatic lipid accumulation, altered gut microbiota, insulin resistance, and hypertrophic white and brown adipose tissues30. Likewise, loss of SIRT3 promoted hyperacetylation of mitochondrial proteins, resulting in metabolic perturbations and increased susceptibility to metabolic disease31. Additionally, SIRT3 was demonstrated to regulate systemic oxidative stress, limit expedited weight gain, and promote metabolic adaptation32. Thus, animal studies suggest that SIRTs may protect against the development of metabolic disease. An ongoing clinical trial is testing the anti-diabetic efficacy of SIRT1 activation in humans33.
The liver has emerged as an important target of HDACs’ metabolic effects. Liver-specific HDAC3 and SIRT6 have been shown to regulate hepatic lipid synthesis, glycolysis, and fatty acid oxidation34, 35. Mice lacking SIRT1 specifically in the liver displayed impaired mTORC2/Akt signaling, resulting in oxidative damage, insulin resistance, and hyperglycemia36. Hepatic SIRT1 deficiency also impaired PPARα signaling and decreased fatty acid beta-oxidation, resulting in hepatic steatosis, hepatic inflammation, and endoplasmic reticulum stress in high fat fed mice37. Conversely, loss of hepatic class IIa HDACs (HDAC4,-5,-7) was metabolically protective, as it lowered fasting blood glucose levels and improved glucose tolerance in diabetic mouse models38.
Skeletal muscle, which is responsible for more than 30% of resting metabolic rate and 80% of whole body glucose uptake39, is also emerging as a target of HDACs. Skeletal muscle is composed of heterogeneous myofibers with characteristic metabolic properties. Class II HDACs have been shown to suppress the formation of type I myofibers, which stimulate insulin-mediated glucose uptake and protect against glucose intolerance, through the repression of MEF2 activity40, 41. Overexpression of HDAC5 was reported to suppress skeletal muscle glucose uptake by repressing GLUT4 gene expression42. Conversely, deletion of SIRT3 in skeletal muscle perturbed mitochondrial function and promoted oxidative stress and insulin resistance43.
Mounting evidence suggests that both peripheral and central mechanisms act in concert to maintain energy balance. The hypothalamic/pituitary axis is essential to the central control of whole-body metabolism. Delivery of a SIRT1 activator, resveratrol, into the central nervous system was shown to attenuate hyperglycemia and hyperinsulinemia in diabetic and DIO mice44. SIRT1 in pro-opiomelanocortin neurons was reported to be required for leptin’s central functions and for energy expenditure adaptations to DIO45. Furthermore, SIRT1 was found to act on steroidogenic factor 1 neurons to protect against the development of DIO and hyperglycemia via promoting energy expenditure and skeletal muscle insulin sensitivity46.
The concept of ‘metabolic memory’ was proposed by Nathan and colleagues in relation to their clinical findings that the benefits of tight glycemic control on micro- and macrovascular complications in diabetic patients might not be immediately obvious but become more evident with time47. Consistently, metabolic memory has been demonstrated in experimental models; Transient hyperglycemia resulted in persistent epigenetic changes leading to aberrant antioxidant and inflammatory gene expressions in VSMC and endothelial cells during subsequent normoglycemia48. With regard to HDACs, SIRT1 was shown to mediate high glucose-induced cellular metabolic memory via the liver kinase B1 (LKB1)/AMPK/ROS pathway49.
HDACs and Inflammation
Inflammation is a pivotal factor that underlies both cardiovascular and metabolic disease, and HDACs have been implicated in regulating both the innate and adaptive immune system. HDAC3, HDAC4, and HDAC9 have been associated with pro-inflammatory responses in macrophages and monocytes. HDAC3 was reported to promote inflammatory gene expression in LPS-stimulated macrophages50, and HDAC4 to contribute to TNFα-induced monocyte adhesion to VSMCs51. HDAC9 was reported to induce inflammatory gene expression in macrophages and prevent polarization to anti-inflammatory M2 phenotype52. With regards to adaptive immunity, HDAC7 and HDAC2 have been reported to maintain B cell and CD4+ T cell identity, respectively53, 54. Interestingly, HDAC9 and HDAC3 were shown to control CD4+ Foxp3+ T regulatory (Treg) cell development and function55, 56. HDAC9 deficient mice exhibited enhanced expression of Foxp3, a master regulator of Treg differentiation55, while deletion of HDAC3 disrupted Treg cell development and function, restored IL-2 production, and upregulated pro-inflammatory IL-656. The pan-HDAC inhibitor SAHA (vorinistat) enhanced oxLDL-induced interleukin-8 and monocyte-chemoattractant protein-1 expression in human vascular endothelial cells57. However, vorinistat is remarkably effective at preventing allogeneic transplant rejection; allogeneic hematopoietic cell transplant patients treated with vorinostat have increased Treg cell numbers with greater suppressive function and reduced plasma levels of proinflammatory cytokines such as IL-1β, TNF-α, IL-6, and IL-858. Thus, pan HDAC-inhibition produces complex effects on the immune system that can potentially modulate pro- and anti-inflammatory pathways in the context of specific diseases.
