Abstract
We evaluated the biocompatibility of a poly(ethylene glycol) and poly(acrylic acid) (PEG/PAA) interpenetrating network hydrogel designed for artificial cornea in a rabbit model. PEG/PAA hydrogel measuring 6 mm in diameter was implanted in the corneal stroma of twelve rabbits. Stromal flaps were created with a microkeratome. Randomly, six rabbits were assigned to bear the implant for 2 months, two rabbits for 6 months, two rabbits for 9 months, one rabbit for 12 months, and one rabbit for 16 months. Rabbits were evaluated monthly. After the assigned period, eyes were enucleated, and corneas were processed for histology and immunohistochemistry. There were clear corneas in three of six rabbits that had implantation of hydrogel for 2 months. In the six rabbits with implant for 6 months or longer, the corneas remained clear in four. There was a high rate of epithelial defect and corneal thinning in these six rabbits. One planned 9-month rabbit developed extrusion of implant at 4 months. The cornea remained clear in the 16-month rabbit but histology revealed epithelial in-growth. Intrastromal implantation of PEG/PAA resulted in a high rate of long-term complications.
Keywords: Keratoprosthesis, artificial cornea, interpenetrating network, poly(ethylene glycol), poly(acrylic acid), hydrogel, biocompatibility
Introduction
Hydrogels are crosslinked hydrophilic polymers that bear a wide range of attractive physical and biological characteristics for biomedical applications. The flexibility of hydrogel materials mimics that of natural tissue. Properties such as water content, swelling behavior, and surface conditions can be tailored for purposes ranging from tissue matrix replacement to drug delivery [1–5]. The optical clarity of hydrogels, in particular, makes them popular candidates for ophthalmic applications [6–10].
An artificial cornea or keratoprosthesis (KPro) may address the shortage of human donor tissue in developing countries and has the potential to replace the need for allogenic transplants. Keratoprostheses currently in clinical use can achieve visual improvement in patients with a poor prognosis with standard penetrating keratoplasty, such as repeated graft failure [11–12]. Since the polymer poly(methyl methacrylate) (PMMA) was found to be tolerated in the eye during World War II, various polymers have been explored as synthetic materials to replace diseased or damaged cornea. In addition to PMMA, hydrophobic polymers that have been explored include nylon, Teflon, Dacron, modified Gore-Tex, polyurethane, and polytetrafluoroethylene (PTFE) [13–19]. The two prevailing designs, Boston KPro and Osteo-Odonto KPro, both employ a PMMA central optic. Recent advances in design have been made to the Boston KPro, with reduction in the complications of retroprosthetic membrane formation, vitritis, and endophthalmitis. However, glaucoma remains a challenge [12, 20–22]. Material properties may play a significant role in modulating these biological responses.
Hydrogels provide an attractive alternative to hard plastics like PMMA, because high water content allows for high permeability to oxygen, glucose, nutrients, and other-soluble metabolites [23–25]. AlphaCor, a device composed of poly(2-hydroxyethyl methacrylate) (PHEMA), has led the way for hydrogel-based keratoprostheses. The clear central optic is prepared with PHEMA at 35% water content, while the opaque porous skirt for tissue integration is prepared with PHEMA at 45% water content [26]. Despite AlphaCor's 1-year retention rate of 80% and good visual outcomes in successful cases, complications such as infection, extrusion, rejection, calcification, and late melting of the anterior corneal lamella have caused a decline in its use [27–33]. An increase in water content may facilitate nutrient diffusion and may better support re-epithelialization over the anterior of the keratoprosthesis. In addition, higher hydrophilicity makes the material more resistant to nonspecific protein adsorption, which may trigger calcification and possibly retroprosthetic membrane formation [29, 34]. Most recently, human recombinant collagen type III scaffold developed by Griffith et al. have been successfully implanted in patients [35–37].
We have designed an interpenetrating polymer network hydrogel based on poly(ethylene) (PEG) and poly(acrylic acid) (PAA). Both PEG and PAA have individually been shown to be biocompatible in multiple biomedical applications. The PEG network is composed of end-linked PEG-diacrylamide macromonomers. When immersed in acrylic acid and polymerized, acrylic acid crosslinks to form a PAA network entangled with the PEG network. The resulting material is a loosely crosslinked, ionizable network with no covalent attachments between PEG and PAA. Although both PEG and PAA are relatively weak materials, when combined to form a mesh-like network, they yield good mechanical properties, while retaining a water content of 70~90% and glucose diffusion coefficients similar to the cornea (~10−6 cm2/s) [25, 38]. In previous work, PEG/PAA was shown to be non-cytotoxic [39]. In addition there had been encouraging results from a short term implantation of a small piece of this material as a corneal inlay in rabbits [40]. This study examines the biocompatibility of the PEG/PAA intrastromal implant in vivo in both the short and long term.
Materials and Methods
Hydrogel Synthesis
PEG/PAA IPN hydrogels were synthesized in a two-step network formation based on UV-initiated free-radical polymerization. Poly(ethylene glycol) diacrylamide was synthesized using a protocol previously described [39]. A precursor solution of polyethylene glycol-diacrylamide (PEG-dAAm) (MW 8000) and a stock solution of photoinitiator, Irgacure 2959 (0.55 mol L−1) in acetone/water (50 v/v%), was prepared and a 5 M excess per PEG-endgroup was added to the polymer solution (50 w/v% in water). This precursor solution was cast between two ethanol-wiped glass plates (1.0-mm thick) separated by a Teflon spacer (250-µm thick, 2 cm inner diameter) and then reacted in a ultra-violet (UV) chamber with a broad range of wavelengths (200–250 nm) for 5 minutes. Upon exposure to the UV light, the precursor solution underwent free-radical induced polymerization to form a water-insoluble network.
To incorporate the second network, the single network hydrogel was removed from the mold and immersed overnight in an aqueous acrylic acid monomer solution (50 v/v%) containing photoinitiator stock solution (0.14 mmol per 1 mL acrylic acid) and triethyleneglycol dimethacrylate/tetraethyleneglycol diallylether as a crosslinking agent (0.04 mmol per 1 mL acrylic acid). The gel was then placed between glass plates separated by Teflon spacers and exposed to the same UV source for 5 minutes. Following UV exposure, a second network, poly(acrylic acid) (PAA), was polymerized and crosslinked in the presence of the first network to form the stable, double network. To remove any unreacted components, the resultant IPN was washed until equilibrium pH was attained in pH 7.4 phosphate buffered saline (PBS). The hydrogel was then washed in pH 7.4 PBS containing 1% antibiotics and antimycotics for 3 days. Hydrogel samples for implantation measured 30~40 µm thick and were cut with a 6 mm diameter trephine.