Potential Role of HDACs in Cardiovascular Diseases
The linkage between HDACs and cardiovascular disease is less well developed compared with metabolic disease. However, the body of data is growing dramatically, and evidence of linkages to human cardiovascular disease is also emerging. HDACs clearly have the potential to regulate many aspects of cardiovascular disease, including inflammation, as discussed above.
HDACs are fundamentally important in cardiac development and regulate hypertrophy, fibrosis, ischemia/reperfusion injury, and other aspects of cardiac function59, 60. HDAC inhibitors have shown promise in experimental studies for their ability to prevent heart failure61 As is the case for metabolic disease and inflammation, the mechanisms whereby HDACs modulate cardiac function are complex. For example, HDACs were recently identified as part of a chromatin repressor complex that inhibits transcription of a long noncoding RNA, which in turn protects the heart against pathological hypertrophy62. It is tempting to speculate that HDACs could play a particularly important role in cardiomyopathy associated with metabolic diseases such as uncontrolled diabetes63, but in vivo experimental data are lacking.
Modulation of inflammation by HDACs has important implications for both cardiac and vascular disease. For example, in spontaneously hypertensive rats, HDAC inhibition (valproic acid) led to reduced left ventricular expression of IL-1β and TNFα, attenuation of cardiac hypertrophy and fibrosis, and improved cardiac function64. Conversely, SIRT1 has been reported to protect against atherosclerosis in part through its anti-inflammatory effects. Its expression in endothelial cells and macrophages was reported to diminish foam cell formation and vascular reactive oxygen species and promote ABCA1-driven reverse cholesterol transport65, 66.
Moreover, SIRT1 expression in vascular smooth muscle cells (VSMC) protected against DNA damage, medial degeneration, and atherosclerosis67. In diabetic patients, incretin therapy was associated with SIRT6 induction, reduced inflammation and oxidative stress, and a more stable plaque phenotype68. Interestingly, mice treated with the HDAC inhibitor TSA showed a significant and dose-dependent improvement in HDL-cholesterol levels and reduced serum glucose, triglycerides, and total cholesterol, suggesting favorable metabolic effects with regard to the pathogenesis of vascular disease69.
A recent GWAS identified HDAC9 to be associated with large vessel ischemic stroke70 and atherosclerosis71. Elevated expression of HDAC9 was also noted in human atherosclerotic plaques. A polymorphism in the intergenic region between HDAC9 and TWIST1/FERD3L in humans was associated with selectively increased HDAC9 expression and an increased incidence of atherosclerosis72. In animal models, HDAC9 gene deficiency was shown to be atheroprotective, favorably modulating inflammatory and lipid homeostatic gene expression while polarizing macrophages towards a protective M2 phenotype52.
Experimental studies have established the relevance of HDACs in hypertension and neointima formation. SIRT1 in VSMC was shown to protect against angiotensin II-induced vascular remodeling, oxidative stress, inflammation, and hypertension in mice73. Conversely, in isolated mesenteric arteries, TSA reversed angiotensin II-induced contraction and increased endothelium-dependent relaxation stimulated by acetylcholine in spontaneously hypertensive rats51. HDAC4 has been implicated in hypertension through its effects on VSMCs51; HDAC4 gene silencing inhibited TNF-induced monocyte adhesion, VCAM-1 expression, transcriptional activity of NF-κB, and oxidative stress in VSMC51. Additionally, HDAC4 has been suggested to control neointima hyperplasia by promoting the activation of p38 mitogen-activated protein kinase/heat shock protein 27 signaling and inducing VSMC proliferation and migration74. In contrast, HDAC7 (unspliced isoform) was shown to suppress VSMC proliferation and neointima formation by preventing β-catenin nuclear translocation and activity75. Class I/II HDAC inhibition increased neointimal thickening in a murine model of post-angioplasty restenosis76, while class IIa HDAC inhibition prevented neointimal hyperplasia in a murine carotid ligation model74. These conflicting results may reflect the diverse functions of HDACs and/or non-specificity of HDAC inhibitors.