Surgical Procedure
This study was approved by the Administrative Panel on Laboratory Animal Care of Stanford University, and conducted in compliance with the Association for Research in Vision and Ophthalmology (ARVO) statement on the use of animals in ophthalmic research. NIH guidelines for the care and use of laboratory animals (NIH Publication #85-23 Rev. 1985) have been followed.
Hydrogel samples were implanted in the cornea stroma in one eye of twelve 3.7 kg female New Zealand Red rabbits for up to 16 months (n = 6 for 2 months, n = 2 for 6 months, n = 2 for 9 months, n = 1 for 12 months, n = 1 for 16 months, these groups were randomly assigned). In one 2-month sham rabbit, the same procedure was followed without placement of the hydrogel. Animals were anesthetized with an intramuscular injection of ketamine (35 mg/kg), xylazine (5 mg/kg), and glycopyrrolate (0.01 mg/kg). Surgery was performed in the right eye of each animal by one surgeon (PH). An Amadeus II microkeratome (Advanced Medical Optics Inc., Santa Ana, CA) was used to create a 12 mm diameter, 3 mm hinge, laser in situ keratomileusis (LASIK) flap at a depth of 250 µm. The hydrogel was placed over the underlying stroma and centered within the flap region. The flap was closed with seven to nine interrupted, 10-0 nylon sutures. A temporary lateral partial tarsorrhaphy was performed using a 6-silk vertical mattress suture. Topical gatifloxacin and 1% prednisolone acetate ophthalmic suspension were administered three times a day to the operated eye for five days. Cornea sutures were removed on the fifth day and the tarsorrhaphy reversed. Clinical examinations were performed monthly using a microscope, and the health and weight of the rabbits were monitored. After rabbits were euthanized, globes were enucleated for histological processing.
Histology
Entire corneas of the operated and non-operated, contralateral eyes were embedded in either paraffin or glycol methacrylate (GMA). For those in paraffin, corneas were fixed in 10% buffered formalin. Slides were stained with hematoxylin and eosin. Since processing for paraffin embedding dehydrates the hydrogel, a modified GMA fixation and embedding protocol was used to visualize the hydrogel structure [41]. After whole globes were fixed in glutaraldehyde for 30 minutes to maintain the natural curvature of the corneas, the corneas were cut away from the rest of the globe using corneal surgical scissors and further fixed for 18–24 hours. Fresh stock-fixative was prepared for each specimen and its pH was adjusted to 7.2. The cornea samples were then processed using a modified glycol methacrylate protocol (Technovit 7100; EMS, Hatfield, PA) which preserves the high water content, pH-sensitive PEG/PAA hydrogel through the histological processing stages. Thin sections (2–4 µm) were taken and stained with cresyl violet.
Immunohistochemistry
Immunohistochemical evaluation of paraffin-embedded specimens was performed using the following antibodies: anti-cytokeratin AE5 (Abcam ab77869. 1:100 dilution), anti-smooth muscle α-actin (Sigma-Aldrich A2547, 1:400 dilution), and anti-rabbit macrophage marker (RAM11, DAKO, 1:50 dilution). The secondary antibodies were anti-mouse Alexafluor 594 immunoglobulin G (Invitrogen, 1:400 dilution) or anti-mouse horseradish peroxidase (Sigma-Aldrich, 1:200 dilution) visualized with 3,3’-diaminobenzidine (DAB) (Sigma-Aldrich).
Results
Clinical Examination
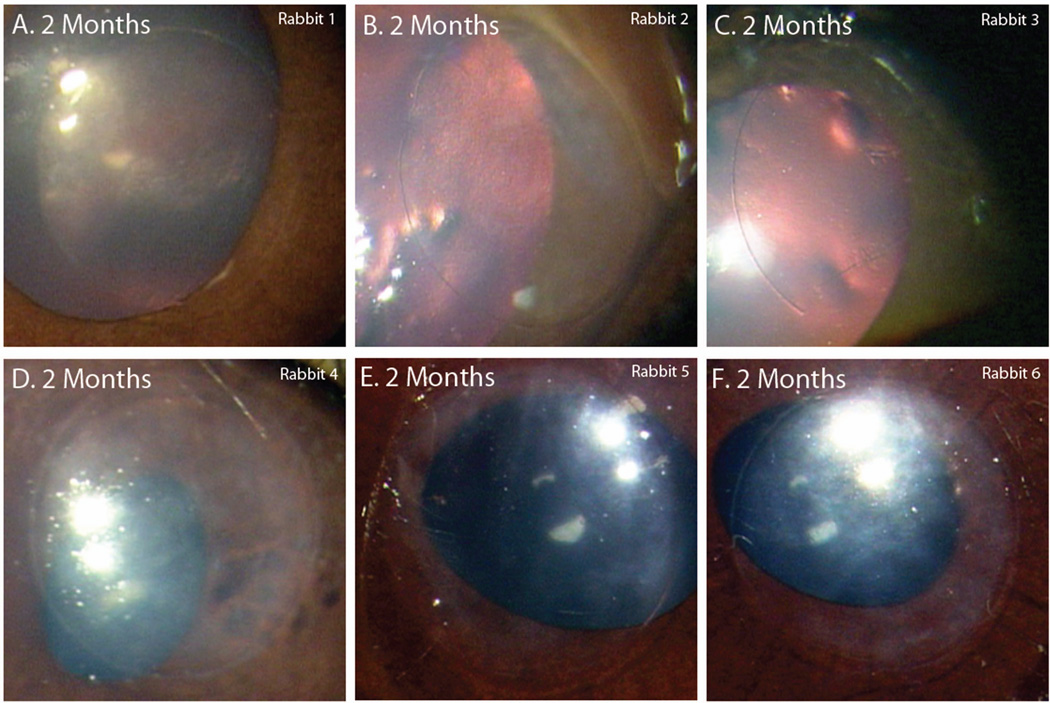
Table 1 summarizes the clinical outcome of all rabbits. Of the six 2-month rabbits (Table 1, Rabbits 1–6), none had extrusion of the polymer or development of neovascularization. One rabbit experienced some corneal thinning over the implant. Half developed light diffuse haze across the implant region. The rest remained clear (Figure 1). Slit lamp examination showed that the haze was localized to the stroma posterior to the implant.
Table 1.
Clinical Examination
| Rabbit | Length of Implantation (Months) |
Corneal Clarity | Epithelial and Stromal Defects | Neovascularization | Polymer Extrusion |
|---|---|---|---|---|---|
| 1 | 2 | Clear | None | No | No |
| 2 | 2 | Clear | None | No | No |
| 3 | 2 | Clear | None | No | No |
| 4 | 2 | Diffuse haze across the implant region | Areas of corneal thinning over the implant (2 mo.) | No | No |
| 5 | 2 | Diffuse haze across the implant region | None | No | No |
| 6 | 2 | Diffuse haze across the implant region | None | No | No |
| 7 | 6 | Clear | None | No | No |
| 8 | 6 | Opacity across the entire cornea | 1 epithelial defect over the polymer edge (3 mo.) | Yes | No |
| 9 | 9 | Moderate haze across the implant region | Areas of corneal thinning over the implant, extrusion (4 mo.) | No | 4 mo |
| 10 | 9 | Clear | 1 epithelial defect over the polymer edge, 1 area of corneal thinning (8 mo.) | No | No |
| 11 | 12 | Clear | 1 epithelial defect over the polymer edge (at 11mo.) | No | No |
| 12 | 16 | Clear | 1 area of corneal thinning over the polymer; several areas of thinning at the polymer edge (14 mo.) | No | No |
| 13-control | 2 | Clear | None | No | No |
Fig. 1.
Photographs of implanted PEG/PAA hydrogels taken prior to collection of tissue. These six rabbits had implants for 2 months. (A–C) The corneas of rabbits 1–3 remained clear. (D–F) Rabbits 4–6 developed mild diffuse haze.
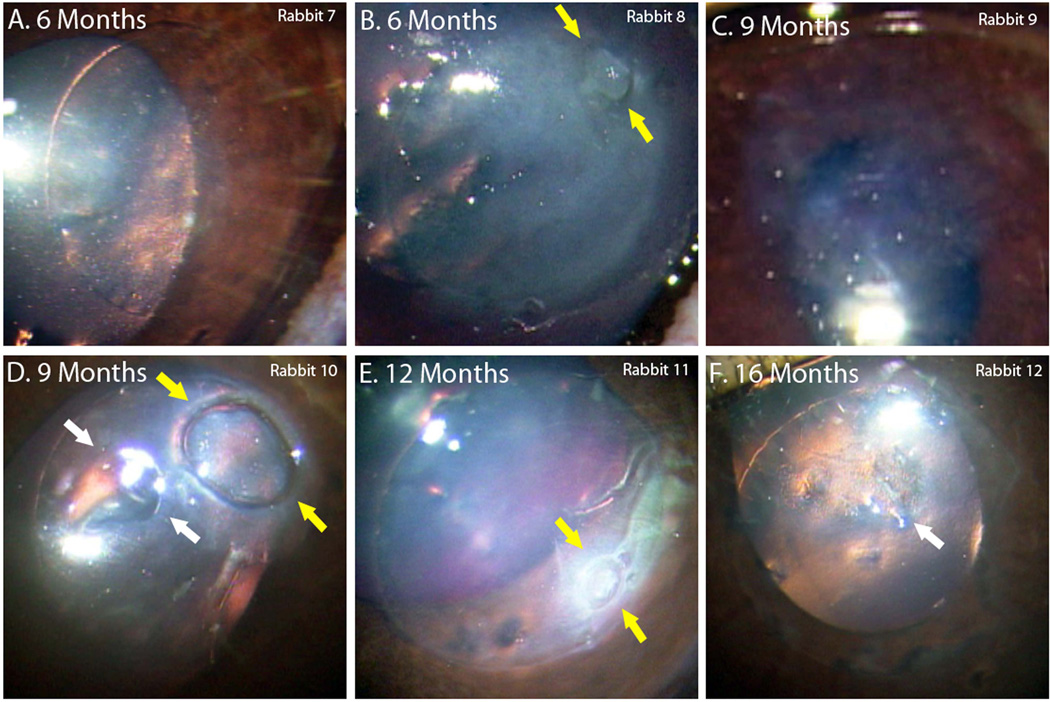
Complications were more common with longer implantations. For rabbits with the implant for 6 months (Table 1, Rabbits 7–8), one remained clear and without epithelial defects (Figure 2A) but the other developed opacity across the entire cornea (Figure 2B). This rabbit was noted to have a non-healing epithelial defect (yellow arrows) which possibly became infected at 3 months and eventually scarred. There was also neovascularization noted in one quadrant of its cornea, but the implant did not extrude.
Fig. 2.
Photographs of implanted PEG/PAA hydrogels taken prior to collection of tissue. (A,B) Rabbit 7 and 8 had implantations for 6 months, however rabbit 8 developed an inflammatory response likely due to infection at 3 months and had subsequent scarring and opacification of its cornea. (C) There was extrusion of the implant 4 months from rabbit 9, leaving a light haze afterwards. (D,E) Rabbits 10 and 11 had implantations for 9 months and 12 months respectively, and both demonstrated epithelial defects (yellow arrows). (F) Rabbit 12 had clear cornea even at 16 months, though histology revealed epithelial ingrowth over part of the implant.
For the planned 9-month group (Table 1, Rabbits 9–10), one of the rabbits developed moderate haze over the implant and corneal thinning several months after implantation. This polymer eventually extruded at 4 months post-implantation. The haze at the implant region improved after extrusion (Figure 2C). The cornea of the other 9-month rabbit remained clear, but developed an epithelial defect (yellow arrows) as well as corneal thinning (white arrows) at 8 months (Figure 2D).
For the 12-month rabbit, the cornea remained clear but an epithelial defect (yellow arrows) developed as well at 11 months (Table 1 Rabbit 11, Figure 2E). Lastly one implantation was carried to 16 months (Table 1, Rabbit 12). Areas of cornea thinning developed at 14 months, but there were no epithelial defects. The areas of the thinning did not increase in size during our observation. Over time, however, cellular deposits (white arrow) were observed in these areas. Around the polymer edge, several divots were also found (Table 1, Figure 2F).