Perspective / Future research
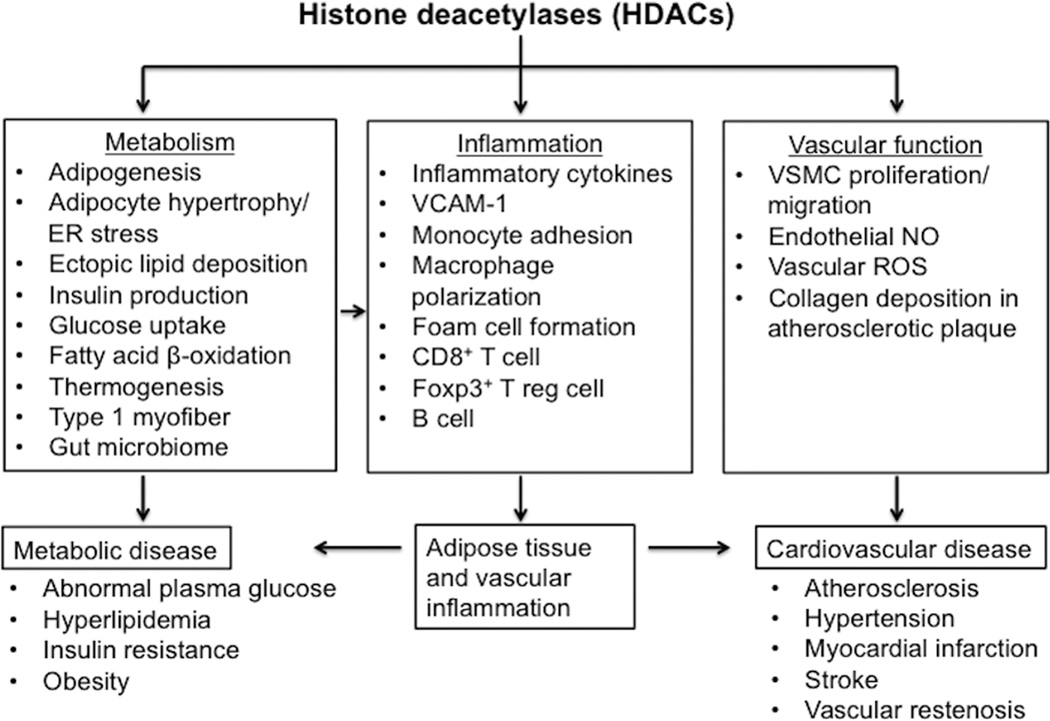
Recent scientific advances have improved our understanding of HDAC function and their potential role in cardiometabolic disease (Figure 1). A number of issues remain to be resolved, however. Most contemporary HDAC inhibitors lack selectivity towards individual HDACs and have limited efficacy against class II HDACs. Non-selectively inhibiting HDACs could yield adverse effects given their broad contributions to cell differentiation, development, and tissue homeostasis. Furthermore, HDACs may produce divergent, cell-specific actions. For instance, endothelial HDAC3 is atheroprotective in response to exposure to disturbed flow, while myeloid HDAC3 prevents collagen deposition and induction of a stable plaque phenotype77,78. Selectively targeting HDAC isoforms in a tissue-specific manner may thus be beneficial but would require identification of tissue-specific mechanisms whereby HDACs function (i.e. histone deacetylase enzymatic activity, transcriptional repression, and interactions with other epigenetic regulatory mechanisms). Subsequently, designing inhibitors to target key HDAC functional domains (rather than full-length protein function) could enhance selectivity and minimize unwanted side effects. Also, designing inhibitors against key HDACs (such as HDAC9), which produce consistent cell-specific actions in metabolic and vascular tissues, is a compelling approach.
Figure 1.
The role of HDACs in cardiometabolic disease. Abbreviations: ER, endoplasmic reticulum; Foxp3+, forkhead box p3; NO, nitric oxide; VCAM-1, vascular cell adhesion protein 1; VSMC, vascular smooth muscle cells; ROS, reactive oxygen species; T reg cells, T regulatory cell.
Further studies are needed to understand the interplay between histone post-translational modifications, DNA methylation, and non-coding RNAs and the consequence of their dysregulation in disease phenotype. Additionally, more work is required to dissect the mechanisms of cellular and transgenerational epigenetic memory. Advancing such studies will likely refine our knowledge of the role of HDACs in cardiometabolic disease and their potential as therapeutic targets.
Supplementary Material
Significance.
Cardiometabolic disease, emerging as a worldwide epidemic, is a combination of metabolic derangements leading to type 2 diabetes and cardiovascular disease. These derangements are generally long-lasting and resistant to conventional therapies. Histone deacetylases (HDACs) are a class of enzymes that alter chromatin structure and epigenetically “reprogram” gene expression, thereby influencing key metabolic pathways such as differentiation and function of pancreatic islet cells, adipocytes, hepatocytes and skeletal muscle. HDACs also play complex roles in regulating the immune and cardiovascular systems. Increasing evidence suggests that HDACs are centrally positioned to regulate the interplay between metabolic disease, inflammation, and cardiovascular risk factors. Understanding the molecular, cellular, and physiological functions of HDACs in cardiometabolic disease is expected to provide insight into disease pathogenesis, risk factor control, and therapeutic development.
Acknowledgements
Acknowledgments: none
Sources of funding: this work was supported by NIH grants HL076684 and HL112640 (to N.L.W.), HL086555 (to Y.T.), DK74932 (to D.Y.H), and an AHA predoctoral fellowship 15PRE21580003 (to K.H.Y).
Footnotes
Disclosures: none
References
- 1.Allfrey VG, Faulkner R, Mirsky AE. Acetylation and methylation of histones and their possible role in the regulation of rna synthesis. Proceedings of the National Academy of Sciences of the United States of America. 1964;51:786–794. doi: 10.1073/pnas.51.5.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brownell JE, Allis CD. An activity gel assay detects a single, catalytically active histone acetyltransferase subunit in tetrahymena macronuclei. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:6364–6368. doi: 10.1073/pnas.92.14.6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taunton J, Hassig CA, Schreiber SL. A mammalian histone deacetylase related to the yeast transcriptional regulator rpd3p. Science. 1996;272:408–411. doi: 10.1126/science.272.5260.408. [DOI] [PubMed] [Google Scholar]
- 4.Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403:41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- 5.Sun Z, Feng D, Fang B, Mullican SE, You SH, Lim HW, Everett LJ, Nabel CS, Li Y, Selvakumaran V, Won KJ, Lazar MA. Deacetylase-independent function of hdac3 in transcription and metabolism requires nuclear receptor corepressor. Molecular cell. 2013;52:769–782. doi: 10.1016/j.molcel.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao X, Sternsdorf T, Bolger TA, Evans RM, Yao TP. Regulation of mef2 by histone deacetylase 4- and sirt1 deacetylase-mediated lysine modifications. Molecular and cellular biology. 2005;25:8456–8464. doi: 10.1128/MCB.25.19.8456-8464.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gregoretti IV, Lee YM, Goodson HV. Molecular evolution of the histone deacetylase family: Functional implications of phylogenetic analysis. Journal of molecular biology. 2004;338:17–31. doi: 10.1016/j.jmb.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Mihaylova MM, Shaw RJ. Metabolic reprogramming by class i and ii histone deacetylases. Trends in endocrinology and metabolism: TEM. 2013;24:48–57. doi: 10.1016/j.tem.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang J, Yan H, Zhuang S. Histone deacetylases as targets for treatment of multiple diseases. Clinical science. 2013;124:651–662. doi: 10.1042/CS20120504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou B, Margariti A, Zeng L, Xu Q. Role of histone deacetylases in vascular cell homeostasis and arteriosclerosis. Cardiovascular research. 2011;90:413–420. doi: 10.1093/cvr/cvr003. [DOI] [PubMed] [Google Scholar]
- 11.Haberland M, Montgomery RL, Olson EN. The many roles of histone deacetylases in development and physiology: Implications for disease and therapy. Nature reviews. Genetics. 2009;10:32–42. doi: 10.1038/nrg2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Houtkooper RH, Pirinen E, Auwerx J. Sirtuins as regulators of metabolism and healthspan. Nature reviews. Molecular cell biology. 2012;13:225–238. doi: 10.1038/nrm3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ridker PM, Buring JE, Cook NR, Rifai N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: An 8-year follow-up of 14 719 initially healthy american women. Circulation. 2003;107:391–397. doi: 10.1161/01.cir.0000055014.62083.05. [DOI] [PubMed] [Google Scholar]
- 14.Albert CM, Ma J, Rifai N, Stampfer MJ, Ridker PM. Prospective study of c-reactive protein, homocysteine, and plasma lipid levels as predictors of sudden cardiac death. Circulation. 2002;105:2595–2599. doi: 10.1161/01.cir.0000017493.03108.1c. [DOI] [PubMed] [Google Scholar]
- 15.Kannel WB. Blood pressure as a cardiovascular risk factor: Prevention and treatment. Jama. 1996;275:1571–1576. [PubMed] [Google Scholar]
- 16.Poss J, Custodis F, Werner C, Weingartner O, Bohm M, Laufs U. Cardiovascular disease and dyslipidemia: Beyond ldl. Current pharmaceutical design. 2011;17:861–870. doi: 10.2174/138161211795428858. [DOI] [PubMed] [Google Scholar]
- 17.Kannel WB, McGee DL. Diabetes and cardiovascular disease. The framingham study. Jama. 1979;241:2035–2038. doi: 10.1001/jama.241.19.2035. [DOI] [PubMed] [Google Scholar]
- 18.Ashrafian H, Frenneaux MP, Opie LH. Metabolic mechanisms in heart failure. Circulation. 2007;116:434–448. doi: 10.1161/CIRCULATIONAHA.107.702795. [DOI] [PubMed] [Google Scholar]