Histology
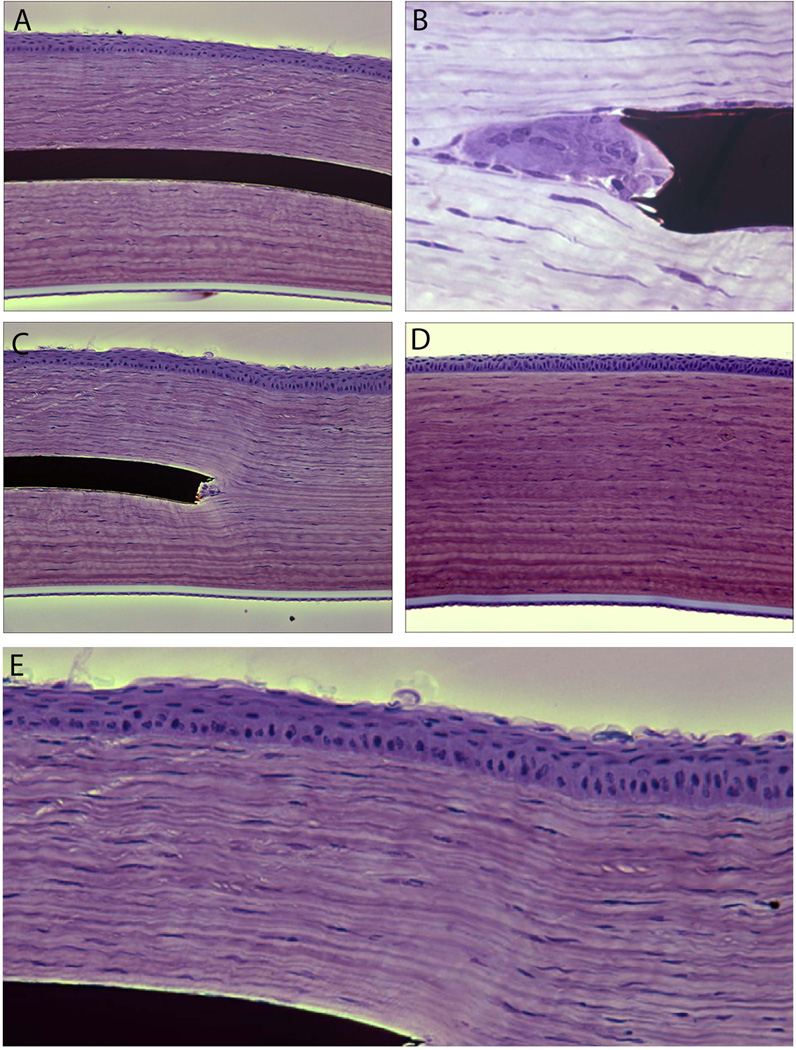
In a majority of cases we found that the implants were well tolerated with no stromal scarring (Figure 3A). We noted that cells aggregated at the edge of the polymer. These cells could be keratocytes filling in the space created by the vertical edge of the polymer (Figure 3B). Immunohistochemical staining with cytokeratin AE5 and smooth muscle α-actin antibodies showed these cells are neither corneal epithelial cells nor myofibroblasts. Stromal tissue otherwise appeared normal in clear corneas. There was no evidence of fibrous capsule formation around the implant. The epithelium over the implant appears to be one to two cell layers thinner compare to normal rabbit epithelium. In some cases, basal epithelial cells had a cuboidal morphology instead of columnar (Figure 3C and E, Table 2). Tissue appearance was otherwise similar to that of the control rabbit (Figure 3D). Examining the rabbit with the longest implantation of 16 months, we noted epithelial in-growth but otherwise clear cornea without inflammation (Figure 4).
Fig. 3.
Cornea of Rabbit 4, sacrificed at 2 months, fixed in GMA, cresyl violet stain. (A) There was no inflammatory response or capsule formation around the hydrogel implant. (B) At the edges there were cells filling the potential space created by the vertical edge of the implant. We believe these are keratocytes as immunostaining with cytokeratin AE5 and smooth muscle α-actin antibodies confirmed these cells are neither epithelial cells nor myofibroblasts. (C,E) The corneal epithelium over the implant appears to be one to two cell layers thinner compare to normal rabbit epithelium. There appears to be more cuboidal morphology of the basal epithelial cells over the implant as compared to control. (D) Control normal cornea.
Table 2.
Histological Evaluation
| Rabbit # | Length of Implantation |
Embedding Material |
Clinical Observations |
Keratocyte Hyperactivity |
Polymorphonuclear Cells |
Epithelial Thinning |
Myofibroblasts (IHC) |
Macrophages (IHC) |
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | GMA | Clear cornea | In stroma underneath the implant | None | Yes | - | - |
| 2 | 2 | GMA | Clear cornea | No | None | No | - | - |
| 3 | 2 | GMA | Clear cornea | No | None | No | - | - |
| 4 | 2 | GMA | Diffuse haze | No | None | Yes | - | - |
| 5 | 2 | Paraffin | Diffuse haze | No | None | No | None | None |
| 6 | 2 | Paraffin | Diffuse haze | Posterior to implant | None | Yes | None | None |
| 7 | 6 | Paraffin | Clear cornea | No | None | No | None | Yes |
| 8 | 6 | Paraffin | Inflammation, opacity, neovascularization, epithelial defect | Throughout the anterior cornea | Yes | Yes | At the peripheral cornea | Yes |
| 9 | 9 | GMA | Corneal thinning and extrusion | No | None | No | - | - |
| 10 | 9 | Paraffin | Epithelial defect | Posterior to implant | Yes | Yes | Posterior to implant | Yes |
| 11 | 12 | GMA | Epithelial defect | In the anterior and posterior stroma | Yes | Yes | - | - |
| 12 | 16 | Paraffin | Corneal thinning, cellular infiltrate | No | None | No | None | Yes |
| 13-control | 2 | Paraffin | Clear cornea | No | None | No | None | None |
Fig. 4.
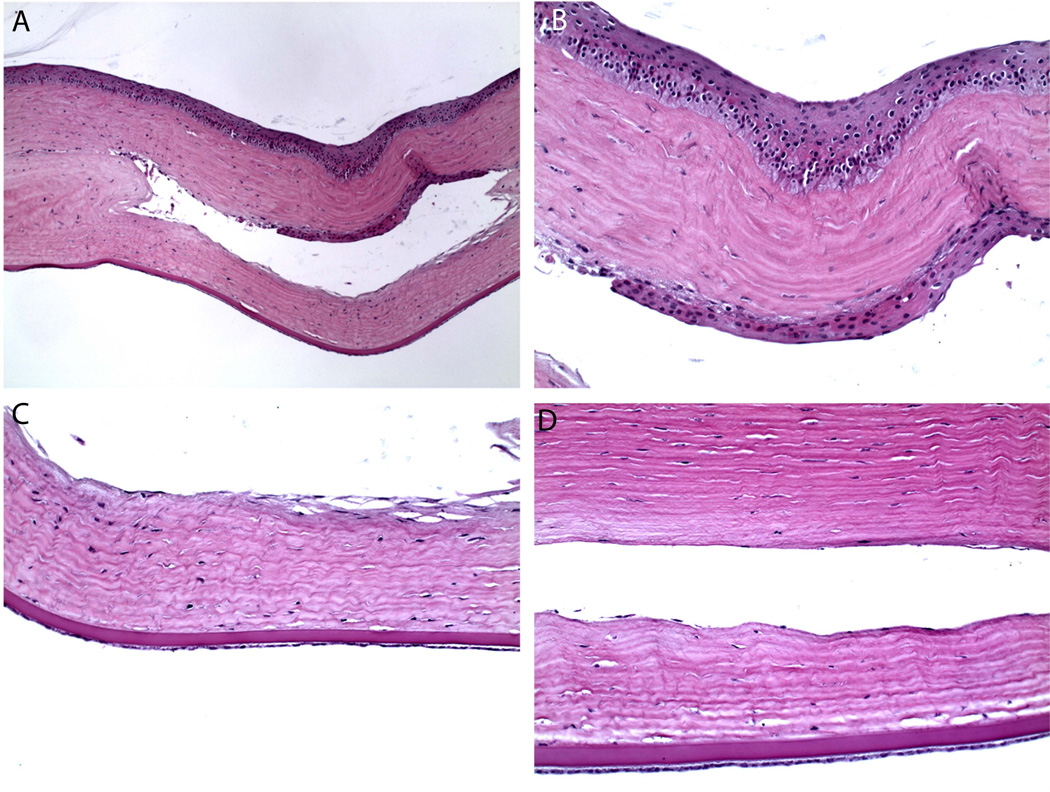
Cornea of Rabbit 12, sacrificed at 16 months post implantation, fixed in paraffin, H&E stain. Paraffin fixation results in dehydration of implant during processing and thus the implant is not visible in these sections. (A) Low magnification view of the pocket that contained implant prior to fixation in the 16 months rabbit. There is noted to be epithelial in growth along the anterior wall of the pocket. Cell type was confirmed by immunohistochemistry. (B) Higher magnification view. (C, D) There was otherwise no significant inflammatory response or capsule formation around the implant.
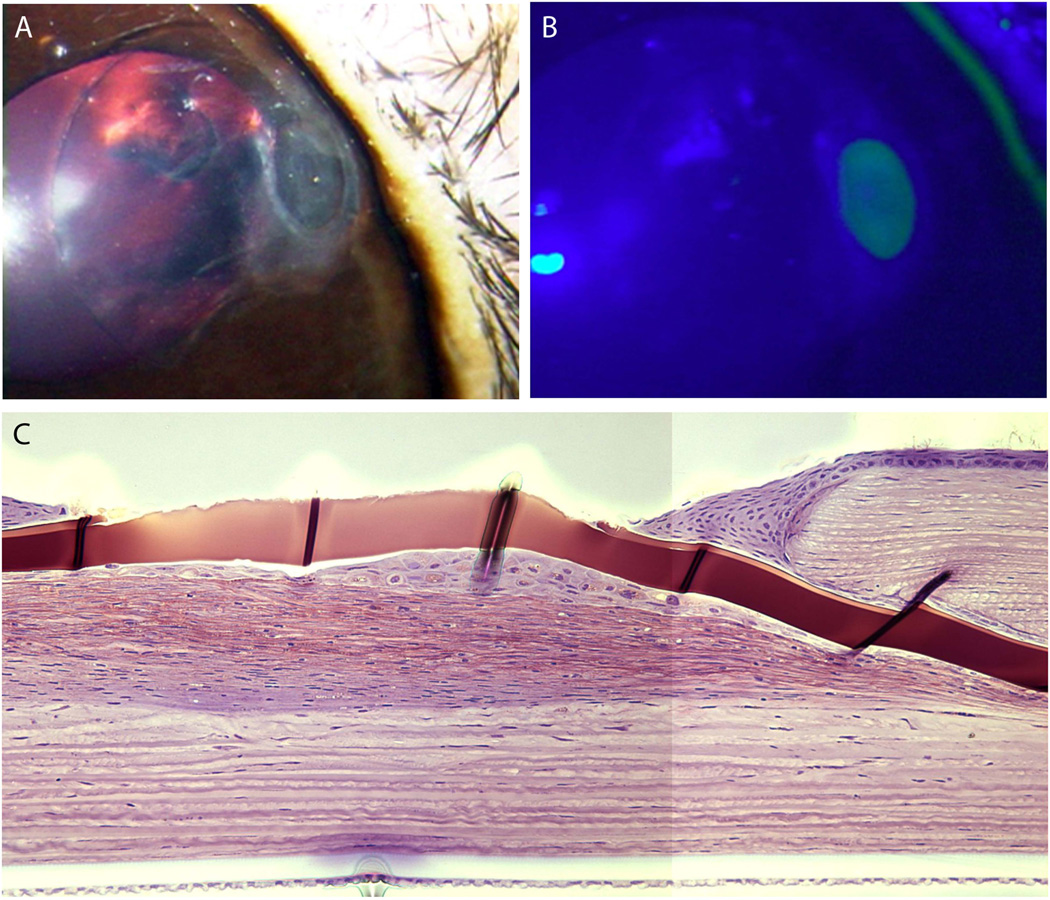
In the four cases of focal defects (Rabbits 8, 9, 10, 11), the stroma anterior to the implant began to deteriorate, exposing the hydrogel to the tear film. The hydrogel surface is hydrophilic and does not support cellular attachment. Therefore, the corneal epithelium is not expected to heal. Meanwhile, due to the ionic differences between the hydrogel and the tear film, the exposed hydrogel may swell locally. Scar tissue formed underneath the hydrogel in Rabbit 10 (Figure 5, Table 2).
Fig. 5.
(A,B) Corneal epithelial defect in rabbit 10 at 8 months, gross picture and fluorescein stain. (C) Histology of exposed implant in Rabbit 11 at 1 year (GMA, cresyl violet stain). Note epithelial cells attempting to smooth the exposed ridge as well as scar formation beneath the implant.