- 19.Lenoir O, Flosseau K, Ma FX, Blondeau B, Mai A, Bassel-Duby R, Ravassard P, Olson EN, Haumaitre C, Scharfmann R. Specific control of pancreatic endocrine beta- and delta-cell mass by class iia histone deacetylases hdac4, hdac5, and hdac9. Diabetes. 2011;60:2861–2871. doi: 10.2337/db11-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen S, Borowiak M, Fox JL, Maehr R, Osafune K, Davidow L, Lam K, Peng LF, Schreiber SL, Rubin LL, Melton D. A small molecule that directs differentiation of human escs into the pancreatic lineage. Nature chemical biology. 2009;5:258–265. doi: 10.1038/nchembio.154. [DOI] [PubMed] [Google Scholar]
- 21.Ma X, Lee P, Chisholm DJ, James DE. Control of adipocyte differentiation in different fat depots; implications for pathophysiology or therapy. Frontiers in endocrinology. 2015;6:1. doi: 10.3389/fendo.2015.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu J, Cohen P, Spiegelman BM. Adaptive thermogenesis in adipocytes: Is beige the new brown? Genes & development. 2013;27:234–250. doi: 10.1101/gad.211649.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang X, Ye X, Guo W, Lu H, Gao Z. Inhibition of hdac3 promotes ligand-independent ppargamma activation by protein acetylation. Journal of molecular endocrinology. 2014;53:191–200. doi: 10.1530/JME-14-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang J, Henagan TM, Gao Z, Ye J. Inhibition of glyceroneogenesis by histone deacetylase 3 contributes to lipodystrophy in mice with adipose tissue inflammation. Endocrinology. 2011;152:1829–1838. doi: 10.1210/en.2010-0828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chatterjee TK, Idelman G, Blanco V, et al. Histone deacetylase 9 is a negative regulator of adipogenic differentiation. The Journal of biological chemistry. 2011;286:27836–27847. doi: 10.1074/jbc.M111.262964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chatterjee TK, Basford JE, Knoll E, Tong WS, Blanco V, Blomkalns AL, Rudich S, Lentsch AB, Hui DY, Weintraub NL. Hdac9 knockout mice are protected from adipose tissue dysfunction and systemic metabolic disease during high-fat feeding. Diabetes. 2014;63:176–187. doi: 10.2337/db13-1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parihar P, Solanki I, Mansuri ML, Parihar MS. Mitochondrial sirtuins: Emerging roles in metabolic regulations, energy homeostasis and diseases. Experimental gerontology. 2015;61C:130–141. doi: 10.1016/j.exger.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 28.Wang F, Tong Q. Sirt2 suppresses adipocyte differentiation by deacetylating foxo1 and enhancing foxo1's repressive interaction with ppargamma. Molecular biology of the cell. 2009;20:801–808. doi: 10.1091/mbc.E08-06-0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qiang L, Wang L, Kon N, Zhao W, Lee S, Zhang Y, Rosenbaum M, Zhao Y, Gu W, Farmer SR, Accili D. Brown remodeling of white adipose tissue by sirt1-dependent deacetylation of ppargamma. Cell. 2012;150:620–632. doi: 10.1016/j.cell.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caron AZ, He X, Mottawea W, Seifert EL, Jardine K, Dewar-Darch D, Cron GO, Harper ME, Stintzi A, McBurney MW. The sirt1 deacetylase protects mice against the symptoms of metabolic syndrome. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2014;28:1306–1316. doi: 10.1096/fj.13-243568. [DOI] [PubMed] [Google Scholar]
- 31.Lombard DB, Alt FW, Cheng HL, et al. Mammalian sir2 homolog sirt3 regulates global mitochondrial lysine acetylation. Molecular and cellular biology. 2007;27:8807–8814. doi: 10.1128/MCB.01636-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Winnik S, Gaul DS, Preitner F, et al. Deletion of sirt3 does not affect atherosclerosis but accelerates weight gain and impairs rapid metabolic adaptation in ldl receptor knockout mice: Implications for cardiovascular risk factor development. Basic research in cardiology. 2014;109:399. doi: 10.1007/s00395-013-0399-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang WJ. Sirtuins: Novel targets for metabolic disease in drug development. Biochemical and biophysical research communications. 2008;373:341–344. doi: 10.1016/j.bbrc.2008.06.048. [DOI] [PubMed] [Google Scholar]
- 34.Sun Z, Miller RA, Patel RT, et al. Hepatic hdac3 promotes gluconeogenesis by repressing lipid synthesis and sequestration. Nature medicine. 2012;18:934–942. doi: 10.1038/nm.2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim HS, Xiao C, Wang RH, Lahusen T, Xu X, Vassilopoulos A, Vazquez-Ortiz G, Jeong WI, Park O, Ki SH, Gao B, Deng CX. Hepatic-specific disruption of sirt6 in mice results in fatty liver formation due to enhanced glycolysis and triglyceride synthesis. Cell metabolism. 2010;12:224–236. doi: 10.1016/j.cmet.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang RH, Kim HS, Xiao C, Xu X, Gavrilova O, Deng CX. Hepatic sirt1 deficiency in mice impairs mtorc2/akt signaling and results in hyperglycemia, oxidative damage, and insulin resistance. The Journal of clinical investigation. 2011;121:4477–4490. doi: 10.1172/JCI46243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Purushotham A, Schug TT, Xu Q, Surapureddi S, Guo X, Li X. Hepatocyte-specific deletion of sirt1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell metabolism. 2009;9:327–338. doi: 10.1016/j.cmet.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mihaylova MM, Vasquez DS, Ravnskjaer K, Denechaud PD, Yu RT, Alvarez JG, Downes M, Evans RM, Montminy M, Shaw RJ. Class iia histone deacetylases are hormone-activated regulators of foxo and mammalian glucose homeostasis. Cell. 2011;145:607–621. doi: 10.1016/j.cell.2011.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Lange P, Moreno M, Silvestri E, Lombardi A, Goglia F, Lanni A. Fuel economy in food-deprived skeletal muscle: Signaling pathways and regulatory mechanisms. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2007;21:3431–3441. doi: 10.1096/fj.07-8527rev. [DOI] [PubMed] [Google Scholar]
- 40.Potthoff MJ, Wu H, Arnold MA, Shelton JM, Backs J, McAnally J, Richardson JA, Bassel-Duby R, Olson EN. Histone deacetylase degradation and mef2 activation promote the formation of slow-twitch myofibers. The Journal of clinical investigation. 2007;117:2459–2467. doi: 10.1172/JCI31960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ryder JW, Bassel-Duby R, Olson EN, Zierath JR. Skeletal muscle reprogramming by activation of calcineurin improves insulin action on metabolic pathways. The Journal of biological chemistry. 2003;278:44298–44304. doi: 10.1074/jbc.M304510200. [DOI] [PubMed] [Google Scholar]
- 42.McGee SL, van Denderen BJ, Howlett KF, Mollica J, Schertzer JD, Kemp BE, Hargreaves M. Amp-activated protein kinase regulates glut4 transcription by phosphorylating histone deacetylase 5. Diabetes. 2008;57:860–867. doi: 10.2337/db07-0843. [DOI] [PubMed] [Google Scholar]
- 43.Jing E, Emanuelli B, Hirschey MD, Boucher J, Lee KY, Lombard D, Verdin EM, Kahn CR. Sirtuin-3 (sirt3) regulates skeletal muscle metabolism and insulin signaling via altered mitochondrial oxidation and reactive oxygen species production. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:14608–14613. doi: 10.1073/pnas.1111308108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ramadori G, Gautron L, Fujikawa T, Vianna CR, Elmquist JK, Coppari R. Central administration of resveratrol improves diet-induced diabetes. Endocrinology. 2009;150:5326–5333. doi: 10.1210/en.2009-0528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramadori G, Fujikawa T, Fukuda M, Anderson J, Morgan DA, Mostoslavsky R, Stuart RC, Perello M, Vianna CR, Nillni EA, Rahmouni K, Coppari R. Sirt1 deacetylase in pomc neurons is required for homeostatic defenses against diet-induced obesity. Cell metabolism. 2010;12:78–87. doi: 10.1016/j.cmet.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramadori G, Fujikawa T, Anderson J, Berglund ED, Frazao R, Michan S, Vianna CR, Sinclair DA, Elias CF, Coppari R. Sirt1 deacetylase in sf1 neurons protects against metabolic imbalance. Cell metabolism. 2011;14:301–312. doi: 10.1016/j.cmet.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B, Diabetes C. Complications Trial/Epidemiology of Diabetes I, Complications Study Research G, Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. The New England journal of medicine. 2005;353:2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reddy MA, Zhang E, Natarajan R. Epigenetic mechanisms in diabetic complications and metabolic memory. Diabetologia. 2015;58:443–455. doi: 10.1007/s00125-014-3462-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zheng Z, Chen H, Li J, Li T, Zheng B, Zheng Y, Jin H, He Y, Gu Q, Xu X. Sirtuin 1-mediated cellular metabolic memory of high glucose via the lkb1/ampk/ros pathway and therapeutic effects of metformin. Diabetes. 