Immunohistochemistry
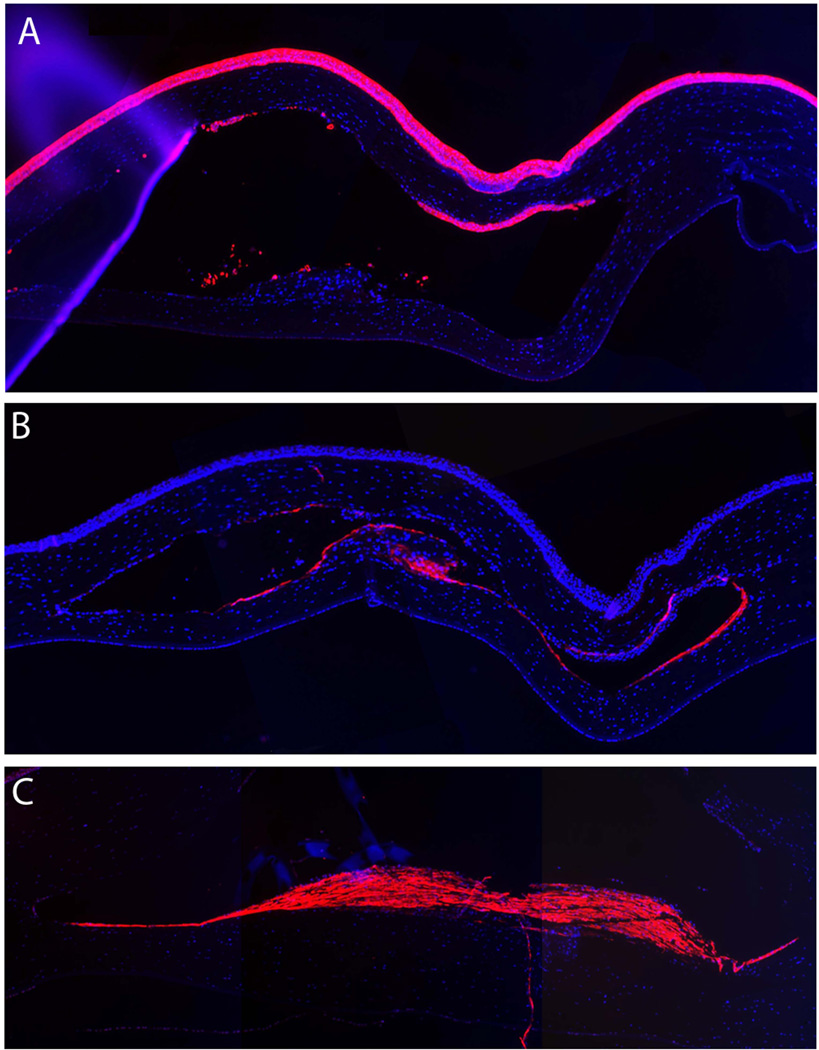
Rabbits 5, 6, 7, 8, 10, and 12 underwent paraffin fixation allowing for immunostaining. Because the required paraffin fixation dehydrates the implant during processing, only half the rabbits, being numbers 5–8, 10, and 12 were evaluated by immunohistochemistry. Myofibroblasts and macrophages were identified in the tissue surrounding the implant. No macrophages were found by immunohistochemistry in any of the 2-month rabbits. However, for animals receiving the implant 6 months and longer, macrophages were identified at the polymer edge (Rabbits 7, 8 and 12) or in the scar tissue formed posterior to the polymer in corneas with focal defects (Rabbit 10). Cytokeratin AE5 staining demonstrated epithelial in-growth in Rabbit 12 (Figure 6, Table 2)
Fig. 6.
Immunohistochemistry studies. Representative images of (A) epithelial marker stain cytokeratin AE5 in Rabbit 12, (B) macrophage marker RAM11 in Rabbit 12 and (C) myofibroblast marker α-actin in Rabbit 10.
Discussion
Implantation of a 6 mm PEG/PAA hydrogel for 16 months was tolerated in one rabbit but there was a high the rate of complications which increased with the duration of implantation. There was clear or light haze without epithelial defects in the 6 corneas with implantation for two months. However, in the rabbits that had implantations for longer, there was a high rate of development of epithelial defect and corneal thinning. One rabbit (Rabbit 9) had extrusion of the implant, and one rabbit (Rabbit 8) developed what was possibly an infection with resultant corneal opacification prior to six months. These likely occurred due to thinning leading to breakdown of the epithelial barrier. One implantation that was carried out to 16 months remained clear but developed epithelial ingrowth and corneal thinning. We note that we are limited by our small sample size in these groups. The rabbits gained weight normally and no behavioral changes were observed that suggested a toxic side effect.
Prior studies have examined this material. Tan et al. implanted a similar but smaller (4 mm diameter) PEG/PAA hydrogel in 13 rabbits for up to 6 months. While three of these 13 rabbits experienced early complications and were terminated, ten subsequent implants were well tolerated for up to 6 months [40]. Their outcomes were more favorable possibly secondary to deeper placement of the implant (750 µm depth versus our 250 µm). Their implant was also smaller (4 mm versus 6 mm). We previously reported the results of three rabbits with a 6 mm diameter implant with cornea remaining clear for up to 175 days [39]. Our current results show a higher rate of complications prior to 6 months as well as a high rate of corneal thinning and associated complications in implantations for longer than 6 months. This suggests that the long-term tolerance of this material is not as good as suggested by the data from prior studies.
All of the focal defects occurred at the periphery of the implant. The location suggested that the defect is not due to poor nutrient diffusion, because the center of the implant would receive the least amount of nutrient flow. It is possible that since the polymer edge was not tapered, the raised edge of the vertical cut might have created an uneven surface topography, making the polymer periphery more vulnerable to epithelial abrasions. Since the hydrogel itself did not support cell adhesion without further surface modification with cell adhesion proteins, the epithelium was unlikely to heal over any exposed surfaces. A tapered implant design may help prevent this occurrence and is of future interest. The cut of the microkeratome may also leave thinner peripheral areas of the corneal flap which may be more prone to lifting.
It is possible that there is the long-term degradation of PEG, the first polymer network. When an inflammatory response is elicited, macrophages infiltrate from the limbus or the tear film if there is a break in the epithelium. The edge of the implant is where macrophages migrating from the limbus first encounter the material. Macrophages release peroxides which leads to chain scission of PEG. PEG oligomers and monomers that break from the long polymer chain may elicit a cellular response from local keratocytes. The effects of macrophages on the polymer and the degree of breakdown, if any, need to be further investigated. In any case, it is crucial to control any inflammatory response. With AlphaCor, topical use of medroxyprogesterone for months postoperatively was found to be associated with fewer corneal stromal melts [42]. In this study, steroids were applied for 5 days post-implantation. It is possible with additional steroid administration, the inflammatory response could be subdued to prevent associated complications.
In creating a lamellar cut in the corneal stroma for placement of the hydrogel, perhaps a femtosecond laser may be superior to a microkeratome. The laser provides better control of flap depth. A more posterior implantation of the hydrogel may result in improved tolerance. A laser-created intrastromal pocket also minimizes the incision width to improve epithelial and stromal healing. There was difficulty centering given the 6 mm size of the implant in the 12 mm size of the pocket. A more closely matched pocket size would help keep the implant in centered position.
Ideally an artificial corneal material would allow for adhesion of corneal epithelial cells and re-innervation to allow eventual coverage by the body's own cells and healing of any exposed areas, and to reduce chances of infection. This has been achieved with recombinant human collagen grafts but does not appear possible in our material without further modification [37]. PEG/PAA hydrogel contains reactive chemical end-groups that can be activated to covalently bind proteins, including extracellular matrix proteins and growth factors. Therefore with further work an improved surface environment can be potentially created for epithelial cell adherence and growth using PEG/PAA material. Because epithelialization is not as important for deep implantations, there is also investigation into using unmodified PEG/PAA material as a deep epikeratoplasty-style inlay [40]. Lastly, we note that the Boston Keratoprosthesis also does not support epithelial attachment but still has wide clinical utility.