2012;61:217–228. doi: 10.2337/db11-0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen X, Barozzi I, Termanini A, Prosperini E, Recchiuti A, Dalli J, Mietton F, Matteoli G, Hiebert S, Natoli G. Requirement for the histone deacetylase hdac3 for the inflammatory gene expression program in macrophages. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E2865–E2874. doi: 10.1073/pnas.1121131109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Usui T, Okada M, Mizuno W, Oda M, Ide N, Morita T, Hara Y, Yamawaki H. Hdac4 mediates development of hypertension via vascular inflammation in spontaneous hypertensive rats. American journal of physiology. Heart and circulatory physiology. 2012;302:H1894–H1904. doi: 10.1152/ajpheart.01039.2011. [DOI] [PubMed] [Google Scholar]
- 52.Cao Q, Rong S, Repa JJ, St Clair R, Parks JS, Mishra N. Histone deacetylase 9 represses cholesterol efflux and alternatively activated macrophages in atherosclerosis development. Arteriosclerosis, thrombosis, and vascular biology. 2014;34:1871–1879. doi: 10.1161/ATVBAHA.114.303393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barneda-Zahonero B, Roman-Gonzalez L, Collazo O, Rafati H, Islam AB, Bussmann LH, di Tullio A, De Andres L, Graf T, Lopez-Bigas N, Mahmoudi T, Parra M. Hdac7 is a repressor of myeloid genes whose downregulation is required for transdifferentiation of pre-b cells into macrophages. PLoS genetics. 2013;9:e1003503. doi: 10.1371/journal.pgen.1003503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boucheron N, Tschismarov R, Goschl L, et al. Cd4(+) t cell lineage integrity is controlled by the histone deacetylases hdac1 and hdac2. Nature immunology. 2014;15:439–448. doi: 10.1038/ni.2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tao R, de Zoeten EF, Ozkaynak E, Chen C, Wang L, Porrett PM, Li B, Turka LA, Olson EN, Greene MI, Wells AD, Hancock WW. Deacetylase inhibition promotes the generation and function of regulatory t cells. Nature medicine. 2007;13:1299–1307. doi: 10.1038/nm1652. [DOI] [PubMed] [Google Scholar]
- 56.Wang L, Liu Y, Han R, Beier UH, Bhatti TR, Akimova T, Greene MI, Hiebert SW, Hancock WW. Foxp3+ regulatory t cell development and function require histone/protein deacetylase 3. The Journal of clinical investigation. 2015;125:1111–1123. doi: 10.1172/JCI77088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dje N'Guessan P, Riediger F, Vardarova K, Scharf S, Eitel J, Opitz B, Slevogt H, Weichert W, Hocke AC, Schmeck B, Suttorp N, Hippenstiel S. Statins control oxidized ldl-mediated histone modifications and gene expression in cultured human endothelial cells. Arteriosclerosis, thrombosis, and vascular biology. 2009;29:380–386. doi: 10.1161/ATVBAHA.108.178319. [DOI] [PubMed] [Google Scholar]
- 58.Choi SW, Gatza E, Hou G, Sun Y, Whitfield J, Song Y, Oravecz-Wilson K, Tawara I, Dinarello CA, Reddy P. Histone deacetylase inhibition regulates inflammation and enhances tregs after allogeneic hematopoietic cell transplantation in humans. Blood. 2015;125:815–819. doi: 10.1182/blood-2014-10-605238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xie M, Hill JA. Hdac-dependent ventricular remodeling. Trends in cardiovascular medicine. 2013;23:229–235. doi: 10.1016/j.tcm.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bush EW, McKinsey TA. Protein acetylation in the cardiorenal axis: The promise of histone deacetylase inhibitors. Circulation research. 2010;106:272–284. doi: 10.1161/CIRCRESAHA.109.209338. [DOI] [PubMed] [Google Scholar]
- 61.Ferguson BS, McKinsey TA. Non-sirtuin histone deacetylases in the control of cardiac aging. Journal of molecular and cellular cardiology. 2015 doi: 10.1016/j.yjmcc.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Han P, Li W, Lin CH, et al. A long noncoding rna protects the heart from pathological hypertrophy. Nature. 2014;514:102–106. doi: 10.1038/nature13596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yu XY, Geng YJ, Liang JL, Lin QX, Lin SG, Zhang S, Li Y. High levels of glucose induce apoptosis in cardiomyocyte via epigenetic regulation of the insulin-like growth factor receptor. Experimental cell research. 2010;316:2903–2909. doi: 10.1016/j.yexcr.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 64.Cardinale JP, Sriramula S, Pariaut R, Guggilam A, Mariappan N, Elks CM, Francis J. Hdac inhibition attenuates inflammatory, hypertrophic, and hypertensive responses in spontaneously hypertensive rats. Hypertension. 