Conclusion
This is the first study to examine the biocompatibility of PEG/PAA for longer than six months and to provide histologic data on the outcomes. Though seven out of 12 rabbits retained clear corneas at the time of sacrifice, a majority developed complications such as haze and epithelial defects, and one implant extruded. This high rate of long-term complications pushes us to improve our material design and properties, and to define the optimal surgical implantation procedure and postoperative treatment.
Acknowledgements
This research was funded by the National Institutes of Health Grant 1 R01 EY016987 and the Singapore Eye Research Institute.
Footnotes
The authors have no disclosures of interest.
References
- 1.Peppas NA, Bures P, Leobandung W, Ichikawa H. Hydrogels in pharmaceutical formulations. European Journal of Pharmaceutics and Biopharmaceutics. 2000;50:27–46. doi: 10.1016/s0939-6411(00)00090-4. [DOI] [PubMed] [Google Scholar]
- 2.Lee KY, Mooney DJ. Hydrogels for tissue engineering. Chemical Reviews. 2001;101:1869–1879. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- 3.Qiu Y, Park K. Environment-sensitive hydrogels for drug delivery. Advanced Drug Delivery Reviews. 2001;53:321–339. doi: 10.1016/s0169-409x(01)00203-4. [DOI] [PubMed] [Google Scholar]
- 4.Hoffman AS. Hydrogels for biomedical applications. Advanced Drug Delivery Reviews. 2002;54:3–12. doi: 10.1016/s0169-409x(01)00239-3. [DOI] [PubMed] [Google Scholar]
- 5.Langer R, Tirrell DA. Designing materials for biology and medicine. Nature. 2004;428:487–492. doi: 10.1038/nature02388. [DOI] [PubMed] [Google Scholar]
- 6.Bozukova D, Pagnoulle C, Jérôme R, Jérôme C. Polymers in modern ophthalmic implants - Historical background and recent advances. Materials Science and Engineering R: Reports. 2010;69:63–83. [Google Scholar]
- 7.Nanjawade BK, Manvi FV, Manjappa AS. In situ-forming hydrogels for sustained ophthalmic drug delivery. Journal of Controlled Release. 2007;122:119–134. doi: 10.1016/j.jconrel.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 8.Bourges JL, Bloquel C, Thomas A, Froussart F, Bochot A, Azan F, Gurny R, BenEzra D, Behar-Cohen F. Intraocular implants for extended drug delivery: Therapeutic applications. Advanced Drug Delivery Reviews. 2006;58:1182–1202. doi: 10.1016/j.addr.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 9.McMahon TT, Zadnik K. Twenty-five years of contact lenses: The impact on the cornea and ophthalmic practice. Cornea. 2000;19:730–740. doi: 10.1097/00003226-200009000-00018. [DOI] [PubMed] [Google Scholar]
- 10.Myung D, Duhamel PE, Cochrane JR, Noolandi J, Ta CN, Frank CW. Development of hydrogel-based keratoprostheses: A materials perspective. Biotechnology Progress. 2008;24:735–741. doi: 10.1021/bp070476n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomaa A, Comyn O, Liu C. Keratoprostheses in clinical practice - a review. Clinical and Experimental Ophthalmology. 2010;38:211–224. doi: 10.1111/j.1442-9071.2010.02231.x. [DOI] [PubMed] [Google Scholar]
- 12.Klufas MA, Colby KA. The boston keratoprosthesis. International Ophthalmology Clinics. 2010;50:161–175. doi: 10.1097/IIO.0b013e3181e20cca. [DOI] [PubMed] [Google Scholar]
- 13.Pintucci S, Pintucci F, Cecconi M, Caiazza S. New Dacron tissue colonisable keratoprosthesis: Clinical experience. British Journal of Ophthalmology. 1995;79:825–829. doi: 10.1136/bjo.79.9.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.White JH, Gona O. "Proplast" for keratoprosthesis. Ophthalmic Surgery. 1988;19:331–333. [PubMed] [Google Scholar]
- 15.Cardona H. The Cardona keratoprosthesis: 40 Years experience. Refractive and Corneal Surgery. 1991;7:468–471. [PubMed] [Google Scholar]
- 16.Pintucci S, Pintucci F, Caiazza S, Cecconi M. The dacron felt colonizable keratoprosthesis: After 15 years. European Journal of Ophthalmology. 1996;6:125–130. doi: 10.1177/112067219600600205. [DOI] [PubMed] [Google Scholar]
- 17.Caldwell DR. The soft keratoprosthesis. Transactions of the American Ophthalmological Society. 1997;95:751–802. [PMC free article] [PubMed] [Google Scholar]
- 18.Barber JC. Keratoprosthesis: past and present. International Ophthalmology Clinics. 1988;28:103–109. doi: 10.1097/00004397-198802820-00002. [DOI] [PubMed] [Google Scholar]
- 19.Legeais JM, Renard G. A second generation of artificial cornea (Biokpro II) Biomaterials. 1998;19:1517–1522. doi: 10.1016/s0142-9612(98)00069-6. [DOI] [PubMed] [Google Scholar]
- 20.Aldave AJ, Kamal KM, Vo RC, Yu F. The Boston Type I Keratoprosthesis. Improving Outcomes and Expanding Indications. Ophthalmology. 2009;116:640–651. doi: 10.1016/j.ophtha.2008.12.058. [DOI] [PubMed] [Google Scholar]
- 21.Ament JD, Stryjewski TP, Ciolino JB, Todani A, Chodosh J, Dohlman CH. Cost-Effectiveness of the Boston Keratoprosthesis. American Journal of Ophthalmology. 2010;149 doi: 10.1016/j.ajo.2009.08.027. [DOI] [PubMed] [Google Scholar]
- 22.Chew HF, Ayres BD, Hammersmith KM, Rapuano CJ, Laibson PR, Myers JS, Jin YP, Cohen EJ. Boston keratoprosthesis outcomes and complications. Cornea. 2009;28:989–996. doi: 10.1097/ICO.0b013e3181a186dc. [DOI] [PubMed] [Google Scholar]
- 23.Cruise GM, Scharp DS, Hubbell JA. Characterization of permeability and network structure of interfacially photopolymerized poly(ethylene glycol) diacrylate hydrogels. Biomaterials. 1998;19:1287–1294. doi: 10.1016/s0142-9612(98)00025-8. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen KT, West JL. Photopolymerizable hydrogels for tissue engineering applications. Biomaterials. 2002;23:4307–4314. doi: 10.1016/s0142-9612(02)00175-8. [DOI] [PubMed] [Google Scholar]
- 25.Myung D, Farooqui N, Waters D, Schaber S, Koh W, Carrasco M, Noolandi J, Frank CW, Ta CN. Glucose-permeable interpenetrating polymer network hydrogels for corneal implant applications: A pilot study. Current Eye Research. 2008;33:29–43. doi: 10.1080/02713680701793930. [DOI] [PubMed] [Google Scholar]
- 26.Chirila TV. An overview of the development of artificial corneas with porous skirts and the use of PHEMA for such an application. Biomaterials. 2001;22:3311–3317. doi: 10.1016/s0142-9612(01)00168-5. [DOI] [PubMed] [Google Scholar]
- 27.Hicks CR, Crawford GJ, Lou X, Tan DT, Snibson GR, Sutton G, Downie N, Werner L, Chirila TV, Constable IJ. Corneal replacement using a synthetic hydrogel cornea, AlphaCor™: Device, preliminary outcomes and complications. Eye. 2003;17:385–392. doi: 10.1038/sj.eye.6700333. [DOI] [PubMed] [Google Scholar]
- 28.Hicks CR, Crawford GJ, Tan DT, Snibson GR, Sutton GL, Downie N, Gondhowiardjo TD, Lam DSC, Werner L, Apple D, Constable IJ. AlphaCor™ cases: Comparative outcomes. Cornea. 2003;22:583–590. doi: 10.1097/00003226-200310000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Hicks CR, Hamilton S. Retroprosthetic membranes in AlphaCor patients: Risk factors and prevention. Cornea. 2005;24:692–698. doi: 10.1097/01.ico.0000154380.13237.ea. [DOI] [PubMed] [Google Scholar]
- 30.Hicks CR, Crawford GJ, Dart JKG, Grabner G, Holland EJ, Stulting RD, Tan DT, Bulsara M. AlphaCor: Clinical outcomes. Cornea. 2006;25:1034–1042. doi: 10.1097/01.ico.0000229982.23334.6b. [DOI] [PubMed] [Google Scholar]
- 31.Holak SA, Holak HM, Bleckmann H. AlphaCor™ keratoprosthesis: Postoperative development of six patients. Graefe's Archive for Clinical and Experimental Ophthalmology. 2009;247:535–539. doi: 10.1007/s00417-008-0964-7. [DOI] [PubMed] [Google Scholar]
- 32.Chalam KV, Chokshi A, Agarwal S, Edward DP. Complications of AlphaCor keratoprosthesis: A clinicopathologic report. Cornea. 2007;26:1258–1260. doi: 10.1097/ICO.0b013e31813e0bd8. [DOI] [PubMed] [Google Scholar]
- 33.Hicks CR, Chirila TV, Werner L, Crawford GJ, Apple DJ, Constable IJ. Deposits in artificial corneas: Risk factors and prevention. Clinical and Experimental Ophthalmology. 2004;32:185–191. doi: 10.1111/j.1442-9071.2004.00781.x. [DOI] [PubMed] [Google Scholar]
- 34.Vijayasekaran S, V.Chirila T, Robertson TA, Lou X, Fitton JH, Hicks CR, Constable IJ. Calcification of poly(2-hydroxyethyl methacrylate) hydrogel sponges implanted in the rabbit cornea: A 3-month study. Journal of Biomaterials Science, Polymer Edition. 2000;11:599–615. doi: 10.1163/156856200743896. [DOI] [PubMed] [Google Scholar]
- 35.Fagerholm P, Lagali NS, Merrett K, Jackson WB, Munger R, Liu Y, Polarek JW, Söderqvist M, Griffith M. A biosynthetic alternative to human donor tissue for inducing corneal regeneration: 24-Month follow-up of a phase 1 clinical study. Science Translational Medicine. 2010;2 doi: 10.1126/scitranslmed.3001022. [DOI] [PubMed] [Google Scholar]
- 36.Liu W, Merrett K, Griffith M, Fagerholm P, Dravida S, Heyne B, Scaiano JC, Watsky MA, Shinozaki N, Lagali N, Munger R, Li F. Recombinant human collagen for tissue engineered corneal substitutes. Biomaterials. 2008;29:1147–1158. doi: 10.1016/j.biomaterials.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 37.Fagerholm P, Lagali NS, Merrett K, Jackson WB, Munger R, Liu Y, Polarek JW, Soderqvist M, Griffith M. A biosynthetic alternative to human donor tissue for inducing corneal regeneration: 24-month follow-up of a phase 1 clinical study. Sci Transl Med. 2010;2:46ra61. doi: 10.1126/scitranslmed.3001022. [DOI] [PubMed] [Google Scholar]
- 38.Myung D, Koh W, Bakri A, Zhang F, Marshall A, Ko J, Noolandi J, Carrasco M, Cochran JR, Frank CW, Ta CN. Design and fabrication of an artificial cornea based on a photolithographically patterned hydrogel construct. Biomedical Microdevices. 2007;9:911–922. doi: 10.1007/s10544-006-9040-4. [DOI] [PubMed] [Google Scholar]
- 39.Hartmann L, Watanabe K, Zheng LL, Kim CY, Beck SE, Huie P, Noolandi J, Cochran JR, Ta CN, Frank CW. Toward the development of an artificial cornea: improved stability of interpenetrating polymer networks. J Biomed Mater Res B Appl Biomater. 2011;98:8–17. doi: 10.1002/jbm.b.31806. [DOI] [PubMed] [Google Scholar]
- 40.Tan XW, Hartman L, Tan KP, Poh R, Myung D, Zheng LL, Waters D, Noolandi J, Beuerman RW, Frank CW, Ta CN, Tan DT, Mehta JS. In vivo biocompatibility of two PEG/PAA interpenetrating polymer networks as corneal inlays following deep stromal pocket implantation. J Mater Sci Mater Med. 2013;24:967–977. doi: 10.1007/s10856-012-4848-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Farroqui N, Myung D, Koh W, Dalal R, Carrasco MR, Noolandi J, Frank CW, Ta CN. Histological Processing of pH-Sensitive Hydrogels Used in Corneal Implant Applications. Journal of Histotechnology. 2007;30:157–163. [Google Scholar]
- 42.Hicks CR, Crawford GJ. Melting after keratoprosthesis implantation: The effects of medroxyprogesterone. Cornea. 2003;22:497–500. doi: 10.1097/00003226-200308000-00001. [DOI] [PubMed] [Google Scholar]