2010;56:437–444. doi: 10.1161/HYPERTENSIONAHA.110.154567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang QJ, Wang Z, Chen HZ, Zhou S, Zheng W, Liu G, Wei YS, Cai H, Liu DP, Liang CC. Endothelium-specific overexpression of class iii deacetylase sirt1 decreases atherosclerosis in apolipoprotein e-deficient mice. Cardiovascular research. 2008;80:191–199. doi: 10.1093/cvr/cvn224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stein S, Matter CM. Protective roles of sirt1 in atherosclerosis. Cell cycle. 2011;10:640–647. doi: 10.4161/cc.10.4.14863. [DOI] [PubMed] [Google Scholar]
- 67.Gorenne I, Kumar S, Gray K, Figg N, Yu H, Mercer J, Bennett M. Vascular smooth muscle cell sirtuin 1 protects against DNA damage and inhibits atherosclerosis. Circulation. 2013;127:386–396. doi: 10.1161/CIRCULATIONAHA.112.124404. [DOI] [PubMed] [Google Scholar]
- 68.Balestrieri ML, Rizzo MR, Barbieri M, et al. Sirtuin 6 expression and inflammatory activity in diabetic atherosclerotic plaques: Effects of incretin treatment. Diabetes. 2014 doi: 10.2337/db14-1149. [DOI] [PubMed] [Google Scholar]
- 69.Sharma S, Taliyan R, Ramagiri S. Histone deacetylase inhibitor, trichostatin a, improves learning and memory in high-fat diet-induced cognitive deficits in mice. Journal of molecular neuroscience : MN. 2015;56:1–11. doi: 10.1007/s12031-014-0461-x. [DOI] [PubMed] [Google Scholar]
- 70.International Stroke Genetics C, Wellcome Trust Case Control C. Bellenguez C, Bevan S, Gschwendtner A, et al. Genome-wide association study identifies a variant in hdac9 associated with large vessel ischemic stroke. Nature genetics. 2012;44:328–333. doi: 10.1038/ng.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Markus HS, Makela KM, Bevan S, Raitoharju E, Oksala N, Bis JC, O'Donnell C, Hainsworth A, Lehtimaki T. Evidence hdac9 genetic variant associated with ischemic stroke increases risk via promoting carotid atherosclerosis. Stroke; a journal of cerebral circulation. 2013;44:1220–1225. doi: 10.1161/STROKEAHA.111.000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Azghandi S, Prell C, van der Laan SW, Schneider M, Malik R, Berer K, Gerdes N, Pasterkamp G, Weber C, Haffner C, Dichgans M. Deficiency of the stroke relevant hdac9 gene attenuates atherosclerosis in accord with allele-specific effects at 7p21.1. Stroke; a journal of cerebral circulation. 2015;46:197–202. doi: 10.1161/STROKEAHA.114.007213. [DOI] [PubMed] [Google Scholar]
- 73.Gao P, Xu TT, Lu J, Li L, Xu J, Hao DL, Chen HZ, Liu DP. Overexpression of sirt1 in vascular smooth muscle cells attenuates angiotensin ii-induced vascular remodeling and hypertension in mice. Journal of molecular medicine. 2014;92:347–357. doi: 10.1007/s00109-013-1111-4. [DOI] [PubMed] [Google Scholar]
- 74.Usui T, Morita T, Okada M, Yamawaki H. Histone deacetylase 4 controls neointimal hyperplasia via stimulating proliferation and migration of vascular smooth muscle cells. Hypertension. 2014;63:397–403. doi: 10.1161/HYPERTENSIONAHA.113.01843. [DOI] [PubMed] [Google Scholar]
- 75.Zhou B, Margariti A, Zeng L, Habi O, Xiao Q, Martin D, Wang G, Hu Y, Wang X, Xu Q. Splicing of histone deacetylase 7 modulates smooth muscle cell proliferation and neointima formation through nuclear beta-catenin translocation. Arteriosclerosis, thrombosis, and vascular biology. 2011;31:2676–2684. doi: 10.1161/ATVBAHA.111.230888. [DOI] [PubMed] [Google Scholar]
- 76.Song S, Kang SW, Choi C. Trichostatin a enhances proliferation and migration of vascular smooth muscle cells by downregulating thioredoxin 1. Cardiovascular research. 2010;85:241–249. doi: 10.1093/cvr/cvp263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zampetaki A, Zeng L, Margariti A, Xiao Q, Li H, Zhang Z, Pepe AE, Wang G, Habi O, deFalco E, Cockerill G, Mason JC, Hu Y, Xu Q. Histone deacetylase 3 is critical in endothelial survival and atherosclerosis development in response to disturbed flow. Circulation. 2010;121:132–142. doi: 10.1161/CIRCULATIONAHA.109.890491. [DOI] [PubMed] [Google Scholar]
- 78.Hoeksema MA, Gijbels MJ, Van den Bossche J, et al. Targeting macrophage histone deacetylase 3 stabilizes atherosclerotic lesions. EMBO molecular medicine. 2014;6:1124–1132. doi: 10.15252/emmm.201404170. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.